Introduction
Breast cancer is the most common cancer diagnosed
among women in the US and the second leading cause of cancer death
among women around the world (1).
Breast tumors are heterogenous, exhibiting notable phenotypic
diversity. Through molecular analysis and gene profiling, breast
tumors are subclassified into three types: HER2 Positive, estrogen
receptor (ER) positive and basal-like breast cancer (2).
HER2, encoded by the ERBB2 gene, is a member of the
human epidermal growth factor receptor family of proteins (3). HER2, a receptor tyrosine kinase,
regulates internal cell activities such as cell proliferation and
survival (4). When HER2 is
overexpressed, it leads to the overactivation of cellular pathways
such as the PI3K and the MAPK pathways, which are known to be
involved in tumorigenesis (5). As
for the estrogen receptor, once activated, it functions as a
transcription factor leading to the transcription of a number of
genes involved in cell proliferation and survival, including c-fos,
insulin-like growth factor binding protein 4 and E2F1 (6). Lastly, progesterone increases breast
cell proliferation through the activation of the DNA replication
machinery (7). All of these
characteristics have led to the high occurrence of aggressive,
invasive and metastatic profiles of breast cancer, with limited
targeted therapeutic options (8).
Cellular motility is a structured process involved
in inflammation, embryogenesis, migration and invasion of cells
(9). Being a vital process, cell
motility is tightly regulated by several proteins, including the
Rho family of small GTPases. These consist of 22 members grouped
into subfamilies according to their sequence homology (10,11). Rho
GTPases are molecular switches that play an important role in
regulating the dynamics of the actin cytoskeleton, impacting
cellular polarity, adhesion and invasion (12,13).
Rho GTPases switch between an active GTP-bound form
and an inactive GDP-bound form. This is regulated by upstream
effectors such as guanine exchange factors (GEFs) and GTPase
activating proteins (GAPs). GEFs are nucleotide exchange factors
that catalyze the dissociation of GDP and its exchange to GTP,
leading to the activation of the Rho GTPase. GAPs are
GTPase-activating proteins, which activate the intrinsic GTPase
activity of the Rho GTPase, leading to the inactivation of the
protein (14,15).
The MAPK family of proteins includes three kinase
types: Extracellular regulated kinases (ERKs), the stress-activated
protein kinases p38 and JNKs (16).
The Raf/MEK/ERK pathway is a signal transduction pathway that
relays signals from cell surface receptors to transcription
factors, therefore regulating gene expression. After activation,
the small GTPase Ras recruits and phosphorylates Raf (MAP3K)
(17). Successively, Raf
phosphorylates a second kinase, MEK (MAP2K), which then
phosphorylates two proteins, ERK1 and ERK2; after phosphorylation,
ERK1/2 are translocated to the nucleus, where they phosphorylate
different transcription factors, altering gene expression (17). The translocation of ERK into the
nucleus affects numerous cell processes, such as proliferation,
cell cycle progression, adhesion, invasion, survival, metabolism
and differentiation (17). The most
notable targets of ERK1/2 are c-Myc, c-Fos, Elk1 and c-Jun
(16,17).
Anthrax lethal toxin (LeTx) is a binary toxin
produced by the Gram-positive bacteria, Bacillus anthracis
(18). B. anthracis contains
two virulence encoding plasmids: pXO1 And pXO2. PXO1 encodes for
three factors: Protective agent (PA), lethal factor (LF) and edema
factor. Although separately non-toxic, a combination of PA and LF
generates LeTx (19). PA (83 kDa)
binds to the host cell surface receptors, tumor endothelial marker
8 (TEM8) or capillary morphogenesis gene 2 (CMG2), with TEM8
demonstrating increased expression in breast tumor tissue (18,19).
Upon binding to TEM8 or CMG2, PA is cleaved by furin-like
proteases, releasing a 20 kDa amino-terminal fragment and yielding
a 63-kDa active PA fragment (PA63) (18,19).
PA63 then oligomerizes, forming a ring-shaped, pre-pore heptameric
or octameric structure (18). The
formation of the PA63 pre-pore complex allows the binding of three
to four LF molecules, depending on whether the PA63 pre-pore
complex is in the heptameric or octameric form, respectively
(18,19). Binding of LF initiates
internalization of the complex into the cell through
receptor-mediated endocytosis; upon acidification of the endosome,
PA63 undergoes a conformational change into a mature pore complex
that subsequently translocates LF into the cytosol (18–20). LF
is a matrix metalloproteinase that cleaves and inactivates all
MEKs, thus inhibiting all three branches of the MAPK pathway
(20).
Previous studies have investigated LeTx as a
potential therapeutic target and abundant literature exists
describing the selective antitumor potential of LeTx in a number of
tumor types, including melanoma and acute myeloid leukemia, both
in vitro and in vivo (21–26). The
tumor selectivity of LeTx derives from the fact that the majority
of normal cells, with the exception of endothelial cells and
macrophages, are not sensitive to the inhibition of the MAPK
pathway (25). Hence, this pathway
is not essential for the survival of the majority of normal cells.
Moreover, not all cancer cells are sensitive to the inhibition of
the MAPK pathways. For example, several studies have shown that
melanoma cells that carry N-Ras mutations are not sensitive to the
LF-mediated inhibition of the MAPK pathway, while those carrying a
V600E B-Raf mutation are sensitive to the inhibition of this
pathway (25,27). Previously, it was also demonstrated
that LeTx successfully decreased cellular motility and invasion of
glioblastoma cells by increasing their adhesion via an increase in
RhoA activity (28).
The aim of the present study was to investigate the
effect of LeTx, a MEK inhibitor, on breast cancer cell motility and
invasion. First, the effect of LeTx on the MAPK pathway was
examined through analyzing the phosphorylation status of ERK.
