Introduction
Myelodysplastic syndrome (MDS) is a malignant clonal
disease with high heterogeneity, which originates from
hematopoietic stem cells. MDS is characterized by decreased numbers
of peripheral blood cells and ineffective hematopoiesis, with a
high risk of transformation to acute myeloid leukemia (AML)
(1,2). In view of the high heterogeneity of
MDS, the World Health Organization (WHO) divides MDS into several
subtypes (3): MDS with multilineage
dysplasia (MDS-MLD), MDS with single lineage dysplasia (MDS-SLD),
MDS with excess blasts-I (MDS-EB-I), MDS with excess blasts-II
(MDS-EB-II), MDS with ring sideroblasts (MDS-RS), MDS,
unclassifiable (MDS-U), and MDS with isolated del(5q). In addition
to differences in morphology and clinical features, there are some
differences in chromosome karyotype and prognosis among the
different WHO subtypes (4). The
annual incidence of MDS is ~4/100,000 population, but increases to
40–50/100,000 for individuals aged ≥70 years, which suggests that
the incidence rate of MDS increases substantially with age
(5,6). The association of MDS with increasing
age may be associated with genetic damage (7). Chromosome karyotype, an independent
prognostic factor for MDS, has important significance in the
diagnosis, treatment and prognosis of MDS management (8). In Asian, European and American
countries, the incidence of MDS chromosomal abnormalities is 40–60%
(9–12). However, the distribution of specific
chromosomal abnormalities in MDS varies among different geographic
regions (11–13).
To better understand the biology of MDS in the
Chinese population, the karyotypes of 665 Chinese patients with MDS
were analyzed in the present study, and the different karyotype
characteristics in different classification/prognosis groups were
evaluated according to the WHO classification, WHO
classification-based Prognostic Scoring System (WPSS) and Revised
International Prognostic Scoring System (IPSS-R).
Materials and methods
Patients
A total of 665 patients with MDS who visited the
Department of Hematology, Xiyuan Hospital, China Academy of Chinese
Medical Sciences (Beijing, China) from January 2011 to September
2019 were included in the study. Criteria for inclusion in the
study were as follows: A confirmed diagnosis of MDS; both bone
marrow and peripheral blood blasts ≤19%; and having not been
treated. Patients with secondary MDS were excluded. Of the 665
patients, 355 were male and 310 were female, corresponding to a
male-to-female ratio of 1.15. The median age of the patients was 51
years (range, 6–86 years). According to the WHO classification, 423
patients (63.6%) were classified as MDS-MLD, 120 patients (18.0%)
as MDS-EB-I, 80 patients (12.0%) as MDS-EB-II, 24 patients (3.8%)
as MDS-SLD, 14 patients (2.0%) as MDS-RS, 2 patients (0.3%) as
MDS-U and 2 patients (0.3%) as MDS with isolated del(5q) (Table I). The study protocol was approved by
the Clinical Research Ethics Committee of Xiyuan Hospital, China
Academy of Chinese Medical Sciences. All patients provided written
informed consent to participate in the study.
 | Table I.Cytogenetic data of patients with MDS
according to the WHO (2016) classification. |
Table I.
Cytogenetic data of patients with MDS
according to the WHO (2016) classification.
|
|
|
| Numerical karyotype
abnormalities, n (%) |
|
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Classification | No. of patients | Normal, karyotypes n
(%) | Single or double | Hypodiploid | Hyperdiploid | Structural
abnormalities, single or double, n (%) | Mixed
abnormalitiesa, n
(%) | Complex
abnormalities, n (%) |
|---|
| MDS (total) | 665 | 367 (55.2) | 96 (14.4) | 23 (3.4) | 5 (0.8) | 105 (15.8) | 14 (2.1) | 55 (8.3) |
| MLD | 423 | 270 (63.8) | 47 (11.1) | 17 (4.0) | 0 | 62 (14.7) | 8 (1.9) | 19 (4.5) |
| SLD | 24 | 16 (66.7) | 3 (12.5) | 1 (4.2) | 1 (4.2) | 2 (8.2) | 0 | 1 (4.2) |
| EB-I | 120 | 42 (35.0) | 27 (22.5) | 3 (2.5) | 2 (1.7) | 21 (17.5) | 3 (2.5) | 22 (18.3) |
| EB-II | 80 | 33 (41.3) | 14 (17.5) | 2 (2.5) | 2 (2.5) | 13 (16.2) | 3 (3.8) | 13 (16.2) |
| MDS-RS | 14 | 5 (35.7) | 4 (28.6) | 0 | 0 | 5 (35.7) | 0 | 0 |
| MDS-U | 2 | 1 (50.0) | 1 (50.0) | 0 | 0 | 0 | 0 | 0 |
| MDS del(5q) | 2 | 0 | 0 | 0 | 0 | 2 (100.0) | 0 | 0 |
Diagnostic and classification
criteria
All patients with MDS were diagnosed on the basis of
the 2007 Vienna criteria (1), and
their disease subtypes were classified according to 2016 WHO
criteria (3).
Cytogenetic prognostic groups
Cytogenetic prognostic groups were established based
on the WPSS (2011) (14) and IPSS-R
(2012) criteria (15). WPSS
cytogenetic categories are as follows: Good-prognosis karyotype
group, including normal, -Y, del(5q) and del(20q);
intermediate-prognosis karyotype group, including other
abnormalities with the exception of those with good-prognosis or
poor-prognosis karyotypes; and poor-prognosis karyotype group,
including complex (≥3 abnormalities) or chromosome 7 abnormalities.
IPSS-R cytogenetic categories are as follows: Very good-prognosis
karyotype group, including-Y and del(11q); good-prognosis karyotype
group, including normal, del(5q), del(12p), del(20q), and del(5q)
with one additional abnormality; intermediate-prognosis karyotype
group, including del(7q), trisomy 8 (+8), +19, i(17q) and any other
single or double independent clones; poor-prognosis karyotype
group, including-7, inv(3)/t(3q)/del(3q), −7/del(7q) with one
additional abnormality and complex abnormalities (3 abnormalities);
very poor-prognosis karyotype group, including complex
abnormalities (>3 abnormalities).
