Introduction
Oral cancer is one of the most common tumors with
the 5-year survival rate that has remained at 50% in the last few
decades (1). Oral cancer is
primarily caused by habits of betel quid chewing, smoking and
alcohol consumption in Southeast Asia (1). Though it is typically regarded as a
disease of the elderly, it has been reported that the numbers of
young patients have been increasing worldwide in recent years
(2). Radical resection and immediate
reconstruction are the mainstays of treatment for oral cancer.
However, the surgical stress response is considered to directly
induce immunosuppression by activating the
hypothalamus-pituitary-adrenal axis and sympathetic nervous system
(3), and residual tumor cells after
surgery are likely to metastasize due to decreased immunity
(4). Meanwhile, some anesthetics
have direct suppressive impacts on innate and adaptive immunity
(5–8). Therefore, selection of suitable
anesthetics is important during the perioperative period.
Dexmedetomidine is an α2-adrenergic receptor agonist with
analgesic, sedative, anxiolytic and anti-sympatholytic properties
(9,10). Several studies have indicated that
dexmedetomidine can regulate perioperative immune response in
radical surgeries of breast, colon and gastric cancer (11–13).
Nevertheless, the influence of dexmedetomidine on immune response
in patients with oral malignant tumors during operation remains
unclear.
The present study aimed to observe the level of
immune cells through flow cytometry and to evaluate the effects of
dexmedetomidine on immune response in patients undergoing radical
operation and immediate reconstruction with forearm flap for oral
cancer.
Materials and methods
Patients and grouping
The study was carried out at the School and Hospital
of Stomatology of Wuhan University and conducted accordance with
the Declaration of Helsinki between September 2018 and January
2019. It was approved by The Ethics Committee of School and
Hospital of Stomatology, Wuhan University (Wuhan, China; approval
no. IRB-2018B23) and registered in the Chinese Clinical Trial
Registry (ChiCTR-1800018367). Written informed consent was provided
by all participants before the trial. Patients classified as
American Society of Anesthesiologist (ASA) physical status I to II
(14) and aged 30–70 years were
enrolled. All patients were scheduled for radical operation and
immediate reconstruction with forearm flap under general
anesthesia. None had a history of endocrine, immune or circulatory
system diseases. Other exclusion criteria included recent or
concurrent chemotherapy and a requirement for perioperative blood
transfusion or perioperative treatment with immunomodulatory
agents. Patients who developed severe surgical complications
including infection or secondary surgery, were also excluded from
study. Patients were randomly allocated to two groups using a
computer-generated randomization list, the procedure of details was
following: Before the patients were allocated into group, random
numbers were generated by computer. Patients who were enrolled in
this study were allocated in the order of random numbers, the even
numbers were divided into control group and the odd numbers were
divided into experimental group (15).
Methods of anesthesia
All patients were premedicated with anintramuscular
injection of 0.01 mg·kg−1 atropine at 30 min before
anesthesia. In the operating room, the right subclavian vein was
cannulated for central venous pressure monitoring and the radial
artery was cannulated for real-time blood pressure monitoring. The
electrocardiography, blood oxygen saturation (SaO2),
end-tidal carbon dioxide (PetCO2), and
cerebral state index were continuously monitored during the
operation. Within 15 min before anesthesia induction,
dexmedetomidine (lot no. 180604BP; Jiangsu Hengrui Medicine Co.
Ltd.) was infused with a 0.5 µg·kg−1 loading dose
followed by a maintenance dose of 0.4
µg·kg−1·h−1 to the end of operation in the
dexmedetomidine group. According to its clinical pharmacokinetics
and pharmacodynamics (10),
dexmedetomidine was actually discontinued 30 min before the
surgery. But in the present study research, it was difficult to
determine 30 min before the operation is completed. Therefore, in
order to reduce deviation, the dexmedetomidine infusion was stopped
immediately after the surgery with the opinion of making the
baseline of every patient the same. The same volume of normal
saline was administered to the patients in the control group.
Anesthesia induction was performed by intravenous injection of
propofol 1.5–2.0 mg·kg−1, sufentanil 0.4
µg·kg−1 and cisatracurium 0.2 mg·kg−1 to
facilitate nasal tracheal intubation. Anesthesia maintenance
included sevoflurane 2–3%, remifentanil 0.2–0.3
µg·kg−1·min−1 and intermittent injection of
cisatracurium 0.1 mg·kg−1. Mechanical ventilation was
performed to maintain the PetCO2 at 35–40
mmHg and SaO2>98%. Blood pressure and heart rate were
fluctuated within ±120% of baseline values. The depth of anesthesia
was monitored to maintain the cerebral state index between 40 and
60. Sufentanil 0.1 µg·kg−1 was administered as a loading
dose for postoperative analgesia in each patient 30 min before the
end of surgery, and patients received the same intravenous
analgesia for postoperative pain therapy.
Peripheral blood mononuclear cell
separation
Saphenous vein blood (2 ml) was obtained at five
time-points: 30 min before induction (T0), 1h after
induction (T1), end of the operation (T2) and
24 (T3) and 48 h (T4) after the operation.
Peripheral blood mononuclear cells (PBMC) were isolated by
centrifugation at 800 × g for 20 min at room temperature using
Lymphoprep™ (Stemcell Technologies, Inc.).
