Introduction
Osteosarcoma is the most prevalent malignant primary
bone tumour in children, teenagers, and young adults (1). Due to limited novel therapeutic
options, the five-year survival rate is 60–70% and has not improved
over the last 30 years (2).
Molecular mechanisms underlying osteosarcoma disease progression
are unclear, however membrane type-1 matrix metalloproteinase
(MT1-MMP; MMP-14) is likely to play a key role. MT1-MMP has been
shown to direct cancer cell invasion in a wide variety of
carcinomas and directs bone metastasis in prostate carcinoma
(3,4). Due to their mesenchymal origin, HT1080
fibrosarcoma cells overexpress MT1-MMP which makes them useful as a
positive control when studying the role of MT1-MMP in sarcoma and
carcinoma. In osteosarcoma, overexpression of MT1-MMP in tumour
tissues has been shown to correlate with poor survival (5), however the potentially important
relevance of this proteinase to cancer progression in bone sarcoma
has not been extensively studied. MT1-MMP is an important member of
the matrix metalloproteinase family. As a zinc dependent
endopeptidase that contains a transmembrane domain, its role in
remodelling the extracellular matrix when localised to the cell
surface membrane has been subject to extensive research (6). However, the cytoplasmic tail of MT1-MMP
has been reported to regulate HIF-1α expression in cancer cells,
suggesting that this protein has an additional intracellular
function (7). Therefore, the aim of
this study was to investigate the expression of MT1-MMP in
osteosarcoma and prostate carcinoma cell lines in response to
hypoxia and also to determine a potential protein interaction
between MT1-MMP and the hypoxic inducible factors 1α and 2α.
Materials and methods
Cell culture
HT1080, U2OS, MDA-MB-231, MCF-7, PC3 and LNCaP cell
lines were purchased from American Type Culture Collection (ATCC).
The two sarcoma cell lines (U2OS and HT1080), two breast cancer
cell lines (MDA-MB-231 and MCF-7) and two prostate carcinoma cell
line (PC3 and LNCaP) were maintained in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM
glutamate, 100 U/ml penicillin, and 10 µg/ml streptomycin. The
human mesenchymal stem cell (MSCs) were grown in α-minimal
essential medium (MEM), supplemented with 20% FBS, 5% L-glutamine
and 8 ng/ml of basic fibroblast growth factor (bFGF).
MSC derivation and culture
Primary MSC cultures were established from 10
individuals aged between 36 and 75 years, undergoing hip
replacement surgery for osteoarthritis. Samples were collected
following appropriate consent and according to approval given by
the Newcastle and North Tyneside 1 Research Ethics Committee (REC
Reference no. 17/NE/0361) and processed within 24 h of surgery.
Trabecular bone fragments were dissected out from the femoral head
and processed over a 1.077 g/ml Lymphoprep™ density gradient medium
(StemCell Technologies). Mononuclear cells including putative MSC
were visible as an opaque band at the Lymphoprep™-marrow
interphase. The mononuclear band was washed in MSC wash buffer (5
mM EDTA/0.2% BSA/1% penicillin-streptomycin) and resuspended in low
glucose DMEM (1,000 mg/ml) (Sigma-Aldrich; Merck KGaA)
reconstituted with 20% fetal calf serum (Gibco®; Life
Technologies), 1% L-glutamine (Sigma-Aldrich; Merck KGaA) and 1%
penicillin-streptomycin (Sigma-Aldrich; Merck KGaA). Healthy MSC
adhere to plastic within 24 h. At this point the cells were washed
with MSC wash buffer to avoid contaminants and fresh media was
added, with the addition of bFGF (Gibco®; Life
Technologies) at 8 ng/ml. MSC were used between passages 2–5 and
within 40 days following surgery.
MSC characterisation
Cells were removed from the T75cm3 flask
with 2 ml Trypsin/EDTA, transferred into a universal container and
diluted up to 20 ml with PBS. The cells were centrifuged at 1,100
rpm and the supernatant removed without disturbing the cell pellet.
The cells were resuspended in 20 ml PBS and centrifuged again at
1,100 rpm. The supernatant was removed and the cells resuspended in
3 ml of flow cytometry buffer (500 ml PBS, 2.5 ml of 0.2 mmol/l
EDTA prepared from anhydrous stock, 2.5 ml of MACS BSA Stock
Solution) and transferred to a flow cytometry tube. The cells were
centrifuged at 1,100 rpm, the supernatant was removed and the cells
were resuspended in 100 µl of flow cytometry buffer. Cells were
then blocked by adding 5 µl human IgG at room temperature for 10
min. Cells were then stained for MSC markers using the Miltenyi
Human MSC Phenotyping Kit (130-125-285; Miltenyi Biotec Ltd.) by
adding 5 µl of the MSC antibody cocktail at room temperature for 30
min. The cells were then washed twice by adding 3 ml flow cytometry
buffer and centrifuging at 1,100 rpm. The cells were then run
through a BD FACSCanto II flow cytometer and the flow cytometry
plots analysed using FACSDiva software.
MSC differentiation assays
For all the below protocols, duplicate cells were
grown in MSC Growth Medium 2 (C-28009; Merck Life Science UK, Ltd.)
only in order to act as negative controls.
