Introduction
Prostate cancer is the third most common oncological
disease in men, according to the incidence and mortality rates in
Slovakia (1). Endogenous sex steroid
hormones, along with environmental and dietary factors, and immune
and inflammatory responses are involved in the pathogenesis of
prostate cancer (2). Prostate cancer
is an androgen-dependent tumor, which notably increases with age.
However, there is consistent evidence that both total and
bioavailable serum testosterone levels significantly decline with
age (3). Circulating testosterone
levels decline with age at a greater extent compared with that in
circulating estradiol, resulting in an elevated ratio of estradiol
to testosterone. The increased ratio might also indirectly reflect
aromatase activity and a higher conversion of testosterone to
estradiol at an older age (4).
Estrogens play an important role in male sex hormone secretion, in
the growth, differentiation and homeostasis of normal prostate
tissue as well as in prostate carcinogenesis (5). Epidemiological studies have not
confirmed the direct association between serum estrogen levels and
prostate cancer risk (6–8), there is a possibility that
intraprostatic estrogen milieu may play a more important role than
circulating estrogen levels.
Estrogen action is commonly mediated by two
receptors, estrogen receptor α (ERα) and estrogen receptor β (ERβ),
which are encoded by separate genes (ESR1 and ESR2, respectively).
Both receptors belong to nuclear receptors, acting as
ligand-activated transcription factors. ERα and ERβ share high
sequence homology, particularly in a DNA-binding domain, allowing
both receptors to recognize the estrogen-responsive element on the
DNA. Lower sequence homology has been described in the
ligand-binding domain, suggesting that both receptors may have
different specific ligands (9).
Estrogen receptors activated by their ligands, act through two
signaling mechanisms. The main mechanism includes diffusion of
estrogens across the cell membrane and their binding to estrogen
receptors. The receptors then dimerize and bind to estrogen
responsible element sequences in the promoter region of the target
genes and such affect gene transcription. The second mechanism is
mediated by membrane-localized estrogen receptors. The binding of
steroid ligands leads to rapid signaling mediated by G protein
activation. This includes the generation of cyclic nucleotides
(cAMP and cGMP) and calcium efflux, which activates kinase cascades
(10).
The exact role of estrogen receptors in prostate
carcinogenesis requires further elucidation. It is hypothesized
that the two types of estrogen receptors have different roles in
prostate cancer. ERα is proposed to contribute to cellular
proliferation, inflammation and has been found to be upregulated in
malignant epithelial prostate tissue (11), while ERβ exhibits antiproliferative,
anti-invasive and proapoptotic effects (12,13), and
its expression declines during the development of prostate cancer
(14).
The ESR2 gene, encoding ERβ, is located on
chromosome 14q23.1 (15) and is
expressed in both stromal and epithelial cells of the prostate. The
loss of ESR2 expression may be considered as a risk factor
for prostate cancer (16). The
precise mechanism of how ESR2 is regulated in prostate
cancer cells is still unknown. Decreased ESR2 expression may
be caused by the methylation of CpG islands, located in the
promoter region (17). The presence
of polymorphisms in the coding regions of the gene may also affect
gene expression levels or transcript stability. Among the most
extensively studied polymorphisms in the ESR2 gene are
rs1256049 and rs4986938; however, the functional significance of
both polymorphisms is still unknown. The rs1256049 is a synonymous
variant located within the ligand binding domain in exon 5
(18). Meta-analysis has shown
significant association between rs1256049 and prostate cancer in
Caucasians, but not in overall population (19). The second polymorphism, rs4986938
represents a G>A transition in the 3′-untranslated region of
exon 8 (20). It is hypothesized
that the untranslated regions of genes are regulatory elements,
controlling translation and may be a target for microRNAs (21). Numerous studies have investigated the
association between the rs4986938 polymorphism and different types
of cancer; it was found to be associated with breast (22,23) and
prostate cancers (24). However, a
meta-analysis conducted to investigate the association of rs4986938
and the overall risk of cancer found no significant associations
(25). The polymorphism rs3020449 is
located near the transcription start site of the promoter 0N of the
ESR2 gene. It is hypothesized that polymorphisms located in
the promoter region could affect transcription factor binding and
affect gene transcription (26). The
association between rs3020449 and prostate cancer has not been
investigated; however, it was found to be associated with other
oncological diseases, such as endometrial (27), ovarian (28) and breast cancers (29).