Cytogenetic analysis
Fresh bone marrow (BM) cells were cultured in
RPMI-1640 (HyClone; Cytiva) supplemented with 20% fetal calf serum
(HyClone; Cytiva) and 3 µg/ml recombinant human granulocyte
colony-stimulating factor (Xiamen Amoytop Biotech Co., Ltd.) at
37°C for 24 h, and G-banding (16)
was used for cytogenetic analysis. Karyotypes were classified and
recorded according to the International System for Human
Cytogenetic Nomenclature (2009) (17). At least 20 metaphases were analyzed
in each sample, and the karyotypes with more than two chromosomal
abnormalities were defined as complex karyotypes.
Statistical analysis
Data reported in the study relate to the time of
diagnosis. All statistical analyses were performed using SPSS 23.0
(IBM Corp.) software. Independent-samples t-test was used to
compare the means of two groups. χ2 tests or the
Fisher's exact tests were used to compare proportions of two or
multiple groups. The Bonferroni correction was used for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference. When the Bonferroni correction was used,
the α-level and P-values were adjusted.
Results
Chromosomal abnormalities and common
abnormal karyotypes in patients with MDS
Among the 665 patients, 367 (55.2%) had a normal
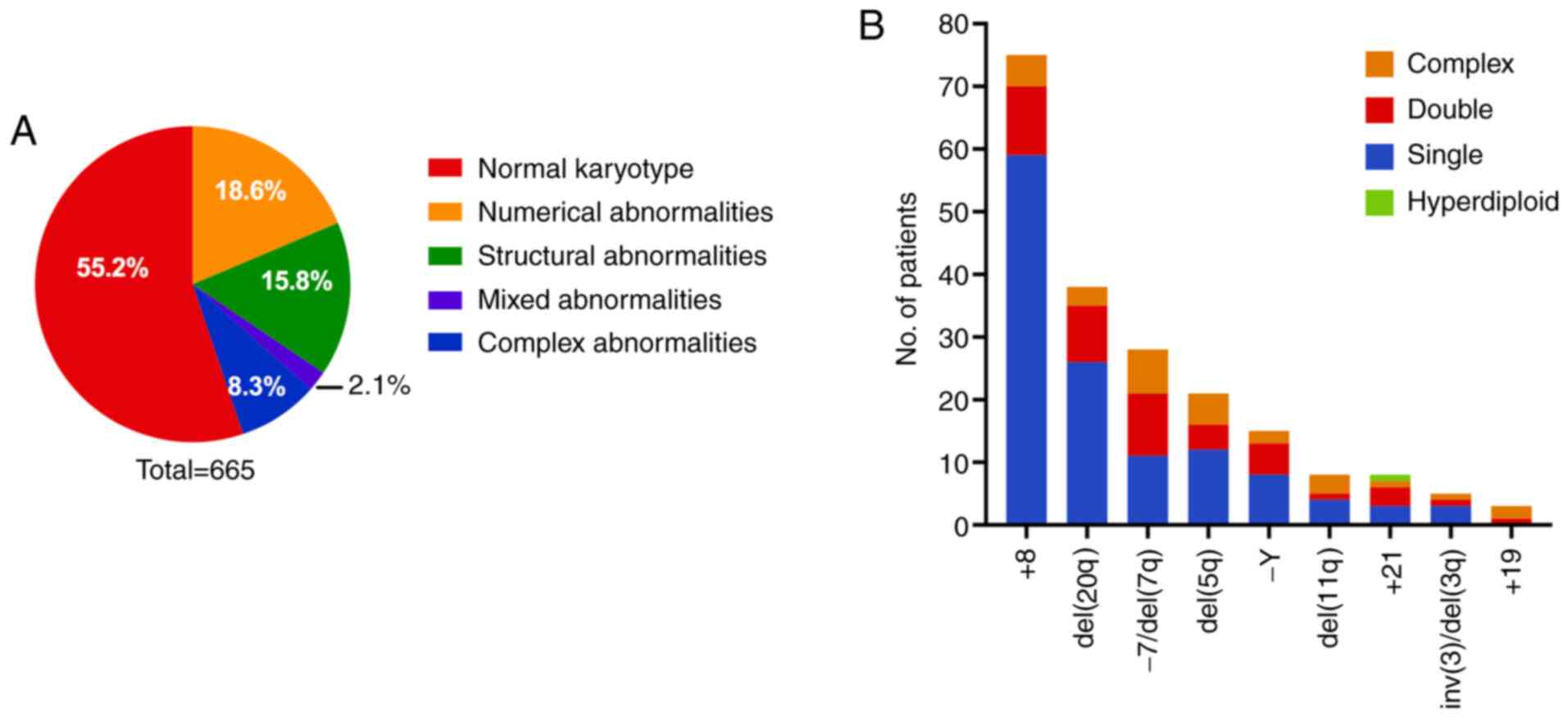
karyotype while 298 (44.8%) had abnormal karyotypes. The patients
with abnormal karyotypes comprised 124 cases (124/665, 18.6%) with
numerical abnormalities, 105 cases (105/665, 15.8%) with structural
abnormalities, 55 cases (55/665, 8.3%) with complex abnormalities
and 14 cases (14/665, 2.1%) with abnormalities both in number and
structure (Table I, Fig. 1A).
Among the patients with single chromosome
abnormalities, there were 75 cases (75/665, 11.3%) with +8, 38
cases (38/665, 5.7%) with del(20q), 27 cases (27/665, 4.1%) with
−7/del(7q), 21 cases (21/665, 3.2%) with del(5q), 15 cases (15/665,
2.3%) with -Y, 8 cases (8/665, 1.2%) with del(11q), 8 cases (8/665,
1.2%) with +21, 5 cases (5/665, 0.8%) with inv(3)/del(3q) and 3
cases (3/665, 0.5%) with +19.
