Introduction
Liver cancer is one of the most common malignant
tumors of the digestive system. Liver cancer ranks sixth in the
global most-common tumors and is the fourth leading cause of
cancer-associated mortality (1). The
incidence and mortality of liver cancer are increasing year by
year. At present, the treatment of liver cancer is mainly surgical
resection, supplemented with radiotherapy and chemotherapy
(2). Although the rapid development
of modern medical technology has significantly improved the
efficiency and safety of resection of liver cancer, the rate of
recurrence and metastasis in postoperative patients with liver
cancer is high, which markedly lowers the patients long-term
survival rate (3). Therefore,
elucidating the mechanism of the development and progression of
liver cancer and finding more effective biomarkers with high
sensitivity and high specificity for early diagnosis and prognosis
of liver cancer are still some of the hot topics of cancer
research.
MicroRNAs (miRNAs or miRs) are endogenous non-coding
small RNAs with 18–22 nucleotides that are found in eukaryotes, and
are involved in regulating a variety of complex pathophysiological
processes in the human body by complementarily binding to the
3-untranslated region (UTR) of the target gene mRNA (4). Previous studies found that ~50% of
miRNAs in chromosomes are localized in tumor-associated fragile
sites (5,6). Abnormal expression of miRNAs can
promote or inhibit the development of malignancies. Previous
studies have reported the roles of a single miRNA in the
development of cancer (7–10). Some studies have found that two or
more different miRNAs cooperatively participate in the biological
process of cancer by targeting the same molecule (4,11,12).
miR-200a and miR-141 are two important members of the miR-200
family, which is aberrantly expressed in liver cancer (13). miR-200a and miR-141 have a similar
sequence, which suggests that both have similar biological
functions (14,15). Although some studies have reported
that miR-200a and miR-141 have low expression in liver cancer and
play important tumor-suppressing roles (16–19),
whether there is a common biological function between miR-200a and
miR-141 in liver cancer and their specific molecular mechanism need
to be further explored.
The main purpose of the present study was to
identify new common molecule STAT4 targeted by both miR-200a and
miR-141 in liver cancer, in order to provide a solid theoretical
foundation for accelerating clinical biomedical transformation and
early implementation of miRNA-based biomarkers for early screening,
diagnosis and prognosis monitoring of liver cancer.
Materials and methods
Human specimens
Blood samples from 30 (22 male and 8 female)
patients with liver cancer aged from 25 to 70 (average, 51.2±12.85)
years old and 30 (22 male and 8 female) normal subjects aged from
22 to 66 (average, 50.37±11.24) years old were collected from the
Affiliated Huashan Hospital of Fudan University (Shanghai, China)
between January 2015 and January 2016, with the written consent of
the patients and approval by the Ethics Committee of Huashan
Hospital (Shanghai, China). Serum was isolated from whole blood by
centrifugation at 4°C and 800 × g for 10 min, and then stored at
−80°C.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from cells with TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.). RT-qPCR assay was
performed to quantify miRNA expression levels using miScript II RT
kit (Qiagen GmbH) and QuantiTect SYBR Green PCR Master Mix (Qiagen
GmbH) according to the manufacturers instructions. Cel-miR-39 was
used as a control, and the primers for each miRNA were purchased
from Qiagen GmbH. To determine the mRNA levels of STAT4,
E-Cadherin, vimentin, GAPDH and U6 small nuclear RNA (RNU6), total
RNA was reversely transcribed using PrimeScript RT Reagent kit
(Takara Biotechnology Co., Ltd.). Reverse transcription reaction
was performed using SYBR Premix Ex Taq (Takara Bio, Inc.) at 37°C
for 15 min and 85°C for 5 sec. qPCR was performed to detect mRNA
expression levels using SYBR Premix Ex Taq (Takara Bio, Inc.) on an
ABI 7500 system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The PCR thermocycling conditions were as follows: 95°C for
30 sec followed by 40 cycles of 95°C for 5 sec and 60°C for 34 sec.
The primers used in this study were: STAT4 forward,
5′-AGCCATCTCGGAGGAATA-3′ and reverse, 5′-CAGACAACCGGCCTTTAT-3′;
E-cadherin forward, 5′-TCCATTTCTTGGTCTACGCC-3′ and reverse,
5′-CACCTTCAGCCAACCTGTTT-3′; vimentin forward,
5′-CGGTTGAGACCAGAGATGGA-3′ and reverse, 5′-TGCTGGTACTGCACTGTTGC-3′;
RNU6 forward, 5′-ATTGGAACGATACAGAGAAGATT-3′ and reverse,
5′-GGAACGCTTCACGAATTTG-3′; and human GAPDH forward,
5′-CCACCCATGGCAAATTCCATGGCA-3′ and reverse,
5′-TCTAGACGGCAGGTCAGGTCCACC-3′. The expression levels of RNU6 or
GAPDH were used as internal controls. The expression levels of
mRNAs in each group were calculated by relative quantification
using the 2−ΔΔCq method (20).
Cell culture and lentivirus
infection
Human liver cancer cell lines (HepG2, Huh7 and
MHCC97H), human normal hepatocyte cell (THLE-2) and 293T cell were
obtained from the American Type Culture Collection, and cultured in
DMEM/high-glucose medium (HyClone; Cytiva) supplemented with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
and 100 µg/ml streptomycin (EMD Millipore), at 37°C and 5%
CO2. The cell lines were characterized by Genetic
Testing Biotechnology Corporation using short tandem repeat profile
analysis. GV309 (Shanghai GeneChem Co., Ltd.) was used as an
expression plasmid for lentiviral packaging. In this construct, the
hU6 promoter is used for miRNA translation, and the ubiquitin
promoter is used for the GFP tag, which is not fused. pHelper 1.0
vector plasmid and pHelper 2.0 vector plasmid was used as
lentiviral auxiliary packaging plasmid (Shanghai GeneChem Co.,
Ltd.). The miR-200a or miR-141 sequence was chemically synthesized
from Guangzhou RiboBio Co., Ltd. (Guangzhou, China) and cloned into
the lentiviral expression vector GV309, and the recombinant plasmid
containing the target fragment was obtained. GV309-GFP served as
negative control (NC). A total of 20 µg recombinant plasmid
(GV309-miR-200a-GFP, GV309-miR-141-GFP or GV309-GFP), 15 µg pHelper
1.0 vector plasmid and 10 µg pHelper 2.0 vector plasmid were
co-transfected into HEK293T cells used by Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) to produce
lentivirus. Virus soups were used to infect liver cancer cells
(HepG2). Infected cells were screened with puromycin (Shanghai
GeneChem Co., Ltd.). Stable cell lines expressing miR-NC, miR-200a,
miR-141 and miR-200a+miR-141 were established and were used for
subsequent functional experiments 72 h after lentiviral
transfection.
Cell proliferation assay
A total of 5×103 miR-NC, miR-200a,
miR-141 or miR-200a+miR-141-transfected cells per well were plated
in 96-well plates, and cultured at 37°C in an incubator with 5%
CO2. Cell proliferation was measured using a Cell
Counting Kit-8 (CCK-8) (Dojindo Molecular Technologies, Inc.)
according to the manufacturers instructions.
Cell migration and invasion
assays
Cell migration and invasion were evaluated using a
Transwell assay (Corning Inc.). miR-NC, miR-200a, miR-141 and
miR-200a+miR-141-transfected HepG2 cells (1×105
cells/100 µl serum-free medium) were placed into the upper chamber.