Adipogenesis differentiation
MSCs were grown in MSC Growth Medium 2 (C-28009;
Merck Life Science UK, Ltd.) for up to 2 days in 6-well plates and
allowed to reach 80–90% confluency. The cells were then induced
with MSC Adipogenic Differentiation Medium 2 (C-28016; Merck Life
Science UK, Ltd.) for 14 days. The medium was changed every third
day taking care not to disturb the cell monolayer. The cells were
gently washed with 1X PBS. The cells were then fixed with 4%
paraformaldehyde for 30 min at room temperature and then washed
twice with distilled water. The distilled water was aspirated and
enough 60% isopropanol was added to cover the cell monolayer. The
cells were incubated at room temperature for 5 min. The 60%
isopropanol was aspirated and enough Oil Red O staining solution
(O1391; Merck Life Science UK, Ltd.) was added to cover the cell
monolayer. The cells were incubated at room temperature for 15 min.
The staining solution was carefully aspirated and the cells washed
several times with distilled water until the water became clear.
The plate was blotted upside down on a paper towel to remove as
much water as possible. The cells were covered with PBS and
promptly analysed as the dye tends to fade upon prolonged light
exposure. Intracellular lipid vesicles in mature adipocytes stained
bright red and were photographed using a light microscope.
Osteogenesis differentiation
MSCs were grown in MSC osteogenic differentiation
medium (C-28013; Merck Life Science UK, Ltd.) for 14 days in
24-well plates. The cells were removed from the incubator and the
medium aspirated. The cells were gently washed with 1X phosphate
buffered saline (PBS). The cells were then fixed with 4%
paraformaldehyde for 30 min at room temperature and then washed
with 1X PBS followed by a further wash with PBS Tween (1X PBS with
0.05% Tween-20). The PBS Tween was aspirated and the cells stained
for alkaline phosphatase with 0.4 ml per well of StemTAG™ AP
solution (CB-306; Cell Biolabs). Following incubation at room
temperature for 30 min in the dark, the solution was aspirated and
the cells washed twice with 1Χ PBS. The cells were then
photographed under a light microscope.
Chondrogenesis differentiation
MSCs were grown in a 96-well U-bottom suspension
culture plate for 2 days to allow spheroid formation. The spheroids
were induced with Chondrogenic Differentiation Medium (C-28012;
Merck Life Science UK, Ltd.) and incubated for 21 days. The medium
was changed every 3 days. The cells were gently washed with 1X
phosphate buffered saline (PBS). The cells were then fixed with 4%
paraformaldehyde for 30 min at room temperature and then twice with
washed distilled water. Immediately before use, Alcian Blue
staining solution (TMS010; Merck Life Science UK, Ltd.) was passed
through a 0.22 µm Millex PES filter. The distilled water was
aspirated and enough filtered Alcian Blue staining solution was
added to generously cover the cartilage spheroids. The cells were
incubated in the dark for 45 min at room temperature. The Alcian
Blue staining solution was aspirated and the cartilage spheroids
washed with the destaining solution for 10 min. The wash step was
repeated twice with distilled water. The spheroids were covered in
PBS and photographed with a light microscope.
Hypoxia and normoxia treatment
Cells undergoing hypoxic treatment were grown in a
37°C incubator set to maintain a hypoxic atmosphere of 1%
O2, 5% CO2, and 94% N2 by
controlled injection of nitrogen. The normoxic condition was met
with standard cell culture conditions (20% O2 and 5%
CO2).
Antibodies
The same antibodies were used for western blot
analysis and for the proximity ligation assay. Note that MT1-MMP is
also classified as MMP14. The antibody to the catalytic domain of
MT1-MMP was mouse monoclonal anti-MMP-14 clone LEM-2/15.8, MAB3328
(Merck Millipore). Rabbit monoclonal anti-HIF-1α (EP1215Y) and
rabbit polyclonal anti-HIF-2α (ab199) were from Abcam. The
reference antibodies were as follows: mouse monoclonal anti-GAPDH
(MAB374) from Merck-Millipore and mouse monoclonal anti-Lamin A/C
clone 4C11 (4777) from Cell Signaling Technology.
Western blot analysis
Cells were harvested into lysis buffer [0.1 M Tris,
pH 7.5-4 M NaCl-10 mM Na3VO4, pH 10
(phosphatase inhibitor) 1% TX-100-1 mM DTT-1 mM PMSF - 1:50
protease inhibitors cocktail (Sigma-Aldrich; Merck KGaA)]. Protein
concentration was determined with a Bradford assay (Thermo Fisher
Scientific, Inc.). The protein concentrations were equalized with
lysis buffer as required. Total protein was subjected to SDS-PAGE
using 10% polyacrylamide gels and transferred to a polyvinylidene
fluoride (PVDF) membrane (Merck-Millipore). Secondary antibodies
were polyclonal goat anti-mouse (for MT1-MMP) or anti-rabbit (for
HIF-1α and 2α) IgG/HRP (horseradish peroxidase conjugated).
Following the addition of the developer Immobilon Western
Chemiluminescent HRP Substrate (Merck Millipore), protein bands
were visualised using Bio-Rad ChemiDoc Imaging System and analysed
using Fiji ImageJ software.
Subcellular protein extraction and
fractionation
In order to analyse protein subcellular
localisation, cytoplasmic and nuclear fractions were isolated from
U2OS cells using the subcellular fractionation kit catalogue no.
78840 (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Exponentially growing cells were
exposed to 1% oxygen (hypoxia) or 20% oxygen (normoxia) for 48 h.
