Introduction
TBC1 domain containing kinase (TBCK), also known as
hematopoietic stem and progenitor cells 302 (HSPC302) or Mammalian
Gene Collection 16169 (MGC16169), was initially discovered in
CD34+ hematopoietic stem cells. It was then reported in
the NEDO human cDNA sequencing project by researchers at the
University of Tokyo (1,2). At that time, TBCK was considered a
hypothetic protein with unknown functions. Since then, several
groups have provided mRNA and peptide data of TBCK in different
cell types. Resing et al (3)
in 2004 identified 5130 proteins in the K562 cell line with high
throughput shotgun proteomics, including two peptides of TBCK;
Tanner et al (4) in 2007 also
identified the expression of TBCK in HEK293 cell line with peptide
mass spectrometry.
It has been shown that TBCK comprised three putative
structural domains: S_TKc, TBC (Tre-2, Bub2 and Cdc16) and RHOD_Kc
(5,6). TBCK included two types of alternatively
spliced isoforms (long TBCK and short TBCK). Long TBCK comprised
all the three conserved motifs, while short TBCK only comprised TBC
and RHOD_Kc domains (Fig. 1A and B).
At present, most of the TBCK-related research focuses on the
relationship between TBCK gene mutations and neuronal development
disorders. Our previous results have demonstrated TBCK's expression
in a cell type-specific manner. The proteins produced by the two
alternative isoforms contribute to differential functions of TBCK.
In the following section, we will summarize the detailed gene
structure, protein expression of TBCK and the important roles of
TBCK in human diseases including cancers.
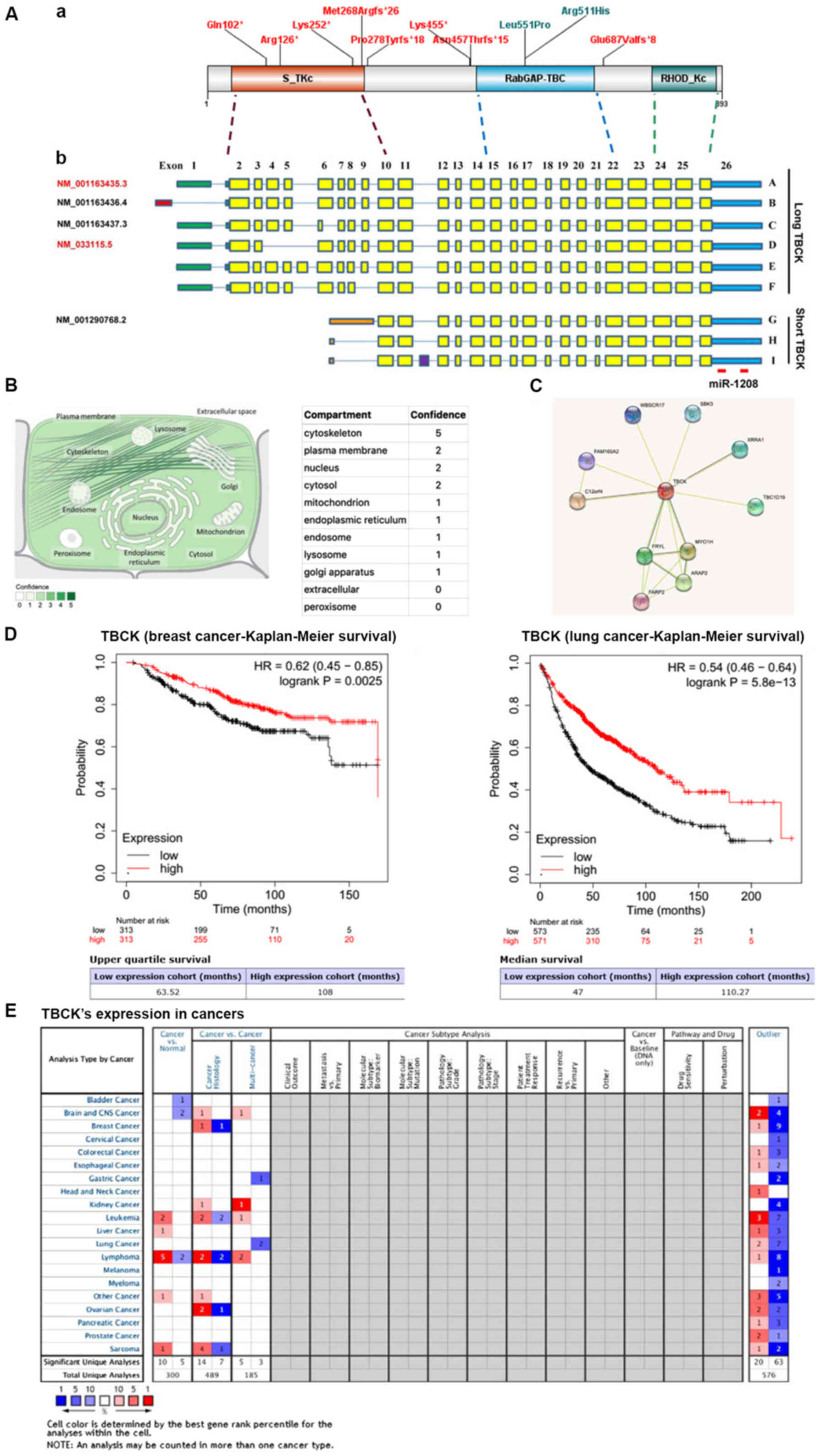 | Figure 1.Molecular structures of TBCK gene and
protein, and potential roles in predicting the prognosis of
patients with breast and lung cancer. (A) Schematic representation
of the two categories of TBCK isoforms and the matching functional
domains. (a) A diagram of TBCK including known domains and the
variants presented in this manuscript. (b) The 5′UTRs are shown as
colored bars. Transcripts A/C/D/E/F share the same 5′UTR region
(308 bp; green), while Transcript B (NM_001163436.4) has a
relatively shorter 5′UTR region (165 bp; red). Besides, for short
TBCK, they have different 5′UTR regions (orange). Differential
transcription initiations and the 3′UTRs are shown as blue bars.