High-glucose DMEM supplemented with 20% FBS (600 µl) was placed
into the lower chamber. Chambers were assembled and incubated at
37°C in the presence of 5% CO2 for 24 h for the
migration assay or 48 h for the invasion assay. After the
incubation, cells from the upper surface of the membranes were
removed, and the migrated cells on the lower surface of the
membranes were fixed with methanol for 10 min at 37°C and stained
with crystal violet at room temperature for 10 min, and counted in
three randomly selected fields using an inverted microscope
(magnification, ×100; Nikon TE2000). Representative photographs
were selected. For the invasion assay, the upper chambers were
pre-incubated with Matrigel for 4 h to form a layer of Matrigel,
and the migrated cells in the lower chamber were counted 48 h after
plating.
Western blot analysis
Cells were lysed by RIPA lysis buffer containing
protease inhibitors (Beyotime Institute of Biotechnology, Inc.).
Protein concentration was determined using bicinchoninic acid assay
kit. Proteins (30 µg per lane) were separated by PAGE using 10%
Bis-Tris gels (Beyotime Institute of Biotechnology, Inc.) and
transferred onto a polyvinylidene fluoride membrane. Immunoblotting
was performed with diluted antibodies against STAT4 (1:1,000; cat.
no. 2653; Cell Signaling Technology, Inc.), E-cadherin (1:1,000;
cat. no. 3195; Cell Signaling Technology, Inc.), and vimentin
(1:1,000; cat. no. 5741; Cell Signaling Technology, Inc.), and an
internal reference antibody against β-actin (1:1,000; cat. no.
4970; Cell Signaling Technology, Inc.) overnight at 4°C. The
membrane was washed with 0.1% Tween-20 in TBST three times and then
incubated with goat anti-rabbit IgG (1:5,000; cat. no. HAF008;
R&D Systems, Inc.) for 1 h at room temperature. Specific
complexes were visualized with an enhanced chemiluminescence
detection system (Thermo Fisher Scientific, Inc.).
Luciferase activity assays
psiCHECK-2 (Promega Corporation) was used as a
luciferase reporter plasmid. The multiple cloning site region was
located behind the T7 promoter in this vector, and contained the
Luc marker of gene expression. The target fragment was inserted
into the XhoI and NotI sites, and the expression was
regulated by the SV40 promoter. Luciferase reporter plasmids
containing the wild-type (WT) or mutant (MUT) sequence of the
STAT4-3′-UTR region were inserted into the psiCHECK-2 luciferase
vector and into a plasmid used as an internal expression control,
and then transfected into 293T/miR-NC, 293T/miR-200a or
293T/miR-141 cells using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). At 48 h after
transfection, luciferase assays were performed using a
Dual-luciferase reporter assay kit (Promega Corporation). The
Renilla luciferase activity was used as internal
reference.
Statistical analysis
Values were obtained from at least three independent
experiments and presented as the mean ± SD. The receiver operating
characteristic (ROC) curve of miR-200a and miR-141 was plotted.
When data conforms to normal distribution, comparison between two
groups was conducted using the Students t-test while comparisons
between multiple groups of data were performed using ANOVA. The
Least Significant Difference post hoc test (three groups) and
Tukeys test (more than three groups) were used for pairwise
comparison between multiple groups. Non-parametric tests
(Mann-Whitney U test and Kruskal-Wallis H test) were performed when
making comparisons in datasets that are not normally distributed.
All statistical analyses were performed using SPSS 19.0 software
(SPSS, Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Downregulation of miR-200a and miR-141
expression in serum of patients with liver cancer is closely
associated with clinicopathological factors
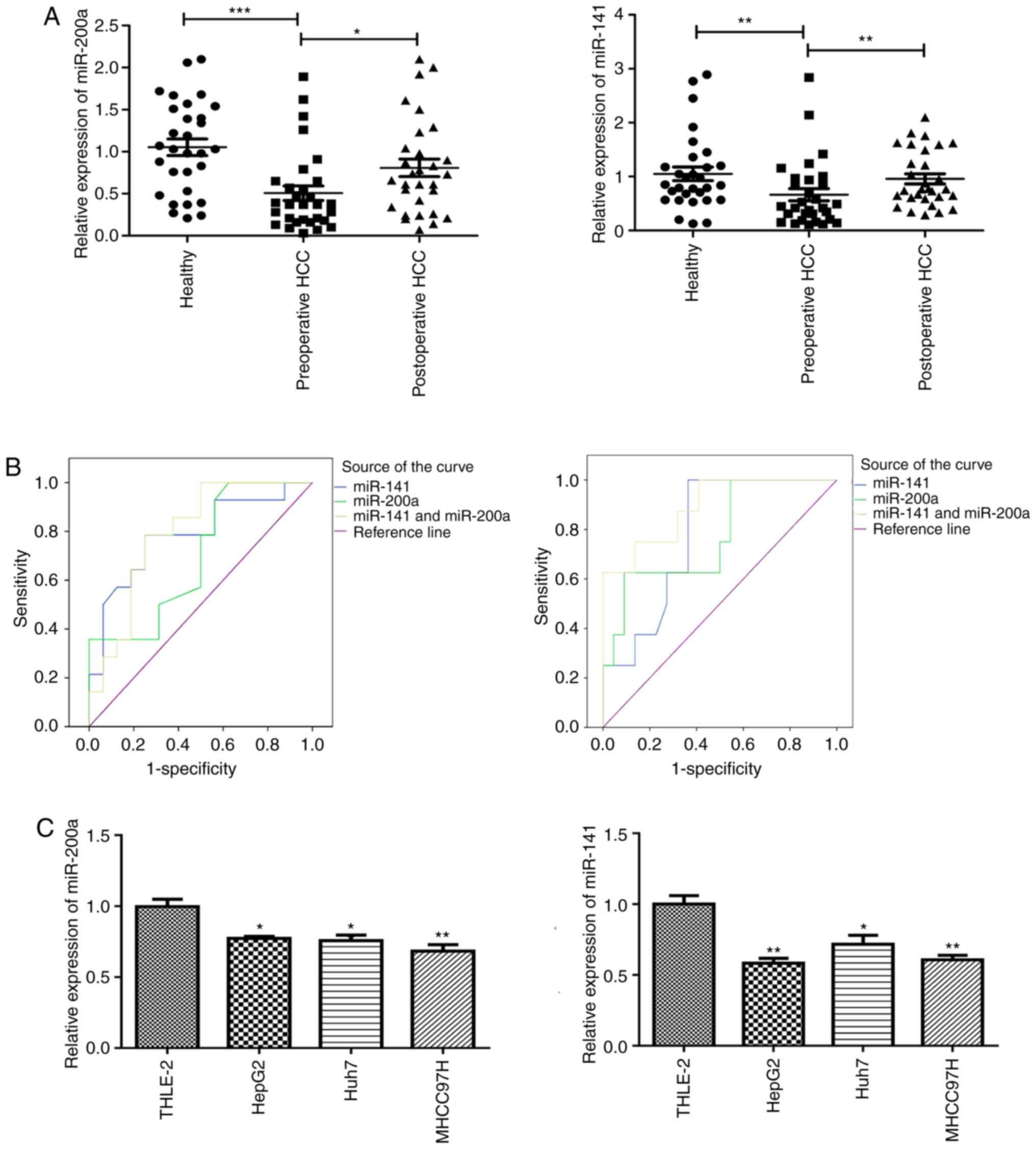
Circulating miR-200a and miR-141 levels were
detected in samples from 30 patients with liver cancer and 30
normal subjects. The expression levels of circulating miR-200a and
miR-141 were markedly decreased in the preoperative serum of
patients with liver cancer compared with those found in healthy
individuals. Besides, the expression level of miR-200a and miR-141
in the postoperative serum of the same patients increased
significantly compared with the levels in preoperative serum
(Fig. 1A). These results indicate
that the expression level of miR-200a or miR-141 can be detected in
blood samples, and may be used as a potential biomarker.
