Introduction
Until several years ago, immunotherapy in the breast
cancer (BC) field was considered as the treatment with anti-HER2
agents such as trastuzumab in combination with conventional
chemotherapy. Although trastuzumab has dramatically changed the
treatment for HER2-type BCs, non-HER2 BCs currently have no
immune-mediated therapies.
Recently, inhibition of interactions between
programmed death 1 (PD-1) on activated T cells and PD-Ligand 1
(PD-L1) on antigen-presenting cells, including tumor cells (TCs),
using anti-PD-1 or anti-PD-L1 antibodies has been shown to induce
robust and durable responses in several cancer types including
melanoma, bladder cancer, and non-small cell lung cancer (NSCLC)
(1–3). BC has been generally considered low
immunogenic due to low mutational load, unlike melanoma and NSCLC
that harbor high mutational loads (4). Nevertheless, tumor-infiltrating
lymphocytes (TILs) are often present around and/or within breast
tumors and are a prognostic factor in early BC based on large
prospective clinical trials of adjuvant therapies (5–7).
Considering that TILs represent the presence of immune responses
against tumors, immune checkpoint inhibitors (ICIs) for BC seemed
to be a novel strategy.
In BC, treatments using ICIs monotherapy or in
combination with chemotherapy have been primarily investigated for
triple-negative (TN) BCs owing to higher immunogenicity derived
from genomic instability and a higher mutational load of TNBCs than
other subtypes (8,9). Interestingly, although monotherapy of
atezolizumab (anti-PD-L1 antibody, Roche) for metastatic TNBC
patients showed low response rate, overall survival (OS) of
patients with PD-L1+ tumors showed durable responses
(2-year OS 25% and 3-year OS 21%), which is a great benefit of ICIs
(9). Recently, a phase III trial
reported that co-treatment with atezolizumab and nab-paclitaxel
improved progression-free survival and overall survival in
metastatic TN patients with PD-L1+ tumors as compared to
nab-paclitaxel treatment alone (10), indicating that immunotherapy
modulating the PD-1/PD-L1 axis is a promising treatment for TNBCs.
However, considering that most BC tumors are not TNBCs, the
development of novel effective immunotherapy regardless of subtype
and identification of new biomarkers are required.
The classification based on TILs levels and PD-L1
status is considered useful for identifying immune phenotypes of
tumors and to indicate the need for immunotherapy (11). TILs indicate the presence of host
immune reactions against tumors. PD-L1 can be upregulated in immune
cells (ICs) and TCs by inflammatory cytokines including interferon
(IFN)-γ produced by TILs. Therefore,
TIL+PD-L1+ tumors indicate the presence of
adaptive immune resistance, and the blockade of PD-1/PD-L1
interaction by ICIs is an effective strategy for immunotherapy.
However, TIL−PD-L1− tumors indicate immune
ignorance, implying the occurrence of fewer antitumor responses. A
recent clinical trial showed that the benefit of atezolizumab was
significantly associated with the high proportion of TILs with
upregulation of PD-L1 in ICs from metastatic TN patients (9,12),
supporting that this concept can be useful. However, this
classification has not been fully applied to BC.
Both antitumor and tumorigenic responses
simultaneously occur in the tumor microenvironment (TME). For
example, CD8+ T cells prevent tumor progression by
eliminating immunogenic TCs. In contrast, CD4+ T helper
2 (TH2) cells and forkhead box (FOX) P3+ T
regulatory cells (Tregs) contribute to tumor progression and
immunosuppression. Notably, tumor-associated macrophages (TAMs),
differentiated from circulating monocytes, enhance tumor
progression and distant metastasis by promoting TC invasion,
migration, and angiogenesis (13).
In particular, TAMs potentially suppress the recruitment of T cells
which attack tumor cells and upregulate immune checkpoint proteins
including PD-L1 and regulate the secretion of inhibitory cytokines
including TGF-β or IL-10 (14).
Furthermore, TAMs reportedly inhibit the efficacy of ICIs through
several mechanisms (15–17). Macrophages are often investigated
within the binary M1 (anti-tumorigenic)-M2 (pro-tumorigenic)
polarization system. While CD68 is frequently used as a
pan-macrophage marker for both M1 and M2 macrophages (18), CD204, a class A scavenger receptor,
has been used as a novel M2-macrophage marker. A high density of
CD204+ macrophages is associated with poor prognosis in
several cancer types (19–21). These data suggest that TAMs play
critical roles to modulate cancer progression. Here, the question
remains what the proportion of suppressive subsets, such as TAMs
and FOXP3+ Tregs, are present in BCs, particularly in
tumors with high PD-L1 expression and high levels of TILs.
The aims of this study were as follows: i) To
classify breast tumors into four groups based on the level of TILs
and PD-L1 status regardless of subtype; and ii) to identify the
presence of suppressive immune subsets, including TAMs and
FOXP3+ Tregs, in primary BCs to assess potential novel
immunotherapeutic targets for breast cancer.
Patients and methods
Patients
Seventy-three patients with invasive BC, who
underwent surgery for stage I to III tumors from January to
November 2017 at Mie University Hospital (Tsu, Japan), were
included in this study. Patients with ductal carcinomas in
situ (DCIS), de novo stage IV tumors, tumors who
received neoadjuvant chemotherapy, and had recurrent tumors were
excluded. Table I shows the clinical
and pathological characteristics of the patients enrolled in this
study.
 | Table I.Characteristics of patients
(n=73). |
Table I.
Characteristics of patients
(n=73).
|
Characteristics | Value |
|---|
| Median age, years
(range) | 57 (26–82) |
| T factor, n
(T1/T2/T3/T4) | 39/32/1/1 |
| N factor, n
(N−/N+) | 42/31 |
| Clinical stage
(I/II/III) | 29/35/9 |
| Subtype, n
(HR+HER2−/HR+HER2+/HR−HER2+/TN) | 45/6/3/19 |
| Histological grade,
n (1/2/3) | 25/23/25 |
| Ki-67 index, n
(≤20/>20%) | 30/43 |
| Histology, n
(invasive ductal carcinoma/othersa) | 63/10 |
This study was conducted in accordance with ethical
principles, including the Helsinki declaration, and was approved by
the Institutional Review Board of Mie University Hospital (No.
3155). The requirement for written informed consent was waived
owing to the retrospective nature of the study.
Histological evaluation
All patients received surgical intervention for
primary breast tumors. All specimens were formalin-fixed,
paraffin-embedded, and cut into 4-µm-thick sections for hematoxylin
and eosin (H&E) staining. Histological grades (HGs) were
assigned based on the Nottingham system (22), using surgical specimens.