The cytoplasmic and nuclear fractions were separated by SDS-PAGE
and analyzed by western immunoblotting with the mouse anti-MMP-14
and rabbit anti-HIF-1α, anti-HIF-2α antibodies as described
above.
Proximity ligation assay (PLA)
The in situ Proximity Ligation Assay (PLA)
was performed using the Duolink II Green kit according to the
manufacturer's instructions (Olink Bioscience). Cells were cultured
in 8 well Lab-Tek chamber slides (Thermo Fisher Scientific, Inc.).
Exponentially growing cells were then exposed to 1% oxygen
(hypoxia) or 20% oxygen (normoxia) for 48 h. Following cell
culture, media were removed and wells were washed with ice cold
PBS/Tween (0.01%). Cells were fixed with 4% paraformaldehyde and
blocked with goat serum solution (Sigma-Aldrich; Merck KGaA) at
room temperature for 20 min. The slides were then incubated
overnight at 4°C with antibodies diluted in goat serum at 1:50. The
reaction with probes, ligation, amplification, and detection were
performed according to the manufacturer's instructions. The slides
were mounted using Duolink in Situ Mounting Medium with DAPI
(Olink) and then analysed using a laser scanning confocal
microscope (Leica Lasertechnik). A 63X 1.4NA oil objective and
sequential scanning with filters 360–460 nm for Dapi (blue) and
495–527 nm for FITC (green) were used.
Representative results and averaged quantitative
values are shown from experiments repeated three times. To quantify
the signal dots representative of protein interaction between
MT1-MMP and HIF-1α or HIF-2α, the number and location (cytoplasmic
or nuclear) of the dots were assessed using Volocity 3D Image
analysis software (Perkin Elmer).
Immunofluorescence and confocal
microscopy on patient sarcoma tissue
Sections were cut from the patient specimen
