Introduction
Intratumoral hypoxia can show potential therapeutic
resistance owing to its adverse impact on the effectiveness of
chemotherapy and radiotherapy. The rapid growth of solid tumors
changes the cellular microenvironment, leading to inadequate oxygen
supply, which results in intratumoral hypoxia (1,2).
Carbon dioxide (CO2) treatment is
reported to have an antitumor effect due to the improvement in
intratumoral hypoxia. The beneficial effects of CO2
treatment include an increase in blood flow and microcirculation,
neocapillary formation depending on nitric oxide, and partial
increase in oxygen pressure in a confined space, known as the Bohr
effect (3), which has been applied
for a treatment for skin disorder (4–6).
Recently, transcutaneous administration or intra-arterial infusion
of CO2 was reported to have an antitumor effect in
animal experiments (7–11). In previous studies, improvement in
intratumoral hypoxia by CO2 treatment was pathologically
proven by decreased hypoxia-inducible factor (HIF-1)α expression
and increased carbonic anhydrase IX expression in the excised
specimens (9,11). However, no study has demonstrated an
improvement in intratumoral hypoxia by CO2 treatment in
living organisms. A noninvasive method of evaluation is required
for future clinical applications of CO2 treatment.
PET-CT using radiolabeled hypoxia-avid compounds is
a noninvasive method that can visualize hypoxia with several types
of hypoxic tracers. 18F-fluoromisonidazole (FMISO), a
labeled 2-nitroimidazole compound, is one of the most widely used
hypoxic tracers (12).
18F-FMISO PET-CT is a well-established method of hypoxic
imaging and has been used not only in animal research but also in
clinical practice (13).
This study aimed to determine the improvement in
intratumoral hypoxia by percutaneous CO2 treatment in
vivo using 18F-FMISO PET images.
Materials and methods
Animal preparation and implantation of
LM8 osteosarcoma cells
This study was approved by the Institutional Animal
Care and Use Committee (permit no. P160903 and 28-043-000) and was
performed in accordance with the Kobe University Animal
Experimentation Regulations and the Osaka University Regulations on
Animal Experiments.
A murine osteosarcoma cell line (LM8; cat. no.
RCB1450; Riken BRC Cell Bank) was used in the present study. The
cells were cultured in a growth medium composed of Dulbecco's
modified Eagle's medium (DMEM; Sigma-Aldrich; Merck KGaA) added to
10% (v/v) fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA) and
100 U/ml penicillin/streptomycin solution (Sigma-Aldrich; Merck
KGaA). The cells were maintained under 37°C in a humidified 5%
CO2 atmosphere.