Flow cytometry
The PBMCs were stained by the following antibodies
at 4°C for 30 min: APC-eFluor 780-conjugated anti-CD45 (clone:
HI30; 1:100), Alexa Fluor 700-conjugated anti-CD3 (clone: UCHT1;
1:50), FITC-conjugated anti-CD4 (clone: RPA-T4; 1:200),
PE-Cy7-conjugated anti-CD8 (clone: SK1; 1:200), PC5.5-conjugated
anti-CD19 (clone: SJ25C1; 1:50), APC-conjugated anti-HLA-DR (clone:
LN3; 1:50), PC5.5-conjugated anti-CD14 (clone: 61D3; 1:50),
PE-conjugated anti-CD15 (clone: HI98; 1:50) and BV421-conjugated
anti-CD11C (clone: 3.9; 1:50). PE-conjugated anti-CD15 were
obtained from Becton, Dickinson and Company; BV421-conjugated
anti-CD11C were obtained from BioLegend, Inc; APC-eFluor
780-conjugated anti-CD45, Alexa Fluor 700-conjugated anti-CD3,
FITC-conjugated anti-CD4, PE-Cy7-conjugated anti-CD8,
PC5.5-conjugated anti-CD19, PC5.5-conjugated anti-CD14 and
APC-conjugated anti-HLA-DR were obtained from eBioscience; Thermo
Fisher Scientific, Inc. Isotype-matched IgG controls (cat. no.
56-0038-41) were purchased from eBioscience; Thermo Fisher
Scientific, Inc., and were incubated at 4°C for 30 min. Dead cells
were excluded using staining with Fixable Viability Dye eFluor™ 506
at 4°C (eBioscience; Thermo Fisher Scientific, Inc.). Flow
cytometry detection were performed on CytoFLEX flow cytometer
(Beckman Coulter). The data were analyzed using FlowJo version 10
(Tree Star, Inc.) and gated by the side scatter and forward scatter
filters.
Observational index
The levels of T lymphocyte subsets, B lymphocytes,
dendritic cells and myeloid-derived suppressor cells (MDSCs) of the
patients in the two groups were observed by flow cytometry. The
level of agitation during emergence was assessed using the Richmond
Agitation-Sedation Scale (RASS), which is defined as RASS score ≥+2
(16). The incidence of emergence
agitation, hypotension and bradycardia during surgery were also
recorded.
Statistical analysis
According to the percentages of CD3+
cells at T4 between two groups from our preliminary
experiment, using Chinese High Intellectualized Statistical
Software, with a type-I error of 5% and a power of 80%, 29 patients
were needed in each group. Anticipating a 10% dropout rate, a total
of 70 patients were recruited. All statistical analyses were
performed using GraphPad Prism 7 (GraphPad Software, Inc.). Data
are expressed as mean ± standard deviation or value. Differences in
sex, ASA status and stage of tumor were compared using
χ2 tests and Fisher's exact test as appropriate.
Student's unpaired t-tests were performed to compare age, body mass
index (BMI), duration of surgery, blood loss and liquid infusion
volume. The differences in percentages of immune cells between
time-points and the two groups are compared using two-way ANOVA and
Bonferroni's correction. P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparison of general data between two
groups
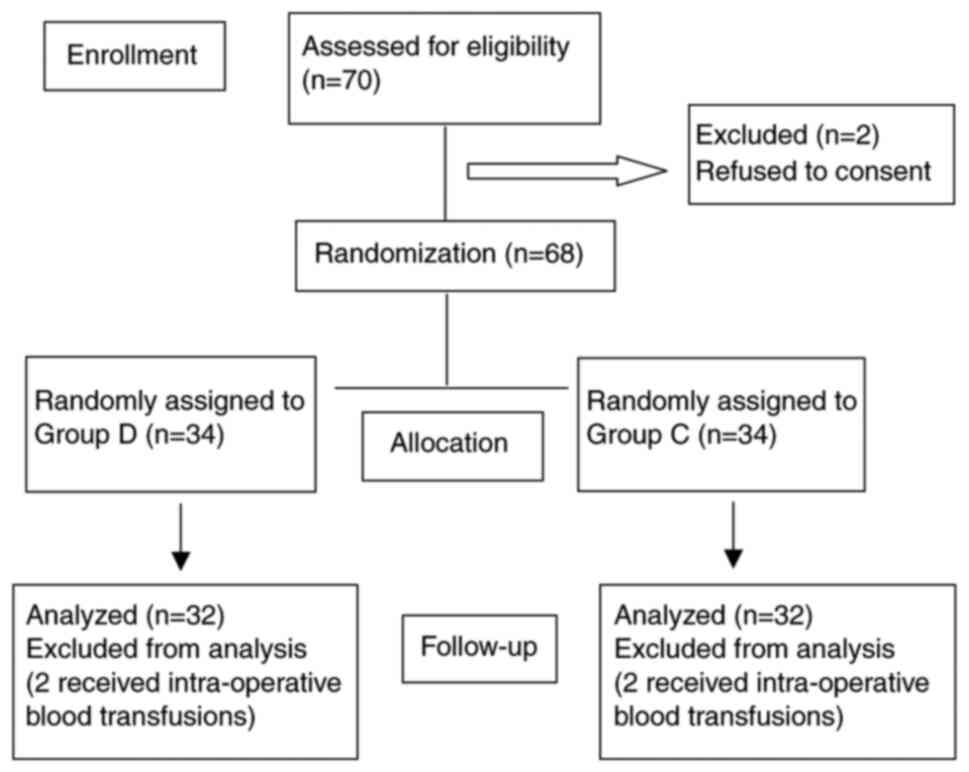
A total of 70 patients were recruited, two refused
to consent and two patients from each group were excluded due to
blood transfusions. Therefore, 32 patients were left in each group
(Fig. 1). The demographics and
surgical profiles of the patients were similar in the two groups.