Formalin-fixed paraffin-embedded (FFPE) tissue block to a thickness
of 3 µm using a rotary microtome, floated on distilled water and
mounted on electrostatically charged microscope slides. Slides were
dried in an incubator at 37°C overnight and left to cool at room
temperature for 30 min before initiating the staining procedure.
For antigen retrieval the slides were loaded into glass slide
holders and dewaxed in 100% xylene (Fisher Scientific UK) followed
by progressively decreasing quantities of ethanol (BDH; Poole) from
100% down to 50% and then distilled water. Antigen retrieval was
then performed in citrate buffer in a decloaking chamber for 30 min
at 125°C. Slides were rinsed in running water and blocked in 4%
BSA/PBS for 1 h. The MT1-MMP primary antibodies (mouse monoclonal
anti-MMP-14 clone LEM-2/15.8, MAB3328 (Merck Millipore and rabbit
polyclonal anti-HIF-2α (ab199) Abcam) were diluted to 1 in 50 in 4%
BSA/PBS. Slides were subsequently incubated at 4°C with the primary
antibody overnight. The secondary antibodies [Invitrogen Alexa
Fluor® 594 goat anti-mouse IgG (H+L) cross-adsorbed
secondary antibody, A-11005 and goat anti-rabbit IgG (H+L) highly
cross-adsorbed secondary antibody, Alexa Fluor 488, A-11034] were
diluted to 1 in 100 in 4% BSA/PBS and added to the slide for
further incubation at room temperature for 30 min in the dark.
Nuclei were mounted and counterstained using Vectashield mounting
medium with DAPI (Vector Laboratories) and visualised on a Leica
SP2 confocal scanning microscope (Leica Lasertechnik).
Statistical analysis
A single comparison between 20% oxygen versus 1%
oxygen was statistically analysed following the PLA to compare
intranuclear interaction. The number and location of the signal