Male BALB/c nude mice [age, 5 weeks; body weight
(mean ± standard deviation), 20.7±0.8 g] were obtained from CLEA
Japan, Inc. The animals were housed under pathogen-free conditions
in accordance with institutional principles. After one week, LM8
cells (1.0×106 cells in 500 µl PBS) were implanted into
the dorsal subcutaneous area of the mice as previously described
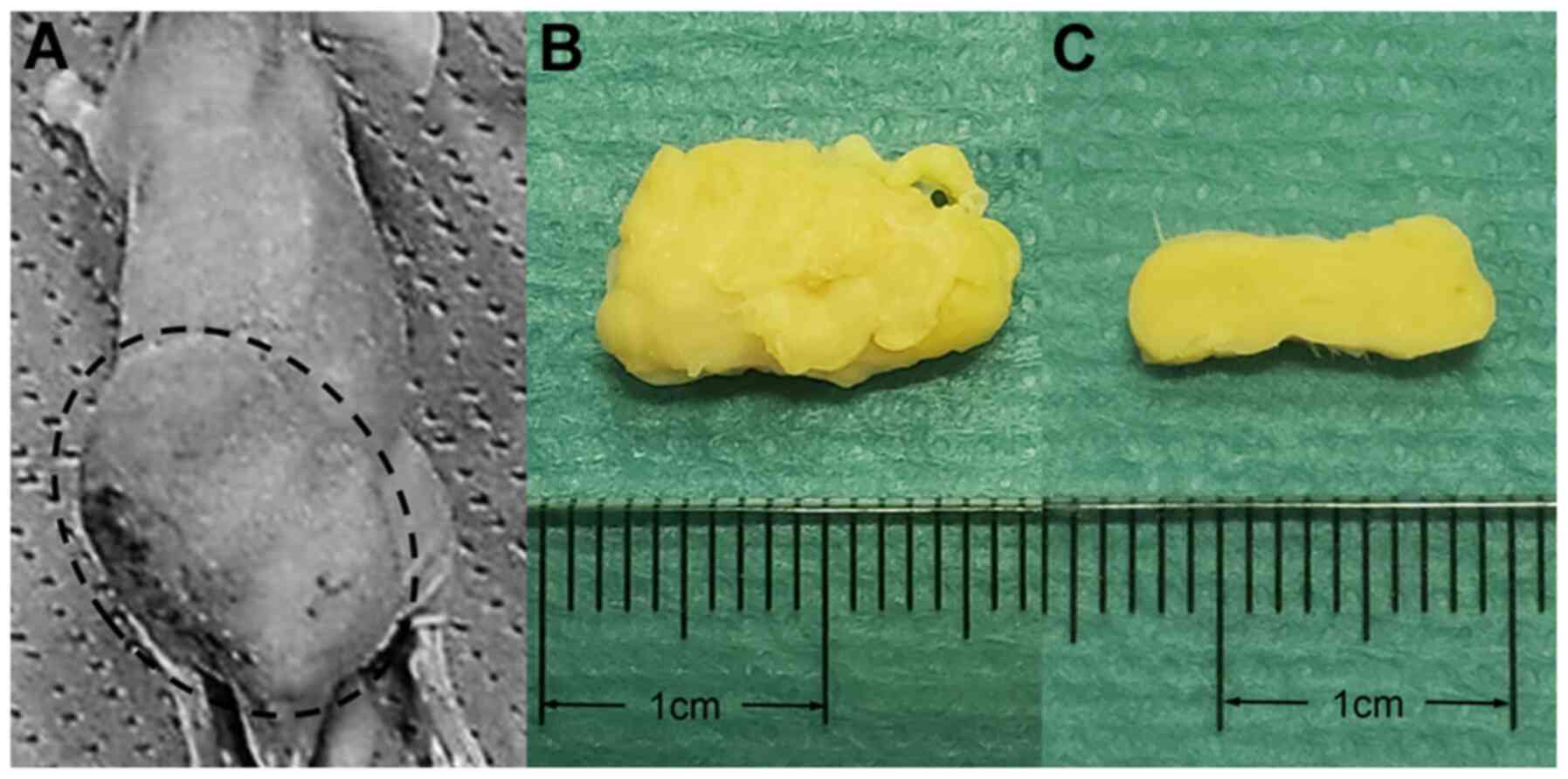
(Fig. 1) (14).
Animal studies
Transcutaneous administration of CO2 and
18F-FMISO PET-CT scans were scheduled at 2 weeks after
LM8 cell implantation. Mice with tumor xenografts (n=12) were
randomly assigned into two groups: CO2-treated group
(n=6) and an untreated group (n=6). Transcutaneous administration
of CO2 was performed in the CO2 treatment
group immediately after intravenous injection of
18F-FMISO, while room air was administered in the
untreated group. Each treatment was performed for 10 min. PET-CT
was performed 2 h after intravenous administration of
18F-FMISO. After the treatment and imaging, all mice
were euthanized by intraperitoneal injection of 200 mg/kg sodium
pentobarbital (Somnopenthyl, 64.8 mg/ml; Kyoritsu Seiyaku).
Transcutaneous CO2
application
Transcutaneous administration of CO2 was
performed as described previously (8,9,15). All mice were anesthetized with 2%
isoflurane in room air. The area of skin surrounding the implanted
tumor was covered with CO2 hydrogel. As this part was
contained, the lower part of the mouse was sealed with a
polyethylene bag, into which 100% CO2 was then
administered. The mice in the untreated group underwent the same
procedure except that CO2 was replaced with room
air.
18F-FMISO PET-CT scan
18F-FMISO was prepared using the same
method as previously mentioned (16). 18F-HF was produced by an
in-house cyclotron (HM-12S; Sumitomo Heavy Industry) and
18F-FMISO was prepared with a UG-M1 synthesizer module
using
3-(2-nitroimidazo-1-yl)-2-Otetrahydropyranyl-1-O-toluenesulfonyl-propanediol
as a labeling precursor. 18F-FMISO PET-CT was
subsequently performed (16,17).
All the mice were anesthetized with 2% isoflurane in
room air, and a Terumo 29-G needle-embedded insulin syringe was
inserted into the tail vein for 18F-FMISO injection. The
mean ± standard error of the mean (SEM) for the injected dose of
18F-FMISO was 19.38±0.82 MBq. In a small-animal PET
system (Inveon PET-CT system; Siemens Medical Solutions), the
animals were placed in a feet-first and supine position under
anesthesia with 2% isoflurane at room air. Static emission scans
were performed at 2 h post-injection, with an emission scan of 10
min, and CT acquisition was performed immediately before PET
acquisition.
