Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second
most common primary hepatic malignant tumor after hepatocellular
carcinoma (HCC) and its age-adjusted incidence rate increased by
almost 20% from 0.75 per 100,000 in 1992 to 0.88 per 100,000 in
1999 (1). It is a highly fatal
malignancy with a median postoperative survival time of 28 months
(2). Once ICC has become
unresectable, the median survival time is only 10 months (3). The main treatments for ICC include
surgery, locoregional therapy and systemic chemotherapy. However,
the efficacy of locoregional therapy for advanced or unresectable
ICC is limited (4,5). A meta-analysis (2) showed that patients with ICC did not
benefit from systemic chemotherapy based on gemcitabine,
fluorouracil or oxaliplatin. Furthermore, the marked heterogeneity
of ICC has led to a lack of effective targeted agents for the
treatment of this fatal disease (5).
Consequently, new drugs or therapeutic strategies are urgently
required to improve the survival prognosis of patients with ICC.
Immunotherapy is a rapidly evolving therapeutic strategy in
oncology. The immune system has the potential to recognise and
eradicate cancer cells, and is regulated by a complex network of
immune checkpoints. Cancers are able to evade host antitumor immune
responses through immune escape mechanisms. Immune checkpoint
inhibitors, including antibodies against programmed cell death
protein 1 (PD-1)/programmed death ligand 1orcytotoxic
T-lymphocyte-associated protein 4, interrupt the mechanisms of
immune resistance and exhibit durable and powerful antitumor
activity in specific subsets of patients across several types of
tumor (6).
Herpesvirus entry mediator (HVEM), also known as
tumor necrosis factor receptor superfamily 14, is widely expressed
on a range of hematopoietic cells, including B cells, T cells, NK
cells, monocytes and immature dendritic cells, and several
non-hematopoietic cells and tissues, including the liver, kidney
and lung (7,8). HVEM is a ligand for cytokines of the
TNF superfamily, including lymphotoxin α and lymphotoxin-related
inducible ligand that competes for glycoprotein D binding to HVEM
on T cells (LIGHT), or a receptor for members of the immunoglobulin
superfamily, including CD160 and B- and T-lymphocyte attenuator
(BTLA), under diverse physiological and pathological conditions.
Interactions of HVEM with different family members occur at
distinct sites, and result in some opposing functions. When HVEM
expressed by antigen-presenting cells interacts with TNF
superfamily cytokines, the activation of T-cell proliferation and
cytokine production is triggered. Conversely, the binding of HVEM
to CD160 or BTLA results in inhibitory signaling to T cells
(9). However, Ritthipichai et
al (10) reported that BTLA
harnesses cytosolic adaptor growth factor receptor-bound protein 2
(GRB2) to provide co-stimulatory signals to CD8+ T
cells. Therefore, it appears that the HVEM/BTLA pathway plays a
dual function in T-cell activation.
In addition to normal hematopoietic and
non-hematopoietic cells, HVEM expression is detected in most cancer
cells, including pancreatic and ampullary cancer (11), HCC (12), oesophageal squamous cell carcinoma
(13), gastric cancer (14), clear cell renal cell carcinoma
(15), ovarian cancer (16,17),
breast cancer (18) and melanoma
(19). In the majority of cases, the
high expression of HVEM is associated with poor prognosis (12–19) and
is associated with low levels of tumor-infiltrating lymphocytes
(TILs) and downregulation of the local immune response (12,13,18).
These previous studies suggest that HVEM is potentially an
independent prognostic marker in patients with specific cancers and
a potential target for antitumor therapy. However, the precise
function of HVEM in ICC has rarely been studied. The present study
aimed to investigate the clinical impact of HVEM in ICC, including
its prognostic value and association with clinicopathological
features and immune status.
Materials and methods
Patients and tissue samples
A total of 102 consecutive patients with ICC who
underwent surgical treatment at Tianjin Medical University Cancer
Institute and Hospital from January 2012 to December 2017 were
evaluated in the present study. Cases with combined HCC-CCA,
composed of typical HCC and representative ICC, were excluded. In
addition, patients who had undergone preoperative treatments, such
as radiofrequency ablation, adjuvant chemotherapy or radiation
therapy, were excluded. All patients were reviewed to confirm the
diagnosis of ICC and to determine the stage according to the
American Joint Committee on Cancer staging system (8th edition,
2017). Baseline clinicopathological features and information were
reviewed and analyzed. All cases were regularly followed up every 3
months to determine whether tumor recurrence occurred. Disease-free
survival (DFS) was measured from the date of surgery to the date of
first recurrence or last follow-up, whereas overall survival (OS)
was defined as the interval between the date of first diagnosis of
ICC and the date of death or last follow-up.
Formalin-fixed paraffin-embedded tissues and
hematoxylin-eosin stained slides from the 102 cases of ICC were
collected. Tissue microarrays (TMAs) with a thickness of 4-µm
consisting of 2-mm cores of tumor samples were constructed by
selecting a typical tumor region and a representative peritumoral
area from each case.
The Medical Ethics Committee of Tianjin Medical
University Cancer Institute and Hospital approved the present study
(approval no. bc2019065), and written informed consent was obtained
from all patients or their legal guardian.
Immunohistochemistry (IHC)
TMA slides were heated at 65°C for 2 h, routinely
dewaxed in xylene and rehydrated in gradient ethanol. After antigen
retrieval, the slides were blocked with 3% hydrogen peroxide
(PV-6002; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.)
for 10 min at room temperature to quench the endogenous peroxidase
activity. The sections were incubated with primary antibodies at
4°C overnight and 37°C for 1 h, followed by HRP-conjugated
secondary antibody at 37°C for 1 h. Then, the sections were
visualised with 3,3′-diaminobenzidine (ZLI-9017; OriGene
Technologies) for 5 min at room temperature and counterstained with
hematoxylin for 1 min at room temperature. Appropriate positive
(formalin-fixed paraffin-embedded sections of human lung tissue)
and negative controls were designed and used for each round. The
sections were observed and recorded using a light microscope (BX61,
Olympus).
