Introduction
Cervical cancer is the fourth most common type of
cancer among women worldwide, and the second most common in low-
and middle-income countries according to the data from GLOBOCAN
2018, with >85% of new cases occurring in developing countries
(1,2). Radiotherapy is widely used,
particularly for locally advanced cervical cancer (3). Following cell cycle arrest, ionizing
radiation-induced apoptosis will occur if DNA damage is not
repaired (4). Strategies to enhance
the expression levels of pro-apoptotic genes could be applied in
gene-radiotherapy (5). Recently,
gene-radiotherapy, which combines gene therapy with radiotherapy,
has shown promising effects (6).
It has been demonstrated that the tumor suppressor
inhibitor of growth 4 (ING4) is deleted in numerous types of cancer
(7). ING4 serves an important role
in cell proliferation, apoptosis, cell cycle arrest, migration and
vascularization, and these are pivotal to tumor progression
(7–11). The radiation-inducible early growth
response 1 (EGR1) promoter, which includes six serum response
elements sensitive to ionizing radiation (12,13), has
attracted particular attentin. Previous studies have indicated that
the EGR1 promoter can enhance the expression of its downstream
genes, such as TNF-α and IFN-γ (14,15).
During the process of tumor development, cells in
hypoxia account for 10–50% of the tumor environment (16). Hypoxic tumor cells cause resistance
to radiotherapy and chemotherapy, leading to tumor recurrence and
distant metastasis (17). Hypoxia
serves a vital role in angiogenesis (18). Furthermore, the hypoxia response
element, upstream of the EGR1 promoter, can enhance the
radiation-induced upregulation of therapeutic genes (19).
The present study combined the EGR1 promoter and
ING4 open reading frame (ORF) as a cassette, which was integrated
into HeLa cells to develop a cell line. Subsequently, the effects
of ING4 on cell cycle arrest and cell proliferation were
investigated when ING4 was induced using various doses of
irradiation. Furthermore, the function of ING4 in hypoxia in HeLa
cells was examined. Radiation and gene-combined treatment, termed
gene-radiotherapy, exhibited a more prominent effect in HeLa
cervical cancer cells in vitro.
Materials and methods
Cell culture and transfection
The human cervical carcinoma HeLa cell line was
purchased from The Cell Bank of Type Culture Collection of Chinese
Academy of Sciences and maintained at 37°C in DMEM (HyClone;
Cytiva) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) and 100 U penicillin-streptomycin. The cells were grown in a
humidified atmosphere of 5% CO2 and 95% air.
HeLa cells in the exponential phase of growth were
treated with trypsin at 37°C for 1 minute. After terminating
digestion with 10% FBS, the cells were resuspended in complete
culture medium. A total of 2×105 cells were inoculated
into each well of a 6-well plate and allowed to grow for 24 h prior
to transfection. Subsequently, the cells in each well were
transiently transfected with 2 µg p-enhanced green fluorescent
protein (EGFP)-N1 (Sigma-Aldrich; Merck KGaA) or pEGR1-EGFP-N1 at
room temperature (pEGR1-N1 was kindly provided by Dr Gerald Thiel
from University of Saarland Medical Center, D-66421 Homburg,
Germany) using 8.0 µl Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The transfection mixture was replaced with fresh
culture medium at 6 h after transfection and was irradiated at 24
h. The fluorescence images were captured at 72 h post-transfection
using a fluorescence microscope (Nikon 80i; Nikon Corporation).
Construction of vectors
The mammalian cellular expression vector, pEGFP-N1
(6085–1), was purchased from Addgene, Inc. The CMV IE promoter was
removed by endonucleases AseI and EcoRI. The promoter
of human EGR1 ranging between −792 and 268 was isolated using
primer pairs pEGR1-AseI-F/pEGR1-EcoRI-R and then
directly cloned into the pEGFP-N1 vector, which was designated as
pEGR1-EGFP-N1.
Similarly, the CMV promoter from construct
pLV-mCherry(2A)puro (VL3405; Inovogen Tech. Co.) was replaced by
the EGR1 promoter using primer pairs
pEGR1-ClaI-F/pEGR1-EcoRI-R and then used to develop
the pEGR1-LV-mCherry(2A)puro vector. Furthermore, the human ING4
was cloned into pEGR1-LV-mCherry(2A)puro by EcoRI and
XhoI with the hING4-EcoRI-F/hING4-XhoI-R
primer pairs and the new construct was named
pEGR1-LV-mCherry(2A)puro-hING4. Additionally,
LV-mCherry(2A)puro-hING4 without any promoter was prepared.
Virus and cell lines
The recombinant lentivirus was obtained from HEK293T
cells (Cell Bank of the Chinese Academy of Science) by transient
transfection of lentivirus construct as well as helper plasmids
psPAX2 (Addgene; cat. no. 12259) and MD2.G (Addgene; cat. no.
12260). Briefly, 1.0×106 293T cells were plated in a
6-cm dish and cultured for 18–24 h. The cells were then
co-transfected with 1.7 µg pLV-mCherry(2A)puro,
pEGR1-LV-mCherry(2A)puro, pEGR1-LV-mCherry(2A)puro-hING4 or
LV-mCherry(2A)puro-hING4, 1.13 µg psPAX2 and 0.57 µg pMD2.G using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.).
293T cells were maintained in DMEM medium with high glucose
(Hyclone, cat. no. SH30243.01) supplemented with 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc; cat. no. 10099141) at
37°C in 5% CO2. The supernatant containing the
lentivirus was harvested at 48 and 72 h and then filtered through a
0.45-µm low protein-binding polysulfonic filter (EMD Millipore).
HeLa cells were inoculated in a 6-well plate in advance at a
density of 1.5×105 cells per well and presented with
~60% confluency following incubation for 20 h. The cells were then
infected with 1 ml lentivirus suspension in the presence of 8 µg/ml
polybrene (Chemicon International; Thermo Fisher Scientific, Inc.).
