Introduction
Prostate cancer (PCa) is the second most frequent
malignancy found in men worldwide (1). There were 1.1 million new cases of PCa
in 2012, accounting for 15% of male cancers (2). The incidence of PCa has been increasing
worldwide in recent years, particularly in Asian countries, such as
China, Japan, Korea and Indian (3).
Statistical data indicates that PCa is the sixth leading cause of
cancer-related deaths that occur in men, with an estimated 307,000
deaths in 2015, which accounted for 6.6% of total male
cancer-associated mortality (4,5). There
are some risk factors which may lead to PCa, such as obesity,
smoking, alcohol consumption, a vasectomy and diet (6). Currently, early diagnosis and efficient
treatment remain obstacles in PCa, owing to the unspecific clinical
symptoms and complex disease pathogenesis (7). Although some advances have been made in
tumor therapeutic strategies, such as surgery, chemotherapy and
radiotherapy, the prognosis in patients with PCa remains poor
(8). In addition, majority of
patients with PCa suffer from severe pain, fractures and abnormal
urination, which seriously reduce the quality of life of the
patients (9). Therefore, it is
necessary to develop novel reliable therapeutic strategies to
improve the treatment of PCa.
MicroRNAs (miRNAs) are a group of non-coding small
RNAs, approximately 18–22 nucleotides in length, that are involved
in numerous important cell processes, such as cell proliferation,
migration, invasion, differentiation and apoptosis (10). miRNAs can directly bind the
3′-untranslated region of target genes leading to inhibition in
gene expression (11). Emerging
studies report that miRNAs serve important regulatory roles in
tumor progression making them potential therapeutic targets in
various human cancers, such as lung and liver cancer (12,13).
Notably, some aberrantly expressed miRNAs have also been detected
in PCa, such as miR-215-5p (14) and
miR-145 (15), which participate in
disease pathogenesis and are associated with the prognosis of PCa.
miR-92b-3p has been investigated in some human malignancies in
previous studies. For instance, Long et al (16) indicated that miR-92b-3p acted as a
tumor suppressor in pancreatic cancer by targeting
Gabra3-associated oncogenic pathways. Notably, a study by Ma et
al (17) found an overexpression
of miR-92b-3p in PCa cell lines, which was associated with PCa
metastasis, but this study did not investigate the clinical
significance and biological function of miR-92b-3p in PCa.
To further improve PCa therapy, the present study
aimed to detect the expression of miR-92b-3p in PCa tissues and
cell lines and evaluate the clinical significance of miR-92b-3p. In
addition, the biological function of miR-92b-3p was also
investigated using gain- and loss-of-function experiments in PCa
cells. The findings of the present study may provide a novel
biomarker to predict PCa prognosis and a potential therapeutic
target for improving the treatment of PCa.
Materials and methods
Patients and tissue collection
A total of 108 patients (average age of 66.7±9.1
years) who had been pathologically diagnosed as PCa in Shengli
Oilfield Central Hospital (Dongying, China) from June 2010 to May
2013 were enrolled in the present study. PCa tissues and adjacent
normal tissues (>2-cm from the edge of tumor) were obtained
during resection and immediately stored in liquid nitrogen at −80°C
for further use. The patients were enrolled in accordance with the
following inclusion criteria: i) The tumor tissues were
histopathologically diagnosed with PCa; ii) none of the patients
had received any antitumor therapy prior to surgery; iii) the age
range of the patients was 18–75 years; iv) had complete demographic
and clinical data; and v) signed informed consent for the use of
clinical samples and data. In addition, the following exclusion
criteria were used: i) Cases with serious heart, liver, respiratory
and kidney diseases; ii) cases with an age <18 or >75; and
iii) cases that had incomplete clinical data or had no follow-up
information. The collected PCa tissues were graded according to the
Gleason grading system (18). In
addition, the Tumor-Node-Metastasis (TNM) stage of the PCa tissues
was determined using the criteria of the American Joint Committee
on Cancer classification (19). In
order to record the survival status of the patients, a 5-year
follow-up survey was conducted, and each patient was followed up
once a month by telephone. Among the 108 patients with PCa, 59
cases received antiandrogen therapy (flutamide) after surgery and
54 patients developed androgen-independent PCa. All patients had
signed an informed consent form and the protocol of this study
received approval from the Ethics Committee of Shengli Oilfield
Central Hospital (Dongying, China; approval no. SLYTh100219).
Cell culture and transfection
Four PCa cell lines DU145, LNCaP, VCaP, 22Rv1 and
one human prostate epithelial cell line RWPE1 were purchased from
the Type Culture Collection of the Chinese Academy of Sciences. The
PCa cells were cultured in RPMI-1640 medium (BioTek China)
supplemented with 10% fetal bovine serum (FBS; Thermo Fisher
Scientific Inc.). RWPE1 cells were cultured in K-SMF medium (Gibco;
Thermo Fisher Scientific Inc.) containing 5 ng/l epidermal growth
factor (Gibco; Thermo Fisher Scientific Inc.) and 50 µg/ml bovine
pituitary extract (Invitrogen; Thermo Fisher Scientific Inc.). All
cells were maintained at 37°C in a humidified incubator with 5%
CO2.