TILs evaluation
H&E-stained slides were reviewed by two
pathologists in accordance with the criteria of the International
TILs Working Group 2014 (23).
Briefly, lymphoplasmacytic infiltration was evaluated in the
stromal area around the invasive tumor, and the average of several
tumor areas was determined, excluding the lymphocyte infiltration
around DCIS and normal lobules (Fig. 1A
and B). TILs were analyzed as a continuous parameter and
categorized into two groups using 10% as a cutoff value, where
<10% stromal TILs were defined as the low TILs group, and
>10% was the high TILs group.
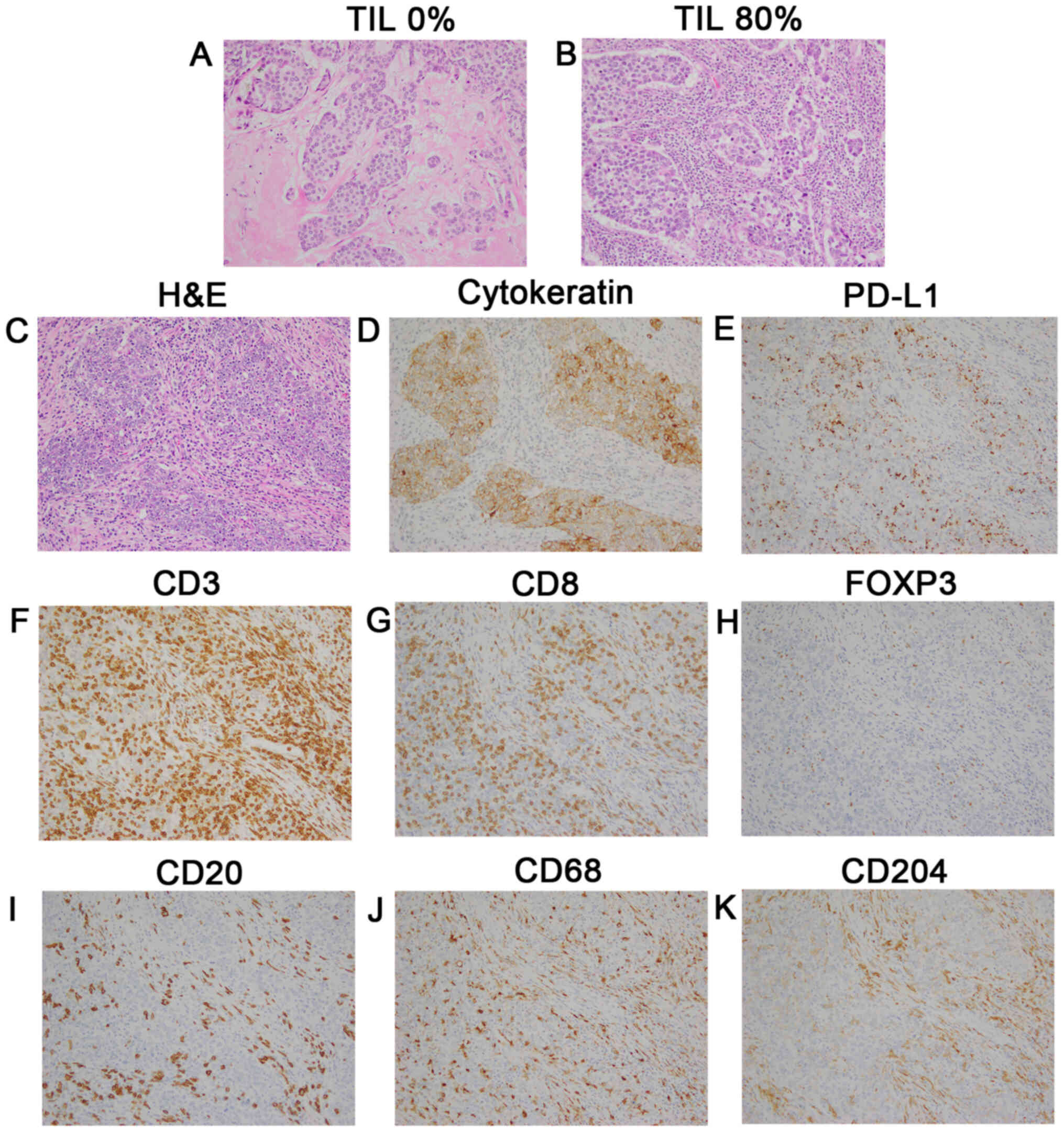 | Figure 1.Representative images of TILs and
immune subsets evaluated by IHC staining. Magnification, ×200. (A)
TILs, 0%; and (B) TILs, 80%. TMA samples of a triple-negative tumor
(stage II; HG 3; Ki-67 80%) according to (C) H&E staining, (D)
cytokeratin, (E) PD-L1 expression (3+ on TCs and 2+ on ICs), (F)
CD3+, (G) CD8+, (H) FOXP3+, (I)
CD20+, (J) CD68+ macrophages, (K)
CD204+ macrophages. TILs, tumor-infiltrating
lymphocytes; IHC, immunohistochemical; TCs, tumor cells; ICs,
immune cells. |
Immunohistochemistry (IHC)
analysis
Estrogen receptor (ER) and progesterone receptor
(PgR) positivity were evaluated against a cutoff of 1%, and HER2
overexpression was evaluated in accordance with the American
Society of Clinical Oncology/College of American Pathologists 2013
guidelines. Fluorescence in situ hybridization for assessing
HER2 amplification was performed whenever equivocal results (2+)
were rendered. Ki-67 expression was assessed via IHC, using the
MIB-1 monoclonal antibody (Table
SI). In the present study, the cutoff of Ki-67 was 20%, which
was the median value in our institute.
IHC was performed using tissue microarrays (TMAs).
TMAs were constructed from tumor blocks of surgical specimens,
using 2.0-mm (diameter) tumor cores from selected blocks. These
cores were assembled in a TMA format, and the paraffin-embedded TMA
blocks were then sectioned at 4-µm thickness and subjected to IHC
analysis.
Primary antibodies targeting CD3+,
CD8+, FOXP3+, CD20+,
CD68+, and CD204+ cells for IHC and the IHC
procedures are described in the supplementary data (Table SI). All IHC staining was performed
using an automatic immunostainer (BenchMark XT; Ventana Medical
Systems, Tucson, AZ, USA).
