Introduction
Lung cancer is currently one of the most common
cancers and one of the most frequent causes of death worldwide
(1). Non-small cell lung cancers
(NSCLCs) constitute ~80% of all lung cancer cases. Adenocarcinoma,
squamous cell carcinoma and large cell carcinoma are the three main
histological subtypes of NSCLC, which account for 40, 25 and 10% of
all the diagnosed cases of NSCLC, respectively (2). The course and prognosis of non-small
cell lung cancers are associated with several factors, such as the
histological type of cancer, the pTNM staging system and the
presence of the EGFR gene mutation or the EML4-ALK fusion gene
(3).
Tesmin (Testis-specific metallothionein-like
protein, also known as metallothionein-like 5 protein, MTL5) is a
60 kDa protein which has cysteine-rich motifs, characteristic of
metallothioneins (MTs) (4). The
human tesmin gene is located at 11q13.3 (5). The specific characteristics of this
protein have been previously presented by us (6). Tesmin was first described in the mouse
testicular tissue and rat testicular and ovarian tissues during the
meiosis of male and female germ cells (7,8). For
that reason, a probable role of tesmin in the regulation of meiosis
was suggested, and the hypothesis on the use of tesmin as a
specific marker for germ cell differentiation was proposed.
Moreover, the translocation from the cytoplasm to the nuclei of
spermatocytes during the G2/M phase of the meiotic division may be
indicative of the role of tesmin in the regulation of other genes
involved in the process of spermatogenesis (7,9). In
addition, the nuclear expression of tesmin is suggested to be a
response to heavy metal ions stress related to the presence of high
concentrations of zinc and cadmium (7,10). The
expression of MTL5 mRNA was noted not only in murine ovaries
and testes, but also in renal, brain, liver, and myocardial tissue.
So far, the expression of tesmin in adult humans has been observed
in prostate and gastric cancer (11,12). An
increased expression of tesmin was also observed in NSCLC, and it
was positively correlated with the expression of the Ki-67 protein
and associated with a poor prognosis (13).
The minichromosome maintenance protein complex (MCM)
is a family of highly conservative, homogenous proteins (MCM2-MCM7)
that play an active role in DNA synthesis (forming the
prereplication-complex, which is a part of the replication forks)
(14). The expression of MCM
proteins is found in the early G1 phase, when the MCM2-MCM7
proteins interact with each other and form stable heterohexamers
(15). MCM proteins expression
increases during the whole cell cycle and decreases during the
differentiation or G0 phase. They participate in the rearrangement
of the chromatin structure, the maintenance of genomic stability,
the cellular response associated with cell cycle checkpoints and
the regulation of transcription (16,17).
Many studies suggest that MCM proteins are expressed
in cancer cells with a high proliferation activity. Consequently,
they are considered as useful markers of proliferation in many
cancers, including NSCLC (18,19).
The aim of this study was to examine the expression
of tesmin (MTL5), MCM5 and MCM7 in NSCLC cases, as well as their
associations with the clinicopathological data of patients.
Materials and methods
Patients' characteristics
The clinical material consisted of 243 paraffin
blocks from patients operated on for non-small cell lung cancer
(NSCLC), including 92 cases of squamous cell lung carcinoma and 151
cases of lung adenocarcinoma. Additionally, 104 paraffin blocks
from the surgical margin were used, which constituted the control
for the analysed cases. In doubtful cases, immunohistochemical
(IHC) anti-p63 (squamous cell lung carcinoma) and anti-TTF-1 (lung
adenocarcinoma) reactions were performed in order to establish the
final histopathological diagnosis. Moreover, the presence of
necrosis in the neoplastic tumour was assessed by determining the
percentage of the area of the histological preparation (stained
with the HE method) covered by necrosis. After surgery, the median
time that patients were followed for a 27 months (range: 0–81
months, mean of 31.82±18.89). Three patients did not report for a
check-up after surgery. During the follow-up period, 95 patients
died.
The tumour fragments and the tissue fragments from
the surgical margin were frozen in liquid nitrogen, then the tumor
cells and the normal lung tissue cells were isolated by laser
microdissection and used to analyse MTL5, MCM5 and
MCM7 mRNA expressions. The median time that these cases were
followed for was 24 months (range: 0–24 months, mean of
20.44±7.137). During the follow-up period, 18 patients died.
All patients were operated on in the years 2010–2016
at the Department of Thoracic Surgery of the Medical University in
Wroclaw.
The specific clinicopathological data of the
patients are presented in Table
I.
 | Table I.Patients and tumour
characteristics. |
Table I.
Patients and tumour
characteristics.
| Parameter | IHC (n=243) | RT-qPCR (n=36) |
|---|
| Mean age, years
(range) | 66.40±7.4
(50–84) | 65.29±7.52
(52–77) |
| Sex, n (%) |
|
Male | 145 (59.67) | 21 (58.33) |
|
Female | 98 (40.33) | 15 (41.67) |
| Tumour size, n
(%) |
| T1 | 76 (31.28) | 8 (22.22) |
| T2 | 124 (51.03) | 20 (55.56) |
| T3 | 24 (9.88) | 5 (13.89) |
| T4 | 5 (2.06) | 1 (2.78) |
| No
data | 14 (5.76) | 2 (5.56) |
| Lymph nodes, n
(%) |
| N0 | 147 (60.49) | 22 (61.11) |
|
N1,N2,N3 | 82 (33.74) | 12 (33.33) |
| No
data | 14 (5.76) | 2 (5.56) |
| Grade, n (%) |
| G1 | 3 (1.23) | 0 (0.00) |
| G2 | 150 (61.73) | 25 (69.44) |
| G3 | 90 (37.04) | 10 (27.78) |
| No
data | 0 (0.00) | 1 (2.78) |
| pTNM, n (%) |
| I | 104 (42.80) | 15 (41.67) |
| II | 76 (31.28) | 15 (41.67) |
|
III | 46 (18.93) | 4 (11.11) |
| IV | 2 (0.82) | 0 (0.00) |
| No
data | 15 (6.17) | 2 (5.56) |
| Stage, n (%) |
|
Early | 180 (74.07) | 30 (83.33) |
|
Advanced | 48 (19.75) | 4 (11.11) |
| No
data | 15 (6.17) | 2 (5.56) |
| Histology, n
(%) |
|
Adeno | 151 (62.14) | 18 (50.00) |
|
SCC | 92 (37.86) | 18 (50.00) |
| p63, n (%) |
|
Positive | 111 (45.68) | 20 (55.56) |
|
Negative | 89 (36.63) | 11 (30.56) |
| No
data | 43 (17.70) | 5 (13.89) |
| TTF-1, n (%) |
|
Positive | 156 (64.20) | 20 (55.56) |
|
Negative | 44 (18.11) | 11 (30.56) |
| No
data | 43 (17.70) | 5 (13.89) |
| Tesmin IHC, n
(%) |
|
IRS |
|
0 | 12 (4.94) | – |
|
1-12 | 231 (95.06) | – |
|
Nuclear |
|
0 | 40 (16.46) | – |
|
1-4 | 203 (83.54) | – |
Tissue microarrays (TMAs)
The TMA method is a accepted method of archiving
material in paraffin blocks with many advantages including economic
aspects, stability of IHC reaction conditions and the time of
evaluation of IHC results with relatively small restrictions
(20). In our study 16 TMAs were
prepared from 243 cases of NSCLC and 104 cases of control lung
tissue from the surgical margin. Prior to performing TMA blocks the
histological slides stained with haematoxylin and eosin were
obtained from whole samples of NSCLC cases archived in the form of
paraffin blocks (donor blocks). The slides were scanned using the
Pannoramic Midi II histological scanner (3DHISTECH Ltd.). After
that by using the Pannoramic Viewer Program (3DHISTECH Ltd.), the
representative areas from the entire sections where selected. In
addition, to increase the representativeness of each case, 3
representative cores with a size of 1.5 mm from the donor block
were selected and then transferred to the TMA ‘recipient’ block
using the TMA Grand Master (3DHISTECH Ltd.).
