Introduction
Immunotherapy in cancer, particularly non-small cell
lung cancer (NSCLC), has undergone remarkable advances in recent
years. Immune checkpoints including programmed death (PD)-1/L1 are
involved in immune escape in patients with cancer. Immune
checkpoint inhibitors are effective in certain proportions of
patients and are now a standard primary treatment for NSCLC
(1,2). However, the effect of a single agent is
limited to ~20% of patients. Therefore, basic research studies have
been initiated to clarify the initial tolerance and acquired
resistance to immune checkpoint inhibitors and various combination
therapies in clinical use. The combination of immune checkpoint
inhibitors with chemotherapy has already been added to the standard
treatment (3–5), and numerous other clinical trials,
including combination therapy with other immunotherapy agents, are
in progress.
The PD-L1 tumor proportion score (TPS) have been
used as a predictive biomarker for immune checkpoint inhibitors
(6); however, this is not adequate
as some patients respond to immune checkpoint inhibitors, even
though the PD-L1 TPS of their tumor is 0% and vice versa. In
addition, the tumor mutation burden (TMB) (7) and tumor infiltrating lymphocytes (TILs)
(8) were previously used to
determine the effects of immune checkpoint inhibitors. TMB reflects
the number of neo-antigens in a tumor, and therefore, in cancer
with high TMB, immune checkpoint inhibitors are likely to have a
positive effect (9). Conversely,
PD-L1 TPS is thought to reflect the production of interferon-γ
(IFN-γ) in the tumor microenvironment (10), as well as being a biomarker for the
tumor expression of PD-L1. It reflects the cytolytic activity of
cytotoxic T cells and natural killer T cells in the tumor
microenvironment. The cancer immune cycle starts with neo-antigen
expression (TMB) followed by the infiltration of lymphocytes into
the tumor (TILs) and finally cytolytic activity (PD-L1 expression).
The associations of TMB and PD-L1 expression with antitumor immune
responses are well-known, but factors associated with TILs are
poorly understood. However, a high number of TILs in NSCLC is a
good prognostic factor (11).
Spranger et al (12,13)
reported that tumors expressing β-catenin in a melanoma model
suppressed dendritic cell recruitment via decreased chemokine
production, which consequently decreased the numbers of TILs.
β-catenin is involved in cell proliferation (14) and the epithelial-mesenchymal
transition (EMT) of cancer cells (15). β-catenin expression was also reported
to be associated with a poor prognosis, even in NSCLC (16), and resistance to chemotherapy
(17) or tyrosine kinase inhibitors
(18). Spranger et al
(12) suggested that β-catenin might
also be involved in tumor immune evasion, thereby promoting the
development of cancer.
However, whether β-catenin is involved in tumor
immune escape mechanisms in lung cancer is unclear. Although NSCLC
cases with abnormal β-catenin expression had low numbers of TILs
(19), it was unclear whether this
was associated with dendritic cells. Therefore, the present study
investigated the association between β-catenin expression, TILs,
and CD11c+ cells in NSCLC. CD11c is a marker of
mononuclear phagocytes (20) and is
expressed on antigen presenting cells (APCs) (21). CD11c, also known as integrin α X, is
a transmembrane protein. CD11c mediates phagocytosis (22) and binds to cell adhesion molecules
(23–25), components of the bacterial wall
(26), complement proteins (22,27) and
matrix proteins (28,29). TMB and PD-L1 TPS were examined for
evaluable cases and their association with β-catenin expression was
clarified. Furthermore, the association between overall survival
and relapse-free survival was examined.
Materials and methods
Patients
A total of 122 patients with NSCLC, who underwent
complete resection between January 2013 and December 2015 at the
Hospital of Fukushima Medical University, were enrolled. No
chemotherapy or immunotherapy was administered before surgery.
Disease staging was evaluated according to the International
Staging System for Lung Tumors, 7th edition (30).
Immunohistochemistry
Paraffin-embedded NSCLC specimens were cut into 3-µm
microtome sections. The sections were deparaffinized in xylene and
dehydrated in a desending alcohol series (99% for 2 min, 90% for 1
min, then 70% for 1 min). Endogenous peroxidase activity was
blocked by a 20-min incubation with 0.3% (v/v) solution of hydrogen
peroxidase (Wako Pure Chemical Industries Ltd.) in 100% methanol.
Following incubation with 5% skimmed milk in PBS for 30 min at room
temperature, the sections were incubated overnight at 4°C with
primary monoclonal antibodies against β-catenin (1:100; cat. no.
UMAB15; OriGene Technologies, Inc.), CD8 (1:50; cat. no. C8/144B;
Agilent Technologies, Inc.), or CD11c (1:200; cat. no. 2F1C10;
ProteinTech Group, Inc.). The primary antibody was then detected
using biotinylated secondary anti-mouse IgG (1:400; cat. no. E0413)
or anti-rabbit IgG antibodies (1:400; cat. no. E0431) (both from
Dako; Agilent Technologies, Inc.) using the avidin-biotin complex
method. The sections were counterstained with Mayer's haematoxylin
(Muto Pure Chemicals Co., Ltd.), dehydrated using 99% alcohol for
three min, and mounted on glass slides.
For each specimen, micrographs of high-power fields
(HPF; magnification, ×400) were captured using a light microscope
(IX73; Olympus Corporation) with a CCD camera (DP73; Olympus
Corporation). Then, micrographs were analyzed by two investigators
without knowledge on the corresponding clinicopathological data.
Specimens containing tumor cells with only membranous staining were
defined as negative (normal) β-catenin expression. Specimens
containing tumor cells with cytoplasmic staining were defined as
positive (abnormal) β-catenin expression. Evaluation of tumor
infiltrating CD8+ cells and CD11c+ cells was
performed according to the visual estimation of these cells in each
visual field. The number of CD8+ cells was evaluated
into one of three groups: Small (<30%), medium (30–60%) and
large (>60%), as previously reported (31). Furthermore, CD8+ and
CD11c+ cell infiltrations into tumor nests were
evaluated as positive or negative.
PD-L1 TPS was evaluated in 68 patients using a PD-L1
IHC 22C3 pharmDx immunohistochemistry assay on the Dako Autostainer
Link 48 at SRL, Inc. PD-L1 TPS was defined as the percentage of
viable tumor cells with partial or complete membrane staining
(32). These 68 patients were
randomly selected in clinical practice from a total of 122
patients.
Sequencing analysis
Whole exome sequencing was conducted in accordance
with the Ethical Guidelines for Human Genome and Genetic Analysis
Research. In 122 patients with NSCLC, whole exome sequencing of 79
tumors and paired normal samples was performed using Ion Amplise
Exome technology and the Ion Proto platform (Thermo Fisher
Scientific, Inc.). Tumor volume was insufficient for the analysis
of the remaining 43 patients. The obtained DNA sequences were
aligned to hg19 of the human genome. The mean coverage depth was
123X and 90.4% of target bases had a coverage of 20X. Sequence
variants in the tumors were named using Ion Reporter v5.0 (Thermo
Fisher Scientific, Inc.) and CLC Genomics Workbench v8.0 software
(Qiagen, Inc.), and the number of non-synonymous coding variants
was counted. The obtained value was designated the TMB. In
addition, gene mutation analysis was conducted for catenin β1
(CTNNB1) and three associated genes: APC regulator of WNT signaling
pathway (APC), axin 1 (AXIN1), and transcription
factor 7 (TCF7).