Evaluation of PD-L1 expression
The PD-L1 was stained using SP142 (Table SI). The PD-L1 status was evaluated
based on the tumor area proportion occupied by PD-L1-expressing
tumor-infiltrating immune cells (%IC) at any intensity or the
percentage of PD-L1-expressing tumor cells (%TC) at any intensity
(Fig. 1C-E). According to the result
of the clinical trial that used SP142 for TNBCs (10), PD-L1 expression was assessed against
a cutoff of 1%.
Evaluation of immune cells
Stained slides were digitized by a slide scanner at
a magnification of ×200 and 2–3 independent areas with the highest
abundance of ICs. All stained cells at both the stroma and tumor
nests, regardless of intensity, were evaluated using ImageJ
software (National Institutes of Health). Tumors were divided into
high or low groups depending on the mean value of the stained area
in each subset.
Statistical analysis
Continuous data were analyzed using the Mann-Whitney
U or Kruskal-Wallis test followed by Dunn's multiple
comparison test. Non-continuous data were compared using Fisher's
exact test (two-sided), and P<0.05 indicated statistically
significant differences. Correlations between the TIL frequency and
the Ki-67 index were evaluated using the Pearson correlation
coefficient. Statistical analyses were performed using Prism 7
software (GraphPad Software, Inc.).
Results
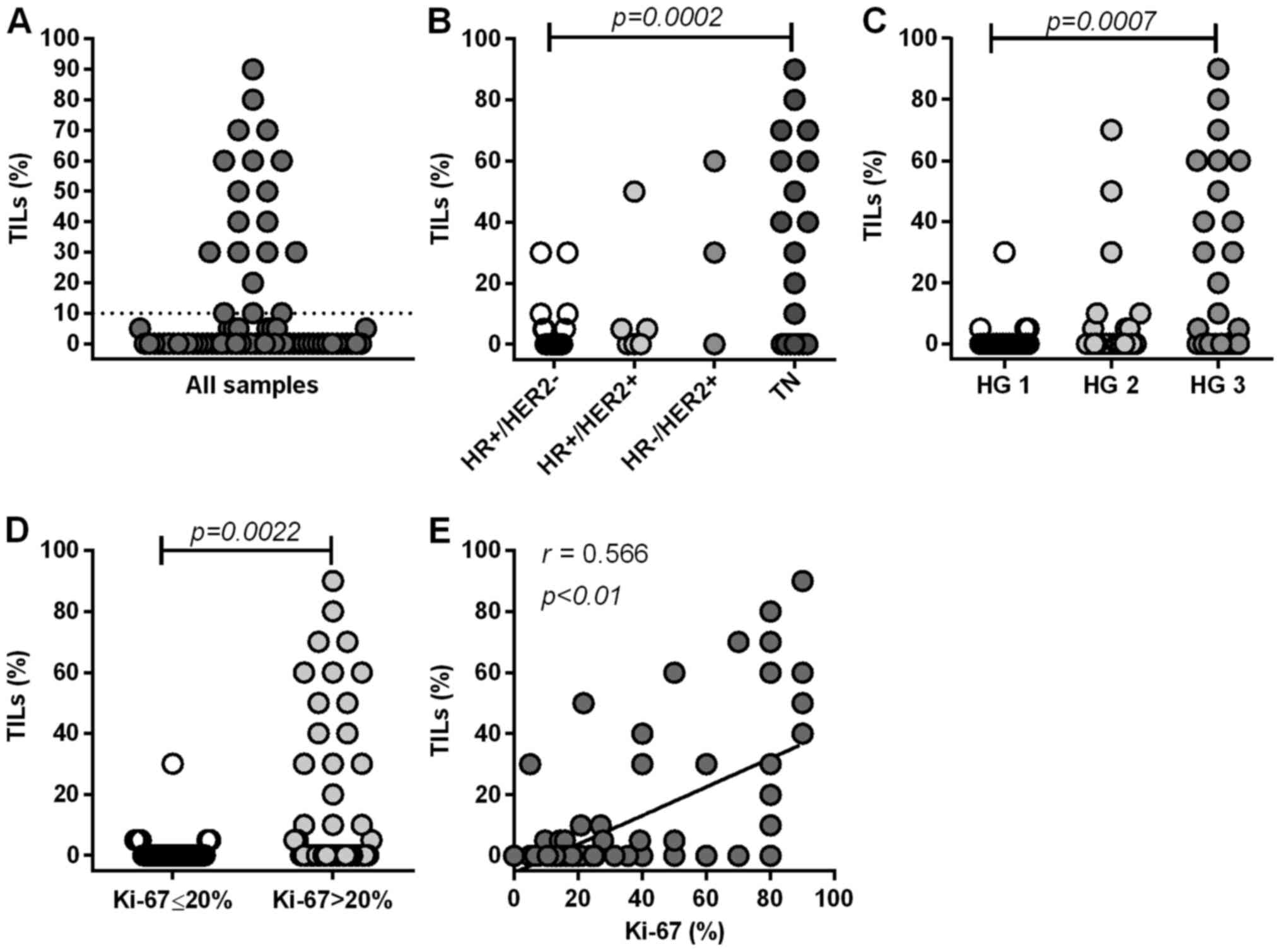
Distribution of TILs in primary
BCs
First, we examined the frequency of TILs in 73
primary breast tumors, of which 12% (9/73) harbored >50% TILs,
14% (10/73) harbored 10% to 50% TILs, and 74% (54/73) harbored
<10% TILs (Fig. 2A). TILs were
significantly more abundant in TN tumors than in
HR+HER2− tumors (P=0.0002) (Fig. 2B). Furthermore, TIL levels were
significantly higher in tumors with a high HG than in tumors with a
low HG (HG3 vs. HG1, P=0.0007) (Fig.
2C), and in more proliferative tumors (Ki-67 >20%) than in
less proliferative tumors (Ki-67 ≤20%) (P=0.0022) (Fig. 2D). TILs levels significantly
correlated with the Ki-67 index (P<0.0001) (Fig. 2E). These results suggest that the
frequency of TILs is highly associated with aggressive features of
primary tumors. Our results are concurrent with previous findings
(6,24). For further analysis, we categorized
tumors into two groups (high or low) in accordance with the
frequency of TILs (10% cutoff) (Fig.
2A).