The present study further evaluated the association
between the preoperative serum level of miR-200a/miR-141 and
clinicopathological factors, which is summarized in Table I. The expression of miR-200a was
significantly associated with tumor size (P=0.001), Barcelona
Clinic Liver Cancer (BCLC) stage (P=0.002), differentiation
(P=0.011), metastasis (P=0.026), invasion (P=0.024) and recurrence
(P=0.029), while the expression of miR-200a was not significantly
associated with sex, age, α-fetoprotein (AFP), virus infection or
cirrhosis (P>0.05). The expression of miR-141 was associated
with the status of liver cancer metastasis and invasion (P<0.01,
P<0.05), but not with sex, age, AFP, virus infection, cirrhosis,
tumor size, BCLC staging, differentiation or recurrence. These
results show that miR-200a and miR-141 are both associated with
metastasis and invasion in patients with liver cancer.
 | Table I.Associations between the expression
levels of miR-200a/miR-141 in preoperative serum, and
clinicopathological factors of patients with hepatocellular
carcinoma. |
Table I.
Associations between the expression
levels of miR-200a/miR-141 in preoperative serum, and
clinicopathological factors of patients with hepatocellular
carcinoma.
|
|
| miR-200a | miR-141 |
|---|
|
|
|
|
|
|---|
| Clinicopathological
parameters | N | Median | P-value | Median | P-value |
|---|
| Sex |
|
|
|
| 0.476 |
|
Male | 22 | 0.370 | 0.781 | 0.435 |
|
|
Female | 8 | 0.425 |
| 0.535 |
|
| Age (years) |
|
|
|
| 0.950 |
|
≤55 | 12 | 0.415 | 0.755 | 0.400 |
|
|
>55 | 18 | 0.370 |
| 0.490 |
|
| AFP (ng/ml) |
|
|
|
| 0.471 |
|
≤20 | 11 | 0.370 | 0.767 | 0.490 |
|
|
>20 | 19 | 0.370 |
| 0.380 |
|
| Viral
infection |
|
|
|
| 0.389 |
|
With | 15 | 0.370 | 0.806 | 0.420 |
|
|
Without | 15 | 0.370 |
| 0.490 |
|
| Cirrhosis |
|
|
|
| 0.432 |
|
With | 17 | 0.370 | 0.483 | 0.310 |
|
|
Without | 13 | 0.370 |
| 0.530 |
|
| Tumor size
(cm) |
|
|
|
| 0.746 |
| ≤5 | 19 | 0.530 | 0.001b | 0.420 |
|
|
>5 | 11 | 0.190 |
| 0.450 |
|
| BCLC stage |
|
|
|
| 0.9510 |
| A | 14 | 0.650 | 0.002b | 0.435 |
|
| B +
C | 16 | 0.210 |
| 0.435 |
|
|
Differentiation |
|
|
|
| 0.6500 |
| Middle
or high | 13 | 0.650 | 0.011a | 0.380 |
|
|
Low | 17 | 0.280 |
| 0.450 |
|
| Migration |
|
|
|
| 0.003b |
|
With | 15 | 0.28 | 0.026a | 0.31 |
|
|
Without | 15 | 0.48 |
| 0.69 |
|
| Invasion |
|
|
|
| 0.018a |
|
With | 8 | 0.170 | 0.024a | 0.315 |
|
|
Without | 22 | 0.380 |
| 0.635 |
|
| Recrudescence |
|
|
|
| 0.781 |
|
With | 6 | 0.115 | 0.029a | 0.435 |
|
|
Without | 24 | 0.380 |
| 0.455 |
|
Furthermore, ROC curve was used to analyze the
sensitivity and specificity of miR-200a and miR-141 (individually
and combined) for evaluating metastasis and invasion in liver
cancer (Fig. 1B). The area under the
curve (AUC) was also calculated. As shown in Table II, the AUC values of miR-200a,
miR-141 and their combination for comparing metastasis of patients
with liver cancer were 0.696 (P>0.05), 0.783 (P<0.01) and
0.795 (P<0.01), respectively. There was no significant
difference between the three AUC values. The sensitivity and
specificity of miR-200a, miR-141 and their combined detection for
diagnosing metastasis of liver cancer were 50.0/50.0, 78.6/68.8 and
78.6/75.0%, respectively (Table
II). Although there was no significant difference between the
three AUC values, the AUC values in the combined group were higher
than those using miR-200a or miR-141 alone. Moreover, the
sensitivity and specificity of the combined group were higher
compared with those of miR-200a or miR-141 alone, which suggests
that the combination of miR-200a and miR-141 can help to improve
the AUC values, specificity and sensitivity of diagnosing
metastasis in patients with liver cancer, and have potential use in
early clinical diagnosis.
 | Table II.AUC value of miR-200a, miR-141 and
their combination for evaluating the metastasis of hepatocellular
carcinoma. |
Table II.
AUC value of miR-200a, miR-141 and
their combination for evaluating the metastasis of hepatocellular
carcinoma.
| Index | AUC (x±s) | P-value | 95% CI | Sensitivity
(%) | Specificity
(%) |
|---|
| miR-200a | 0.696±0.097 | 0.067 | 0.506–0.887 | 50.0 | 50.0 |
| miR-141 | 0.783±0.087 | 0.008 | 0.613–0.954 | 78.6 | 68.8 |
|
miR-200a+miR-141 | 0.795±0.083 | 0.006 | 0.632–0.957 | 78.6 | 75.0 |
As shown in Table
III, the AUC values of miR-200a, miR-141 and their combination
for comparing tumor invasion in patients with liver cancer were
0.773 (P<0.05), 0.781 (P<0.05) and 0.892 (P<0.01),
respectively. There was no significant difference between the three
AUC values. The sensitivity and specificity of miR-200a, miR-141
and their combined detection for diagnosing tumor invasion in liver
cancer were 62.5/77.3, 62.5/72.7 and 75.0/86.4%, respectively
(Table III). Although there were
no significant differences between the three AUC values, the AUC
values in the combined group were higher compared with those using
miR-200a or miR-141 alone. Moreover, the sensitivity and
specificity of the combined group were higher than those of
miR-200a or miR-141 alone, which suggests that the combination of
miR-200a and miR-141 can help to improve the AUC values,
specificity and sensitivity of diagnosing tumor invasion in
patients with liver cancer, and have a potential use in early
clinical diagnosis.
 | Table III.Area under the curve value of
miR-200a, miR-141 and their combination for evaluating the invasion
of hepatocellular carcinoma. |
Table III.
Area under the curve value of
miR-200a, miR-141 and their combination for evaluating the invasion
of hepatocellular carcinoma.
| Index | AUC (x±s) | P-value | 95% CI | Sensitivity
(%) | Specificity
(%) |
|---|
| miR-200a | 0.773±0.099 | 0.024 | 0.579–0.967 | 62.5 | 77.3 |
| miR-141 | 0.781±0.085 | 0.020 | 0.616–0.947 | 62.5 | 72.7 |
|
miR-200a+miR-141 | 0.892±0.065 | 0.001 | 0.764–1.000 | 75.0 | 86.4 |
The results of ROC curve showed that the combination
of miR-200a and miR-141 can improve the sensitivity and specificity
of diagnosis for patients with liver cancer exhibiting metastasis
or tumor invasion. Therefore, miR-200a and miR-141 may be involved
in the development of liver cancer, especially the processes of
metastasis and invasion.