Flow cytometry
Tumor infiltrating CD45+ cells in two
patients with NSCLC were analyzed using flow cytometry. There was
one patient with squamous cell carcinoma and the other had
adenocarcinoma of the lung. Tumor specimens from both patients were
β-catenin negative by immunohistochemistry. CD8+ and
CD11c+ cell infiltrations into tumor nests were positive
in both cases. A 7-mm square of tissue was obtained from each
patient's tumor at surgical resection. The tissue was dissociated
into a single-cell suspension using a gentleMACS Dissociator
(Miltenyi Biotec GmBH). Blood cells were separated and collected by
CD45 MicroBeads and autoMACS separator (Miltenyi Biotec) and then
stained with the following antibodies: PE anti-human CD11c (1:20;
cat. no. 301605; BioLegend, Inc.), PE/Cy7 anti-human HLA-DR (1:20;
cat. no. 307615; BioLegend, Inc.), PerCP/Cy5.5 anti-human CD163
(1:20; cat. no. 326511; BioLegend, Inc.), APC/Cy7 anti-human CD14
(1:20; cat. no. 301819; BioLegend, Inc.), APC anti-human CD86
(1:20; cat. no. 305411; BioLegend, Inc.), and FITC anti-human CD20
(1:20; cat. no. 130-113-373; Miltenyi Biotec GmBH). Samples were
acquired with a FACSCanto II (BD Biosciences) and analysed using
FlowJo software v10.0 (FlowJo LLC). The samples were gated on
single cells, then the lymphocytes and monocytes were selected and
analyzed.
Statistical analysis
T-test, Fisher's exact test, χ2 test, and
log-rank test were performed using GraphPad Prism software v8.4.3
(GraphPad Software, Inc.). Continuous variables are presented as
the mean ± SEM. P<0.05 was considered to indicate a
statistically significant difference. Survival curves were drawn
using the Kaplan-Meier method, and differences in survival were
evaluated using the log-rank test. The Cox proportional regression
model using the forward stepwise likelihood ratio method was
performed to select prognostic factors of survival using SPSS
software v27 (IBM Corp.).
Results
β-catenin expression and patient
characteristics
Patient characteristics are presented in Table I. Tumor β-catenin expression was
positive in 29 patients (24%). The β-catenin-positive group had
higher smoking status and larger tumor size compared with the
β-catenin-negative group. β-catenin was more commonly found in
squamous cell carcinoma (16%) compared with adenocarcinoma (8%).
There was no difference in age, sex, or pathological stage between
β-catenin-positive and -negative groups.
 | Table I.Patient characteristics (n=122). |