The aim of the present study was to determine the
ESR2 expression levels in hyperplastic and malignant
prostate tissues and analyze the possible association of three
polymorphisms in the ESR2 gene (rs3020449, rs4986938 and
rs1256049) with prostate cancer development and progression.
Materials and methods
Study population
The case-control study included 510 patients with
histologically verified prostate cancer and 184 healthy men. Tissue
samples from 22 patients with prostate cancer and 12 patients with
benign prostatic hyperplasia (BHP) were collected during routine
surgery, placed into RNA stabilization solution and stored at −80°C
until further analysis. All patients were recruited at the
Department of Urology, University Hospital Martin in Slovakia,
between 2005 and 2019. Healthy volunteers were selected from men
attending routine urological examination and were confirmed to have
no history of cancer or any prostate disease. The present study was
approved by the Ethics Committee of Comenius University in
Bratislava, Jessenius Faculty of Medicine in Martin and all men
provided written informed consent to participate in the study. The
clinical characteristics of the study groups are summarized in
Table I.
 | Table I.Characteristics of patients with
prostate cancer and healthy subjects. |
Table I.
Characteristics of patients with
prostate cancer and healthy subjects.
|
Characteristics | Healthy controls
(n=184) | Prostate cancer
(n=510) |
|---|
| Age, years |
|
|
| Mean ±
SD | 57.61±10.39 | 67±8.26 |
| PSA, ng/ml |
|
|
| Median
(25–75th percentile) | 0.81
(0.49–1.60) | 10.57
(5.84–28.76) |
| Gleason score |
|
|
| ≤7 | NA | 270 |
|
>7 | NA | 136 |
| Mean ±
SD | NA | 7.28±1.25 |
|
Missing | NA | 104 |
| Pathological
stage |
|
|
|
pT1/pT2 | NA | 88 |
|
pT3/pT4 | NA | 148 |
|
Missing | NA | 274 |
Genotyping
Genomic DNA samples from the individuals were
isolated from whole blood using The Wizard® Genomic DNA
Purification kit (Promega Corporation) according to the
manufacturer's protocol and stored at −20°C until further analysis.
The ESR2 gene polymorphism, rs3020449 was analyzed using
tetra-primer amplification refractory mutation system PCR approach
(30) allowing allele-specific
amplification using the following primers: IP1,
5′-GCATTGTCCTTTTTACATATTGTTAGGGTA-3′; IP2,
5′-AATTCTCAAGGAAATTTTAGCAAAGCC-3′; OP1,
5′-TAGATTTTGTCAAACACTTTTGGTGGAT-3′; OP2,
5′-CCAAATGATTAAGGAGAAATAACAGCAG-3′. The PCR Master mix contained
100 ng genomic DNA, 2.4 µl 5X HOT FIREPol® Blend Master
Mix RTL (Solis BioDyne OÜ), 0.5 µl each primer and 6.6 µl
nuclease-free water. The following thermocycling conditions were
used: Initial denaturation at 95°C for 15 min followed by 35 cycles
at 95°C for 20 sec, 56°C for 50 sec and 72°C for 1 min, with a
final extension at 72°C for 5 min. The PCR products were separated
using 2% agarose gel electrophoresis. The allele-specific product
size for rs3020449 was 231 or 193 bp for the A and G alleles,
respectively).