dots were analysed and compared using the one sample Student's
t-test (SPSS version 21). For ease of presentation the graph was
generated with percentages on the y-axis.
Results
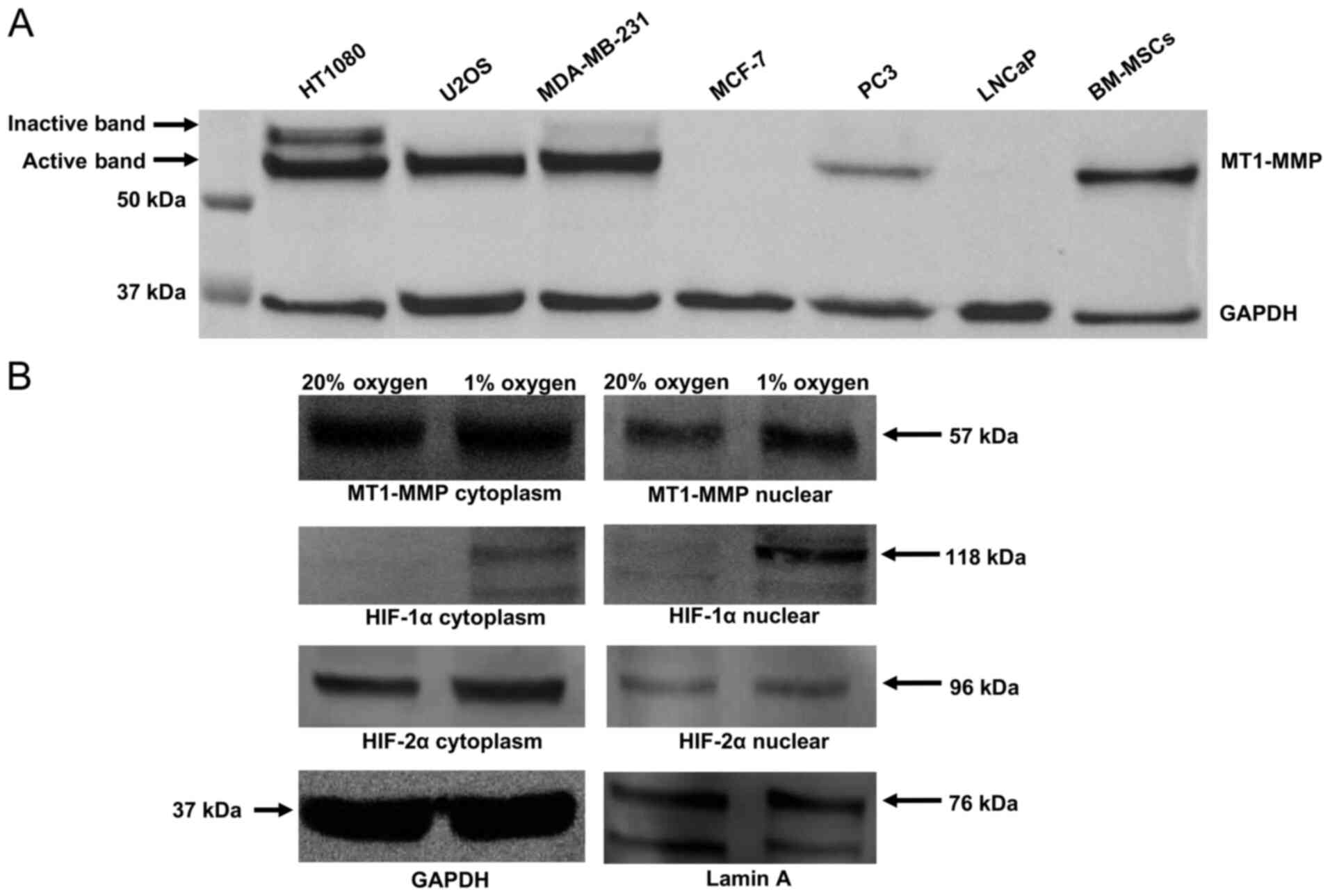
Protein expression of MT1-MMP in a
panel of cell lines and subcellular localisation of MT1-MMP in the
U2OS line
MT1-MMP expression in total cell lysate was assessed
using western blotting in a panel of cell lines which included the
HT1080 fibrosarcoma line, the U2OS osteosarcoma line, two breast
carcinoma lines (MDA-MB-231 and MCF-7), two prostate carcinoma
lines (PC3 and LNCaP) and a bone marrow derived mesenchymal stem
cell population (Fig. 1A). The blot
demonstrates high expression of MT1-MMP in the HT1080 cell line
(positive control) and an absence of MT1-MMP protein expression in
the MCF-7 line (negative control). A differing degree of MT1-MMP
expression was shown across the rest of the cell lines in the
panel, with the active 57 kDa form present in HT1080, U2OS,
MDA-MB-231, PC3 and MSCs. Expression of the inactive 64 kDa
pro-peptide is clearly evident in the HT1080 and MDA-MB-231 cell
lines but not in U2OS, PC3 or MSCs (Fig.
1A).
Subcellular fraction analysis of the U2OS cell line
(Fig. 1B) in 20% oxygen versus 1%
oxygen demonstrates increased nuclear localisation of MT1-MMP and
HIF-1α in hypoxia. HIF-2α expression is similar in all compartments
regardless of oxygen tension.
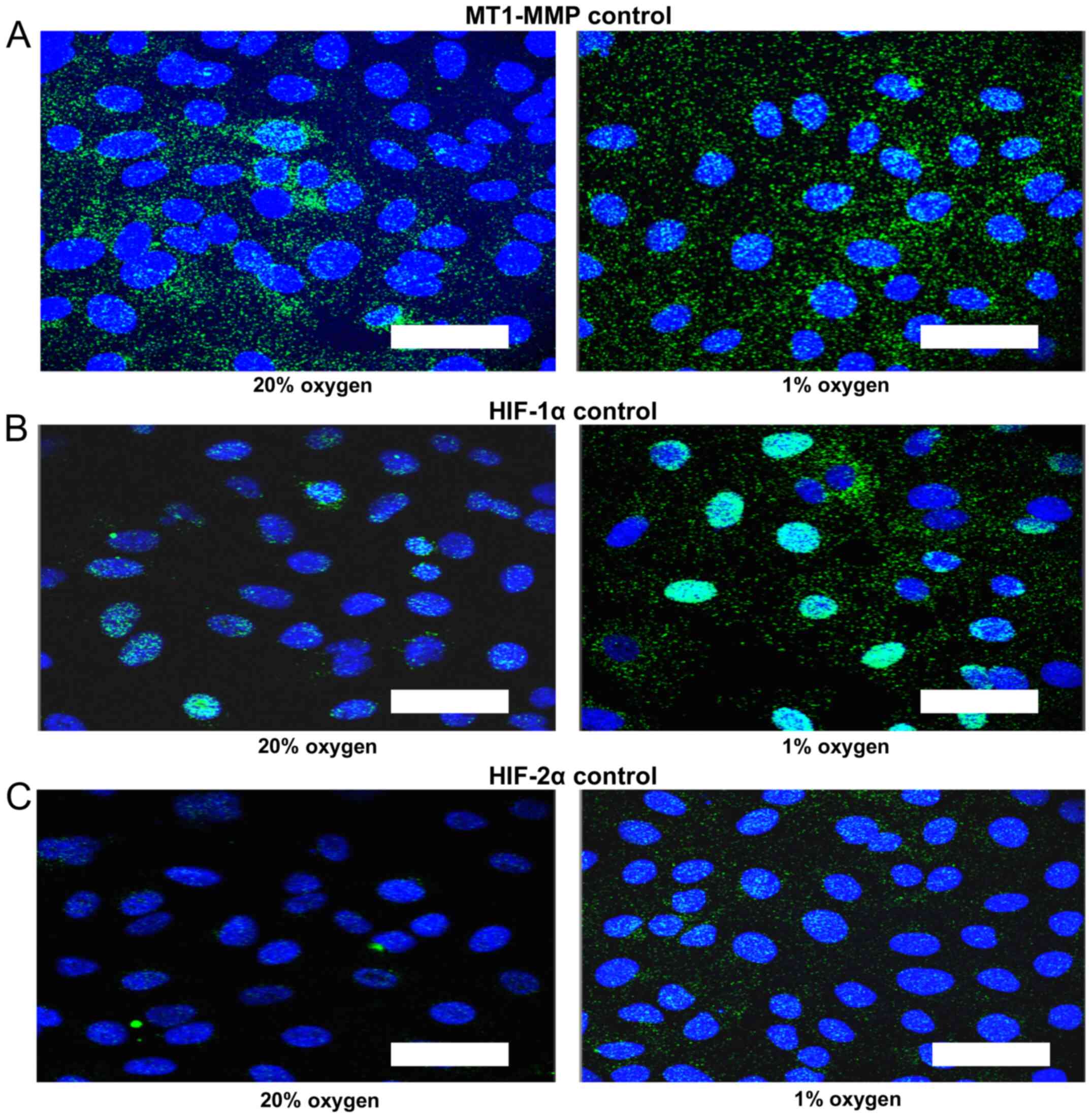
Control proximity ligation assay
As a control for the proximity ligation assay
increased number of foci and increased signal intensity in the
nucleus of all three proteins was demonstrated for cells treated
with 1% oxygen (Fig. 2). The MT1-MMP
signal is distributed in both cytoplasmic and nuclear compartments