The patients in the two groups showed no significant differences in
age, sex, BMI, ASA, stage of tumor, duration of surgery, blood loss
or liquid infusion volume (all P>0.05; Table I).
 | Table I.Demographics and surgical profiles of
the patients. |
Table I.
Demographics and surgical profiles of
the patients.
| Variables | Group D | Group C | P-value |
|---|
| Age, years | 49±10 | 51±11 | 0.454 |
| Sex,
male/female | 25/7 | 24/8 | 0.768 |
| BMI,
kg/m2 | 22.9±3.5 | 21.81±2.7 | 0.159 |
| ASA status,
I/II | 22/10 | 23/9 | 0.784 |
| Stage of tumor |
|
| 0.963 |
|
T1N0M0 | 12 | 11 |
|
|
T2N0M0 | 15 | 16 |
|
|
T3N0M0 | 5 | 5 |
|
| Duration of
surgery, h | 6.6±0.5 | 6.4±0.6 | 0.108 |
| Blood loss, ml | 302±53 | 280±54 | 0.080 |
| Liquid infusion
volume, ml | 3006±320 | 3072±295 | 0.336 |
Comparison of T lymphocyte subsets
between the two groups
No significant differences in the percentages of
CD3+ and CD4+ cells and the
CD4+/CD8+ ratios were observed between the
two groups before anesthesia induction (P>0.05). The percentages
of CD3+ and CD4+ cells, and the
CD4+/CD8+ ratios significantly decreased at
T1–4 in the two groups compared with the baseline value
at T0 and significantly increased at T1–4 in
the dexmedetomidine group compared with the control group (all
P<0.05). No significant difference in the percentage of
CD8+ cells was found between the two groups at