PD-L1 expression on TCs and ICs in
primary BCs
We analyzed PD-L1 expression in both TCs and ICs
(Table II). For all subtypes, 16.4%
(12/73) of tumors were positive for PD-L1 in TCs, while 30.1%
(22/73) were positive in ICs. Among HR+HER2−
tumors, 6.7% (3/45) and 15.6% (7/45) were positive for PD-L1 in TCs
and ICs, respectively. Among HR+HER2+tumors,
0% (0/6) and 33.3% (2/6) were positive for PD-L1 in TCs and ICs,
respectively. Among HR−HER2+ tumors, 33.3%
(1/3) and 66.7% (2/3) tumors were positive for PD-L1 in TCs and
ICs, respectively. In contrast, among TN tumors, 42.1% (8/19) and
57.9% (11/19) of tumors were positive for PD-L1 in TCs and ICs,
respectively. PD-L1 expression levels tended to be higher in ICs
than in TCs, and higher in the TN type than in the
HR+HER2− type, concurrent with previous
reports (25). Furthermore, PD-L1
expression in both TCs and ICs was significantly associated with
high HG (HG 3 vs. HG1-2) (TC: P=0.0002; IC: P=0.0001), high Ki-67
index (Ki-67 >20%) (TC: P=0.0215; IC: P=0.0037), and high
frequency of TILs (TC: P<0.0001; IC: P<0.0001) (Table III).
 | Table II.PD-L1 expression in TCs and ICs. |
Table II.
PD-L1 expression in TCs and ICs.
|
|
| PD-L1 expression in
TCs | PD-L1 expression in
ICs |
|---|
|
|
|
|
|
|---|
| Subtype | No. | Negative, n
(%) |
Positivea, n (%) | Negative, n
(%) |
Positivea, n (%) |
|---|
|
HR+HER2− | 45 | 42 (93.3) | 3 (6.7) | 38 (84.4) | 7 (15.6) |
|
HR+HER2+ | 6 | 6 (100.0) | 0 (0) | 4 (66.7) | 2 (33.3) |
|
HR−HER2+ | 3 | 2 (66.7) | 1 (33.3) | 1 (33.3) | 2 (66.7) |
| TN | 19 | 11 (57.9) | 8 (42.1) | 8 (42.1) | 11 (57.9) |
| All | 73 | 61 (83.6) | 12 (16.4) | 51 (69.9) | 22 (30.1) |
 | Table III.Association between PD-L1 expression
in TCs/ICs and clinicopathological parameters. |
Table III.
Association between PD-L1 expression
in TCs/ICs and clinicopathological parameters.
|
|
| PD-L1 in TCs | PD-L1 in ICs |
|---|
|
|
|
|
|
|---|
| Variables | No. | Negative, n | Positive,
na |
P-valueb | Negative, n | Positive, n |
P-valueb |
|---|
| HG |
|
1-2 | 48 | 46 | 2 | 0.0002 | 41 | 7 | 0.0001 |
| 3 | 25 | 15 | 10 |
| 10 | 15 |
|
| Ki-67, % |
|
≤20 | 30 | 29 | 1 | 0.0218 | 26 | 3 | 0.0037 |
|
>20 | 43 | 33 | 10 |
| 25 | 19 |
|
| TILs, % |
|
<10 | 54 | 51 | 2 | <0.0001 | 48 | 6 | <0.0001 |
|
≥10 | 19 | 10 | 10 |
| 3 | 16 |
|
Classification of tumors based on TILs
and PD-L1 status in primary BCs
Based on the TILs and PD-L1 status, we categorized
73 primary BCs into four groups (Table
IV). As shown in Table II, we
considered the PD-L1 status in ICs as the entire PD-L1 status in
tumor sites. Among all tumors examined, 21.9% (16/73) of tumors
were classified as TIL+PD-L1+, and 65.7%
(48/73) were classified as TIL−PD-L1−.
TIL+PD-L1− group and
TIL−PD-L1+ group was 4.1% (3/73) and 8.3%
(6/73), respectively. Among TIL+PD-L1+ group,
18.7% (3/16), 0% (0/16), 12.5% (2/16), and 68.7% (11/16) were
HR+HER2−, HR+HER2+,
HR−HER+ and TN type, respectively. In
contrast, among TIL−PD-L1− group, 77.1%
(37/48), 6.3% (3/48), 2.0% (1/48), and 14.6% (7/48) were
HR+HER2−, HR+HER2+,
HR−HER2+ and TN types, respectively.
Conversely, 82.2% (37/45) of HR+HER2− tumors
were TIL−PD-L1−, while 57.9% (11/19) of TN
tumors were TIL+PD-L1+ tumors.
 | Table IV.Classification of primary breast
cancer based on TIL levels and PD-L1 status. |
Table IV.
Classification of primary breast
