Lung carcinoma is the most diagnosed cancer type
worldwide, and the leading cause of cancer-associated mortality
(1). In 2018, 2.1 million new lung
carcinoma cases were diagnosed and 1.8 million mortalities were
registered, and almost one fifth of the mortalities were by cancer
cases (2). Applications of immune
checkpoint inhibitors (ICIs) aimed at blocking programmed cell
death 1 (PD-1), programmed cell death ligand 1 (PD-L1) and
anti-cytotoxic T lymphocyte antigen-4 (CTLA-4) have improved
overall prognosis compared with traditional chemotherapy and
radiotherapy (3–5). PD-L1 expression is a common biomarker
to predict response to ICIs and is detected in patients with
advanced non-small cell lung cancer (NSCLC). The positivity of
PD-L1 has been associated with high probability of response
(6). However, other studies have
failed to identify an association been PD-L1 expression and ICIs
treatment outcomes (3,7,8). A
meta-analysis reported that PD-L1 expression is associated with
poor prognosis (9). Recently, tumor
mutational burden (TMB) has demonstrated prospects in clinical
response. The objective response rate (ORR) and progression-free
survival (PFS) in ICIs therapy are associated with high-TMB (TMB-H)
(10). However, no significance
association has been identified between TMB and overall prognosis
(11). Neither PD-L1 nor TMB can
specifically aid patient selection (12). Thus, it remains necessary to identify
reliable biomarkers to improve ICIs therapy.
Immunotherapy targets the immune system or tumor
microenvironment and actives the immune system against tumors by
different immune cells (13).
Lymphocytes that infiltrate the tumor (tumor-infiltrating
lymphocytes, TILs) play an important role (13). Previous studies (14,15) have
demonstrated that TILs density, particularly CD8+TILs
and regulatory T cells (Tregs), may predict clinical outcomes for
ICIs therapy. In most types of human cancer, consistent
intra-tumoral infiltration of CD8+ TILs and helper T
cells 1 (Th1) are associated with favorable prognosis and better
survival outcomes (16).
Pretreatment incidence of CD8+ T cells density at the
invasive margin is associated with the PD-1/PD-L1 pathway and is an
important determinant of improved outcomes in patients with
melanoma (14). Apart from TILs
density and distribution, their phenotypes also play key roles. A
study demonstrated that high numbers of pre-existing memory T cells
are potential biomarkers in patients with melanoma treated with
CTLA-4 antibody (17). Another
randomized trial in a hepatocellular carcinoma cohort that received
preoperative nivolumab or ipilimumab treatment demonstrated that
increased effector T cells were associated with complete response
(CR) (18). The present study
summarizes TILs phenotypes in tumor tissues, lymphocyte subtypes
and serum cytokines in peripheral blood (PB). Their associations
with clinical outcomes and probable prognostic biomarkers for
patients with NSCLC in ICIs therapy are also summarized.
TILs are a group of tumor invasive cells that exert
antigenic effects, which were initially discovered in tumor tissue
of mice in 1986 (19). PD-L1 is
expressed on both TILs and tumor tissues (20). Recently, a model of tumor immune
microenvironment based on TIL status and PD-L1 expression has been
established (21). In this model,
tumor microenvironments were classified into four types (21), including type I (PD-L1 positive with
TILs positive), type II (PD-L1 negative with TILs negative), type
III (PD-L1 positive with TILs negative) and type IV (PD-L1 negative
with TILs positive). The type I tumor microenvironment has
favorable response to PD-1/PD-L1 checkpoint inhibitors therapy
(21). Furthermore, TILs phenotypes
in tumor tissues play a crucial role in ICIs therapy (22) (Table
I).
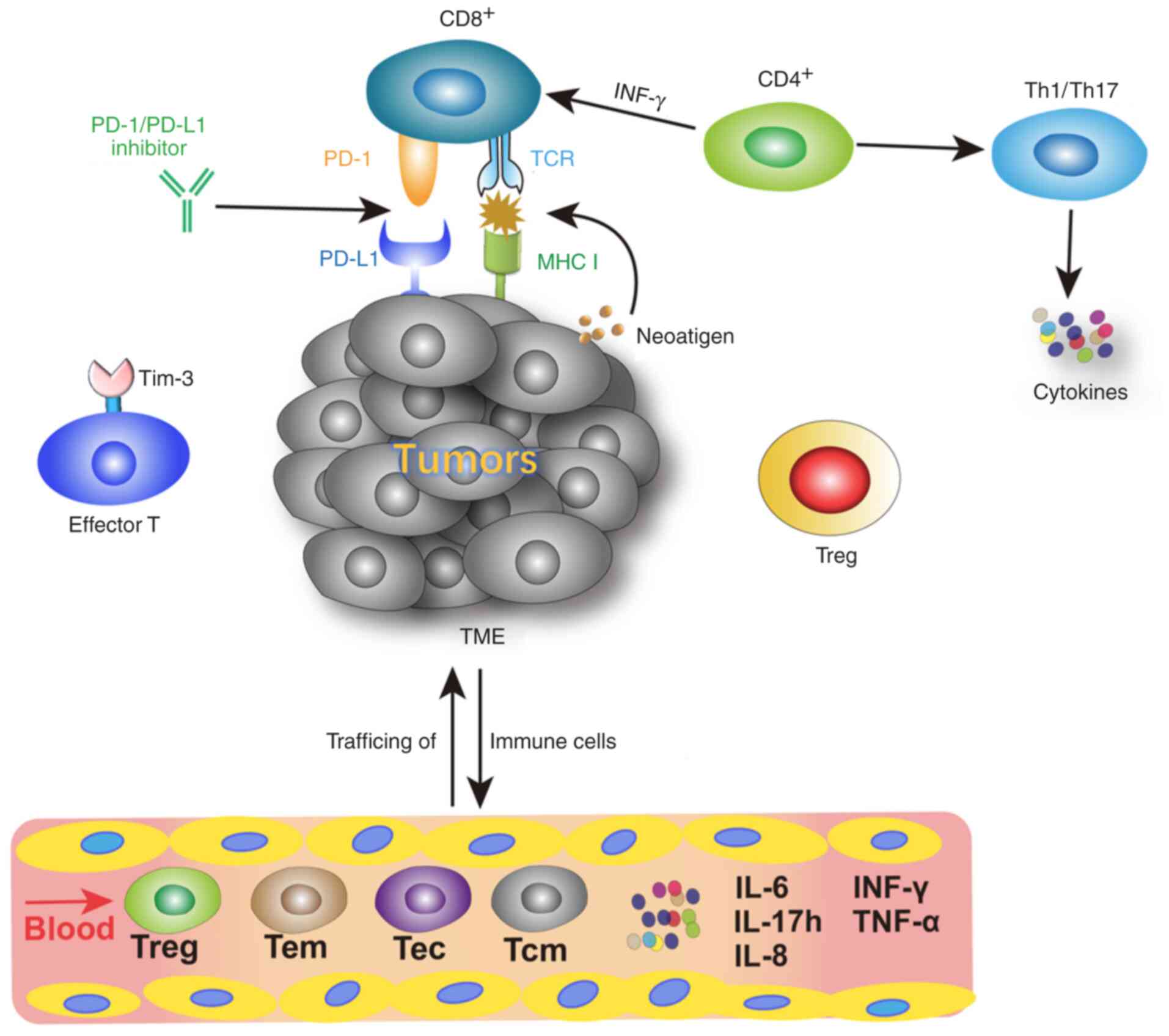
The activation of T cells involves antigen delivery
from the major histocompatibility complex (MHC) molecules on
antigen-presenting cells to the corresponding T cell receptor (TCR)
on naïve T cells (23). MHC I
molecules combine with CD8+ T cells and MHC II molecules