Overall, +8 was the most frequent single abnormal
karyotype in the present cohort. Of the 75 patients with +8, 59
(59/665, 8.9%) had only the +8 abnormal karyotype, 11 (11/665,
1.7%) had +8 with one additional abnormality, and 5 (5/665, 0.8%)
had +8 with complex abnormalities. Among the 27 patients with
chromosome 7 abnormality, 8 (8/665, 1.2%) had isolated-7, 3 (3/665,
0.5%) had isolated del(7q), 10 (10/665, 1.5%) had-7 or del(7q) with
one additional abnormality, and 6 (6/665, 0.9%) had-7 or del(7q)
with complex abnormalities. Among the 21 patients with del(5q), 12
(12/665, 1.8%) had del(5q) in isolation, 4 (4/665, 0.6%) had
del(5q) with one additional abnormality, and 5 (5/665, 0.8%) had
del(5q) with complex abnormalities (Fig.
1B). Among the 55 patients with complex karyotypes, 26 (26/665,
3.9%) had 3 chromosomal abnormalities and 29 (29/665, 4.4%) had
>3 abnormalities.
Cytogenetic risk based on two MDS
prognostic scoring systems
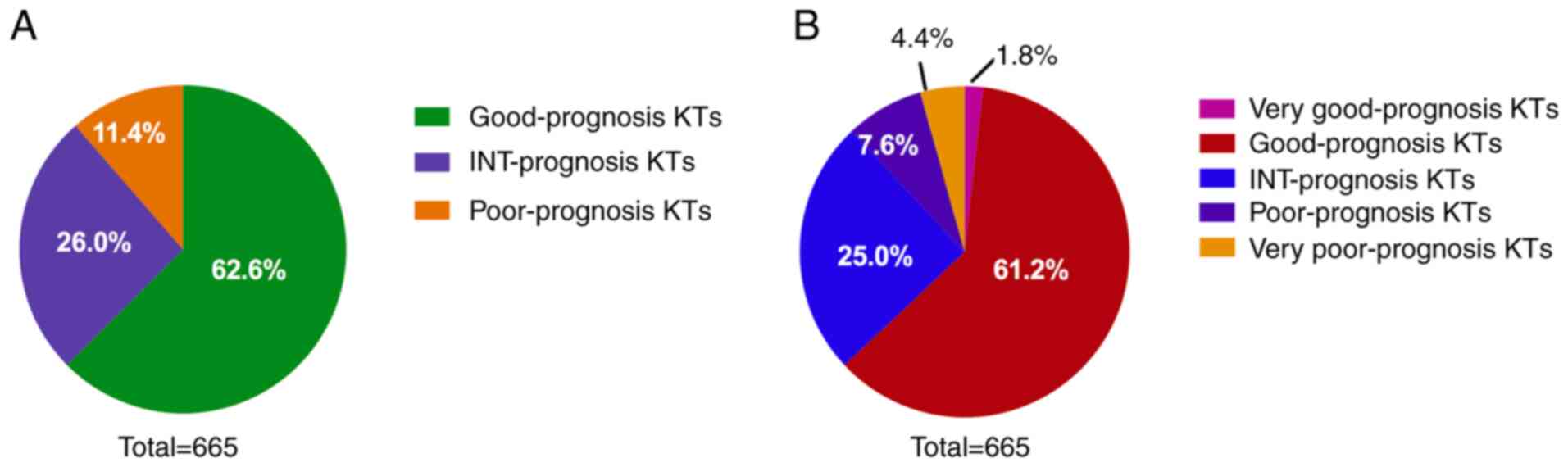
When the prognoses of the patients were evaluated on
the basis of their karyotype analysis using WPSS criteria, 416
(416/665, 62.6%) of all patients were classified as having a good
prognosis, 173 (173/665, 26.0%) as having intermediate prognosis
and 76 (76/665, 11.4%) as having a poor prognosis (Fig. 2A). The patient cohort was also
evaluated using the IPSS-R system, which classified 12 (12/665,
1.8%) patients as having very good cytogenetic prognosis, 407
(407/665, 61.2%) as having a good prognosis, 166 (166/665, 25.0%)
as having an intermediate prognosis, 51 (51/665, 7.6%) as having a
poor prognosis and 29 (29/665, 4.4%) as having a very poor
prognosis (Fig. 2B).
Incidence of chromosomal abnormalities
in different age groups
Chromosomal abnormalities were examined in patients
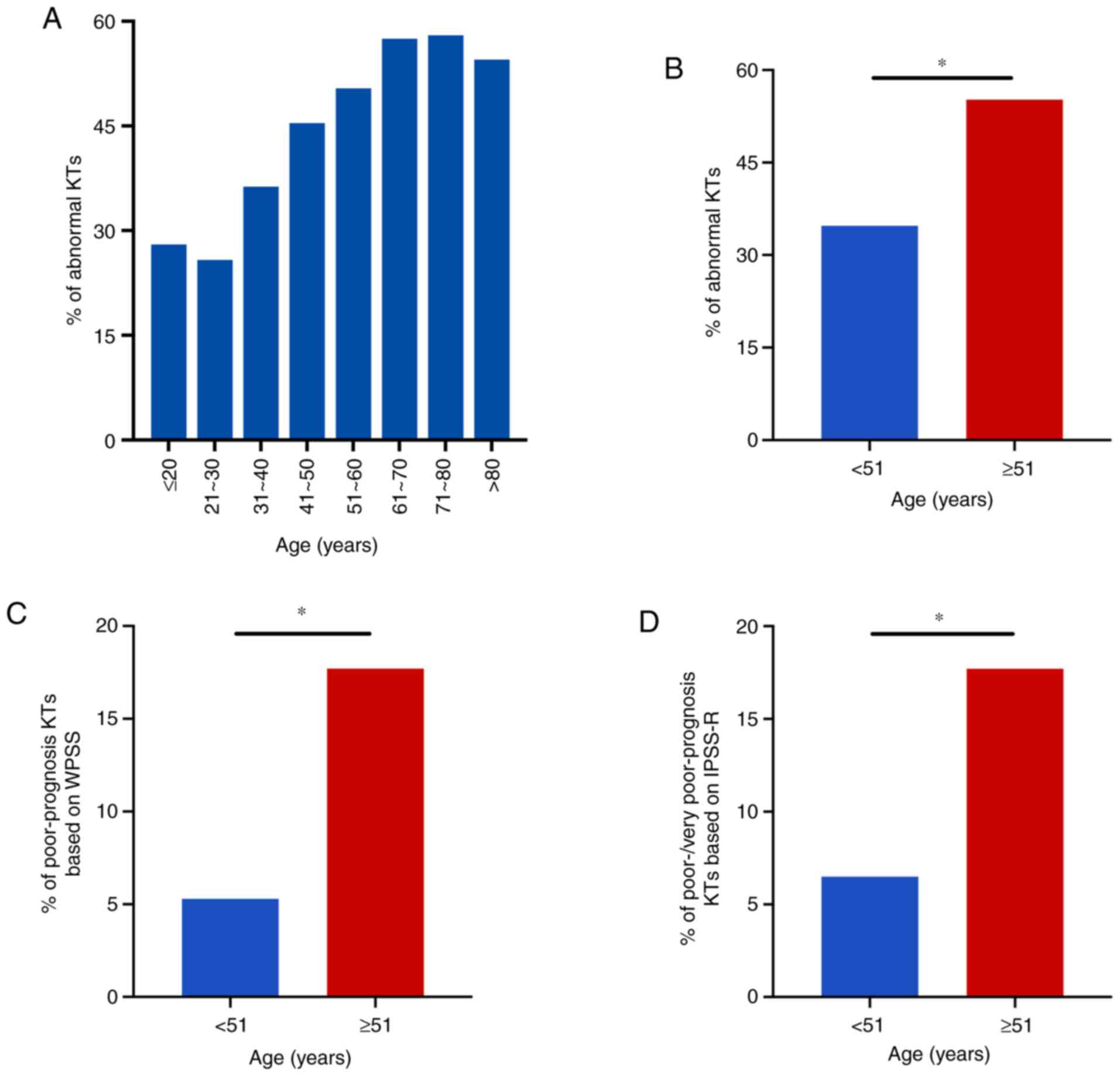
with MDS of different age groups, and differences were observed in
the incidence of abnormal karyotypes across the age groups. The
incidence of abnormal karyotypes was 28% (14/50) in ≤20-year-old
patients, 25.8% (24/93) in patients 21–30 years old, 36.3% (33/91)
in patients 31–40 years old, 45.4% (44/97) in patients 41–50 years
old, 50.4% (62/123) in patients 51–60 years old, 57.5% (69/120) in
patients 61–70 years old, 58.0% (40/69) in patients 71–80 years
old, and 54.5% (12/22) in >80-year-old patients (Fig. 3A).
All patients were divided into two age groups using
the median age of 51 years as the cutoff. Among the 331 patients
aged <51 years, 115 (115/331, 34.7%) had abnormal karyotypes, 18
(18/331, 5.4%) had poor-prognosis karyotypes based on WPSS criteria
and 22 (22/331, 6.6%) had poor-/very poor-prognosis karyotypes