All PET-CT images were reconstructed with
3-dimensional ordered-subset expectation maximization followed by
maximum a posteriori (OSEM3D-MAP) (16 subsets, 2 OSEM3D and 18 MAP
iterations) and CT-based attenuation correction. The image matrix
was 128 × 128 × 159, which produced a voxel size of 0.776 × 0.776 ×
0.796 mm.
Image analysis
All images were analyzed on a clinical Osirix MD
workstation (Pixmeo) using Metavol software (http://www.metavol.org/) (18).
The tumor volume was calculated based on the volume
measured on the CT images. 18F-FMISO uptake by the tumor
was evaluated quantitatively using the maximum standardized uptake
value (SUVmax), tumor-to-liver ration (TLR),
tumor-to-muscle ration (TMR), metabolic tumor volume (MTV) and
total lesion glycolysis (TLG). A polygonal volume of interest
(VOI), which included the entire lesion in the axial, sagittal, and
coronal planes, was also examined. The parameters were defined as
the following: SUVmax, the SUV of the single highest
voxel in the tumor; TMR, the ratio of SUVmax of the
tumor to the mean SUV of the gluteal muscle; TLR, the
SUVmax of the tumor/the mean SUV of the liver; MTV, the
volume of the tumor that exhibited FDG uptake; and TLG, the mean
SUV of the tumor × MTV. To determine the MTV, images of the tumor
were segmented using a fixed-threshold (SUV, 0.4), referring to the
past report (19). The threshold
value was determined by measuring 40% of the mean SUVmax
of all tumors (mean ± SEM, 1.07±0.28).
Statistical analysis
All parameters were compared between the two groups.
Data are expressed as the mean ± SEM, unless indicated otherwise.
The difference between the two groups was evaluated using the
Mann-Whitney U-test. All statistical analyses were performed using