bind to CD4+T cells to assist antigen recognition
(23). CD8+T cells are
the primary cytotoxic cells and critical for cell-mediated
antitumor immune responses (23).
CD4+ T cells are predominantly expressed on helper T
cells (Th), which regulate or help other lymphocytes (23) (Fig.
1).
Blood-based biomarkers are easily accessible during
therapy, as disruption via intra- and inter-tumor heterogeneity is
prevented (50). Some vital
peripheral lymphocyte subgroups associated with responses to
immunotherapy are presented in Fig.
1. Previous studies of circulating biomarkers for ICIs therapy
include different T cells subtypes and cytokines (Table I).
Most T cells extend to differentiate into short-term
living effector T cells (Tec), which disappear quickly in the
contraction phase (61).
Antigen-specific memory T cells maintain high frequencies and can
respond to antigenic stimulation with rapid effector function and
proliferation (61). There are two
main subsets of memory cells, effector memory T cells (Tem) and
central memory T cells (Tcm) (61).
Effector and memory T cells take up residency and become resident
memory T cells (Trm), which inhabit peripheral tissues (61). Memory T cells infiltration in lymph
node metastases is an independent positive prognostic factors in
patients with 1NSCLC (62). Higher
levels of memory CD4+T, memory CD8+T and less
naïve CD4+ T cells have been in observed in responders
compared with non-responders in lung metastases, treated with
stereotactic body radiotherapy (63). Julia et al (53) demonstrated that in patients with
NSCLC treated with either 6 cycles of nivolumab or 4 cycles of
pembrolizumab, the percentage of CD4+Tcm
(CD4+CD45RO+CCR7+) prior to
treatment is higher in stable disease (SD) and partial response
(PR) patients compared with progressive disease (PD) patients,
while a decrease in naïve CD4+ T cells was observed in
SD and PR patients. The PD cohort was defined as patients whose sum
of the maximum diameters of targeted lesions increased at least
20%, or patients displaying new metastasis (53). In a cohort of non-squamous NSCLC
patients (n=22) treated with nivolumab, high CD4+ and
CD8+ Tcm/Tec ratios were associated with inflamed tumors
and extended PFS (64). A tendency
towards a higher percentage of
CD45RA+CCR7−CD8+ T cells was
detected in PR groups compared with PD groups at baseline and
several time points during treatment with nivolumab (65). Higher percentages of
CD95+CD60−CD8+ T cells were also
observed (65). It was speculated
that the local antigen encounter may have induced T cell
differentiation and tissue egression of CD8+ T cells in
PR patients (65).
CD8+CD103+ serves as a marker of Trm
(66). A study demonstrated that
high CD103+ TILs density is associated with the survival
of patients with NSCLC at an early stage, and CD103+ may
be a potential biomarker of tumor reactive CD8+ TIL
(66). In advanced NSCLC patients
(n=74) treated with nivolumab, the frequency of exhausted
CD8+ T cells
(CD8+PD1+Eomes+) prior to
treatment notably decreases in patients whose disease progress is
well controlled (54). In addition,
a higher frequency of exhausted CD8+ T cells in PB is
associated with a longer OS time (54).
The nuclear protein, Ki67, functions in assessing
cell proliferation and measuring the proliferative capacity of
cells (67). Although Ki67
expression may not be visible at all active stages of cell
division, the principles of irreparable DNA repair or the quiescent
step are not yet available (67). In
NSCLC patients treated with ICIs, the highest fold change of Ki-67
proliferation occurred among CD8+T cells (66). Thus, the activity of cycling
CD8+ T cells increased in 70% of patients following ICIs
therapy, which may be associated with clinical outcomes (66). A study aiming to monitor occupancy of
PD-1 on T cells with nivolumab demonstrated that Ki67 positivity
rates of both nivolumab-binding and total T cells decreased in
patients with non-responding tumors (68). Ki67 significantly decreases at the
time of disease progression (68).