Cell lines LNCaP and DU145 were selected to perform
the transfection experiments due to significantly higher expression
of miR-92b-3p in the two cell lines compared with the normal cells.
To regulate the expression of miR-92b-3p, 50 nM of miR-92b-3p mimic
and mimic negative control (NC), and 100 nM of miR-92b-3p inhibitor
and inhibitor NC were synthesized by Guangzhou RiboBio Co., Ltd..
The above sequences were separately transfected into PCa cells
using Lipofectamine 2000® (Invitrogen; Thermo Fisher
Scientific Inc.) at 37°C following the manufacturer's instructions.
Untransfected cells were used as controls. The sequences of
transfection vectors were as follows: miR-92b-3p Mimic
5′-UAUUGCACUUGUCCCGGCCUGU-3′; mimic NC 5′-UUCUCCGAACGUGUCACGU-3′;
miR-92b-3p inhibitor 5′-ACAGGCCGGGACAAGUGCAAUA-3′; inhibitor NC
5′-CAGUACUUUUGUGUAGUACAA-3′. After 48 h of cell transfection, the
subsequent cell experiments were performed.
RNA extraction and real-time
quantitative (RT-q)PCR
Total RNA from the PCa tissues and all cell lines
were extracted by using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific Inc.) according to the manufacturer's
instructions. cDNA synthesis was performed using the PrimeScript RT
reagent kit (Takara Bio Inc.) according to the manufacturer's
instructions. The expression of miR-92b-3p was assessed using
RT-qPCR, which was performed with the SYBR Green I Master Mix kit
(Invitrogen; Thermo Fisher Scientific Inc.) on a 7500 Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific Inc.) with
following thermocycling conditions: 95°C For 10 min and 40 cycles
of 95°C for 20 sec, 58°C for 15 sec, 72°C for 20 sec. U6 was used
as an internal control and the 2−ΔΔCq method (20) was used to calculate the final
expression level of miR-92b-3p. The sequences of primers used were
as follows: miR-92b-3p forward 5′-GCCGAGTATTGCACTTGTCC-3′,
miR-92b-3p reverse 5′-CTCAACTGGTGTCGTGGA-3′; U6 forward
5′-CTCGCTTCGGCAGCACA-3′, U6 reverse 5′-AACGCTTCACGAATTTGCGT-3′.
Cell proliferation assay
DU145 and LNCaP cells were selected to perform the
cell experiments following transfection with miR-92b-3p mimic,
miR-92b-3p inhibitor or NCs as aforementioned. After the cells grew
into a stable phase, they were seeded in 96-well plates at a
density of 5×103 cells/well and cell proliferation was
assessed using the MTT assay. The cells were incubated at 37°C for
3 days and 10 µl MTT (5 mg/ml; Sigma-Aldrich; Merck KGaA) was added
every 24 h followed by subsequent 4 h incubation at 37°C.
Subsequently, 150 µl of DMSO was added to each well and the
absorbance of cells was measured using a microplate reader at 570
nm.
Cell migration and invasion
assays
PCa cell migration and invasion abilities were
measured using Transwell chambers (Corning Inc.). Membranes were
precoated with Matrigel at 37°C for 6 h for invasion assay. Serum
free RPMI-1640 medium without any drug treatment was added to the
upper chambers and the lower chambers were filled with RPMI-1640
medium supplemented with 10% FBS. DU145 and LNCaP cells
(5×105 cells/well) were seeded in the upper chambers.
Following 48 h incubation at 37°C, the cells in the lower chambers
were stained using 0.2% crystal violet for 10 min at room
temperature and counted under an inverted light microscope (Olympus
Corporation) with a magnification of ×200.