Table I.
Patient characteristics (n=122).
|
| β-catenin |
|
|---|
|
|
|
|
|---|
|
Characteristics | Positive | Negative | P-value |
|---|
| Total, n (%) | 29 (24) | 93 (76) |
|
| Age, years | 69.6±10.3 | 68.9±9.4 |
0.7469 |
| Sex, n (%) |
|
|
0.2643 |
|
Male | 22 (76) | 59 (63) |
|
|
Female | 7
(24) | 34 (37) |
|
| Smoking, pack
years | 47.3±36.9 |
27.0±29.5 |
0.0098 |
| p-Stage, n (%) |
|
|
0.0785 |
| 1 | 18 (62) | 75 (81) |
|
| 2 | 5
(17) | 11 (12) |
|
| 3 | 6
(20) | 7 (8) |
|
| Tumor size, mm | 37±21 |
29±16 |
0.0472 |
| Histology, n
(%) |
|
| <0.0001 |
| Ad | 10 (34) | 80 (86) |
|
| Sq | 19 (66) | 13 (14) |
|
β-catenin expression in tumors, and
tumor infiltrating lymphocytes and CD11c+ cells
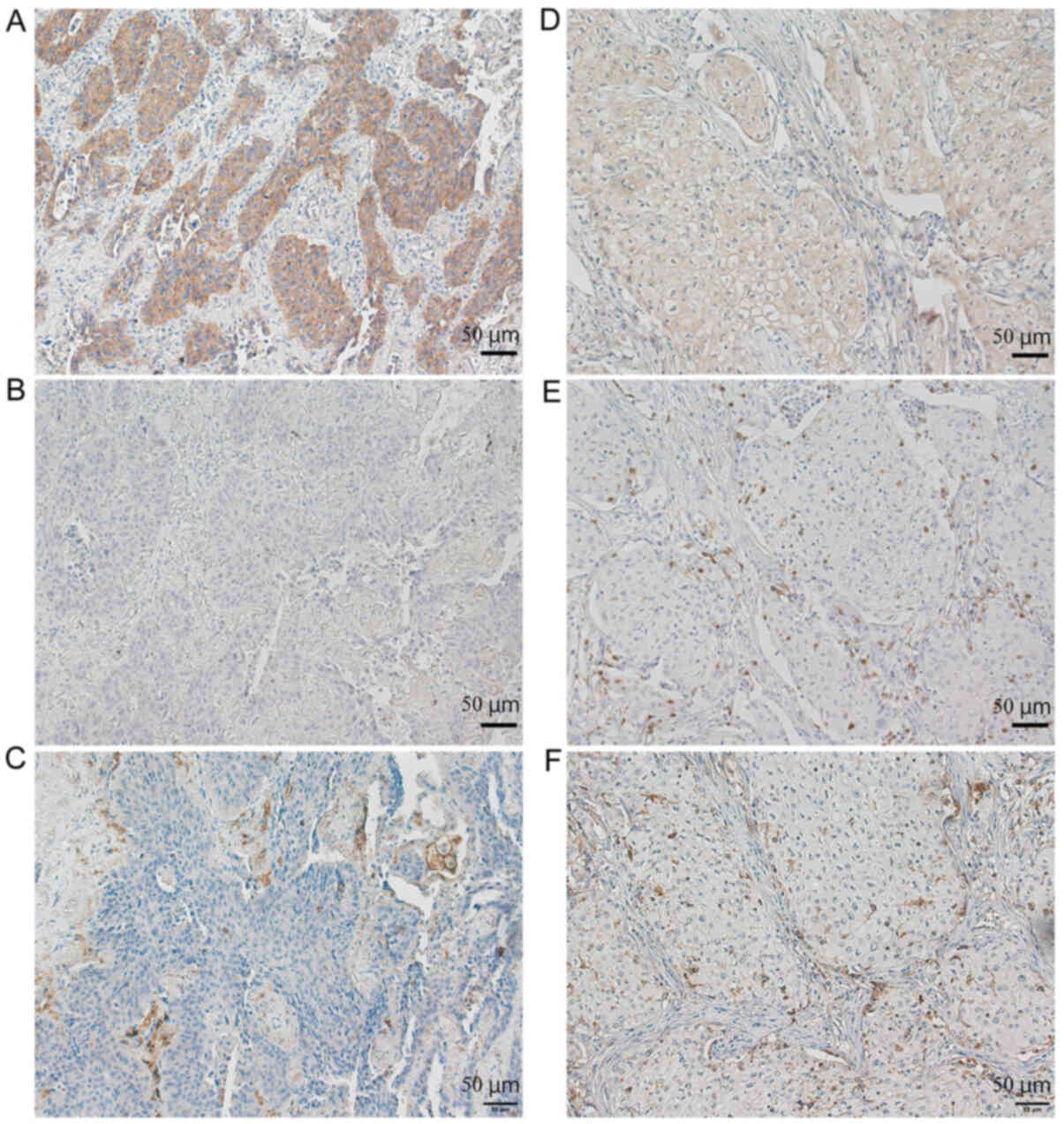
β-catenin positive NSCLC patients had low
CD8+ and CD11c+ cell infiltration into tumors
(Fig. 1). There were two types of
tumor infiltrating lymphocytes: Those present in the stroma; and
those that directly infiltrated into the tumor nests (Fig. 1E). β-catenin expression was
associated with CD8+ and CD11c+ cell
infiltration into tumor nests regardless of the total number of
CD8+ cells in the tumor sites (Table II). There was a significant
association between CD8+ and CD11c+ cell
infiltration (P<0.0001). The infiltrations of CD8+
and CD11c+ cells into tumor nests were good prognostic
factors. The group without CD8+ cell infiltration into
tumor nests had a significantly shorter overall survival time
(P=0.0234) and tended to have a shorter recurrence-free survival
time compared with the CD8+ cell infiltration group
(P=0.1362; Fig. 2A and B). The group
without CD11c+ cell infiltration into the tumor nests
also tended to have short overall survival time (P=0.0932) and
recurrence-free survival time (P=0.1619; Fig. 2C and D). Conversely, the total number
of CD8+ cells in tumor sites did not have a clear
association with overall survival (P=0.4065) or recurrence-free
survival (P=0.5851; Fig. 2E and F).
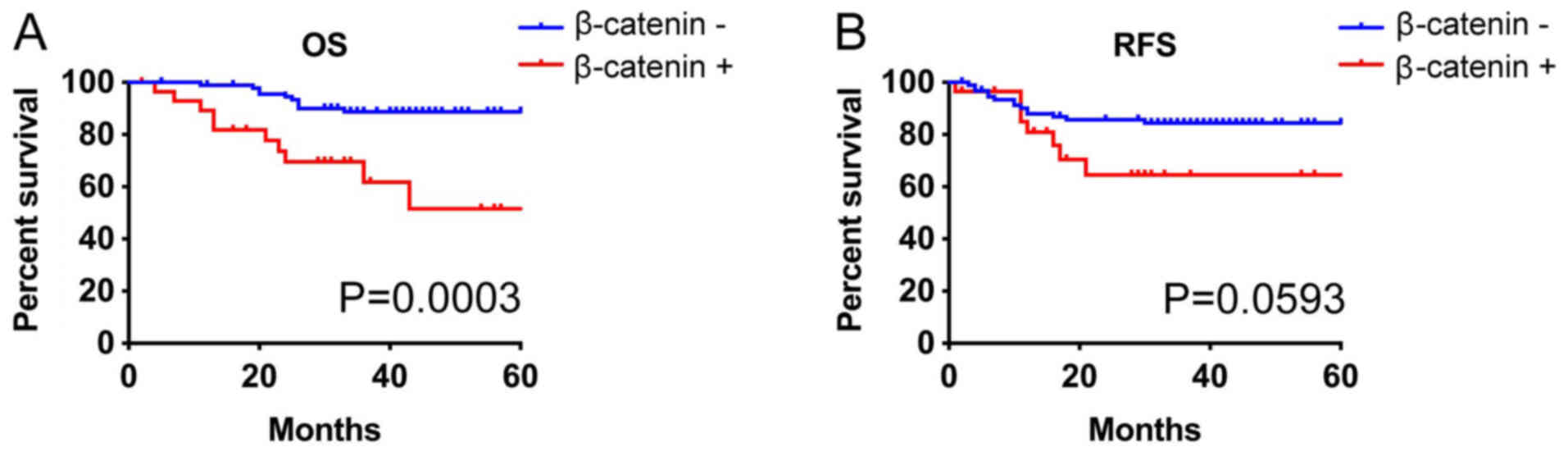
The median overall and recurrence-free survival was not reached in
all groups. NSCLC with β-catenin expression tended to have short
overall survival (P=0.0003) and recurrence-free survival (P=0.0593;
Fig. 3). Multivariate analysis
indicated that tumor size, β-catenin, and sex were poor prognosis
factors for overall survival although tumor size, histology, and
pathological stage were associated with recurrence-free survival
(Tables III and IV).