In a study by Kim et al (69), the proliferative percentage of Ki67
among cycle PD-1+CD8+ T cells was assessed
prior to treatment (D0) and 7 days after patients received the
first dose of pembrolizumab or nivolumab (D7). The higher
fold-change (Ki-67D7/D0) revealed prolonged survival.
Ki-67D7/D0>2.8 predicted better PFS and OS, both in
the independent tested and validated cohorts (69). Furthermore, some studies have
demonstrated that ICIs treatment efficacy is associated with
frequencies of PD1+Ki67+CD8+
T-lymphocytes or the ratio of
PD1+KI67+CD8+ T lymphocytes to
tumor burden (70,71).
T cell immunoglobulin and mucin-domain containing-3
(Tim-3) is a co-inhibitory receptor expressed on IFN-r-producing T
cells, FOXP3+ Treg cells and innate immune cells
(72). It functions in inhibiting
Th1 responses and the expression of cytokines (72). Tim-3 regulates T cell exhaustion in
TILs (72). Early accumulation of
lymphocytes with high Tim-3 expression is associated with both
primary and secondary resistance (73). Koyama et al (74) demonstrated that upregulation of Tim-3
was an indication of anti-PD1 treatment resistance in both the
animal model and pleural effusion of patients with NSCLC. High
Tim-3 levels expressed on CD8+ T cells are associated
with poor tumor progression (75).
In patients with lung adenocarcinoma who undergo surgery, positive
Tim-3 expression is associated with positive PD-1 expression and
high CD8+ TIL density (76). In addition, positive Tim-3 expression
is associated with poor recurrence-free survival (RFS) and OS
(76). In the study by Julia et
al (53), Tim-3 expression in
CD4+ and CD8+ T cells increased in PD
patients, while the levels decreased in the SD and PR groups
following anti-PD1 therapy (53).
Taken together, these results suggest that Tim-3 may be an
underlying negative biomarker of disease progression following
immunotherapy.
TCR specifically targets antigenic peptides on the
cell surface of human leukocyte antigen class I/β-2-microglobulin
complexes (77). TCR and its
signaling molecules accumulate in T cells or tumor cells, resulting
in the formation and transmission cascade of immune synapse (IS),
and perform cytotoxic T lymphocyte (CTL) effector functions
(77). Activation of the host immune
response against cancer cells includes recognition of neoantigen
peptides by clonally proliferating TCRs. The highly variable
complementarity determining region 3 (CDR3) determines the
specificity and diversity of TCR (77). Recently, TCR-based biomarkers have
been investigated and some predictive responses have been
demonstrated as follows. In patients with melanoma who are treated
with ipilimumab, the clinical outcome is positive in relation to a
higher pretreatment abundance of TCR repertoire richness (78). The level of CDR3 diversity in
patients with lung cancer is inferior compared with healthy
individuals (79). Patients with a
more advanced disease state or poorer immune status have
substantially lower CDR3 diversity (79). Peripheral
PD1+CD8+ TCR diversity may predict clinical
benefits of PD-1/PD-L1 blockade therapy, which was calculated based
on the Shannon-Wiener index (80) by
sequencing CDR3 semi quantitatively with multiplex PCR (81). A high diversity of TCR provides more
opportunities for tumor neoantigen recognition and improves immune
response. Patients with high diversity of TCR have a significantly
longer PFS than those with low diversity (79,81).
Cytokines are small molecular proteins with
extensive biological activity secreted by immune cells, which are
associated with physical activity, toxicity of drugs, resistance of
treatment and infection (82). In a
lung cancer mouse model induced by K-Ras oncogene, IL-6 was
demonstrated to suppress initiation of lung carcinoma by activating
cytotoxic CD8+ T cells; however, it improved tumor
growth by inducing cell proliferation (83). These findings were consistent with
the negative prognostic role of IL-6 in patients with NSCLC
(84). In addition, the expression
levels of IL-17A and IL-8 are significantly associated with disease
progression (85,86). However, inflammatory cytokines are
associated with cachexia, pain, toxicity of drugs, resistance to
treatment and physical activity, which are all prognostic factors
of clinical outcomes and may be influenced by these factors
(87). Excluding granule exocytosis
and Fas ligand-mediated apoptosis induction, CTL also releases
interferon-γ (IFN-γ) and tumor necrosis factor α (TNF-α) to induce
cytotoxicity in tumor cells (88).
Thus, these two cytokines may be important in predicting efficacy.
Another study demonstrated that four IFN-γ signatures from genetic
studies at baseline tumor biopsies were predictive biomarkers for
longer OS and PFS following treatment with durvalumab (89). In baseline NSCLC tumors, higher IFN-γ
signature thresholds are associated with higher ORR, longer median
OS and median PFS (89). In an
open-label, phase II, randomized controlled trial, the efficacy of
atezolizumab vs. docetaxel in 287 patients with NSCLC was assessed.
The results demonstrated that tumor patients with a higher
expression of T-effector/IFN-γ gene signature had a longer OS in
the atezolizumab treatment group (90). Karachaliou et al (91) reported the association between high
IFN-γ gene expression in baseline and PFS following treatment with
anti-PD1, as well as the tendency between high IFN-γ gene
expression and OS in patients with NSCLC. In patients treated with
ICIs, such as pembrolizumab, nivolumab and atezolizumab, disease
progression and interstitial pneumonitis were observed if the IFN-γ
levels in the blood were <10 IU/ml (92). Levels of IFN-γ in the PB of advanced
or metastatic patients with NSCLC were associated with better
treatment response at 3 months following anti-PD1 therapy. In
addition, statistical analysis demonstrated that different levels
of IFN-γ resulted in different PD-L1 expression levels (93).