The ESR2 gene polymorphisms, rs4986938 and
rs1256049 were determined using the PCR-restriction fragment length
polymorphism method and the following primers: rs4986938 forward,
5′-GACCTGCTGCTGGAGATGCT-3′ and reverse, 5′-AATGAGGGACCACACAGCA-3′;
and rs1256049 forward, 5′-TCTTGCTTTCCCCAGGCTTT-3′ and reverse,
5′-ACCTGTCCAGAACAAGATCT-3′. The PCR Master mix contained 100 ng
genomic DNA, 6 µl Dream Taq Green PCR master mix (2X) (Thermo
Fisher Scientific Inc.), 30 ng forward and reverse primers and
nuclease-free water to a total volume of 12 µl. The following
thermocycling conditions were used: Initial denaturation at 95 C
for 5 min followed by 35 cycles at 95°C for 20 sec, 58°C for 50 sec
for rs4986938 or 56°C for 50 sec for rs1256049, and 72°C for 1 min,
with a final extension at 72°C for 5 min. The PCR products of the
rs4986938 polymorphism were digested with AluI, which
produced a 234 bp sized band for the GG genotype; 168 and 66 bp
sized bands for the AA genotype and 234, 168 and 66 bp sized bands
for the GA genotype. The PCR products of the rs1256049 polymorphism
were digested with RsaI, which produced a 156 bp sized band
for the GG genotype; 110 and 46 bp sized bands for the AA genotype
and 156, 110 and 46 bp sized bands for the GA genotype.
Gene expression analysis
Isolation of total RNA was performed using an
AllPrep DNA/RNA/miRNA Universal kit (Qiagen GmbH) according to the
manufacturer's protocol. For each sample, an equal quantity of RNA
(1 µg) was used for reverse transcription into cDNA with a RT2
First Strand kit, following the standard protocol (Qiagen GmbH).
Reverse transcription-quantitative PCR analysis of the ESR2
expression level was performed using Custom RT2 Profiler
PCR array (Qiagen GmbH). GAPDH and actin served as
housekeeping genes.
Statistical analysis
Genotype frequencies were calculated for the
patients with prostate cancer and the healthy controls. Observed
genotype frequencies were tested for Hardy-Weinberg equilibrium in
the control group. Dominant, codominant and recessive genetic
models were evaluated. The comparison of the genotype distribution
and association with selected clinical data was performed using a
Fisher's exact test. Fisher's exact test, calculation of odds
ratios and 95% confidence intervals (CIs) were performed using the
StatsDirect statistical package (v2.7.0.2). The test for linkage
disequilibrium of the selected polymorphisms was performed using
Haploview 4.2 software.
The relative quantification method was used for the
analysis of ESR2 expression levels. The Cq values
of the ESR2 gene were compared with the average
Cq values of the two housekeeping genes to obtain
ΔCq values. The fold-change was calculated as
2−ΔΔCq (31). The data
are represented in the figures as median ± IQR. The Mann-Whitney
test was used for the comparison of the ESR2 mRNA expression
levels between patients with prostate cancer and with BHP. The
Mann-Whitney test with Bonferroni correction was used for the
comparison of the ESR2 mRNA expression levels between
rs3020449 genotypes. All P-values were derived from two-sided tests
and P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed using
the StatsDirect statistical package (v2.7.0.2).