The primary antibodies used in the present study
were as follows: HVEM/TNFRSF14 (MAB3561; concentration, 16 µg/ml;
R&D Systems, Inc.), CD4 (ab133616; dilution, 1:500; Abcam), CD8
(ab17147; dilution, 1:50; Abcam) andCD45RO (sc-1183; dilution,
1:500; Santa Cruz Biotechnology, Inc.).
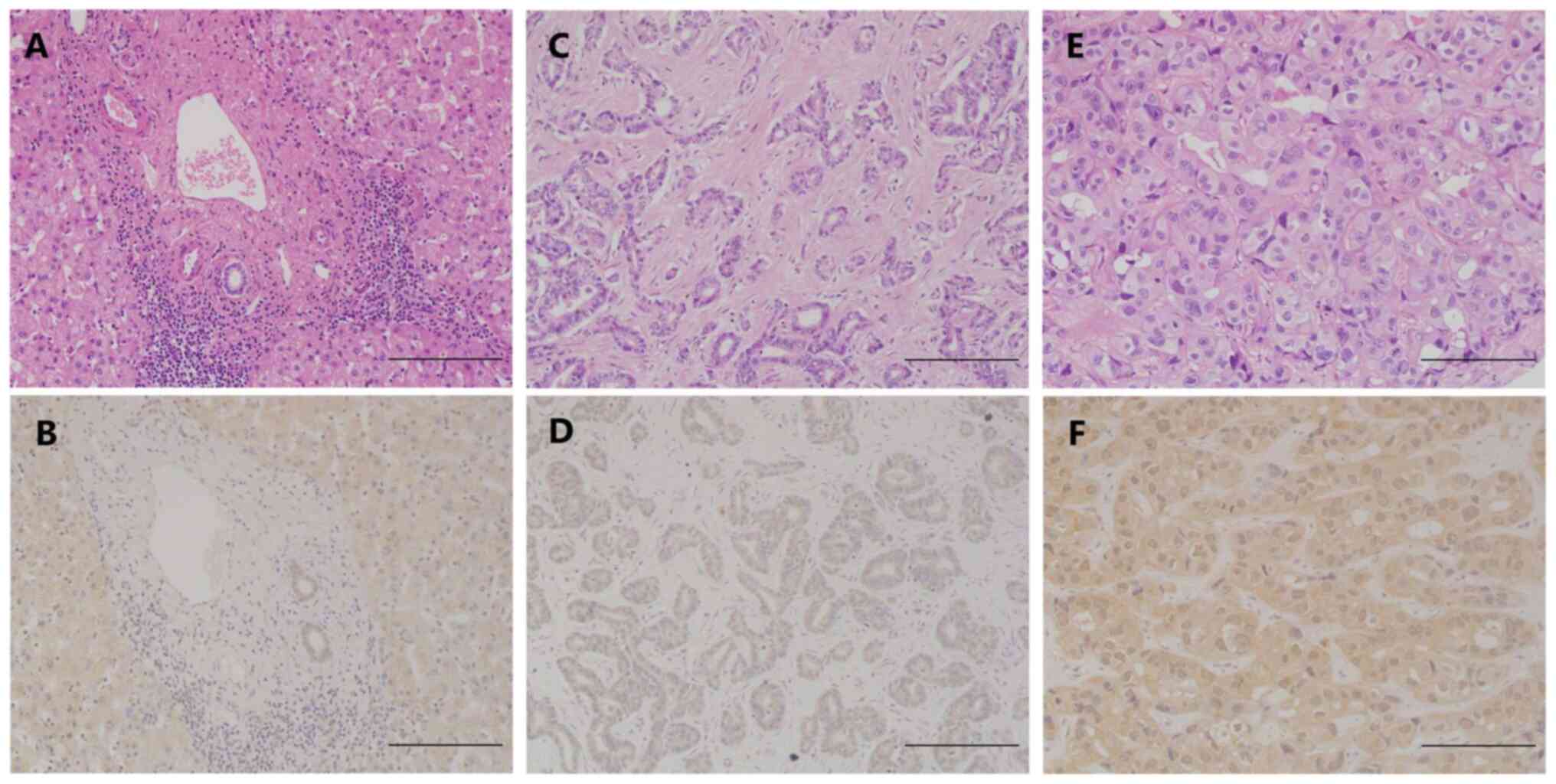
The staining pattern revealed that HVEM was
expressed in the cytoplasm and cell membrane. The staining was
evaluated semiquantitatively by multiplying scores for the
intensity and percentage of positive tumor cells; representative
images are presented in Fig. 1. The
intensity of staining was divided into four subgroups: Score 0,
negative; score 1, mild; score 2, moderate; and score 3, strong.
The percentage of staining was determined as follows: Score 0,
<1%; score 1, 1–25%; score 2, 26–50%; score 3, 51–75% and score
4, 76–100% (17). Total scores of ≤6
and ≥8 were defined as low and high HVEM expression,
respectively.