Immunohistochemistry
The paraffin blocks with NSCLC cases were cut into
4-µm sections. The IHC reactions were performed using anti-tesmin
rabbit polyclonal antibody (Novus Biologicals; cat. no. NBP2-13624)
in a 1:400 dilution, anti-MCM5 (Santa Cruz Biotechnology, Inc.;
sc-165994) in a 1:100 dilution, anti-MCM7 (Santa Cruz
Biotechnology, Inc.; sc-9966) in a 1:50 dilution and anti-Ki-67
mouse monoclonal antibody Clone MIB-1 (Dako) ready-to-use. The IHC
reactions were performed using the DAKO Autostainer Link 48 (Dako).
The visualization of the reactions was carried out using EnVision™
FLEX High pH (Link) reagents (Dako), according to the
manufacturer's instructions. Positive IHC reaction for the tesmin
antigen was assessed using the immunoreactive score (IRS) scale by
Remmele and Stegner (21). This
scale evaluates the percentage of positive cancer cells (A) and the
staining intensity of the reaction (B). The final result is the
product of these two values (AxB). Following the antibody
manufacturer instructions, before carrying out the IHC experiments,
we performed reactions to the positive and negative controls.
Additionally, the nuclear expression intensity for
the Ki-67, MCM5 and MCM7 proteins was determined using a scale that
analyses the percentage of the number of cancer cells with positive
nuclear expression of the antigens studied, according to the
following scale: 0%, 0 p.; 1–10%, 1 p.; 11–25%, 2 p.; 26–50%, 3 p.;
51–100%, 4 p. All specimens were assessed using an OLYMPUS BX-41
light microscope (Olympus) by two independent pathologists. In
cases with divergent scores the evaluation was reassessed and
discussed until consensus was reached. The final result was the
mean of the IHC expression scores of 3 TMA cores. Moreover, p63 and
TTF-1 antigen expressions were used to confirm the histological
type of the tumor (TTF-1(+) adenocarcinoma; p63(+) squamous cell
carcinoma).
Cell line
Lung cancer cell line NCI-H1703 (lung squamous cell
carcinoma) was obtained from the American Type Culture Collection.
RPMI-1640 cell culture medium was used. The medium was additionally
supplemented with L-glutamine to a final concentration of 2 mM, and
with foetal bovine serum to a final concentration of 10%. All of
the cell culture media and reagents were provided by Sigma-Aldrich;
Merck KGaA. In addition, we performed in vitro knock down
experiments on the NCI-H1703 NSCLC cell line using Tesmin Silencer
siRNAs s18519 and s18520 (Thermo Fisher Scientific, Inc.),
receiving the silencing of the tesmin expression at a height of ~55
kDa, as presented in our previous work (13). All experiments were performed in
duplicate.
Cell cycle analyses by flow cytometry
(FACS)
The count of cultured cells (NCI-H1703) in the
phases of the cell cycle was evaluated using the FACSCanto II flow
cytometer (BD Biosciences) and FxCycle PI/RNase Staining Solution
(Life Technologies; Thermo Fisher Scientific, Inc.), according to
the manufacturer's instructions.
Laser capture microdissection
(LCM)
Frozen tissue samples of 36 NSCLC cases and 8 cases
of non-malignant lung tissue (NMLT) (control) were used for RNA
extraction. Tissue sections of 10-µm thickness were prepared with
use of a Leica CM1950 cryostat (Leica Microsystems) and placed on a
polyethylene terephthalate membrane slide (MMI). The slides were
fixed in 70% isopropyl alcohol and stained with HE by using the
H&E Staining Kit for LCM (MMI). LCM was performed using the MMI
CellCut Plus System (MMI). The dissected neoplastic and normal
cells were collected onto the adhesive lid of 500-µl tubes (MMI).
Total RNA was isolated from the tissue samples by using the RNeasy
Micro Kit (Qiagen) according to the manufacturer's instructions.
The protocol included on-column DNase digestion to eliminate
genomic DNA. First-strand cDNA was synthesized using the QuantiTect
Reverse Transcription Kit (Qiagen), according to the manufacturer's
instructions.
Reverse transcription-quantitative PCR
(RT-qPCR)
The mRNA expression of MTL5, MCM5 and
MCM7 was studied on 36 cases of NSCLC and 8 control cases
(18 cases of adenocarcinoma, 18 cases of squamous cell carcinoma
and 8 cases of NMLT), as well as on cell cultures of NCI-H1703.
Total RNA was extracted from the studied tissues and the cell line
with the use of the RNeasy Mini Kit (Qiagen), according to the
manufacturer's protocol. In order to eliminate genomic DNA
contamination, on-column DNase digestion was performed using the
RNase-Free DNase Set (Qiagen). The concentration and quality of the
RNA samples were assessed by spectrophotometry using NanoDrop 1000
(Thermo Fisher Scientific, Inc.). First-strand cDNA was synthesized
with the High Capacity cDNA Reverse Transcription Kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). RT-qPCR was performed
using the 7900HT Fast Real-Time PCR System and the TaqMan Gene
Expression Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.). β-actin (ACTB) was used as an endogenous
control. The following sets of primers and TaqMan probes were used
in the studies: Hs01127481_m1 for MTL5, Hs01052148_m1 for
MCM5, Hs00428518_m1 for MCM7 and Hs99999903_m1 for
ACTB (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The reactions were conducted in triplicates under the following
conditions: polymerase activation at 50°C for 2 min, denaturation
at 94°C for 10 min followed by 40 cycles of denaturation at 94°C
for 15 sec and annealing with synthesis at 60°C for 1 min. The
relative expression of MTL5 mRNA was calculated using the
ΔΔCq method (22).
