Introduction
Breast cancer is the second most common cause of
cancer-associated mortality in women globally, accounting for 16%
of cancer-associated mortalities in adult women annually (1–3). Triple
negative breast cancer (TNBC) is the most aggressive subtype of
breast cancer and is characterized by a lack of hormone receptors,
such as estrogen receptor (ER), progesterone receptor (PR) and
human epidermal growth factor receptor 2 (HER2) (4). Given that this subtype cannot be
treated with molecular targeted therapies towards these
receptors/hormones, the treatment of TNBC is challenging and these
patients typically exhibit a poor prognosis (5,6).
Currently, surgery, chemotherapy and radiation therapy are the
standard methods of treatment for breast cancer; however, the
5-year survival rate for patients with breast cancer of advanced
stages remains low from 2010–2015 (7). Additionally, treatments for patients
with advanced breast cancer result in undesirable therapeutic
effects, since relapse often occurs after treatment (1). Therefore, the development of effective
anti-cancer drugs with minimum side effects is critical.
Traditional Chinese Medicine has provided multiple
medicinal resources and materials for the treatment of a variety of
cancers. Berbamine (BBM) is derived from Berberis
amunrensis. As a small molecule of natural origin, BBM has been
widely used to treat leukopenia caused by chemotherapy and/or
radiotherapy, without any obvious side effects (8). Previous studies reported that BBM
possesses biological activities including anti-oxidation,
anti-inflammation and protective effects against
ischemia/reperfusion injury (9–11). In
addition, BBM exhibits anti-tumor activity and inhibits the
proliferation of breast cancer cell lines (8,12).
Although previous studies have demonstrated that BBM downregulates
the protein levels of Bax and Bcl-2 (12), the molecular mechanism underlying its
anti-tumor function is still unclear. In addition, few studies have
explored the effects and mechanism of BBM in breast cancer.
Hyperactivation of the phosphatidylinositol 3
kinase/protein kinase B (PI3K/Akt) signaling pathway is associated
with tumor growth, maintenance and chemotherapy resistance in
several types of cancer, such as breast cancer, endometrial cancer
and liver cancer (13–21). Dysregulation of signaling via the
PI3K/Akt signaling pathway is one of the most frequent oncogenic
aberrations associated with TNBC (22). Pierobon et al (21) reported that breast cancer with liver
metastasis may be associated with increased incidence of PIK3CA
mutations and activation of the PI3K/Akt/mTOR signaling pathway.
These studies suggest that the mechanism underlying the effect of
BBM treatment on tumors may involve the PI3K/Akt pathway.
Therefore, it was hypothesized that BBM may inhibit the
proliferation, invasion and metastasis of TNBC cells via the
PI3K/Akt signaling pathway. Murine double minute 2 (MDM2) and mTOR
were the downstream targets of Akt (4,23), MDM2
is a master regulator of the p53 tumor suppressor (24). Li et al (10) reported that HBXIP promotes human
breast cancer growth by activating phosphorylated (p)-Akt, which in
turn phosphorylates MDM2, thus enhancing the interaction between
MDM2 and p53 and resulting in p53 degradation. Rinaldi et al
(25) found that the mTOR pathway is
related to tumor growth. Therefore, MDM2 and mTOR may serve an
important role in the anti-breast cancer effects of BBM. Lysyl
oxidase (LOX) and cyclooxygenase (COX)-2 are also downstream
targets of the PI3K/Akt pathway (26,27). Lox
is overexpressed in patients with TNBC and is closely associated
with tumor metastasis (28).
Moreover, the expression of LOX is enhanced by the activation of
PI3K (26). COX-2 is also
overexpressd in patients with TNBC (29) and COX-2 promotes migration in
osteosarcoma MG-63 cells via the PI3K/Akt signal pathway (27). Previous studies have demonstrated
that BBM may inhibit the proliferation and metastasis of tumor
cells by regulating the expression levels of several proteins,
including p-Akt (30–33). However, to the best of our knowledge,
there are have not been previous studies which indicate that BBM
inhibits breast cancer by inhibiting the expression of PI3K, and
there are no studies investigating the association between the
downstream targets of Akt mentioned above (including MDM2, mTOR,
LOX, and COX-2) and BBM. The present study will investigate the
effects of BBM on TNBC cell proliferation, invasion and migration
and will explore its underlying mechanisms. The current study will
examine whether BBM inhibits the proliferation, migration and
invasion of TNBC cells via regulating the PI3K/Akt pathway and
downstream targets such as MDM2, p53, mTOR, COX-2 and LOX.
Materials and methods
Drugs and reagents
BBM (purity ≥98%) was purchased from Shanghai
Macklin Biochemical Technology Co., Ltd. and was dissolved in
dimethyl sulfoxide (DMSO) at a concentration of 100 mM as a stock
solution and diluted into indicated concentrations using DMEM
medium (Gibco; Thermo Fisher Scientific, Inc.), as previously
described (30,31). The final DMSO concentration was
<0.1% in all experiments. Fetal bovine serum (FBS) and
Dulbecco's modified Eagle's Medium (DMEM) were purchased from
Gibco; Thermo Fisher Scientific, Inc. Matrigel was purchased from
BD Biosciences. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl
tetrazolium bromide (MTT) and Cell Death Detection ELISA kits were
purchased from Sigma-Aldrich; Merck KGaA. Primary antibodies
against PI3K, MDM2, Akt, phosphorylated (p)-Akt, p53 and GAPDH, and
the ELISA kit for mTOR, were purchased from Abcam. LOX, COX-2 and
β-actin antibodies were purchased from Santa Cruz Biotechnology,
Inc. Trypan Blue assay kits and all other reagents for western
blotting were purchased from Beyotime Institute of
Biotechnology.
Cell culture
The human TNBC cell lines MDA-MB-231 and MCF-7 were
purchased from the Cell Bank of the Chinese Academy of Sciences.
The cells were cultured in DMEM medium containing 10% FBS and 1%
penicillin-streptomycin and stored in a humidified incubator at
37°C with 5% CO2.
MTT assay
MDA-MB-231 cells and MCF-7 cells were seeded into a
96-well plate (5×104 cells/well) and treated with
vehicle (DMEM medium with 0.08% DMSO) or different concentrations
of BBM (1.25, 2.5, 5, 10, 20, 40, and 80 µM) for 24 h at 37°C. MTT
solution (5 mg/ml) was added to each well and the plate was
incubated for another 4 h at 37°C. Next, the supernatant was
removed and DMSO solution was added. The absorbance was read by a
Multi-Well Micro-Plate Reader (Bio-Rad Laboratories, Inc.) at 560
nm. Cell viability was expressed as a percentage of the vehicle
(Ctrl) group.
Colony formation assay
The colony formation assay was performed as
previously described (29).
MDA-MB-231 cells and MCF-7 cells were cultured in a 6-well plate
(1×103 cells/well) and treated with vehicle or different
concentrations of BBM (10, 20 and 40 µM) for 24 h at 37°C. The
medium was replaced by a drug-free, complete DMEM (with 10% FBS).
The medium was changed every 3 days for MAD-MB-231 cells and every
2 days for MCF-7 cells. After 7 days, the cells were fixed with 4%
paraformaldehyde for 30 min at 25°C and stained with crystal violet
solution (0.05% w/v) for 20 min at 25°C. The number of clones
>10 cells was counted under a light microscope (Leica DMI 4000;
magnification, ×100).