EZR (Saitama Medical Center, Jichi Medical University), a graphical
user interface for R (The R Foundation for Statistical Computing;
version 2.13.0). It is a modified version of R commander (version
1.6–3), designed with additional statistical functions used more
frequently in biostatistics (20).
P<0.05 was considered to indicate a statistically significant
difference.
Results
A total of 12 mice [age, 8 weeks; body weight (mean
± SEM), 23.86±0.39 g] were randomly assigned into two groups: The
CO2-treated group (n=6) and the control group (n=6).
The animal characteristics are summarized in
Table I. There were no significant
differences between the two groups with respect to weight, injected
dose of 18F-FMISO, and the tumor volume.
 | Table I.Characteristics of the animals in the
present study. |
Table I.
Characteristics of the animals in the
present study.
|
Characteristics |
CO2-treated group, n=6 | Control group,
n=6 | P-value |
|---|
| Body weight, g |
|
| 0.818 |
| Mean ±
SEM | 23.98±0.55 | 23.73±0.59 |
|
|
Range | 22.62–26.06 | 21.92–25.79 |
|
| Injected dose of
18F-FMISO, MBq |
|
| 0.937 |
| Mean ±
SEM | 19.69±1.00 | 19.08±1.39 |
|
|
Range | 16.84–22.91 | 13.03–22.55 |
|
| Tumor volume,
cm3 |
|
| 0.485 |
| Mean ±
SEM | 1.178±0.450 | 1.368±0.295 |
|
|
Range | 0.361–3.148 | 0.433–2.151 |
|
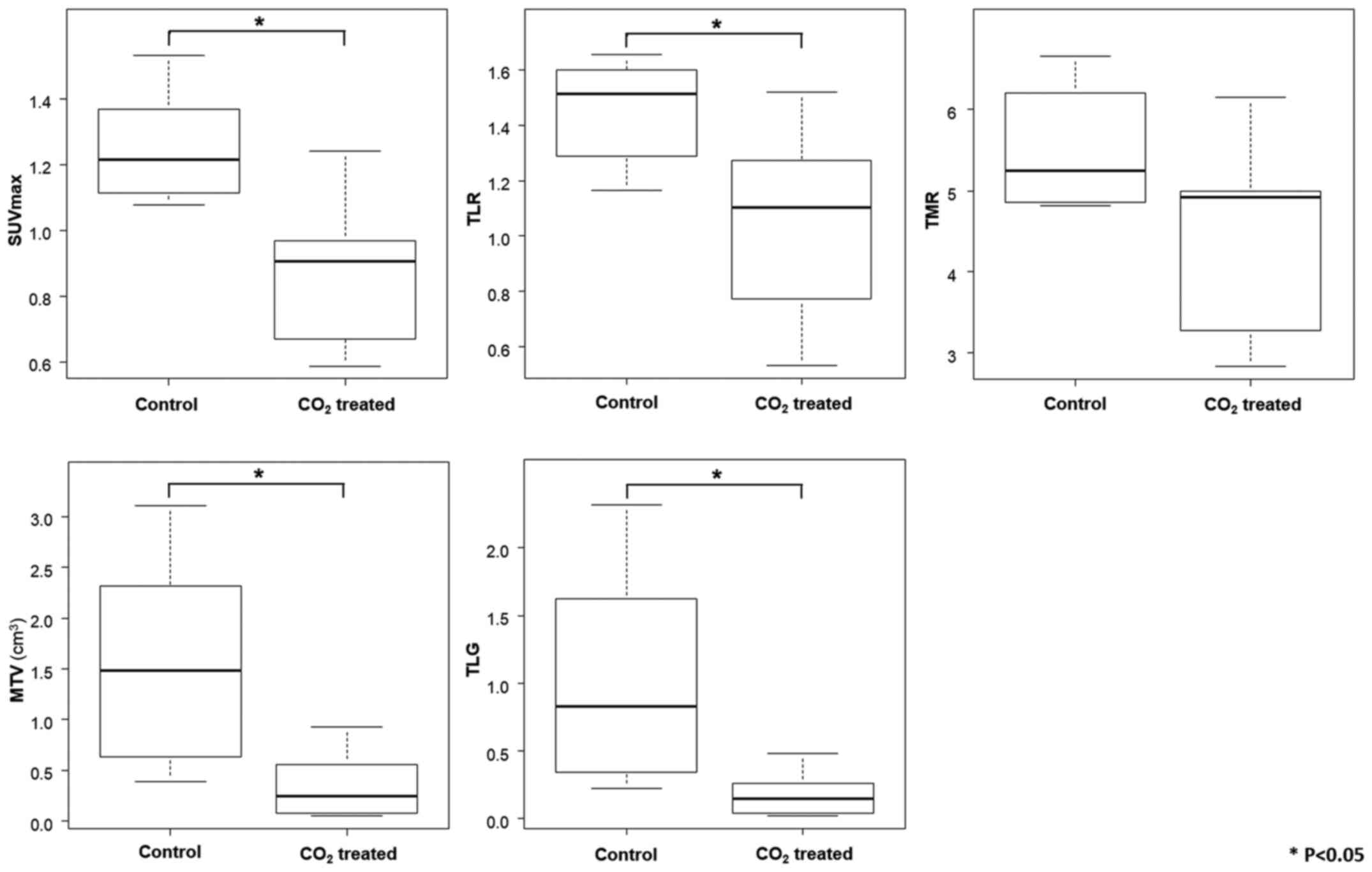
Fig. 2 shows the
representative cases in each group. The mean value of
SUVmax of the tumor, TMR, TLR, MTV and TLG are
summarized in Table II.
SUVmax of the tumor, TLR, MTV and TLG in the
CO2-treated group were significantly lower compared with
those in the control group. No significant difference in TMR was
observed between the two groups (Fig.
3).