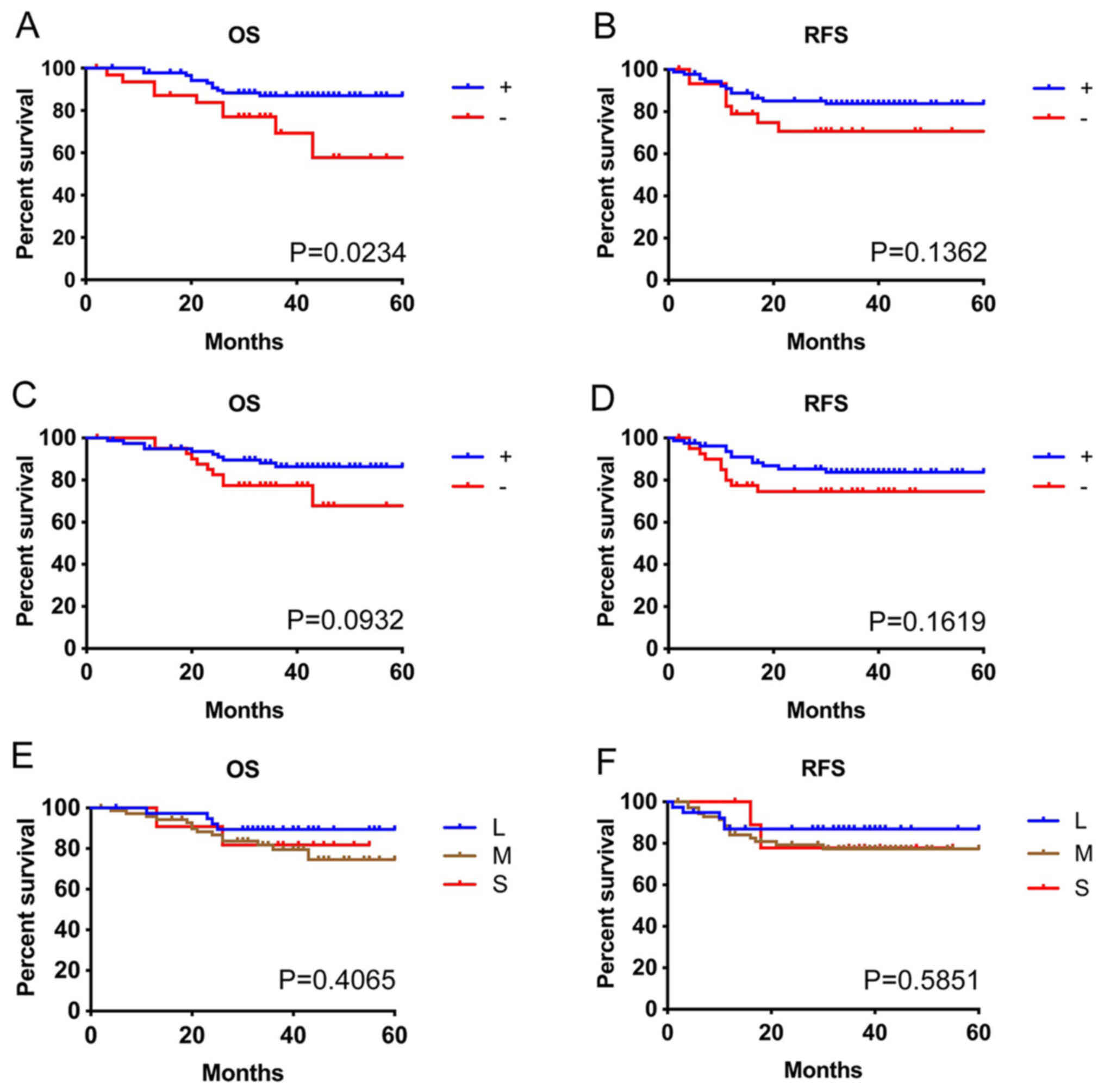 | Figure 2.Survival and tumor infiltrating
immune cells. Analysis between OS and RFS, and (A and B) tumor
epithelial infiltration of CD8+ lymphocytes, (C and D)
CD11c+ cells, and (E and F) total number of
CD8+ cells in the tumor tissues, including the stroma,
respectively. Infiltration into the tumor epithelium, but not
numbers of tumor infiltrating lymphocytes, was associated with
prognosis. Infiltration of CD11c+ cells into the tumor
epithelium tended to be similar to that of CD8+
lymphocytes. +, cases with tumor epithelial infiltration of cells;
-, cases without tumor epithelial infiltration of cells; L, large;
M, medium; S, small; OS, overall survival; RFS, relapse-free
survival. |
 | Table II.Number of patients associated with
β-catenin expression, tumor-infiltrating CD8+ cells, and
tumor infiltrating CD11c+ cells (n=122). |
Table II.
Number of patients associated with
β-catenin expression, tumor-infiltrating CD8+ cells, and
tumor infiltrating CD11c+ cells (n=122).
|
| β-catenin |
|
|---|
|
|
|
|
|---|
|
Characteristics | Positive | Negative | P-value |
|---|
| CD8+
cell infiltration into tumor nests, n (%) | 10 (8) | 80 (66) | <0.0001 |
| + | 19
(16) | 13 (11) |
|
| − |
|
|
|
| CD11c+
cell infiltration into tumor nests, n (%) |
|
|
0.0788 |
| + | 15
(12) | 65 (53) |
|
| − | 14
(11) | 28 (23) |
|
| Total number of
CD8+ cells in tumor sites, n (%) |
|
|
0.8781 |
|
Large | 9
(7) | 30 (25) |
|
|
Medium | 18
(15) | 54 (44) |
|
|
Small | 2
(2) | 9 (7) |
|
 | Table III.Univariate and Cox regression
multivariable stepwise procedure of overall survival. |
Table III.
Univariate and Cox regression
multivariable stepwise procedure of overall survival.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
|
Characteristics | Number | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (<69
years) | 56 | 0.925 | 0.383–2.232 | 0.862 |
|
|
|
| Sex (female) | 41 | 0.297 | 0.087–1.016 | 0.053 | 0.289 | 0.081–1.030 | 0.055 |
| Smoking
(<32.5) | 61 | 0.467 | 0.186–1.171 | 0.104 |
|
|
|
| p-Stage (1) | 93 | 0.282 | 0.117–0.679 | 0.005 |
|
|
|
| Tumor size (<28
mm) | 59 | 0.108 | 0.025–0.467 | 0.003 | 0.121 | 0.028–0.522 | 0.005 |
| Histology (Ad) | 90 | 0.324 | 0.134–0.786 | 0.013 |
|
|
|
| β-catenin (−) | 93 | 0.229 | 0.095–0.551 | 0.001 | 0.264 | 0.109–0.640 | 0.001 |
 | Table IV.Univariate and Cox regression
multivariable stepwise procedure of recurrence-free survival. |
Table IV.
Univariate and Cox regression
multivariable stepwise procedure of recurrence-free survival.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
|
Characteristics | Number | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (<69
years) | 56 | 0.933 | 0.403–2.160 |
0.872 |
|
|
|
| Sex (female) | 41 | 0.871 | 0.355–2.137 |
0.763 |
|
|
|
| Smoking
(<32.5) | 61 | 0.633 | 0.270–1.479 |
0.291 |
|
|
|
| p-Stage (1) | 93 | 0.127 | 0.053–0.303 | <0.001 | 0.204 | 0.081–0.513 | 0.001 |
| Tumor size (<28
mm) | 59 | 0.086 | 0.020–0.369 |
0.001 | 0.132 | 0.028–0.608 | 0.009 |
| Histology (Ad) | 90 | 1.543 | 0.522–4.566 |
0.433 | 3.460 | 1.149–10.42 | 0.027 |
| β-catenin (−) | 93 | 0.443 | 0.184–1.063 |
0.068 |
|
|
|
Subsets of tumor infiltrating
CD11c+ cells
Flow cytometry revealed that most tumor infiltrating
CD45+ cells were HLA-DR positive. Numerous
CD11c+ cells were CD163 positive and CD20 negative. The
CD11c+ subset also contained cells that were positive
and negative for CD14 and CD86 (Fig.
S1).