5-Ethynyl-2′-deoxyuridine (EdU)
staining assay
EdU staining assay was performed using the EdU
Apollo-567 in vitro kit (Guangzhou RiboBio Co., Ltd; cat.
no. C10310-1). Briefly, MDA-MB-231 cells and MCF-7 cells were
seeded into 96-well plates (1×104 cells/well) and
cultured overnight at 37°C. EdU (10 µM) was added into each well
and incubated for 2 h at 37°C. After the cells were fixed with 4%
polyoxymethylene at 25°C for 30 min and decolorized with glycine (2
mg/ml) at 25°C for 5 min, the cell nuclei were stained with Hoechst
33342 at 25°C for 30 min. The fluorescence of cells was observed
using an inverted fluorescence microscope (magnifiation, ×100;
Leica Microsystems GmbH). The EdU ratio was calculated as follows:
Number of EdU-positive cells/number of Hoechst 33342-positive cells
×100%.
Trypan Blue Dye assay
MDA-MB-231 cells (1×105 cells/well) were
seeded into 6-well plates in the presence or absence of different
concentrations of BBM (10, 20 and 40 µM) and cultured at 37°C for
48 h. The cells were collected and re-suspended using 0.4% Trypan
blue at 25°C. Then the cells visualized under a light microscope
(Leica DMI 4000; magnification, ×100).
Apoptosis assay
Cell apoptosis was detected using a photometric
enzyme immunoassay (Cell Death Detection ELISA kit, cat. no.
11544675001; Sigma-Aldrich Merck KGaA), as previously described
(30). This is based on the
quantitative sandwich immunoassay employing antibodies against
histone and DNA. MDA-MB-231 cells and MCF-7 cells (1×105
cells/well) were seeded into a 6-well plate in the presence or
absence of different concentrations of BBM (10, 20 and 40 µM) and
cultured for 24 h at 37°C. The cells were washed with PBS and then
lysed in RIPA lysis buffer (containing 1 mM PMSF). The supernatant
was collected via centrifugation at 12,000 × g for 15 min at 4°C.
and used for testing. The mono- and oligonucleosomal fragmented DNA
was measured according to the instructions of the manufacturer.
Wound healing assay
MDA-MB-231 cells and MCF-7 cells were seeded
(2×105 cells/well) into 6-well plates and cultured for
48 h at 37°C. Once the cells reached a confluence of 90%, a 200 µl
plastic tip was used to make a straight line in the monolayer, and
the plate was washed with PBS. Fresh serum-free medium with vehicle
or different concentrations of BBM (10, 20 and 40 µM) and mitomycin
(2 µg/ml) was added into each well. The cells were observed under
an inverted microscope coupled to a camera (100×, Leica DMI 4000)
at 25°C, and this time was designated at 0 h, then the cells were
placed in an incubator with 5% CO2 for 24 h (MDA-MB-231
cells) or 72 h (MCF-7 cells) at 37°C. The cells were observed under
an inverted microscope coupled to a camera (100×, Leica DMI 4000)
at 25°C. The migration distance was measured using Image J
(National Institutes of Health, USA, v 1.5.1), and the migration
percentage was calculated, the percentage of wound closure=(the
scratch area of 0 h-the scratch area of 24 h)/the scratch area of 0
h.
Transwell assay
MDA-MB-231 cells and MCF-7 cells (8×104
cells) in the presence or absence of different concentrations of
BBM (10, 20 and 40 µM) were suspended in serum-free medium and then
seeded into the upper chamber of a transwell chamber with an 8-µm
pore (Corning, Inc.). The upper chambers were pre-coated at 37°C
with (Transwell invasion assay) or without (Transwell migration
assay) Matrigel (1 mg/ml), and the lower chambers contained medium
with 20% FBS. The chambers were incubated for 36 h (MDA-MB-231) or
48 h (MCF-7) at 37°C. The cells on the surface of the membrane were
fixed with 4% polyoxymethylene at 25°C for 30 min and stained with
crystal violet staining solution (1%) at 25°C for 5 min. Mitomycin
(2 µg/ml) was added when the cells were seeded into the upper
chambers to exclude the proliferation of the cells. Cells
visualized under a light microscope (Leica DM 4000; magnification,
×100).
Western blotting assay
Cells were lysed using RIPA lysis buffer. Protein
concentration was determined using a BCA assay kit (Beyotime
Institute of Biotechnology; cat. no. P0012). Lysate proteins (20
µg) were then separated by 10% SDS-PAGE and transferred to a PVDF
membrane (EMD Millipore). The membranes were blocked using TBST
(containing tween 20, 0.05%) containing 5% non-fat milk at room
temperature for 2 h and then incubated with primary antibodies
against PI3K (cat. no. ab70912; 1:1,000), MDM2 (cat. no. ab16895;
1:500), Akt (cat. no. ab8805; 1:1,000), p-Akt (cat. no. ab38449;
1:1,000) COX-2 (cat. no. sc-19999; 1:200), LOX (cat. no. sc-373995;
1:500), GAPDH (Abcam; cat. no. ab9845; 1:1,000) and β-actin (cat.
no. sc-8432; 1:1,000) at 4°C overnight. After washing with TBST,
the membranes were incubated with secondary antibodies (GenScript;
cat. no. A00098; 1:10,000) conjugated to horseradish peroxidase at
room temperature for 2 h. Bands were detected using THE
Hypersensitive ECL chemiluminescence kit (Beyotime Institute of
Biotechnology; cat. no. P10018FS). Optical density of bands
(relative to GAPDH or β-actin) were quantified using Image J
(National Institutes of Health; v1.5.1).
ELISA
mTOR (pSer2448) level was assayed using the mTOR
ELISA kit (Abcam, cat. no. ab176657). Cells were lysed in RIPA
buffer as described above and mTOR was detected according to the
manufacturer's instructions.
Statistical analysis
All statistical analyses were performed using SPSS
21.0 (IBM Corp.). Data are presented as mean ± standard deviation
(SD). Significance differences were determined using one-way ANOVA,
followed by Dunnett's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
BBM inhibits the cell viabilities of
MDA-MB-231 and MCF-7 cells
MTT assay was used to detect the effects of BBM on
the proliferation of human TNBC cell lines. The results
demonstrated that BBM significantly inhibited the cell viabilities
of MDA-MB-231 cells and MCF-7 cells in a time- and dose-dependent
manner; Fig. 1A). In MDA-MB-231
cells, after incubation for 24, 48, and 72 h, the minimum
concentration of BBM inhibited cell proliferation was 10 µM.
Moreover, with the extension of incubation time, the proliferation
rate of cells inhibited by BBM was significantly decreased at the
same concentration. In MCF-7 cells, the minimum concentration of
BBM inhibited cell proliferation was 20 µM for 24 h treated cells,
10 µM for 48 h, and 2.5 µM for 72 h. In addition, with the
extension of incubation time, the proliferation rate of cells
inhibited by BBM was significantly decreased as well. The
IC50 values of BBM for 72 h on MDA-MB-231 cells and
MCF-7 cells were 22.7±3.3, and 20.9±1.5 µM, respectively (Fig. 1B). IC50 value of 72 h was selected as
the basis to exclude the effect of cell proliferation. Thus, BBM of
10, 20 and 40 µM were selected for the subsequent assays.