T0–4 (P>0.05) (Fig. 2
and Fig. S1). These results
indicated that cellular immunity was suppressed in the two groups
after anesthesia and dexmedetomidine may be associated with less
impairment of cellular immunity in these patients.
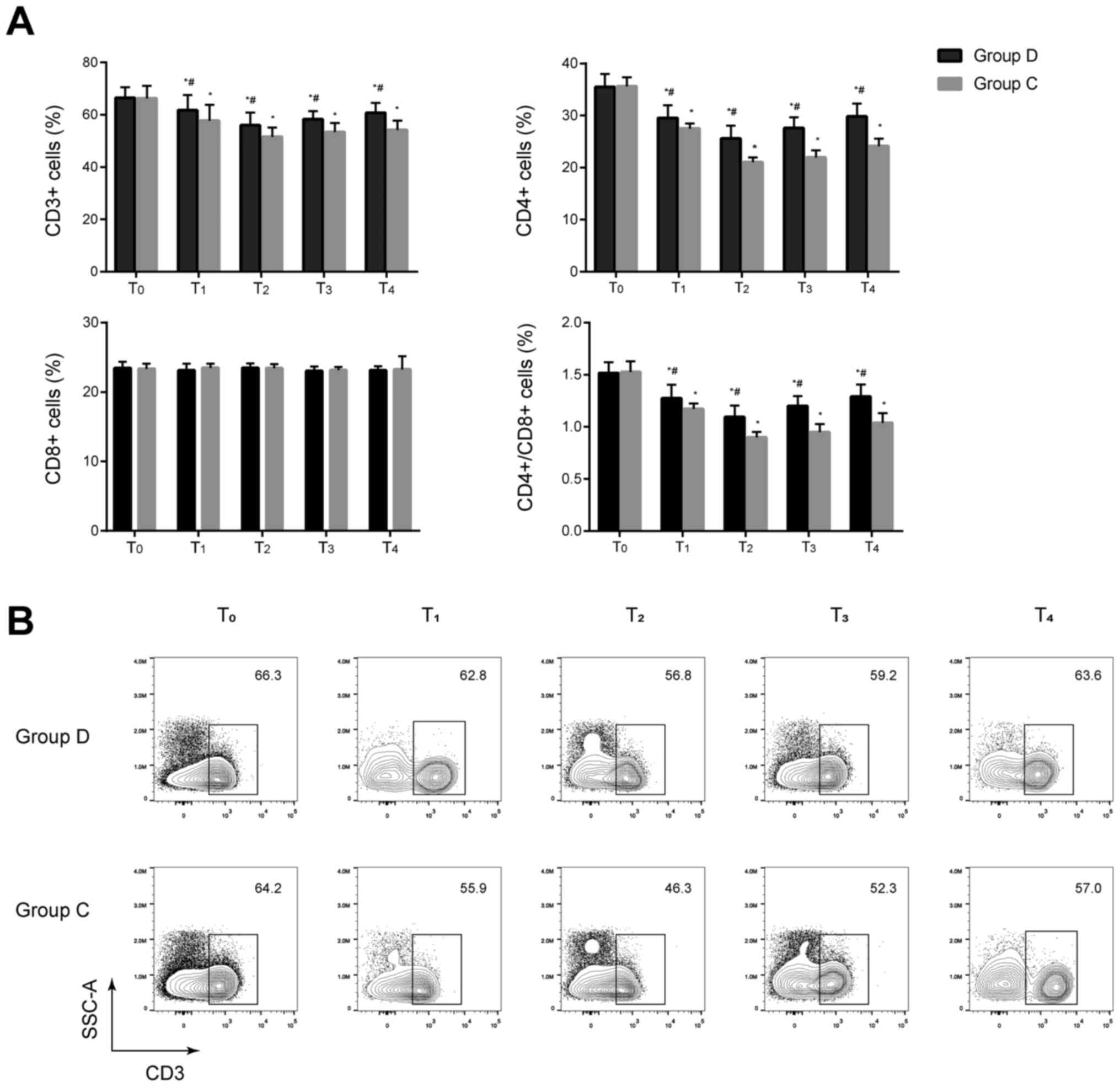 | Figure 2.Comparison of T lymphocyte subsets at
different time-points between the two groups. (A) T lymphocyte
subsets including CD3+, CD4+ and
CD8+ cells were analyzed by flow cytometry. Percentages
of CD3+ and CD4+ cells, and the
CD4+/CD8+ ratios significantly decreased at
T1–4 in the two groups compared with the baseline value
at T0 and significantly increased at T1–4 in
the dexmedetomidine group compared with the control group.
*P<0.05 vs. T0, #P<0.05 vs. group C. (B)
Representative flow cytometry contour plots of CD3+ T
cells in CD45+ cells of group C and group D at
T0–4. D, dexmedetomidine; C, control; T0, 30
min before induction; T1, 1 h after induction;
T2, end of the operation; T3, 24 h after
operation; T4, 48 h after operation. |
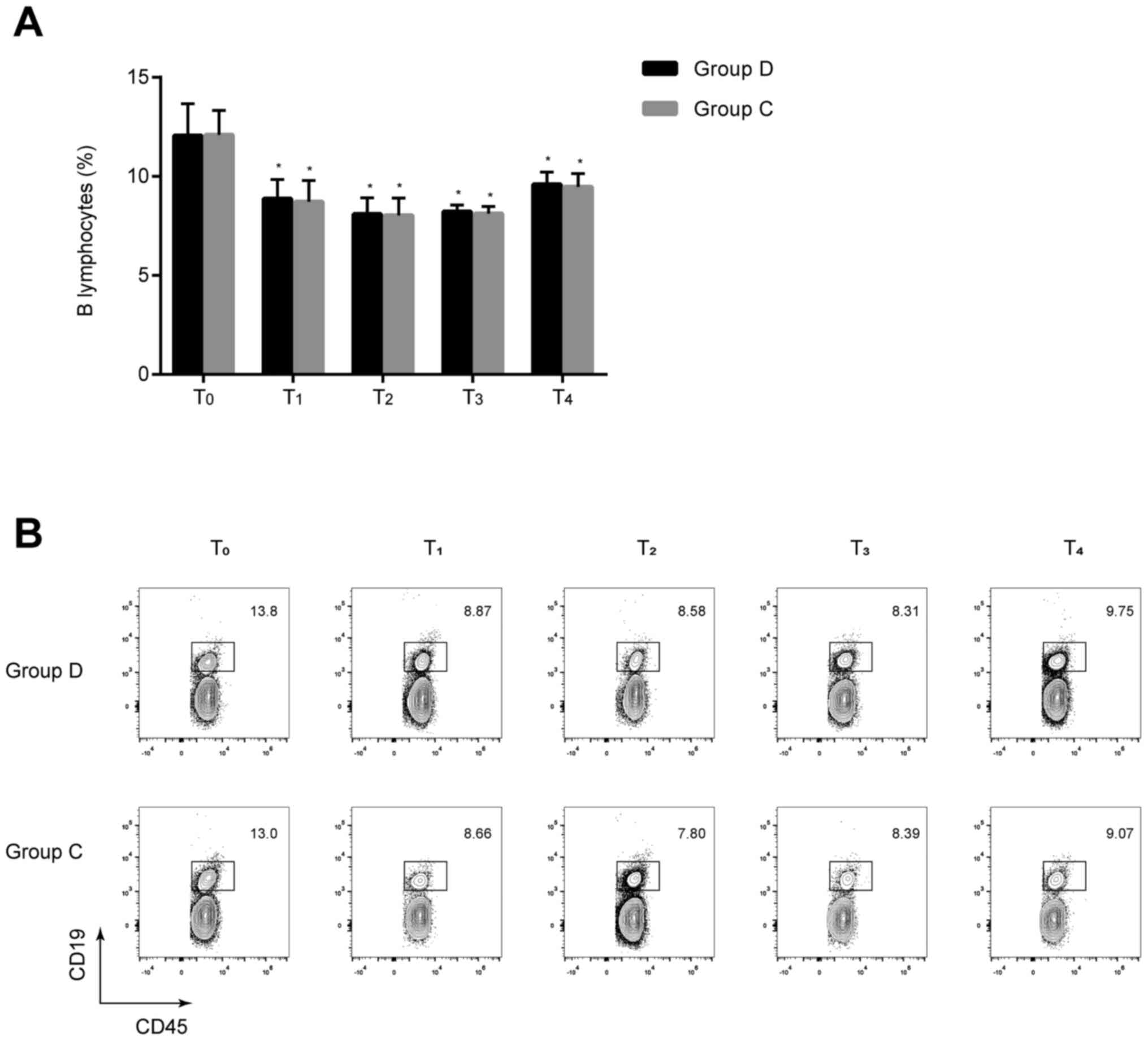
Comparison of B lymphocytes between
the two groups
The percentages of B lymphocytes at T1–4
were significantly lower compared with those at T0 in
the two groups (all P<0.05), but no statistically significant
differences were found between the two groups at the same
time-points (P>0.05) (Fig. 3).