cancer based on TIL levels and PD-L1 status.
|
| Classification of
BCs, n (%) |
|---|
|
|
|
|---|
| Subtype | TIL+aPD-L1+b |
TIL−PD-L1− |
TIL+PD-L1− |
TIL−PD-L1+ |
|---|
|
HR+HER2− | 3 (18.7) | 37 (77.1) | 1 (33.3) | 4 (66.7) |
|
HR+HER2+ | 0 (0) | 3 (6.3) | 1 (33.3) | 2 (33.3) |
|
HR−HER2+ | 2 (12.5) | 1 (2.0) | 0 (0) | 0 (0) |
| TN | 11 (68.8) | 7 (14.6) | 1 (33.3) | 0 (0) |
| Total | 16 (21.9) | 48 (65.7) | 3 (4.1) | 6 (8.3) |
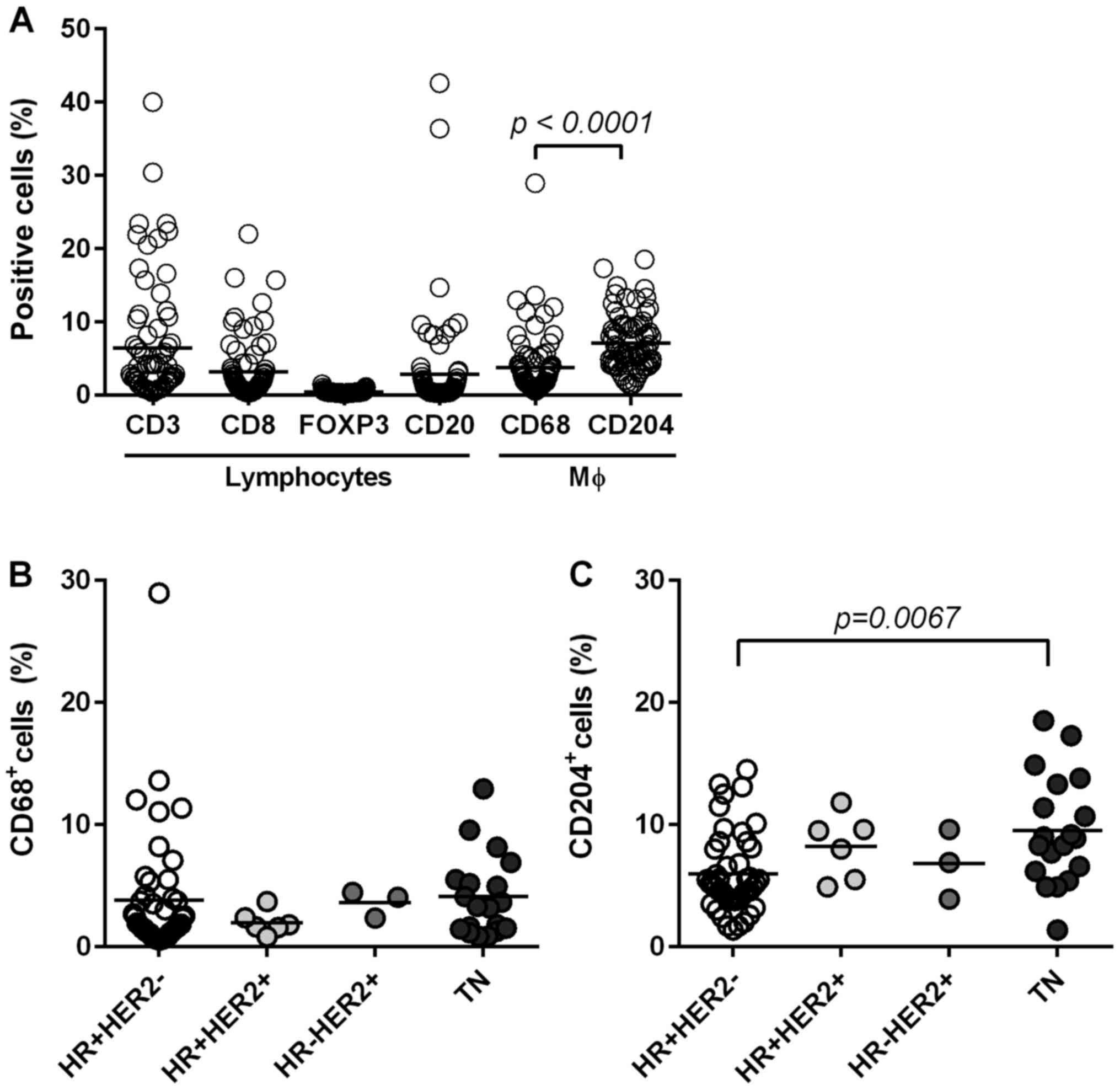
Lymphocyte composition in breast
cancer tissues
We identified lymphocytes (CD3+,
CD8+ T cells, FOXP3+ Tregs and
CD20+ B cells) and macrophages (CD68+ and
CD204+ cells) in tumor tissues via IHC (Fig. 1F-K) to examine local immune TME.
Stained-cell areas were counted using ImageJ software and the
distribution was evaluated for each subset (Fig. 3A). As shown in Fig. 3A, CD3+ T cells (total T
cells) were highly accumulated (mean: 6.4%) with nearly half of the
T cells determined to be CD8+ T cells (mean: 3.1%).
CD20+ B cells were also accumulated in certain tumors
(mean: 2.8%). Since we identified FOXP3+ Tregs through
nuclear staining, the positive area seemed smaller (mean: 0.4) than
that in other cells evaluated through surface staining.
Nevertheless, we observed the presence of FOXP3+ cells
in some tumors by this method (Fig.
1H). We categorized tumors into a ‘high’ or ‘low’ group based
on the mean value of each subset. PD-L1 negativity in ICs and TCs
was significantly associated with a low number of CD3+ T
cells, CD8+ T cells, CD20+ B cells and
FOXP3+ Tregs (Table
V).
 | Table V.Association between PD-L1 expression
in TCs/ICs and immune subsets. |
Table V.
Association between PD-L1 expression
in TCs/ICs and immune subsets.
|
|
| PD-L1 in TCs | PD-L1 in ICs |
|---|
|
|
|
|
|
|---|
| Variables | No. | Negative, n |
Positivea, n |
P-valueb | Negative, n |
Positivea, n |
P-valueb |
|---|
| CD3 |
|
|
| 0.0006 |
|
| <0.0001 |
|
Lowc | 51 | 48 | 3 |
| 46 | 6 |
|
|
High | 22 | 13 | 9 |
| 6 | 16 |
|
| CD8 |
|
|
| 0.0014 |
|
| <0.0001 |
|
Low | 54 | 50 | 4 |
| 46 | 9 |
|
|
High | 19 | 11 | 8 |
| 6 | 13 |
|
| FOXP3 |
|
|
| 0.0419 |
|
| 0.0317 |
|
Low | 50 | 45 | 5 |
| 39 | 11 |
|
|
High | 23 | 16 | 7 |
| 12 | 11 |
|
| CD20 |
|
|
| <0.0001 |
|
| <0.0001 |
|
Low | 59 | 55 | 4 |
| 48 | 11 |
|
|
High | 14 | 6 | 8 |
| 3 | 11 |
|
| CD68 |
|
|
| 0.5132 |
|
| 0.0145 |
|
Low | 49 | 42 | 7 |
| 39 | 10 |
|
|
High | 24 | 19 | 5 |
| 12 | 12 |
|
| CD204 |
|
|
| 0.0024 |
|
| <0.0001 |
|
Low | 43 | 41 | 2 |
| 39 | 4 |
|
|
High | 31 | 20 | 10 |
| 12 | 18 |
|
Macrophage composition in breast
cancer tissue and its association with clinicopathological
factors
Next, we stained macrophages with two different
markers to clarify the characteristics of TAMs in BC tissues: CD68
(a marker of pan-macrophages) and CD204 (a marker of M2-type
macrophages) (Fig. 1J and K). Both
macrophages were present in the tumor stroma and nest.
CD204+ macrophages occupied significantly larger areas
(mean, 7.1%) compared to CD68+ macrophages (mean, 3.7%)
in BC tissues (P<0.0001; Fig.
3A). Both types of macrophages were associated with high levels
of TILs, CD3+ T cells, and CD8+ T cells, but
not CD20+ B cells (Table
VI).
 | Table VI.Association between CD68 or
CD204-positive macrophages and clinicopathological parameters or
immune subsets. |
Table VI.