and tends to increase in the nuclear compartment in 1% oxygen
(Fig. 2A). The intra-nuclear signal
is most intense for HIF-1α in 1% oxygen (Fig. 2B). There is also a trend towards an
increase in intra-nuclear HIF-2α signal in hypoxia (Fig. 2C).
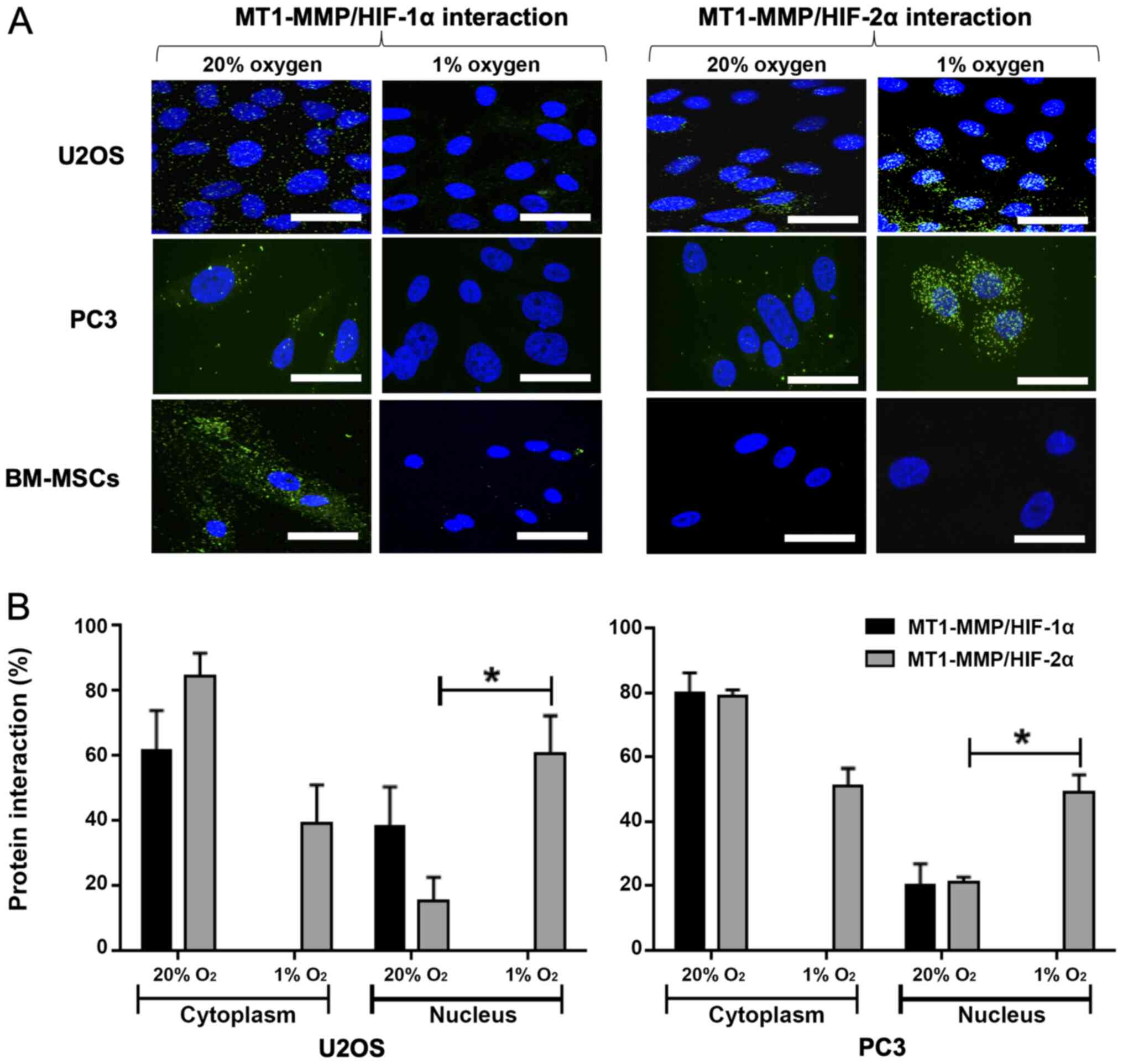
Proximity ligation assay indicates an
interaction between MT1-MMP and HIF-2α in the nucleus in U2OS and
PC3 cells
The interaction between MT1-MMP and HIF-1α in 20%
oxygen demonstrates signal in the cytoplasm for U2OS, PC3 and MSCs
(Fig. 3A). There is no evidence in
either cell type of an interaction between MT1-MMP and HIF-1α in 1%
oxygen. Furthermore, there is no evidence of interaction between
MT1-MMP and HIF-2α in the MSCs regardless of oxygen tension. In
U2OS and PC3 cells cultured in 20% oxygen, the signal indicates a
low level interaction between MT1-MMP and HIF-2α, mainly within the
cytoplasm. Compared with 20% oxygen, the MT1-MMP/HIF-2α interaction
in cells cultured in 1% oxygen is markedly increased with a larger
number and amplified intensity of interaction signals in the
nucleus. Quantitative and statistical analysis using the Student's
t-test showed this increase in intra-nuclear interaction to be
significant; P=0.040 for U2OS and P=0.028 for PC3 (Fig. 3B).
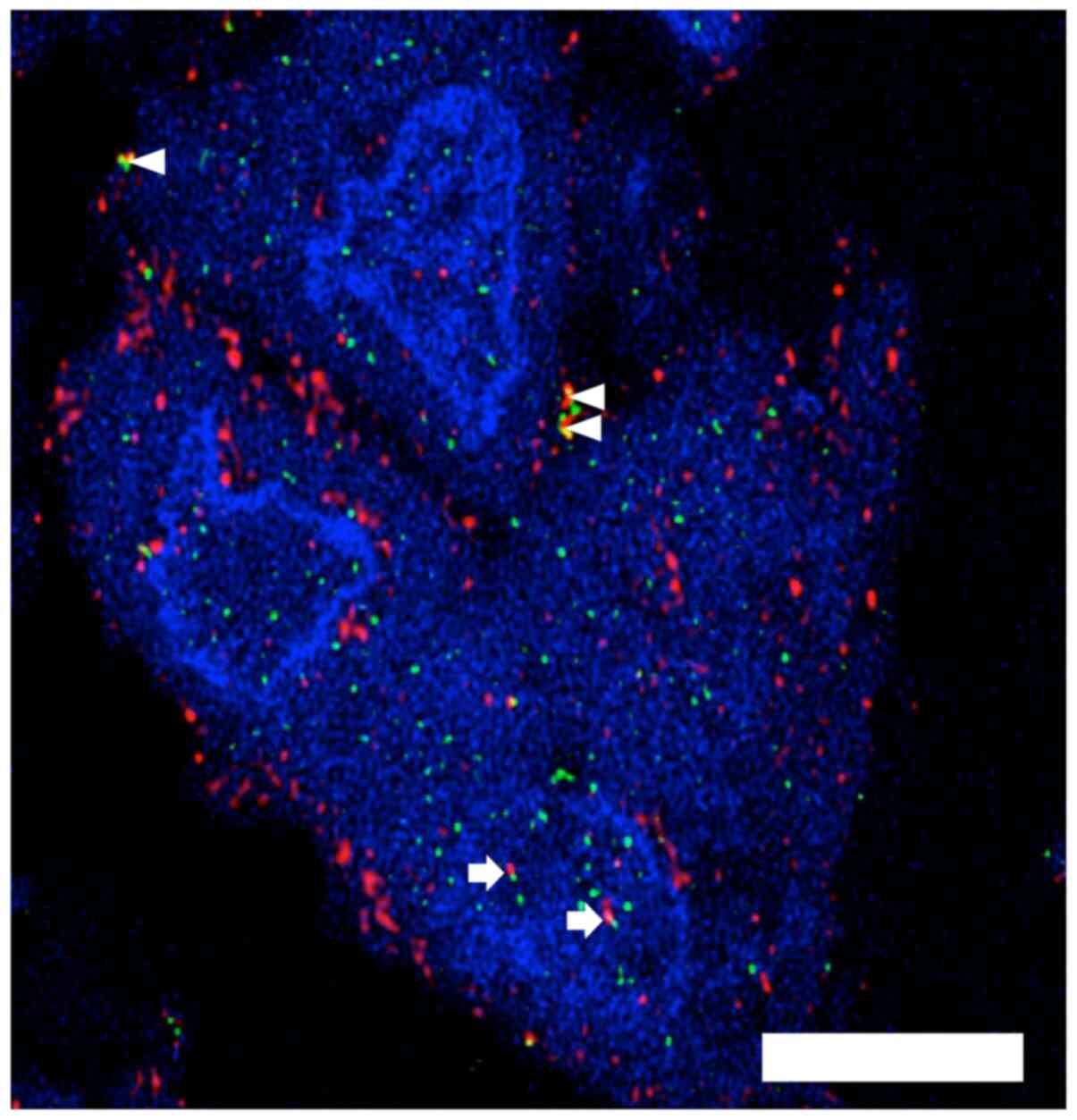
Confirmation of intra-nuclear MT1-MMP
in patient tumour cells using immunofluorescence and confocal
microscopy
A patient sarcoma specimen with evidence of possible
intra-nuclear MT1-MMP on conventional immunohistochemistry was
selected from our archive. Confocal microscopy demonstrates the
presence of MT1-MMP in the nucleus of most of the tumour cells in
the representative image along with evidence of MT1-MMP and HIF-2α
co-expression in the cytoplasm and the nucleus (Fig. 4).