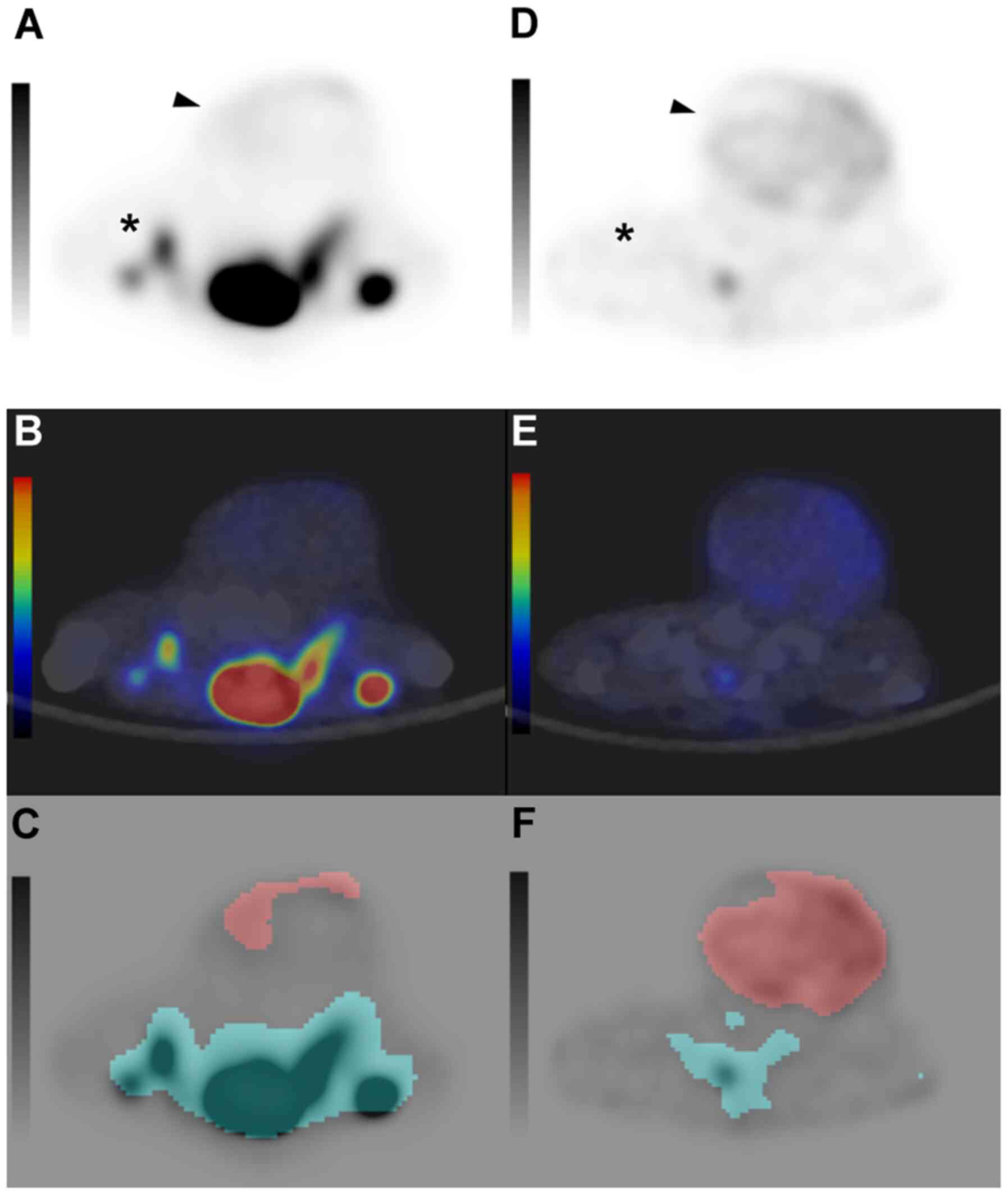 | Figure 2.Axial images of 18F-FMISO
PET-CT. (A-C) Axial images of 18F-FMISO PET, PET-CT
fusion, and the MTV measurement in the CO2-treated group
and (D-F) control group. 18F-FMISO PET and PET-CT fusion
image using a color map shows 18F-FMISO uptake in the
tumor located in the gluteal region of mice (arrowhead). Red area
on the MTV measurement image indicates a tumoral area with a SUV
exceeding 0.4. 18F-FMISO uptake in the
CO2-treated group (SUVmax, 0.669; MTV, 0.56
cm3) is lower compared with the control group
(SUVmax, 1.532; MTV, 3.111 cm3). The dorsal
uptake of the tumor (*) is physiological. 18F-FMISO,
18F- fluoromisonidazole; PET-CT positron emission
tomography/computed tomography; MTV, metabolic tumor volume; SUV,
standardized uptake value; SUVmax, maximum SUV. |
 | Table II.Mean SUVmax, TMR, TLR, MTV
and TLG values in the present study. |
Table II.
Mean SUVmax, TMR, TLR, MTV
and TLG values in the present study.
| Variables |
CO2-treated group, n=6 | Control group,
n=6 | P-value |
|---|
|
SUVmax |
|
| 0.015 |
| Mean ±
SEM | 0.880±0.095 | 1.253±0.071 |
|
|
Range | 0.587–1.241 | 1.076–1.532 |
|
| TLR |
|
| 0.041 |
| Mean ±
SEM | 1.063±0.147 | 1.455±0.078 |
|
|
Range | 0.533–1.521 | 1.165–1.654 |
|
| TMR |
|
| 0.240 |
| Mean ±
SEM | 4.520±0.503 | 5.504±0.310 |
|
|
Range | 2.836–6.144 | 4.816–6.656 |
|
| MTV,
cm3 |
|
| 0.015 |
| Mean ±
SEM | 0.353±0.139 | 1.569±0.438 |
|
|
Range | 0.073–0.934 | 0.391–3.111 |
|
| TLG |
|
| 0.015 |
| Mean ±
SEM | 0.182±0.070 | 1.028±0.338 |
|
|
Range | 0.022–0.483 | 0.220–2.318 |
|
Discussion
This study demonstrated the improvement in
intratumoral hypoxia by percutaneous CO2 treatment; this
was later ascertained using 18F-FMISO PET images in the
living state. As most tumors cannot be diagnosed clinically,
18F-FMISO PET may contribute to the clinical use of
CO2 treatment by measuring the response of various
tumors to CO2 administration.