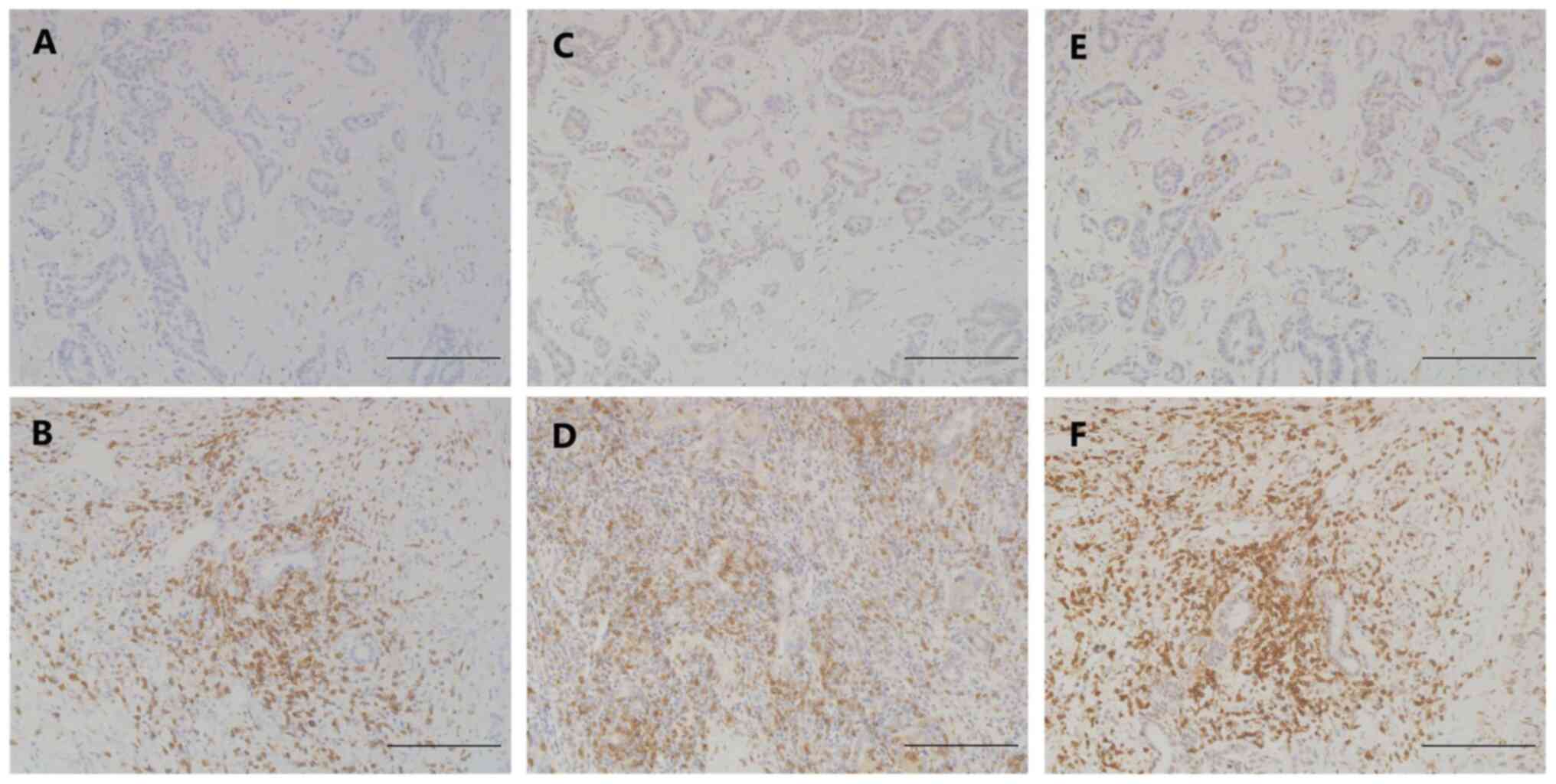
In the case of lymphocyte subset staining, the
numbers of CD4+, CD8+ and CD45RO+
T cells that infiltrated into the ICC tissues were counted
manually. Four randomly selected areas were counted for each sample
at a magnification of ×200 (Fig. 2).
The average value was recorded for each sample.
All IHC scoring was performed independently by two
investigators without knowledge of the clinical data. Final
agreement on the scores was reached through full discussion.
Analysis of histological
classification and frequent mutations in ICC
Our previous study (20) classified this cohort into two
subtypes, namely large-duct and small-duct types with regard to
histological characteristics, S100P expression and Alcian blue
scores (Data S1). The number of cases identified as large-duct and
small-duct types of ICC were 21 (20.6%) and 81 (79.4%)
respectively. Frequently mutated genes in ICC, including isocitrate
dehydrogenase 1/2 (IDH1/2), BRCA1 associated protein 1 (BAP1),
AT-rich interaction domain 1A (ARID1A) and polybromo 1 (PBRM1) were
also analyzed (Data S1). DNA sequencing showed that 17 cases
(16.7%) harboured IDH1/2 mutations, whereas IHC analysis showed the
loss of expression of BAP1, ARID1A and PBRM1 in 46 (45.1%), 20
(19.6%) and 33 (32.4%) of cases, respectively.
Statistical analysis
Categorical variables (clinicopathological factors)
are presented as total numbers and frequencies and were evaluated
by Chi-square test. Continuous variables (number of TILs) are
presented as medians with ranges and were compared using
Mann-Whitney U test. Survival analysis was calculated by the
Kaplan-Meier method and assessed by log-rank test for univariate
analysis. Cox proportional hazards model was used for analyzing the
prognostic value of HVEM expression and other clinical factors. The
results are presented as the hazard ratio (HR) and 95% confidence
intervals (CIs). A two-tailed P<0.05 was considered to indicate
a statistically significant difference. SPSS Statistics 24 (IBM
Corp.) was used for data analysis.
Results
HVEM expression in ICC and adjacent
liver tissues
HVEM expression in the ICC tissues exhibited
significant heterogeneity. No HVEM staining was detected in the
tumor tissues of 10 cases (9.8%), whereas the tumor tissues of the
other cases exhibited mild-to-strong staining. According to the
aforementioned classification criteria, low and high HVEM
expression was detected in 50 and 52 of the 102 specimens,
respectively. In addition, the peritumoral liver tissues in all
cases presented mild-to-moderate staining of HVEM (Fig. 1, Table
I).
 | Table I.Association between HVEM expression
and clinicopathological characteristics in 102 patients with
intrahepatic cholangiocarcinoma. |
Table I.