Separated by introns shown by blue lines, exons are indicated by
solid rectangles with yellow for known exons and purple for a
newly-identified exon. A total of five transcripts are listed in
the NCBI database with indicated accession numbers. One currently
identified miRNA was matched to the 3′UTR region of TBCK mRNA. (B)
Subcellular locations from COMPARTMENTS. The subcellular
localizations are derived from database annotations, automatic text
mining of the biomedical literature and sequence-based predictions.
(C) Candidate interaction partners of TBCK were identified using
STRING. (D) In breast and lung cancer tissues in the Kaplan-Meier
Plotter database, high TBCK expression was associated with good
prognosis. (E) Oncomine enabled the systematic analysis of TBCK
expression in multiple cancer types. HR, hazard ratio; miR,
microRNA; TBCK, TBC1 domain containing kinase; UTR, untranslated
region. |
General properties of TBCK
Molecular features of TBCK
TBCK is commonly and abundantly expressed in
mammalian cells according to the human protein atlas (https://www.proteinatlas.org/ENSG00000145348-TBCK/summary/rna)
(7–9). Based on an in silico analysis,
homologues of TBCK in 12 Bilateria, and the conservation of these
homologues was quite high. The percentage of protein identity in
the top 7 species surpassed 90% (Table
I), indicating that TBCK might participate in important
activities.
 | Table I.Homologues of TBCK in different
species. |
Table I.
Homologues of TBCK in different
species.
| Gene Species | Identity, %
Symbol | Protein | DNA |
|---|
| H.
sapiens | TBCK |
|
|
| vs. P.
troglodytes | TBCK | 99.6 | 99.4 |
| vs. M.
mulatta | TBCK | 97.2 | 97.0 |
| vs. C.
lupus | TBCK | 96.8 | 93.2 |
| vs. B.
taurus | TBCK | 96.5 | 92.7 |
| vs. M.
musulus | Tbck | 95.4 | 89.1 |
| vs. R.
norvegicus | Tbck | 94.1 | 87.6 |
| vs. G.
gallus | TBCK | 87.2 | 79.2 |
| vs. X.
tropicalis | tbck | 81.5 | 73.9 |
| vs. D.
rerio | tbck | 76.7 | 69.2 |
| vs. D.
melanogaster | CD4041 | 47.9 | 49.3 |
| vs. A.
gambiae |
AgaP_AGAP000552 | 46.3 | 47.5 |
| vs. C.
elegans | Tbck-1 | 33.9 | 44.9 |
TBCK has three separate functional domains:
N-terminal Serine/Threonine kinase domain, central TBC domain, and
C-terminal rhodanese homology domain (RHOD). It has been reported
that the kinase domain could bind GTP and possessed protein kinase
activities (10). TBCK was
discovered to possess the ability to selectively support coupling
of active EGFR to ERK1/2 regulation (11) and positively correlated highly with
rapamycin activity, indicating that TBCK might be a
Serine/Threonine protein kinase (12). The TBC domain was identified as a
conserved sequence in the three proteins including Tre-2, BUB2p and
Cdc16p. These proteins have been proven to be functional domains of
Rab GAP, which could catalyze GTP hydrolysis of Rab GTPase via a
dual-finger mechanism (13). For
containing the conserved TBC domain, TBCK was considered to be a
member of the RabGAP family. However, a yeast two-hybrid assay
showed that TBCK had no physical interaction with any one of the 60
known Rab proteins (14). The RHOD
domain is a homologous domain of rhodanese, but little is known
about its function.