Statistical analysis
Data in the present study was analyzed using SPSS
21.0 software (IBM Corp.) and GraphPad Prism 7.0 software (GraphPad
Software, Inc.) and were expressed as mean ± SD. Each experiment
was repeated at least three times. A Chi-square test was performed
to analyze the association between miR-92b-3p and the
clinicopathological characteristics of patients with PCa. The
differences between groups were assessed using a paired Student's
t-test or one-way ANOVA followed by a post hoc Tukey's test. The
Kaplan-Meier method was used to generate the survival curves of
patients with PCa and the differences between survival curves were
analyzed using the log-rank test. The prognostic significance of
miR-92b-3p was evaluated using Cox regression analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
miR-92b-3p is upregulated in PCa
tissues and cell lines
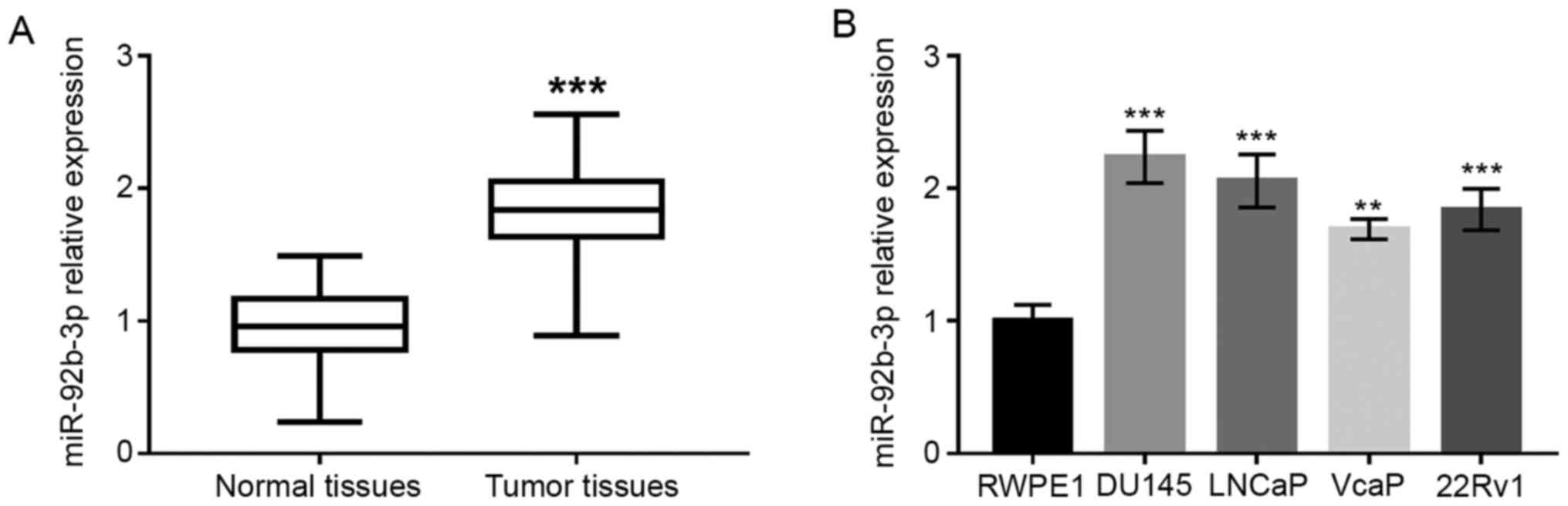
As shown in Fig. 1A,
the expression of miR-92b-3p in PCa tissues was significantly
upregulated compared to adjacent normal tissues (P<0.001).
Upregulated expression of miR-92b-3p was also found in PCa cell
lines compared with normal human prostate epithelial cells
(P<0.01 or P<0.001; Fig.
1B).
Association of miR-92b-3p with
clinicopathological characteristics of patients with PCa
Since miR-92b-3p was found to be upregulated in PCa
tissues and cells, it was hypothesized that miR-92b-3p may be
related to PCa development. Therefore, the association between
miR-92b-3p expression and clinicopathological characteristics of
patients with PCa were investigated. For this analysis, patients
with PCa were divided into a low miR-92b-3p expression group (n=50)
and high miR-92b-3p expression group (n=58) based on the mean
expression value (1.824). The data were analyzed using a Chi-square
test and presented in Table I. The
expression of miR-92b-3p was associated with prostate-specific
antigen (PSA), bone metastasis, Gleason score and TNM stage (all
P<0.05; Table I). However, no
relationship was found between miR-92b-3p and age (P>0.05;
Table I).
 | Table I.Association of miR-92b-3p expression
and clinicopathological features of 108 patients with PCa. |
Table I.
Association of miR-92b-3p expression
and clinicopathological features of 108 patients with PCa.
|
|
|
| miR-92b-3p
expression |
|
|---|
|
|
|
|
|
|
|---|
| Features | Category | Total patients | Low, n=50 | High, n=58 | P-value |
|---|
| Age, years |
|
|
|
| 0.685 |