Association between HVEM expression
and clinicopathological characteristics in 102 patients with
intrahepatic cholangiocarcinoma.
|
| HVEM
expression |
|
|---|
|
|
|
|
|---|
| Variables | Low | High | P-value |
|---|
| Sex |
|
| 0.439 |
|
Male | 26 (45.6) | 31 (54.4) |
|
|
Female | 24 (53.3) | 21 (46.7) |
|
| Age (years) |
|
| 0.411 |
|
≤60 | 30 (52.6) | 27 (47.4) |
|
|
>60 | 20 (44.4) | 25 (55.6) |
|
| Hepatitis B |
|
| 0.397 |
|
Negative | 32 (52.5) | 29 (47.5) |
|
|
Positive | 18 (43.9) | 23 (56.1) |
|
| Neu
(×109/l) |
|
| 0.723 |
|
≤5.0 | 41 (48.2) | 44 (51.8) |
|
|
>5.0 | 9 (52.9) | 8 (47.1) |
|
| Lym
(×109/l) |
|
| 0.031 |
|
≤2.0 | 38 (56.7) | 29 (43.3) |
|
|
>2.0 | 12 (34.3) | 23 (65.7) |
|
| Mon
(×109/l) |
|
| 0.727 |
|
≤0.6 | 33 (47.8) | 36 (52.2) |
|
|
>0.6 | 17 (51.5) | 16 (48.5) |
|
| CEA (µg/l) |
|
| 0.036 |
| ≤5 | 36 (43.9) | 46 (56.1) |
|
|
>5 | 14 (70.0) | 6 (30.0) |
|
| CA19-9 (U/ml) |
|
| 0.570 |
|
≤39 | 28 (46.7) | 32 (53.3) |
|
|
>39 | 22 (52.4) | 20 (47.6) |
|
| Histological
grade |
|
| 0.868 |
|
G1-G2 | 29 (48.3) | 31 (51.7) |
|
| G3 | 21 (50.0) | 21 (50.0) |
|
| T category |
|
| 0.193 |
|
T1-T2 | 42 (46.7) | 48 (53.3) |
|
|
T3-T4 | 8 (66.7) | 4 (33.3) |
|
| N category |
|
| 0.141 |
| N0 | 19 (51.4) | 18 (48.6) |
|
| N1 | 13 (72.2) | 5 (27.8) |
|
| M category |
|
| 0.156 |
| M0 | 44 (46.8) | 50 (53.2) |
|
| M1 | 6 (75.0) | 2 (25.0) |
|
| TNM stage |
|
| 0.043 |
|
I–II | 16 (48.5) | 17 (51.5) |
|
|
III–IV | 16 (76.2) | 5 (23.8) |
|
| Histological
subtype |
|
| 0.021 |
|
Large-duct | 15 (71.4) | 6 (28.6) |
|
|
Small-duct | 35 (43.2) | 46 (56.8) |
|
| IDH1/2 |
|
| 0.479 |
| Wild
type | 43 (50.6) | 42 (49.4) |
|
|
Mutant | 7 (41.2) | 10 (58.8) |
|
| BAP1 |
|
| 0.010 |
|
Lost | 29 (63.0) | 17 (37.0) |
|
|
Retained | 21 (37.5) | 35 (62.5) |
|
| Sex |
|
| 0.439 |
| IRID1A |
|
| 0.273 |
|
Lost | 12 (60.0) | 8 (40.0) |
|
|
Retained | 38 (46.3) | 44 (53.7) |
|
| PBRM1 |
|
| 0.727 |
|
Lost | 17 (51.5) | 16 (48.5) |
|
|
Retained | 33 (47.8) | 36 (52.2) |
|
Association between HVEM expression
and clinicopathological characteristics in patients with ICC
The study cohort included 57 males (55.9%) and 45
females (44.1%), with a mean age of 57.7±9.4 years (range, 28–77
years). Fifty-five cases (53.9%) underwent lymphadenectomy.
Patients with high HVEM expression had an increased
peripheral blood lymphocyte (PBL) concentration (P=0.031),
decreased CEA concentration (P=0.036), low TNM stage (P=0.043) and
high frequencies of small-duct histological subtype (P=0.021) and
BAP1 retained expression (P=0.010) (Table I). The association between HVEM
expression and PBL concentration indicated that HVEM expression
might enhance the immune response by increasing the number of PBLs
in patients with intrahepatic cholangiocarcinoma.
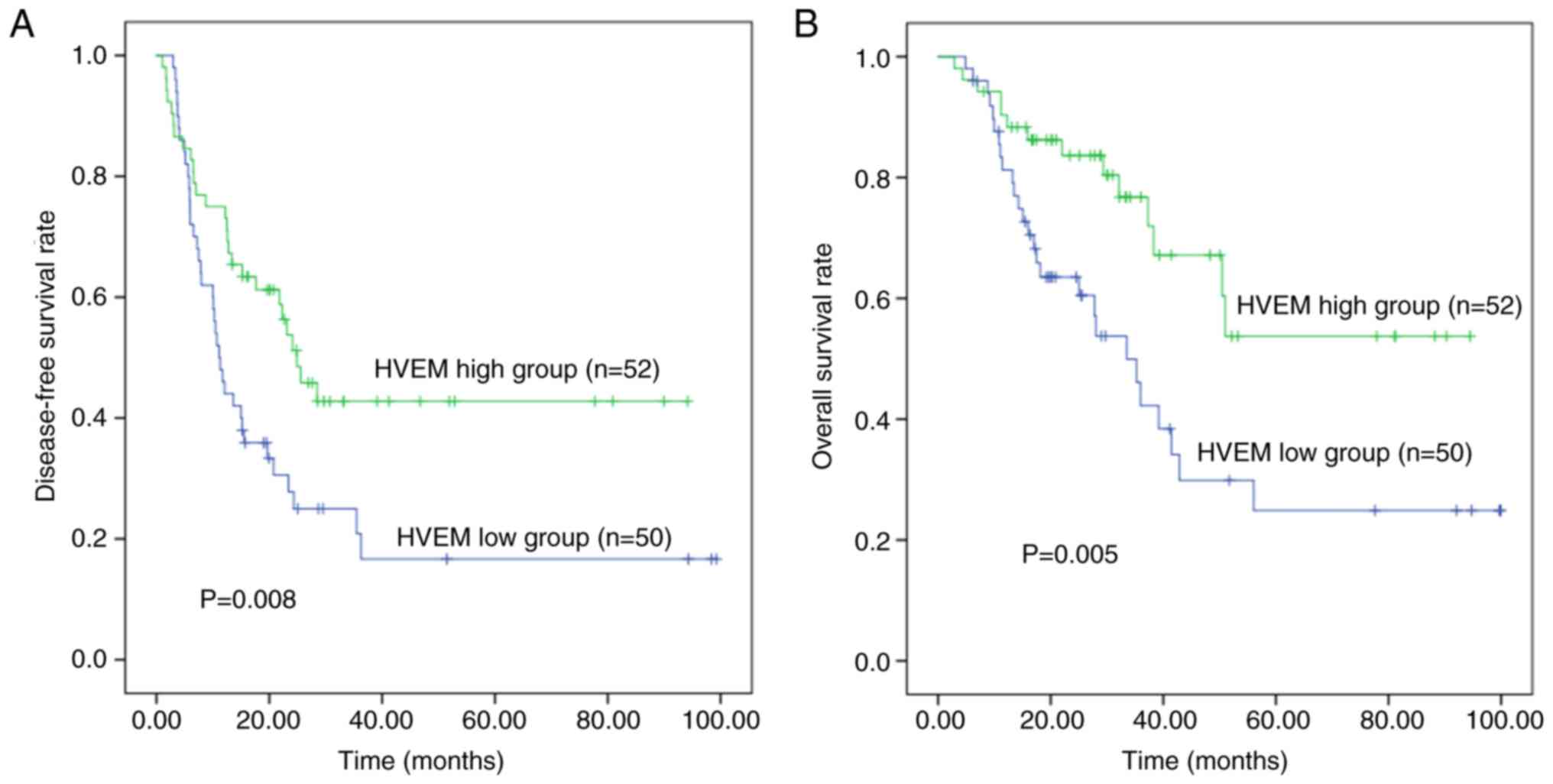
Prognostic significance of HVEM in
patients with ICC
Survival analysis revealed 3-year DFS and OS rates
of 30.4 and 59.4%, respectively. The median survival time was 42.9
months, and the median follow-up period was 25.1 months (range,
4.9–100.0 months).
Univariate analysis showed that patients with ICC
and high HVEM expression had significantly improved the survival
DFS time (P=0.008) and OS (P=0.005) than patients with low HVEM
expression. In addition, the neutrophil-lymphocyte ratio, CA19-9
concentration, T category and M category were prognostic factors
for DFS and OS in ICC (P<0.020). Multivariate analysis included