According to the NCBI Core Nucleotide and UCSC
Genome Browser database, 6 TBCK transcripts were listed. Jin and
his colleagues have provided evidence for these transcripts in 4
different cell types (A431, HeLa, HepG2 and HEK293FT) using
multiple primer sets covering the whole ORF region of TBCK.
Furthermore, three more transcripts were identified and all
isoforms were categorized as long and short types based on the mRNA
sequence. The long isoforms (6 members) contained STYKc kinase,
TBC, and RHOD domains, whereas the short isoforms (3 members)
lacked the region of STYKc kinase. These two distinctive types were
most likely products of differential transcription initiation
(Fig. 1A) (6). Although the proteins representing the
short isoforms of TBCK were not recognized in the above-mentioned
four cell types, additional bands with a similar molecular mass
were observed in HepG2 and HEK293FT cells, which were possibly
generated by alternative splicing or post-translational
modifications (6). Moreover, Chong
et al (15) demonstrated that
two major bands with molecular weights of ~101 and 71 kDa, which
represented long and short TBCK respectively, were observed in two
control fibroblast lines, and the full-length isoform was more
abundant than the short TBCK.
Distribution and interaction partners
of TBCK in mammalian cells
It has been approximately 7 years since the first
protein evidence for TBCK was raised (5). Immunofluorescence analysis for
endogenous TBCK revealed that TBCK was clearly colocalized with
γ-tubulin in addition to punctate distribution in HEK293 cells.
TBCK appeared to be not substantially colocalized with the
endoplasmic reticulum, Golgi and endosomes in both HEK293 and HeLa
cells (5). However, GFP-tagged TBCK
showed cell cycle-dependent distribution in HeLa cells. TBCK is
mainly localized in the cytoplasm during interphase, while a
portion of TBCK accumulated at the mitotic apparatus and
colocalized with centrosomes and spindle fibers as shown by the
fluorescent staining of α-tubulin. At the end of mitosis, a clear
midbody staining of TBCK was usually observed between the two
daughter cells (6). These
inconsistent results might be due to the specificity of the chosen
TBCK antibody. The TBCK antibody used in 2013 was generated by KLH
conjugated peptide (LFEDGESFGQGRDRSSLLDDT), which was located
adjacent to GAP domain and was not suitable for distinguishing the
long and short isoforms of TBCK. GFP-tagged TBCK only reflected the
distribution of long isoforms of TBCK.
Moreover, TBCK was also probably localized to plasma
membrane, nucleus, and mitochondrion according to the COMPARTMENTS
subcellular localization database (https://compartments.jensenlab.org/Entity?figures=subcell_cell_%%&knowledge=10&textmining=10&predictions=10&type1=9606&type2=−22&id1=ENSP00000273980)
(Fig. 1B) (16).
As a poor-explored protein, no evidence has been
raised for identifying the interaction partners of TBCK. Based on
the public STRING database (https://string-db.org/cgi/network.pl?taskId=K08rYosvQxGl),
several proteins exhibited higher possibility to be interaction
partners of TBCK (Fig. 1C) (17–26).
Besides, our recent research has uncovered 17 candidate proteins of
TBCK using RNAi-mediated TBCK silencing in combination with
2-DE-DIGE assays (data not shown). These candidates played
important roles in multiple activities, such as protein folding,
post-translational modification, and the cytoskeleton. These
candidates await further investigation.
TBCK and neurodevelopmental diseases
Although there is a long way to go to fully
understand the function of TBCK, recent research indicates that
TBCK plays an important role in brain development. Mutations to the
TBCK gene could cause neurological developmental disorders. Until
now, a total of 17 mutations were reported to be associated with
neurodevelopmental diseases (Fig. 1A
and Table II).
 | Table II.Characteristics of TBCK mutations
associated with neurogenetic disorders. |
Table II.
Characteristics of TBCK mutations
associated with neurogenetic disorders.