Overexpression of miR-200a and miR-141
has a combined effect on inhibiting the proliferation, migration
and invasion of liver cancer cells
The results of serology revealed that miR-200a and
miR-141 are downregulated in the preoperative serum of patients
with liver cancer and can be used as potential non-invasive
markers. To further explore the expression of miR-200a and miR-141
in hepatocarcinoma cell lines, qPCR was used to analyze the
expression levels of miR-200a and miR-141 in a human normal
hepatocyte cell line (THLE-2) and four hepatoma cell lines (HepG2,
Huh7 and MHCC97H). The results showed that the expression levels of
miR-200a and miR-141 in these four hepatocarcinoma cell lines were
downregulated to different degrees compared with those in THLE-2
(Fig. 1C). As HepG2 cells are easy
to be cultured and are commonly used for co-transfection, HepG2
cells were selected for subsequent experiments.
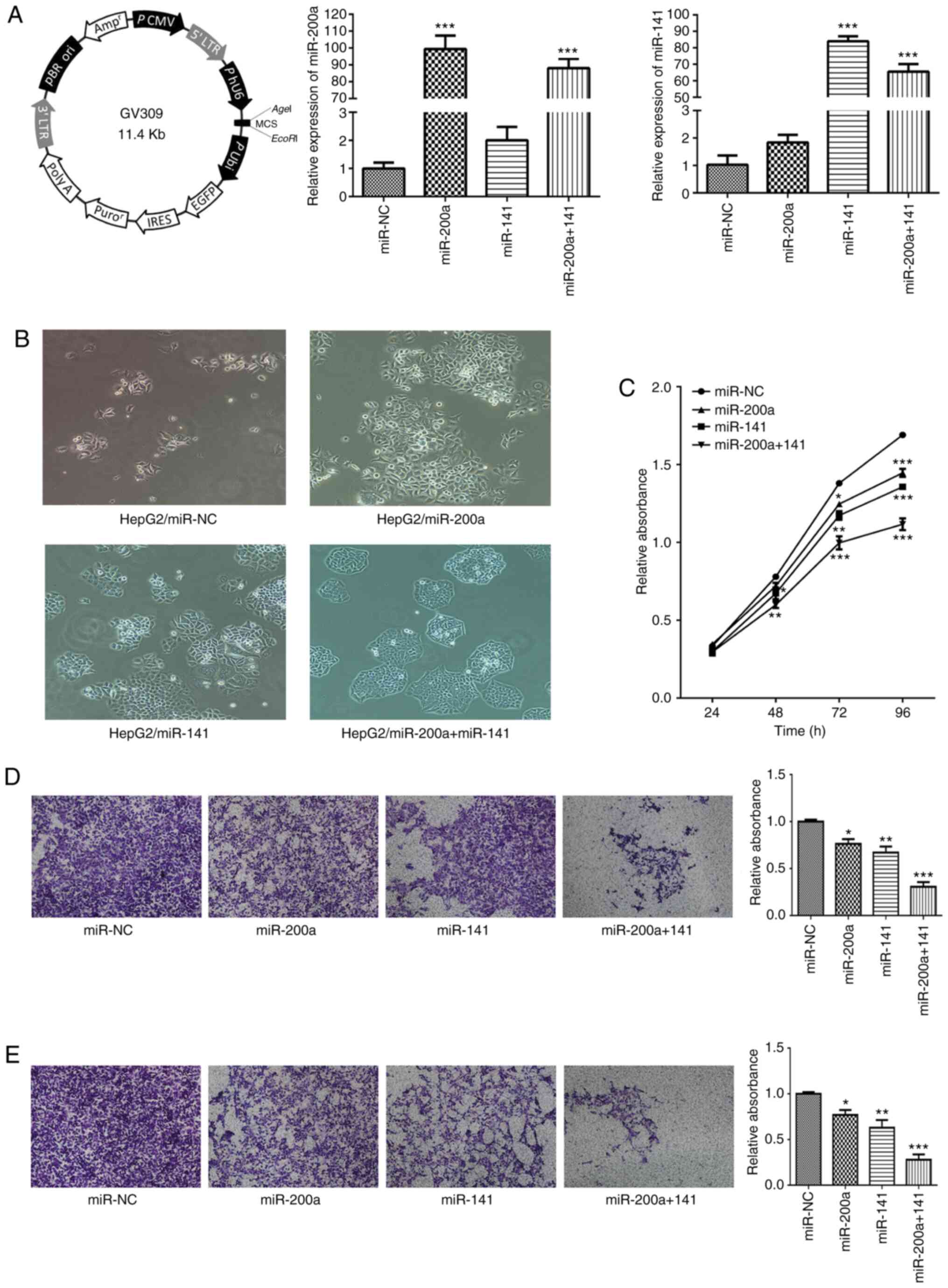
To evaluate the function of miR-200a and miR-141 in
liver cancer cells and to explore whether there is a combined
effect of miR-200a and miR-141, GV309 (whose map is shown in
Fig. 2A) was used as an expression
plasmid for lentiviral packaging. Stable cell lines expressing NC
(HepG2/miR-NC), miR-200a (HepG2/miR-200a), miR-141 (HepG2-miR/141)
and miR-200a+miR-141 (HepG2/miR-200a+miR-141) were established by
lentiviral transduction. The expression levels of miR-200a and
miR-141 in each stable strain were analyzed by qPCR. The results
showed that the levels of miR-200a in stable HepG2/miR-200a and
HepG2/miR-200a+miR-141 cells were significantly higher compared
with those in the control group (P<0.001 both), which indicates
that stable cell lines expressing miR-200a were successfully
constructed (Fig. 2A). Similarly,
the expression levels of miR-141 in the stable strains
HepG2/miR-141 and HepG2/miR-200a+miR-141 were significantly higher
compared with those in the control group (P<0.001 both), which
showed that stable cell lines expressing miR-141 were successfully
constructed (Fig. 2A).
In the present study, cellular morphology was
monitored. There were some differences between miR-200a- or
miR-141-overexpressing cells and control cells. HepG2/miR-NC cells
were loosely adhered to each other, slender and looked
spindle-like, showing the appearance of mesenchymal cells.
HepG2/miR-200a, HepG2/miR-141 or HepG2/miR-200a+miR-141 cells were
closely connected to each other and polygonal arranged in a
cobblestone-like arrangement, which is a typical epithelial cell
morphology (Fig. 2B).
Meanwhile, the effects of miR-200a and miR-141 on
cell proliferation, migration and invasion were also assessed.
Overexpression of miR-200a or miR-141 in liver cancer cells
attenuated cell proliferation (Fig.
2C). Overexpression of miR-200a or miR-141 in liver cancer
cells decreased migration and invasion compared with the effects
observed in miR-NC-transfected cells (Fig. 2D and E). It is noteworthy that the
inhibition of cell function was most obvious in the stable cell
line overexpressing simultaneously miR-200a and miR-141.
The results of the above biological function
experiments showed that miR-200a and miR-141 exert synergistic
inhibitory effects on the proliferation, migration and invasion of
hepatocarcinoma cells.