these five factors with P<0.02, and revealed that high HVEM
expression was a favorable independent predictor of OS (P=0.034,
HR=0.486, 95% CI=0.249–0.945) in ICC. However, HVEM expression only
tended to be an independent prognostic factor for DFS (P=0.054,
HR=0.604, 95% CI=0.361–1.009) in ICC, because the association with
DFS did not reach statistical significance (Fig. 3, Table
II).
 | Table II.Univariate and multivariate analysis
for prognostic factors in intrahepatic cholangiocarcinoma. |
Table II.
Univariate and multivariate analysis
for prognostic factors in intrahepatic cholangiocarcinoma.
|
| DFS | OS |
|---|
|
|
|
|
|---|
|
|
| Multivariate |
| Multivariate |
|---|
|
|
|
|
|
|
| Variables | Univariate
P-value | HR | 95% CI | Univariate
P-value | P-value | HR | 95% CI | P-value |
|---|
| NLR, ≤3 vs.
>3 | 0.012 | 1.380 | 0.699–2.727 | 0.353 | 0.004 | 1.798 | 0.785–4.115 | 0.165 |
| CA19-9, ≤40 vs.
>40 U/ml | 0.017 | 1.446 | 0.857–2.441 | 0.167 | 0.019 | 1.549 | 0.794–3.022 | 0.200 |
| T category, T1-T2
vs. T3-T4 | 0.013 | 1.628 | 0.757–3.500 | 0.212 | 0.000 | 2.707 | 1.155–6.344 | 0.022 |
| M category, M0 vs.
M1 | 0.000 | 2.601 | 1.054–6.419 | 0.038 | 0.009 | 1.281 | 0.453–3.620 | 0.641 |
| HVEM expression,
low vs. high | 0.008 | 0.604 | 0.361–1.009 | 0.054 | 0.005 | 0.486 | 0.249–0.945 | 0.034 |
Association of HVEM expression with
TILs in ICC
TILs were investigated by IHC to explore the
underlying mechanism of the predictive value of HVEM expression in
ICC. The number of CD4+, CD8+ and
CD45RO+ TILs in different ICC tissue samples varied
significantly and did not follow a normal distribution. The results
revealed that HVEM expression was not significantly associated with
CD4+ (P=0.512), CD8+ (P=0.750) or
CD45RO+ (P=0.078) TILs (Fig.
2, Table III). The results
indicate that HVEM expression has no significant effect on the
infiltration of T-cell subsets into ICC tissues.
 | Table III.Association between tumor HVEM
expression and TILs in intrahepatic cholangiocarcinoma. |
Table III.
Association between tumor HVEM
expression and TILs in intrahepatic cholangiocarcinoma.
|
| HVEM
expression |
|
|---|
|
|
|
|
|---|
| Variables | Low | High | P-value |
|---|
| CD4+ TILs | 55 (1–1,196) | 44 (2–842) | 0.512 |
| CD8+ TILs | 20 (1–573) | 17 (1–599) | 0.750 |
| CD45RO+ TILs | 58 (3–782) | 36 (2–774) | 0.078 |
Prognostic value of PBL and TIL
subsets in ICC
Survival analysis demonstrated that patients with
high PBL concentration shad a significantly prolonged OS time
(P=0.048) and an increased DFS time that was not statistically
significant (P=0.097) in ICC (Fig.
S1). Meanwhile, CD4+, CD8+ and
CD45RO+ TILs were not significantly associated with DFS
(P=0.934, P=0.717 and P=0.816, respectively) or OS (P=0.958,
P=0.485 and P=0.416, respectively) in patients with ICC (Figs. S2–S4).
Discussion
Immunotherapeutic strategies aim to enhance the host
antitumor immune response and restrain the ability of tumors to
evade that response by blocking co-inhibitory signals. Given the
limited efficacy of the PD-1-targeting antibody pembrolizumab in
the treatment of biliary tract cancer in clinical trials (21), targeting HVEM could be a
complementary or alternative strategy to improve the outcome of
patients with ICC. However, it is necessary to determine the
expression status of HVEM and its association with clinical outcome
in patients with ICC in order to support this suggestion.