Along with other proinflammatory factors, TNF-α
recruits neutrophils, macrophages and lymphocytes to the place of
damage and infection and subsequently actives these cells (82). TNF-α levels in the plasma are
positively associated with the expression levels of PD-L1 and PD-L2
in total T cells, Th cells and CTL (94). Excluding IFN-γ and ILs, Boutsikou
et al (93) demonstrated that
increased TNF-α levels in the PB result in better response and
longer survival time following anti-PD1 therapy.
Although high PD-L1 expression and TMB-H are
currently recognized as beneficial signals in the application of
ICIs therapy in patients with lung cancer, >50% of patients do
not exhibit durable responses (95).
Thus, it remains unclear if patients will benefit from ICIs
therapy. The present review summarized different potential
biomarkers. CD8+TILs are considered important cytotoxic
immune cells, and the accumulation of Tregs is associated with
immune suppression (23). Memory and
effector T lymphocytes in the PB exert antitumor activity (61), and TCR diversity indicates the
probability of neoantigen recognition (77). Furthermore, cytokines reflect immune
levels and are closely associated with survival (82). Although these biomarkers are
controversial and their specific molecular mechanism remain
unclear, a promising tumor immune landscape has been established.
Neither tissues or blood biomarkers alone can accurately predict
disease progression and clinical outcomes. Prospective studies
should investigate additional potential biomarkers both in
vitro and in vivo. Future studies should also focus on
the development and validation of multi-marker assays. Notably,
clinical experiments with large sample sizes should be designed and
their accuracy should be verified.
Not applicable.
Not applicable.
Not applicable.
XW conceived and designed the present review,
performed the literature review, and prepared and drafted the
initial manuscript. LG helped perform the literature review. WH
contributed to the design, manuscript writing and editing. All
authors have read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brahmer J, Reckamp KL, Baas P, Crino L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Borghaei H BJ, Horn L, Ready N, Steins M,
Felip E, Paz-Ares L, Xx X, Barlesi F, Antonia S and Fayette J:
P2.35: nivolumab vs docetaxel in advanced NSCLC: CheckMate 017/057
2-Y update and exploratory cytokine profile analysis. Track:
Immunotherapy J Thorac Oncol. 11:S237–S238. 2016.
|
|
8
|
Sharma P, Retz M, Siefker-Radtke A, Baron
A, Necchi A, Bedke J, Plimack ER, Vaena D, Grimm MO, Bracarda S, et
al: Nivolumab in metastatic urothelial carcinoma after platinum
therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial.
Lancet Oncol. 18:312–322. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang M, Li G and Wang Y and Wang Y, Zhao
S, Haihong P, Zhao H and Wang Y: PD-L1 expression in lung cancer
and its correlation with driver mutations: A meta-analysis. Sci
Rep. 7:102552017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goodman AM, Kato S, Bazhenova L, Patel SP,
Frampton GM, Miller V, Stephens PJ, Daniels GA and Kurzrock R:
Tumor mutational burden as an independent predictor of response to
immunotherapy in diverse cancers. Mol Cancer Ther. 16:2598–2608.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carbone DP, Reck M, Paz-Ares L, Creelan B,
Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F,
et al: First-line nivolumab in stage IV or recurrent non-small-cell
lung cancer. N Engl J Med. 376:2415–2426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu Y, Zeng D, Ou Q, Liu S, Li A, Chen Y,
Lin D, Gao Q, Zhou H, Liao W and Yao H: Association of survival and
immune-related Biomarkers with immunotherapy in patients with
non-small cell lung cancer. A meta-analysis and individual
patient-level analysis. JAMA Netw Open. 2:e1968792019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kitamura T, Qian BZ and Pollard JW: Immune
cell promotion of metastasis. Nat Rev Immunol. 15:73–86. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tumeh PC, Harview CL, Yearley JH, Shintaku
IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu
V, et al: PD-1 blockade induces responses by inhibiting adaptive
immune resistance. Nature. 515:568–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Geng Y, Shao Y, He W, Hu W, Xu Y, Chen J,
Wu C and Jiang J: Prognostic role of tumor-infiltrating lymphocytes
in lung cancer: A meta-analysis. Cell Physiol Biochem.
37:1560–1571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fridman WH, Pages F, Sautes-Fridman C and
Galon J: The immune contexture in human tumours: Impact on clinical
outcome. Nat Rev Cancer. 12:298–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Subrahmanyam PB, Dong Z, Gusenleitner D,
Giobbie-Hurder A, Severgnini M, Zhou J, Manos M, Eastman LM,
Maecker HT and Hodi FS: Distinct predictive biomarker candidates
for response to anti-CTLA-4 and anti-PD-1 immunotherapy in melanoma
patients. J Immunother Cancer. 6:182018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaseb AO, Vence L, Blando J, Yadav SS,
Ikoma N, Pestana RC, Vauthey JN, Allison JP and Sharma P:
Immunologic correlates of pathologic complete response to
preoperative immunotherapy in hepatocellular carcinoma. Cancer
Immunol Res. 7:1390–1395. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rosenberg SA, Spiess P and Lafreniere R: A
new approach to the adoptive immunotherapy of cancer with
tumor-infiltrating lymphocytes. Science. 233:1318–1321. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He Y, Rozeboom L, Rivard CJ, Ellison K,
Dziadziuszko R, Yu H, Zhou C and Hirsch FR: PD-1, PD-L1 protein
expression in non-small cell lung cancer and their relationship
with tumor-infiltrating lymphocytes. Med Sci Monit. 23:1208–1216.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teng MW, Ngiow SF, Ribas A and Smyth MJ:
Classifying cancers based on T-cell Infiltration and PD-L1. Cancer
Res. 75:2139–2145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park J, Kwon M, Kim KH, Kim TS, Hong SH,
Kim CG, Kang SG, Moon JH, Kim EH, Park SH, et al: Immune checkpoint
inhibitor-induced reinvigoration of tumor-infiltrating
CD8+ T cells is determined by their differentiation
status in glioblastoma. Clin Cancer Res. 25:2549–2559.