|
| <60 | 41 | 20 | 21 |
|
|
| ≥60 | 67 | 30 | 37 |
|
| PSA, ng/ml |
|
|
|
| 0.009 |
|
| <10 | 34 | 22 | 12 |
|
|
| ≥10 | 74 | 28 | 46 |
|
| Bone metastasis |
|
|
|
| 0.033 |
|
| Negative | 55 | 31 | 24 |
|
|
| Positive | 53 | 19 | 34 |
|
| Gleason score |
|
|
|
| 0.001 |
|
| ≤7 | 59 | 36 | 23 |
|
|
| >7 | 49 | 14 | 35 |
|
| TNM stage |
|
|
|
| 0.015 |
|
| I–II | 47 | 28 | 19 |
|
|
| III–IV | 61 | 22 | 39 |
|
Relationship between miR-92b-3p and
overall survival of patients with PCa
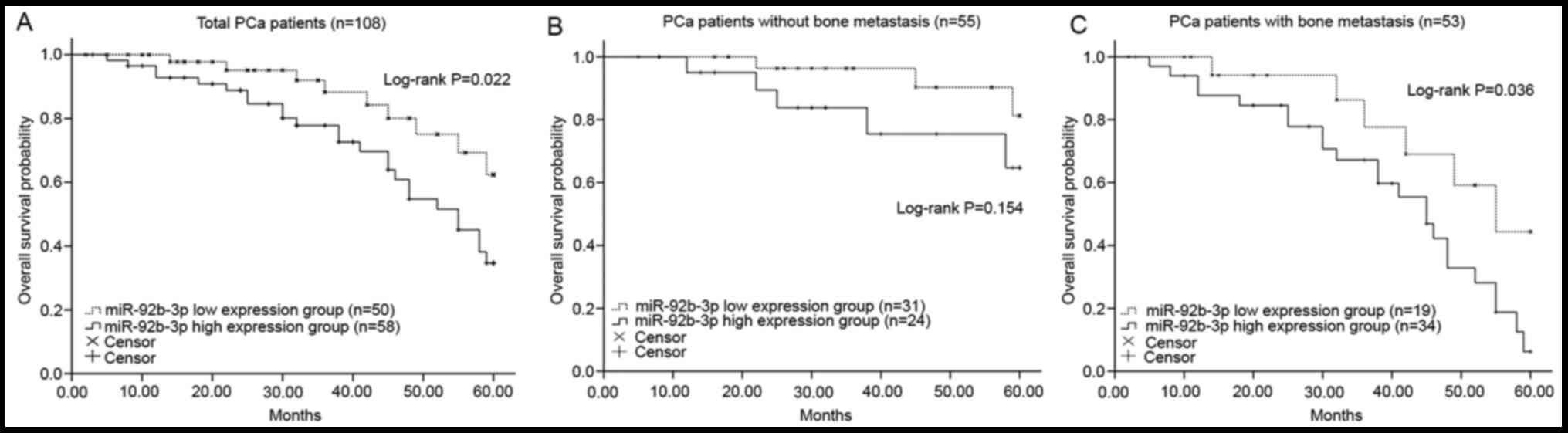
Kaplan-Meier curves revealed that patients with
higher miR-92b-3p expression had a shorter overall survival rate
compared with those with lower miR-92b-3p expression (log-rank
P=0.022; Fig. 2A). In addition, the
predictive value of miR-92b-3p for the prognosis of patients with
PCa with different status of bone metastasis was evaluated. As
shown in Fig. 2B and C, patients
with PCa with high expression of miR-92b-3p had a shorter survival
rate under both negative and positive metastasis status compared
with those patients with low levels of miR-92b-3p, and high
miR-92b-3p expression in patients with positive bone metastasis was
significantly associated with poor overall survival (log-rank
P=0.036; Fig. 2C). However, the
difference of survival time between high and low miR-92b-3p groups
was not statistically significant in patients with negative bone
metastasis (log-rank P=0.154; Fig.
2B). Furthermore, the Cox regression analysis data revealed
that the overexpression of miR-92b-3p was an independent prognostic
factor for the overall survival rate of patients with PCa [hazard
ratio (HR)=2.346; 95% confidence interval (CI)=1.185–5.276;
P-value=0.025; Table II].
 | Table II.Multivariate analysis for overall
survival of patients with PCa using the Cox regression model. |
Table II.
Multivariate analysis for overall
survival of patients with PCa using the Cox regression model.
| Variables | HR | 95% CI | P-value |
|---|
| Age | 1.431 | 0.589–3.425 | 0.421 |
| PSA | 1.715 | 0.845–3.503 | 0.140 |
| Bone
metastasis | 1.985 | 1.018–3.742 | 0.049 |
| Gleason score | 2.161 | 1.069–4.370 | 0.032 |
| TNM stage | 2.085 | 1.042–4.185 | 0.040 |
| miR-92b-3p | 2.346 | 1.185–5.276 | 0.025 |
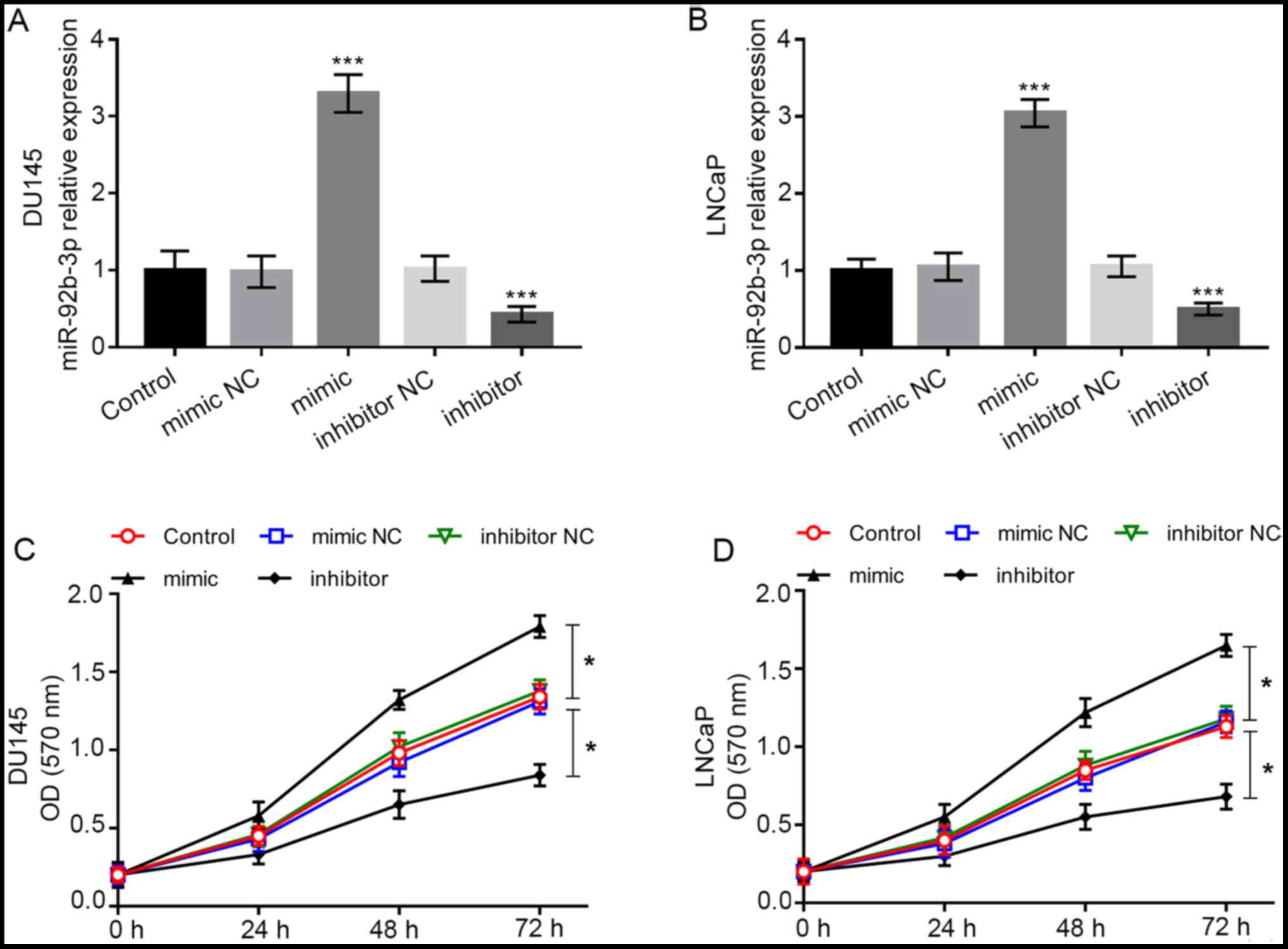
Overexpression of miR-92b-3p promotes
PCa cell proliferation
To further investigate the biological function of
miR-92b-3p in PCa cells, cell experiments were conducted. Cell