Furthermore, the effect of LeTx on the migration, adhesion and
invasion of MDA-MB-231, a highly aggressive and invasive type of
breast cancer cell line, was studied.
Materials and methods
Cell culture
The human epithelial triple negative breast cancer
cell line MDA-MB-231 was cultured adherently in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS) and 100 units penicillin/streptomycin (all Invitrogen; Thermo
Fisher Scientific, Inc.) at 37°C and 5% CO2 in a
humidified chamber.
Drug concentrations
We have previously shown that the MDA-MB-231 cell
line was not sensitive to LeTx, hence its viability and
proliferation were not affected by the maximum LeTx concentration
used in the present assay (Abi-Habib et al, unpublished
data). Therefore, a concentration of LeTx consisting of
10−8 M PA and 10−9 M LF was used for cell
treatment for 24 h. Cells were also treated with the MEK1/2
inhibitor U0126 (Sigma-Aldrich; Merck KGaA) at a final
concentration of 50 µM for 24 h.
Pull-down assay
Cell lysates were collected from breast cancer cells
following treatment with LeTx (10−8 M PA/10−9
M LF) or U0126 (50 µM) for 24 h. The RhoA/Rac1/Cdc42 Activation
Assay Combo kit (cat. no. STA-405; Cell Biolabs, Inc.) was used for
a pull-down assay following the manufacturer's instructions.
Briefly, the cells were lysed with lysis buffer (25 mM HEPES, 1%
Igepal, 150 mM NaCl, 10 mM MgCl2, 10% glycerol, 1 mM
EDTA, 1 mM NaVO4, 20 mM NaF, 1 mM PMSF, 100 lg/ml
aprotinin and 5 lM leupeptin). Lysates were cleared by
centrifugation for 1 min at 1,500 × g at 4°C and incubated with
GST-RBD beads (20 µg) or GST-CRIB beads (20 µg) provided in the
aforementioned kit for 1 h at 4°C with gentle shaking. Then,
samples were centrifuged for 2 min at 1,000 × g at 4°C, and the
pellet was washed 3 times with PBS. Beads alone samples (without
cell lysate) were used as a negative control and the total cell
lysate (before incubation with the beads) were blotted for total
RhoA, Cdc42 and β-actin.
Western blotting
Proteins were extracted using the 1X lysis buffer
(25 mM HEPES, 1% Igepal, 150 mM NaCl, 10 mM MgCl2, 10%
glycerol, 1 mM EDTA, 1 mM NaVO4, 20 mM NaF, 1 mM PMSF,
100 lg/ml aprotinin and 5 lM leupeptin). Protein concentrations
were determined using the Bradford assay (Bio-Rad Laboratories,
Inc.). Proteins (25 µg/ml) were separated by 9% SDS-PAGE and
transferred onto PVDF membranes. Membranes were blocked with 5% BSA
(Sigma-Aldrich; Merck KGaA) in PBS for 1 h at room temperature and
then incubated with the corresponding primary antibodies overnight
at 4°C and HRP-conjugated secondary antibodies for 1 h at room
temperature the following day. GTP-RhoA and GTP-Cdc42 were detected
using anti-RhoA (1:500) or anti-Cdc42 (1:500), provided in the
aforementioned RhoA/Rac1/Cdc42 Activation Assay Combo kit. Mouse
monoclonal anti-ERK (1:200; cat. no. ab54230), mouse monoclonal
anti-phospho-Erk1 (pT202/pY204) + phospho-Erk2 (pT185/pY187)
(1:200; cat. no. ab50011) and rabbit polyclonal anti-β-actin
antibodies (1:500; cat. no. ab8227) were purchased from Abcam.
Anti-rabbit (cat. no. W4011) and anti-mouse (cat. no. W4021)
HRP-conjugated secondary antibodies (both 1:1,000) were obtained
from Promega Corporation. Finally, the bands were visualized with
chemiluminescent reagent ECL (GE Healthcare Life Sciences) using
the Chemidoc imaging system (Bio-Rad Laboratories, Inc.). The
protein expression levels were compared by densitometry using
ImageJ v1.51k (National Institutes of Health) (29).
Wound healing
Cells were cultured to confluence on culture plates.
After 24 h, a wound was made in the monolayer with a sterile
pipette tip. Cells were then washed twice with PBS to remove debris
and new medium was added. Images were captured at 0 and 72 h. Wound
widths were measured at 13 different points for each wound, and the
average rate of wound closure was calculated (in µm/h) using ImageJ
(29). The assay was done using
infinity-corrected optics on a Zeiss Observer Z1 microscope
supplemented with a computer-driven Roper cooled CCD camera and
operated by Zen Blue 2.5 software (all Zeiss AG).
Random cell motility assay
(time-lapse)
Cells treated as indicated were imaged randomly
moving in DMEM (with 10% FBS and 1% Penicillin/Streptomycin) in
their respective plates that were placed on a heated stage (37°C)
with controlled CO2 levels (5%). Cell images were
collected every min for 2 h using a 20× objective lens on the Zeiss
Observer Z1 microscope. The total distance traveled by the cells
was quantified using the ROI tracker plugin in ImageJ (29). The rate (µm/min) of at least 10
randomly selected cells per condition was then calculated by
dividing the total distance traveled over time. Finally, the
difference in cell motility was also expressed as fold change of
the treated cells normalized to the control (29).