2019.PubMed/NCBI
|
|
23
|
Natarajan K, Jiang J, May NA, Mage MG,
Boyd LF, McShan AC, Sgourakis NG, Bax A and Margulies DH: The role
of molecular flexibility in antigen presentation and T cell
receptor-mediated signaling. Front Immunol. 9:16572018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tokito T, Azuma K, Kawahara A, Ishii H,
Yamada K, Matsuo N, Kinoshita T, Mizukami N, Ono H, Kage M and
Hoshino T: Predictive relevance of PD-L1 expression combined with
CD8+ TIL density in stage III non-small cell lung cancer patients
receiving concurrent chemoradiotherapy. Eur J Cancer. 55:7–14.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
El-Guindy DM, Helal DS, Sabry NM and Abo
El-Nasr M: Programmed cell death ligand-1 (PD-L1) expression
combined with CD8 tumor infiltrating lymphocytes density in
non-small cell lung cancer patients. J Egypt Natl Canc Inst.
30:125–131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim SH, Go SI, Song DH, Park SW, Kim HR,
Jang I, Kim JD, Lee JS and Lee GW: Prognostic impact of CD8 and
programmed death-ligand 1 expression in patients with resectable
non-small cell lung cancer. Br J Cancer. 120:547–554. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin G, Fan X, Zhu W, Huang C, Zhuang W, Xu
H, Lin X, Hu D, Huang Y, Jiang K, et al: Prognostic significance of
PD-L1 expression and tumor infiltrating lymphocyte in surgically
resectable non-small cell lung cancer. Oncotarget. 8:83986–83994.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Donnem T, Hald SM, Paulsen EE, Richardsen
E, Al-Saad S, Kilvaer TK, Brustugun OT, Helland A, Lund-Iversen M,
Poehl M, et al: Stromal CD8+ T-cell density-a promising
supplement to tnm staging in non-small cell lung cancer. Clin
Cancer Res. 21:2635–2643. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Al-Shibli KI, Donnem T, Al-Saad S, Persson
M, Bremnes RM and Busund LT: Prognostic effect of epithelial and
stromal lymphocyte infiltration in non-small cell lung cancer. Clin
Cancer Res. 14:5220–5227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kawai O, Ishii G, Kubota K, Murata Y,
Naito Y, Mizuno T, Aokage K, Saijo N, Nishiwaki Y, Gemma A, et al:
Predominant infiltration of macrophages and CD8+ T cells
in cancer nests is a significant predictor of survival in stage IV
non-small cell lung cancer. Cancer. 113:1387–1395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sepesi B, Cuentes EP, Canales JR, Behrens
C, Correa A, Antonoff M, Gibbons DL, Heymach WH, Mehran R, Rice DC,
et al: Tumor-infiltrating lymphocytes and overall survival in
surgically resected tage II and III non-small cell lung cancer. Int
J Rad Oncology Biol Phys. 98:2232017. View Article : Google Scholar
|
|
33
|
Mazzaschi G, Madeddu D, Falco A,
Bocchialini G, Goldoni M, Sogni F, Armani G, Lagrasta CA, Lorusso
B, Mangiaracina C, et al: Low PD-1 expression in cytotoxic
CD8+ tumor-infiltrating lymphocytes confers an
immune-privileged tissue microenvironment in NSCLC with a
prognostic and predictive value. Clin Cancer Res. 24:407–419. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bauml JM, Mick R, Ciunci C, Aggarwal C,
Davis C, Evans T, Deshpande C, Miller L, Patel P, Alley E, et al:
Pembrolizumab after completion of locally ablative therapy for
oligometastatic non-small cell lung cancer: A Phase 2 Trial. JAMA
Oncol. 5:1283–1280. 2019. View Article : Google Scholar
|
|
35
|
Nakazawa N, Yokobori T, Kaira K, Turtoi A,
Baatar S, Gombodorj N, Handa T, Tsukagoshi M, Ubukata Y, Kimura A,
et al: High stromal TGFBI in lung cancer and intratumoral
CD8-positive T cells were associated with poor prognosis and
therapeutic resistance to immune checkpoint inhibitors. Ann Surg
Oncol. 27:933–942. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kinoshita T, Kudo-Saito C, Muramatsu R,
Fujita T, Saito M, Nagumo H, Sakurai T, Noji S, Takahata E, Yaguchi
T, et al: Determination of poor prognostic immune features of
tumour microenvironment in non-smoking patients with lung
adenocarcinoma. Eur J Cancer. 86:15–27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koh J, Go H, Keam B, Kim MY, Nam SJ, Kim
TM, Lee SH, Min HS, Kim YT, Kim DW, et al: Clinicopathologic
analysis of programmed cell death-1 and programmed cell
death-ligand 1 and 2 expressions in pulmonary adenocarcinoma:
Comparison with histology and driver oncogenic alteration status.
Mod Pathol. 28:1154–1166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zeng DQ, Yu YF, Ou QY, Li XY, Zhong RZ,
Xie CM and Hu QG: Prognostic and predictive value of
tumor-infiltrating lymphocytes for clinical therapeutic research in
patients with non-small cell lung cancer. Oncotarget.