Association between CD68 or
CD204-positive macrophages and clinicopathological parameters or
immune subsets.
|
|
| CD68-positive
cells | CD204-positive
cells |
|---|
|
|
|
|
|
|---|
| Variables | No. | Lowa, n | Higha, n |
P-valueb | Lowa, n | Higha, n |
P-valueb |
|---|
| HG |
|
|
| 0.6055 |
|
| 0.0246 |
|
1-2 | 48 | 31 | 17 |
| 33 | 15 |
|
| 3 | 25 | 18 | 7 |
| 10 | 15 |
|
| Ki-67, % |
|
|
| 0.8012 |
|
| 0.0152 |
|
≤20 | 29 | 21 | 9 |
| 23 | 7 |
|
|
>20 | 44 | 28 | 15 |
| 20 | 23 |
|
| TILs, % |
|
|
| 0.0472 |
|
| <0.0001 |
|
<10 | 54 | 40 | 14 |
| 40 | 14 |
|
|
≥10 | 19 | 9 | 10 |
| 3 | 16 |
|
| CD3 |
|
|
| 0.0004 |
|
| <0.0001 |
|
Low | 51 | 41 | 10 |
| 38 | 13 |
|
|
High | 22 | 8 | 14 |
| 5 | 17 |
|
| CD8 |
|
|
| <0.0001 |
|
| <0.0001 |
|
Low | 54 | 44 | 10 |
| 40 | 14 |
|
|
High | 19 | 5 | 14 |
| 3 | 16 |
|
| FOXP3 |
|
|
| 0.0001 |
|
| 0.0796 |
|
Low | 50 | 41 | 9 |
| 33 | 17 |
|
|
High | 23 | 8 | 15 |
| 10 | 13 |
|
| CD20 |
|
|
| 0.0547 |
|
| 0.0706 |
|
Low | 59 | 43 | 16 |
| 38 | 21 |
|
|
High | 14 | 6 | 8 |
| 5 | 9 |
|
Furthermore, CD204+ M2-type macrophages
were significantly higher in TN type compared to in
HR+HER2− type (P=0.0067), whereas the
frequency of CD68+ macrophages was the same among all
subtypes (Fig. 3B and C).
Furthermore, high levels of CD204+ macrophages were
significantly associated with high HG (P=0.0246), and high Ki-67
(P=0.0152). In contrast, CD68+ macrophages were
not associated with these factors (Table VI). Interestingly, CD204+
M2-macrophages significantly accumulated in tumors with PD-L1
upregulation in both TCs and ICs (Table
V).
Suppressive subsets in
TIL+PD-L1+ and
TIL−PD-L1− tumors
Finally, we quantified the suppressive cells,
FOXP3+ Tregs, and CD204+ M2-type macrophages
in each of the four groups categorized by TILs and PD-L1 status.
The frequency of tumors with high FOXP3+ Tregs, and high
CD204+ macrophages was 62.5% (10/16) and 87.5% (14/16)
among TIL+PD-L1+ tumors, respectively
(Table VII). By contrast, among
TIL−PD-L1− tumors, the frequency of tumors
with high FOXP3+ Tregs and high CD204+
macrophages were 27.1% (13/48) and 20.8% (10/48), respectively.
These results indicate that TIL+PD-L1+
tumors, considered responsible for ICIs, contain abundant
suppressive cells as infiltrating cell subsets.
 | Table VII.Distribution of suppressive subsets
in each of the four groups categorized by TIL level and PD-L1
status (n=73). |
Table VII.
Distribution of suppressive subsets
in each of the four groups categorized by TIL level and PD-L1
status (n=73).
|
| Classification of
BCs, n (%) |
|---|
|
|
|
|---|
| Suppressive
subset | TIL+a PD-L1+b | TIL−
PD-L1− | TIL+
PD-L1− | TIL−
PD-L1+ |
|---|
|
FOXP3-lowc | 6 (37.5) | 35 (72.9) | 1 (33.3) | 5 (83.3) |
|
FOXP3-highd | 10 (62.5) | 13 (27.1) | 2 (66.7) | 1 (16.7) |
|
CD204-lowe | 2 (12.5) | 38 (79.2) | 1 (33.3) | 2 (33.3) |
|
CD204-highf | 14 (87.5) | 10 (20.8) | 2 (66.7) | 4 (66.7) |
Discussion
In this study, we categorized 73 primary breast
tumors into four different groups based on TILs and PD-L1 status.
Consequently, 22% of the tumors were classified as
TIL+PD-L1+ and 66% were as
TIL−PD-L1−. Furthermore, suppressive subsets,
CD204+M2-type macrophages and FOXP3+ Tregs
were highly accumulated in TIL+PD-L1+
tumors.
As expected, the TIL+PD-L1+
group primarily comprised TN types (69%) known to be highly
immunogenic, whereas the TIL−PD-L1− group
primarily included HR+HER2− cells (77%),
suggesting that the classification of BC tumors based on TILs and
the PD-L1 status mostly corresponded to the classification by
subtype. However, these results show that approximately one-third
of TIL+PD-L1+ tumors were non-TNBCs and could
benefit from immunotherapy, though this population is very small.
Conversely, 23% of TIL−PD-L1− tumors were
represented with HR+HER2+,
HR−HER2+, and TN types.
The molecular classifications of TN tumors have
recently been reported. Lehmann et al (26) proposed TN tumors into four stable
transcriptional subtypes, basal-like 1 (BL1), basal-like 2 (BL2),
mesenchymal (M), and luminal androgen receptor (LAR). Furthermore,
Harano et al (27) reported
that the rate of immunomodulatory signature was the highest in BL1
(48%) and the lowest in M (0%). We showed that PD-L1 expression was
enhanced in aggressive tumors featuring high HG and Ki-67 index
with abundant immune cells. Notably, this tendency became more
significant in highly proliferative tumors (data not shown). These
clinical results may suggest that among TN types, most
TIL+PD-L1+ tumors may have molecular
characteristics of basal-like types which show highly proliferative
phenotypes. By contrast, some TN tumors categorized as
TIL−PD-L1− may have other characteristics
such as mesenchymal, or LAR type. In fact, one of the
TIL−PD-L1− TN tumor in this study was a
metaplastic carcinoma that presented with a mesenchymal
characteristic (data not shown). Besides, the sample size was too
small to sufficiently assess the association between two types of
HER2-positive tumors and PD-L1 expression in this study. A larger
study is required to further address this issue.
TIL−PD-L1+ tumors indicate
that PD-L1 is upregulated through intrinsic oncogenic induction,
such as the effect of the loss of tumor suppression by phosphatase
and tensin homolog (PTEN) signals (28). This tumor type is not considered an
effective target for ICIs owing to its lack of T cells. Therefore,
T cells should be recruited through other approaches such as
chemotherapy and irradiation therapy to induce immune reactions
through immunogenic cell death (29).