Protein isolation, SDS-PAGE and
western blotting
The Western blot technique (WB) was used to
determine tesmin, MCM5 and MCM7 expression levels on the cell
cultures of NCI-H1703. Whole protein lysates from the cell culture
samples were obtained using the T-PER Tissue Protein Extraction
Reagent (Thermo Fisher Scientific, Inc.) with the addition of the
Halt™ Protease Inhibitor Cocktail (Thermo Fisher Scientific, Inc.)
and 0.2 mM PMSF (Sigma-Aldrich; Merck KGaA). Protein concentrations
were quantified using the Pierce BCA Protein Assay Kit (Thermo
Fisher Scientific, Inc.) and the NanoDrop™ 1000 (Thermo Fisher
Scientific) spectrophotometer. Equal amounts of total protein (30
µg) were mixed with Laemmli sample buffer and resolved on 10%
acrylamide gel by SDS-PAGE. After electrophoresis, the samples were
transferred to Immobilon-P polyvinylidene difluoride (PVDF)
membranes (Merck KGaA) in the XCell SureLock™ Mini-Cell
Electrophoresis System (Thermo Fisher Scientific, Inc.). Next, the
membranes were blocked in 4% bovine serum albumin solution (Merck
KGaA) in TBST buffer (0.2 M Tris; 1.5 M NaCl; 0.1% Tween-20). After
blocking, the membranes were incubated overnight at 4°C with the
primary rabbit anti-human tesmin polyclonal antibody (NBP2-13624;
Novus Biologicals), diluted at 1:200, anti-MCM5 antibody (Santa
Cruz Biotechnology, Inc.; sc-165994) diluted at 1:200 and anti-MCM7
(Santa Cruz Biotechnology, Inc.; sc-9966) antibody diluted at
1:200. Further, the membranes were incubated with secondary
HRP-conjugated donkey anti-rabbit antibody (715-035-152; Jackson
ImmunoResearch), diluted at 1:3,000 for 1 h at room temperature.
Finally, the membranes were rinsed and treated with the Luminata
Classico (Merck KGaA) chemiluminescent substrate. The reactions
were visualized using the ChemiDoc Imaging System (Bio-Rad
Laboratories). β-actin detected with primary rabbit anti-human
β-actin antibody (4970; Cell Signaling Technology) diluted at
1:1,000 and secondary HRP-conjugated donkey anti-rabbit antibody
(711-035-152; Jackson ImmunoResearch) diluted at 1:3,000 were used
as an internal control to normalize the amount of tesmin. A
densitometric analysis of the results obtained was performed with
the use of the Image Lab software (Bio-Rad Laboratories).
Statistical analysis
The Kolmogorov-Smirnov test was used to evaluate the
normality assumption of the examined groups. The Student's t-test,
unpaired t-tests, Mann-Whitney, Kruskal-Wallis test with Dunn's
multiple comparison test and MANOVA with Bonferroni post hoc tests
were used to compare the differences in the expression of the
examined markers in in vitro results and in all groups of
patients and the clinicopathological data. Additionally, the
Spearmans correlation test was used to analyse the existing
correlations. The Kaplan-Meier method was used to construct
survival curves. The Gehan-Breslow-Wilcoxon method and the
univariate and multivariate Cox analyses of survival were performed
to evaluate the analysis of survival. All statistical analyses were
conducted using Prism 5.0 (GraphPad Software) and Statistica 13.3
(Tibco Software, Inc.). The results were considered statistically
significant when P<0.05.
Results
Cell line (in vitro tests)
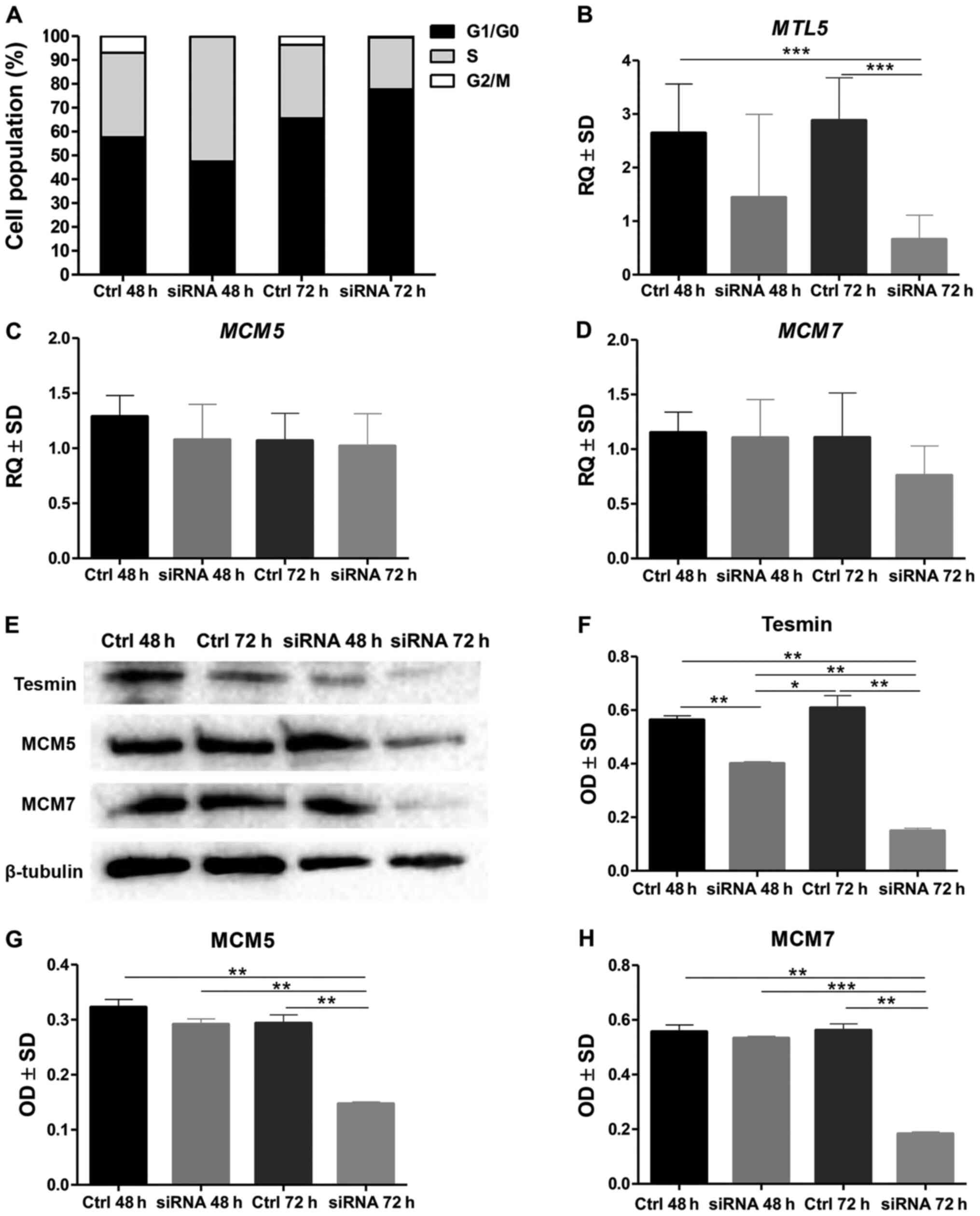
The analysis of the cell cycle by means of the
assessment of the DNA content in the NCI-H1703 cells stained with
propidium diodide and then analysed in the flow cytometer showed
that in the NCI-H1703 cell line with a silenced MTL5
expression the percentage of cells in the G1/G0 phase increased
significantly, while decreasing in the G2/M phase (at 72 h of
incubation) (P<0.0001; MANOVA, Bonnferoni post hoc test;
Fig. 1A).