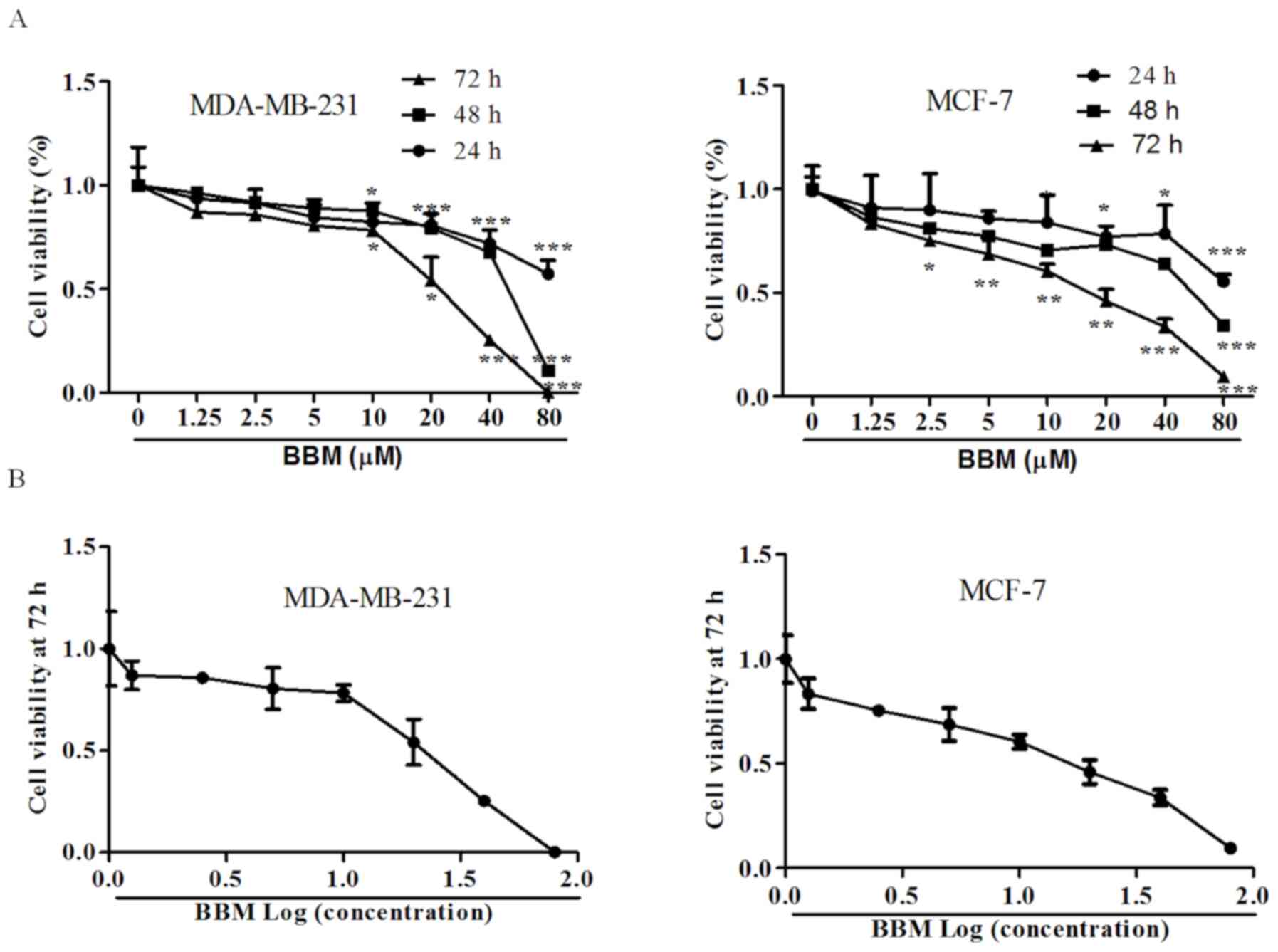 | Figure 1.BBM is cytotoxic to triple negative
breast cancer cells. The cells were treated with applied
concentrations (1.25, 2.5, 5, 10, 20, 40 or 80 µM) of BBM, cell
viability was tested using MTT assay. (A) Effects of different
concentrations of BBM on cell proliferation at different times. (B)
The effect of BBM on cell proliferation after treatment for 72 h.
The Ctrl group was the cells treated with solvent (medium
containing DMSO, DMSO <0.8‰). Data are presented as the mean ±
standard deviation (n=3). *P<0.05, **P<0.01, ***P<0.001
vs. Ctrl group. BBM, Berbamine; Ctrl, control. |
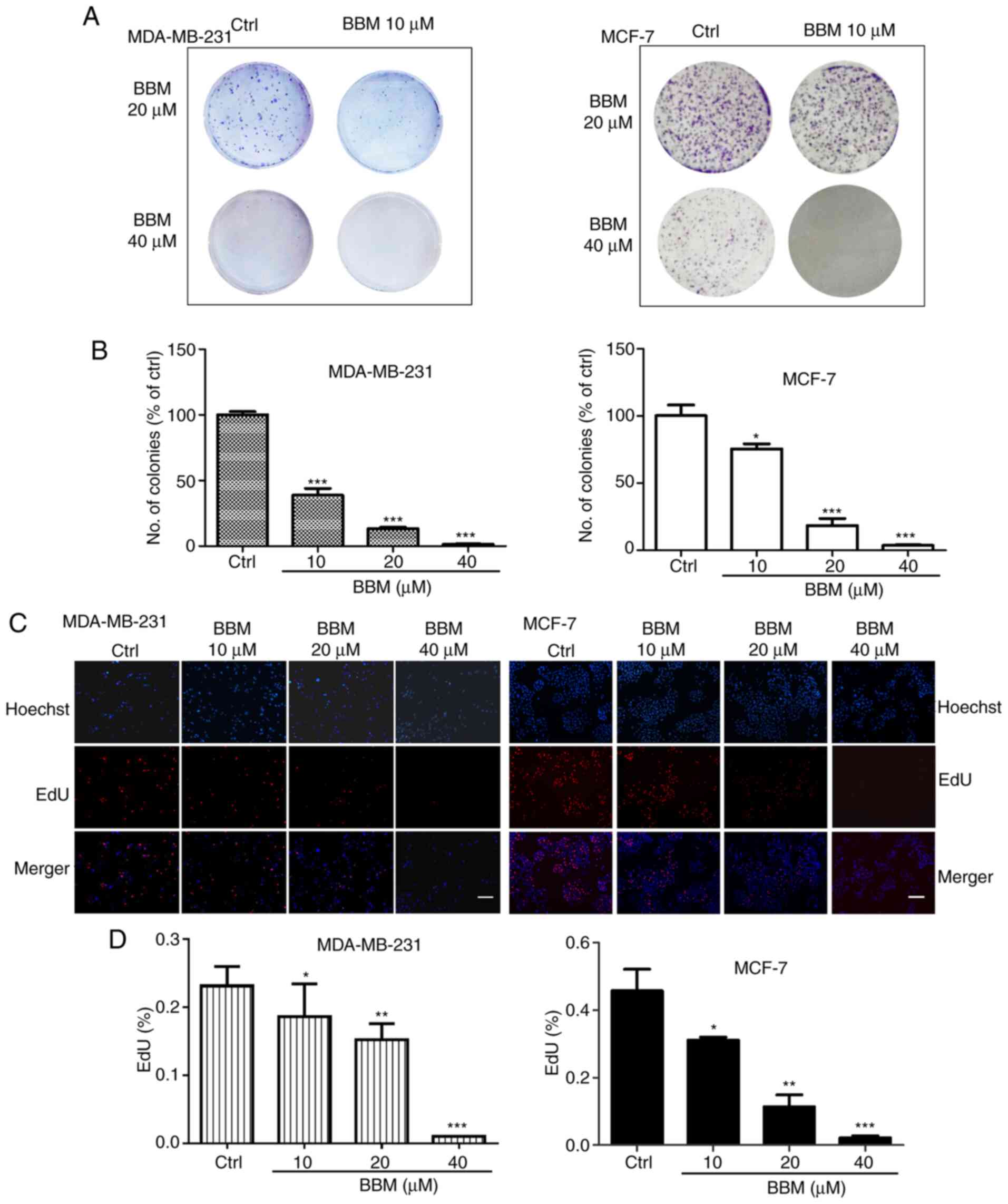
BBM inhibits the proliferation of
MDA-MB-231 and MCF-7 cells
The effects of BBM on cell proliferation were
evaluated using colony formation and EdU staining assays. In
MDA-MB-231 cells, compared with the Ctrl group, BBM at 10, 20 and
40 µM decreased the number of the colonies in a dose-dependent
manner (P<0.001; Fig. 2A and B).