Adhesion assay
Collagen Solution, Type I (Sigma-Aldrich; Merck
KGaA) was used to coat 96-well plates overnight at 37°C then washed
with washing buffer (0.1% BSA in RPMI-1620 AQ media). The plates
were then blocked with 0.5% BSA in RPMI-1620 AQ media at 37°C in a
CO2 incubator for 1 h. This was followed by washing the
plates and chilling them on ice. Meanwhile, the cells were
trypsinized and counted to 4×105 cells/ml. In total, 50
µl of cells were added in each well and incubated at 37°C in a
CO2 incubator for 30 min. The plates were then shaken
and washed three times with PBS Cells were then fixed with 4%
paraformaldehyde at room temperature for 10 min, washed and stained
with crystal violet (5 mg/ml in 2% ethanol) for 10 min at room
temperature. Following the staining, the plates were washed
extensively with water and left to dry completely. Crystal violet
was solubilized by incubating the cells with 2% SDS for 30 min at
room temperature. The absorption of the plates was read at 550 nm
using a Varioskan Flash Multimode reader (Thermo Fisher Scientific,
Inc.).
Invasion assay
Cells were treated with LeTx or left untreated as
control, and the invasion assay was performed following the
treatment period using the collagen-based invasion assay kit (cat.
no. ECM551; EMD Millipore) according to the manufacturer's
instructions. Briefly, 24 h prior to the assay, cells were starved
with serum-free medium. Cells were harvested, centrifuged at 600 ×
g for 5 min at 4°C and then resuspended in quenching medium
(without serum). Cells were then counted using a hemocytometer and
brought to a concentration of 1×106 cells/ml. In the
meantime, the kit inserts (collagen-coated 8-µm pore size
polycarbonate membrane) were rehydrated with prewarmed 300 µl of
serum-free medium for 30 min at room temperature. After
rehydration, 250 µl of medium was removed from the inserts, and 250
µl of cell suspension was added. Inserts were then placed in a
24-well plate, and 500 µl of complete medium (with 10% serum) was
added to the lower wells. Plates were incubated for 48 h at 37°C in
a CO2 incubator. Following the incubation period,
inserts were stained for 20 min at room temperature with 400 µl of
cell stain provided with the kit. The stain was then extracted with
extraction buffer (also provided). The extracted stain (100 µl) was
then transferred to a 96-well plate suitable for colorimetric
measurement using a plate reader. Optical density was then measured
at 560 nm.
Statistical analysis
The results reported represent mean values from
three independent experiments. The error estimates are given as ±
SEM. The P-values were calculated by a one-way ANOVA or unpaired
t-test. Tukey's post hoc test was used for comparing all possible
group pairings to check if the changes observed in the results were
significant. P<0.05 was considered to indicate a statistically
significant difference.
Results
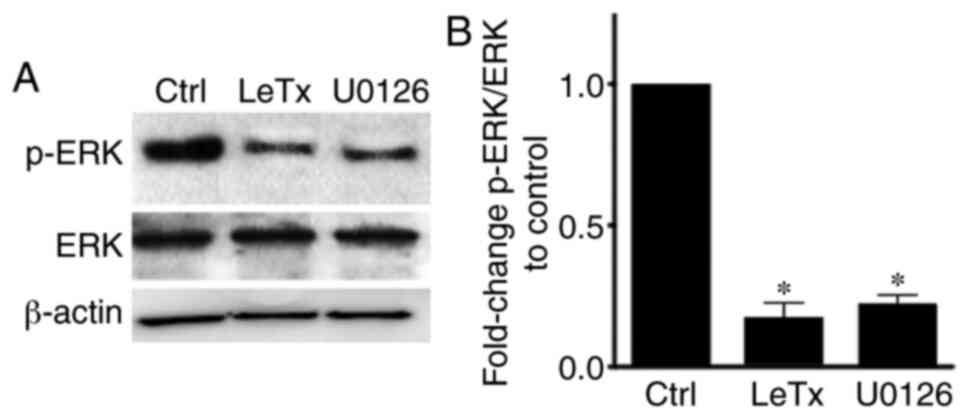
LeTx treatment leads to a decrease in
phosphorylated (p-)ERK in breast cancer cells
In the ERK MAPK module, MEK1/2 (a MAPK kinase)
phosphorylates and subsequently activates ERK (a MAP kinase)
(20,21). LeTx is known to cleave MEK by
degrading it in order to inhibit the MAPK pathway in cells
(26). The present results showed a
decrease in the level of p-ERK upon treatment of MBA-MD-231 cells
with LeTx and the MEK1/2 inhibitor U0126 in comparison with
control, while leaving the total ERK expression intact (Fig. 1).