These results implied that dexmedetomidine exerts minimal effect on
the humoral immune response of patients undergoing radical and
reconstructive surgery for oral cancer.
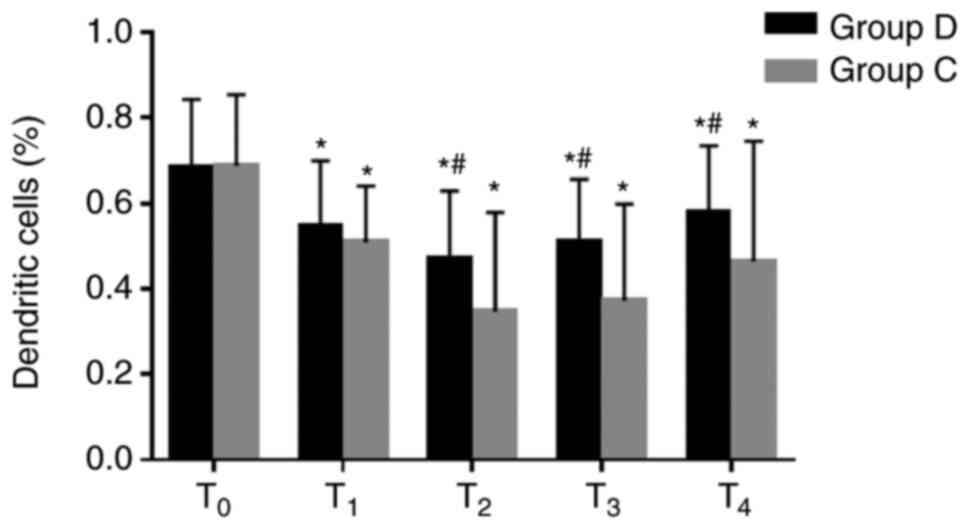
Comparison of dendritic cells between
the two groups
The percentages of dendritic cells were
significantly decreased at T1–4 in the two groups
compared with the baseline value at T0, and they were
significantly higher at T2–4 in the dexmedetomidine
group compared with the respective control group (0.5±0.2% vs.
0.3±0.2% at T2, 0.5±0.1% vs. 0.4±0.2% at T3,
0.6±0.2% vs. 0.5±0.3% at T4; all P<0.05; Fig. 4). These results suggested that
dexmedetomidine may attenuate the inhibition of immune response and
may be beneficial to antitumor therapy.
Comparison of MDSCs between the two
groups
The percentages of MDSCs significantly decreased at
T1–3 in the two groups compared with the respective
baseline value at T0, and they were significantly lower
at T2–4 in the dexmedetomidine group compared with the
respective control group (2.6±1.4% vs. 3.4±1.4% at T2,
3.2±1.1% vs. 4.0±1.1% at T3 and 4.2±1.1% vs. 4.7±1.% at
T4; all P<0.05; Fig.
5). These results suggested that dexmedetomidine may decrease
the percentages of MDSCs and improve the immunosuppressive state of
patients.