Following transduction for 48 h, HeLa cells were selected with 2.0
µg/ml puromycin for 10 days until all blank control cells were
dead. The puromycin-resistant cells were stably infected with
exogenous genes.
Cell cycle analysis
HeLa cells stably integrated with
LV-mCherry(2A)puro-hING4 or pEGR1-LV-mCherry(2A)puro-hING4 were
inoculated in a 6-well plate and were allowed to grow for 24 h. The
cells were then exposed to X-rays with a dose ranging between 0 and
8 Gy (2 Gy gradient). Following growth for a further 48 h, the
cells were treated with trypsin at 37°C for 1 min and subjected to
centrifugation at 800 × g for 5 min at 4°C. After rinsing with 1X
PBS, the cells were fixed in cold (4°C) 70% ethanol and stored at
−20°C overnight. Following RNA removal using 50 µg/ml RNaseA (cat.
no. R4875; Sigma-Aldrich; Merck KGaA) for 30 min at 37°C, cells
were stained with 500 µg/ml propidium iodide (eBioscience™, Thermo
Fisher Scientific, Inc.) at room temperature for 15 min.
Subsequently, the cells were subjected to fluorescence-activated
cell sorting (FACS)-based cell cycle analysis using a flow
cytometer (FACSCalibur; BD Immunocytometry Systems) with CellQuest
software (version 5.1). The percentages of cells in the
sub-G1 phase and different cell cycle phases were used
as an index to evaluate the levels of cell apoptosis and cell cycle
distribution, respectively.
Western blot analysis
Cells were lysed in 2X SDS buffer consisting of 0.1
M Tris·Cl, 0.2 M DTT, 4% SDS and 20% Glycerol and subsequently
prepared for three cycles of boiling (95–100°C, 5–10 min) and
cooling (4°C, 5–10 min). The proteins were quantified using the
Bradford method and subsequently mixed with 0.2% Bromophenol blue.
A total of 15 µg protein was loaded into each lane. Whole cell
lysates were subjected to SDS-PAGE for protein separation and then
electrophoretically transferred to a nitrocellulose membrane
(Axygen; Corning Inc.), followed by blocking using PBS containing
5% fat-free milk. The nitrocellulose membranes were incubated with
a rabbit polyclonal antibody against ING4 (cat. no. ab113425;
Abcam), an antibody against hypoxia-inducible factor 1α (HIF-1α;
cat. no. 14179; Cell Signaling Technology, Inc.) and a rabbit
polyclonal antibody against β-actin (cat. no. 103030001; HarO,
http://www.lifeqho.com/pd.jsp?id=6#_jcp=2) overnight
at 4°C. Subsequently, the membranes were incubated with a
HRP-conjugated rabbit IgG secondary antibody (cat. no. 7074; Cell
Signaling Technology, Inc. http://www.cellsignal.com/products/secondary-antibodies/anti-rabbit-igg-hrp-linked-antibody/7074?Ntk=Products&Ntt=7074)