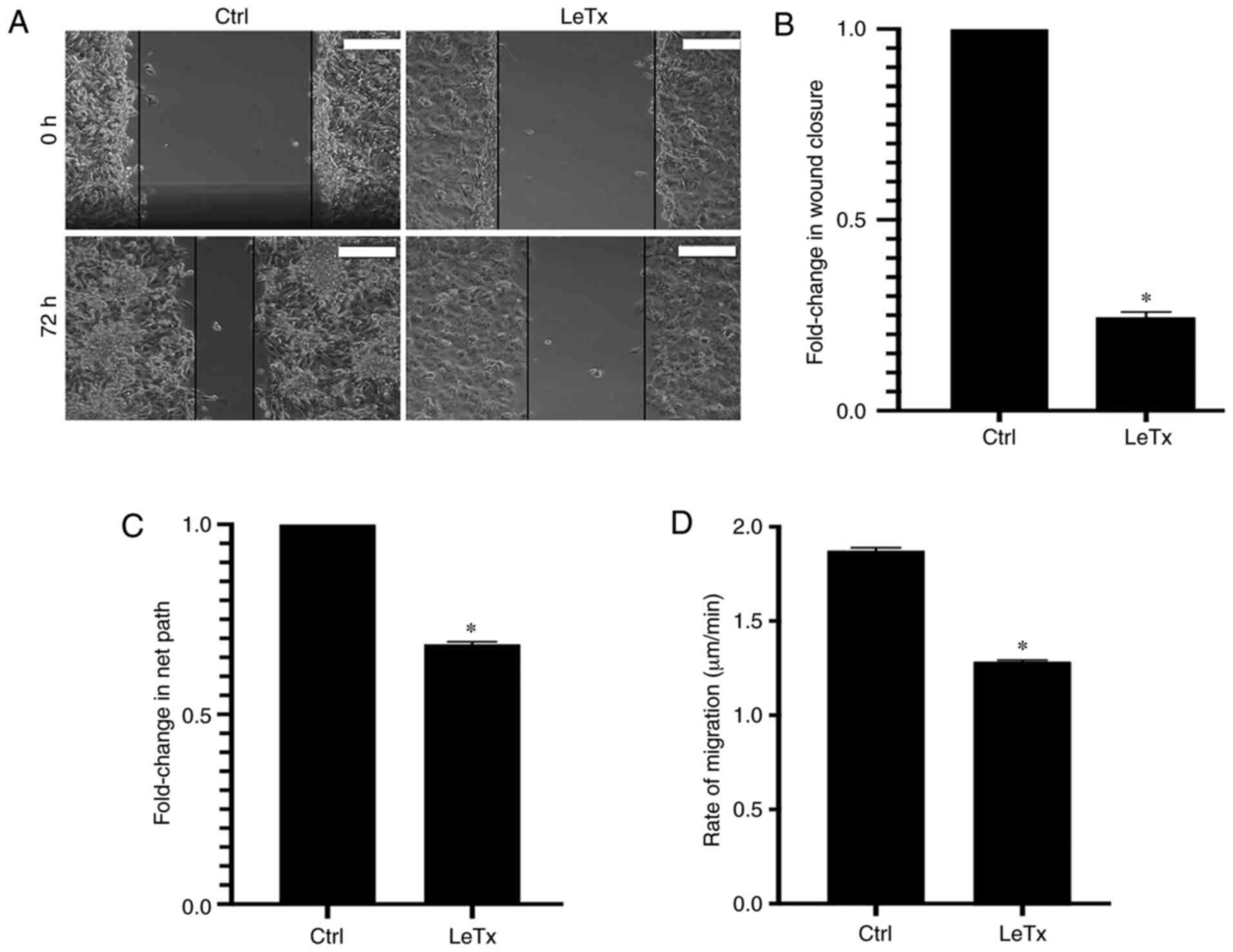
LeTx treatment leads to a decrease in
migration in breast cancer cells
In order to study the effect of LeTx on MDA-MB-231
cell migration, a 2D wound closure assay was performed. The rate of
wound closure was calculated over the span of 72 h. Treatment with
LeTx caused a decrease in the rate of wound closure from 3.8 to 0.9
µm/h (~2.4-fold decrease in wound closure rate) (Fig. 2A and B).
In order to eliminate the confounding effect of cell
proliferation, a time lapse examination of individual cells
undergoing random migration in serum was also performed (29). Consistently with the results obtained
in the wound healing, a 0.75-fold decrease in the net path and rate
of migration was observed in cells treated with LeTx (Fig. 2C and D; Videos S1 and S2).
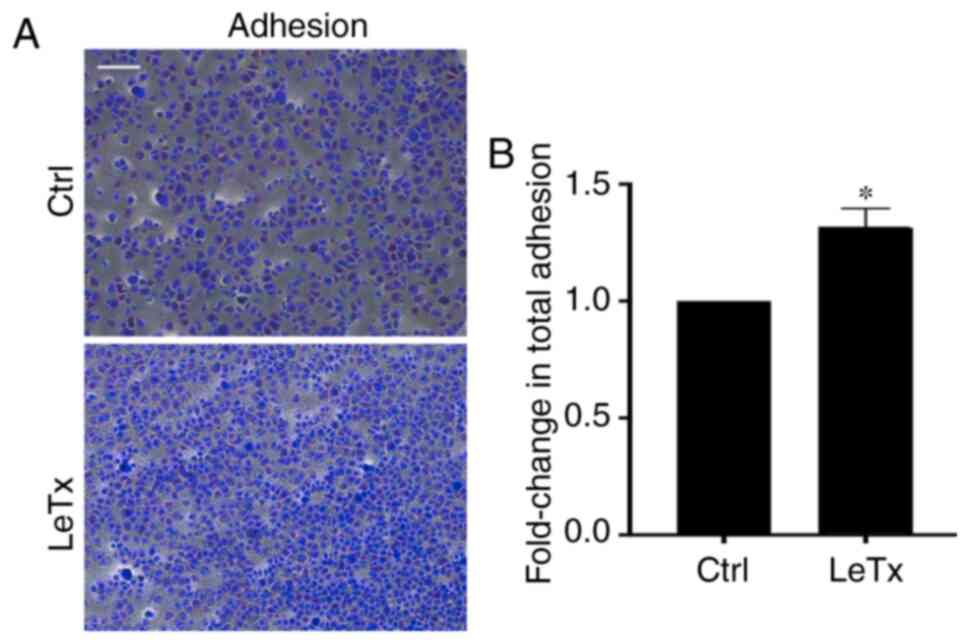
LeTx treatment leads to an increase in
adhesion in breast cancer cells
Since treated cells exhibited a reduction in total
net path and rate of migration, it was hypothesized that this
reduction in migration may be due to an increase in MDA-MB-231
adhesion to the underlying matrix, as previously seen in another
tumor model (28). Indeed, cells
treated with LeTx displayed a ~0.7-fold increase in adhesion
(Fig. 3).