based on IPSS-R criteria. Among the 334 patients who were ≥51 years
old, 183 (183/334, 54.8%) had abnormal karyotypes, 58 (58/334,
17.4%) had poor-prognosis karyotypes based on WPSS criteria and 58
(58/334, 17.4%) had poor-/very poor-prognosis karyotypes based on
IPSS-R criteria. The incidence of abnormal karyotypes was
significantly higher in patients aged≥51 years compared with those
aged <51 years (54.8 vs. 34.7%, respectively; P<0.05), and
the incidence of poor-prognosis karyotypes based on WPSS criteria
(17.4 vs. 5.4%, respectively) and of poor-/very poor-prognosis
karyotypes based on IPSS-R criteria (17.4 vs. 6.6%, respectively)
were also significantly higher (P<0.05) in patients aged≥51
years than in younger patients (Fig.
3B-D).
The mean ages of patients with normal and abnormal
karyotypes were also compared, and the results revealed that the
mean age of patients with abnormal karyotypes was significantly
higher compared with that of patients with a normal karyotype
(53.54±17.94 vs. 45.03±19.19 years, respectively; P<0.001;
Table II).
 | Table II.Comparison of median age and PB
blasts between normal and abnormal karyotype groups. |
Table II.
Comparison of median age and PB
blasts between normal and abnormal karyotype groups.
| Group | Age, years | PB blasts, % |
|---|
| Normal
karyotype | 45.03±19.19 | 3.26±4.34 |
| Abnormal
karyotypes | 53.54±17.94 | 3.99±4.59 |
| P-value | <0.001 | 0.395 |
Incidence of chromosomal abnormalities
across different WHO classification groups
When classified using the WHO (2016) criteria, the
cohort of 665 patients with MDS was segregated into the following
groups: SLD, 24 cases including 8 cases (8/24, 33.3%) with abnormal
karyotypes; MLD, 423 cases including 153 cases (153/423, 36.2%)
with abnormal karyotypes; RS, 14 cases including 9 cases (9/14,
64.3%) with abnormal karyotypes; EB-I, 120 cases including 78 cases
(78/120, 65.0%) with abnormal karyotypes; EB-II, 80 cases including
47 cases (47/80, 58.7%) with abnormal karyotypes; MDS with isolated
del(5q), 2 cases including 1 case with isolated del(5q) and 1 case
with one additional abnormality; and MDS-U, 2 cases including 1
case (1/2, 50%) with one abnormality.
As shown in Table
III, the +8 abnormality was found in all WHO subtypes with the
exception of MDS-U and MDS with isolated del(5q), and was more
prevalent than other abnormalities in those subtypes; del(20q) was
found in all subtypes with the exception of MDS-U; and −7/del(7q)
and del(5q) were more common in EB-I and EB-II than in other
subtypes.
 | Table III.Frequency of common chromosomal
abnormalities among WHO subtypes of MDS. |
Table III.