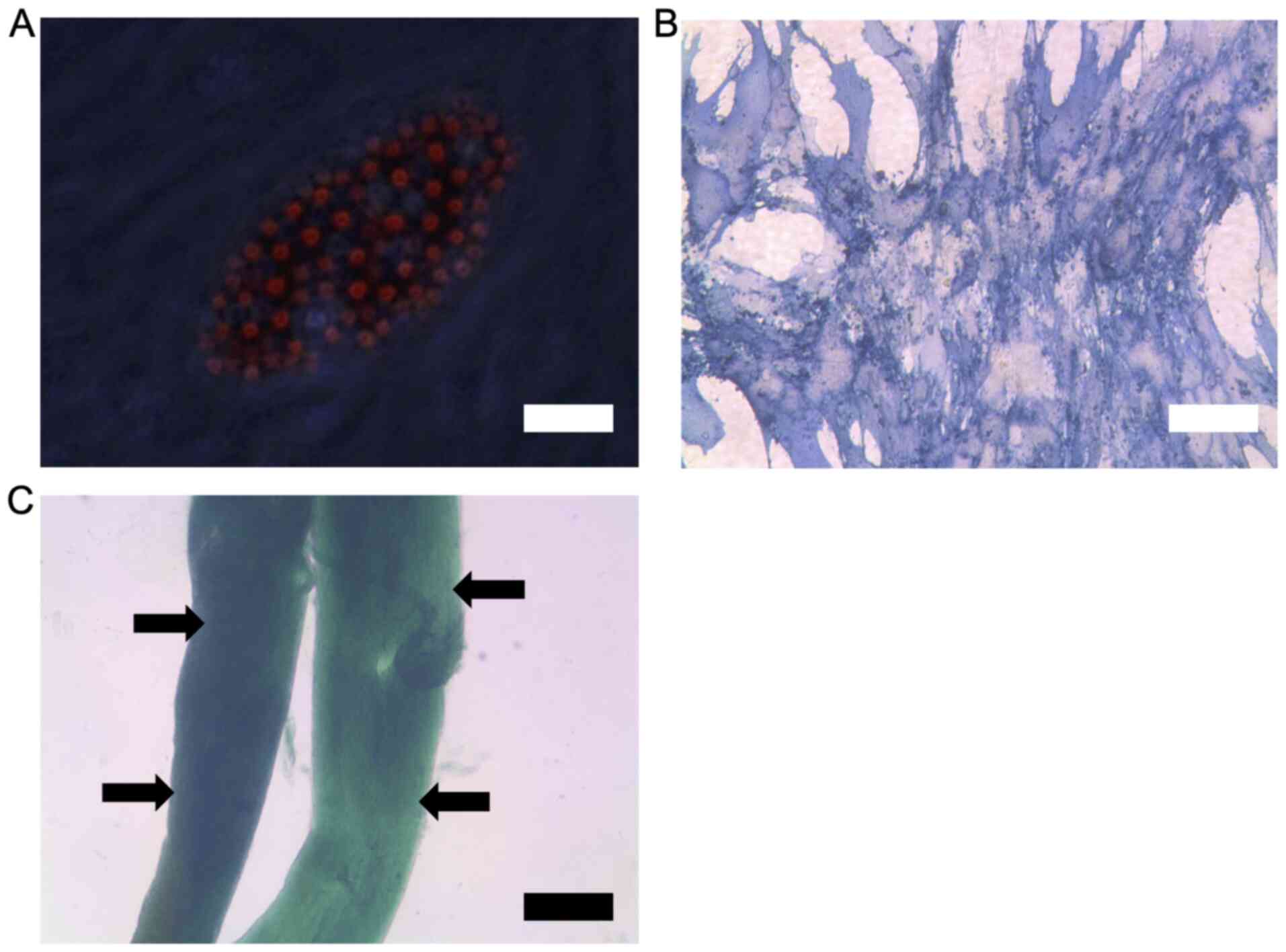
MSC characterisation using FACS to
demonstrate positive surface expression of stem cell markers
FACS data showed that the human derived MSCs had
positive surface expression for MSC-associated surface markers
CD73, CD90 and CD105 (data readily available upon request).
Confirmation of stem cell phenotype
using differentiation assays
The data demonstrates that the human derived MSCs
were able to differentiate into adipocytes, osteoblasts and
chondrocytes in culture. Successful tri-lineage differentiation was
confirmed by the relevant staining for lineage markers followed by
microscopy (Fig. 5). From the
results, adipocytes show lipid vesicles upon oil red staining
(Fig. 5A), osteoblasts show positive
staining for alkaline phosphatase (Fig.