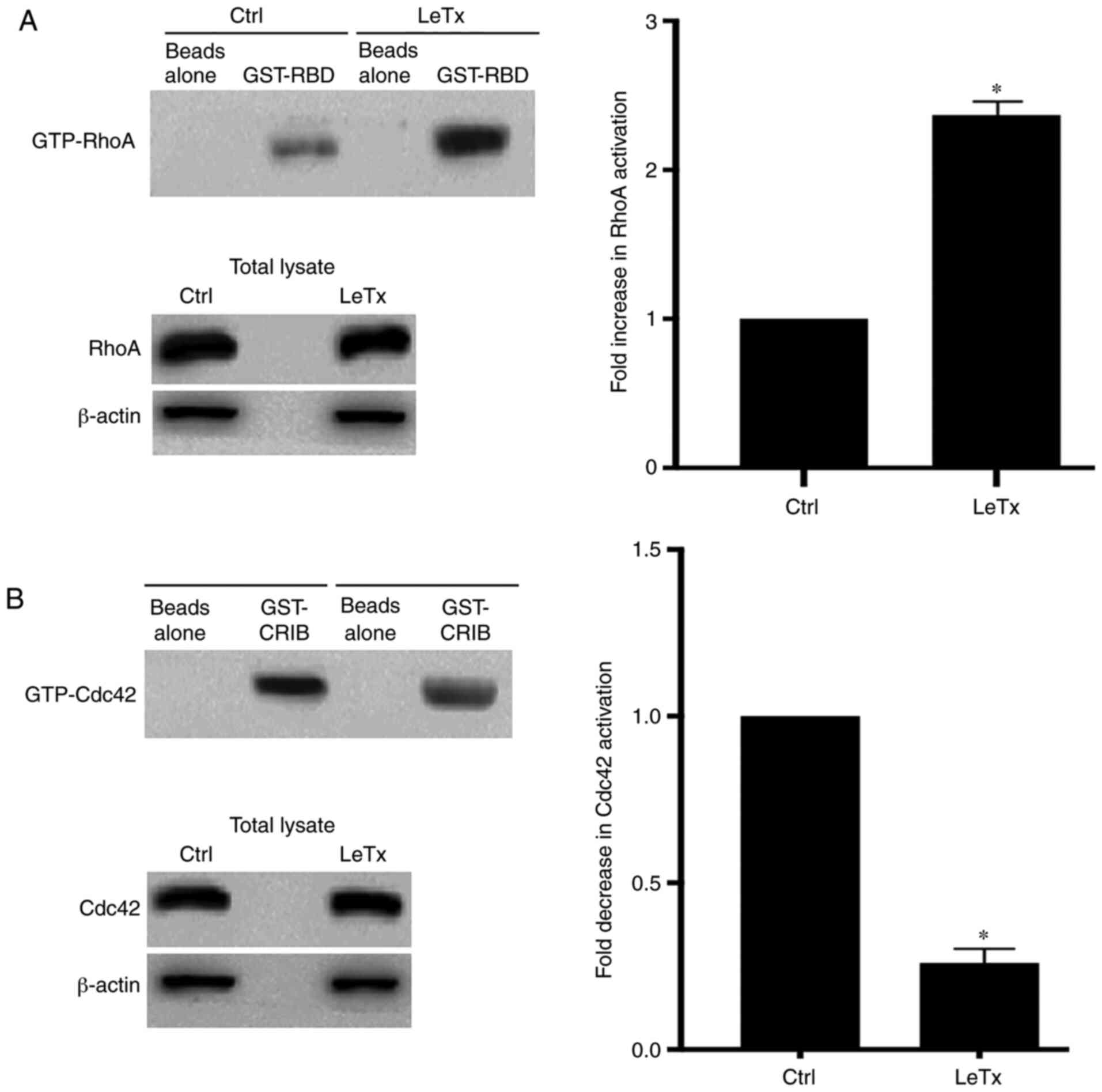
LeTx treatment leads to an alteration
of Rho GTPase activation
Since LeTx-treated MBA-MD-231 cells exhibited an
increase in cell adhesion, it was analyzed whether this was due to
an increase in RhoA activation. RhoA at the leading edge of cells
is needed for the maturation of the focal adhesions that anchor the
cell to the underlying extracellular matrix (29). Performing a pull-down assay, using
GST-RBD and GST-PAK beads, revealed an increase in RhoA activation
in the LeTx-treated cells, which is consistent with the increase in
total adhesion (Fig. 4A). In
addition, there was a decrease in Cdc42 activation, which might
additionally explain the decrease in cell migration in response to
LeTx treatment (Fig. 4B).