Frequency of common chromosomal
abnormalities among WHO subtypes of MDS.
|
|
| No. of frequent
chromosomal abnormalities (%) |
|---|
|
|
|
|
|---|
| Subtypes | No. of
patients | Abnormal | +8 | del(20q) | −7/del(7q) | del(5q) |
|---|
| MLD | 423 | 153 (36.2) | 39 (9.2) | 24 (5.7) | 15 (3.5) | 8 (1.9) |
| SLD | 24 | 8
(33.3) | 1
(4.2) | 1
(4.2) | 0 | 0 |
| EB-I | 120 | 78
(65.0) | 20 (16.7) | 6
(5.0) | 6 (5.0) | 5 (4.2) |
| EB-II | 80 | 47
(58.7) | 11 (13.8) | 3
(3.8) | 5 (6.3) | 5 (6.3) |
| MDS-RS | 14 | 9
(64.3) | 4
(28.6) | 3 (21.4) | 0 | 1 (7.1) |
| MDS-U | 2 | 1
(50.0) | 0 | 0 | 1 (50.0) | 0 |
| MDS del(5q) | 2 | 2
(100.0) | 0 | 1 (50.0) | 0 | 2 (100.0) |
The incidence of abnormal karyotypes in cases of
EB-I (65.0%) and EB-II (58.7%) was significantly higher compared
with that in MLD (36.2%) (adjusted P<0.005). Furthermore, the
incidence of abnormal karyotypes in EB-I (65.0%) was significantly
increased compared with that in SLD (33.3%) (adjusted P<0.005).
However, no significant differences were identified in the
incidence of abnormal karyotypes among the other subtypes of MDS
(Fig. 4A).
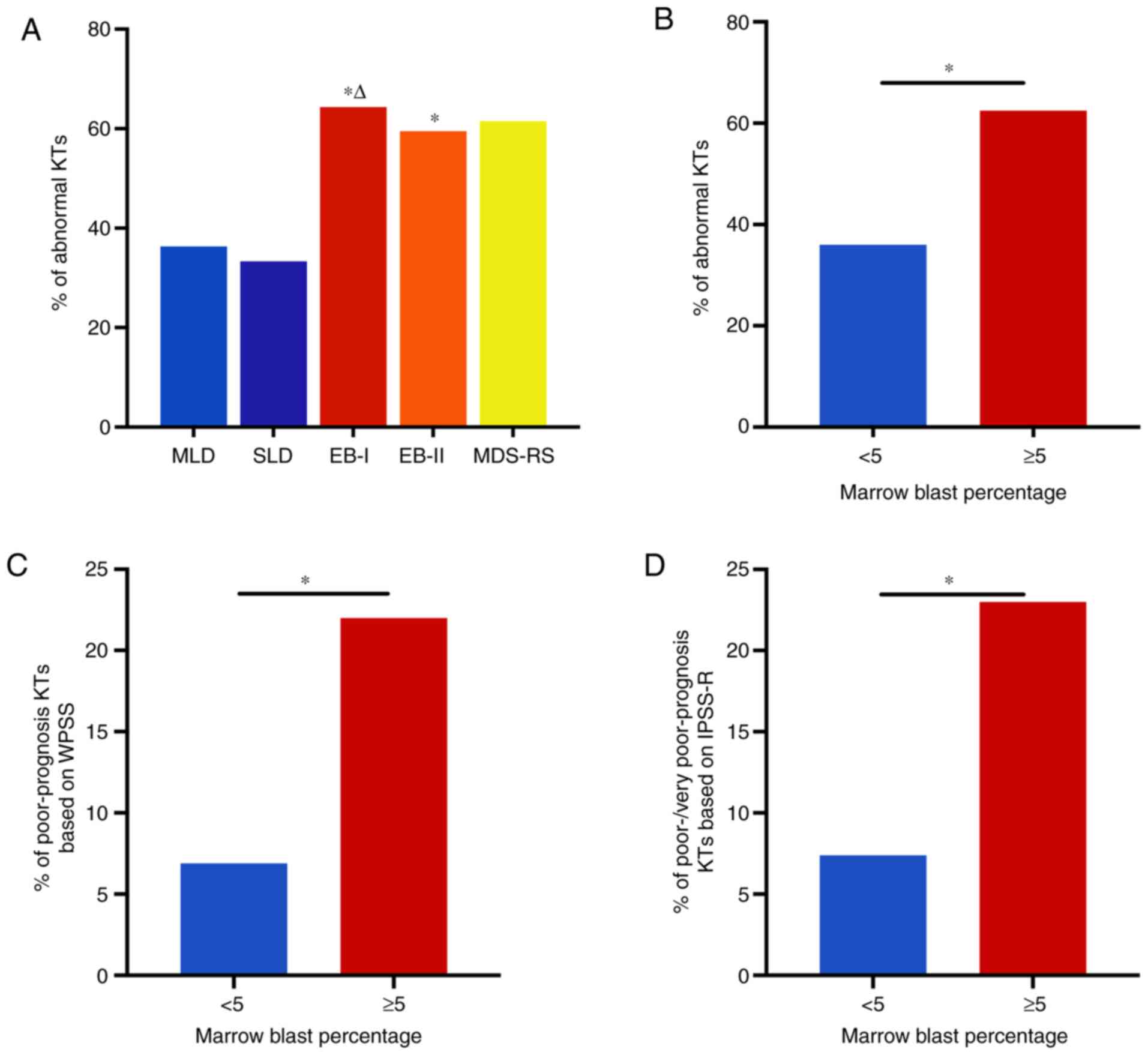 | Figure 4.Incidence of chromosomal abnormalities
across different WHO classification groups. (A) Comparison of
different WHO subtypes of myelodysplastic syndrome. P<0.001
among all WHO subtypes (4 degrees of freedom). Pairwise comparisons
of different WHO subtypes: MLD vs. SLD, P=0.778; MLD vs. EB-I,
P<0.001; MLD vs. EB-II, P<0.001; MLD vs. MDS-RS, P=0.032; SLD
vs. EB-I, P=0.004; SLD vs. EB-II, P=0.029; SLD vs. MDS-RS, P=0.094;
EB-I vs. EB-II, P=0.371; EB-I vs. MDS-RS, P=1.000; EB-II vs.