lines DU-145 and LNCaP were included in cell transfection as they
had significantly high miR-92b-3p expression compared with the
normal cell line RWPE1. The expression of miR-92b-3p was
dramatically higher in cells transfected with miR-92b-3p mimic,
while it was dramatically lower in cells transfected with
miR-92b-3p inhibitor compared with the cells in control group (all
P<0.001; Fig. 3A and B). Using
the MTT assay, it was found that the overexpression of miR-92b-3p
promoted tumor cell proliferation, whereas downregulation of
miR-92b-3p inhibited the cell proliferation of DU-145 and LNCaP
cells (all P<0.05; Fig. 3C and
D).
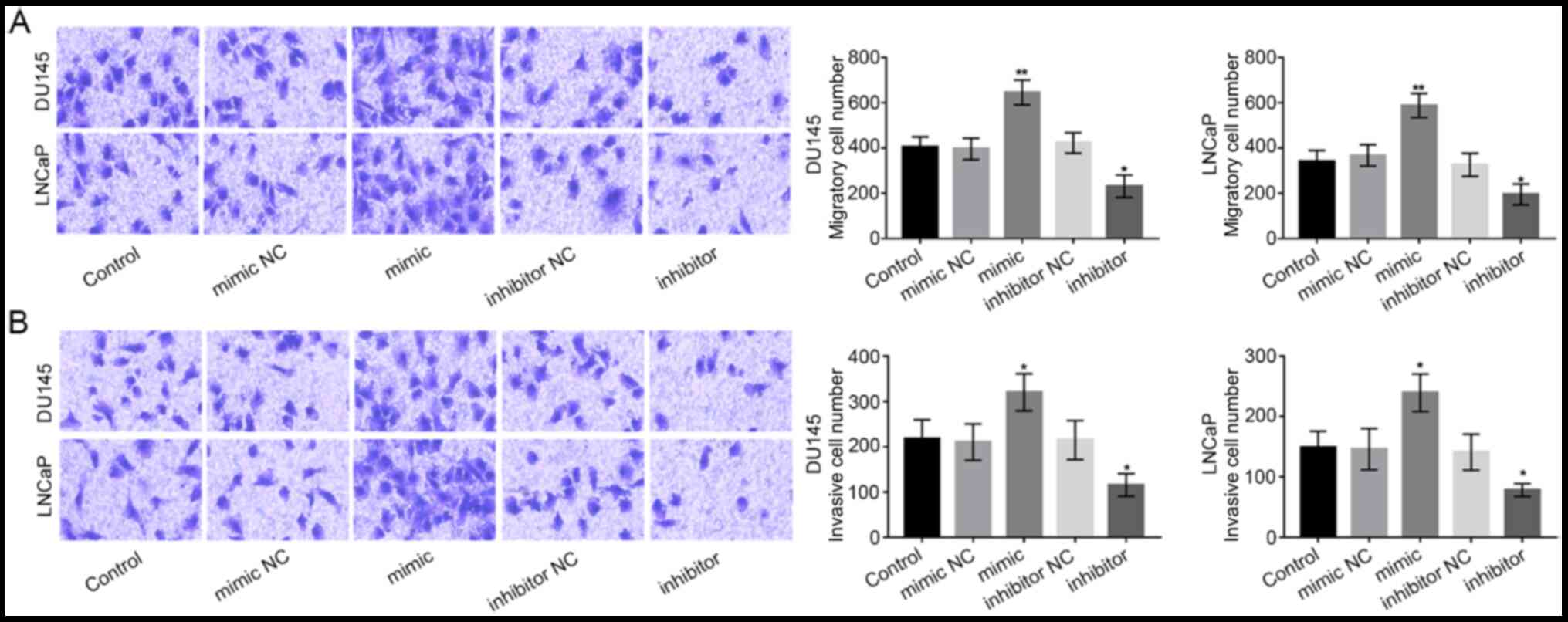
Overexpression of miR-92b-3p promotes
cell migration and invasion of PCa cells
Subsequently, Transwell chambers were used to
measure the migration and invasion abilities of DU-145 and LNCaP
cells. The migration ability of PCa cells was promoted by
miR-92b-3p expression overexpression, while was inhibited by the
downregulation of miR-92b-3p (P<0.05 or P<0.01; Fig. 4A). As for the invasion ability, we
found that the overexpression of miR-92b-3p significantly boosted
PCa cell invasion, while the downregulation of miR-92b-3p
significantly reduced PCa cell invasion (all P<0.05; Fig. 4B).
Discussion
There is growing evidence that indicates that
miRNAs, which can transmit signals and regulate intracellular gene
expression, serve important roles in tumor development and
progression (21). Additionally,
some studies have also found that miRNAs serve as tumor suppressors
or oncogenes involved in tumor progression (22,23). For
example, a study by Wang et al (24) revealed that miR-66a-3p expression in
gastric cancer tissues and cells was upregulated, which may
function as an oncogene by targeting the Hippo pathway.
Additionally, miR-506 was downregulated in cervical cancer tissues,
which further showed that miR-506 was able to suppress tumor cell
proliferation and can serve as a novel therapeutic target of
cervical cancer (25). Similarly, in
PCa tissues, some miRNAs with ectopic expression have also been
found. For instance, a study by Zhang et al (26) found that downregulation of miR-410-3p
can accelerate PCa cell apoptosis and suppress cell proliferation,
migration and epithelial-mesenchymal transition progress and exert
oncogenic functions by downregulating PTEN. A study also showed
that the downregulation of miR-375 presented better discriminating