Downregulation of miR-200a and miR-141
enhances the epithelial-mesenchymal transition (EMT) through
targeting STAT4
To further understand the molecular mechanism of
miR-200a and miR-141 in synergistically inhibiting tumorigenesis,
potential common targets of miR-200a and miR-141 were searched
using dedicated software. First, published literature on the
miR-200 family was searched through PubMed (http://www.ncbi.nlm.nih.gov/pubmed), and the miRBase
(http://www.mirbase.org/index.shtml)
online tool was used to obtain the base sequence, chromosome
location and target prediction of miR-200a and miR-141. Next,
TargetScan (www.targetscan.org), picTar
(https://pictar.mdc-berlin.de/) and miRDB
(www.mirdb.org) were applied to predict the common
targets of miR-200a and miR-141, and their intersection was
considered as the target gene set for further analysis. The three
calculation methods predicted that STAT4 is a potential novel
direct target of miR-200a and miR-141 with the binding site at its
3′-UTR region. Reporter luciferase vectors containing the WT or MUT
miR-200a/miR-141 binding site of STAT4 3′-UTR were constructed
(Fig. 3A). psiCHECK-2, whose map is
shown in Fig. 3B, was used as a
luciferase reporter plasmid. Luciferase activity assay showed that
overexpression of miR-200a and miR-141 markedly decreased the
activity of the WT reporter to 24.89 and 31.58%, respectively
(P<0.01 both), but not of the MUT reporter (Fig. 3B), suggesting that miR-200a and
miR-141 both inhibited the 3-UTR function of STAT4, and the point
mutation of this target sequence abolished the effect of miR-200a
or miR-141. The present study found that overexpression of miR-200a
or miR-141 significantly downregulated the mRNA and protein levels
of STAT4 in liver cancer cell lines (Fig. 3C and D).
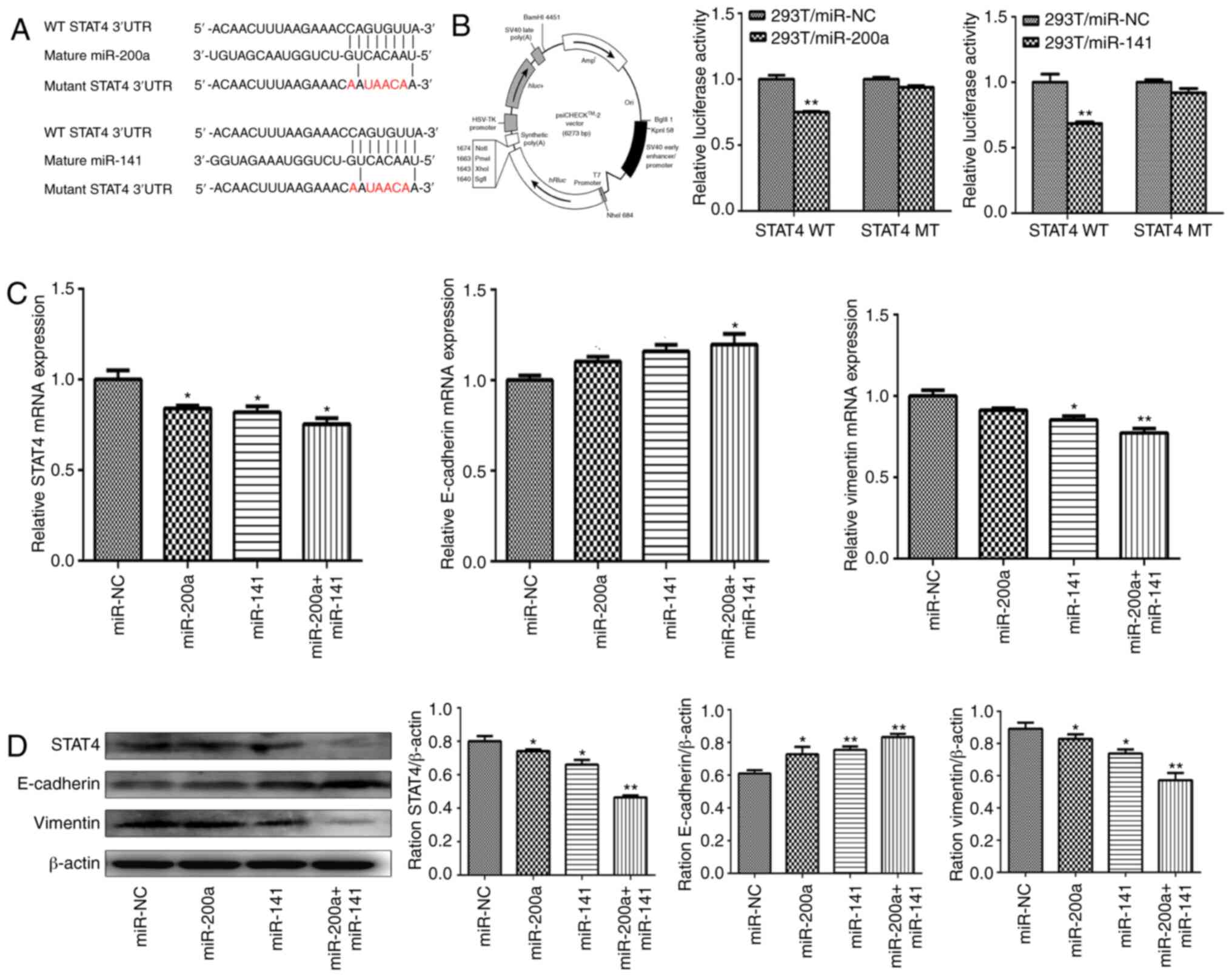 | Figure 3.miR-200a and miR-141 regulate
epithelial-mesenchymal transition progression in liver cancer
through targeting STAT4. (A) Schematic diagram of the putative
miR-200a/miR-141 binding site in the 3′-UTR of the human STAT4
gene. The mutated nucleotides of the STAT4 3′-UTR are labeled in
red. (B) The psiCHECK-2 vector map is shown (left) and 293T cells
were co-transfected with miR-NC, miR-200a or miR-141 mimics along
with a wild-type or mutant STAT4 luciferase reporter (middle and
right). Luciferase activities were measured 48 h after transfection
using the dual-luciferase reporter assay system. (C) The mRNA
expression levels of STAT4 (left), E-cadherin (middle) and vimentin
(right) were measured by reverse transcription-quantitative PCR in
HepG2 cells stably overexpressing miR-NC, miR-200a, miR-141 and
miR-200a+miR-141, and were normalized to the level of GAPDH. (D)
The protein expression levels of STAT4, E-cadherin and vimentin
were determined by western blotting in HepG2 stably overexpressing
miR-NC, miR-200a, miR-141 and miR-200a+miR-141. β-actin was used as
an internal control. *P<0.05; **P<0.01. UTR, untranslated
region; STAT4, signal transducer and activator of transcription 4;
NC, negative control; miR, microRNA. |
EMT is an important way for epithelial cells to
obtain migration and invasion abilities, which plays an important
role in tumor metastasis and invasion. The change in E-cadherin and
vimentin protein is an important molecular characteristic of the
EMT process. Consistent with the decrease in STAT4, overexpression
of miR-200a and miR-141 in HepG2 cells also inhibited EMT progress
(Fig. 3C and D). Furthermore, this
inhibition was most pronounced in the combined group.