MDS-RS, P=0.697. After Bonferroni correction, the adjusted
P<0.005 was used to indicate a statistically significant
difference among the multiple pairwise comparisons of different WHO
subtypes. *P<0.005 vs. MLD, ΔP<0.005 vs. SLD. (B)
Incidence of (B) abnormal KTs, (C) poor-prognosis KTs according to
WPSS criteria and (D) poor- and very poor-prognosis KTs according
to IPSS-R criteria in patients divided into two groups according to
the percentage of bone marrow blasts. *P<0.05. WHO, World Health
Organization; MLD, multilineage dysplasia; SLD, single lineage
dysplasia; EB-I, excess blasts-I; EB-II, excess blasts-II; MDS-RS,
MDS with ring sideroblasts; KTs, karyotypes; WPSS, WHO
classification-based Prognostic Scoring System; IPSS-R, Revised
International Prognostic Scoring System. |
The incidence of abnormal karyotypes in patients
with high percentages of BM blasts (EB-I + EB-II, ≥5% blasts) was
significantly higher compared with that in patients with low
percentages of BM blasts (SLD + MLD, <5% blasts), at 62.5 and
36.0%, respectively (P<0.05). The incidence of poor-prognosis
karyotypes based on WPSS criteria was significantly higher in
patients with high percentages of BM blasts than in those with low
BM blast percentages (22.0 vs. 6.9%, respectively; P<0.05), as
was the incidence of poor-/very poor-prognosis karyotypes based on
IPSS-R criteria (23.0 vs. 7.4%, respectively; P<0.05) (Fig. 4B-D).
In addition to BM blasts, the mean percentages of
peripheral blood blasts were also compared between patients with
normal and abnormal karyotypes, and no significant difference was
identified (Table II).
Discussion
The median age of MDS onset differs between Asian
and Western countries. MDS is considered to be a disease associated
with aging in Western countries, and the median and mean ages at
diagnosis in Western countries are ~70 years (7,18,19).
However, the median age at which patients are diagnosed with MDS in
Asian countries is lower, and ranges from 49 to 58 years in China
(11,20). In the present study, the median age
was 51 years, which is consistent with previous studies.
According to the literature, 40–60% of patients with
MDS possess cytogenetic abnormalities in both Chinese and Western
populations (10–12). The results of the present study
indicate that the incidence of chromosomal abnormalities in Chinese
patients with MDS was 44.8%, corroborating previous data.
Previous studies have shown that the most common
karyotype abnormalities differ between patients with MDS in China
and those in Western countries. While +8 is the most common
karyotype abnormality in patients with MDS from China and other
Asian countries, the most common karyotype abnormality in Western
patients with MDS is del(5q) (21–23). In
the present study, 75 patients had the +8 abnormality, accounting
for 25.2% of the abnormal karyotypes. This genetic difference may
partially explain the differences in clinical characteristics and
prognosis between Asian and Western patients with MDS, and suggests
that targeted treatments should be considered for different patient
groups with different karyotype abnormalities.
There are other differences in the distribution of
prognostic karyotypes between Chinese and Western patients with
MDS. In data from Western populations (15), the IPSS-R cytogenetic categories were
found to be distributed as follows: 4% of patients had very
good-prognosis karyotypes; 72% of patients had good-prognosis
karyotypes; 13% of patients had intermediate-prognosis karyotypes;
4% of patients had poor-prognosis karyotypes; and 7% of patients
had very poor-prognosis karyotypes. By comparison, Qu et al
(24) reported that in Chinese
populations, the distributions of the IPSS-R cytogenetic categories
were as follows: 2% of patients had very good-prognosis karyotypes;
43% had good-prognosis karyotypes; 36% had intermediate-prognosis
karyotypes; 7% had poor-prognosis karyotypes; and 12% had very
poor-prognosis karyotypes. The data from the present study revealed
that very good-prognosis karyotypes were present in 1.8% of
patients, good-prognosis karyotypes in 61.2%,
intermediate-prognosis karyotypes in 25.0%, poor-prognosis
karyotypes in 7.6% and very poor-prognosis karyotypes in 4.4%. The
differences in the data between Chinese and Western patients with
MDS are mainly in the karyotypes with good and intermediate
prognosis, which may be because del(5q) is the most common
abnormality in the West while +8 is the most common abnormality in
Chinese patients. However, the data in the present study differ
from the results reported by Qu et al (24), suggesting that further study using
larger patient cohorts is warranted.
Previous studies have shown that aging is an adverse
prognostic factor for MDS (25,26). The
present study identified that the mean age of patients with
abnormal karyotypes was higher than that of patients with a normal
karyotype. Furthermore, the incidence rates of chromosomal
abnormalities and karyotypes with poor prognosis were both
significantly increased in ≥51-year-old patients. Thus, it may be
inferred that both chromosomal abnormalities and karyotypes with
poor prognosis are more common in Chinese patients with MDS who are
≥51 years old than in younger patients. Therefore, it appears that
the adverse effects of aging on prognosis may be associated with
the higher incidence of poor-prognosis karyotypes in older
patients.
As the disease progresses, the incidence of
chromosomal abnormalities in patients with MDS gradually increases
from 30–40% in the early stage of the disease to 50–70% in the
advanced stage (12,20). The progression of MDS involves an
increase in the number of blasts and a reduction in the number of
peripheral blood cells, which can eventually develop into AML or BM
failure. A previous study has proposed that the incidence of
abnormal karyotypes in patients with early MDS is lower than that
in patients with advanced MDS, and secondary chromosomal
abnormalities are likely to occur in advanced cases (27). Chromosomal abnormalities can also
affect the development of AML in patients with MDS (15,27).
According to the WHO classification, the BM blast percentages in
the SLD and MLD subtypes of MDS are <5%, while in the EB-I and
EB-II subtypes they are ≥5%. The data in the present study showed
clear differences in the incidence of chromosomal abnormalities and
of poor-prognosis karyotypes between the subtypes with <5% BM
blasts and those with ≥5% blasts. Specifically, chromosomal
abnormalities and poor-prognosis karyotypes were more common in the
patients with a high percentage of blasts, which is consistent with
previous reports (23,24).
The evolution of chromosome karyotype is closely
associated with the progression of MDS, which may be related to the
genetic instability caused by chromosomal aberrations (7,28). As a
result of genomic alteration and clonal expansion, some malignant
clones may gain proliferation advantages and be prone to
transformation into leukemic clones, while other clones may be
eliminated by selection mechanisms (7,29).