performance compared with prostate-specific antigen indicating that
miR-375 had stronger diagnostic accuracy and can be used as a
non-invasive biomarker for PCa screening (27). The aforementioned studies
demonstrated the importance of identifying novel miRNAs that affect
tumor progression to improve the treatment of PCa.
The present study focused on the expression and
functional role of miR-92b-3p in PCa. miR-92b-3p has been
previously investigated in some cancers. For example, the
overexpression of miR-92b-3p was detected in gastric cancer
SGC-7901 cells, which inhibited SGC-7901 cell proliferation,
migration and invasion via downregulating matrix
metalloproteinase-2/9 expression and targeting homeobox D10
(28). Another study by Gong et
al (29) revealed that
miR-92b-3p inhibition prevented colorectal carcinoma cell
proliferation, invasion, and migration by upregulating F-box with
WD repeated domain-containing 7 (FBXW7). Notably, a previous study
by Ma et al has reported miR-92b-3p was deregulated in PCa
cells and this may be related with chemoresistance of tumor cells
(17). Nevertheless, the expression
of miR-92b-3p in PCa tissues and its clinical and functional role
in PCa progression remain largely elusive. In the present study,
miR-92b-3p was upregulated in PCa tissues and cell lines compared
with normal tissues and normal cells, and the expression of
miR-92b-3p was associated with PSA, bone metastasis, TNM stage and
Gleason score of patients with PCa, which suggested that miR-92b-3p
might be involved in PCa development. In addition, the recorded
5-year follow-up survival information analyzed by Kaplan-Meier
survival curves demonstrated that the patients with higher
miR-92b-3p expression had shorter overall survival rates compared
with those patients with lower miR-92b-3p levels. Besides, in
patients with PCA who had positive bone metastasis, high levels of
miR-92b-3p were also associated with shorter overall survival time
when compared to patients with low levels of miR-92b-3p. Although
the survival time in patients without bone metastasis was also
shorter when they had high expression of miR-92b-3p, but the
difference did not reach statistically significance, which may due
to the limited sample size. In addition, Cox regression analysis
further revealed that miR-92b-3p was an independent prognostic
factor for PCa. According to these findings of the present study,
miR-92b-3p may serve as a biomarker for PCa prognosis.