Firstly, the present study confirmed that HVEM was
expressed in 90.2% of 102 ICC specimens using IHC, and found that
high HVEM expression was a favorable independent predictor of
postoperative survival and associated with a trend towards low
CD45RO+ TIL concentrations in patients with ICC. These
data are consistent with a recent study by Sideras et al
(11), who showed that high tumor
expression of HVEM (P=0.001) and a high CD8/FoxP3 TIL ratio
(P=0.006) were significantly associated with improved
cancer-specific survival in patients with pancreatic and ampullary
cancer. In another study, a bioinformatic analysis using The Cancer
Genome Atlas database confirmed that the expression of HVEM mRNA
was positively associated with OS in bladder cancer (22). However, these data are inconsistent
with results obtained in previous studies on other tumor types
(12–19). Thus, the function of HVEM in tumors
is complex and may be tumor-type specific. Notably, HVEM is an
immune checkpoint molecule that provides bidirectional signals in
T-cell activation based on the ligands and intracytoplasmic
effectors it interacts with. A previous study suggested that the
co-stimulatory HVEM/LIGHT signaling pathway facilitates the
activation of tumor-specific T cells, leading to the eradication of
tumors (23). BTLA is predominantly
located in tumor-specific T cells, and its binding to HVEM in tumor
cells suppresses the response of tumor-specific CD8+ T
cells, resulting in tumor immune evasion (9). BTLA also provides co-stimulatory
signals in CD8+ T cells by harnessing the cytosolic
adaptor GRB2 (10). However, the
immunosuppressive function of HVEM appears to be predominantly
effective in carcinoma. In several other malignancies, the high
expression of HVEM has been found to be associated with low levels
of tumor-infiltrating T cells and the decreased expression of
interferon-γ, perforin and granzyme B (12,13,18).
HVEM gene silencing can significantly inhibit the proliferation and
growth of tumor cells by inducing CD8+ cells and
upregulating the local immune response in esophageal squamous cell
carcinoma (13). Knockdown of HVEM
has been shown to increase the sensitivity of activated T cells in
ovarian cancer (13,16). In addition to having a modulating
effect on immune function, HVEM may act directly on tumor cells. In
oesophageal cancer (13) and clear
cell renal cell carcinoma (15),
HVEM gene silencing was shown to significantly reduce cell
proliferation activity in vitro and in vivo. Thus,
HVEM may drive tumor development via multiple pathways. However,
the results of the present study of ICC do not appear to support
these theories that high expression of HVEM can inhibit the immune
function of T cells, resulting in tumor development. In the present
study, high HVEM expression was associated with decreased CEA
levels (P=0.036), low TNM stage (P=0.043) and improved survival
outcome (P<0.01). This may be because, firstly the
co-stimulatory signals from HVEM play a dominant role in the
progression of ICC. In a study of melanoma, the tumor expression of
HVEM was shown to be positively associated with the expression of
BTLA on TILs by co-immunofluorescence analysis (19). Therefore, HVEM/BTLA may inhibit tumor
progression primarily by interacting with the cytoplasmic adapter
GRB2 to stimulate T-cell activation in ICC. Furthermore, increased
immune responses may occur due to the binding of HVEM with LIGHT or
other checkpoint regulators that have not yet been confirmed in
patients with ICC. Secondly, mutation of HVEM in the extracellular
or cytoplasmic domain may prevent it from binding to its ligands or
influence the recruitment of adaptor protein (9), resulting in distinct roles of HVEM
expression in different types of tumors. The HVEM signaling network
in cancer remains unknown. Given the evident genetic and
histological heterogeneity of ICC, further study is required to
reveal the precise signaling pathways in which HVEM participates,
and determine whether HVEM may be an effective therapeutic target
in ICC.
The immune activity of HVEM can be indirectly
demonstrated by the numbers of PBLs and TILs, which are closely
associated with patient survival in solid tumors. Previous studies
showed a significant association of high HVEM expression with low
levels of CD4+, CD8+ and CD45RO+
TIL and poor prognosis in HCC (12)
and esophageal squamous cell carcinoma (13). Tsang et al (18) observed that outcomes were poor in
patients with breast cancer with low levels of TIL and
HVEM-positive tumors. These findings indicate that HVEM suppresses
TILs and promotes tumor progression. However, the present study
found that HVEM expression was not significantly associated with
CD4+ (P=0.512), CD8+ (P=0.750) or
CD45RO+ (P=0.078) TILs, indicating that HVEM expression
has no significant effect on the infiltration of T-cell subsets
into ICC tissues. In addition, these findings do not support the
suggestion that HVEM is a favorable prognostic marker for ICC.
Therefore, the prognostic value of these TIL subsets was further
determined and the results revealed that CD4+,
CD8+ and CD45RO+ TILs were not significantly
associated with DFS or OS in patients with ICC (P>0.05).
Furthermore, the effect of HVEM on PBLs was investigated, and it
was found that high HVEM expression was associated with an
increased PBL concentration (P=0.031). The association between PBL
concentration and prognosis was further investigated, and survival
analysis showed that patients with high PBL concentration shad a
significantly prolonged OS (P=0.048) and an increased DFS that was
not statistically significant (P=0.097) in ICC. Thus, it appears
that the role of HVEM involves the activation of tumor immunity by
increasing the number of PBLs in patients with ICC. This finding
indicates that HVEM may be a favorable prognostic marker for ICC,
although it also appears to be inconsistent with results obtained
in previous studies on HCC. In one study, the levels of membrane
and soluble HVEM were downregulated and upregulated on PBLs,
respectively, and the latter was positively associated with
advanced tumor stages, suggesting that the role of HVEM maybe
immunosuppressive (24).
Furthermore, in another study, blockade of the BTLA/HVEM pathway
increased IFN-γ production in the circulating T cells of patients
with HCC (25).