While chromosome karyotype is important, it is just
one of several independent factors affecting the prognosis of MDS.
It should be considered together with other prognostic factors,
including the degree of cytopenia in peripheral blood, the
percentage of BM blasts and WHO subtypes. It is clear that the
incidence and types of chromosomal abnormalities vary among
different regions of the world. Some countries have proposed a more
suitable prognosis scoring system for their own populations
(30,31). However, at present, there is no MDS
prognosis score system suitable for Chinese populations. The
present study data, together with larger datasets collected in
future studies, will collectively contribute to the formulation of
a more accurate MDS prognosis scoring system for Chinese patients
in the future.
Acknowledgements
The authors would like to thank Professor Xiaosheng
Wu (Department of Biochemistry and Molecular Biology, Mayo Clinic,
Rochester, MN, USA) for English language editing.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81673821 and
81774142) and the Special Research Foundation of Central Level
Public Scientific Research Institutes (grant no. ZZ10-016).
Availability of data and materials
All data and materials analyzed during the current
study are included in this published article.
Authors' contributions
XH designed the study; XW, WL, MW and XG analyzed
the data and wrote the manuscript; XW, XG and XY performed the
cytogenetic analyses; XH gave the final karyotype approval; WL, TF,
YML, HX, HW, SZ, RQ, CL, XT, YL, ZC, LL, YX and RM collected the
clinical data, analyzed and interpreted the data; HW, YX and RM
performed the morphologic review and gave the final approval. XH
checked the final manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Clinical
Research Ethics Committee of Xiyuan Hospital, China Academy of
Chinese Medical Sciences. All patients provided written informed
consent to participate in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vanlet P, Horny HP, Bennet JM, Fonatsch C,
Germing U, Greenberg P, Haferlach T, Haase D, Kolb HJ, Krieger O,
et al: Definitions and standards in the diagnosis and treatment of
myelodysplastic syndromes: Consensus statements and report from a
working conference. Leuk Res. 31:727–736. 2007. View Article : Google Scholar
|
|
2
|
AdèS L, Itzykson R and Fenaux P:
Myelodysplastic syndromes. Lancet. 383:2239–2252. 2014. View Article : Google Scholar
|
|
3
|
Arber DA, Orazi A, Hasserjian R, Thiele J,
Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M and Vardiman JW:
The 2016 revision to the World Health Organization classification
of myeloid neoplasms and acute leukemia. Blood. 127:2391–2405.
2016. View Article : Google Scholar
|
|
4
|
Malcovati L, Germing U, Kuendgen A, Della
Porta MG, Pascutto C, Invernizzi R, Giagounidis A, Hildebrandt B,
Bernasconi P, Knipp S, et al: Time-dependent prognostic scoring
system for predicting survival and leukemic evolution in
myelodysplastic syndromes. J Clin Oncol. 25:3503–3510. 2007.
View Article : Google Scholar
|
|
5
|
Neukirchen J, Schoonen WM, Strupp C,
Gattermann N, Aul C, Haas R and Germing U: Incidence and prevalence
of myelodysplastic syndromes: Data from the Düsseldorf
MDS-registry. Leuk Res. 35:1591–1596. 2011. View Article : Google Scholar
|
|
6
|
Zeidan AM, Shallis RM, Wang R, Davidoff A
and Ma XM: Epidemiology of myelodysplastic syndromes: Why
characterizing the beast is a prerequisite to taming it. Blood Rev.
34:1–15. 2019. View Article : Google Scholar
|
|
7
|
Corey SJ, Minden MD, Barber DL, Kantarjian
H, Wang JC and Schimmer AD: Myelodysplastic syndromes: The
complexity of stem-cell diseases. Nat Rev Cancer. 7:118–129. 2007.
View Article : Google Scholar
|
|
8
|
Schanz J, Tüchler H, Solé F, Mallo M,
Luño E, Cervera J, Granada I, Hildebrandt B, Slovak ML, Ohyashiki
K, et al: New comprehensive cytogenetic scoring system for primary
myelodysplastic syndromes (MDS) and oligoblastic acute myeloid
leukemia after MDS derived from an international database merge. J
Clin Oncol. 30:820–829. 2012. View Article : Google Scholar
|
|
9
|
Bernasconi P, Klersy C, Boni M, Cavigliano
PM, Calatroni S, Giardini I, Cavigliano PM, Calatroni S, Giardini
I, Rocca B, et al: World Health Organization classification in
combination with cytogenetic markers improves the prognostic
stratification of patients with de novo primary myelodysplastic
syndromes. Br J Haematol. 137:193–205. 2007. View Article : Google Scholar
|
|
10
|
Xiao Y, Wei J, Chen Y, Zhang KJ, Zhou JF
and Zhang YC: Trisomy 8 is the most frequent cytogenetic
abnormality in de novo myelodysplastic syndrome in China.
Onkologie. 35:100–106. 2012. View Article : Google Scholar
|
|
11
|
Wang H, Wang XQ, Xu XP and Lin GW:
Cytogenetic features and prognosis analysis in Chinese patients
with myelodysplastic syndrome: A multicenter study. Ann Hematol.
89:535–544. 2010. View Article : Google Scholar
|
|
12
|
Sole F, Espinet B, Sanz GF, Cervera J,
Calasanz MJ, Luño E, Prieto F, Granada I, Hernández JM, Cigudosa
JC, et al: Incidence, characterization and prognostic significance
of chromosomal abnormalities in 640 patients with primary
myelodysplastic syndromes. Grupo Cooperativo Español de
Citogenética Hematológica. Br J Haematol. 108:346–356. 2000.
View Article : Google Scholar
|
|
13
|
Matsuda A, Germing U, Jinnai I, Misumi M,
Kuendgen A, Knipp S, Aivado M, Iwanaga M, Miyazaki Y, Tsushima H,
et al: Difference in clinical features between Japanese and German
patients with refractory anemia in myelodysplastic syndromes.