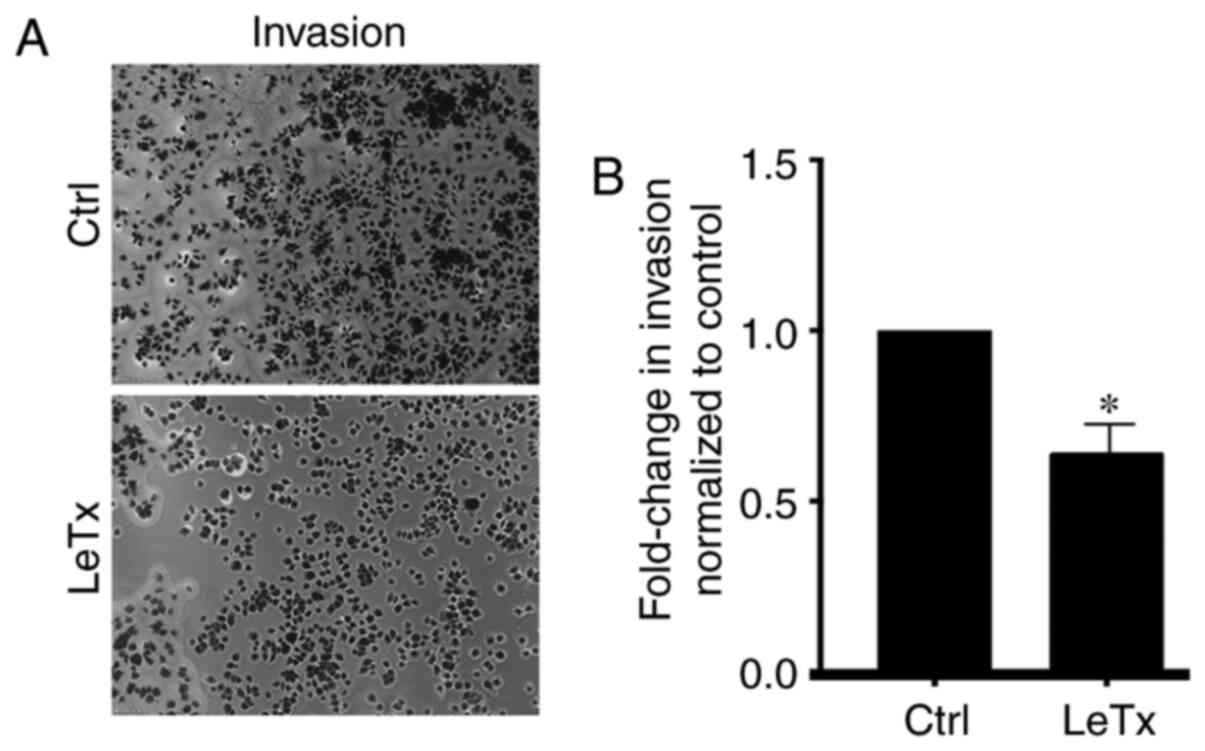
LeTx treatment leads to a decrease in
breast cancer invasion
Cdc42 is a potent regulator of invadopodia and
cancer cell invasion (30).
Accordingly, having detected an inhibition of Cdc42 in response to
LeTx treatment in these cells, a decrease in invasion was expected.
Indeed, the results showed a decrease in invasion upon treating
cells with LeTx (Fig. 5A and B).
Collectively, these results consolidated LeTx as a potential potent
therapeutic agent to target breast cancer migration and invasion
through the inhibition of cell migration regulators.
Discussion
Metastatic lesions attack vital organs leading to a
poor prognosis of patients with breast cancer (31). The MAPK pathway has been described to
play a vital role in cellular proliferation, survival and
migration. ERK, a member of the MAPK cascade has been shown to lead
to an increased cell proliferation upon overactivation in a number
of different cancer types, including pancreatic cancer, colon
cancer, melanoma and breast cancer (32,33).
Previous studies have established that inactivation of the MAPK
pathway could lead to the inhibition of cancer cell survival as
well as the inhibition of migration and invasion (28,34).
LeTx is a binary toxin composed of two proteins: PA
And LF. LF is a zinc-activated metalloprotease that inhibits the
MAPK pathway by cleaving MEKs (35,36).
Based on the ability of LeTx to cause cell death or inhibition of
migration and invasion due to MAPK inhibition, breast cancer cells
were treated with LeTx, and the migratory and invasive capabilities
of the cells were examined in the present study.
Previous research has shown the ability of LeTx to
impair migration of cells and disrupt their polarity (37). The present study treated breast
cancer cells with LeTx, which led to a decrease in 2D cell motility
as shown in the quantification of the wound healing assay.
Consequently, it was suspected that there was a relationship
between LeTx and an increase in cellular adhesion resulting in a
decreased net path and rate of migration. After treatment with the
toxin, cells exhibited an increase in adhesion as shown in the
adhesion assay. We have previously shown that overexpression of
RhoA leads to an increase in cell adhesion in breast cancer cells,
among other tumor types (28,29,38–41).
RhoA regulates focal adhesion dynamics via its downstream effector
Rho-associated protein kinase 1 and the formation of stress fibers
needed for migration (28,38–41). At
focal contact points with the underlying extracellular matrix,
cells recruit RhoA, which in turn recruits actin to form premature
adhesions (28,40–43).
RhoA activity then mediates the maturation of these contacts into
mature adhesions (40). In parallel,
as previously mentioned, ERK, through Fos-related antigen 1, leads
to the inhibition of RhoA (31,42).
Thus, the disruption of the inhibition of the MAPK pathway via LeTx
should relieve inhibition of RhoA activation. The current study
reported that, indeed, the increased adhesion phenotype was due to
an increase in RhoA activation upon LeTx treatment, in addition to
a decrease in Cdc42 activation, which might be the mechanism behind
the decrease in cell migration.
Lastly, the present study explored the effect of
LeTx on invasion of MDA-MB-231 cells. Treating the cells with LeTx
led to a decrease in invasion. Cdc42 is known to play a role in
invasion of cells by contributing to the formation of invadopodia
by activating actin-related protein 2/3 via Neural Wiskott-Aldrich
syndrome protein and by exerting an effect on matrix
metalloproteinases, which is necessary for their translocation to
invadopodia (43,44). Having observed decreased Cdc42
activation in treated cells in pull-down assays, it was
hypothesized that the effect exerted by LeTx on invasion was
through Cdc42.
Overall, the present study has demonstrated the
effect of LeTx on the migration and invasive capabilities of breast
cancer cells via the MAPK pathway. By inhibiting MAPK, cells
demonstrated decreased motility, increased adhesion and decreased
invasion, which are characteristics attributed to cancer metastasis
(45). The current data has been
indicative of the importance of Rho GTPases in these cellular
processes and offers an insight on the potential use of LeTx in
therapeutic approaches for breast cancer. Although few studies have
linked Rho GTPases and the MAPK pathway (46,47), the
exact mechanism involved and the crosstalk between these requires
further investigation. Future studies should investigate the
effectiveness of LeTx treatment on the inhibition of metastasis
in vivo.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Dr Isabelle Fakhoury
(Department of Natural Sciences, School of Arts and Sciences,
Lebanese American University, Beirut, Lebanon) for help with the
experiments.