TIL+PD-L1− tumors indicate
the presence of immune tolerance by suppressive factors other than
adaptive resistance induced by the PD-1/PD-L1 interaction.
Lymphocytes in these tumors may become suppressed through several
mechanisms such as those involving M2-polarized macrophages,
myeloid-derived suppressor cells, or metabolites like indoleamine
2, 3-dioxygenase (IDO) (11).
Herein, we focused on the presence of TAMs to
comprehend the local TME. CD68 and CD163 are frequently used
markers to identify macrophages in tumor tissue (18,30). In
particular, CD163+ macrophages are reportedly correlated
with a poor prognosis (18,31). Furthermore, recent studies have
reported that a high density of CD204+ macrophages is
strongly associated with poor prognosis of BC patients (19,20) as
well as in NSCLC (32), gastric
cancer (33), and esophageal cancer
(21,34). Therefore, we analyzed the presence of
CD204+ macrophages comparing it to CD68+
macrophages. We observed that CD204+ cells accumulated
in more aggressive tumors than did CD68+ cells in BC
tissue, in line with a previous study (19). Furthermore, we found that
CD204+ macrophages were likely to be present in tumors
with high PD-L1 expression, indicating that CD204+
macrophages may be one of the immune subsets that express PD-L1,
thus preventing the function of PD-1 expressing CD8+ T
cells. We also found that TIL+PD-L1+ BC
tumors, considered responsible for ICIs, included abundant TAMs in
tumor tissue, thus potentially accounting for the weak efficacy of
ICIs alone for TNBCs (35,36). Currently, clinical trials targeting
TAMs, e.g., targeting colony-stimulating factor 1 (CSF1), and
chemokine receptor 2 (CCR2), alone or in combination with
chemotherapy and/or checkpoint inhibitors are underway (14). Future studies are required to
evaluate whether CD204+ cells in BC is an efficacious
therapeutic target.
One of the limitations of our study is the small
number of cases. In particular, data obtained for HER2-positive
tumors were inconclusive owing to the small number of cases
enrolled (6 HR+HER2−, 3
HR−HER2+ tumors). Furthermore, since cutoff
values for immune subsets have not yet been established, we
determined high/low values in accordance with our own conditions. A
standardized evaluation system is required for immune subsets like
TILs (23). Furthermore, we did not
determine the prognostic effects of these classifications and high
density of CD204+ macrophages/FOXP3+ Tregs,
given that no data on survival are currently available.
In conclusion, we classified breast tumors into
four groups based on TILs and PD-L1 status irrespective of subtype
and found that 22% of tumors were classified as
TIL+PD-L1+ tumors (69% was TN type), whereas
66% of tumors were classified as TIL−PD-L1−
tumors (77% was HR+HER2− type). Furthermore,
we found that CD204+ macrophages and FOXP3+
Tregs were highly accumulated in TIL+PD-L1+
tumors, which may contribute to immunotherapy resistance. The
present results suggest that the evaluation of TILs, PD-L1
expression, and TAMs would help effectively select candidate
immunotherapies and the development of novel strategies depending
on the immune microenvironment in BC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
KS designed the study. MN, KS, AN, NI, MS, MK,
MakI, YT, HO, MikI, TM and TO contributed to acquisition of data.
YK, MasI and HY contributed to pathological analysis. KS, MN, YK
and TM contributed to analysis and interpretation of data. KS and
MN drafted the manuscript. TO and NK were involved in analysis and
interpretation of data, and revised the manuscript critically for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Mie University Hospital (approval no. 3155). The
requirement for written informed consent was waived due to the
retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wolchok JD, Kluger H, Callahan MK, Postow
MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K,
et al: Nivolumab plus ipilimumab in advanced melanoma. N Engl J
Med. 369:122–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Powles T, Eder JP, Fine GD, Braiteh FS,
Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et
al: MPDL3280A (anti-PD-L1) treatment leads to clinical activity in
metastatic bladder cancer. Nature. 515:558–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alexandrov LB, Nik-Zainal S, Wedge DC,
Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A,
Børresen-Dale AL, et al: Signatures of mutational processes in
human cancer. Nature. 500:415–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adams S, Gray RJ, Demaria S, Goldstein L,
Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, et
al: Prognostic value of tumor-infiltrating lymphocytes in
triple-negative breast cancers from two phase III randomized
adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin
Oncol. 32:2959–2966. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Loi S, Sirtaine N, Piette F, Salgado R,
Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, et al:
Prognostic and predictive value of tumor-infiltrating lymphocytes
in a phase III randomized adjuvant breast cancer trial in
node-positive breast cancer comparing the addition of docetaxel to
doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin
Oncol. 31:860–867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Loi S, Michiels S, Salgado R, Sirtaine N,
Jose V, Fumagalli D, Kellokumpu-Lehtinen PL, Bono P, Kataja V,
Desmedt C, et al: Tumor infiltrating lymphocytes are prognostic in
triple negative breast cancer and predictive for trastuzumab
benefit in early breast cancer: Results from the FinHER trial. Ann
Oncol. 25:1544–1550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adams S, Loi S, Toppmeyer D, Cescon DW, De
Laurentiis M, Nanda R, Winer EP, Mukai H, Tamura K, Armstrong A, et
al: Pembrolizumab monotherapy for previously untreated,
PD-L1-positive, metastatic triple-negative breast cancer: Cohort B
of the phase II KEYNOTE-086 study. Ann Oncol. 30:405–411. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Emens LA, Cruz C, Eder JP, Braiteh F,
Chung C, Tolaney SM, Kuter I, Nanda R, Cassier PA, Delord JP, et
al: Long-term clinical outcomes and biomarker analyses of
atezolizumab therapy for patients with metastatic triple-negative
breast cancer: A phase 1 study. JAMA Oncol. 5:74–82. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schmid P, Adams S, Rugo HS, Schneeweiss A,
Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Shaw Wright G, et al:
Atezolizumab and nab-paclitaxel in advanced triple-negative breast
cancer. N Engl J Med. 379:2108–2121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Teng MW, Ngiow SF, Ribas A and Smyth MJ:
Classifying cancers based on T-cell Infiltration and PD-L1. Cancer
Res. 75:2139–2145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Emens LA, Loi S, Rugo HS, Schneeweiss A,
Diéras V, Iwata H, Barrios CH, Nechaeva M, Molinero L, Duc AN, et
al: Abstract GS1-04: IMpassion130: Efficacy in immune biomarker
subgroups from the global, randomized, double-blind,
placebo-controlled, phase III study of atezolizumab +
nab-paclitaxel in patients with treatment-naïve, locally advanced
or metastatic triple-negative breast cancer. Cancer Res. 79 (4
Suppl):GS1–04. 2019.
|
|
13
|
Noy R and Pollard JW: Tumor-associated
macrophages: From mechanisms to therapy. Immunity. 41:49–61. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
DeNardo DG and Ruffell B: Macrophages as
regulators of tumour immunity and immunotherapy. Nat Rev Immunol.