Blood. 106:2633–2640. 2005. View Article : Google Scholar
|
|
14
|
Malcovati L, Della Porta MG, Strupp C,
Ambaglio I, Kuendgen A, Nachtkamp K, Travaglino E, Invernizzi R,
Pascutto C, Lazzarino M, et al: Impact of the degree of anemia on
the outcome of patients with myelodysplastic syndromes and its
integration into the WHO classification-based Prognostic Scoring
System (WPSS). Haematologica. 96:1433–1440. 2011. View Article : Google Scholar
|
|
15
|
Greenberg PL, Tuechler H, Schanz J, Sanz
G, Garcia-Manero G, Solé F, Bennett JM, Bowen D, Fenaux P, Dreyfus
F, et al: Revised international prognostic scoring system for
myelodysplastic syndromes. Blood. 120:2454–2465. 2012. View Article : Google Scholar
|
|
16
|
Seabright M: A rapid banding technique for
human chromosomes. Lancet. 2:971–972. 1971. View Article : Google Scholar
|
|
17
|
Shaffer LG, Slovak ML and Campbell LJ:
ISCN: An International System for Human Cytogenetic Nomenclature.
S. Karger; Basel: 2009
|
|
18
|
Pozdnyakova O, Miron PM, Tang G, Walter O,
Raza A, Woda B and Wang SA: Cytogenetic abnormalities in a series
of 1029 patients with primary myelodysplastic syndromes: A report
from the US with a focus on some undefined single chromosomal
abnormalities. Cancer. 113:3331–3340. 2008. View Article : Google Scholar
|
|
19
|
Germing U, Gattermann N, Strupp C, Aivado
M and Aul C: Validation of the WHO proposals for a new
classification of primary myelodysplastic syndromes: A
retrospective analysis of 1600 patients. Leuk Res. 24:983–992.
2000. View Article : Google Scholar
|
|
20
|
Chen B, Zhao WL, Jin J, Xue YQ, Cheng X,
Chen XT, Cui J, Chen ZM, Cao Q, Yang G, et al: Clinical and
cytogenetic features of 508 Chinese patients with myelodysplastic
syndrome and comparison with those in Western countries. Leukemia.
19:767–775. 2005. View Article : Google Scholar
|
|
21
|
Wu L, Song L, Xu L, Chang C, Xu F, Wu D,
He Q, Su J, Zhou L, Xiao C, et al: Genetic landscape of recurrent
ASXL1, U2AF1, SF3B1, SRSF2, and EZH2 mutations in 304 Chinese
patients with myelodysplastic syndromes. Tumour Biol. 37:4633–4640.
2016. View Article : Google Scholar
|
|
22
|
Lai YY, Huang XJ, Li J, Zou P, Xu ZF, Sun
H, Shao ZH, Zhou DB, Chen FP, Liu ZG, et al: Standardized
fluorescence in situ hybridization testing based on an appropriate
panel of probes more effectively identifies common cytogenetic
abnormalities in myelodysplastic syndromes than conventional
cytogenetic analysis: A multicenter prospective study of 2302
patients in China. Leuk Res. 39:530–535. 2015. View Article : Google Scholar
|
|
23
|
Haase D, Germing U, Schanz J, Pfeilstöcker
M, Nösslinger T, Hildebrandt B, Kundgen A, Lübbert M, Kunzmann R,
Giagounidis AA, et al: New insights into the prognostic impact of
the karyotype in MDS and correlation with subtypes: Evidence from a
core dataset of 2124 patients. Blood. 110:4385–4395. 2007.
View Article : Google Scholar
|
|
24
|
Qu S, Xu Z, Zhang Y, Qin T, Cui R and Xiao
Z: Impacts of cytogenetic categories in the Revised International
Prognostic Scoring System on the prognosis of primary
myelodysplastic syndromes: Results of a single-center study. Leuk
Lymphoma. 53:940–946. 2012. View Article : Google Scholar
|
|
25
|
Xu Y, Li Y, Xu Q, Chen Y, Lv N, Jing Y,
Dou L, Bo J, Hou G, Guo J, et al: Implications of mutational
spectrum in myelodysplastic syndromes based on targeted
next-generation sequencing. Oncotarget. 8:82475–82490. 2017.
View Article : Google Scholar
|
|
26
|
Gangat N, Mudireddy M, Lasho TL, Finke CM,
Nicolosi M, Szuber N, Patnaik MM, Pardanani A, Hanson CA,
Ketterling RP and Tefferi A: Mutations and prognosis in
myelodysplastic syndromes: Karyotype-adjusted analysis of targeted
sequencing in 300 consecutive cases and development of a genetic
risk model. Am J Hematol. 93:691–697. 2018. View Article : Google Scholar
|
|
27
|
Bernasconi P, Klersy C, Boni M, Cavigliano
PM, Giardini I, Rocca B, Zappatore R, Dambruoso I, Calvello C,
Caresana M and Lazzarino M: Does cytogenetic evolution have any
prognostic relevance in myelodysplastic syndromes? A study on 153
patients from a single institution. Ann Hematol. 89:545–551. 2010.
View Article : Google Scholar
|
|
28
|
Willman CL: Molecular genetic features of
myelodysplastic syndromes (MDS). Leukemia. 12 (Suppl 1):S2–S6.
1998.
|
|
29
|
Greenberg PL: Apoptosis and its role in
the myelodysplastic syndromes: Implications for disease natural
history and treatment. Leuk Res. 22:1123–1136. 1998. View Article : Google Scholar
|
|
30
|
Solé F, Luño E, Sanzo C, Espinet B, Sanz
GF, Cervera J, Calasanz MJ, Cigudosa JC, Millà F, Ribera JM, et
al: Identification of novel cytogenetic markers with prognostic
significance in a series of 968 patients with primary
myelodysplastic syndromes. Haematologica. 90:1168–1178. 2005.
|
|
31
|
Kantarjian H, O'Brien S, Ravandi F, Cortes
J, Shan J, Bennett JM, List A, Fenaux P, Sanz G, Issa JP, et al:
Proposal for a new risk model in myelodysplastic syndrome that
accounts for events not considered in the original International
Prognostic Scoring System. Cancer. 113:1351–1361. 2008. View Article : Google Scholar
|