for 1.5 h at room temperature. The immunolabeled proteins were
detected using an immobilon western chemiluminescent HRP Substrate
(WBKLS0500; MilliporeSigma™).
Anchorage-independent growth
analysis
HeLa cells infected with LV-mCherry(2A)puro-hING4 or
pEGR1-LV-mCherry(2A)puro-hING4 were trypsinized and suspended in
culture medium. A total of 900 cells were seeded in each well of a
6-well plate with complete medium and were grown for 24 h. The
cells were then radiated by X-rays at a dose of 8, 6, 4, 2 and 0
Gy. When visible clones were observed for cells free from
irradiation, all treated cells were subjected to fixation by 100%
methanol at 4°C for 10 min and then stained for 1 h with 0.1%
crystal violet at room temperature. After removing the dye,
colonies containing >50 cells were counted (captured using a
digital camera and the colonies in the photos were counted).
Cobalt chloride-imitated hypoxia
Cobalt chloride is an additive and widely used to
imitate hypoxia in cell culture (20). The present study used cobalt chloride
(cat. no. 232696; Sigma-Aldrich; Merck KGaA) at 100 µM to treat the
HeLa cells (at a density of 1.5×105/ml), maintained at
37°C in a humidified atmosphere with 5% CO2, following
X-ray exposure.
Statistical analysis
Statistical analysis was performed using SPSS 20.0
software (IBM Corp). All experiments were performed in triplicate
wells for each condition and repeated at least twice. Data are
presented as the mean ± standard error. Error bars indicate the
standard deviation. Generally, the one-way ANOVA method was used to
evaluate the differences among treatments. Multiple comparisons
among the groups were performed using the Bonferroni method.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Promoter of EGR1 is sensitive to X-ray
irradiation
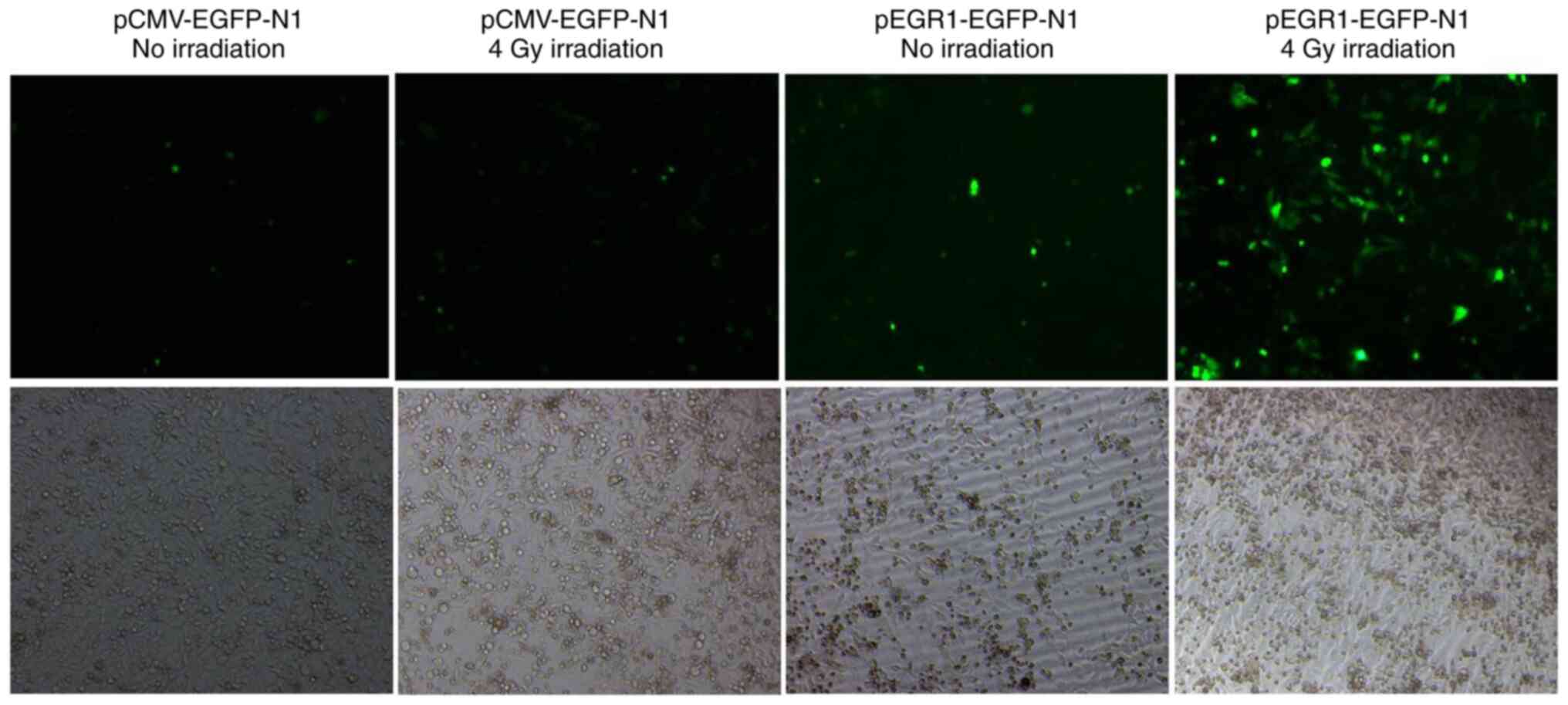
The pEGFP-N1 or pEGR1-EGFP-N1 plasmid, in which the
CMV promoter was replaced by the promoter of the EGR1 gene, was
transfected into HeLa cells. At 24 h after transfection, cells were
subjected to irradiation with X-rays at 4 Gy and fluorescence was
observed at 48 h post-exposure to X-rays. The results indicated
that the CMV promoter did not respond to irradiation induction;
however, the EGR1 promoter was sensitive to X-ray exposure
(Figs. 1 and S1). Therefore, the EGR1 promoter was
inducible by radiation and promoted the transcription and
expression of its downstream gene.
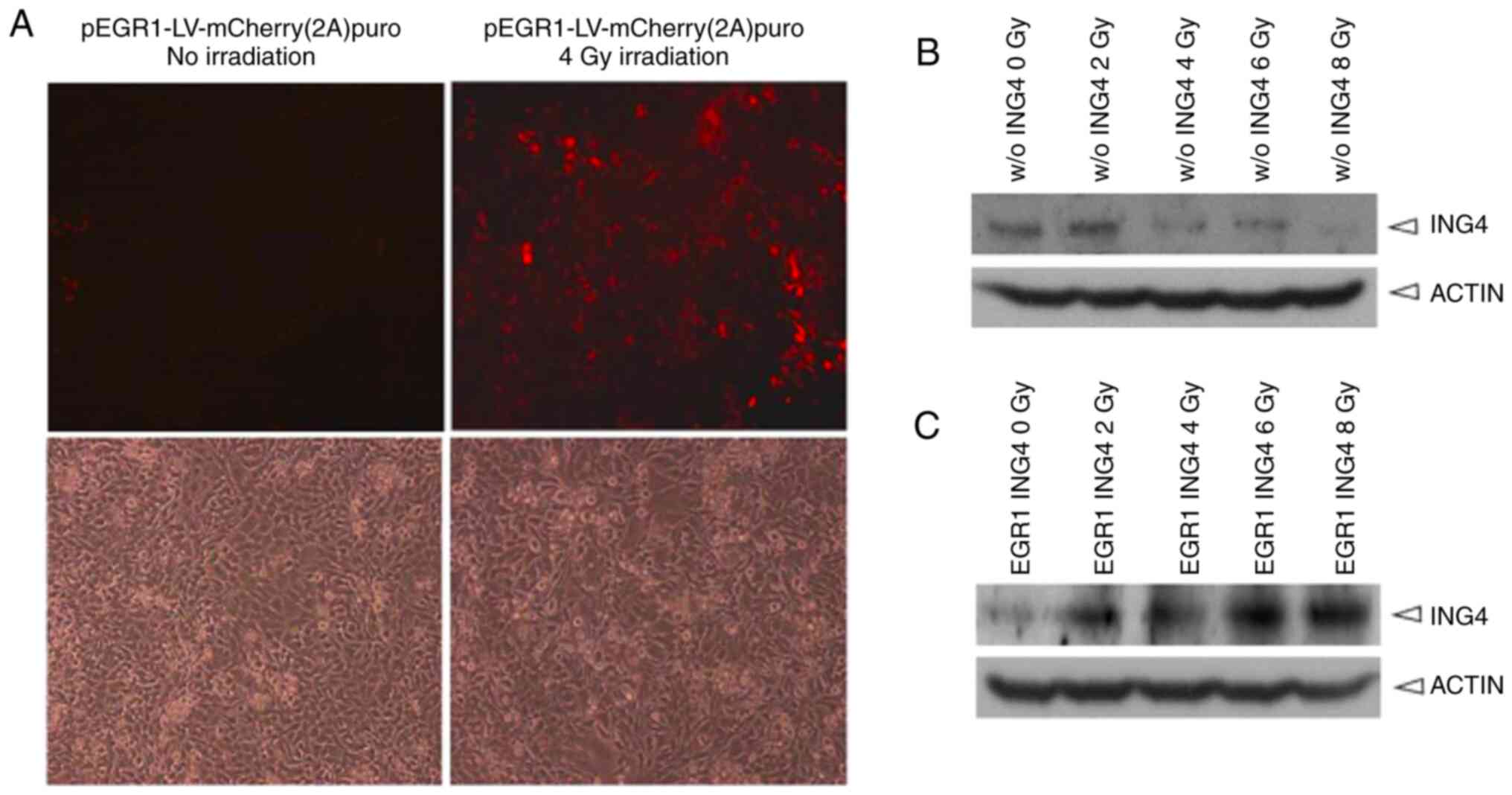
HeLa cells stably integrated with the EGR1 promoter
and the ING4 ORF cassette were screened and set up as a cell line.