The reoxygenation and anticancer effects of
CO2 therapy were previously reported (9). Transcutaneous CO2
application significantly decreased tumor growth in mice implanted
with LM8 cells, the same experimental platform used in the present
study. Apoptotic activity increased and intratumoral hypoxia
improved with decreased expression of HIF-1α. Pimonidazole, a
2-nitroimidazole that is activated specifically in hypoxic cells,
was used to assess intratumoral hypoxia directly. HIF-1α
accumulates under hypoxic conditions; along with its downstream
genes such as matrix metalloproteinases, it confers poor prognosis
in many types of tumors (9). The
previous studies were based on histological analysis alone; this
study suggests that the hypoxic condition actually improves in the
living state.
18F-FMISO has high affinity for hypoxic
cells with functional nitro reductase enzymes (21). The rate of 18F-FMISO
binding increases when the partial pressure of oxygen is less than
10 mmHg (22). Animal and human
model studies have demonstrated a significant correlation between
18F-FMISO uptake and the oxygen status of several tumors
including gliomas, non-small cell lung cancer, head and neck
cancer, cervical cancer, and rhabdomyosarcoma (12,14,23–25).
Recently, the 18F-FMISO uptake has been found to be
associated with prognosis after treatment in certain tumors, such
as in head and neck cancer (26,27).
18F-FMISO PET-CT may be utilized not only for evaluation
of the hypoxic state of the tumor before and after CO2
therapy, but also to predict the therapeutic effect.
In the present study, five quantitative parameters
were used for the analysis of 18F-FMISO PET-CT. PET
images are usually converted to SUVmax, which is the
most common conventional parameter. However, its use has some
limitations, in that it is affected by many patient-associated and
technical factors. Patient-associated factors include body size,
body fat percentage, blood glucose level and tumor type, while
technical factors include the signal-to-noise properties of PET
scanners, accuracy of the image reconstruction algorithm, and time
from administration to image acquisition. Without accounting for
all these sources of error, the SUVmax calculation could
show >50% error (28). Therefore,
other incremental parameters are commonly used to evaluate PET
imaging. The tumor-to-background ratio (TBR) is the ratio of
SUVmax of the tumor to the SUV of the non-tumoral area.
A blood pool from the left ventricle and aorta were used as a
background tissue in an 18F-FMISO study (17). However, the left ventricle and aorta
in mice are too small to set an accurate VOI; hence, the gluteal
muscle and liver were used as a background tissue. No significant
difference in TMR was found; this could have been due to the
oxygenation of the gluteal muscles by percutaneous CO2
treatment. TLR was considered to be more reliable than TMR in
measuring the therapeutic effect of CO2 in this study as
CO2 was not administered in the liver.
In some studies, single-voxel-based parameters such
as SUVmax and TBR could not evaluate the entire tumor
concentration, because most malignant tumors are heterogenous. The
18F-FMISO concentration in this study was not
homogeneous. This may reflect not only the heterogeneity of the
cancer cell, but also the existence of necrotic areas. Volume-based
parameters such as MTV and TLG may reveal overall tumor
concentrations. Some studies reported that MTV and TLG were
superior to single-voxel-based parameters in predicting treatment
response in many tumors. In the present study, both,
single-voxel-based and volume-based parameters decreased after
CO2 treatment, indicating an improvement in the hypoxic
condition of the tumor.
There are some limitations to the present study.
Firstly, 18F-FMISO was used, in which several hours are
required after administration for obtaining the sufficient
tumor/normal tissue ratio needed for visualization; due to its slow
clearance and high nonspecific accumulation in normal tissues.