Moreover, the statistical analysis of the results
obtained by RT-qPCR showed a significantly lower expression of
MTL5 mRNA in the line with the silenced expression of this
gene compared to the control line, as well as no differences in the
expression of MCM5 and MCM7 in the line with silenced
MTL5 expression compared to the control line (Fig. 1B-D). In contrast, the expression of
the tesmin, MCM5 and MCM7 proteins (analysed by means of optical
density obtained by WB method) was lower in the line with silenced
MTL5 expression than in the control line (Fig. 1E-H).
RT-qPCR
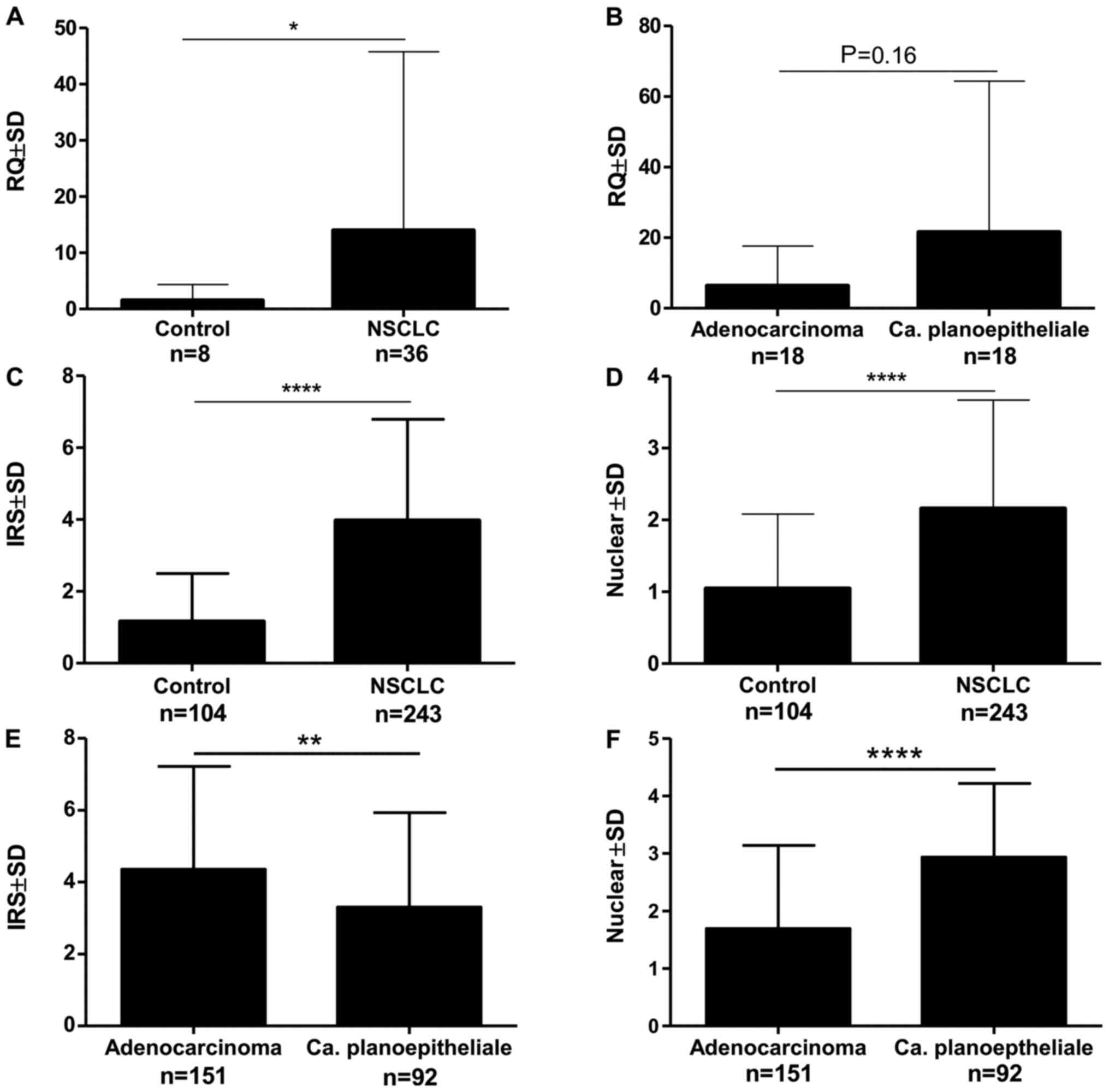
The analysis of the expression of the mRNA isolated
by laser microdissection showed the expression of MTL5, MCM5
and MCM7 in 91.66% of cases. This expression was
significantly higher in NSCLC compared to the control (Fig. 2A). There was no significant
relationship between the expression of MTL5 and the
histological type (Fig. 2B).
Correlation analysis of mRNA expression: MTL5 and
MCM5 as well as MTL5 and MCM7 showed
statistically significant mean positive correlations (Table II).
 | Table II.Correlations of IHC tesmin expression
and MTL5 mRNA expression in cancer cells of non-small cell
lung cancer (n=243 for IHC analysis; n=36 for reverse
transcription-quantitative PCR analysis; Spearman correlation
test). |
Table II.
Correlations of IHC tesmin expression
and MTL5 mRNA expression in cancer cells of non-small cell
lung cancer (n=243 for IHC analysis; n=36 for reverse
transcription-quantitative PCR analysis; Spearman correlation
test).
| Markers | r | P-value |
|---|
| Tesmin IRS vs.
tesmin nuclear | −0.007 | 0.9086 |
| Tesmin IRS vs.
Ki-67 | 0.015 | 0.8060 |
| Tesmin IRS vs.
MCM5 | 0.049 | 0.4436 |
| Tesmin IRS vs.
MCM7 | 0.034 | 0.5954 |
| Tesmin nuclear vs.
Ki-67 | 0.239 | <0.0010 |
| Tesmin nuclear vs.
MCM5 | 0.336 | <0.0001 |
| Tesmin nuclear vs.
MCM7 | 0.315 | <0.0001 |
| Ki-67 vs. MCM5 | 0.634 | <0.0001 |
| Ki-67 vs. MCM7 | 0.603 | <0.0001 |
| MCM5 vs. MCM7 | 0.722 | <0.0001 |
| MTL5 vs.
MCM5 | 0.421 | <0.0500 |
| MTL5 vs.
MCM7 | 0.553 | <0.0100 |
| MCM5 vs.