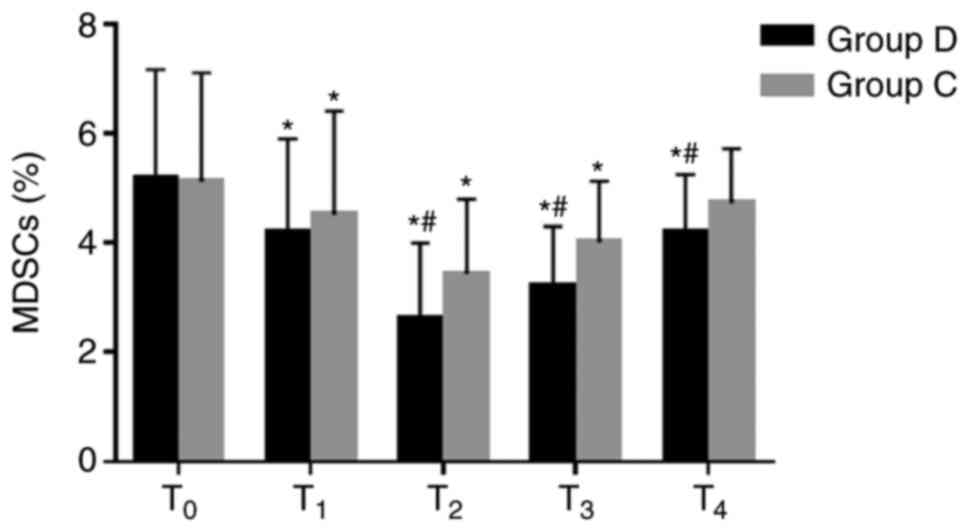 | Figure 5.Comparison of MDSCs at different
time-points between the two groups. MDSCs were analyzed using flow
cytometry. Percentages of MDSCs significantly decreased at T1-3 in
the two groups compared with the baseline value at T0, and they
were significantly lower at T2-4 in the dexmedetomidine group
compared with the control group. *P<0.05 vs. T0,
#P<0.05 vs. group C. MDSCs, myeloid-derived
suppressor cells; D, dexmedetomidine; C, control; T0, 30 min before
induction; T1, 1 h after induction; T2, end of the operation; T3,
24 h after operation; T4, 48 h after operation. |
Comparison of the incidence of
emergence agitation, hypotension and bradycardia between the two
groups
The incidence of emergence agitation was lower in
the dexmedetomidine group compared with the control group (31.2 vs.
59.4%; P=0.044). However, the incidence of hypotension and
bradycardia during surgery were significantly higher in the
dexmedetomidine group compared with in the control group
(P<0.05) (Table II).
 | Table II.Incidence of emergence agitation,
hypotension and bradycardia between the D (n=32) and C (n=32)
groups. |
Table II.
Incidence of emergence agitation,
hypotension and bradycardia between the D (n=32) and C (n=32)
groups.
| Variable | Group D, n (%) | Group C, n (%) | P-value |
|---|
| Emergence
agitation | 10 (31.2) | 19 (59.4) | 0.044 |
| Hypotension in
OR | 14 (43.8) | 5 (15.6) | 0.027 |
| Bradycardia in
OR | 12 (37.5) | 3 (9.4) | 0.016 |
Discussion
The present results showed that dexmedetomidine
alleviated the decrease in the percentages of CD3+,
CD4+ and dendritic cells as well as the
CD4+/CD8+ ratios and reduced the percentages
of MDSCs, indicating that dexmedetomidine can attenuate
immunosuppression in patients undergoing radical and reconstructive
surgery for oral cancer.
Oral cancer is a major life-threatening disease with
a high incidence in Southeast Asian countries (1), and radical operation and immediate
reconstruction are the common treatment for patients. The surgical
stress response is considered to directly induce immunosuppression
by activating the hypothalamus-pituitary-adrenal axis and
sympathetic nervous system, which results in the increased
production of glucocorticoids and catecholamines (3). Glucocorticoids are known to decrease
the number and activity of natural killer cells and reduce T cell
proliferation in a dose-dependent manner (17). Catecholamines can also inhibit T cell
proliferation by decreasing IL-2 expression and secretion and
reduce natural killer cell activity (3). Meanwhile, it is reported that some
anesthetics have direct suppressive impacts on innate and adaptive
immunity (5–8). Sevoflurane is a popular inhaled
anesthetic that can inhibit the activity of natural killer cells
and induce the apoptosis of T and B lymphocytes (8). Opioids are commonly used analgesic
agents in surgery and have immunosuppressive effects by suppressing
the activity of natural killer cells, neutrophils and macrophages
and the proliferation of T lymphocytes (7). Surgery and anesthesia-induced
immunosuppression have been implicated in the development of
post-operative septic complications and tumor metastasis (3,4).
Considering that surgery is necessary in cancer
treatment, extensive research has been conducted to determine the
effect of anesthetics on immune cell population (5–8).
Dexmedetomidine is a highly selectiveα2-adrenoceptor
agonist with sedative, anxiolytic, analgesic and anti-sympatholytic
properties (9,10). Ebert et al (18) reported that dexmedetomidine reduces
the concentration of circulating plasma catecholamines by 60–80% in
a dose-dependent manner, and this reduction is consistent with
long-lasting anti-sympathetic effect. Other studies have indicated
that dexmedetomidine exerts anti-inflammatory effects and
organ-protective effects against ischemic and hypoxic injury, which
can also regulate immune response (10,13,19).
Cellular immunity is closely associated with
antitumor effects and cancer metastasis surveillance (6,20).