| Author, year | Disease type | Research
target | Research
approach | Variation of
TBCK | Mapping region | Mutation type | (Refs.) |
|---|
| Alazami et
al, 2015 | Neurogenetic
disorders | 143 multiplex
consanguineous families | Whole-exome
sequencing | NM_033115:
c.1708+1G>A | RabGap-TBC | Splicing
(frameshift) | (27) |
| Bhoj et al,
2016 | Syndrome of
intellectual disability and hypotonia | 13 individuals from
nine unrelated families | Whole-exome
sequencing | NM_001163435.2:
c.1897+1G>A; | RabGap-TBC | Splicing
(frameshift) | (28) |
|
|
|
|
| c.831_832insTA | NA | Insertion |
|
|
|
|
|
|
(p.Pro278Tyrfs*18) |
| (frameshift) |
|
|
|
|
|
| c.1652T>C | RabGap-TBC | Missense |
|
|
|
|
|
| (p.Leu551Pro) |
|
|
|
|
|
|
|
|
c.[2060-2A>G] | NA | Splicing |
|
|
|
|
|
|
|
| (frameshift) |
|
|
|
|
|
| c.803_806delTGAA,
p.[=];[Met268fsArg*26] | S_TKc | Frameshift |
|
|
|
|
|
| c.376C>T
(p.Arg126*) | S_TKc | Nonsense |
|
|
|
|
|
| c.1370delA | NA | Frameshift |
|
|
|
|
|
|
(p.Asn457Thrfs*15) | S_TKc | Splice |
|
|
|
|
|
| c.455+4 C>G |
| (skipping of exons
3 and 4) |
|
|
|
|
|
|
c.[(658+1_659-1)_(2059+1_2060-1) del] | S_TKc | Deletion of exons
7–22 |
|
| Chong et al,
2016 | Infantile syndromic
encephalopathy | Four unrelated
families | Whole-exome
sequencing | c.376C>T
(p.Arg126*) | S_TKc | Nonsense | (15) |
|
|
|
|
| c.1363A>T | RabGap-TBC | Nonsense |
|
|
|
|
|
| [p.Lys455*] |
|
|
|
|
|
|
|
| c.1532G>A | RabGap-TBC | Missense |
|
|
|
|
|
| (p.Arg511His) |
|
|
|
| Guerreiro et
al, 2016 | Recessive
developmental disorder | A family with 3
siblings affected by a severe, yet viable, congenital disorder | Whole-genome
genotyping and whole-exome sequencing | NM_033115:
c.614_617del: p.205_206del | S_TKc | Frameshift | (29) |
| Mandel et
al, 2017 | TBCK-related
intellectual disability syndrome | Two siblings born
to an Arab-Moslem family living in northern Israel | Whole-exome
sequencing | NM_001163435.2:
c.1854delT | RabGap-TBC | Frameshift | (30) |
| Ortiz-Gonzalez
et al, 2018 |
TBCK-encephalo-neuronopathy | Children (n=8) of
Puerto Rican (Boricua) descent affected with homozygous TBCK
p.R126X mutations | Whole-exome
sequencing | c.376C>T
(p.Arg126*) | S_TKc | Nonsense | (37) |
| Zapata-Aldana et
al, 2019 | TBCK-infantile
hypotonia | A family with two
siblings who presented with a novel TBCK mutation | Whole-exome
sequencing | NM_001163435.2:
c.753dup; p.(Lys252*) | S_TKc | Nonsense | (31) |
| Beck-Wodl et
al, 2018 | New type of
neuronal ceroid lipofuscinosis | Two siblings born
in 1972 and 1974 suffering from the disease | Sanger
sequencing/whole exome sequencing | NM_001163435.2:
c.304C >T, p.(Gln102*) | S_TKc | Nonsense | (32) |
| Sumathipala et
al, 2019 | TBCK
encephalo-neuropathy | A family with two
siblings who presented with a novel TBCK mutation | Whole genome
sequencing |
p.Glu687Valfs*8 | NA | Splicing
(frameshift) | (33) |
| Tsang et al,
2020 | Pediatric-onset
mitochondrial diseases | A family with two
siblings who presented with a novel TBCK mutation | Whole genome
sequencing | c.976dupT,
p.(Tyr326Leufs*10) | NA | Missense | (35) |
|
|
| 66 patients with
pre-biopsy MDC scores of 3–8 were recruited | Whole-exome
sequencing | c.478G>T,
p.(Glu160*) | S_TKc | Nonsense |
|
| Saredi et
al, 2020 | Muscle disease and
severe psychomotor delay | Two sisters
diagnosed with muscle disease and severe psychomotor delay | Whole-exome
sequencing | c.535_554del,
p.(Leu179ArgfsTer10) | S_TKc | Missense | (34) |
| Hartley et
al, 2018 | Inherited
peripheral neuropathies | A cohort of 50
families affected individuals with a molecularly undiagnosed IPN
features | Whole genome
sequencing | c.1652T>C
(p.Leu551Pro) | RabGap-TBC | Missense | (36) |
Most of the mutations were nonsense mutations,
generating premature stop codons. After categorization, it can be
found that 73.3% (11/15) of mutations were located in the region
containing the first two domains, affecting the translation of
full-length TBCK. It is worth noting that the two missense
mutations happened in the RabGap domain, indicating that the RabGap
domain might be the most important functional unit for proper brain
development. However, the underlying molecular mechanisms still
remain unknown.