MCM7 | 0.861 | <0.0001 |
IHC
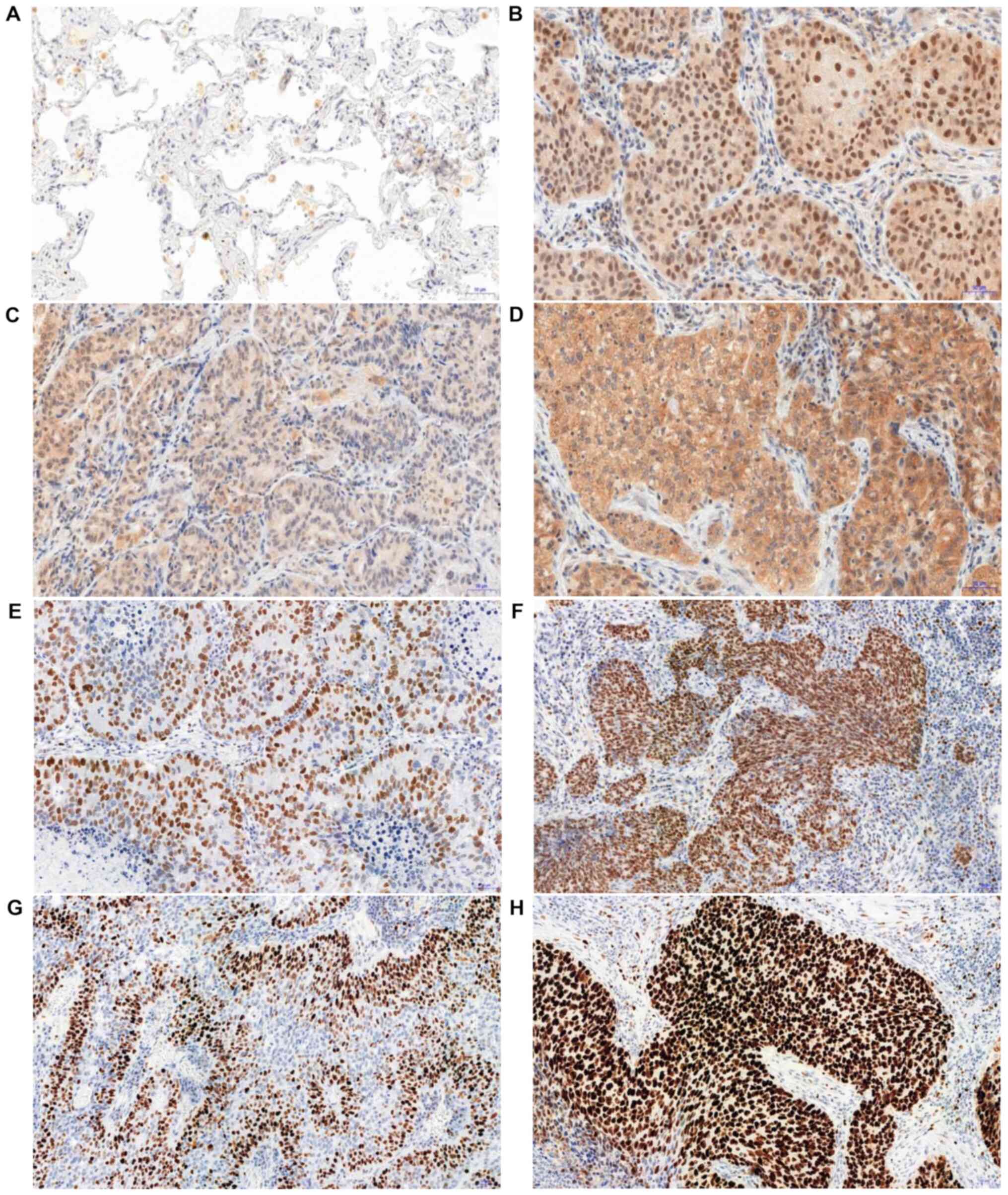
In the analysed NSCLC cases, positive cytoplasmic
(95.6%) and nuclear (83.4%) IHC expression of tesmin were
demonstrated (Fig. 3A-D). Both
nuclear and cytoplasmic tesmin expressions were significantly
higher in NSCLC cases compared to the control (Fig. 2C and D). Comparing the expression of
tesmin in cases of squamous cell carcinoma showed a significantly
higher nuclear expression of this protein in this histological type
than in adenocarcinoma cases, while the cytoplasmic expression was
significantly higher in cases of adenocarcinoma in comparison to
squamous cell carcinoma cases (Fig. 2E
and F). An analysis of the comparison of the expression of
tesmin with the clinicopathological data of the NSCLC cases showed
that nuclear tesmin expression was significantly lower in pT1 cases
compared to pT2-T4 cases in both the whole NSCLC group and the
squamous cell carcinoma group, but not in the adenocarcinoma group
(respectively: 1.85±1.53 vs. 2.37±1.48, P<0.05; 1.49±1.45 vs.
2.98±1.25, P<0.0001; 1.85±1.53 vs. 1.84±1.46, P>0.05).
Moreover, a mean positive correlation between the tesmin nuclear
expression and the percentage of the area of the preparation
covered by necrosis was demonstrated (r=0.299, P<0.0001), which
was not observed for the cytoplasmic expression. The nuclear
expression of tesmin in the NSCLC group correlated positively with
the expression of the marker p63 (r=0.464, P<0.0001), and
negatively with the expression of the marker TTF-1 (r=−0.382,
P<0.0001). On the other hand, the cytoplasmic expression of
tesmin in the NSCLC group correlated with the expression of the
marker TTF-1 (r=0.244, P<0.001), while showing no correlation
with the expression of the marker p63 (r=−0.178, P<0.05).
Additionally, no correlation was found between the
expression of tesmin (both cytoplasmic and nuclear) and other
clinicopathological data such as patient's age, sex, tumour size,
pN, pM, clinical stage or tobacco smoking status (Fig. S1).
The analysis of the correlation of the intensity of
the expression of tesmin (IHC) and the proliferation markers showed
that the nuclear (but not the cytoplasmic) expression of tesmin
correlated moderately positively with the nuclear expression of
MCM5, MCM7 and Ki-67 (Fig. 3E-H;
Table II).