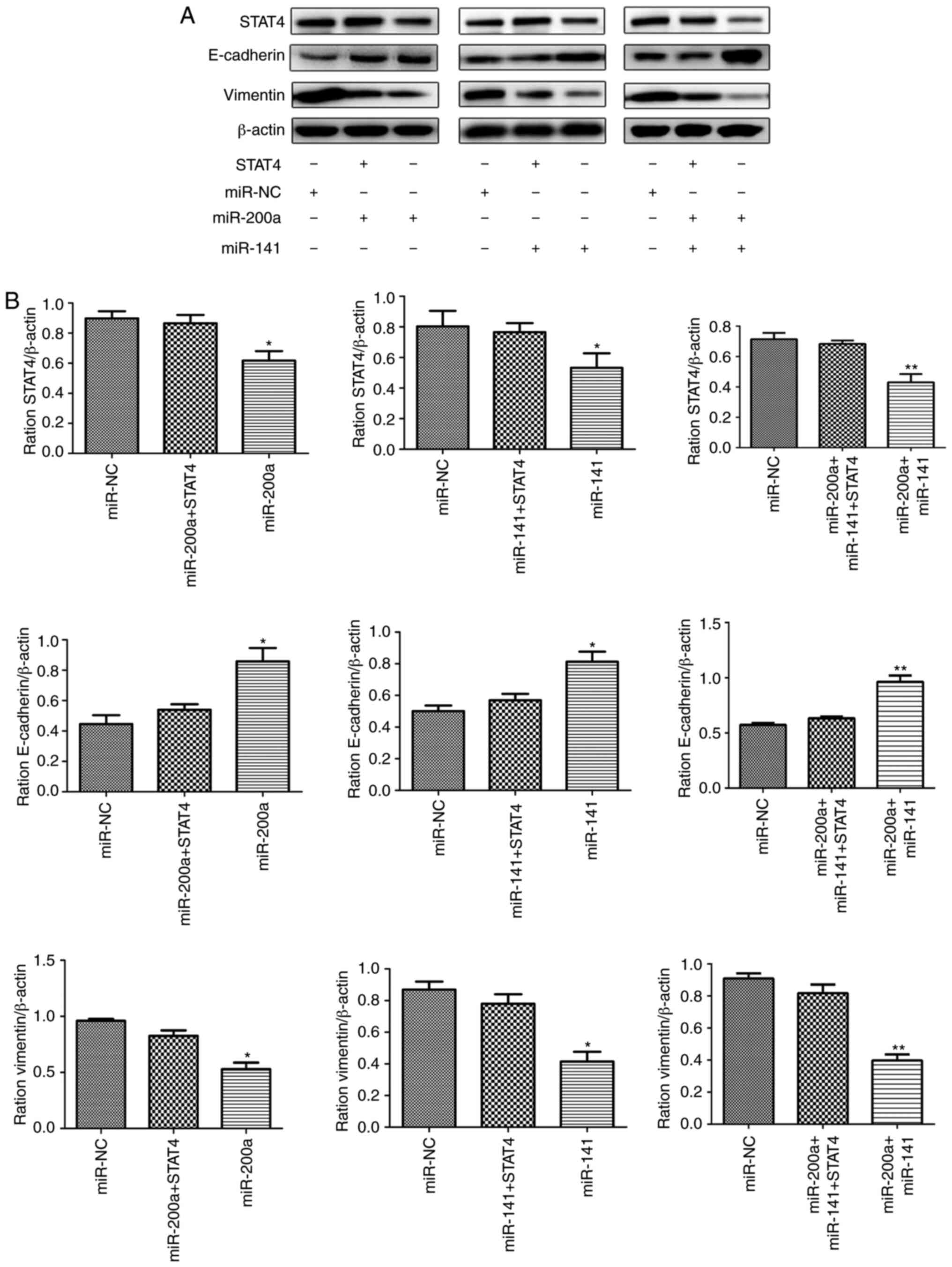
Forced expression of STAT4 restores
miR-200a/miR-141-inhibited EMT progress
Some studies have shown that abnormal expression of
STAT4 causes changes in EMT-associated molecules. Therefore, the
present study further explored whether miR-200a and miR-141
regulate the downstream EMT process via targeting STAT4. Stable
cell lines expressing miR-200a, miR-141 and miR-200a+miR-141 were
transfected with lentivirus carrying STAT4. The proteins of each
group were extracted, and the expression of E-cadherin, vimentin
and STAT4 was detected by western blotting. The results showed that
restoring the expression level of STAT4 partially reverses the
inhibitory effect produced by miR-200a and miR-141 on the EMT
process (Fig. 4).
Discussion
The major finding of the present study is that
miR-200a and miR-141 synergistically inhibit the expression of the
same target gene, STAT4, thereby restraining the EMT process of
liver cancer. Several studies have demonstrated that miR-141
retards liver cancer cell growth or enhances chemical sensitivity
by targeting sperm associated antigen 9 (SPAG9) (21), zinc finger E-box binding (ZEB1)
(22), kelch like ECH associated
protein 1 (Keap1) (23) or
hepatocyte nuclear factor-3β (HNF-3β) (24), whereas miR-200a does so by targeting
MET transcriptional regulator MACC1 (17), Grb2-associated binding protein 1
(GAB1) (16) and dual-specific
phosphatase 6 (DUSP6) (25), in
liver cancer. The association between miRNA and target can be
one-to-many or many-to-one; that is, one miRNA can inhibit many
proteins, and one protein can be regulated by many miRNAs. Previous
studies have found that the miR-200 family can directly target the
E-box-binding transcription regulators ZEB1 and ZEB2, causing
abnormal expression of E-cadherin and vimentin in cancer cell
lines, thereby inhibiting the tumor EMT process (26,27).
Although the present study showed that the common target genes ZEB1
and ZEB2 of the miR-200 family play a crucial role in the EMT
process, they did not clearly clarify the specific synergy. The
present study provides a better insight into the synergetic role of
miR-141 and miR-200a by co-targeting STAT4 in liver cancer cells.
To the best of our knowledge, this is the first study to
demonstrate the cooperative effect of miR-200a and miR-141 on
inhibiting the EMT of liver cancer by targeting a new gene (STAT4)
directly.
miR-200a and miR-141 belong to the same miRNA
family, miR-200, which is one of the miRNAs families found to be
expressed abnormally in numerous tumors (28–30). In
the present study, the expression levels of miR-200a and miR-141
were first detected in serum. It was found that, compared with
those in the serum of healthy subjects and in the postoperative
serum of patients with liver cancer, the expression levels of
miR-200a and miR-141 were downregulated in the preoperative serum
of patients. The present study further analyzed the association
between the expression levels of miR-200a and miR-141 in the
preoperative serum of patients and clinicopathological features.
The results showed that the relative expression levels of miR-200a
and miR-141 were significantly associated with metastasis and
invasion in patients with liver cancer. The combination of miR-200a
and miR-141 can improve the sensitivity and specificity of the
diagnosis of liver cancer with metastasis and invasion. These
results suggest that miR-200a and miR-141 may play important roles
in mediating the genesis and development of liver cancer. The
combined detection of miR-200a and miR-141 in serum has potential
clinical application for early screening, diagnosis and prognosis
of liver cancer.
Previous studies have shown that miRNAs from the
same cluster can play a synergistic role in cancer (31,32).
Although miR-200a and miR-141 are located on chromosomes 1p36.33
and12p13.31, respectively, they share the same target sequence
(AACACUG in 5 to 3 orientation) (33,34). The
present and other studies have reported that miR-200a and miR-141
have low expression in liver cancer and play important roles in
suppressing tumors. Thus, the present study aimed to investigate
whether there is a common biological function in the process of
hepatocellular growth, metastasis and invasion.