The cells expressed an intense red fluorescence protein, mCherry,
following 4 Gy irradiation. By contrast, no red fluorescence was
observed when the cells were not exposed to X-rays (Fig. 2A).
The present study further examined whether the EGR1
promoter was sensitive to X-ray irradiation based on translation
levels. HeLa cells with an EGR1-driven ING4 ORF were exposed to
irradiation at the 0, 2, 4, 6 or 8 Gy for 48 h, and proteins were
then harvested and used to detect ING4 expression via western blot
analysis. ING4 protein exhibited a dose-dependent expression
pattern after the cells were irradiated with X-rays (Fig. 2C). However, HeLa cells integrated
only with ING4 ORF did not exhibit enhanced ING4 protein expression
following X-ray irradiation (Fig.
2B).
Therefore, it was concluded that the EGR1 promoter
was sensitive to X-ray induction according to transient
transfection-based green fluorescence protein, stably
integration-based red fluorescence protein and ING4 protein
expression. Since the purpose of the present study was to examine
the responsiveness of the EGR1 promoter but not ING4 to
irradiation, other negative controls, such as pEGR1-0, pCMV–ING4
and pING4 were not additionally evaluated.
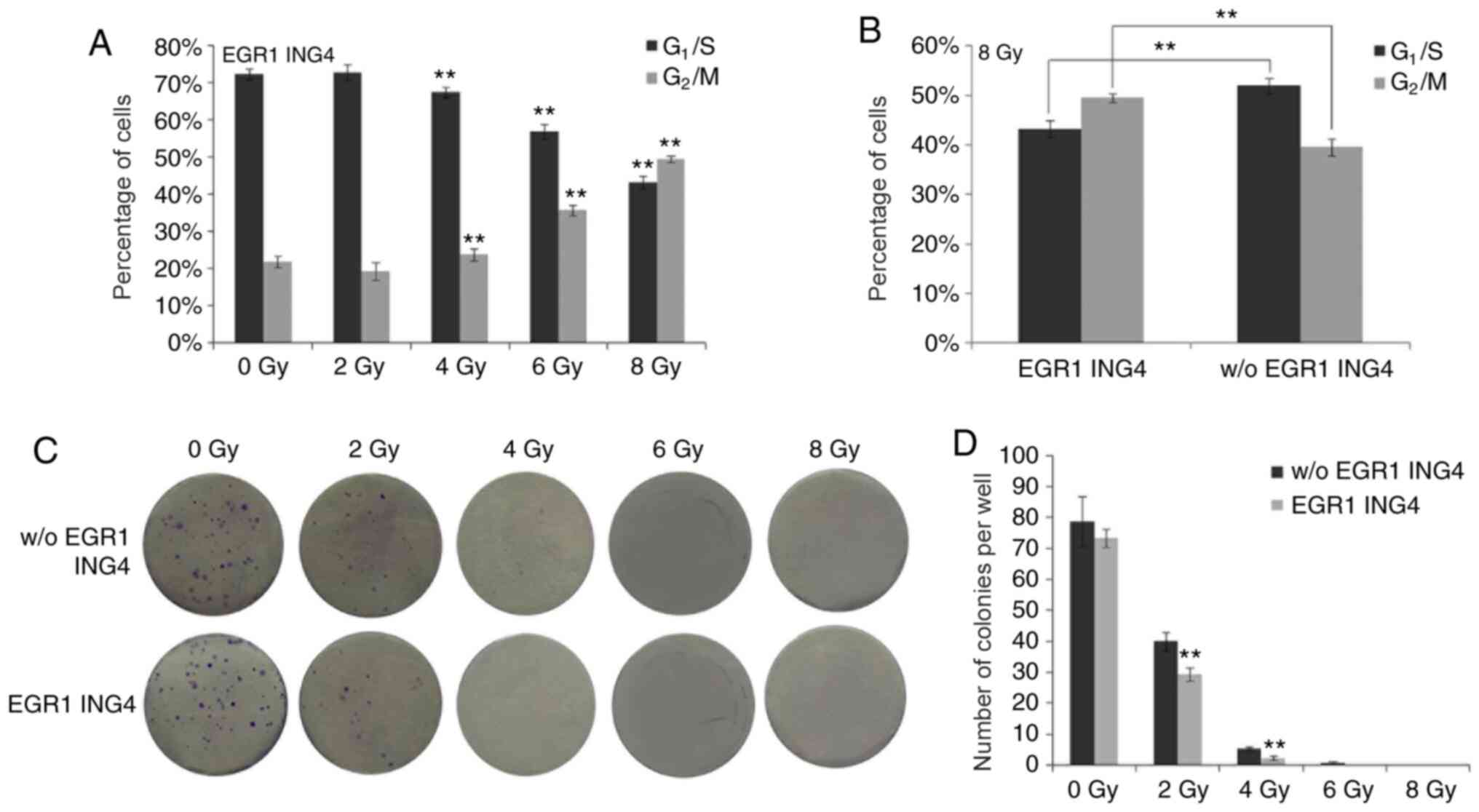
ING4 induces G2/M phase
arrest
With the increment of ING4 expression caused by the
increasing doses of irradiation, the percentage of cells in the
G2/M phase gradually increased along with a sequential
decrease in the number of cells in the G1/S phase
(P<0.001; Figs. 3A and 4). In particular, the percentage of cells
in the G2/M phase was statistically significantly higher
in the ING4-expressing HeLa cells than in the HeLa cells without
ING4 expression when 8 Gy irradiation was employed (P=0.005). By
contrast, the number of cells in the G1/S phase was
lower in the ING4-expressing HeLa cells than in the HeLa cells
without ING4 expression (P=0.008; Figs.