A number of studies have provided evidence for the
therapeutic potential of miRNAs in a wide variety of human cancers,
including PCa (30,31). The proposed functional miRNAs exert
therapeutic potential by regulating tumor cell biological
processes, such as cell proliferation, migration and invasion
(32). Thus, cell experiments were
conducted in the present study to investigate the functional role
of miR-92b-3p in PCa progression. The expression of miR-92b-3p was
regulated by miR-92b-3p mimic or inhibitor following transfection.
The MTT assay findings of the present study revealed that the
overexpression of miR-92b-3p promoted cell proliferation, migration
and invasion, while the downregulation of miR-92b-3p led to
opposite results, which suggested that miR-92b-3p may function as
an oncogene in PCa progression. The oncogenic role of miR-92b-3p
has also been demonstrated in other malignancies, such as
colorectal carcinoma and gastric cancer (28,29).
FBXW7 has been identified as a tumor suppressor in the progression
of non-small cell lung carcinoma (NSCLC), and was related with the
chemoresistance of NSCLC (33,34).
Whether miR-92b-3p could regulate FBXW7 in NSCLC is unclear, and
whether miR-92b-3p could be involved in the chemoresistance of
NSCLC through targeting FBXW7 is also uncertain.
There were some limitations to the present study.
First, the sample size was relatively small, which may limit the
accuracy of analysis results, such as the Kaplan-Meier survival
analysis for patients with PCa without bone metastasis. Second,
although the potential target genes of miR-92b-3p were identified,
the exact target of miR-92b-3p in PCa was not explored. Thus, the
results and conclusion should be confirmed and improved by further
studies with a larger study population and mechanism-related
investigations.
In conclusion, the present study found that
miR-92b-3p was upregulated in PCa tissues and cells compared with
normal controls. The overexpression of miR-92b-3p predicted poor
prognosis of patients with PCa and can be used as an independent
prognostic biomarker. Downregulation of miR-92b-3p is able to
suppress cell proliferation, migration and invasion of PCa cells.
Based on these findings, miR-92b-3p may act as a potential
therapeutic target for patients with PCa.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and material
All data analyzed during this study are included in
the published article.
Authors' contributions
GW, BC and WL conducted this study, analyzed the
clinical data and wrote the manuscript. RJ and BT performed the
cell experiments and analyzed the corresponding data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
All patients provided signed informed consent and
the present study received approval from the Ethics Committee of
Shengli Oilfield Central Hospital (Dongying, China; approval no.
SLYTh100219).
Patient consent for publication
Consent for publication was obtained from the
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Castillejos-Molina RA and
Gabilondo-Navarro FB: Prostate cancer. Salud Publica Mex.
58:279–284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pu YS, Chiang HS, Lin CC, Huang CY, Huang
KH and Chen J: Changing trends of prostate cancer in Asia. Aging
Male. 7:120–132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kimura T and Egawa S: Epidemiology of
prostate cancer in Asian countries. Int J Urol. 25:524–531. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Perdana NR, Mochtar CA, Umbas R and Hamid
AR: The risk factors of prostate cancer and its prevention: A
literature review. Acta Med Indones. 48:228–238. 2016.PubMed/NCBI
|
|
7
|
Redman JM, Gulley JL and Madan RA:
Combining immunotherapies for the treatment of prostate cancer.
Urol Oncol. 35:694–700. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gasnier A and Parvizi N: Updates on the
diagnosis and treatment of prostate cancer. Br J Radiol.
90:201701802017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Powell Gray B, Kelly L, Ahrens DP, Barry
AP, Kratschmer C, Levy M and Sullenger BA: Tunable cytotoxic
aptamer-drug conjugates for the treatment of prostate cancer. Proc
Natl Acad Sci USA. 115:4761–4766. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rupaimoole R, Calin GA, Lopez-Berestein G
and Sood AK: miRNA deregulation in cancer cells and the tumor
microenvironment. Cancer Discov. 6:235–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Markou A, Zavridou M and Lianidou ES:
miRNA-21 as a novel therapeutic target in lung cancer. Lung Cancer
(Auckl). 7:19–27. 2016.PubMed/NCBI
|
|
13
|
Wu HM and Kim SG: miRNA-324, a potential
therapeutic target for paracetamol-induced liver injury. Stem Cell
Investig. 3:672016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen JY, Xu LF, Hu HL, Wen YQ, Chen D and
Liu WH: miRNA-215-5p alleviates the metastasis of prostate cancer
by targeting PGK1. Eur Rev Med Pharmacol Sci. 24:639–646.