19:369–382. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu Y, Knolhoff BL, Meyer MA, Nywening TM,
West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC and
DeNardo DG: CSF1/CSF1R blockade reprograms tumor-infiltrating
macrophages and improves response to T-cell checkpoint
immunotherapy in pancreatic cancer models. Cancer Res.
74:5057–5069. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arlauckas SP, Garris CS, Kohler RH,
Kitaoka M, Cuccarese MF, Yang KS, Miller MA, Carlson JC, Freeman
GJ, Anthony RM, et al: In vivo imaging reveals a tumor-associated
macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci
Transl Med. 9:eaal36042017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lo Russo G, Moro M, Sommariva M, Cancila
V, Boeri M, Centonze G, Ferro S, Ganzinelli M, Gasparini P, Huber
V, et al: Antibody-Fc/FcR interaction on macrophages as a mechanism
for hyperprogressive disease in non-small cell lung cancer
subsequent to PD-1/PD-L1 blockade. Clin Cancer Res. 25:989–999.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Medrek C, Pontén F, Jirström K and
Leandersson K: The presence of tumor associated macrophages in
tumor stroma as a prognostic marker for breast cancer patients. BMC
Cancer. 12:3062012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miyasato Y, Shiota T, Ohnishi K, Pan C,
Yano H, Horlad H, Yamamoto Y, Yamamoto-Ibusuki M, Iwase H, Takeya M
and Komohara Y: High density of CD204-positive macrophages predicts
worse clinical prognosis in patients with breast cancer. Cancer
Sci. 108:1693–1700. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimada H, Hasebe T, Sugiyama M, Shibasaki
S, Sugitani I, Ueda S, Gotoh Y, Yasuda M, Arai E, Osaki A and Saeki
T: Fibrotic focus: An important parameter for accurate prediction
of a high level of tumor-associated macrophage infiltration in
invasive ductal carcinoma of the breast. Pathol Int. 67:331–341.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hatogai K, Kitano S, Fujii S, Kojima T,
Daiko H, Nomura S, Yoshino T, Ohtsu A, Takiguchi Y, Doi T and
Ochiai A: Comprehensive immunohistochemical analysis of tumor
microenvironment immune status in esophageal squamous cell
carcinoma. Oncotarget. 7:47252–47264. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salgado R, Denkert C, Demaria S, Sirtaine
N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL,
Penault-Llorca F, et al: The evaluation of tumor-infiltrating
lymphocytes (TILs) in breast cancer: Recommendations by an
International TILs working group 2014. Ann Oncol. 26:259–271. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mohammed ZM, Going JJ, Edwards J,
Elsberger B, Doughty JC and McMillan DC: The relationship between
components of tumour inflammatory cell infiltrate and
clinicopathological factors and survival in patients with primary
operable invasive ductal breast cancer. Br J Cancer. 107:864–873.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miglietta F, Griguolo G, Guarneri V and
Dieci MV: Programmed cell death ligand 1 in breast cancer:
Technical aspects, prognostic implications, and predictive value.
Oncologist. 24:e1055–e1069. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lehmann BD, Jovanović B, Chen X, Estrada
MV, Johnson KN, Shyr Y, Moses HL, Sanders ME and Pietenpol JA:
Refinement of triple-negative breast cancer molecular subtypes:
Implications for neoadjuvant chemotherapy selection. PLoS One.
11:e01573682016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Harano K, Wang Y, Lim B, Seitz RS, Morris
SW, Bailey DB, Hout DR, Skelton RL, Ring BZ, Masuda H, et al: Rates
of immune cell infiltration in patients with triple-negative breast
cancer by molecular subtype. PLoS One. 13:e02045132018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Parsa AT, Waldron JS, Panner A, Crane CA,
Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, et
al: Loss of tumor suppressor PTEN function increases B7-H1
expression and immunoresistance in glioma. Nat Med. 13:84–88. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kroemer G, Galluzzi L, Kepp O and Zitvogel
L: Immunogenic cell death in cancer therapy. Annu Rev Immunol.
31:51–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mahmoud SM, Lee AH, Paish EC, Macmillan
RD, Ellis IO and Green AR: Tumour-infiltrating macrophages and
clinical outcome in breast cancer. J Clin Pathol. 65:159–163. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tiainen S, Tumelius R, Rilla K, Hämäläinen
K, Tammi M, Tammi R, Kosma VM, Oikari S and Auvinen P: High numbers
of macrophages, especially M2-like (CD163-positive), correlate with
hyaluronan accumulation and poor outcome in breast cancer.
Histopathology. 66:873–883. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Z, Maeda D, Yoshida M, Umakoshi M,
Nanjo H, Shiraishi K, Saito M, Kohno T, Konno H, Saito H, et al:
The intratumoral distribution influences the prognostic impact of
CD68- and CD204-positive macrophages in non-small cell lung cancer.
Lung Cancer. 123:127–135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ichimura T, Abe H, Morikawa T, Yamashita
H, Ishikawa S, Ushiku T, Seto Y and Fukayama M: Low density of
CD204-positive M2-type tumor-associated macrophages in Epstein-Barr
virus-associated gastric cancer: A clinicopathologic study with
digital image analysis. Hum Pathol. 56:74–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yagi T, Baba Y, Okadome K, Kiyozumi Y,
Hiyoshi Y, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Watanabe
M, et al: Tumour-associated macrophages are associated with poor
prognosis and programmed death ligand 1 expression in oesophageal
cancer. Eur J Cancer. 111:38–49. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nanda R, Chow LQ, Dees EC, Berger R, Gupta
S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD, et al:
Pembrolizumab in patients with advanced triple-negative breast
cancer: Phase Ib KEYNOTE-012 study. J Clin Oncol. 34:2460–2467.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Adams S, Schmid P, Rugo HS, Winer EP,
Loirat D, Awada A, Cescon DW, Iwata H, Campone M, Nanda R, et al:
Pembrolizumab monotherapy for previously treated metastatic
triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086
study. Ann Oncol. 30:397–404. 2019. View Article : Google Scholar : PubMed/NCBI
|