Alazami et al (27) identified 69 genes related to
neurogenic diseases through whole exome sequencing of 143 multiplex
consanguineous families, of which an insertion mutation at 1709ntin
the TBCK coding region was verified to cause a frameshift and
further influence disease progression. This insertion was also
detected in 13 individuals from nine unrelated families, likely
being pathogenic variants of TBCK. Eight other mutations of the
TBCK gene were reported to be the main cause of mental retardation
and hypotonic syndrome, and L-type leucine-mediated activation of
the mTOR signaling pathway helped alleviate related symptoms
(28). In the meantime, another
group verified two novel mutations of TBCK genes (c.1363A>T
[p.Lys455*] and c.1532G>A [p.Arg511His) via whole-exome
sequencing of infants with encephalopathy in 4 unrelated families,
of which the former mutation would induce a stop codon and lead to
the deletion of long TBCK, while the latter mutation was located in
the TBC conserved domain and might affect the RabGap activity of
TBCK (15). Unlike the overgrowth of
the brain caused by mTOR pathway disorders, a gradually decrease of
the brain volume of infants with encephalopathy would be caused by
TBCK deficiency (29). Furthermore,
six more mutations (either resulting in nonsense or frameshift)
affecting the TBCK expression were reported by six different groups
(Table II) (30–35).
It should be noted that four common mutations have
been reported in different patients from at least two different
groups (Table II): c.1897+1G>A
(27,28); c.1652T>C (28,36);
c.803_806delTGAA (28,29); and c.376C>T (15,28,37). All
of the mutations would ablate the expression of full-length TBCK
and cause TBCK-related developmental and neurological diseases.
However, TBCK function has been poorly explored. Previous research
shows that TBCK played a role in cell growth and actin organization
by enhancing the signaling pathways of mammalian target of
rapamycin (mTOR), presumably at a transcriptional or
post-transcriptional level (5).
Besides, TBCK deficiency would disturb activation of the mTOR
complex 1 (mTORC1), thus, affecting the autophagy process and
further leading to autophagosomal-lysosomal dysfunction (37). Nevertheless, does TBCK directly or
indirectly affect the mTOR signaling pathway? Which domain
contributes the most? What are the binding partners of TBCK? These
open questions await further studies.
TBCK and tumorigenesis
TBCK was expressed universally in almost all human
tissues, except a relatively low expression in heart, brain,
skeletal muscle, and peripheral blood leukocytes (data not shown).
Besides, TBCK was proven to be down-regulated in 55.6% of paired
gastric carcinoma and 75.0% pair-matched esophageal carcinomas.
Overexpression of TBCK in HeLa cells could remarkably inhibit cell
growth and arrest cells at S phase, which was indicative of tumor
suppressive function (6). After
analyzing the clinical information collected from TCGA, the
five-year survival rates for patients with high-level TBCK was
significantly higher than that of patients with low-level TBCK in
renal cancer (P=3.20E-4) and pancreatic cancer (P=3.67E-2). A
similar phenomenon could be found in breast cancer (P=2.50E-3) and
lung cancer (P=4.70E-13) (Fig. 1D)
using Kaplan-Meier Plotter database [https://kmplot.com/analysis/] (38). This implied that TBCK might also
possess the potential to be a viable prognosis marker for treatment
of some cancer types.
However, TBCK might also exhibit tumor-promoting
functions in certain cancer types. Based on the Oncomine database
(Fig. 1E), it has been shown that
TBCK exhibit the tumor-promoting functions in leukemia, lymphoma,
liver cancer and sarcoma, in addition, individual experiments also
validated that exhibit the functions in squamous cell carcinoma and
renal cancers (11,39). In a human kinase mapping study using
the entire kinome siRNA library targeting over 600 related genes,
TBCK-specific RNAi decreased the phosphorylation of ERK1/2 and
increased the phosphorylation of STAT3. TBCK was further proven to
selectively support coupling of active EGFR to ERK1/2 regulation
(11). A very recent study on TBCK
showed that TBCK was a direct target of miR-1208, and that the
miR-1208/TBCK interaction had an important role in the regulation
of apoptosis, as well as in the enhancement of cisplatin or TRAIL
sensitivities in renal cancer cells (39). However, how TBCK involved in both
tumor promotion and inhibition in different cancer types is
unknown, and requires further investigation.
Future prospects of TBCK research
Previous results indicated that the eukaryotic
protein kinase comprised of 12 essential conserved subdomains to
maintain its kinase activity (40).
Due to its lack of two important motifs (GXGXXG motif and VAIK
motif) responsible for ATP binding, and the replacement of those
motifs with mutated HRD motif that was essential for catalytic
activity, TBCK was considered a pseudokinase (5,41,42).
Itis implied that TBCK might phosphorylate the ERK1/2 protein
(11). However, further direct
kinase assays should be performed to clarify whether TBCK has
kinase activity or not. The positive answer also provides evidence
for differential functions between long and short isoforms of TBCK
(6).
TBC domain-containing proteins usually function as a
RabGap (Rab GTPase-activating protein) to negatively regulate Rab
functions through accelerating GTP hydrolysis via a ‘dual-finger’
mechanism (13,43–45).