To further clarify these questions, a series of
stable cell lines of liver cancer overexpressing miR-200a,
overexpressing miR-141, and simultaneously overexpressing miR-200a
and miR-141, were successively constructed. The biological function
and common mechanism of miR-200a and miR-141 were studied at the
cellular level, respectively. The results showed that
overexpression of miR-200a or miR-141 in hepatocarcinoma cell lines
significantly inhibited cell proliferation, migration, invasion and
EMT. In vitro inhibition was most pronounced in cell lines
overexpressing miR-200a and miR-141 simultaneously, indicating that
miR-200a and miR-141 have synergistic effects on inhibiting the
biological function of liver cancer. Next, the potential molecular
mechanism of this phenomenon was further studied. STAT4, a member
of the STAT family, is an important transcription factor that
regulates the expression of various molecules in vivo, and
mediates cell proliferation, apoptosis, metastasis and other
processes (35–37). In recent years, numerous studies have
shown that single nucleotide polymorphisms of STAT4 are closely
associated with the occurrence and development of liver cancer
(38,39). The present study used TargetScan,
PicTar, microRNA and other biological information software to
predict that STAT4 may be a common target gene of miR-200a and
miR-141. Although it has been shown that miR-141 can inhibit the
growth of gastric cancer by targeting STAT4 (40), whether miR-200a can target STAT4 and
whether miR-141 can also mediate the expression of STAT4 in liver
cancer are still not reported. The present study used
bioinformatics software, luciferase reporter gene assay, qPCR and
western blotting to predict and confirm that STAT4 is a direct and
common target gene of miR-200a and miR-141. As an important
transcription factor, STAT4 is upregulated in multiple tumors, and
promotes tumor metastasis and invasion through various mechanisms,
especially regulation of EMT. Zhao et al (41) found that activated STAT4 was
overexpressed in epithelial cells of ovarian cancer, and mediated
the metastasis and invasion of ovarian cancer via the EMT process.
EMT is a process by which epithelial cells transform into
interstitial cells in a dynamic way. This change causes cells to
lose polarity; that the adhesion molecules on the cell surface are
expressed abnormally; and that cells acquire some characteristics
of mesenchymal cells. This process is also an important way to make
cells involved in metastasis and invasion. The abnormal expression
of E-cadherin and vimentin is one of the most important features of
EMT progression. Thus, the present study aimed to clarify whether
miR-200a and miR-141 affect the expression levels of two downstream
EMT-associated important molecules such as E-cadherin and vimentin
to participate in the process of EMT by targeting STAT4, which
promotes the progression of tumors. For that purpose, stable cell
lines overexpressing miR-200a, miR-141 and miR-200a+miR-141 were
cultured with lentivirus carrying the STAT4 gene, which restored
the expression of STAT4 inhibited by miR-200a or miR-141 in stable
strains. The changes in expression of E-cadherin and vimentin in
the stable strains were detected by western blotting. The results
showed that restoring the expression levels of STAT4 could partly
reverse the expression changes of downstream EMT-related
characteristic molecules.
In summary, this study conducted investigations at a
clinical and cellular level, and showed that the expression levels
of miR-200a and miR-141 in the preoperative serum of patients with
liver cancer were downregulated. The expression levels of miR-200a
and miR-141 were reported to be closely associated with
clinicopathological features of liver cancer, especially metastasis
and invasion. This study is the first to report that STAT4 is the
new common target gene of miR-200a and miR-141. miR-200a and
miR-141 were confirmed to inhibit the expression of E-cadherin and
vimentin in EMT synergistically to regulate the proliferation,
migration and invasion of hepatocarcinoma cells by targeting STAT4
at a cellular level. These results enrich the knowledge on the
tumor suppressor mechanism of the miR-200 family, and also provide
a new experimental and theoretical basis for the use of miRNAs for
the early diagnosis, prognosis and thorough treatment of liver
cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Key
Research and Development Plan of China (grant no. 2018YFC2000200),
the National Natural Science Foundation of China (grant no.
81772673), the Shanghai Sailing Program (grant no. 19YF1405500) and
the Initial Scientific Research Fund of the Huashan Hospital
Affiliated to Fudan University (grant no. 2019QD003).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors contributions
YM, WC, CL and HW collected the case material and
collected the data. SC, JZ, JC and XC performed the experiments and
analyzed the data. SC, YM, WC, CL and HW drafted the manuscript and
revised the manuscript. QC, YuL and YoL conceived and designed the
study. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by The Ethics Committee of
Huashan Hospital Affiliated to Fudan University (approval no. 557,
2019). All the healthy individuals and patients provided written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Villanueva A: Hepatocellular carcinoma. N
Engl J Med. 380:1450–1462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marrero JA, Kulik LM, Sirlin CB, Zhu AX,
Finn RS, Abecassis MM, Roberts LR and Heimbach JK: Diagnosis,
staging, and management of hepatocellular carcinoma: 2018 practice
guidance by the American association for the study of liver
diseases. Hepatology. 68:723–750. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ye J, Wu S, Pan S, Huang J and Ge L: Risk
scoring based on expression of long non-coding RNAs can effectively
predict survival in hepatocellular carcinoma patients with or
without fibrosis. Oncol Rep. 43:1451–1466. 2020.PubMed/NCBI
|
|
4
|
Bracken CP, Scott HS and Goodall GJ: A
network-biology perspective of microRNA function and dysfunction in
cancer. Nat Rev Genet. 17:719–732. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M
and Croce CM: Human microRNA genes are frequently located at
fragile sites and genomic regions involved in cancers. Proc Natl
Acad Sci USA. 101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heusschen R, van Gink M, Griffioen AW and
Thijssen VL: MicroRNAs in the tumor endothelium: Novel controls on
the angioregulatory switchboard. Biochim Biophys Acta. 1805:87–96.
2010.PubMed/NCBI
|
|
7
|
Xu WP, Liu JP, Feng JF, Zhu CP, Yang Y,
Zhou WP, Ding J, Huang CK, Cui YL, Ding CH, et al: MiR-541
potentiates the response of human hepatocellular carcinoma to
sorafenib treatment by inhibiting autophagy. Gut. 69:1309–1321.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin J, Shen J, Yue H and Cao Z:
MiRNA-183-5p.1 promotes the migration and invasion of gastric
cancer AGS cells by targeting TPM1. Oncol Rep. 42:2371–2381.
2019.PubMed/NCBI
|
|
9
|
Zhang J, Xu S, Xu J, Li Y, Zhang J, Zhang
J and Lu X: MiR-767-5p inhibits glioma proliferation and metastasis
by targeting SUZ12. Oncol Rep. 42:55–66. 2019.PubMed/NCBI
|
|
10
|
Velazquez-Torres G, Shoshan E, Ivan C,
Huang L, Fuentes-Mattei E, Paret H, Kim SJ, Rodriguez-Aguayo C, Xie
V, Brooks D, et al: A-to-I miR-378a-3p editing can prevent melanoma
progression via regulation of PARVA expression. Nat Commun.
9:4612018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khella HW, Bakhet M, Allo G, Jewett MA,
Girgis AH, Latif A, Girgis H, Von Both I, Bjarnason GA and Yousef
GM: MiR-192, miR-194 and miR-215: A convergent microRNA network
suppressing tumor progression in renal cell carcinoma.