Survival analysis
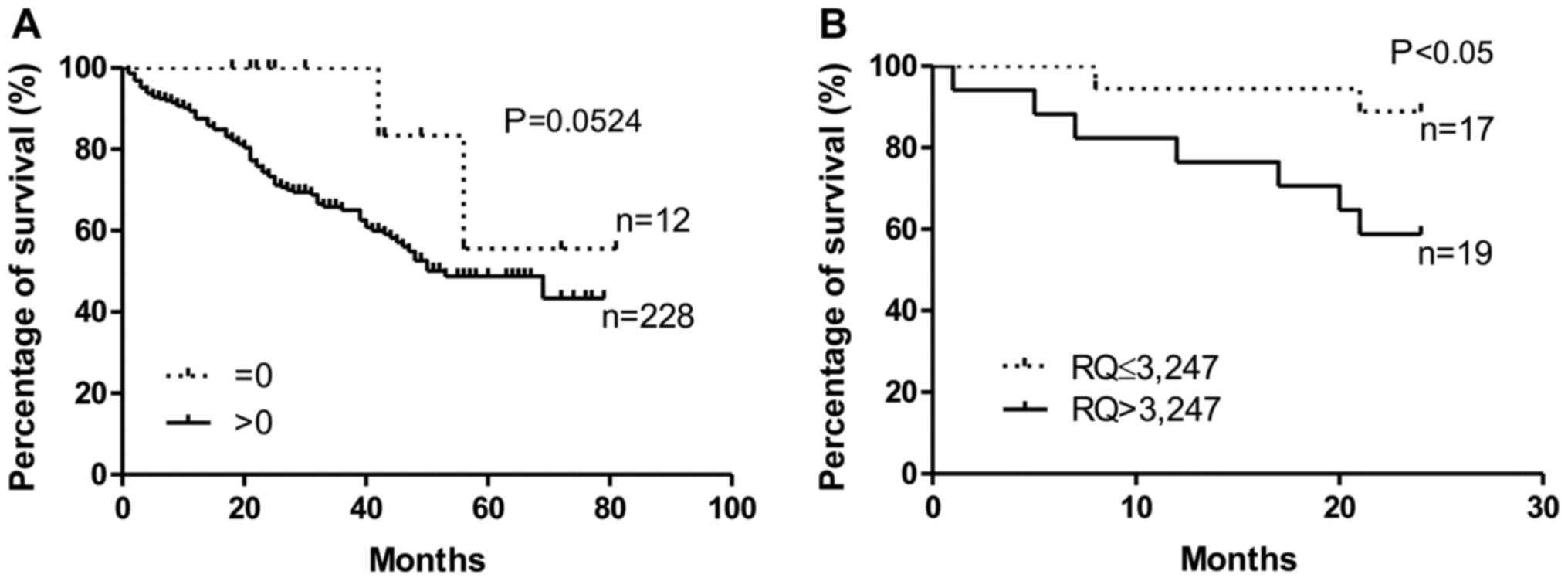
The analysis of the survival of the NSCLC cases
showed that patients without confirmed (by the IHC method)
cytoplasmic expression of tesmin in the tumour cells lived longer
that patients with confirmed tesmin expression (P=0.052,
Gehan-Breslow-Wilcoxon test; Fig.
4A). This relationship was not demonstrated for the nuclear
expression of tesmin (IHC). Similarly, patients with low
MTL5 mRNA expression lived significantly longer than
patients with high MTL5 mRNA expression (P<0.05,
Gehan-Breslow-Wilcoxon test; Fig.
4B). Moreover, univariate and multivariate Cox survival
analyses showed that in NSCLC cases, only pT and pN are independent
prognostic factors (Tables
III–V).
 | Table III.Univariate and multivariate Cox
analyses of overall survival of patients with non-small cell lung
cancer. |
Table III.
Univariate and multivariate Cox
analyses of overall survival of patients with non-small cell lung
cancer.
|
| Univariate Cox
analysis of survival | Multivariate Cox
analysis of survival |
|---|
|
|
|
|
|---|
|
Characteristics | P-value | Hazard ratio | 95% CI lower | 95% CI upper | P-value | Hazard ratio | 95% CI lower | 95% CI upper |
|---|
| p63 (negative vs.
positive) | 0.7645 | 1.0680 | 0.6945 | 1.6424 |
|
|
|
|
| TTF-1 (negative vs.
positive) | 0.8613 | 1.0484 | 0.6168 | 1.7822 |
|
|
|
|
| Histological type
(adeno vs. SCC) | 0.1486 | 0.7301 | 0.4765 | 1.1187 |
|
|
|
|
| Clinical stage
(I–II vs. III–IV) | 0.0003 | 1.5542 | 1.2215 | 1.9775 | 0.6312 | 1.1109 | 0.7231 | 1.7064 |
| pT (pT1-2 vs.
pT3-4) | 0.0024 | 1.5170 | 1.1593 | 1.9852 | 0.0061 | 1.7928 | 1.1809 | 2.7216 |
| pN (pN0 vs.
pN1-3) | 0.0023 | 1.9072 | 1.2604 | 2.8861 | 0.0068 | 1.4608 | 1.1105 | 1.9216 |
| Necrosis (%)
(continuous) | 0.3824 | 0.9458 | 0.8347 | 1.0718 |
|
|
|
|
| Histological grade
(G1 vs. G2-3) | 0.1886 | 1.3095 | 0.8761 | 1.9572 |
|
|
|
|
| Tesmin IRS (0 vs.
1–12) | 0.1184 | 3.0555 | 0.7521 | 12.4124 |
|
|
|
|
| Tesmin nuclear (0
vs. 1–4) | 0.3831 | 1.2978 | 0.7225 | 2.3310 |
|
|
|
|
| MCM-5 (0–2 vs.
3–4) | 0.4159 | 0.8169 | 0.5019 | 1.3297 |
|
|
|
|
| MCM-7 (0–2 vs.
3–4) | 0.5114 | 1.3192 | 0.5770 | 3.0160 |
|
|
|
|
| Ki-67 (0–2 vs.
3–4) | 0.8906 | 0.9641 | 0.5730 | 1.6224 |
|
|
|
|
 | Table V.Univariate Cox analysis of overall
survival of patients with squamous cell carcinoma of the lung. |
Table V.
Univariate Cox analysis of overall
survival of patients with squamous cell carcinoma of the lung.
|
| Univariate Cox
analysis of survival |
|---|
|
|
|
|---|
|
Characteristics | P-value | Hazard ratio | 95% CI lower | 95% CI upper |
|---|
| TTF-1 (negative vs.
positive) | 0.7255 | 0.8774 | 0.4227 | 1.8213 |
| Clinical stage
(I–II vs. III–IV) | 0.0614 | 1.6079 | 0.9775 | 2.6447 |
| pT (pT1-2 vs.
pT3-4) | 0.0206 | 1.9206 | 1.1052 | 3.3377 |
| pN (pN0 vs.
pN1-3) | 0.4125 | 1.3751 | 0.6420 | 2.9452 |
| Necrosis, %
(continuous) | 0.6559 | 0.9575 | 0.7908 | 1.1592 |
| Histological grade
(G1 vs. G2-3) | 0.3497 | 0.5671 | 0.1727 | 1.8619 |
| Tesmin IRS (0 vs.
1–12) | 0.4507 | 1.7353 | 0.4143 | 7.2686 |
| Tesmin nuclear (0
vs. 1–4) | 0.5495 | 1.8384 | 0.2503 | 13.5045 |
| MCM-5 (0–2 vs.
3–4) | 0.9938 | 0.9920 | 0.1321 | 7.4486 |
| MCM-7 (0–3 vs.
4) | 0.3132 | 0.8703 | 0.5433 | 1.4610 |
| Ki-67 (0–2 vs.