Association of β-catenin expression
with tumor mutation burden and PD-L1 expression
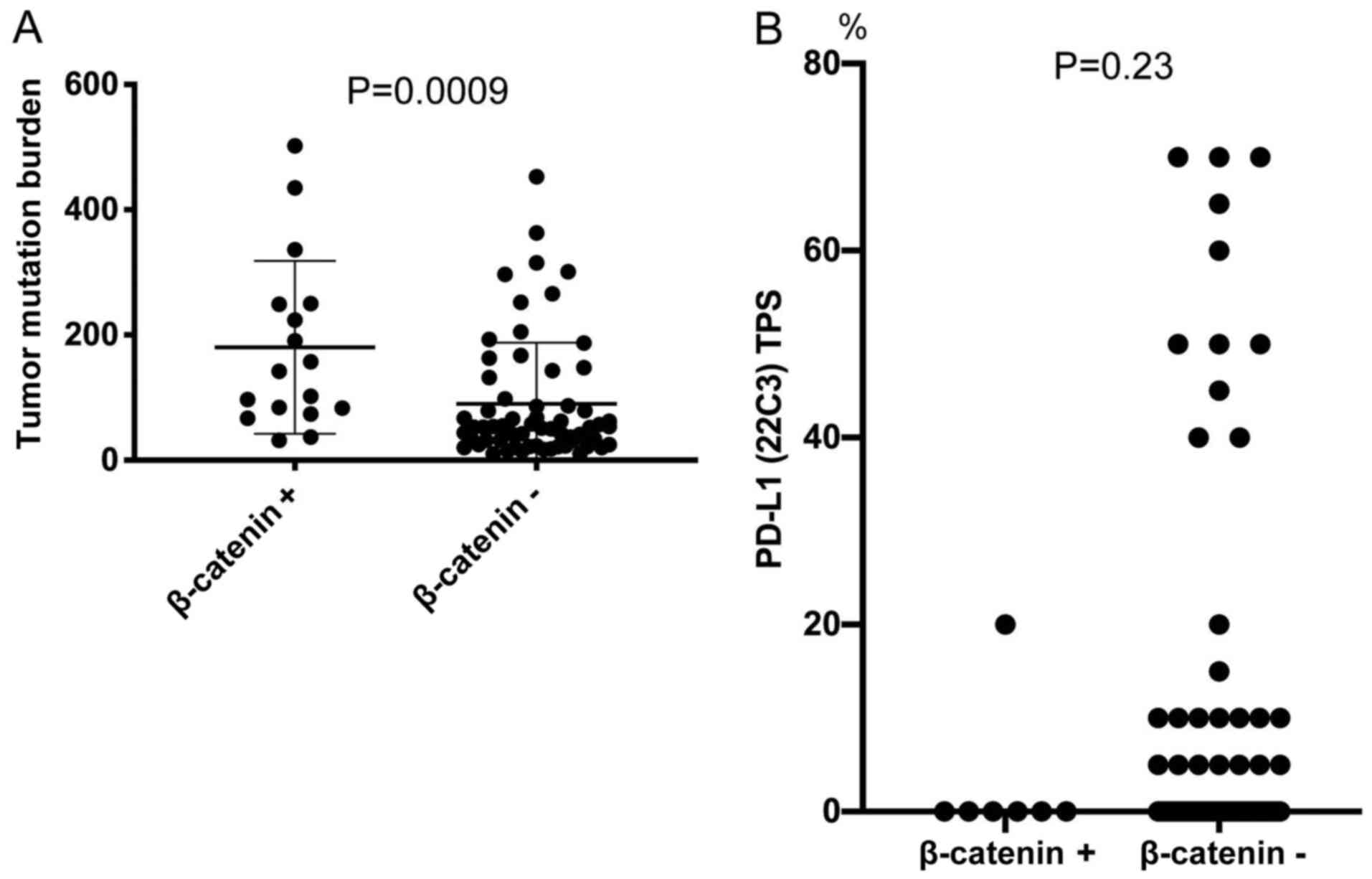
The median TMB was 142 (range, 32–502; n=17) in the
β-catenin-positive group and 53 (range, 10–453; n=62) in the
β-catenin-negative group. NSCLC-expressing β-catenin had a
significantly higher TMB than non-β-catenin-expressing lung cancer
(Fig. 4A). Conversely,
NSCLC-expressing β-catenin tended to have a lower PD-L1 TPS
compared with β-catenin-negative NSCLC (Fig. 4B). The median PD-L1 TPS was 0%
(range, 0–20; n=7) in the β-catenin-positive group and 0% (range,
0–70; n=61) in the β-catenin-negative group.
Whole exome sequencing of CTNNB1 and
related genes
Whole exome sequencing showed that three
β-catenin-negative tumors (assessed using immunohistochemistry) had
a CTNNB1 mutation. An APC mutation and an AXIN1 mutation were found
in one β-catenin positive tumor each (assessed using
immunohistochemistry). No tumors had a TCF7 mutation.
Discussion
The present study investigated how β-catenin
expression in NSCLC tumors influenced antitumor immune responses.
It was found that β-catenin expression in tumors was negatively
associated with TILs in NSCLC, which were associated with the
suppression of CD11c+ cell recruitment. This result
supports the findings of previous reports (12,13).
Furthermore, to the best of our knowledge, the present study is the
first to reveal that NSCLC-expressing β-catenin had high TMB,
whereas PD-L1 TPS tended to be low. This suggests that
NSCLC-expressing β-catenin might express numerous neo-antigens;
however, there were few TILs, and IFN-γ production was low in the
tumor microenvironment. The MYSTIC trial reported that history of
smoking and squamous histology was associated with high TMB;
however, PD-L1 expression status was not associated with TMB
(33). This data also supports the
result in the present study. Lung squamous cell carcinoma was
reported to have a high TMB (34)
and has a greater association with smoking history compared with
lung adenocarcinoma. Therefore, it is likely that smoking history
and squamous histology are associated with a high TMB. The present
results suggest that IFN-γ produced by TILs is associated with
PD-L1 expression in NSCLC. This indicates that NSCLC-expressing
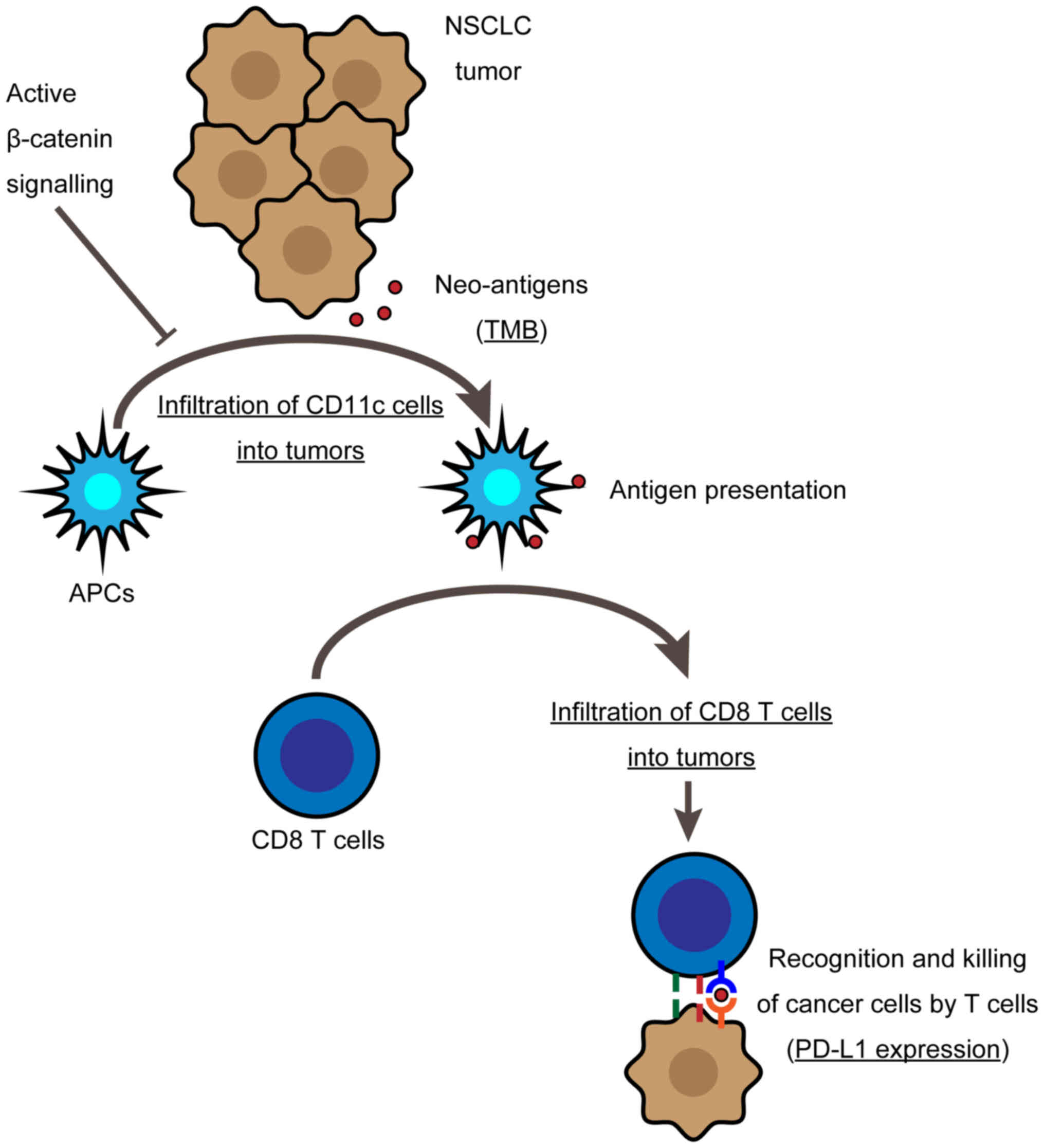
β-catenin prevents the cancer immune cycle from progressing,
possibly by suppressing lymphocyte infiltration into tumor sites.
Furthermore, an immune evasion mechanism might suppress antigen
presentation by decreasing APC recruitment (Fig. 5).
Previously, tumors with low immunogenicity, or
‘cold’ tumors, were thought to have low levels of neo-antigens or
have lost the expression of HLA class I. However, Spranger et
al (12) used a melanoma model
to show that β-catenin signaling induced the expression of
activating transcription factor 3 (ATF3), which suppressed the C-C
motif chemokine ligand 4 (CCL4) gene (Ccl4), resulting in
the decreased recruitment of CD103+ dendritic cells.
Although the production of ATF3 or CCL4 was not examined, tumor
evasion might occur by a similar mechanism in NSCLC.
Another finding of the present study was that the
expression of β-catenin was associated with lymphocyte infiltration
into tumor nests, but not the total number of TILs in tumor nests
or the tumor stroma. In contrast to the present findings, a
previous study reported that the number of TILs was associated with
the abnormal expression of β-catenin in NSCLC (19). Even if CD8+ cells are
present in the tumor stroma, they may not be able to attack tumor