In MCF-7 cells, BBM at 10, 20 and 40 µM decreased the number of the
colonies in a dose-dependent manner as well (P<0.05, P<0.001
and P<0.001, respectively (Fig. 2A
and B). To further elucidate the effect of BMM on the cell
proliferation of TNBC cells, EdU staining assay was performed. The
results indicated that BBM inhibited cell proliferation in a
concentration-dependent manner (Fig. 2C
and D). In MDA-MB-231 cells, BBM at 10, 20 and 40 µM
significantly inhibited cell proliferation compared with the Ctrl
group (P<0.05, P<0.01 and P<0.001, respectively). In MCF-7
cells, BBM at 10, 20, and 40 µM significantly inhibited cell
proliferation compared with the Ctrl group (P<0.05, P<0.01
and P<0.001, respectively).
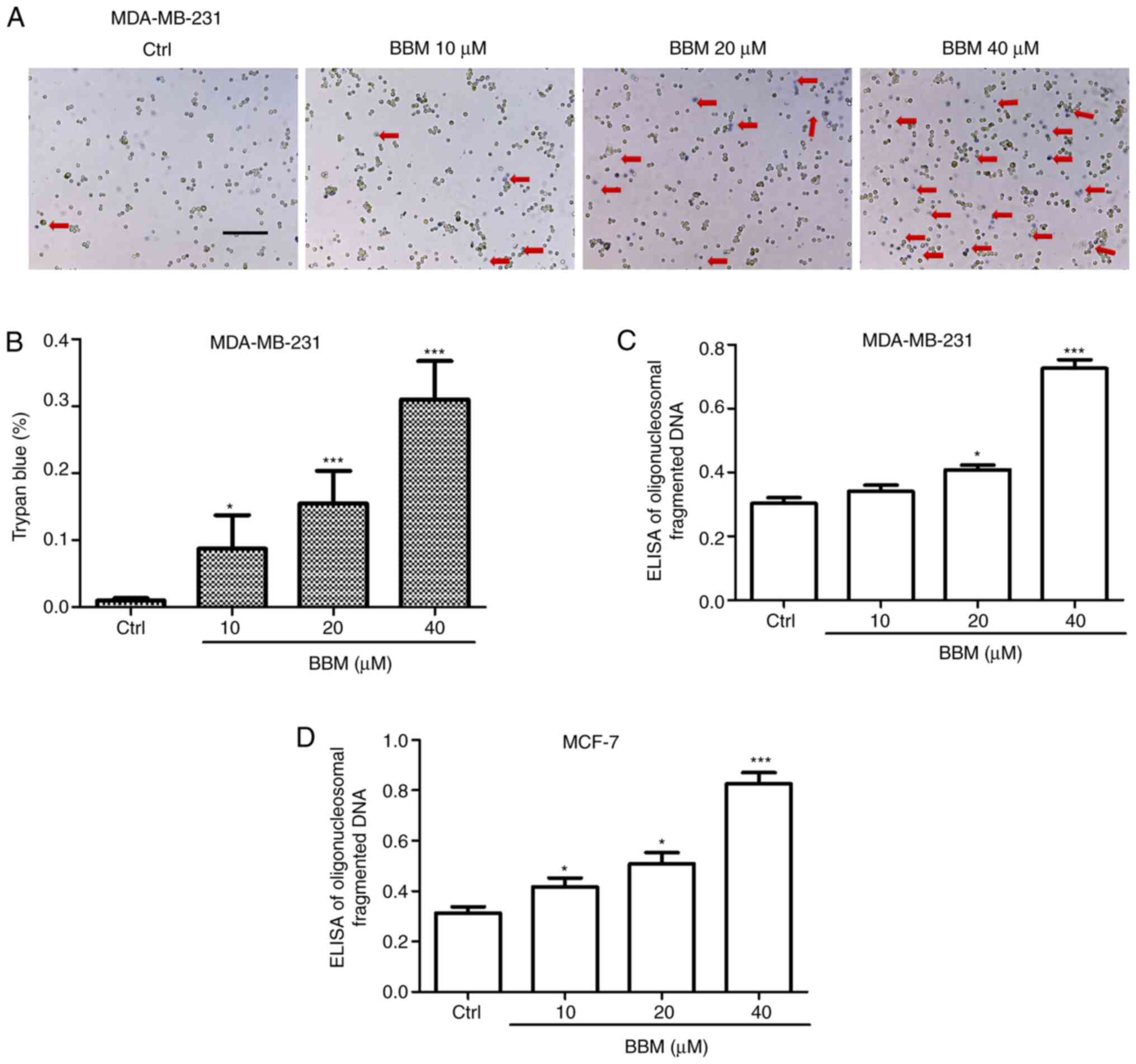
BBM induces apoptosis of MDA-MB-231
and MCF-7 cells
To investigate the effects of BBM on TNBC cell
apoptosis, trypan blue and Cell Death Detection ELISA assays were
performed. The results showed that BBM induced cell death in a
concentration-dependent manner. BBM at 10, 20, and 40 µM
significantly induced cell death compared to the Ctrl group
(P<0.05, P<0.001 and P<0.001, respectively; Fig. 3A and B). In addition, the ELISA assay
revealed that BBM at 20 and 40 µM significantly induced death of
MDA-MB-231 cells compared with the Ctrl group (P<0.05 and
P<0.001, respectively; Fig. 3C).
Moreover, BBM at 10, 20, and 40 µM induced the cell death of MCF-7
cells compared with the Ctrl group (Fig.
3D) (P<0.05, P<0.05 and P<0.001, respectively).