Carcinogenesis. 34:2231–2239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang W, Qian P, Zhang X, Zhang M, Wang H,
Wu M, Kong X, Tan S, Ding K and Perry JK: Autocrine/paracrine human
growth hormone-stimulated MicroRNA 96-182-183 cluster promotes
epithelial-mesenchymal transition and invasion in breast cancer. J
Biol Chem. 290:13812–13829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng X, Wang Z, Fillmore R and Xi Y:
MiR-200, a new star miRNA in human cancer. Cancer Lett.
344:166–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Belgardt BF, Ahmed K, Spranger M,
Latreille M, Denzler R, Kondratiuk N, von Meyenn F, Villena FN,
Herrmanns K, Bosco D, et al: The microRNA-200 family regulates
pancreatic beta cell survival in type 2 diabetes. Nat Med.
21:619–627. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim YK, Wee G, Park J, Kim J, Baek D, Kim
JS and Kim VN: TALEN-based knockout library for human microRNAs.
Nat Struct Mol Biol. 20:1458–1464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Song W, Shen W, Yang X, Sun W, Qu
S, Shang R, Ma B, Pu M, Tao K, et al: MicroRNA-200a suppresses cell
invasion and migration by directly targeting GAB1 in hepatocellular
carcinoma. Oncol Res. 25:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feng J, Wang J, Chen M, Chen G, Wu Z, Ying
L, Zhuo Q, Zhang J and Wang W: MiR-200a suppresses cell growth and
migration by targeting MACC1 and predicts prognosis in
hepatocellular carcinoma. Oncol Rep. 33:713–720. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hou X, Yang L, Jiang X, Liu Z, Li X, Xie
S, Li G and Liu J: Role of microRNA-141-3p in the progression and
metastasis of hepatocellular carcinoma cell. Int J Biol Macromol.
128:331–339. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao Y, Xu Z, Zhou J and Yang H: MiR-141
inhibits proliferation, migration and invasion in human
hepatocellular carcinoma cells by directly downregulating TGFβR1.
Oncol Rep. 42:1656–1666. 2019.PubMed/NCBI
|
|
20
|
Mansini AP, Pisarello MJ, Thelen KM,
Cruz-Reyes M, Peixoto E, Jin S, Howard BN, Trussoni CE, Gajdos GB,
LaRusso NF, et al: MicroRNA (miR)-433 and miR-22 dysregulations
induce histone-deacetylase-6 overexpression and ciliary loss in
cholangiocarcinoma. Hepatology. 68:561–573. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lou G, Dong X, Xia C, Ye B, Yan Q, Wu S,
Yu Y, Liu F, Zheng M, Chen Z and Liu Y: Direct targeting
sperm-associated antigen 9 by miR-141 influences hepatocellular
carcinoma cell growth and metastasis via JNK pathway. J Exp Clin
Cancer Res. 35:142016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng L, Xu M, Xu J, Wu K, Fang Q, Liang
Y, Zhou S, Cen D, Ji L, Han W and Cai X: ELF3 promotes
epithelial-mesenchymal transition by protecting ZEB1 from
miR-141-3p-mediated silencing in hepatocellular carcinoma. Cell
Death Dis. 9:3872018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu L, Pan C, Wei X, Shi Y, Zheng J, Lin X
and Shi L: lncRNA KRAL reverses 5-fluorouracil resistance in
hepatocellular carcinoma cells by acting as a ceRNA against
miR-141. Cell Commun Signal. 16:472018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin L, Liang H, Wang Y, Yin X, Hu Y, Huang
J, Ren T, Xu H, Zheng L and Chen X: MicroRNA-141 inhibits cell
proliferation and invasion and promotes apoptosis by targeting
hepatocyte nuclear factor-3β in hepatocellular carcinoma cells. BMC
Cancer. 14:8792014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee H, Kim C, Kang H, Tak H, Ahn S, Yoon
SK, Kuh HJ, Kim W and Lee EK: MicroRNA-200a-3p increases
5-fluorouracil resistance by regulating dual specificity
phosphatase 6 expression. Exp Mol Med. 49:e3272017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park SM, Gaur AB, Lengyel E and Peter ME:
The miR-200 family determines the epithelial phenotype of cancer
cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes
Dev. 22:894–907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Paterson EL, Kazenwadel J, Bert AG,
Khew-Goodall Y, Ruszkiewicz A and Goodall GJ: Down-regulation of
the miRNA-200 family at the invasive front of colorectal cancers
with degraded basement membrane indicates EMT is involved in cancer
progression. Neoplasia. 15:180–191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gibbons DL, Lin W, Creighton CJ, Rizvi ZH,
Gregory PA, Goodall GJ, Thilaganathan N, Du L, Zhang Y,
Pertsemlidis A and Kurie JM: Contextual extracellular cues promote
tumor cell EMT and metastasis by regulating miR-200 family
expression. Genes Dev. 23:2140–2151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang GL, Sun J, Lu Y, Liu Y, Cao H, Zhang
H and Calin GA: MiR-200 family and cancer: From a meta-analysis
view. Mol Aspects Med. 70:57–71. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cimmino A, Calin GA, Fabbri M, Iorio MV,
Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S and Dono M:
MiR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl
Acad Sci USA. 102:13944–13949. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Villadsen SB, Bramsen JB, Ostenfeld MS,
Wiklund ED, Fristrup N, Gao S, Hansen TB, Jensen TI, Borre M,
Ørntoft TF, et al: The miR-143/-145 cluster regulates plasminogen
activator inhibitor-1 in bladder cancer. Br J Cancer. 106:366–374.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Altuvia Y, Landgraf P, Lithwick G, Elefant
N, Pfeffer S, Aravin A, Brownstein MJ, Tuschl T and Margalit H:
Clustering and conservation patterns of human microRNAs. Nucleic
Acids Res. 33:2697–2706. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Michael MZ, OConnor SM, van Holst
Pellekaan NG, Young GP and James RJ: Reduced accumulation of
specific microRNAs in colorectal neoplasia. Mol Cancer Res.
1:882–891. 2003.PubMed/NCBI
|
|
35
|
Miklossy G, Hilliard TS and Turkson J:
Therapeutic modulators of STAT signalling for human diseases. Nat
Rev Drug Discov. 12:611–629. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu H and Jove R: The STATs of cancer--new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang YS, Xin DE, Wang Z, Song X, Sun Y,
Zou QC, Yue J, Zhang C, Zhang JM, Liu Z, et al: STAT4 activation by
leukemia inhibitory factor confers a therapeutic effect on
intestinal inflammation. EMBO J. 15:e995952019.
|
|
38
|
Zhang L, Xu K, Liu C and Chen J:
Meta-analysis reveals an association between signal transducer and
activator of transcription-4 polymorphism and hepatocellular
carcinoma risk. Hepatol Res. 47:303–311. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li J, Liang L, Liu Y, Luo Y, Liang X, Luo
D, Feng Z, Dang Y, Yang L and Chen G: Clinicopathological
significance of STAT4 in hepatocellular carcinoma and its effect on
cell growth and apoptosis. Onco Targets Ther. 9:1721–1734.
2016.PubMed/NCBI
|
|
40
|
Zhou Y, Zhong JH, Gong FS and Xiao J:
MiR-141-3p suppresses gastric cancer induced transition of normal
fibroblast and BMSC to cancer-associated fibroblasts via targeting
STAT4. Exp Mol Pathol. 107:85–94. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao L, Ji G, Le X, Luo Z, Wang C, Feng M,
Xu L, Zhang Y, Lau WB, Lau B, et al: An integrated analysis
identifies STAT4 as a key regulator of ovarian cancer metastasis.
Oncogene. 36:3384–3396. 2017. View Article : Google Scholar : PubMed/NCBI
|