Funding
The present study was supported by intramural
funding at the Lebanese American University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DEC and MAH performed the experiments and analyzed
the data. RAH and MES are the principal investigators on the
project who designed and supervised the project, wrote the
manuscript and provided the resources. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeSantis CE, Fedewa SA, Goding Sauer A,
Kramer JL, Smith RA and Jemal A: Breast cancer statistics, 2015:
Convergence of incidence rates between black and white women. CA
Cancer J Clin. 66:31–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Callahan R and Hurvitz S: Human epidermal
growth factor receptor-2-positive breast cancer: Current management
of early, advanced, and recurrent disease. Curr Opin Obstet
Gynecol. 23:37–43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sauter G, Lee J, Bartlett JMS, Slamon DJ
and Press MF: Guidelines for human epidermal growth factor receptor
2 testing: Biologic and methodologic considerations. J Clin Oncol.
27:1323–1333. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoeferlin LA, E Chalfant C and Park MA:
Challenges in the treatment of triple negative and
HER2-overexpressing breast cancer. J Surg Sci. 1:3–7.
2013.PubMed/NCBI
|
|
6
|
Fragomeni SM, Sciallis A and Jeruss JS:
Molecular subtypes and local-regional control of breast cancer.
Surg Oncol Clin N Am. 27:95–120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Daniel AR, Hagan CR and Lange CA:
Progesterone receptor action: Defining a role in breast cancer.
Expert Rev Endocrinol Metab. 6:359–369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Toft DJ and Cryns VL: Minireview:
Basal-Like breast cancer: From molecular profiles to targeted
therapies. Mol Endocrinol. 25:199–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hanna S and El-Sibai M: Signaling networks
of Rho GTPases in cell motility. Cell Signal. 25:1955–1961. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zandvakili I, Lin Y, Morris JC and Zheng
Y: Rho GTPases: Anti- or pro-neoplastic targets? Oncogene.
36:3213–3222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Al-Koussa H, Atat OE, Jaafar L, Tashjian H
and El-Sibai M: The role of Rho GTPases in motility and invasion of
glioblastoma cells. Anal Cell Pathol (Amst).
2020:92740162020.PubMed/NCBI
|
|
12
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zgheib P, Daher CF, Mroueh M, Nasrallah A,
Taleb RI and El-Sibai M: Daucus carota pentane/diethyl ether
fraction inhibits motility and reduces invasion of cancer cells.
Chemotherapy. 60:302–309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nasrallah A, Saykali B, Al Dimassi S,
Khoury N, Hanna S and El-Sibai M: Effect of StarD13 on colorectal
cancer proliferation, motility and invasion. Oncol Rep. 31:505–515.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Perry JA and Maddox AS: Uncovering the
secret life of Rho GTPases. Elife. 8:e532762019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li L, Zhao GD, Shi Z, Qi LL, Zhou LY and
Fu ZX: The Ras/Raf/MEK/ERK signaling pathway and its role in the
occurrence and development of HCC. Oncol Lett. 12:3045–3050. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shapiro P: Ras-MAP kinase signaling
pathways and control of cell proliferation: Relevance to cancer
therapy. Crit Rev Clin Lab Sci. 39:285–330. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu S, Moayeri M and Leppla SH: Anthrax
lethal and edema toxins in anthrax pathogenesis. Trends Microbiol.
22:317–325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bachran C and Leppla S: Tumor targeting
and drug delivery by anthrax toxin. Toxins (Basel). 8:1972016.
View Article : Google Scholar
|
|
20
|
Agrawal A and Pulendran B: Anthrax lethal
toxin: A weapon of multisystem destruction. Cell Mol Life Sci.
61:2859–2865. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Frankel AE, Koo HM, Leppla SH, Duesbery NS
and Vande Woude GF: Novel protein targeted therapy of metastatic
melanoma. Curr Pharm Des. 9:2060–2066. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kassab E, Darwish M, Timsah Z, Liu S,
Leppla SH, Frankel AE and Abi-Habib RJ: Cytotoxicity of anthrax
lethal toxin to human acute myeloid leukemia cells is nonapoptotic
and dependent on extracellular signal-regulated kinase 1/2
activity. Transl Oncol. 6:25–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koo HM, VanBrocklin M, McWilliams MJ,
Leppla SH, Duesbery NS and Vande Woude GF: Apoptosis and
melanogenesis in human melanoma cells induced by anthrax lethal
factor inactivation of mitogen-activated protein kinase kinase.