In general, PBLs should infiltrate into the tumor
microenvironment at an early stage of tumor progression to play a
part in immune surveillance. Therefore, elevated PBL and TILs
should be associated with improved prognosis (9,10,13,18),
which our studies in ICC did not support. However, tumor signaling
pathways are complex, andthe antitumor effect of HVEM in ICC may be
achieved through other signaling pathways, rather than by direct
alteration of the infiltration of lymphocytes. Alternatively, an
association of TIL subsets with HVEM expression and prognosis may
exist in ICC; however, the association did not reach statistical
significance in the present study, which may be due to the small
sample size. The HVEM network is complicated, and its effect on
TILs and PBLs in ICC requires further study.
The association of HVEM expression with several
clinicopathological characteristics was investigated in the present
study, and it was revealed for the first time, to the best of our
knowledge, that HVEM expression is positively associated with the
frequencies of small-duct type (P=0.021) and BAP1 retained
expression (P=0.010) in ICC. ICCs are heterogeneous tumors that can
be histologically subdivided into two types based on the size of
the biliary duct, namely small-duct and large-duct types (26). In addition, this classification
individuates ICC subgroups with different clinicopathological and
molecular characteristics (26,27).
Therefore, high HVEM expression appears to be a specific feature of
small-duct type ICC. BAP1 is a commonly mutated gene involved in
histone modification and chromatin remodelling in ICC (28), and its mutation is strongly
associated with loss of expression of the corresponding protein
(29). The association identified
between BAP1 mutation and HVEM expression in ICC progression may
provide a new direction for study of the HVEM network in tumor
immunity.
The present study has some limitations. Firstly, the
small sample of data from a single institution may lead to false
negative results in the statistical analysis. Secondly, the effects
of HVEM blockade on immunity and tumor progression were not
investigated in vitro and in vivo due to the lack of
available HVEM inhibitors.
In conclusion, the present study demonstrated for
the first time, to the best of our knowledge, that high HVEM
expression was significantly associated with improved postoperative
survival and low TNM stage in ICC, indicating that HVEM might be a
favorable prognostic marker for ICC. Furthermore, the
co-stimulatory signals from HVEM may play a dominant role in the
progression of ICC, which may be explained by an increase in the
number of PBLs rather than a change in the number of TILs. However,
the function of the HVEM network in ICC progression is complex and
requires further study.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was supported by funding from National
Natural Fund Project (grant no. 81572434), Ministry of Science and
Technology, National Science and Technology Major Special Project:
Prevention and Treatment of Major Infectious Diseases such as AIDS
and Viral Hepatitis (grant no. 2018ZX10723204-007-001), ‘Young
Medical Elites’, Tianjin Health Commission (duration of grant
2018.12–2020.11), ‘Young Innovative Talents’, Tianjin Medical
University Cancer Institute and Hospital (duration of grant
2017.1–2019.12), Science and Technology Development Plan of Weifang
in 2020 (Medical Science).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BM was responsible for study conception and design,
data collection and analysis, performing experiments, writing the
manuscript and statistical analysis. HM contributed to study
conception and design, data analysis and writing the manuscript. YT
performed data interpretation and provided technical and material
support. YW, TS, TZ, QW, YC and HL performed data collection and
data analysis. WZ and QL contributed to study conception, data
collection and data interpretation, and critically revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Medical Ethics Committee of Tianjin Medical
University Cancer Institute and Hospital approved the present study
(approval no. bc2019065), and written informed consent was obtained
from all patients or their legal guardian.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ICC
|
intrahepatic cholangiocarcinoma
|
|
HVEM
|
herpesvirus entry mediator
|
|
Mon
|
monocyte
|
|
TIL
|
tumor-infiltrating lymphocyte
|
|
DFS
|
disease-free survival
|
|
OS
|
overall survival
|
|
TMAs
|
tissue microarrays
|
|
IHC
|
immunohistochemistry
|
|
Lym
|
lymphocyte
|
|
HR
|
hazard ratio
|
|
CIs
|
confidence intervals
|
|
PBL
|
peripheral blood lymphocyte
|
|
Neu
|
neutrophil
|
|
NLR
|
neutrophil-lymphocyte ratio
|
References
|
1
|
Bergquist A and von Seth E: Epidemiology
of cholangiocarcinoma. Best Pract Res Clin Gastroenterol.
29:221–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mavros MN, Economopoulos KP, Alexiou VG
and Pawlik TM: Treatment and prognosis for patients with
intrahepatic cholangiocarcinoma: Systematic review and
meta-analysis. JAMA Surg. 149:565–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gonzalez-Carmona MA, Bolch M, Jansen C,
Vogt A, Sampels M, Mohr RU, van Beekum K, Mahn R, Praktiknjo M,
Nattermann J, et al: Combined photodynamic therapy with systemic
chemotherapy for unresectable cholangiocarcinoma. Aliment Pharmacol
Ther. 49:437–447. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bridgewater J, Galle PR, Khan SA, Llovet
JM, Park JW, Patel T, Pawlik TM and Gores GJ: Guidelines for the
diagnosis and management of intrahepatic cholangiocarcinoma. J
Hepatol. 60:1268–1289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rizvi S, Khan SA, Hallemeier CL, Kelley RK
and Gores GJ: Cholangiocarcinoma-evolving concepts and therapeutic
strategies. Nat Rev Clin Oncol. 15:95–111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martin-Liberal J, Ochoa de Olza M, Hierro
C, Gros A, Rodon J and Tabernero J: The expanding role of
immunotherapy. Cancer Treat Rev. 54:74–86. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Montgomery RI, Warner MS, Lum BJ and Spear
PG: Herpes simplex virus-1 entry into cells mediated by a novel
member of the TNF/NGF receptor family. Cell. 87:427–436. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kwon BS, Tan KB, Ni J, Oh KO, Lee ZH, Kim
KK, Kim YJ, Wang S, Gentz R, Yu GL, et al: A newly identified
member of the tumor necrosis factor receptor superfamily with a
wide tissue distribution and involvement in lymphocyte activation.