Results
Genotype analysis
The genotype frequencies of the three analyzed
ESR2 variants did not deviate from the Hardy-Weinberg
equilibrium and their genotyping success rates were over 95%. The
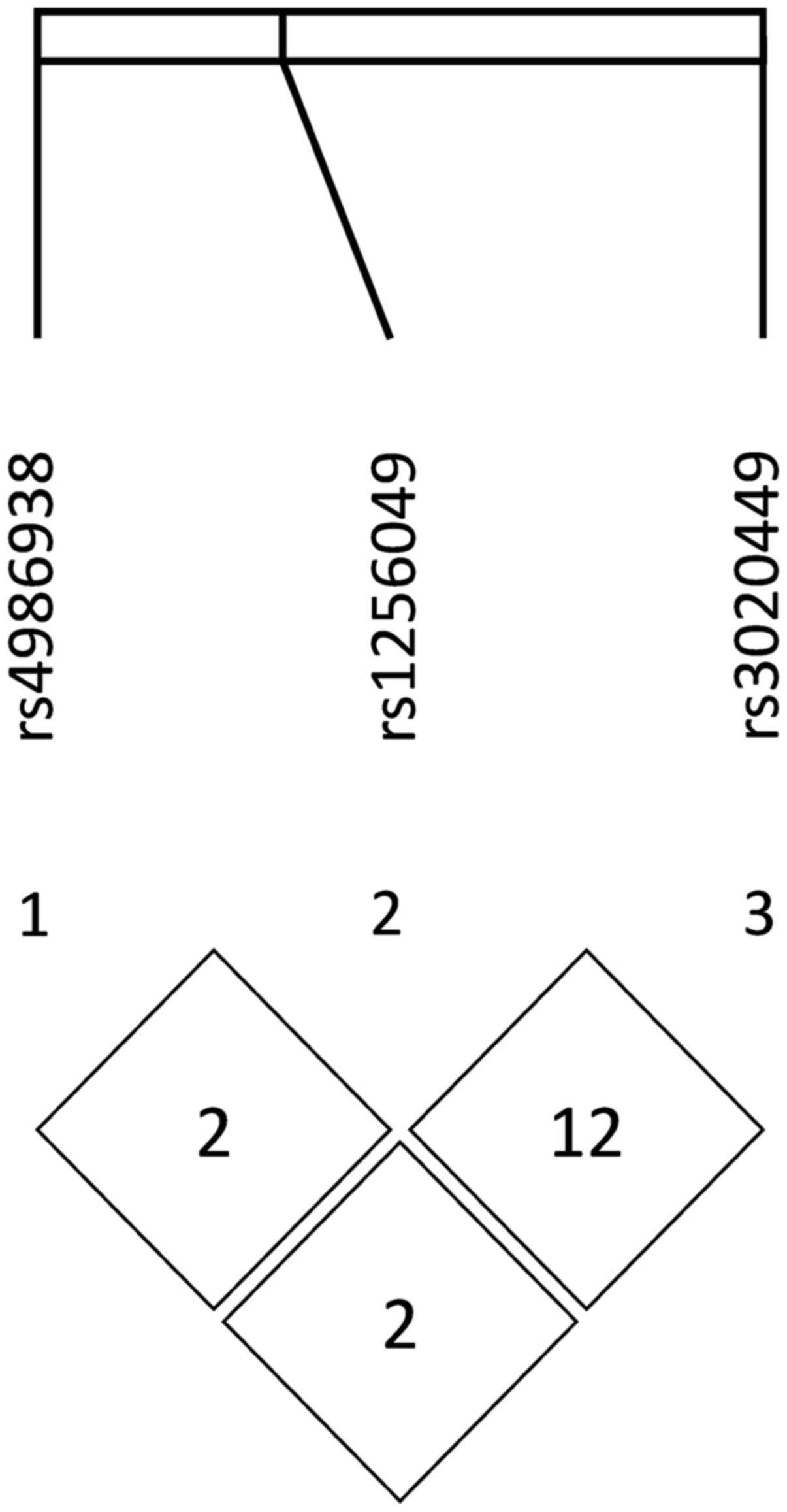
analyzed polymorphisms were not found to be in linkage
disequilibrium, while the estimated R2 values were 0.02,
0.02 and 0.12, respectively (Fig.
1). The distribution of the genotypes and alleles of the three
analyzed ESR2 polymorphisms in both the control group and in
the patients with prostate cancer are summarized in Table II. Dominant, codominant and
recessive genetic models were evaluated.
 | Table II.Distribution of the ESR2
genotypes and alleles and their association with the risk of
prostate cancer. |
Table II.