cells, suggesting immune exclusion.
A high number of TILs in NSCLC was reported to be a
good prognostic factor (11,31). Although studies have investigated the
association between TILs and NSCLC prognosis, whether TILs are
present in tumor nests, tumor stroma, or a combination thereof
differs between studies. A previous meta-analysis did not determine
the site at which TILs can affect the prognosis (35). However, in the current study, TILs in
tumor nests were associated with NSCLC prognosis. In a study of
colorectal cancer, TILs in the tumor nest, but not the stroma, were
associated with prognosis (36),
similar to the present findings.
According to the model of Spranger and Gajewski
(37), decreased CCL4 secretion in
β-catenin-activated-NSCLC suppressed dendritic cell recruitment,
which in turn decreased the production of C-X-C motif chemokine
ligand (CXCL) 9/10 from dendritic cells, preventing lymphocyte
infiltration into the tumor microenvironment. The present results
are consistent with these findings. It was found that lymphocyte
infiltration into tumor epithelium, but not the total number of
TILs, was associated with the expression of β-catenin. This type of
lymphocyte infiltration has been termed an “excluded” phenotype
(38). Although cytotoxic T
lymphocytes are thought to undergo antigen presentation in tertiary
lymphoid structures or regional lymph nodes, a second round of T
cell-dendritic cell crosstalk is necessary for T cells to attack
tumor cells (39). The activation of
β-catenin and suppression of APC infiltration might suppress this
crosstalk. Tumor β-catenin activation blocks antigen presentation
and crosstalk, creating a state of low antigenicity, which explains
the remaining low PD-L1 TPS. Therefore, the activation of tumor
β-catenin might be a resistance mechanism of immune checkpoint
blockers (37).
Tumor Wnt/β-catenin signaling is involved in immune
evasion and various carcinogenic and cancer developmental
mechanisms including: i) maintenance of a stem cell-like state; ii)
activation of EMT; iii) tumor vascularization; and iv) treatment
resistance (40). During immune
evasion, Wnt ligands released by cancer cells induced canonical and
non-canonical Wnt signaling in dendritic cells, resulting in the
secretion of the anti-inflammatory cytokine interleukin-10 and
indoleamine 2,3-dioxygenase 1 (IDO1), which impaired the ability of
dendritic cells to cross-prime cytotoxic T lymphocytes via
regulatory T cells (41,42). A previous study revealed that
increased regulatory T cells were associated with a poor prognosis
in NSCLC (43,44).
The role of β-catenin has been studied in settings
other than immune evasion, and β-catenin has been a drug discovery
target for several decades (45).
However, in recent years, the activation of β-catenin has been
considered a tumor-cell-intrinsic mechanism of primary and adaptive
resistance to immunotherapy via T cell exclusion, and has therefore
attracted increasing attention as a target for drug discovery
(40). If β-catenin inhibitors can
turn a ‘cold’ tumor into a ‘hot’ tumor (with numerous tumor
infiltrating lymphocytes), the effect of immunotherapy might be
further enhanced.
In addition to these β-catenin inhibitors,
stimulator of interferon genes (STING) agonists are also attracting
attention. STING is a receptor in the endoplasmic reticulum that
propagates the innate immune sensing of cytosolic pathogen-derived
and self DNA. The STING pathway is critical for IFN-β production
and dendritic cell cross-presentation (13,46).