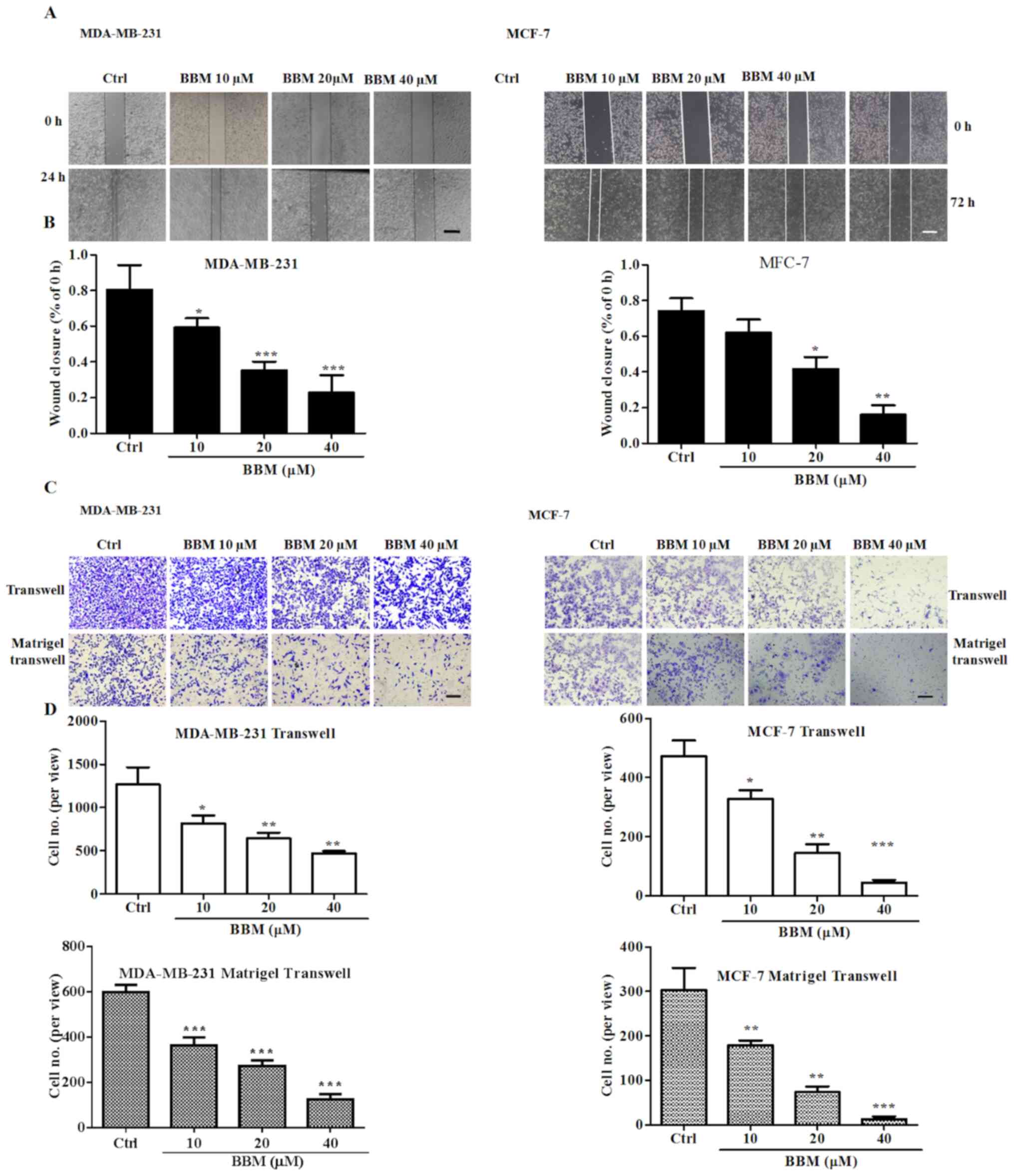
BBM inhibits cell migration and
invasion in MDA-MB-231 and MCF-7 cells
Wound healing and transwell assays were performed to
evaluate the effects of BBM on cell migration and invasion. The
results showed that BBM inhibited wound closure in a
concentration-dependent manner (Fig. 4A
and B). BBM at 10, 20, and 40 µM significantly inhibited the
migration of MDA-MB-231 cells compared to the Ctrl group
(P<0.05, P<0.001 and P<0.001, respectively). BBM at 20,
and 40 µM significantly inhibited the migration of MCF-7 cells
compared to the Ctrl group (P<0.05 and P<0.01,
respectively).
It was also revealed that BBM significantly
inhibited cell migration and invasion of TNBC cells in a
concentration-dependent manner (Fig. 4C
and D). In MDA-MB-231 cells, BBM at 20, and 40 µM significantly
reduced the number of the migrated cells compared to the Ctrl group
(P<0.05, P<0.01 and P<0.01, respectively). Furthermore,
BBM at 20, and 40 µM significantly reduced the number of invaded
cells compared to the Ctrl group (all P<0.001). In MCF-7 cells,
BBM at 20, and 40 µM significantly reduced the number of migrated
cells compared to the Ctrl group (P<0.05, P<0.01 and
P<0.001, respectively). BBM at 10, 20, and 40 µM significantly
reduced the number of invaded cells compared to the Ctrl group
(P<0.01, P<0.01 and P<0.001, respectively). These results
indicated that BBM inhibited the cell migration and invasion in
TNBC cells.
BBM regulates the PI3K/Akt/MDM2/p53
and PI3K/Akt/mTOR signaling pathways in MDA-MB-231 and MCF-7
cells
The present results revealed that BBM exhibited
strong inhibitory effects on cell growth, proliferation, metastasis
and invasion in TNBC cells. It was speculated that BBM may serve an
anti-cancer role in breast cells via regulation of the
PI3K/Akt/MDM2/p53 and PI3K/Akt/mTOR signaling pathways. To verify
this hypothesis, the expression levels of PI3K, p-Akt/Akt, MDM2 and
p53 were evaluated using western blotting. BBM at 10, 20 and 40 µM
significantly inhibited the expression of PI3K (Fig. 5A and B) both in MDA-MB-231 cells
(P<0.05) and in MCF-7 cells (P<0.05). BBM at 20 and 40 µM
significantly inhibited the expression of MDM2 (Fig. 5A and C) both in MDA-MB-231 cells
(P<0.01) and in MCF-7 cells (P<0.05). BBM significantly
inhibited the expression levels of p-Akt/Akt in MDA-MB-231 cells at
the concentrations of 20 and 40 µM (P<0.01) and in MCF-7 cells
at the concentration of 10, 20 and 40 µM (P<0.001) (Fig. 5A and D). BBM significantly inhibited
the expression of COX-2 in MDA-MB-231 cells at the concentrations
of 20 and 40 µM (P<0.001) and in MCF-7 cells at the
concentration of 10, 20 and 40 µM (P<0.001) (Fig. 5A and E). BBM at 20 and 40 µM
significantly inhibited the expression of LOX both in MDA-MB-231
cells (P<0.001) and MCF-7 cells (P<0.001) (Fig. 5A and F). Notably, BBM at 20 and 40 µM
increased the expression p53 in MDA-MB-231 (P<0.05) cells in
MCF-7 cells (P<0.05) (Fig. 5A and
G). The expression level of mTOR was tested by ELISA. BBM
significantly inhibited the expression of mTOR in MDA-MB-231 cells
at of the doses of 10 µM (P<0.05), 20 µM (P<0.01) and 40 µM
(P<0.01) and in MCF-7 cells at the dose of 20 µM (P<0.01) and
40 µM (P<0.01) (Fig. 5H). In
summary, the results of the western blot assay indicated that the
expression levels of PI3K, MDM2, p-Akt/Akt, COX-2, LOX and mTOR
were significantly downregulated by BBM, while the expression of
p53 was upregulated by BBM.