3B and 4). Therefore,
irradiation-induced ING4 expression led to G2/M phase
arrest, which may be responsible for the growth retardation
(decreased number of the cells) of these HeLa cells (Fig. 3D).
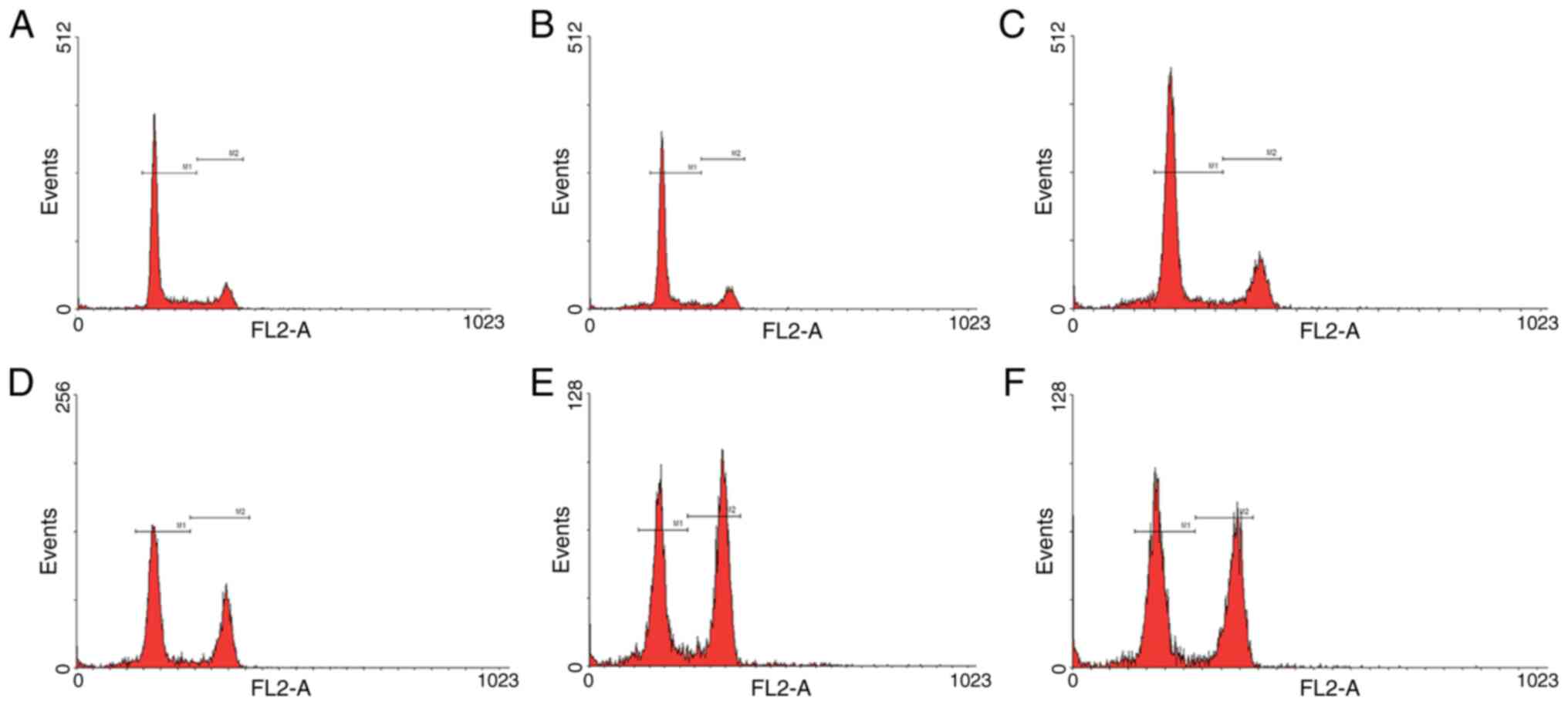 | Figure 4.Cell cycle analyses of HeLa cells by
flow cytometry under the control of different doses of irradiation.
The cell counts of HeLa cells stably integrated with EGR1 promoter
and ING4 ORF, irradiated by (A) 0, (B) 2, (C) 4, (D) 6 and (E) 8
Gy, in G1/S and G2/M phase. (F) The cell
counts of HeLa cells stably integrated with ING4 ORF without
irradiation-induced EGR1 promoter, and irradiated by 8 Gy, in
G1/S and G2/M phase. Events, cell counts; M1,
G1/S phase; M2, G2/M phase; EGR1, early
growth response factor-1; ING4, inhibitor of growth 4; ORF, open
reading frame. |
ING4 inhibits the proliferation of
HeLa cells
With the increase in the irradiation dose, ING4
expression was increased in HeLa cells, which resulted in less
colonies being formed compared with in the HeLa cells not
expressing ING4 (2 Gy, P=0.009; 4 Gy, P=0.003). By contrast, no
statistically significant differences were observed in the number
of colonies between the groups of HeLa cells not exposed to
irradiation (0 Gy; Fig. 3C and D).
No obvious colonies were observed following irradiation with 6 Gy
or 8 Gy.