7:13765–13781. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hiraoka K, Miyamoto M, Cho Y, Suzuoki M,
Oshikiri T, Nakakubo Y, Itoh T, Ohbuchi T, Kondo S and Katoh H:
Concurrent infiltration by CD8+ T cells and
CD4+ T cells is a favourable prognostic factor in
non-small-cell lung carcinoma. Br J Cancer. 94:275–280. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wakabayashi O, Yamazaki K, Oizumi S,
Hommura F, Kinoshita I, Ogura S, Dosaka-Akita H and Nishimura M:
CD4+ T cells in cancer stroma, not CD8+ T
cells in cancer cell nests, are associated with favorable prognosis
in human non-small cell lung cancers. Cancer Sci. 94:1003–1009.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hu-Lieskovan S, Lisberg A, Zaretsky JM,
Grogan TR, Rizvi H, Wells DK, Carroll J, Cummings A, Madrigal J,
Jones B, et al: Tumor characteristics associated with benefit from
pembrolizumab in advanced non-small cell lung cancer. Clin Cancer
Res. 25:5061–5068. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nishikawa H and Sakaguchi S: Regulatory T
cells in cancer immunotherapy. Curr Opin Immunol. 27:1–7. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Petersen RP, Campa MJ, Sperlazza J, Conlon
D, Joshi MB, Harpole DH Jr and Patz EF Jr: Tumor infiltrating
Foxp3+ regulatory T-cells are associated with recurrence
in pathologic stage I NSCLC patients. Cancer. 107:2866–2872. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Barua S, Fang P, Sharma A, Fujimoto J,
Wistuba I, Rao AUK and Lin SH: Spatial interaction of tumor cells
and regulatory T cells correlates with survival in non-small cell
lung cancer. Lung Cancer. 117:73–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu SP, Liao RQ, Tu HY, Wang WJ, Dong ZY,
Huang SM, Guo WB, Gou LY, Sun HW, Zhang Q, et al: Stromal
PD-L1-positive regulatory T cells and PD-1-positive CD8-positive T
cells define the response of different subsets of non-small cell
lung cancer to PD-1/PD-L1 blockade immunotherapy. J Thorac Oncol.
13:521–532. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Giatromanolaki A, Banham AH, Harris AL and
Koukourakis MI: FOXP3 infiltrating lymphocyte density and PD-L1
expression in operable non-small cell lung carcinoma. Exp Lung Res.
45:76–83. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sakai K, Maeda S, Yamada Y, Chambers JK,
Uchida K, Nakayama H, Yonezawa T and Matsuki N: Association of
tumour-infiltrating regulatory T cells with adverse outcomes in
dogs with malignant tumours. Vet Comp Oncol. 16:330–336. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jackute J, Zemaitis M, Pranys D,
Sitkauskiene B, Miliauskas S, Bajoriunas V, Lavinskiene S and
Sakalauskas R: The prognostic influence of tumor infiltrating
Foxp3+CD4+, CD4+ and
CD8+ T cells in resected non-small cell lung cancer. J
Inflamm (Lond). 12:632015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kinoshita F, Takada K, Yamada Y, Oku Y,
Kosai K, Ono Y, Tanaka K, Wakasu S, Oba T, Osoegawa A, et al:
Combined Evaluation of tumor-nfiltrating CD8 + and FoxP3 +
lymphocytes provides accurate prognosis in stage IA lung
adenocarcinoma. Ann Surg Oncol. 27:2102–2109. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
De Mattos-Arruda L, Cortes J, Santarpia L,
Vivancos A, Tabernero J, Reis-Filho JS and Seoane J: Circulating
tumour cells and cell-free DNA as tools for managing breast cancer.
Nat Rev Clin Oncol. 10:377–389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang WJ, Tao Z, Gu W and Sun LH: Variation
of blood T lymphocyte subgroups in patients with non- small cell
lung cancer. Asian Pac J Cancer Prev. 14:4671–4673. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu L, Chen D, Lu C, Liu X, Wu G and Zhang
Y: Advanced lung cancer is associated with decreased expression of
perforin, CD95, CD38 by circulating CD3+CD8+ T lymphocytes. Ann
Clin Lab Sci. 45:528–532. 2015.PubMed/NCBI
|
|
53
|
Julia EP, Mando P, Rizzo MM, Cueto GR,
Tsou F, Luca R, Pupareli C, Bravo AI, Astorino W, Mordoh J, et al:
Peripheral changes in immune cell populations and soluble mediators
after anti-PD-1 therapy in non-small cell lung cancer and renal
cell carcinoma patients. Cancer Immunol Immunother. 68:1585–1596.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ottonello S, Genova C, Cossu I, Fontana V,
Rijavec E, Rossi G, Biello F, Dal Bello MG, Tagliamento M, Alama A,
et al: Association between response to nivolumab treatment and
peripheral blood lymphocyte subsets in patients with non-small cell
lung cancer. Front Immunol. 11:1252020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Inomata M, Kado T, Okazawa S, Imanishi S,
Taka C, Kambara K, Hirai T, Tanaka H, Tokui K, Hayashi K, et al:
Peripheral PD1-positive CD4 T-lymphocyte count can predict
progression-free survival in patients with non-small cell lung
cancer receiving immune checkpoint inhibitor. Anticancer Res.
39:6887–6893. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Thompson RH, Dong H, Lohse CM, Leibovich
BC, Blute ML, Cheville JC and Kwon ED: PD-1 is expressed by
tumor-infiltrating immune cells and is associated with poor outcome
for patients with renal cell carcinoma. Clin Cancer Res.