Although the crystallographic structure of TBCK has not been
reported, Chong et al (15)
generated a homology model of the TBC1 domain of TBCK using
DeepView and the SwissModel server (46) and uncovered a structural impact of
the disease-causing amino acid substitution (p.Arg511His). Other
reports also demonstrated that the TBC domain in TBCK included the
key conserved amino acid residues required for RabGAP activity in
functional RabGAPs (5,13). However, the direct substrate of TBCK
was failed to be identified in a systematical screening for target
Rabs (60 Rab proteins) of TBC domain-containing proteins (40
proteins including TBCK) based on their Rab-binding activity
(14). Thus, it is necessary to
carry out critical experiments to figure out the physiological
target of TBCK, which shall provide direct evidence for the RabGap
activity of TBCK.
In addition, previous studies have shown that TBCK
mutations would cause neurogenetic disorders. The mTOR pathway and
mTOR-mediated autophagy might play important roles in such
processes (28,37). However, it is still unclear how TBCK
affects the mTOR signaling pathway, what the interacting proteins
of TBCK are and whether there are other pathways involved remains
unsolved. Our current research has uncovered 17 candidate proteins
of TBCK using RNAi-mediated TBCK silencing in combination with
2-DE-DIGE assays. These candidates played important roles in
multiple activities, such as protein folding, post-translational
modification, and the cytoskeleton etc. (data not shown). More work
on the mechanism of action needs to be completed in order to
clearly clarify the roles of TBCK in neurogenetic disorders and
tumor development.
Concluding remarks
An important finding for TBCK function in recent
years was that TBCK functions as a candidate RabGAP. Deleterious
mutations of TBCK would ablate the function of TBCK and cause
severe infantile syndromic encephalopathy or other neurogenetic
disorders. These mutation sites were found in the whole exons
covering three conserved domains. Abnormal function of TBCK would
destroy the mTOR signaling pathway and its mTOR-mediated autophagy
process, which was considered the major cause of TBCK-related
neurogenetic disorders. In addition, two types of TBCK isoforms
were verified, and the kinase domain might account for the
functional differences among TBCK isoforms. Limited research also
suggested that the distribution of TBCK was cell cycle-dependent,
and the role of TBCK in tumors was cell line-dependent. Overall,
the function of TBCK is poorly explored and awaits further
investigation.
Acknowledgements
The authors would like to thank Mr. Alexander
Caradori (Center for Genetics and Pharmacology, Roswell Park
Comprehensive Cancer Cente) for providing constructive suggestions
and manuscript proofreading.
Funding
This work was supported by funding from the National
Science Foundation of China (grant no. 81101490 to GL).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW and GL wrote the manuscript and performed article
revision. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yudate HT, Suwa M, Irie R, Matsui H,
Nishikawa T, Nakamura Y, Yamaguchi D, Peng ZZ, Yamamoto T, Nagai K,
et al: HUNT: Launch of a full-length cDNA database from the Helix
Research Institute. Nucleic Acids Res. 29:185–188. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ota T, Suzuki Y, Nishikawa T, Otsuki T,
Sugiyama T, Irie R, Wakamatsu A, Hayashi K, Sato H, Nagai K, et al:
Complete sequencing and characterization of 21,243 full-length
human cDNAs. Nat Genet. 36:40–45. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Resing KA, Meyer-Arendt K, Mendoza AM,
Aveline-Wolf LD, Jonscher KR, Pierce KG, Old WM, Cheung HT, Russell
S, Wattawa JL, et al: Improving reproducibility and sensitivity in
identifying human proteins by shotgun proteomics. Anal Chem.
76:3556–3568. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tanner S, Shen Z, Ng J, Florea L, Guigó R,
Briggs SP and Bafna V: Improving gene annotation using peptide mass
spectrometry. Genome Res. 17:231–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Y, Yan X and Zhou T: TBCK influences
cell proliferation, cell size and mTOR signaling pathway. PLoS One.
8:e713492013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu J, Li Q, Li Y, Lin J, Yang D, Zhu G,
Wang L, He D, Lu G and Zeng C: A long type of TBCK is a novel
cytoplasmic and mitotic apparatus-associated protein likely
suppressing cell proliferation. J Genet Genomics. 41:69–72. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uhlén M, Zhang C, Lee S, Sjöstedt E,
Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z, Edfors F, et
al: A pathology atlas of the human cancer transcriptome. Science.
357:eaan25072017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thul PJ, Akesson L, Wiking M, Mahdessian
D, Geladaki A, Blal HA, Alm T, Asplund A, Björk L, Breckels LM, et
al: A subcellular map of the human proteome. Science.