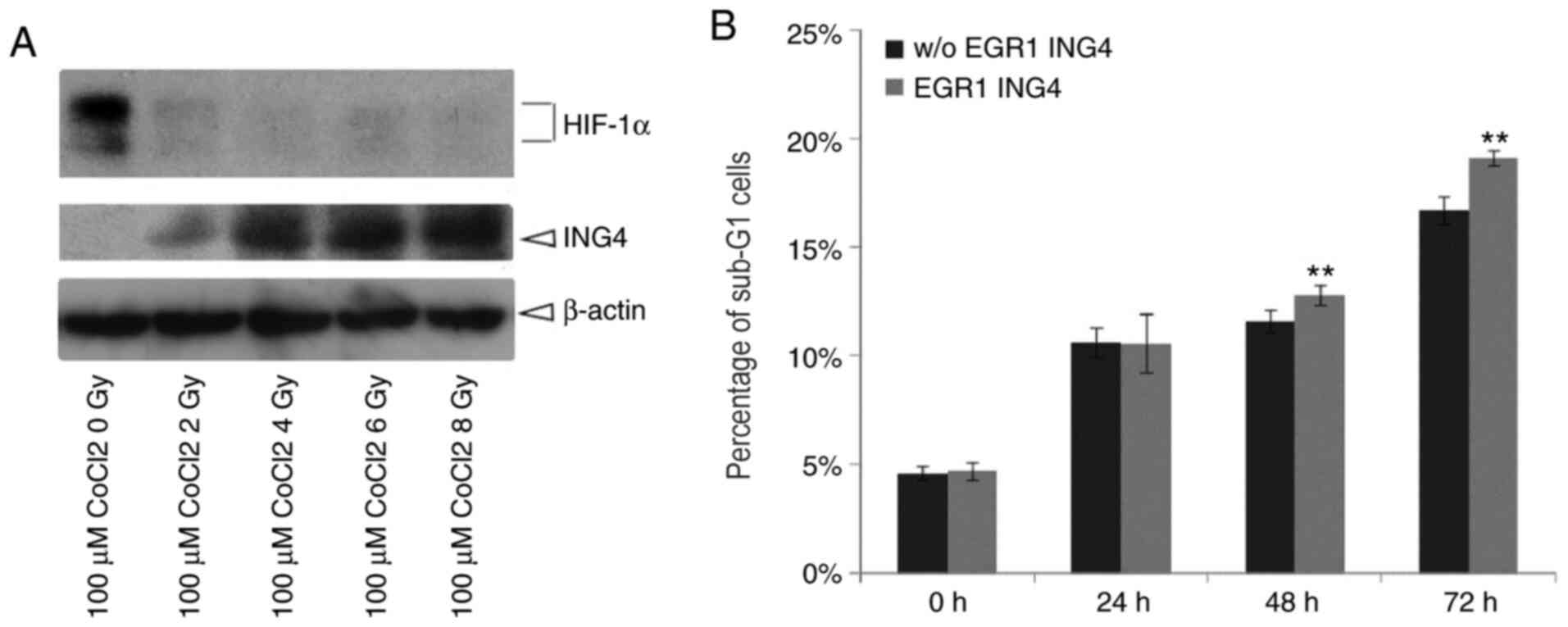
ING4 inhibits HIF-1α expression
In the present study, following irradiation with
X-rays at various doses, HeLa cells were treated with 100 µM cobalt
chloride for 24, 48 or 72 h. Proteins were then collected from HeLa
cells. HIF-1α expression markedly decreased while ING4 expression
increased (Fig. 5A).
ING4 promotes irradiation-induced
apoptosis under hypoxic conditions
Furthermore, the proportions of HeLa cells in the
sub-G1 phase under hypoxic conditions and following
X-ray exposure were detected. At 48 and 72 h, ING4 increased the
proportion of cells in the sub-G1 phase following 8 Gy
irradiation, which indicated that a greater number of HeLa cells
was apoptotic (P=0.04 and P=0.03, respectively; Figs. 5B and 6). These results demonstrate that ING4
could promote cell apoptosis under hypoxic conditions.
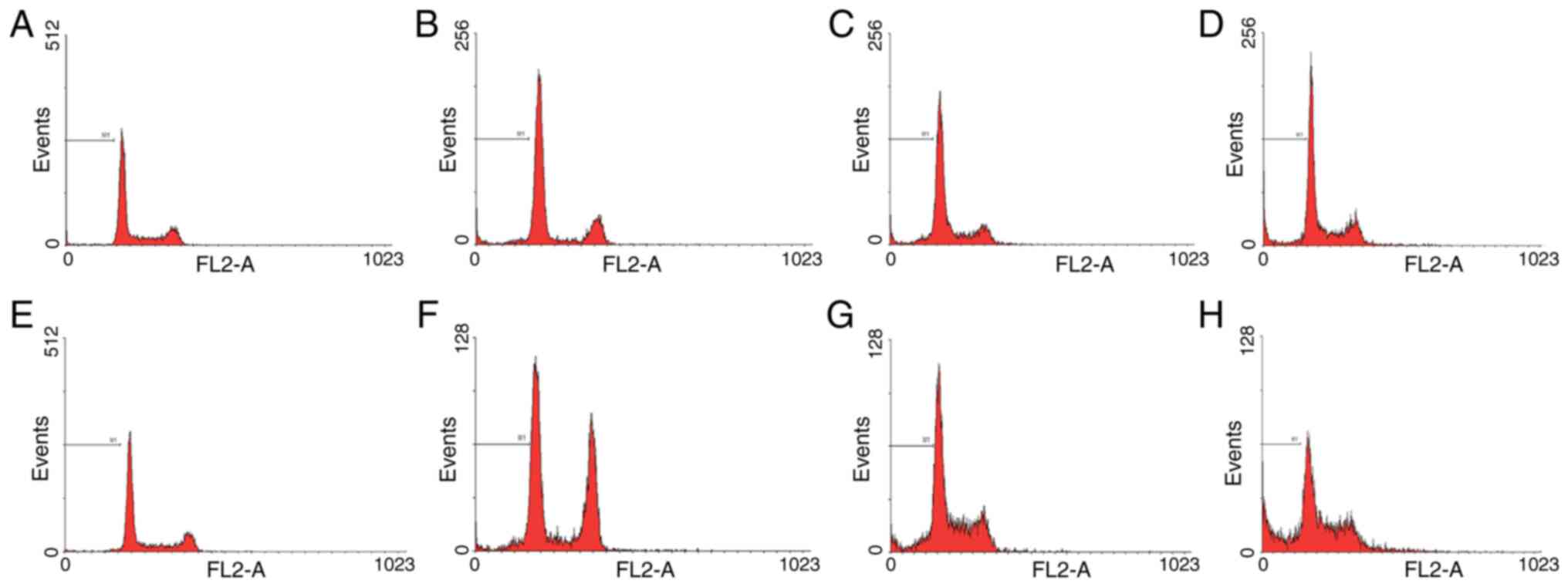 | Figure 6.Apoptosis analysis of HeLa cells by
flow cytometry with irradiation under hypoxia. Percentages of
sub-G1 phase HeLa cells stably integrated with ING4 ORF
without irradiation-induced EGR1 promoter, irradiated and analyzed
at (A) 0, (B) 24, (C) 48 and (D) 72 h post-irradiation. Percentages
of sub-G1 phase HeLa cells stably integrated with EGR1
promoter and ING4 ORF, irradiated and analyzed at (E) 0, (F) 24,
(G) 48 and (H) 72 h post-irradiation. M1, percentages of
sub-G1 phase cells (apoptotic cells); EGR1, early growth
response factor-1; ING4, inhibitor of growth 4; ORF, open reading
frame. |
Discussion
Hypoxic cancer cells are more resistant to
irradiation compared with fully oxygenated cells (21). Therefore, the extent of tumor hypoxia
is one of the most crucial biological factors affecting the
outcomes of radiotherapy (22).