Proc Natl Acad Sci USA. 99:3052–3057. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abi-Habib RJ, Singh R, Liu S, Bugge TH,
Leppla SH and Frankel AE: A urokinase-activated recombinant anthrax
toxin is selectively cytotoxic to many human tumor cell types. Mol
Cancer Ther. 5:2556–2562. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abi-Habib RJ, Urieto JO, Liu S, Leppla SH,
Duesbery NS and Frankel AE: BRAF status and mitogen-activated
protein/extracellular signal-regulated kinase kinase 1/2 activity
indicate sensitivity of melanoma cells to anthrax lethal toxin. Mol
Cancer Ther. 4:1303–1310. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duesbery NS, Resau J, Webb CP, Koochekpour
S, Koo HM, Leppla SH and Vande Woude GF: Suppression of
ras-mediated transformation and inhibition of tumor growth and
angiogenesis by anthrax lethal factor, a proteolytic inhibitor of
multiple MEK pathways. Proc Natl Acad Sci USA. 98:4089–4094. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alfano RW, Leppla SH, Liu S, Bugge TH,
Herlyn M, Smalley KS, Bromberg-White JL, Duesbery NS and Frankel
AE: Cytotoxicity of the matrix metalloproteinase-activated anthrax
lethal toxin is dependent on gelatinase expression and B-RAF status
in human melanoma cells. Mol Cancer Ther. 7:1218–1226. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Al-Dimassi S, Salloum G, Saykali B, Khoury
O, Liu S, Leppla SH, Abi-Habib R and El-Sibai M: Targeting the MAP
kinase pathway in astrocytoma cells using a recombinant anthrax
lethal toxin as a way to inhibit cell motility and invasion. Int J
Oncol. 48:1913–1920. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khalil BD, Hanna S, Saykali BA, El-Sitt S,
Nasrallah A, Marston D, El-Sabban M, Hahn KM, Symons M and El-Sibai
M: The regulation of RhoA at focal adhesions by StarD13 is
important for astrocytoma cell motility. Exp Cell Res. 321:109–122.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Al Haddad M, El-Rif R, Hanna S, Jaafar L,
Dennaoui R, Abdellatef S, Miskolci V, Cox D, Hodgson L and El-Sibai
M: Differential regulation of rho GTPases during lung
adenocarcinoma migration and invasion reveals a novel role of the
tumor suppressor StarD13 in invadopodia regulation. Cell Commun
Signal. 18:1442020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jin X and Mu P: Targeting breast cancer
metastasis. Breast Cancer (Auckl). 9 (Suppl 1):S23–S34. 2015.
|
|
32
|
Shebaby WN, Mroueh M, Bodman-Smith K,
Mansour A, Taleb RI, Daher CF and El-Sibai M: Daucus carota
pentane-based fractions arrest the cell cycle and increase
apoptosis in MDA-MB-231 breast cancer cells. BMC Complement Altern
Med. 14:3872014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang SH, Sharrocks AD and Whitmarsh AJ:
MAP kinase signalling cascades and transcriptional regulation.
Gene. 513:1–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tawil M, Bekdash A, Mroueh M, Daher CF and
Abi-Habib RJ: Wild carrot oil extract is selectively cytotoxic to
human acute myeloid leukemia cells. Asian Pac J Cancer Prev.
16:761–767. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bekdash A, Darwish M, Timsah Z, Kassab E,
Ghanem H, Najjar V, Ghosn M, Nasser S, El-Hajj H, Bazerbachi A, et
al: Phospho-MEK1/2 and uPAR expression determine sensitivity of AML
blasts to a urokinase-activated anthrax lethal toxin (PrAgU2/LF).
Transl Oncol. 8:347–357. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Duesbery NS, Webb CP, Leppla SH, Gordon
VM, Klimpel KR, Copeland TD, Ahn NG, Oskarsson MK, Fukasawa K,
Paull KD and Vande Woude GF: Proteolytic inactivation of
MAP-kinase-kinase by anthrax lethal factor. Science. 280:734–737.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
During RL, Li W, Hao B, Koenig JM,
Stephens DS, Quinn CP and Southwick FS: Anthrax lethal toxin
paralyzes neutrophil actin-based motility. J Infect Dis.
192:837–845. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hernández SE, Settleman J and Koleske AJ:
Adhesion-dependent regulation of p190RhoGAP in the developing brain
by the Abl-related gene tyrosine kinase. Curr Biol. 14:691–696.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hanna S, Khalil B, Nasrallah A, Saykali
BA, Sobh R, Nasser S and El-Sibai M: StarD13 is a tumor suppressor
in breast cancer that regulates cell motility and invasion. Int J
Oncol. 44:1499–1511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Al-Koussa H, Al-Haddad M, Abi-Habib R and
El-Sibai M: Human recombinant arginase I [HuArgI
(Co)-PEG5000]-induced arginine depletion inhibits colorectal cancer
cell migration and invasion. Int J Mol Sci. 20:60182019. View Article : Google Scholar
|
|
41
|
El-Sibai M, Pertz O, Pang H, Yip SC,
Lorenz M, Symons M, Condeelis JS, Hahn KM and Backer JM:
RhoA/ROCK-mediated switching between Cdc42- and Rac1-dependent
protrusion in MTLn3 carcinoma cells. Exp Cell Res. 314:1540–1552.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou X and Zheng Y: Cell type-specific
signaling function of RhoA GTPase: Lessons from mouse gene
targeting. J Biol Chem. 288:36179–36188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sadok A and Marshall CJ: Rho GTPases:
Masters of cell migration. Small GTPases. 5:e297102014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sakurai-Yageta M, Recchi C, Le Dez G,
Sibarita J-B, Daviet L, Camonis J, D'Souza-Schorey C and Chavrier
P: The interaction of IQGAP1 with the exocyst complex is required
for tumor cell invasion downstream of Cdc42 and RhoA. J Cell Biol.
181:985–998. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
National Institutes of Health (US), .
Understanding Cancer. NIH Curriculum Supplement Series [Internet].
National Institutes of Health (US). 2007, [cited 2020 Apr 10].
Available from:. simplehttps://www.ncbi.nlm.nih.gov/books/NBK20362/
|
|
46
|
Faltas B: Cornering metastases:
Therapeutic targeting of circulating tumor cells and stem cells.
Front Oncol. 2:682012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nicolas S, Abdellatef S, Haddad MA,
Fakhoury I and El-Sibai M: Hypoxia and EGF stimulation regulate
VEGF expression in human glioblastoma multiforme (GBM) Cells by
differential regulation of the PI3K/Rho-GTPase and MAPK pathways.
Cells. 8:13972019. View Article : Google Scholar
|