5B) and chondrocytes show clear formation of cartilage layers
upon Alcian blue staining (Fig.
5C).
Discussion
MT1-MMP has been extensively investigated as a
potential target for carcinoma therapy with several extracellular
roles described including the facilitation of cancer cell invasion,
migration, angiogenesis and metastasis (6,8,9). These features are all relevant to
osteosarcoma, particularly as this type of cancer metastasises via
the haematogenous route. Whilst the extracellular role of MT1-MMP
is important and has been identified as a therapeutic target, the
intra-nuclear presence of MT1-MMP has also previously been reported
in hepatocellular carcinoma and therefore warrants further
investigation (10). Our study
confirms the intra-nuclear presence of MT1-MMP and provides
evidence that there is a protein-protein interaction with HIF-2α in
the nucleus, particularly in osteosarcoma and prostate carcinoma
cells under hypoxic conditions. Furthermore, there is evidence that
intra-nuclear MT1-MMP in macrophages results in transactivation of
gene networks (11). The
significance of this could be important because the nuclear
presence of MT1-MMP in cancer cells may alter gene expression.
Therefore, improved understanding of the intra-nuclear role of
MT1-MMP may open up novel avenues for targeting the diverse
activities of this enzyme. This is particularly relevant for
osteosarcoma which severely lacks additional therapeutic
options.
Acknowledgements
Not applicable.
Funding
This work was supported by the Newcastle upon Tyne
NHS Trust Healthcare Charity (grant no. BH111959).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors of this paper contributed significantly
to the work stated and in the writing of this manuscript. CDC, EJH,
HAT, KJR and KSR drafted the manuscript, designed the study and
planned the experiments. CDC, EJH, ZG, JB, MAB and SN were involved
in undertaking the majority of the laboratory experiments and data
collection, including the imaging and analyses. EJH, ZG, CHG, CNR
and JL made substantial contributions to the analysis and
interpretation of the data. CDC, CHG, CNR, JL and KSR were involved
in writing, processing the figures and editing the manuscript. All
authors discussed the results and commented on the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Appropriate informed consent was obtained and
approved by the Newcastle and North Tyneside 1 Research Ethics
Committee (REC Reference no. 17/NE/0361).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Longhi A, Errani C, De Paolis M, Mercuri M
and Bacci G: Primary bone osteosarcoma in the pediatric age: State
of the art. Cancer Treat Rev. 32:423–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Allison DC, Carney SC, Ahlmann ER,
Hendifar A, Chawla S, Fedenko A, Angeles C and Menendez LR: A
meta-analysis of osteosarcoma outcomes in the modern medical era.
Sarcoma. 2012:7048722012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Castro-Castro A, Marchesin V, Monteiro P,
Lodillinsky C, Rossé C and Chavrier P: Cellular and molecular
mechanisms of MT1-MMP-dependent cancer cell invasion. Annu Rev Cell
Dev Biol. 32:555–576. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bonfil RD, Dong Z, Trindade Filho JC,
Sabbota A, Osenkowski P, Nabha S, Yamamoto H, Chinni SR, Zhao H,
Mobashery S, et al: Prostate cancer-associated membrane type
1-matrix metalloproteinase: A pivotal role in bone response and
intraosseous tumor growth. Am J Pathol. 170:2100–2111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uchibori M, Nishida Y, Nagasaka T, Yamada
Y, Nakanishi K and Ishiguro N: Increased expression of
membrane-type matrix metalloproteinase-1 is correlated with poor
prognosis in patients with osteosarcoma. Int J Oncol. 28:33–42.
2006.PubMed/NCBI
|
|
6
|
Poincloux R, Lizárraga F and Chavrier P:
Matrix invasion by tumour cells: A focus on MT1-MMP trafficking to
invadopodia. J Cell Sci. 122:3015–3024. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakamoto T, Weng JS, Hara T, Yoshino S,
Kozuka-Hata H, Oyama M and Seiki M: Hypoxia-inducible factor 1
regulation through cross talk between mTOR and MT1-MMP. Mol Cell
Biol. 34:30–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sabeh F, Shimizu-Hirota R and Weiss SJ:
Protease-dependent versus -independent cancer cell invasion
programs: Three-dimensional amoeboid movement revisited. J Cell
Biol. 185:11–19. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perentes JY, Kirkpatrick ND, Nagano S,
Smith EY, Shaver CM, Sgroi D, Garkavtsev I, Munn LL, Jain RK and
Boucher Y: Cancer cell-associated MT1-MMP promotes blood vessel
invasion and distant metastasis in triple-negative mammary tumors.
Cancer Res. 71:4527–4538. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ip YC, Cheung ST and Fan ST: Atypical
localization of membrane type 1-matrix metalloproteinase in the
nucleus is associated with aggressive features of hepatocellular
carcinoma. Mol Carcinog. 46:225–230. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimizu-Hirota R, Xiong W, Baxter BT,
Kunkel SL, Maillard I, Chen XW, Sabeh F, Liu R, Li XY and Weiss SJ:
MT1-MMP regulates the PI3Kδ·Mi-2/NuRD-dependent control of
macrophage immune function. Genes Dev. 26:395–413. 2012. View Article : Google Scholar : PubMed/NCBI
|