Furthermore, HIF-1 has been identified to serve a pivotal role in
hypoxia-mediated radioresistance (23). Research on the effects of hypoxia has
increased in recent years; however, limited progress has been
achieved in previous studies. On the other hand, dose escalation
that aims to increase tumor control may observed the adverse
effects of the normal tissues nearby. Therefore, enhancing the
radiosensitivity of tumor cells, including overcoming hypoxia, is
considered critical in order to achieve successful radiation
therapy (24).
The strategies used to enhance radiosensitivity in
the present study included three parts. At first, ING4 suppressed
HIF-1 expression, which exerts a promoting effect on tumor growth
under hypoxic conditions (21).
Secondly, ING4 induced G2/M phase arrest and the
apoptosis of HeLa cells. Several previous studies have reported
similar results (25–28). The third part is that a lentiviral
gene therapy vector was constructed, containing human ING4 and its
upstream promoter, EGR1, which share the radiation-inducible
characteristics to activate the transcription of downstream genes.
When the experimental model was exposed to external irradiation,
ING4 was activated by the EGR1 promoter and targeted to be
expressed in irradiated sites. Therefore, the irradiation-sensitive
promoter, EGR1, facilitated therapeutic gene expression under the
control of ionizing radiation. Thus, the promoting effect on tumor
growth by hypoxic cells was inhibited when HIF-1 was suppressed by
ING4. Combined with the role of ING4 in regulating the cell cycle,
synergetic radiosensitizing effects were achieved simultaneously
with radiation therapy. The findings of the present study may
provide novel strategies for the application and efficacy
evaluation of radiosensitization in cervical cancer.
Increasing evidence has indicated that ING4 serves
an important role in cancer progression as a tumor suppressor
(5). In lung cancer tissues,
decreased ING4 expression is present in ~50% of cases and is
associated with lymph node metastasis (29). Together with the results of other
surveys, ING4 is a promising target in gene-radiotherapy (30–32).
Radiotherapy combined with tumor suppressor genes is
increasingly being used in tumor therapy (5). To achieve combined therapy, the genetic
modification of tumor cells is critical. In the present study,
ING4, as a tumor suppressor, was introduced into HeLa cells by
lentiviral transfection under the control of the EGR1 promoter. The
EGR1 promoter is sensitive to radiation. Therefore, the combination
of ING4 and radiation was more effective in the cellular model of
human cervical cancer treatment. The findings of the present study
may provide promising strategies for use in the treatment of other
types of cancer. However, these findings warrant further
investigation using in vivo models.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Shanghai Pujiang
Program (grant no. 2019PJD029)
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
HPX and YNJ were involved in design of the work. TM
was involved in acquisition of data and drafting the initial
manuscript. HPX, XW and YNJ were the major contributors in revising
the manuscript for important intellectual content. TM and HPX gave
final approval of the version to be published. TM, XW, RG, WTS and
MZ performed experimental research and analyzed experimental data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bhatla N, Berek JS, Fredes MC, Denny LA,
Grenman S, Karunaratne K, Kehoe ST, Konishi I, Olawaiye AB, Prat J,
et al: Revised FIGO staging for carcinoma of the cervix uteri. Int
J Gynaecol Obstet. 145:129–135. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chino J, Annunziata CM, Beriwal S,
Bradfield L, Erickson BA, Fields EC, Fitch K, Harkenrider MM,
Holschneider CH, Kamrava M, et al: Radiation therapy for cervical
cancer: Executive summary of an ASTRO clinical practice guideline.
Pract Radiat Oncol. 10:220–234. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lukas J, Lukas C and Bartek J: Mammalian
cell cycle checkpoints: Signalling pathways and their organization
in space and time. DNA Repair (Amst). 3:997–1007. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao Y, Li Z, Sheng W, Miao J and Yang J:
Radiosensitivity by ING4-IL-24 bicistronic adenovirus-mediated gene
cotransfer on human breast cancer cells. Cancer Gene Ther.
20:38–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang X, Zhang QL, Tian YH, Huang JC and
Ma GL: RNA interference-mediated gene silencing of cyclophilin A
enhances the radiosensitivity of PAa human lung adenocarcinoma
cells in vitro. Oncol Lett. 13:1619–1624. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Yu L, Wang Y, Zhang Y, Wang Y and
Zhang G: Expression of tumor suppressor gene ING4 in ovarian
carcinoma is correlated with microvessel density. J Cancer Res Clin
Oncol. 138:647–655. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gunduz M, Nagatsuka H, Demircan K, Gunduz
E, Cengiz B, Ouchida M, Tsujigiwa H, Yamachika E, Fukushima K,
Beder L, et al: Frequent deletion and down-regulation of ING4, a
candidate tumor suppressor gene at 12p13, in head and neck squamous
cell carcinomas. Gene. 356:109–117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coles AH and Jones SN: The ING gene family
in the regulation of cell growth and tumorigenesis. J Cell Physiol.