Improving perioperative cellular immune status contributes to
attenuation of immunosuppression and resistance to metastasis. T
lymphocytes are important in cellular immunity (20). All mature peripheral T lymphocytes,
labeled by CD3+, comprise of CD4+ and
CD8+. The former represents cellular immune function,
whereas the latter recognizes and kills tumor cells. The reduction
of CD4+/CD8+ ratio implies that the cellular
immune function is downregulated (6). The present study showed that the
percentages of CD3+ and CD4+ cells and the
CD4+/ CD8+ ratios significantly decreased
from T1 to T4 in the two groups and
significantly decreased in the control group. This result implied
that cellular immunity was suppressed in the two groups after
anesthesia and dexmedetomidine may be associated with less
impairment of cellular immunity in these patients. These results
are aligned with previous reports. For example, Yang et al
(11) found a significant difference
in lymphocyte count after anesthesia between the dexmedetomidine
and control groups in radical mastectomy. A recent clinical trial
on 141 patients receiving radical operation of colon carcinoma has
revealed that dexmedetomidine can decrease the inhibition of T
lymphocyte subsets and reduce the secretion of inflammatory factors
(12). Another study by Wang et
al (13) indicated that
dexmedetomidine can preserve the balance of T helper (h)1/Th2 ratio
24 h after surgery and attributed this result to the increased
response of Th1 in patients undergoing radical gastrectomy. The
decrease in Th1/Th2 ratio after surgery suggests a suppressed
cell-mediated immunity (13).
B lymphocytes producing antibodies mediate humoral
immunity (6). Surgery-induced
immunosuppression is mainly caused by the effect on the cellular
immune system, and T lymphocytes are most affected with B
lymphocytes numbers changing little (3). In the present study, the percentage of
B lymphocytes was slightly decreased in the two groups after
anesthesia, and there was no significant difference between the two
groups at T1–4. The results suggested that
dexmedetomidine exerts minimal effect on the humoral immune
response of patients undergoing radical and reconstructive surgery
for oral cancer. Further studies are needed to find the influence
of dexmedetomidine on the humoral immunity of patients with
malignant tumors during operation.
Dendritic cells, first identified by Steinman in
1973, are antigen-presenting cells that are considered a critical
factor in anti-tumor immunity (21).
As an immune surveillance cell, dendritic cells can efficiently
cluster and activate T cells inhibiting the occurrence and
development of tumors (22).
Clinical studies on dendritic cells have focused on therapeutic
vaccination against cancer, immunotherapy applying ex
vivo-generated and tumor antigen-loaded dendritic cells has
been successfully introduced in clinical vaccination protocols and
has proven to be feasible and effective (23). The present study demonstrated that
the percentages of dendritic cells were significantly higher in the
dexmedetomidine group compared within the control group from
T2to T4. This result suggested that
dexmedetomidine may attenuate the inhibition of immune response and
may be beneficial to antitumor therapy.
MDSCs are a subset of immune cells that have a
myeloid origin with immunosuppressive abilities (24). Studies have reported that MDSCs
suppress CD8+ T cells and IFN-γ and IL-2 production by T
cells (24,25). In addition, MDSCs inhibit B cell
proliferation and antibody production (25) and the expansion of MDSCs is
associated with tumor progression (26). The present study showed that the
percentages of MDSCs significantly decreased from T1 to
T3 in the two groups, indicating that surgical resection
of the tumor has an anti-tumor effect. Meanwhile, the percentages
of MDSCs in the dexmedetomidine group significantly decreased at
T2 to T4, suggesting that dexmedetomidine may
decrease the percentages of MDSCs and improve the immunosuppressive
state of patients.
The level of agitation during emergence was assessed
using RASS scores (16). The
incidence of emergence agitation was lower in the dexmedetomidine
group compared within the control group. This result may be
associated with the sedation and analgesia effects of
dexmedetomidine. Thus, patients are likely to tolerate tracheal
catheter and other discomfort during the recovery period. However,
the incidence of hypotension and bradycardia was higher in the
dexmedetomidine group compared within the control. This result is
in accordance with previous research and the reason is related to
the inhibitory effect of dexmedetomidine on the sympathetic system
(27,28).
The present study has some limitations. The clinical
outcomes and cancer-free survival time were not measured. In
addition, the specific mechanisms of immunosuppressive effects of
dexmedetomidine were not explored. Therefore, cellular and
molecular level studies are also needed to resolve these underlying
mechanisms. Immune cells could be analyzed to detect mRNA
expression levels of transcription factors and chemokines.
In conclusion, dexmedetomidine alleviated the
decrease in the percentages of CD3+, CD4+ and
dendritic cells as well as the CD4+/CD8+
ratios and reduced the percentages of MDSCs. Thus, dexmedetomidine
can attenuate immunosuppression in patients undergoing radical and
reconstructive surgery for oral cancer.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank their colleague Dr
Liang Mao (School and Hospital of Stomatology, Wuhan University,
Wuhan, Hubei, China) for his excellent technical assistance.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LH designed the study, performed the experiments and
wrote the manuscript. CQ performed the experiments and revised the
manuscript. LW designed the study and revised the manuscript. TZ
collected and analyzed the data. JL designed the study and revised
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by The Ethics Committee of
School and Hospital of Stomatology, Wuhan University (Wuhan, China;
approval noIRB-2018B23) and registered in the Chinese Clinical
Trial Registry (ChiCTR-1800018367). Written informed consent was
provided by all participants before the trial.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kumar M, Nanavati R, Modi TG and Dobariya
C: Oral cancer: Etiology and risk factors: A review. J Cancer Res
Ther. 12:458–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hussein AA, Helder MN, de Visscher JG,
Leemans CR, Braakhuis BJ, de Vet HCW and Forouzanfar T: Global
incidence of oral and oropharynx cancer in patients younger than 45
years versus older patients: A systematic review. Eur J Cancer.