356:eaal33212017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Uhlén M, Fagerberg L, Hallström BM,
Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C,
Sjöstedt E, Asplund A, et al: Proteomics. Tissue-based map of the
human proteome. Science. 347:12604192015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Manning G, Whyte DB, Martinez R, Hunter T
and Sudarsanam S: The protein kinase complement of the human
genome. Science. 298:1912–1934. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Komurov K, Padron D, Cheng T, Roth M,
Rosenblatt KP and White MA: Comprehensive mapping of the human
kinome to epidermal growth factor receptor signaling. J Biol Chem.
285:21134–21142. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen G, Yang N, Wang X, Zheng SY, Chen Y,
Tong LJ, Li YX, Meng LH and Ding J: Identification of p27/KIP1
expression level as a candidate biomarker of response to rapalogs
therapy in human cancer. J Mol Med (Berl). 88:941–952. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pan X, Eathiraj S, Munson M and Lambright
DG: TBC-domain GAPs for Rab GTPases accelerate GTP hydrolysis by a
dual-finger mechanism. Nature. 442:303–306. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Itoh T, Satoh M, Kanno E and Fukuda M:
Screening for target Rabs of TBC (Tre-2/Bub2/Cdc16)
domain-containing proteins based on their Rab-binding activity.
Genes Cells. 11:1023–1037. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chong JX, Caputo V, Phelps IG, Stella L,
Worgan L, Dempsey JC, Nguyen A, Leuzzi V, Webster R, Pizzuti A, et
al University of Washington Center for Mendelian Genomics, :
Recessive inactivating mutations in TBCK, encoding a rab
GTPase-activating protein, cause severe infantile syndromic
encephalopathy. Am J Hum Genet. 98:772–781. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Binder JX, Pletscher-Frankild S, Tsafou K,
Stolte C, ODonoghue SI, Schneider R and Jensen LJ: COMPARTMENTS:
Unification and visualization of protein subcellular localization
evidence. Database (Oxford). Feb 25–2014.(Epub ahead of print).
doi: 10.1093/database/bau012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Szklarczyk D, Gable AL, Lyon D, Junge A,
Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork
P, et al: STRING v11: Protein-protein association networks with
increased coverage, supporting functional discovery in genome-wide
experimental datasets. Nucleic Acids Res. 47:D607–D613. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szklarczyk D, Morris JH, Cook H, Kuhn M,
Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al:
The STRING database in 2017: Quality-controlled protein-protein
association networks, made broadly accessible. Nucleic Acids Res.
45:D362–D368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Franceschini A, Szklarczyk D, Frankild S,
Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering
C, et al: STRING v9.1: Protein-protein interaction networks, with
increased coverage and integration. Nucleic Acids Res.
41:D808–D815. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Szklarczyk D, Franceschini A, Kuhn M,
Simonovic M, Roth A, Minguez P, Doerks T, Stark M, Muller J, Bork
P, et al: The STRING database in 2011: Functional interaction
networks of proteins, globally integrated and scored. Nucleic Acids
Res. 39:D561–D568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jensen LJ, Kuhn M, Stark M, Chaffron S,
Creevey C, Muller J, Doerks T, Julien P, Roth A, Simonovic M, et
al: STRING 8 - a global view on proteins and their functional
interactions in 630 organisms. Nucleic Acids Res. 37:D412–D416.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
von Mering C, Jensen LJ, Kuhn M, Chaffron
S, Doerks T, Krüger B, Snel B and Bork P: STRING 7 - recent
developments in the integration and prediction of protein
interactions. Nucleic Acids Res. 35:D358–D362. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
von Mering C, Jensen LJ, Snel B, Hooper
SD, Krupp M, Foglierini M, Jouffre N, Huynen MA and Bork P: STRING:
Known and predicted protein-protein associations, integrated and
transferred across organisms. Nucleic Acids Res. 33:D433–D437.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
von Mering C, Huynen M, Jaeggi D, Schmidt
S, Bork P and Snel B: STRING: A database of predicted functional
associations between proteins. Nucleic Acids Res. 31:258–261. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Snel B, Lehmann G, Bork P and Huynen MA:
STRING: A web-server to retrieve and display the repeatedly
occurring neighbourhood of a gene. Nucleic Acids Res. 28:3442–3444.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alazami AM, Patel N, Shamseldin HE, Anazi
S, Al-Dosari MS, Alzahrani F, Hijazi H, Alshammari M, Aldahmesh MA,
Salih MA, et al: Accelerating novel candidate gene discovery in
neurogenetic disorders via whole-exome sequencing of prescreened
multiplex consanguineous families. Cell Rep. 10:148–161. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bhoj EJ, Li D, Harr M, Edvardson S,