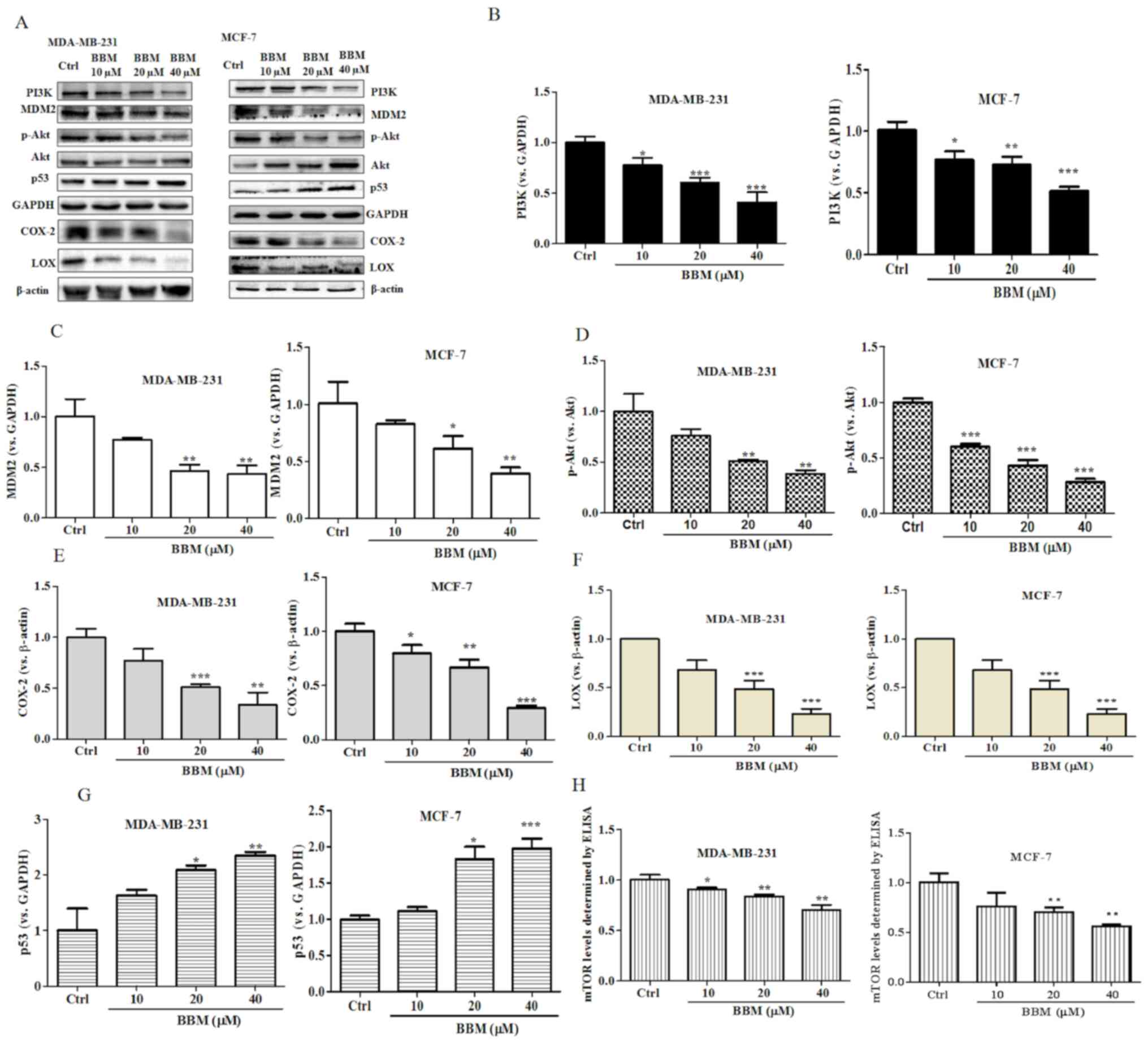 | Figure 5.BBM disrupts the PI3K/Akt/MDM2/p53
and PI3K/Akt/mTOR signaling pathways in triple negative breast
cancer cells. Cells were untreated (Ctrl) or treated with BBM (10,
20 and 40 µM). (A) Western blots were performed on both cell lines.
The expression levels of (B) PI3K, (C) MDM2, (D) p-Akt/Akt, (E)
COX-2, (F) LOX and (G) p53 were quantified. (H) The expression of
p-mTOR was tested using an ELISA. The Ctrl group was untreated
cells. Data are presented as the mean ± standard deviation (n=3).
*P<0.05, **P<0.01, ***P<0.001 vs. Ctrl group. BBM,
Berbamine; Ctrl, control; p-, phosphorylated-. |
Discussion
TNBC is the most aggressive subtype of breast cancer
and accounts for 15~20% of all breast cancers worldwide (34). Metastasis is the primary cause of
mortality in patients with breast cancer (35). BBM is a traditional medicine derived
from Berberis amurensis. Previous studies have demonstrated
the anti-tumor effects of BBM in a variety of cancers (36,37). Jin
and Wu (38) demonstrated that BBM
significantly downregulates the expressions of apoptosis-related
proteins including Bcl-2 and Bcl-xL and inhibits cell proliferation
in pancreatic carcinoma. Du et al (30) found that BBM induces cell apoptosis
and inhibits cell proliferation via the PI3K/Akt pathway in
lymphoma. In addition, BBM inhibits cell proliferation and destroys
mitochondria in prostate cancer cells (39). These studies support the potential of
BBM as a drug for cancer treatment.
In the present study, the effect of BBM on the
proliferation, apoptosis, invasion and migration of TNBC cells was
evaluated. The present study indicated that the cell proliferation
of MAD-MB-231cells and MCF cells was inhibited by BBM. Moreover,
BBM induces the apoptosis of TNBC cells. Subsequent experiments
revealed that BBM inhibits the invasion and metastasis of TNBC
cells. These results are consistent with previous studies (8,12,17)
showing that BBM has inhibitory effects on tumors. Western blot was
used to detect the effect of BBM on protein expression in TNBC
cells to elucidate the possible mechanism of BBM inhibition of
proliferation, invasion and metastasis of TNBC cells.
The PI3K/Akt pathway is involved in cell
proliferation, apoptosis invasion, and migration, and aberrant
activation of this pathway is associated with the development of
many types of cancers, breast cancer and lung cancer (40). The present results revealed that cell
proliferation, invasion and migration of TNBC cells were inhibited
by BBM, and apoptosis was induced by BBM. In addition, the
expressions of PI3K and p-Akt/Akt were downregulated following BBM
treatment. These results, combined with previous studies, indicate
that BBM may serve an anti-tumor role in TNBC by inhibiting the
PI3K/Akt signaling pathway. Several studies have reported that
proteins downstream of Akt regulate cancer cell apoptosis and other
cellular events (41–43). For instance, MDM2 is downstream of
Akt and inhibits the expression of p53. Overexpression of MDM2 may
induce breast cancer via the activation of other oncogenes, such as
by promoting ubiquitination/degradation of E-cadherin, resulting in
increased invasion of cancer cells (44).
COX-2 is overexpressed in TNBC (28) and may increase the phosphorylation of
MDM2, resulting in impaired p53 function (45). Zhang et al (27) reported that COX-2 promotes
angiogenesis and tissue invasion in osteosarcoma. The current
results revealed that the invasion of TNBC cells was inhibited by
BBM. And the expressions of MDM2 and COX-2 were downregulated by
BBM treatment, while the expression of p53 was upregulated.
Combined with the aforementioned studies, these findings suggest
that BBM inhibits the invasion of TNBC cells via regulating the
MDM2-p53 pathway, and the pathway may also be regulated by COX-2.
In addition, overexpression of Akt2 induces cell metastasis and
invasion via upregulation of integrin β1 in breast and ovarian
cancers (3,4). mTOR is an important effector of the
PI3K/Akt signaling pathway and is expressed in most mammalian
cells, resulting in a rise of cellular protein mass and inhibition
of autophagy (8). Gao et al
(46) found that cepharanthine can
induce apoptosis and autophagy by inhibiting the Akt/mTOR signaling
pathway (46). Cepharanthine is a
natural product and used for >70 years in Japan to treat a
variety of diseases, including leukopenia (42). These characteristics of cepharanthine
are very similar to BBM (47).
Moreover, cepharanthine was reported to inhibit the metastasis and
invasion, and induce apoptosis of breast cancer cells (46,48).
Therefore, we hypothesized that BBM also served an anti-tumor role
via the Akt/mTOR signaling pathway. Furthermore, LOX is high
expressed in patients with TNBC, a previous study revealed that LOX
promotes cell proliferation and inhibits apoptotic cell death, and
this effect may be achieved by activation of the Akt/mTOR signaling
pathway (49). The present results
indicate that BBM induced the apoptosis of TNBC cells, and the
expressions of mTOR and LOX were downregulated following treatment
with BBM. Collectively, the current results suggest that BBM may
induce the apoptosis of TNBC cells by inhibiting the expressions of
mTOR and LOX. MDM2 and mTOR are downstream targets of the PI3K/Akt
signaling pathway (4,21,50,51).
Thus, it was hypothesized that BBM may induce the apoptosis of TNBC
cells via the PI3K/Akt/MDM2/p53 and PI3K/Akt/mTOR signal
pathways.