82:115–127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hogan BV, Peter MB, Shenoy HG, Horgan K
and Hughes TA: Surgery induced immunosuppression. Surgeon. 9:38–43.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim R: Anesthetic technique and cancer
recurrence in oncologic surgery: Unraveling the puzzle. Cancer
Metastasis Rev. 36:159–177. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kurosawa S and Kato M: Anesthetics, immune
cells, and immune responses. J Anesth. 22:263–277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang T, Fan Y, Liu K and Wang Y: Effects
of different general anaesthetic techniques on immune responses in
patients undergoing surgery for tongue cancer. Anaesth Intensive
Care. 42:220–227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Plein LM and Rittner HL: Opioids and
immune system-friend or foe. Br J Pharmacol. 175:2717–2725. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stollings LM, Jia LJ, Tang P, Dou H, Lu B
and Xu Y: Immune modulation by volatile anesthetics.
Anesthesiology. 125:399–411. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mahmoud M and Mason KP: Dexmedetomidine:
Review, update, and future considerations of pediatric
perioperative and periprocedural applications and limitations. Br J
Anaesth. 115:171–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maud AS, Michel MR, Laura NH, Clements RM,
Anthony RA and Pieter C: Clinical pharmacokinetics and
pharmacodynamics of dexmedetomine. Clin Pharmacokinet. 56:893–913.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang XH, Bai Q, Lv MM, Fu HG, Dong TL and
Zhou Z: Effect of dexmedetomidine on immune function of patients
undergoing radical mastectomy. Eur Rev Med Pharmacol Sci.
21:1112–1116. 2017.PubMed/NCBI
|
|
12
|
Wang K and Li C: Effects of
dexmedetomidine on inflammatory factors, T lymphocyte subsets and
expression of NF-κB in peripheral blood mononuclear cells in
patients receiving radical surgery of colon carcinoma. Oncol Lett.
15:7153–7157. 2018.PubMed/NCBI
|
|
13
|
Wang Y, Xu X, Liu H and Ji F: Effects of
dexmedetomine on patients undergoing radical gastrectomy. J Surg
Res. 194:147–153. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mayhew D, Mendonca V and Murthy BVS: A
review of ASA physical status-historical perspectives and modern
developments. Anesthesia. 74:373–379. 2019. View Article : Google Scholar
|
|
15
|
Scott NW, McPherson GC, Ramsay CR and
Campbell MK: The method of minimization for allocation to clinical
trials: A review. Control Clin Trials. 23:662–674. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barr J, Fraser GL, Puntillo K, Ely EW,
Gélinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, et
al: Clinical practice guidelines for the management of pain,
agitation, and delirium in adult patients in the Intensive Care
Unit. Crit Care Med. 41:263–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen L, Jondal M and Yakimchuk K:
Regulatory effects of dexamethasone on NK and T cell immunity.
Inflammopharmacology. 26:1331–1338. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ebert TJ, Hall JE, Barnev JA, Uhrich TD
and Colinco MD: The effects of increasing plasma concentrations of
dexmedetomidine in humans. Anesthesiology. 93:382–394. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiang H, Hu B, Li Z and Li J:
Dexmedetomine controls systemic cytokine levels through the
cholinergic anti-inflammatory pathway. Inflammation. 37:1763–1770.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goldfarb Y, Sorski L, Benish M, Levi B,
Melamed R and Ben-Eliyahu S: Improving postoperative immune status
and resistance to cancer metastasis: A combined perioperative
approach of immunostimulation and prevention of excessive surgical
stress responses. Ann Surg. 253:798–810. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Steinman RM: Decisions about dendritic
cells: Past, present, and future. Annu Rev Immunol. 30:1–22. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mildner A and Jung S: Development and
function of dendritic cell subsets. Immunity. 40:642–656. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Den Brok MH, Nierkens S, Figdor CG, Ruers
TJ and Adema GJ: Dendritic cells: Tools and targets for antitumor
vaccination. Expert Rev Vaccines. 4:699–710. 2005. View Article : Google Scholar
|
|
24
|
Dai J, El Gazzar M, Li GY, Moorman JP and
Yao ZQ: Myeloid-derived suppressor cells: Paradoxical roles in
infection and immunity. J Innate Immun. 7:116–126. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tamadaho RSE, Hoerauf A and Layland LE:
Immunomodulatory effects of myeloid-derived suppressor cells in
diseases: Role in cancer and infections. Immunobiology.
223:432–442. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marvel D and Gabrilovich DI:
Myeloid-derived suppressor cells in the tumor microenvironment:
Expect the unexpected. J Clin Invest. 125:3356–3364. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang X, Li Z, Gao C and Liu R: Effect of
dexmedetomidine on preventing agitation and delirium after
microvascular free flap surgery: A randomized, double-blind,
control study. J Oral Maxillofac Surg. 73:1065–1072. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Riker RR, Shehabi Y, Bokesch PM, Ceraso D,
Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Ely EW, et
al: Dexmedetomidine vs. midazolam for sedation of critically ill
patients: A randomized trial. JAMA. 301:489–499. 2009. View Article : Google Scholar : PubMed/NCBI
|