218:45–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu M, Xie Y, Sheng W, Miao J and Yang J:
Adenovirus-mediated ING4 gene transfer in osteosarcoma suppresses
tumor growth via induction of apoptosis and inhibition of tumor
angiogenesis. Technol Cancer Res Treat. 14:369–378. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan R, He L, Li Z, Han X, Liang J, Si W,
Chen Z, Li L, Xie G, Li W, et al: SCF(JFK) is a bona fide E3 ligase
for ING4 and a potent promoter of the angiogenesis and metastasis
of breast cancer. Genes Dev. 29:672–685. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weichselbaum RR and Kufe D: Translation of
the radio- and chemo-inducible TNFerade vector to the treatment of
human cancers. Cancer Gene Ther. 16:609–619. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kufe D and Weichselbaum R: Radiation
therapy: Activation for gene transcription and the development of
genetic radiotherapy-therapeutic strategies in oncology. Cancer
Biol Ther. 2:326–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu LL, Smith MJ, Sun BS, Wang GJ, Redmond
HP and Wang JH: Combined IFN-gamma-endostatin gene therapy and
radiotherapy attenuates primary breast tumor growth and lung
metastases via enhanced CTL and NK cell activation and attenuated
tumor angiogenesis in a murine model. Ann Surg Oncol. 16:1403–1411.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang W and Li XY: Anti-tumor effect of
pEgr-interferon-gamma-endostatin gene-radiotherapy in mice bearing
Lewis lung carcinoma and its mechanism. Chin Med J (Engl).
118:296–301. 2005.PubMed/NCBI
|
|
16
|
Arai M, Kawachi T, Setiawan A and
Kobayashi M: Hypoxia-selective growth inhibition of cancer cells by
furospinosulin-1, a furanosesterterpene isolated from an Indonesian
marine sponge. ChemMedChem. 5:1919–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Toma-Daşu I, Daşu A and Karlsson M: The
relationship between temporal variation of hypoxia, polarographic
measurements and predictions of tumour response to radiation. Phys
Med Biol. 49:4463–4475. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hickey MM and Simon MC: Regulation of
angiogenesis by hypoxia and hypoxia-inducible factors. Curr Top Dev
Biol. 76:217–257. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leskov KS, Criswell T, Antonio S, Li J,
Yang CR, Kinsella TJ and Boothman DA: When X-ray-inducible proteins
meet DNA double strand break repair. Semin Radiat Oncol.
11:352–372. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rovetta F, Stacchiotti A, Faggi F,
Catalani S, Apostoli P, Fanzani A and Aleo MF: Cobalt triggers
necrotic cell death and atrophy in skeletal C2C12 myotubes. Toxicol
Appl Pharmacol. 271:196–205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rockwell S, Dobrucki IT, Kim EY, Marrison
ST and Vu VT: Hypoxia and radiation therapy: Past history, ongoing
research, and future promise. Curr Mol Med. 9:442–458. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Begg AC, Stewart FA and Vens C: Strategies
to improve radiotherapy with targeted drugs. Nat Rev Cancer.
11:239–253. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moeller BJ and Dewhirst MW: HIF-1 and
tumour radiosensitivity. Br J Cancer. 95((1)): 1–5. 2006.(Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaanders JH, Bussink J and van der Kogel
AJ: ARCON: A novel biology-based approach in radiotherapy. Lancet
Oncol. 3:728–737. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Z, Xie Y, Sheng W, Miao J, Xiang J and
Yang J: Tumor-suppressive effect of adenovirus-mediated inhibitor
of growth 4 gene transfer in breast carcinoma cells in vitro and in
vivo. Cancer Biother Radiopharm. 25:427–437. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Du Y, Cheng Y and Su G: The essential role
of tumor suppressor gene ING4 in various human cancers and
non-neoplastic disorders. Biosci Rep. 39:BSR201807732019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie Y, Zhang H, Sheng W, Xiang J, Ye Z and
Yang J: Adenovirus-mediated ING4 expression suppresses lung
carcinoma cell growth via induction of cell cycle alteration and
apoptosis and inhibition of tumor invasion and angiogenesis. Cancer
Lett. 271:105–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
You Q, Wang XS, Fu SB and Jin XM:
Downregulated expression of inhibitor of growth 4 (ING4) in
advanced colorectal cancers: A non-randomized experimental study.
Pathol Oncol Res. 17:473–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang QS, Li M, Zhang LY, Jin Y, Tong DD,
Yu Y, Bai J, Huang Q, Liu FL, Liu A, et al: Down-regulation of ING4
is associated with initiation and progression of lung cancer.
Histopathology. 57:271–281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Galal El-Shemi A, Mohammed Ashshi A, Oh E,
Jung BK, Basalamah M, Alsaegh A and Yun CO: Efficacy of combining
ING4 and TRAIL genes in cancer-targeting gene virotherapy strategy:
First evidence in preclinical hepatocellular carcinoma. Gene Ther.
25:54–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang H, Zhou X, Xu C, Yang J, Xiang J,
Tao M and Xie Y: Synergistic tumor suppression by
adenovirus-mediated ING4/PTEN double gene therapy for gastric
cancer. Cancer Gene Ther. 23:13–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Yang J, Sheng W, Xie Y and Liu J:
Adenovirus-mediated ING4/PTEN double tumor suppressor gene
co-transfer modified by RGD enhances antitumor activity in human
nasopharyngeal carcinoma cells. Int J Oncol. 46:1295–1303. 2015.
View Article : Google Scholar : PubMed/NCBI
|