Therefore, although the present study showed that CO2
treatment improved hypoxia, the temporal changes in intratumoral
hypoxia after treatment, including the duration of CO2
action, were not evaluated in the present study. Dynamic PET
studies or the use of newer tracers with higher clearance and
greater accumulation in the hypoxic region may help overcome this
limitation. Secondly, evaluation of true hypoxia is challenging in
areas of poor blood flow due to difficulty in 18F-FMISO
infusion. Thirdly, bone tumor cells were transplanted
subcutaneously, and the small animals used were different from the
patients encountered in actual clinical practice. It may be
necessary to conduct future research under conditions similar to
those in actual clinical practice. Finally, no immunohistochemical
assessment was conducted in this study; however, as mentioned,
immunohistochemical assessment had already been previously
performed using the same cell lines, and therefore were omitted
from the present study.
In conclusion, 18F-FMISO PET revealed
that CO2 treatment improved intratumoral hypoxia in
vivo. This technique enables assessment of the therapeutic
effect by imaging and may contribute to the clinical application of
CO2 treatment.
Acknowledgements
Not applicable.
Funding
This work was supported by JSPS KAKENHI (grant no.
JP18K15547).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KM, TO, TU and KI conducted all experiments. HI, YK,
KSa and TG contributed to the animal experiments. KM, EU, KSo, MN,
MY and KSu contributed to data interpretation or data analysis. TO,
YS, JH and TM conceived and designed this study. KM and TO wrote
the manuscript. All authors contributed to the writing of the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committees (approval nos. P160903 and
28-043-000) and performed in accordance with Animal Experimentation
Regulations of Kobe University Graduate School of Medicine and
Osaka University Graduate School of Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Laking G and Price P: Radionuclide imaging
of perfusion and hypoxia. Eur J Nucl Med Mol Imaging. 37 (Suppl
1):S20–S29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vaupel P: The role of hypoxia-induced
factors in tumor progression. Oncologist. 9 (Suppl 5):10–17. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jensen FB: Red blood cell pH, the Bohr
effect, and other oxygenation-linked phenomena in blood
O2 and CO2 transport. Acta Physiol Scand.
182:215–227. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mimeault M and Batra SK: Hypoxia-inducing
factors as master regulators of stemness properties and altered
metabolism of cancer- and metastasis-initiating cells. J Cell Mol
Med. 17:30–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hartmann BR, Bassenge E, Pittler M and
Hartmann BR: Effect of carbon dioxide-enriched water and fresh
water on the cutaneous microcirculation and oxygen tension in the
skin of the foot. Angiology. 48:337–343. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang J, Kang D, Wang Y, Yu Y, Fan J and
Takashi E: Carbonate ion-enriched hot spring water promotes skin
wound healing in nude rats. PLoS One. 10:e01171062015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakai Y, Miwa M, Oe K, Ueha T, Koh A,
Niikura T, Iwakura T, Lee SY, Tanaka M and Kurosaka M: A novel
system for transcutaneous application of carbon dioxide causing an
‘artificial Bohr effect’ in the human body. PLoS One. 6:e241372011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Onishi Y, Kawamoto T, Ueha T, Kishimoto K,
Hara H, Fukase N, Toda M, Harada R, Minoda M, Sakai Y, et al:
Transcutaneous application of carbon dioxide (CO2) induces
mitochondrial apoptosis in human malignant fibrous histiocytoma in
vivo. PLoS One. 7:e491892012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harada R, Kawamoto T, Ueha T, Minoda M,
Toda M, Onishi Y, Fukase N, Hara H, Sakai Y, Miwa M, et al:
Reoxygenation using a novel CO2 therapy decreases the
metastatic potential of osteosarcoma cells. Exp Cell Res.
319:1988–1997. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ueshima E, Yamaguchi M, Ueha T, Muradi A,
Okada T, Idoguchi K, Sofue K, Akisue T, Miwa M, Fujii M, et al:
Inhibition of growth in a rabbit VX2 thigh tumor model with
intraarterial infusion of carbon dioxide-saturated solution. J Vasc
Interv Radiol. 25:469–476. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Katayama N, Sugimoto K, Okada T, Ueha T,
Sakai Y, Akiyoshi H, Mie K, Ueshima E, Sofue K, Koide Y, et al:
Intra-arterially infused carbon dioxide-saturated solution for
sensitizing the anticancer effect of cisplatin in a rabbit VX2
liver tumor model. Int J Oncol. 51:695–701. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dubois L, Landuyt W, Haustermans K, Dupont
P, Bormans G, Vermaelen P, Flamen P, Verbeken E and Mortelmans L:
Evaluation of hypoxia in an experimental rat tumour model by
[(18)F]fluoromisonidazole PET and immunohistochemistry. Br J
Cancer. 91:1947–1954. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sato J, Kitagawa Y, Yamazaki Y, Hata H,
Okamoto S, Shiga T, Shindoh M, Kuge Y and Tamaki N:
18F-fluoromisonidazole PET uptake is correlated with
hypoxia-inducible factor-1α expression in oral squamous cell
carcinoma. J Nucl Med. 54:1060–1065. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Valk PE, Mathis CA, Prados MD, Gilbert JC
and Budinger TF: Hypoxia in human gliomas: demonstration by PET
with fluorine-18-fluoromisonidazole. J Nucl Med. 33:2133–2137.