3–4) | 0.4511 | 0.7507 | 0.3562 | 1.5824 |
Discussion
In the presented study, we have demonstrated for the
first time a relationship between an increased nuclear expression
of tesmin and a higher expression of MCM5 and MCM7 at the protein
and mRNA levels in NSCLC. A positive correlation between the
expression of tesmin and Ki-67 in NSCLC, which we have as well
demonstrated in this study, was also previously observed by us
(13). This indirectly indicates a
relationship between the expression of tesmin and the proliferation
process of NSCLC cells. The relationship of tesmin with the process
of cellular proliferation has already been described on many
occasions in the case of cells undergoing meiotic division
(4,6–9). A
higher expression of this protein and its translocation from the
cytoplasm to the cell nucleus were observed, suggesting its
participation in the organization of chromatin in chromosomes
(6). Furthermore, this phenomenon
was intensified by the presence of zinc and cadmium ions (6–9).
The role of tesmin in the process of proliferation
of NSCLC cells is additionally confirmed by an in vitro
experiment in which the silencing of the expression of tesmin with
siRNA causes a reduction in the percentage of cells in the G2 phase
of the cell cycle and a reduction of the expression of MCM5 and
MCM7, which are confirmed proliferation markers also in NSCLC
(16,17).
Despite this, the role of tesmin in cancer is not
fully understood. An increased expression of this protein in NSCLC
cells compared to the control tissue has already been described by
us (13). However, contrary to the
previous study, this time we were also able to show for the first
time a difference in the localization of tesmin expression
depending on the histological subtype of NSCLC. In cases of lung
adenocarcinoma, the cytoplasmic expression of tesmin was higher
than in cases of squamous cell carcinoma. In contrast, in cases of
squamous cell carcinoma, the nuclear expression of tesmin was
higher than in the adenocarcinomas. Similarly, the nuclear
expression of MCM5 and MCM7 was also significantly higher in
squamous cell carcinoma than in adenocarcinoma (data not shown in
the study). This confirms that NSCLCs are a heterogeneous group of
neoplasms, and squamous cell carcinomas and adenocarcinomas may
have different regulatory mechanisms, something that has already
been emphasized on numerous occasions (23–26).
This may also indirectly indicate the participation of tesmin, MCM5
and MCM7 in the same mechanisms that influence carcinogenesis and
progression in NSCLC, resulting, among others, in a higher
proliferative potential of the cells for this type of tumour. A
stronger nuclear expression of tesmin in squamous cell carcinoma
and the alleged biological role of tesmin in the cell nucleus may
suggest that tesmin plays a more important role in this subtype of
NSCLC. This is confirmed by the fact that a different profile of
genes and a different expression of markers in adenocarcinoma and
squamous cell lung carcinoma have already been indicated numerous
times (26).
The positive correlations between the expression of
tesmin (MLT5) and MCM5 and MCM7 at the level of the protein
(IHC) and the mRNA isolated from tumour cells by using the laser
microdissection method may indicate the mutual regulation of these
proteins. Sanada et al (27)
suggested that the miR-143 molecule is involved in the
regulation of the expression of MTL5 and another member of
the MCM-MCM4 protein family, among others. Taking into account the
already known function of the proteins from the MCM family in DNA
replication, perhaps tesmin is also involved in this process, all
the more so because there are reports that suggest a possible role
of tesmin in the organization of chromatin during meiotic divisions
(4). It is possible that tesmin has
a similar function in carcinogenesis and mitotic divisions of NSCLC
cells.
Due to the fact that tesmin is indicated as a
co-activator that differentiates the activity of the ligands
aldosterone and deoxycorticosterone for the mineralocorticoid
receptor (this receptor is a ligand-dependent transcription
factor), it can be assumed that tesmin, as a co-activator, may
regulate the expression of other genes, e.g. those related to the
proliferation process (28).
However, this hypothesis would require further, more detailed
research.
Moreover, as in our previous study, we were also
able to demonstrate that an increased expression of tesmin is a
negative prognostic factor in NSCLC, both at the mRNA and the IHC
levels (13). A similar role of the
expression of MTL5 was indicated by Sanada et al
(27). This proves that tesmin may
have a significant effect on the progression of NSCLC.
One of the limitation of the study was lack of
clinical data in the form of presence of the EGFR gene mutation.
This is due the fact that the mutation testing of NSCLC in Poland
is not routinely performed in cases of I and II stage of non-small
cell lung cancer. The cases in these stages that are mostly treated
surgically and constitute the dominant group of cases in the
presented study. Unfortunately, we also do not have such clinical
data for a much smaller group of patients in stage III and IV.
Because the main cause of the EGFR gene mutation is smoking we
compared the expression of tesmin in smokers and non-smokers but we
did not reveal any significant differences in the expression of
tesmin in these groups.
The present study was carried out using the
NCI-H1703 squamous cell carcinoma because the analysis of
MTL5 mRNA expression isolated by laser capture
microdissection from NSCLC cases shown that the MTL5
expression was higher in squamous cell carcinoma cases than in
adenocarcinoma cases. The results of IHC analysis also shown that
the nuclear expression of tesmin was significantly higher in
squamous cell carcinoma cases than in adenocarcinoma cases.
Moreover, it seems that the biological activity of the tesmin
protein is dominant in the cell nucleus (7,28,29). In
addition, on the basis of previous research in which we showed the
highest nuclear expression of tesmin in the NCI-H1703 cell line we
assumed that this cell line would be most suitable for our research
(13). However using only one cell
line could be also limitation of our study
In conclusion, the role of tesmin in NSCLC seems to
be related to the cell proliferation process of this tumour, and a
worse prognosis of NSCLC patients with a higher expression of it
may indicate the role of this protein in the progression of
NSCLC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Science Centre, Poland, under the programme ‘Preludium 12’ project
no. 2016/23/N/NZ5/02570.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JG designed the study, evaluated the IHC reaction,
prepared the database, performed the statistical analysis, analysed
the results and created a draft of the manuscript. PSG evaluated
the IHC reaction and prepared the database. AG performed
microdissection, isolation of mRNA and quantitative PCR analysis.
MO, KR and NGP prepared all in vitro experiments and
analysed the results. AP prepared the IHC analysis and analysed the
results. MPO, BW and AR obtained in vivo material from
patients, collected clinical data of patients, prepared the
database and analysed the results. MPO and PD reviewed and edited
the manuscript. PD supervised methodical all experiments, analysed
the results, prepared, reviewed and edited manuscript. All authors
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The experiment was performed in accordance with the
ethical standards and following approval of the Ethics Committee of
Wroclaw Medical University (decision no. KB 455/2009 and KB
40/2017) and all patients provided a written statement of informed
consent for the use of the material samples for scientific
research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brambilla E and Travis WD: Lung cancer.