Elpeleg O, Chisholm E, Juusola J, Douglas G, Guillen Sacoto MJ,
Siquier-Pernet K, et al: Mutations in TBCK, encoding
TBC1-domain-containing kinase, lead to a recognizable syndrome of
intellectual disability and hypotonia. Am J Hum Genet. 98:782–788.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guerreiro RJ, Brown R, Dian D, de Goede C,
Bras J and Mole SE: Mutation of TBCK causes a rare recessive
developmental disorder. Neurol Genet. 2:e762016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mandel H, Khayat M, Chervinsky E, Elpeleg
O and Shalev S: TBCK-related intellectual disability syndrome: Case
study of two patients. Am J Med Genet A. 173:491–494. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zapata-Aldana E, Kim DD, Remtulla S,
Prasad C, Nguyen CT and Campbell C: Further delineation of TBCK -
Infantile hypotonia with psychomotor retardation and characteristic
facies type 3. Eur J Med Genet. 62:273–277. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Beck-Wödl S, Harzer K, Sturm M, Buchert R,
Riess O, Mennel HD, Latta E, Pagenstecher A and Keber U: Homozygous
TBC1 domain-containing kinase (TBCK) mutation causes a novel
lysosomal storage disease - a new type of neuronal ceroid
lipofuscinosis (CLN15)? Acta Neuropathol Commun. 6:1452018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sumathipala D, Strømme P, Gilissen C,
Corominas J, Frengen E and Misceo D: TBCK encephaloneuropathy with
abnormal lysosomal storage: Use of a structural variant
bioinformatics pipeline on whole-genome sequencing data unravels a
20-year-old clinical mystery. Pediatr Neurol. 96:74–75. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Saredi S, Cauley ES, Ruggieri A, Spivey
TM, Ardissone A, Mora M, Moroni I and Manzini MC: Myopathic changes
associated with psychomotor delay and seizures caused by a novel
homozygous mutation in TBCK. Muscle Nerve. 62:266–271. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tsang MH, Kwong AK, Chan KL, Fung JL, Yu
MH, Mak CC, Yeung KS, Rodenburg RJ, Smeitink JA, Chan R, et al:
Delineation of molecular findings by whole-exome sequencing for
suspected cases of paediatric-onset mitochondrial diseases in the
Southern Chinese population. Hum Genomics. 14:282020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hartley T, Wagner JD, Warman-Chardon J,
Tétreault M, Brady L, Baker S, Tarnopolsky M, Bourque PR,
Parboosingh JS, Smith C, et al FORGE Canada Consortium; Care4Rare
Canada Consortium, : Whole-exome sequencing is a valuable
diagnostic tool for inherited peripheral neuropathies: Outcomes
from a cohort of 50 families. Clin Genet. 93:301–309. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ortiz-González XR, Tintos-Hernández JA,
Keller K, Li X, Foley AR, Bharucha-Goebel DX, Kessler SK, Yum SW,
Crino PB, He M, et al: Homozygous boricua TBCK mutation causes
neurodegeneration and aberrant autophagy. Ann Neurol. 83:153–165.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nagy Á, Lánczky A, Menyhárt O and Győrffy
B: Validation of miRNA prognostic power in hepatocellular carcinoma
using expression data of independent datasets. Sci Rep. 8:1–9.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim EA, Jang JH, Sung EG, Song IH, Kim JY
and Lee TJ: MiR-1208 increases the sensitivity to cisplatin by
targeting TBCK in renal cancer cells. Int J Mol Sci. 20:35402019.
View Article : Google Scholar
|
|
40
|
Hanks SK and Hunter T: Protein kinases 6.
The eukaryotic protein kinase superfamily: Kinase (catalytic)
domain structure and classification. FASEB J. 9:576–596. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Boudeau J, Miranda-Saavedra D, Barton GJ
and Alessi DR: Emerging roles of pseudokinases. Trends Cell Biol.
16:443–452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Scheeff ED, Eswaran J, Bunkoczi G, Knapp S
and Manning G: Structure of the pseudokinase VRK3 reveals a
degraded catalytic site, a highly conserved kinase fold, and a
putative regulatory binding site. Structure. 17:128–138. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Barr F and Lambright DG: Rab GEFs and
GAPs. Curr Opin Cell Biol. 22:461–470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gavriljuk K, Gazdag EM, Itzen A, Kötting
C, Goody RS and Gerwert K: Catalytic mechanism of a mammalian
Rab·RabGAP complex in atomic detail. Proc Natl Acad Sci USA.
109:21348–21353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cherfils J and Zeghouf M: Regulation of
small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 93:269–309.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Biasini M, Bienert S, Waterhouse A, Arnold
K, Studer G, Schmidt T, Kiefer F, Gallo Cassarino T, Bertoni M,
Bordoli L, et al: SWISS-MODEL: Modelling protein tertiary and
quaternary structure using evolutionary information. Nucleic Acids
Res. 42:W252–W258. 2014. View Article : Google Scholar : PubMed/NCBI
|