2020.PubMed/NCBI
|
|
15
|
Zhang X and Wu J: Prognostic role of
microRNA-145 in prostate cancer: A systems review and
meta-analysis. Prostate Int. 3:71–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Long M, Zhan M, Xu S, Yang R, Chen W,
Zhang S, Shi Y, He Q, Mohan M, Liu Q and Wang J: miR-92b-3p acts as
a tumor suppressor by targeting Gabra3 in pancreatic cancer. Mol
Cancer. 16:1672017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma H, Wang LY, Yang RH, Zhou Y, Zhou P and
Kong L: Identification of reciprocal microRNA-mRNA pairs associated
with metastatic potential disparities in human prostate cancer
cells and signaling pathway analysis. J Cell Biochem.
120:17779–17790. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Epstein JI, Egevad L, Amin MB, Delahunt B,
Srigley JR and Humphrey PA; Grading Committee, : The 2014
international society of urological pathology (ISUP) consensus
conference on gleason grading of prostatic carcinoma: Definition of
grading patterns and proposal for a new grading system. Am J Surg
Pathol. 40:244–252. 2016.PubMed/NCBI
|
|
19
|
Singletary SE, Allred C, Ashley P, Bassett
LW, Berry D, Bland KI, Borgen PI, Clark GM, Edge SB, Hayes DF, et
al: Staging system for breast cancer: Revisions for the 6th edition
of the AJCC cancer staging manual. Surg Clin North Am. 83:803–819.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao Y, Song Y, Yao L, Song G and Teng C:
Circulating microRNAs: Promising biomarkers involved in several
cancers and other diseases. DNA Cell Biol. 36:77–94. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Steinberg BA and Fang JC: Long-term
outcomes of acute heart failure: Where are we now? J Am Coll
Cardiol. 70:2487–2489. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao L, Yu H, Yi S, Peng X, Su P, Xiao Z,
Liu R, Tang A, Li X, Liu F and Shen S: The tumor suppressor
miR-138-5p targets PD-L1 in colorectal cancer. Oncotarget.
7:45370–45384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang L, Li B, Zhang L, Li Q, He Z, Zhang
X, Huang X, Xu Z, Xia Y, Zhang Q, et al: miR-664a-3p functions as
an oncogene by targeting Hippo pathway in the development of
gastric cancer. Cell Prolif. 52:e125672019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gong M, Chen C, Zhao H, Sun M and Song M:
miR-506 suppresses cervical cancer cell proliferation both in vitro
and in vivo. Neoplasma. 65:331–338. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Zhang D, Lv J, Wang S and Zhang
Q: miR-410-3p promotes prostate cancer progression via regulating
PTEN/AKT/mTOR signaling pathway. Biochem Biophys Res Commun.
503:2459–2465. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kachakova D, Mitkova A, Popov E, Popov I,
Vlahova A, Dikov T, Christova S, Mitev V, Slavov C and Kaneva R:
Combinations of serum prostate-specific antigen and plasma
expression levels of let-7c, miR-30c, miR-141, and miR-375 as
potential better diagnostic biomarkers for prostate cancer. DNA
Cell Biol. 34:189–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li C, Huo B, Wang Y and Cheng C:
Downregulation of microRNA-92b-3p suppresses proliferation,
migration, and invasion of gastric cancer SGC-7901 cells by
targeting Homeobox D10. J Cell Biochem. 120:17405–17412. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gong L, Ren M, Lv Z, Yang Y and Wang Z:
miR-92b-3p promotes colorectal carcinoma cell proliferation,
invasion, and migration by inhibiting FBXW7 in vitro and in vivo.
DNA Cell Biol. 37:501–511. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arisan ED, Rencuzogullari O, Freitas IL,
Radzali S, Keskin B, Kothari A, Warford A and Uysal-Onganer P:
Upregulated Wnt-11 and miR-21 expression trigger epithelial
mesenchymal transition in aggressive prostate cancer cells. Biology
(Basel). 9:522020.
|
|
31
|
Krebs M, Solimando AG, Kalogirou C,
Marquardt A, Frank T, Sokolakis I, Hatzichristodoulou G, Kneitz S,
Bargou R, Kübler H, et al: miR-221-3p regulates VEGFR2 expression
in high-risk prostate cancer and represents an escape mechanism
from sunitinib in vitro. J Clin Med. 9:6702020. View Article : Google Scholar
|
|
32
|
Zheng C, Guo K, Chen B, Wen Y and Xu Y:
miR-214-5p inhibits human prostate cancer proliferation and
migration through regulating CRMP5. Cancer Biomark. 26:193–202.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hidayat M, Mitsuishi Y, Takahashi F,
Tajima K, Yae T, Miyahara K, Hayakawa D, Winardi W, Ihara H,
Koinuma Y, et al: Role of FBXW7 in the quiescence of
gefitinib-resistant lung cancer stem cells in EGFR-mutant non-small
cell lung cancer. Bosn J Basic Med Sci. 19:355–367. 2019.PubMed/NCBI
|
|
34
|
Xiao G, Li Y, Wang M, Li X, Qin S, Sun X,
Liang R, Zhang B, Du N, Xu C, et al: FBXW7 suppresses
epithelial-mesenchymal transition and chemo-resistance of
non-small-cell lung cancer cells by targeting snai1 for
ubiquitin-dependent degradation. Cell Prolif. 51:e124732018.
View Article : Google Scholar : PubMed/NCBI
|