Therefore, STING agonists provide a therapeutic strategy to
initiate endogenous T cell priming.
The main limitation of the present study was that
APCs were not detected by multiple colour immunohistochemistry.
CD11c is expressed by dendritic cells and other APCs. The flow
cytometry analysis of tumor infiltrating cells revealed that most
tumor infiltrating blood cells were HLA-DR positive. Most
CD11c+ cells were CD163-positive M2 macrophages, but
there were a few CD11c-positive and CD163-negative cells. The
CD11c+ subset contained cells positive and negative for
CD14 and CD86. These results indicate that CD11c was expressed by
various type of APCs (20,21). Since only two patients were used for
these analyses, the results can only be assumed in this
instance.
A second study limitation was that the gene
expressions of β-catenin and ATF3, or CCL4 chemokine production
were not examined. The whole exome data indicated mutations in
CTNNB1 and other associated genes. However, these did not
seem to be associated with β-catenin expression, as assessed by
immunohistochemistry. Therefore, the cause of β-catenin expression
was not determined. In addition, other mechanisms associated with
β-catenin-associated cancer types, such as cell proliferation and
EMT, were not investigated. Despite these limitations, the present
results indicate that β-catenin activation influences immune
evasion by decreasing the recruitment of CD11c+ cells
and TILs in NSCLC. The development of novel therapeutic approaches
targeting β-catenin is likely to enhance the immunotherapy of
NSCLC.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank the Biostatistical
Consulting Service at the Clinical Research Center, Fukushima
Medical University, and in particular Dr Noriko Tanaka, assisted
with the interpretation of the results of the statistical analysis.
The authors would also like to thank Ms Eiko Ohtomo, Ms Mie Ohtsuki
and Ms Yukiko Kikuta (Department of Chest Surgery, Fukushima
Medical University, Japan) for their technical assistance.
Funding
This study was supported by a Grant-in-Aid for Young
Scientists (JSPS KAKENHI; grant no. JP19K18222).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SM and HS designed the study. SM, YO, HY, HM, HT,
MW, TIn, TY, MF, NO, YM, TH, JO, MHo, MHi and YS prepared
surgically resected samples for the study. SM and YO planned and
performed experiments. SM and YO confirm the authenticity of all
the raw data. SM, YO, HN, JII, TIs and SW analysed the data. SM and
HS wrote the paper.
Ethics approval and consent to
participate
The Human Ethics Committee at Fukushima Medical
University approved this study (approval no. 30161). Written
informed consent was provided from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WEE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gandhi L, Rodríguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al KEYNOTE-189 Investigators, : Pembrolizumab plus
chemotherapy in metastatic non-small-cell lung cancer. N Engl J
Med. 378:2078–2092. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paz-Ares L, Luft A, Vicente D, Tafreshi A,
Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, et
al KEYNOTE-407 Investigators, : Pembrolizumab plus chemotherapy for
squamous non-small-cell lung cancer. N Engl J Med. 379:2040–2051.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Socinski MA, Jotte RM, Cappuzzo F, Orlandi
F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D,
Thomas CA, Barlesi F, et al IMpower150 Study Group, : Atezolizumab
for first-line treatment of metastatic nonsquamous NSCLC. N Engl J
Med. 378:2288–2301. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Herbst RS, Soria J-C, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rizvi NA, Hellmann MD, Snyder A, Kvistborg
P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al: Cancer
immunology. Mutational landscape determines sensitivity to PD-1
blockade in non-small cell lung cancer. Science. 348:124–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tumeh PC, Harview CL, Yearley JH, Shintaku
IP, Taylor EJM, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu
V, et al: PD-1 blockade induces responses by inhibiting adaptive
immune resistance. Nature. 515:568–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ready N, Hellmann MD, Awad MM, Otterson
GA, Gutierrez M, Gainor JF, Borghaei H, Jolivet J, Horn L, Mates M,
et al: First-line nivolumab plus ipilimumab in advanced
non-small-cell lung cancer (CheckMate 568): Outcomes by programmed
death ligand 1 and tumor mutational burden as biomarkers. J Clin
Oncol. 37:992–1000. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blank C, Kuball J, Voelkl S, Wiendl H,
Becker B, Walter B, Majdic O, Gajewski TF, Theobald M, Andreesen R,
et al: Blockade of PD-L1 (B7-H1) augments human tumor-specific T
cell responses in vitro. Int J Cancer. 119:317–327. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bremnes RM, Busund L-T, Kilvær TL,
Andersen S, Richardsen E, Paulsen EE, Hald S, Khanehkenari MR,
Cooper WA, Kao SC, et al: The role of tumor-infiltrating
lymphocytes in development, progression, and prognosis of non-small
cell lung cancer. J Thorac Oncol. 11:789–800. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spranger S, Bao R and Gajewski TF:
Melanoma-intrinsic β-catenin signalling prevents anti-tumour
immunity. Nature. 523:231–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Spranger S, Dai D, Horton B and Gajewski
TF: Tumor-residing Batf3 dendritic cells are required for effector
t cell trafficking and adoptive t cell therapy. Cancer Cell.
31:711–723.e4. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rijsewijk F, van Deemter L, Wagenaar E,
Sonnenberg A and Nusse R: Transfection of the int-1 mammary
oncogene in cuboidal RAC mammary cell line results in morphological
transformation and tumorigenicity. EMBO J. 6:127–131. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yook JI, Li XY, Ota I, Fearon ER and Weiss
SJ: Wnt-dependent regulation of the E-cadherin repressor snail. J
Biol Chem. 280:11740–11748. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim Y, Jin D, Lee BB, Cho EY, Han J, Shim
YM, Kim HK and Kim DH: Overexpression of β-catenin and cyclin D1 is
associated with poor overall survival in patients with stage IA-IIA
squamous cell lung cancer irrespective of adjuvant chemotherapy. J
Thorac Oncol. 11:2193–2201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang H, Zhang G, Zhang H, Zhang F, Zhou B,
Ning F, Wang HS, Cai SH and Du J: Acquisition of
epithelial-mesenchymal transition phenotype and cancer stem
cell-like properties in cisplatin-resistant lung cancer cells
through AKT/β-catenin/Snail signaling pathway. Eur J Pharmacol.
723:156–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoo SB, Kim YJ, Kim H, Jin Y, Sun P-L,
Jheon S, Lee JS and Chung JH: Alteration of the
E-cadherin/β-catenin complex predicts poor response to epidermal
growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI)
treatment. Ann Surg Oncol. 20 (Suppl 3):S545–S552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye L, Li H, Wang H, Liu H, Lv T, Zhang F
and Song Y: Abnormal β-catenin expression and reduced
tumor-infiltrating T cells are related to poor progression in
non-small cell lung cancer. Int J Clin Exp Pathol. 10:11572–11579.