In conclusion, the proliferation, migration and
invasion of TNBC cells were suppressed following treatment with
BBM, and this effect may be achieved by modulating the
PI3K/Akt/MDM2/p53 and PI3K/Akt/mTOR signaling pathways. However,
this study is only a preliminary in vitro study, future
studies such as in vivo experiments may need to be performed
to verify that BBM has low toxicity, and either alone or in
combination with other chemotherapy may represent a novel treatment
strategy for breast cancer.
Acknowledgements
Not applicable.
Funding
The present work was supported by grants including
‘Clinical Medical Science and Technology Development Fund Project’
from Jiangsu University (grant no. JLY2016118), The Integrated
Traditional Chinese and Western Medicine Research Fund from Suzhou
(grant no. SYSD2016173), the Science and Technology Project from
Zhangjiagang (grant no. ZKS1638), and the science and technology
development plan from Suzhou (grant no. SYSD2019165).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
JY and LL designed the present study, analyzed the
data, drafted the initial manuscript and revised it for important
intellectual content. YC and YY performed the experiments. BY, LT,
XS and LL were responsible for acquiring, analyzing and
interpretating the data. SW designed the present study and revised
the manuscript for important intellectual content. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Guo Y and Pei X: Tetrandrine-induced
autophagy in MDA-MB-231 Triple-negative breast cancer cell through
the Inhibition of PI3K/AKT/mTOR signaling. Evid Based Complement
Alternat Med. 20:75174312019.
|
|
2
|
Jin F, Wu Z, Hu X, Zhang J, Gao Z, Han X,
Qin J, Li C and Wang Y: The PI3K/Akt/GSK-3β/ROS/eIF2B pathway
promotes breast cancer growth and metastasis via suppression of NK
cell cytotoxicity and tumor cell susceptibility. Cancer Biol Med.
16:38–54. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nassan MA, Soliman MM, Ismail SA and
El-Shazly S: Effect of Taraxacum officinale extract on PI3K/Akt
pathway in DMBA-induced breast cancer in albino rats. Biosci Rep.
38:BSR201803342018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pascual J and Turner NC: Targeting the
PI3-kinase pathway in triple-negative breast cancer. Ann Oncol.
30:1051–1060. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jouali F, Marchoudi N, Talbi S, Bilal B,
El Khasmi M, Rhaissi H and Fekkak J: Detection of PIK3/AKT pathway
in Moroccan population with triple negative breast cancer. BMC
Cancer. 18:9002018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou T, Xu D, Tang B, Ren Y, Han Y, Liang
G, Wang J and Wang L: Expression of programmed death ligand-1 and
programmed death-1 in samples of invasive ductal carcinoma of the
breast and its correlation with prognosis. Anticancer Drugs.
29:904–910. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sarveazad A, Babahajian A, shamsadin J and
Bahardoust M: 5-year survival rates and prognostic factors in
patients with synchronus and metachronus breast cancer from 2010 to
2015. Asian Pac J Cancer Prev. 19:3489–3493. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fu R, Deng Q, Zhang H, Hu X, Li Y, Liu Y,
Hu J, Luo Q, Zhang Y, Jiang X, et al: A novel autophagy inhibitor
berbamine blocks SNARE-mediated autophagosome-lysosome fusion
through upregulation of BNIP3. Cell Death Dis. 9:2432018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simonetti RG, Camma C, Fiorello F, Politi
F, D'Amico G and Pagliaro L: Hepatocellular carcinoma. A worldwide
problem and the major risk factors. Dig Dis Sci. 36:962–972. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li H, Wang Z, Jiang M, Fang RP, Shi H,
Shen Y, Cai XL, Liu Q, Ye K, Fan SJ, et al: The oncoprotein HBXIP
promotes human breast cancer growth through down-regulating p53 via
miR-18b/MDM2 and pAKT/MDM2 pathways. Acta Pharmacol Sin.
39:1787–1796. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu YJ, Zheng B, Wang HY and Chen L: New
knowledge of the mechanisms of sorafenib resistance in liver
cancer. Acta Pharmacol Sin. 38:614–622. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu H, Ruan S, Jia F, Chu J, Zhu Y, Huang
Y and Liu G: In vitro and in vivo superior radiosensitizing effect
of berbamine for head and neck squamous cell carcinoma. Onco
Targets Ther. 11:8117–8125. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leal-Orta E, Ramirez-Ricardo J,
Cortes-Reynosa P, Galindo-Hernandez O and Salazar EP: Role of
PI3K/Akt on migration and invasion of MCF10A cells treated with
extracellular vesicles from MDA-MB-231 cells stimulated with
linoleic acid. J Cell Commun Signal. 13:235–244. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tian L, Cheng F, Wang L, Qin W, Zou K and
Chen J: CLE-10 from Carpesium abrotanoides L. Suppresses the Growth
of human breast cancer cells (MDA-MB-231) in vitro by inducing
apoptosis and pro-death autophagy via the PI3K/Akt/mTOR signaling
pathway. Molecules. 24:10912019. View Article : Google Scholar
|
|
15
|
Xia E, Zhou X, Bhandari A, Zhang X and
Wang O: Synaptopodin-2 plays an important role in the metastasis of
breast cancer via PI3K/Akt/mTOR pathway. Cancer Manag Res.
10:1575–1583. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan W, Ma X, Zhao X and Zhang S: Baicalein
induces apoptosis and autophagy of breast cancer cells via
inhibiting PI3K/AKT pathway in vivo and vitro. Drug Des Devel Ther.
12:3961–3972. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang S, Liu Q, Zhang Y, Liu K, Yu P, Liu
K, Luan J, Duan H, Lu Z, Wang F, et al: Suppression of growth,
migration and invasion of highly-metastatic human breast cancer
cells by berbamine and its molecular mechanisms of action. Mol
Cancer. 8:812009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stephen H and Hare AJ: mTOR function and
therapeutic targeting in breast cancer. Am J Cancer Res. 7:383–404.
2017.PubMed/NCBI
|
|
19
|
Zheng S, Lv P, Su J, Miao K, Xu H and Li
M: Overexpression of CBX2 in breast cancer promotes tumor
progression through the PI3K/AKT signaling pathway. Am J Transl
Res. 11:1668–1682. 2019.PubMed/NCBI
|
|
20
|
Fan L, Zhang Y, Zhou Q, Liu Y, Gong B, Lü
J, Zhu H, Zhu G, Xu Y and Huang G: Casticin inhibits breast cancer
cell migration and invasion by down-regulation of PI3K/Akt
signaling pathway. Biosci Rep. 38:BSR201807382018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pierobon M, Ramos C, Wong S, Hodge KA,
Aldrich J, Byron S, Anthony SP, Robert NJ, Northfelt DW, Jahanzeb
M, et al: Enrichment of PI3K-AKT-mTOR pathway activation in hepatic
metastases from breast cancer. Clin Cancer Res. 23:4919–4928. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qiang P, Shao Y, Sun YP, Zhang J and Chen
LJ: Metformin inhibits proliferation and migration of endometrial
cancer cells through regulating PI3K/AKT/MDM2 pathway. Eur Rev Med
Pharmacol Sci. 23:1778–1785. 2019.PubMed/NCBI
|
|
23
|
Ghoneum A and Said N: PI3K-AKT-mTOR and
NFκB pathways in ovarian cancer: Implications for targeted
therapeutics. Cancers (Basel). 11:9492019. View Article : Google Scholar
|
|
24
|
Qin JJ, Wang W and Zhang R: Experimental
therapy of advanced breast cancer: Targeting NFAT1-MDM2-p53
pathway. Prog Mol Biol Transl Sci. 151:195–216. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rinaldi L, Sepe M, Delle Donne R, Conte K,
Arcella A, Borzacchiello D, Amente S, De Vita F, Porpora M, Garbi
C, et al: Mitochondrial AKAP1 supports mTOR pathway and tumor
growth. Cell Death Dis. 8:e28422017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoshikawa Y, Takano O, Kato I, Takahashi
Y, Shima F and Kataoka T: Ras inhibitors display an anti-metastatic
effect by downregulation of lysyl oxidase through inhibition of the
Ras-PI3K-Akt-HIF-1α pathway. Cancer Lett. 410:82–91. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang X, Qu P, Zhao H, Zhao T and Cao N:
COX2 promotes epithelialmesenchymal transition and migration in
osteosarcoma MG63 cells via PI3K/AKT/NF-κB signaling. Mol Med Rep.