Word Cancer Report 2014. Stewart BW and Wild CP: World Health
Organization; Lyon: pp. 350–361. 2014
|
|
2
|
Ettinger DS, Akerley W, Bepler G, Blum MG,
Chang A, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Ganti AK, et
al NCCN non-small cell lung cancer panel members, : Non-small cell
lung cancer. J Natl Compr Canc Netw. 8:740–801. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Camidge DR, Dziadziuszko R, Peters S, Mok
T, Noe J, Nowicka M, Gadgeel SM, Cheema P, Pavlakis N, de Marinis
F, et al: Updated efficacy and safety data and impact of the
EML4-ALK fusion variant on the efficacy of alectinib in untreated
ALK-positive advanced non-small cell lung cancer in the global
phase III ALEX study. J Thorac Oncol. 14:1233–1243. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sutou S, Miwa K, Matsuura T, Kawasaki Y,
Ohinata Y and Mitsui Y: Native tesmin is a 60-kilodalton protein
that undergoes dynamic changes in its localization during
spermatogenesis in mice. Biol Reprod. 68:1861–1869. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
HUGO Gene Nomenclature Committee.
simplehttp://www.genenames.orgMay
30–2020
|
|
6
|
Grzegrzółka J, Podhorska-Okołów M,
Krawczuk Z and Dzięgiel P: The role of tesmin in the physiology and
pathogenesis of human diseases. Postepy Hig Med Dosw. 73:762–767.
2019. View Article : Google Scholar
|
|
7
|
Matsuura T, Kawasaki Y, Miwa K, Sutou S,
Ohinata Y, Yoshida F and Mitsui Y: Germ cell-specific
nucleocytoplasmic shuttling protein, tesmin, responsive to heavy
metal stress in mouse testes. J Inorg Biochem. 88:183–191. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Olesen C, Møller M and Byskov AG: Tesmin
transcription is regulated differently during male and female
meiosis. Mol Reprod Dev. 67:116–126. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coyle P, Philcox JC, Carey LC and Rofe AM:
Metallothionein: The multipurpose protein. Cell Mol Life Sci.
59:627–647. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maremanda KP, Khan S and Jena G: Zinc
protects cyclophosphamide-induced testicular damage in rat:
Involvement of metallothionein, tesmin and Nrf2. Biochem Biophys
Res Commun. 445:591–596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oh JH, Yang JO, Hahn Y, Kim MR, Byun SS,
Jeon YJ, Kim JM, Song KS, Noh SM, Kim S, et al: Transcriptome
analysis of human gastric cancer. Mamm Genome. 16:942–954. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fic M, Pula B, Rogala K and Dziegiel P:
Role of metallothionein expression in gastrointestinal cancers.
Postepy Biol Komorki. 40:5–20. 2013.
|
|
13
|
Grzegrzolka J, Gomulkiewicz A, Olbromski
M, Glatzel-Plucinska N, Piotrowska A, Ratajczak-Wielgomas K,
Rzechonek A, Podhorska-Okolow M, Krawczuk Z and Dziegiel P:
Expression of tesmin (MTL5) in non small cell lung cancer: A
preliminary study. Oncol Rep. 42:253–262. 2019.PubMed/NCBI
|
|
14
|
Jankowska-Konsur A, Kobierzycki C, Reich
A, Grzegrzolka J, Maj J and Dziegiel P: Expression of MCM-3 and
MCM-7 in primary cutaneous T-cell lymphomas. Anticancer Res.
35:6017–6026. 2015.PubMed/NCBI
|
|
15
|
Yu S, Wang G, Shi Y, Xu H, Zheng Y and
Chen Y: MCMs in Cancer: Prognostic Potential and Mechanisms. Anal
Cell Pathol (Amst). 2020:37502942020.PubMed/NCBI
|
|
16
|
Honeycutt KA, Chen Z, Koster MI, Miers M,
Nuchtern J, Hicks J, Roop DR and Shohet JM: Deregulated
minichromosomal maintenance protein MCM7 contributes to oncogene
driven tumorigenesis. Oncogene. 25:4027–4032. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mukherjee G, Muralidhar B, Bafna UD,
Laskey RA and Coleman N: MCM immunocytochemistry as a first line
cervical screening test in developing countries: A prospective
cohort study in a regional cancer centre in India. Br J Cancer.
96:1107–1111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu YZ, Wang BS, Jiang YY, Cao J, Hao JJ,
Zhang Y, Xu X, Cai Y and Wang MR: MCMs expression in lung cancer:
Implication of prognostic significance. J Cancer. 8:3641–3647.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Zhou C, Su B, Teng G, Zheng Y,
Zhou X, Guo L, Xu F and Wang X: MCM7 expression is correlated with
histological subtypes of lung adenocarcinoma and predictive of poor
prognosis. Int J Clin Exp Pathol. 10:11747–11753. 2017.PubMed/NCBI
|
|
20
|
Kobierzycki C, Pula B, Wojnar A,
Podhorska-Okolow M and Dziegiel P: Tissue microarray technique in
evaluation of proliferative activity in invasive ductal breast
cancer. Anticancer Res. 32:773–777. 2012.PubMed/NCBI
|
|
21
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nowinska K, Jablonska K, Pawelczyk K,
Piotrowska A, Partynska A, Gomulkiewicz A, Ciesielska U, Katnik E,
Grzegrzolka J, Glatzel-Plucinska N, et al: Expression of
Irisin/FNDC5 in Cancer Cells and Stromal Fibroblasts of Non-small
Cell Lung Cancer. Cancers (Basel). 11:112019. View Article : Google Scholar
|
|
24
|
Olbromski M, Rzechonek A, Grzegrzolka J,
Glatzel-Plucinska N, Chachaj A, Werynska B, Podhorska-Okolow M and
Dziegiel P: Influence of miR-7a and miR-24-3p on the SOX18
transcript in lung adenocarcinoma. Oncol Rep. 39:201–208.
2018.PubMed/NCBI
|
|
25
|
Olbromski M, Grzegrzolka J,
Jankowska-Konsur A, Witkiewicz W, Podhorska-Okolow M and Dziegiel
P: MicroRNAs modulate the expression of the SOX18 transcript in
lung squamous cell carcinoma. Oncol Rep. 36:2884–2892. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vidarsdottir H, Tran L, Nodin B, Jirström
K, Planck M, Jönsson P, Mattsson JSM, Botling J, Micke P and
Brunnström H: Immunohistochemical profiles in primary lung cancers
and epithelial pulmonary metastases. Hum Pathol. 84:221–230. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sanada H, Seki N, Mizuno K, Misono S,
Uchida A, Yamada Y, Moriya S, Kikkawa N, Machida K, Kumamoto T, et
al: Involvement of dual strands of miR-143 (miR-143-5p and
miR-143-3p) and their target oncogenes in the molecular
pathogenesis of lung adenocarcinoma. Int J Mol Sci. 20:44822019.
View Article : Google Scholar
|
|
28
|
Fuller PJ: Novel interactions of the
mineralocorticoid receptor. Mol Cell Endocrinol. 408:33–37. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rogerson FM, Yao YZ, Young MJ and Fuller
PJ: Identification and characterization of a ligand-selective
mineralocorticoid receptor coactivator. FASEB J. 28:4200–4210.
2014. View Article : Google Scholar : PubMed/NCBI
|