2017.PubMed/NCBI
|
|
20
|
Hogg N, Takacs L, Palmer DG, Selvendran Y
and Allen C: The p150,95 molecule is a marker of human mononuclear
phagocytes: Comparison with expression of class II molecules. Eur J
Immunol. 16:240–248. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hume DA: Macrophages as APC and the
dendritic cell myth. J Immunol. 181:5829–5835. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Malhotra V, Hogg N and Sim RB: Ligand
binding by the p150,95 antigen of U937 monocytic cells: Properties
in common with complement receptor type 3 (CR3). Eur J Immunol.
16:1117–1123. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Diamond MS, Garcia-Aguilar J, Bickford JK,
Corbi AL and Springer TA: The I domain is a major recognition site
on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct
adhesion ligands. J Cell Biol. 120:1031–1043. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Blackford J, Reid HW, Pappin DJC, Bowers
FS and Wilkinson JM: A monoclonal antibody, 3/22, to rabbit CD11c
which induces homotypic T cell aggregation: Evidence that ICAM-1 is
a ligand for CD11c/CD18. Eur J Immunol. 26:525–531. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ihanus E, Uotila LM, Toivanen A, Varis M
and Gahmberg CG: Red-cell ICAM-4 is a ligand for the
monocyte/macrophage integrin CD11c/CD18: Characterization of the
binding sites on ICAM-4. Blood. 109:802–810. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ingalls RR and Golenbock DT: CD11c/CD18, a
transmembrane signaling receptor for lipopolysaccharide. J Exp Med.
181:1473–1479. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Myones BL, Dalzell JG, Hogg N and Ross GD:
Neutrophil and monocyte cell surface p150,95 has iC3b-receptor
(CR4) activity resembling CR3. J Clin Invest. 82:640–651. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Loike JD, Sodeik B, Cao L, Leucona S,
Weitz JI, Detmers PA, Wright SD and Silverstein SC: CD11c/CD18 on
neutrophils recognizes a domain at the N terminus of the A alpha
chain of fibrinogen. Proc Natl Acad Sci USA. 88:1044–1048. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Garnotel R, Rittié L, Poitevin S,
Monboisse J-C, Nguyen P, Potron G, Maquart F-X, Randoux A and
Gillery P: Human blood monocytes interact with type I collagen
through alpha × beta 2 integrin (CD11c-CD18, gp150-95). J Immunol.
164:5928–5934. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L;
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions, : The
IASLC Lung Cancer Staging Project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schalper KA, Brown J, Carvajal-Hausdorf D,
McLaughlin J, Velcheti V, Syrigos KN, Herbst RS and Rimm DL:
Objective measurement and clinical significance of TILs in
non-small cell lung cancer. J Natl Cancer Inst. 107:dju4352015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Roach C, Zhang N, Corigliano E, Jansson M,
Toland G, Ponto G, Dolled-Filhart M, Emancipator K, Stanforth D and
Kulangara K: Development of a companion diagnostic PD-L1
immunohistochemistry assay for pembrolizumab therapy in
non-small-cell lung cancer. Appl Immunohistochem Mol Morphol.
24:392–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft
A, Ahn MJ, van den Heuvel MM, Cobo M, Vicente D, Smolin A, et al
MYSTIC Investigators, : Durvalumab with or without tremelimumab vs
standard chemotherapy in first-line treatment of metastatic
non-small cell lung cancer: The MYSTIC Phase 3 randomized clinical
trial. JAMA Oncol. 6:661–674. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schumacher TN and Schreiber RD:
Neoantigens in cancer immunotherapy. Science. 348:69–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zeng DQ, Yu YF, Ou QY, Li XY, Zhong RZ,
Xie CM and Hu QG: Prognostic and predictive value of
tumor-infiltrating lymphocytes for clinical therapeutic research in
patients with non-small cell lung cancer. Oncotarget.
7:13765–13781. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Naito Y, Saito K, Shiiba K, Ohuchi A,
Saigenji K, Nagura H and Ohtani H: CD8+ T cells
infiltrated within cancer cell nests as a prognostic factor in
human colorectal cancer. Cancer Res. 58:3491–3494. 1998.PubMed/NCBI
|
|
37
|
Spranger S and Gajewski TF:
Tumor-intrinsic oncogene pathways mediating immune avoidance.
OncoImmunology. 5:e10868622015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Galluzzi L, Chan TA, Kroemer G, Wolchok JD
and López-Soto A: The hallmarks of successful anticancer
immunotherapy. Sci Transl Med. 10:102018. View Article : Google Scholar
|
|
39
|
Garris CS, Arlauckas SP, Kohler RH, Trefny
MP, Garren S, Piot C, Engblom C, Pfirschke C, Siwicki M,
Gungabeesoon J, et al: Successful anti-PD-1 cancer immunotherapy
requires T cell-dendritic cell crosstalk involving the cytokines
IFN-gamma and IL-12. Immunity. 49:1148–1161.e7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Galluzzi L, Spranger S, Fuchs E and
López-Soto A: WNT signaling in cancer immunosurveillance. Trends
Cell Biol. 29:44–65. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yaguchi T, Goto Y, Kido K, Mochimaru H,
Sakurai T, Tsukamoto N, Kudo-Saito C, Fujita T, Sumimoto H and
Kawakami Y: Immune suppression and resistance mediated by
constitutive activation of Wnt/β-catenin signaling in human
melanoma cells. J Immunol. 189:2110–2117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Holtzhausen A, Zhao F, Evans KS, Tsutsui
M, Orabona C, Tyler DS and Hanks BA: Melanoma-derived Wnt5a
promotes local dendritic-cell expression of IDO and
immunotolerance: Opportunities for pharmacologic enhancement of
immunotherapy. Cancer Immunol Res. 3:1082–1095. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hasegawa T, Suzuki H, Yamaura T, Muto S,
Okabe N, Osugi J, Hoshino M, Higuchi M, Ise K and Gotoh M:
Prognostic value of peripheral and local forkhead box
P3+ regulatory T cells in patients with non-small-cell
lung cancer. Mol Clin Oncol. 2:685–694. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Muto S, Owada Y, Inoue T, Watanabe Y,
Yamaura T, Fukuhara M, Okabe N, Matsumura Y, Hasegawa T, Osugi J,
et al: Clinical significance of expanded Foxp3+
Helios− regulatory T cells in patients with non-small
cell lung cancer. Int J Oncol. 47:2082–2090. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sheridan C: Wnt is back in drugmakers'
sights, but is it druggable? Nat Biotechnol. 36:1028–1029. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Woo SR, Fuertes MB, Corrales L, Spranger
S, Furdyna MJ, Leung MY, Duggan R, Wang Y, Barber GN, Fitzgerald
KA, et al: STING-dependent cytosolic DNA sensing mediates innate
immune recognition of immunogenic tumors. Immunity. 41:830–842.
2014.Erratum in: Immunity 42: 199, 2015. View Article : Google Scholar : PubMed/NCBI
|