13:1757–1761. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Muenst S, Soysal SD, Gao F, Obermann EC,
Oertli D and Gillanders WE: The presence of programmed death 1
(PD-1)-positive tumor-infiltrating lymphocytes is associated with
poor prognosis in human breast cancer. Breast Cancer Res Treat.
139:667–676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hsu MC, Hsiao JR, Chang KC, Wu YH, Su IJ,
Jin YT and Chang Y: Increase of programmed death-1-expressing
intratumoral CD8 T cells predicts a poor prognosis for
nasopharyngeal carcinoma. Mod Pathol. 33:1393–1403. 2010.
View Article : Google Scholar
|
|
59
|
Mazzaschi G, Facchinetti F, Missale G,
Canetti D, Madeddu D, Zecca A, Veneziani M, Gelsomino F, Goldoni M,
Buti S, et al: The circulating pool of functionally competent NK
and CD8+ cells predicts the outcome of anti-PD1 treatment in
advanced NSCLC. Lung Cancer. 127:153–163. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhuo M, Chen H, Zhang T, Yang X, Zhong J,
Wang Y, An T, Wu M, Wang Z, Huang J and Zhao J: The potential
predictive value of circulating immune cell ratio and tumor marker
in atezolizumab treated advanced non-small cell lung cancer
patients. Cancer Biomark. 22:467–476. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Schenkel JM and Masopust D:
Tissue-resident memory T cells. Immunity. 41:886–897. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kilvaer TK, Paulsen EE, Khanehkenari MR,
Al-Saad S, Johansen RM, Al-Shibli K, Bremnes RM, Busund LT and
Donnem T: The presence of intraepithelial CD45RO+ cells in resected
lymph nodes with metastases from NSCLC patients is an independent
predictor of disease-specific survival. Br J Cancer. 114:1145–1151.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liu C, Hu Q, Xu B, Hu X, Su H, Li Q, Zhang
X, Yue J and Yu J: Peripheral memory and naïve T cells in non-small
cell lung cancer patients with lung metastases undergoing
stereotactic body radiotherapy: Predictors of early tumor response.
Cancer Cell Int. 19:1212019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Manjarrez-Orduno N, Menard LC, Kansal S,
Fischer P, Kakrecha B, Jiang C, Cunningham M, Greenawalt D, Patel
V, Yang M, et al: Circulating T cell subpopulations correlate with
immune responses at the tumor site and clinical response to PD1
inhibition in non-small cell lung cancer. Front Immunol.
9:16132018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kunert A, Basak EA, Hurkmans DP, Balcioglu
HE, Klaver Y, van Brakel M, Oostvogels AAM, Lamers CHJ, Bins S,
Koolen SLW, et al: CD45RA+CCR7- CD8 T cells lacking co-stimulatory
receptors demonstrate enhanced frequency in peripheral blood of
NSCLC patients responding to nivolumab. J Immunother Cancer.
7:1492019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Djenidi F, Adam J, Goubar A, Durgeau A,
Meurice G, de Montpréville V, Validire P, Besse B and Mami-Chouaib
F: CD8+CD103+ tumor-infiltrating lymphocytes
are tumor-specific tissue-resident memory T cells and a prognostic
factor for survival in lung cancer patients. J Immunol.
194:3475–3486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Iatropoulos MJ and Williams GM:
Proliferation markers. Exp Toxicol Pathol. 48:175–181. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Osa A, Uenami T, Koyama S, Fujimoto K,
Okuzaki D, Takimoto T, Hirata H, Yano Y, Yokota S, Kinehara Y, et
al: Clinical implications of monitoring nivolumab immunokinetics in
non-small cell lung cancer patients. JCI Insight. 3:e591252018.
View Article : Google Scholar
|
|
69
|
Kim KH, Cho J, Ku BM, Koh J, Sun JM, Lee
SH, Ahn JS, Cheon J, Min YJ, Park SH, et al: The first-week
proliferative response of peripheral blood
PD-1+CD8+ T cells predicts the response to
Anti-PD-1 therapy in solid tumors. Clin Cancer Res. 25:2144–2154.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Huang AC, Postow MA, Orlowski RJ, Mick R,
Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, et al: T-cell
invigoration to tumour burden ratio associated with anti-PD-1
response. Nature. 545:60–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kamphorst AO, Pillai RN, Yang S, Nasti TH,
Akondy RS, Wieland A, Sica GL, Yu K, Koenig L, Patel NT, et al:
Proliferation of PD-1+ CD8 T cells in peripheral blood after
PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci
USA. 114:4993–4998. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kuchroo VK, Umetsu DT, DeKruyff RH and
Freeman GJ: The TIM gene family: Emerging roles in immunity and
disease. Nat Rev Immunol. 3:454–462. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Limagne E, Richard C, Thibaudin M, Fumet
JD, Truntzer C, Lagrange A, Favier L, Coudert B and Ghiringhelli F:
Tim-3/galectin-9 pathway and mMDSC control primary and secondary
resistances to PD-1 blockade in lung cancer patients.
Oncoimmunology. 8:e15645052019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Koyama S, Akbay EA, Li YY, Herter-Sprie
GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ,
Asahina H, et al: Adaptive resistance to therapeutic PD-1 blockade
is associated with upregulation of alternative immune checkpoints.
Nat Commun. 7:105012016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Japp AS, Kursunel MA, Meier S, Mälzer JN,
Li X, Rahman NA, Jekabsons W, Krause H, Magheli A, Klopf C, et al:
Dysfunction of PSA-specific CD8+ T cells in prostate
cancer patients correlates with CD38 and Tim-3 expression. Cancer
Immunol Immunother. 64:1487–1494. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Su H, Xie H, Dai C, Ren Y, She Y, Xu L,
Chen D, Xie D, Zhang L, Jiang G and Chen C: Characterization of
TIM-3 expression and its prognostic value in patients with
surgically resected lung adenocarcinoma. Lung Cancer. 121:18–24.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kuhns MS, Davis MM and Garcia KC:
Deconstructing the form and function of the TCR/CD3 complex.