1992.PubMed/NCBI
|
|
15
|
Onishi Y, Kawamoto T, Ueha T, Hara H,
Fukase N, Toda M, Harada R, Sakai Y, Miwa M, Nishida K, et al:
Transcutaneous application of carbon dioxide (CO2)
enhances chemosensitivity by reducing hypoxic conditions in human
malignant fibrous histiocytoma. J Cancer Sci Ther. 4:72012.
View Article : Google Scholar
|
|
16
|
Watabe T, Kanai Y, Ikeda H, Horitsugi G,
Matsunaga K, Kato H, Isohashi K, Abe K, Shimosegawa E and Hatazawa
J: Quantitative evaluation of oxygen metabolism in the intratumoral
hypoxia: 18F-fluoromisonidazole and 15O-labelled gases
inhalation PET. EJNMMI Res. 7:162017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koyasu S, Tsuji Y, Harada H, Nakamoto Y,
Nobashi T, Kimura H, Sano K, Koizumi K, Hamaji M and Togashi K:
Evaluation of tumor-associated stroma and its relationship with
tumor hypoxia using dynamic contrast-enhanced CT and (18)F
misonidazole PET in murine tumor models. Radiology. 278:734–741.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hirata K, Kobayashi K, Wong KP, Manabe O,
Surmak A, Tamaki N and Huang SC: A semi-automated technique
determining the liver standardized uptake value reference for tumor
delineation in FDG PET-CT. PLoS One. 9:e1056822014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Im HJ, Bradshaw T, Solaiyappan M and Cho
SY: Current methods to define metabolic tumor volume in positron
emission tomography: Which one is better? Nucl Med Mol Imaging.
52:5–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee ST and Scott AM: Hypoxia positron
emission tomography imaging with 18F-fluoromisonidazole.
Semin Nucl Med. 37:451–461. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Krohn KA, Link JM and Mason RP: Molecular
imaging of hypoxia. J Nucl Med. (Suppl 2):49:129S–148S. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eschmann SM, Paulsen F, Reimold M,
Dittmann H, Welz S, Reischl G, Machulla HJ and Bares R: Prognostic
impact of hypoxia imaging with 18F-misonidazole PET in
non-small cell lung cancer and head and neck cancer before
radiotherapy. J Nucl Med. 46:253–260. 2005.PubMed/NCBI
|
|
24
|
Gagel B, Reinartz P, Demirel C, Kaiser HJ,
Zimny M, Piroth M, Pinkawa M, Stanzel S, Asadpour B, Hamacher K, et
al: [18F] fluoromisonidazole and [18F] fluorodeoxyglucose positron
emission tomography in response evaluation after
chemo-/radiotherapy of non-small-cell lung cancer: A feasibility
study. BMC Cancer. 6:512006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zimny M, Gagel B, DiMartino E, Hamacher K,
Coenen HH, Westhofen M, Eble M, Buell U and Reinartz P: FDG - a
marker of tumour hypoxia? A comparison with [18F]fluoromisonidazole
and pO2-polarography in metastatic head and neck cancer.
Eur J Nucl Med Mol Imaging. 33:1426–1431. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kikuchi M, Yamane T, Shinohara S, Fujiwara
K, Hori SY, Tona Y, Yamazaki H, Naito Y and Senda M:
18F-fluoromisonidazole positron emission tomography
before treatment is a predictor of radiotherapy outcome and
survival prognosis in patients with head and neck squamous cell
carcinoma. Ann Nucl Med. 25:625–633. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Asano A, Ueda S, Kuji I, Yamane T,
Takeuchi H, Hirokawa E, Sugitani I, Shimada H, Hasebe T, Osaki A,
et al: Intracellular hypoxia measured by
18F-fluoromisonidazole positron emission tomography has
prognostic impact in patients with estrogen receptor-positive
breast cancer. Breast Cancer Res. 20:782018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mah K and Caldwell CB: Biological target
volume. PET-CT in Radiotherapy Treatment Planning. Paulino AC and
Teh BS: Content Repository Only; Philadelphia: pp. 52–89. 2008,
View Article : Google Scholar
|