J Biol Chem. 272:14272–14276. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Šedý JR and Ramezani-Rad P: HVEM network
signaling in cancer. Adv Cancer Res. 142:145–186. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ritthipichai K, Haymaker CL, Martinez M,
Aschenbrenner A, Yi X, Zhang M, Kale C, Vence LM, Roszik J,
Hailemichael Y, et al: Multifaceted Role of BTLA in the control of
CD8(+) T-cell Fate after Antigen Encounter. Clin Cancer Res.
23:6151–6164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sideras K, Biermann K, Yap K, Mancham S,
Boor PPC, Hansen BE, Stoop HJA, Peppelenbosch MP, van Eijck CH,
Sleijfer S, et al: Tumor cell expression of immune inhibitory
molecules and tumor-infiltrating lymphocyte count predict
cancer-specific survival in pancreatic and ampullary cancer. Int J
Cancer. 141:572–582. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hokuto D, Sho M, Yamato I, Yasuda S, Obara
S, Nomi T and Nakajima Y: Clinical impact of herpesvirus entry
mediator expression in human hepatocellular carcinoma. Eur J
Cancer. 51:157–165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Migita K, Sho M, Shimada K, Yasuda S,
Yamato I, Takayama T, Matsumoto S, Wakatsuki K, Hotta K, Tanaka T,
et al: Significant involvement of herpesvirus entry mediator in
human esophageal squamous cell carcinoma. Cancer. 120:808–817.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lan X, Li S, Gao H, Nanding A, Quan L,
Yang C, Ding S and Xue Y: Increased BTLA and HVEM in gastric cancer
are associated with progression and poor prognosis. Onco Targets
Ther. 10:919–926. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang M, Cao X, Li Y, Li GQ, He QH, Li SJ,
Chen J, Xu GL and Zhang KQ: High expression of herpes virus entry
mediator is associated with poor prognosis in clear cell renal cell
carcinoma. Am J Cancer Res. 9:975–987. 2019.PubMed/NCBI
|
|
16
|
Zhang T, Ye L, Han L, He Q and Zhu J:
Knockdown of HVEM, a lymphocyte regulator gene, in ovarian cancer
cells increases sensitivity to activated T cells. Oncol Res.
24:189–196. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fang Y, Ye L, Zhang T, He QZ and Zhu JL:
High expression of herpesvirus entry mediator (HVEM) in ovarian
serous adenocarcinoma tissue. J BUON. 22:80–86. 2017.PubMed/NCBI
|
|
18
|
Tsang JYS, Chan KW, Ni YB, Hlaing T, Hu J,
Chan SK, Cheung SY and Tse GM: Expression and clinical significance
of herpes virus entry mediator (HVEM) in breast cancer. Ann Surg
Oncol. 24:4042–4050. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Malissen N, Macagno N, Granjeaud S,
Granier C, Moutardier V, Gaudy-Marqueste C, Habel N, Mandavit M,
Guillot B, Pasero C, et al: HVEM has a broader expression than
PD-L1 and constitutes a negative prognostic marker and potential
treatment target for melanoma. Oncoimmunology. 8:e16659762019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma B, Meng H, Tian Y, Wang Y, Song T,
Zhang T, Wu Q, Cui Y, Li H, Zhang W and Li Q: Distinct clinical and
prognostic implication of IDH1/2 mutation and other most frequent
mutations in large duct and small duct subtypes of intrahepatic
cholangiocarcinoma. BMC Cancer. 20:3182020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kang J, Jeong JH, Hwang HS, Lee SS, Park
DH, Oh DW, Song TJ, Kim KH, Hwang S, Hwang DW, et al: Efficacy and
Safety of Pembrolizumab in patients with refractory advanced
biliary tract cancer: Tumor proportion score as a potential
biomarker for response. Cancer Res Treat. 52:594–603. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dobosz P, Stempor PA, Roszik J, Herman A,
Layani A, Berger R, Avni D, Sidi Y and Leibowitz-Amit R: Checkpoint
genes at the cancer side of the immunological synapse in bladder
cancer. Transl Oncol. 13:193–200. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tamada K, Shimozaki K, Chapoval AI, Zhu G,
Sica G, Flies D, Boone T, Hsu H, Fu YX, Nagata S, et al: Modulation
of T-cell-mediated immunity in tumor and graft-versus-host disease
models through the LIGHT co-stimulatory pathway. Nat Med.
6:283–289. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao Q, Zhang GL, Zhu X, Su D, Huang ZL,
Hu ZX and Peng L: The paradoxical changes of membrane and soluble
herpes virus entry mediator in hepatocellular carcinoma patients. J
Gastroenterol Hepatol. 32:1520–1524. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu J, Li J, He M, Zhang GL and Zhao Q:
Distinct changes of BTLA and HVEM expressions in circulating
CD4+ and CD8+ T cells in hepatocellular
carcinoma patients. J Immunol Res. 2018:45615712018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Komuta M, Govaere O, Vandecaveye V, Akiba
J, Van Steenbergen W, Verslype C, Laleman W, Pirenne J, Aerts R,
Yano H, et al: Histological diversity in cholangiocellular
carcinoma reflects the different cholangiocyte phenotypes.
Hepatology. 55:1876–1888. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liau JY, Tsai JH, Yuan RH, Chang CN, Lee
HJ and Jeng YM: Morphological subclassification of intrahepatic
cholangiocarcinoma: Etiological, clinicopathological, and molecular
features. Mod Pathol. 27:1163–1173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiao Y, Pawlik TM, Anders RA, Selaru FM,
Streppel MM, Lucas DJ, Niknafs N, Guthrie VB, Maitra A, Argani P,
et al: Exome sequencing identifies frequent inactivating mutations
in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat
Genet. 45:1470–1473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peña-Llopis S, Vega-Rubín-de-Celis S, Liao
A, Leng N, Pavía-Jiménez A, Wang S, Yamasaki T, Zhrebker L,
Sivanand S, Spence P, et al: BAP1 loss defines a new class of renal
cell carcinoma. Nat Genet. 44:751–759. 2012. View Article : Google Scholar : PubMed/NCBI
|