20:3811–3819. 2019.PubMed/NCBI
|
|
28
|
Chan N, Willis A, Kornhauser N, Ward MM,
Lee SB, Nackos E, Seo BR, Chuang E, Cigler T, Moore A, et al:
Influencing the tumor microenvironment: A phase II study of copper
depletion using tetrathiomolybdate in patients with breast cancer
at high risk for recurrence and in preclinical models of lung
metastases. Clin Cancer Res. 23:666–676. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mosalpuria K, Hall C, Krishnamurthy S,
Lodhi A, Hallman DM, Baraniuk MS, Bhattacharyya A and Lucci A:
Cyclooxygenase-2 expression in non-metastatic triple-negative
breast cancer patients. Mol Clin Oncol. 2:845–850. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Du HP, Shen JK, Yang M, Wang YQ, Yuan XQ,
Ma QL and Jin J: 4-Chlorobenzoyl berbamine induces apoptosis and
G2/M cell cycle arrest through the PI3K/Akt and NF-kappaB signal
pathway in lymphoma cells. Oncol Rep. 23:709–716. 2010.PubMed/NCBI
|
|
31
|
Zhang L, Tong J, He X, Liang Y, Zhu L, Xu
R and Zhao X: Novel synthetic 4-chlorobenzoyl berbamine inhibits
c-Myc expression and induces apoptosis of diffuse large B cell
lymphoma cells. Ann Hematol. 97:2353–2362. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang SS, Lv Y, Xu XC, Zuo Y, Song Y, Wu
GP, Lu PH, Zhang ZQ and Chen MB: Triptonide inhibits human
nasopharyngeal carcinoma cell growth via disrupting Lnc-RNA
THOR-IGF2BP1 signaling. Cancer Lett. 443:13–24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cummins CB, Wang X, Xu J, Hughes BD, Ding
Y, Chen H, Zhou J and Radhakrishnan RS: Antifibrosis effect of
novel oridonin analog CYD0618 via suppression of the NF-κB pathway.
J Surg Res. 232:283–292. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Saatci O, Kaymak A, Raza U, Ersan PG,
Akbulut O, Banister CE, Sikirzhytski V, Tokat UM, Aykut G, Ansari
SA, et al: Targeting lysyl oxidase (LOX) overcomes chemotherapy
resistance in triple negative breast cancer. Nat Commun.
11:24162020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meng F, Wu L, Dong L, Mitchell AV, James
Block C, Liu J, Zhang H, Lu Q, Song WM, Zhang B, et al: EGFL9
promotes breast cancer metastasis by inducing cMET activation and
metabolic reprogramming. Nat Commun. 10:50332019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu R, Zhang Y, Chen Y, Qi J, Ren S, Xushi
MY, Yang C, Zhu H and Xiong D: A novel calmodulin antagonist
O-(4-ethoxyl-butyl)-berbamine overcomes multidrug resistance in
drug-resistant MCF-7/ADR breast carcinoma cells. J Pharm Sci.
99:3266–3275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cao Y, Cao J, Yu B, Wang S, Liu L, Tao L
and Sun W: Berbamine induces SMMC-7721 cell apoptosis via
upregulating p53, downregulating survivin expression and activating
mitochondria signaling pathway. Exp Ther Med. 15:1894–1901.
2018.PubMed/NCBI
|
|
38
|
Jin X and Wu Y: Berbamine enhances the
antineoplastic activity of gemcitabine in pancreatic cancer cells
by activating transforming growth factor-β/Smad signaling. Anat Rec
(Hoboken). 297:802–809. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao Y, Lv JJ, Chen J, Jin XB, Wang MW, Su
ZH, Wang LY and Zhang HY: Berbamine inhibited the growth of
prostate cancer cells in vivo and in vitro via triggering intrinsic
pathway of apoptosis. Prostate Cancer Prostatic Dis. 19:358–366.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ding X, Wang Q, Tong L, Si X and Sun Y:
Long non-coding RNA FOXO1 inhibits lung cancer cell growth through
down-regulating PI3K/AKT signaling pathway. Iran J Basic Med Sci.
22:491–498. 2019.PubMed/NCBI
|
|
41
|
Deng S, Dai G, Chen S, Nie Z, Zhou J, Fang
H and Peng H: Dexamethasone induces osteoblast apoptosis through
ROS-PI3K/AKT/GSK3β signaling pathway. Biomed Pharmacother.
110:602–608. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Leszczynska KB, Foskolou IP, Abraham AG,
Anbalagan S, Tellier C, Haider S, Span PN, O'Neill EE, Buffa FM and
Hammond EM: Hypoxia-induced p53 modulates both apoptosis and
radiosensitivity via AKT. J Clin Invest. 125:2385–2398. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yue X, Li M, Chen D, Xu Z and Sun S:
UNBS5162 induces growth inhibition and apoptosis via inhibiting
PI3K/AKT/mTOR pathway in triple negative breast cancer MDA-MB-231
cells. Exp Ther Med. 16:3921–3928. 2018.PubMed/NCBI
|
|
44
|
Wang W, Wu J, Fei X, Chen W, Li Y, Shen K
and Zhu L: CHD1L promotes cell cycle progression and cell motility
by up-regulating MDM2 in breast cancer. Am J Transl Res.
11:1581–1592. 2019.PubMed/NCBI
|
|
45
|
Liu XH, Kirschenbaum A, Yu K, Yao S and
Levine AC: Cyclooxygenase-2 suppresses hypoxia-induced apoptosis
via a combination of direct and indirect inhibition of p53 activity
in a human prostate cancer cell line. J Biol Chem. 280:3817–3823.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gao S, Li X, Ding X, Qi W and Yang Q:
Cepharanthine induces autophagy, apoptosis and cell cycle arrest in
breast cancer cells. Cell Physiol Biochem. 41:1633–1648. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Haginaka J, Kitabatake T, Hirose I,
Matsunaga H and Moaddel R: Interaction of cepharanthine with
immobilized heat shock protein 90α (Hsp90α) and screening of Hsp90α
inhibitors. Anal Biochem. 434:202–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bailly C: Cepharanthine: An update of its
mode of action, pharmacological properties and medical
applications. Phytomedicine. 62:1529562019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim BR, Dong SM, Seo SH, Lee JH, Lee JM,
Lee SH and Rho SB: Lysyl oxidase-like 2 (LOXL2) controls
tumor-associated cell proliferation through the interaction with
MARCKSL1. Cell Signal. 26:1765–1773. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang Y, Sun H, Xiao Z, Zhang G, Zhang D,
Bao X, Li F, Wu S, Gao Y and Wei N: DNA damage and apoptosis
induced by a potent orally podophyllotoxin derivative in breast
cancer. Cell Commun Signal. 16:522018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kanaizumi H, Higashi C, Tanaka Y, Hamada
M, Shinzaki W, Azumi T, Hashimoto Y, Inui H, Houjou T and Komoike
Y: PI3K/Akt/mTOR signalling pathway activation in patients with
ER-positive, metachronous, contralateral breast cancer treated with
hormone therapy. Oncol Lett. 17:1962–1968. 2019.PubMed/NCBI
|