Immunity. 24:133–139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Postow MA, Manuel M, Wong P, Yuan J, Dong
Z, Liu C, Perez S, Tanneau I, Noel M, Courtier A, et al: Peripheral
T cell receptor diversity is associated with clinical outcomes
following ipilimumab treatment in metastatic melanoma. J Immunother
Cancer. 3:232015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liu YY, Yang QF, Yang JS, Cao RB, Liang
JY, Liu YT, Zeng YL, Chen S, Xia XF, Zhang K and Liu L:
Characteristics and prognostic significance of profiling the
peripheral blood T-cell receptor repertoire in patients with
advanced lung cancer. Int J Cancer. 145:1423–1431. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ruggiero E, Nicolay JP, Fronza R, Arens A,
Paruzynski A, Nowrouzi A, Ürenden G, Lulay C, Schneider S, Goerdt
S, et al: High-resolution analysis of the human T-cell receptor
repertoire. Nat Commun. 6:80812015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Han J, Duan J, Bai H, Wang Y, Wan R, Wang
X, Chen S, Tian Y, Wang D, Fei K, et al: TCR repertoire diversity
of peripheral PD-1+CD8+ T cells predicts
clinical outcomes after immunotherapy in patients with non-small
cell lung cancer. Cancer Immunol Res. 8:146–154. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kaplansky G and Bongrand P: Cytokines and
chemokines. Cell Mol Biol (Noisy-le-grand). 74:569–574. 2001.
|
|
83
|
Qu Z, Sun F, Zhou J, Li L, Shapiro SD and
Xiao G: Interleukin-6 prevents the initiation but enhances the
progression of lung cancer. Cancer Res. 75:3209–3215. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Silva EM, Mariano VS, Pastrez PRA, Pinto
MC, Castro AG, Syrjanen KJ and Longatto-Filho A: High systemic IL-6
is associated with worse prognosis in patients with non-small cell
lung cancer. PLoS One. 12:e01811252017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Pan B, Che D, Cao J, Shen J, Jin S, Zhou
Y, Liu F, Gu K, Man Y, Shang L and Yu Y: Interleukin-17 levels
correlate with poor prognosis and vascular endothelial growth
factor concentration in the serum of patients with non-small cell
lung cancer. Biomarkers. 20:232–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Sanmamed MF, Perez-Gracia JL, Schalper KA,
Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, Oñate C, Perez G, Alfaro
C, Martín-Algarra S, et al: Changes in serum interleukin-8 (IL-8)
levels reflect and predict response to anti-PD-1 treatment in
melanoma and non-small-cell lung cancer patients. Ann Oncol.
28:1988–1995. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Seruga B, Zhang HB, Bernstein LJ and
Tannock IF: Cytokines and their relationship to the symptoms and
outcome of cancer. Nat Rev Cancer. 8:887–899. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Thomas DA and Massagué J: TGF-beta
directly targets cytotoxic T cell functions during tumor evasion of
immune surveillance. Cancer Cell. 8:369–380. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Higgs BW, Morehouse CA, Streicher K,
Brohawn PZ, Pilataxi F, Gupta A and Ranade K: Interferon gamma
messenger RNA Signature in tumor biopsies predicts outcomes in
patients with non-small cell lung carcinoma or urothelial cancer
treated with durvalumab. Clin Cancer Res. 24:3857–3866. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Fehrenbacher L, Spira A, Ballinger M,
Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D,
Artal-Cortes A, Lewanski C, et al: Atezolizumab versus docetaxel
for patients with previously treated non-small-cell lung cancer
(POPLAR): A multicentre, open-label, phase 2 randomised controlled
trial. Lancet. 387:1837–1846. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Karachaliou N, Gonzalez-Cao M, Crespo G,
Drozdowskyj A, Aldeguer E, Gimenez-Capitan A, Teixido C,
Molina-Vila MA, Viteri S, De Los Llanos Gil M, et al: Interferon
gamma, an important marker of response to immune checkpoint
blockade in non-small cell lung cancer and melanoma patients. Ther
Adv Med Oncol. 10:17588340177497482018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Hirashima T, Kanai T, Suzuki H, Yoshida H,
Matsushita A, Kawasumi H, Samejima Y, Noda Y, Nasu S, Tanaka A, et
al: The Levels of Interferon-gamma release as a biomarker for
non-small-cell lung cancer patients receiving immune checkpoint
inhibitors. Anticancer Res. 39:6231–6240. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Boutsikou E, Domvri K, Hardavella G,
Tsiouda D, Zarogoulidis K and Kontakiotis T: Tumour necrosis
factor, interferon-gamma and interleukins as predictive markers of
antiprogrammed cell-death protein-1 treatment in advanced non-small
cell lung cancer: A pragmatic approach in clinical practice. Ther
Adv Med Oncol. 10:17588359187682382018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Arrieta O, Montes-Servin E,
Hernandez-Martinez JM, Cardona AF, Casas-Ruiz E, Crispin JC, Motola
D, Flores-Estrada D and Barrera L: Expression of PD-1/PD-L1 and
PD-L2 in peripheral T-cells from non-small cell lung cancer
patients. Oncotarget. 8:101994–102005. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Patel SP and Kurzrock R: PD-L1 Expression
as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther.
14:847–856. 2015. View Article : Google Scholar : PubMed/NCBI
|