Distribution of the ESR2
genotypes and alleles and their association with the risk of
prostate cancer.
|
|
|
| Controls vs.
Prostate cancer |
|---|
|
|
|
|
|
|---|
| Genotype | Healthy controls,
n | Prostate cancer,
n | OR (95% CI) | P-value |
|---|
| ESR2
rs3020449 |
|
|
|
|
|
Codominant model |
|
|
|
|
|
AA | 75 | 173 | 1.00 (ref.) |
|
|
AG | 90 | 234 | 1.13
(0.77–1.65) | 0.58 |
|
GG | 19 | 103 | 2.35
(1.31–4.36) | 0.002a |
|
Dominant model |
|
|
|
|
|
AA | 75 | 173 | 1.00 (ref.) |
|
|
AG + GG | 109 | 337 | 1.34
(0.93–1.92) | 0.11 |
|
Recessive model |
|
|
|
|
|
AA + AG | 165 | 407 | 1.00 (ref.) |
|
|
GG | 19 | 103 | 2.20
(1.29–3.92) | 0.002a |
|
Allele |
|
|
|
|
|
A | 240 | 580 | 1.00 (ref.) |
|
|
G | 128 | 440 | 1.42
(1.10–1.84) | 0.005a |
| ESR2
rs1256049 |
|
|
|
|
|
Codominant model |
|
|
|
|
|
GG | 166 | 460 | 1.00 (ref.) |
|
|
GA | 18 | 47 | 0.94
(0.52–1.78) | 0.88 |
|
AA | 0 | 3 | NA | NA |
|
Dominant model |
|
|
|
|
|
GG | 166 | 460 | 1.00 (ref.) |
|
|
GA + AA | 18 | 50 | 1.00
(0.56–1.88) | 1.00 |
|
Allele |
|
|
|
|
|
G | 350 | 967 | 1.00 (ref.) |
|
|
A | 18 | 53 | 1.07
(0.60–1.96) | 0.89 |
| ESR2
rs4986938 |
|
|
|
|
|
Codominant model |
|
|
|
|
|
GG | 88 | 228 | 1.00 (ref.) |
|
|
GA | 77 | 229 | 1.15
(0.79–1.67) | 0.47 |
|
AA | 19 | 49 | 1.00
(0.54–1.89) | >0.99 |
|
Dominant model |
|
|
|
|
|
GG | 88 | 228 | 1.00 (ref.) |
|
|
GA + AA | 96 | 278 | 1.12
(0.79–1.59) | 0.55 |
|
Recessive model |
|
|
|
|
|
GG + GA | 165 | 457 | 1.00 (ref.) |
|
|
AA | 19 | 49 | 0.93
(0.52–1.73) | 0.77 |
|
Allele |
|
|
|
|
|
G | 253 | 685 | 1.00 (ref.) |
|
|
A | 115 | 327 | 1.05
(0.81–1.37) | 0.74 |
There was a statistically significant association
between the rs3020449 GG genotype [odds ratio (OR), 2.35; 95% CI
1.31–4.36; P=0.002] compared with that in the AA genotype, as well
as in the recessive model (OR, 2.20; 95% CI 1.23–3.92; P=0.002) and
the higher risk of prostate cancer development. The frequency of
the rs3020449 G allele (OR, 1.42; 95% CI 1.10–1.84; P=0.005) was
also significantly higher in the patients with prostate cancer
(Table II). The other two analyzed
ESR2 variants, rs4986938 and rs1256049, were not found to be
associated with the risk of prostate cancer development.
To detect the possible associations between the
ESR2 polymorphisms and the selected clinical features,
patients were stratified according to Gleason score (≤7 and >7),
pathological T stage (pT1/pT2 and pT3/pT4) and prostate-specific
antigen levels (<10 and ≥10 ng/ml). There was a statistically
significant association between the rs3020449 GG genotype and a
higher risk of development of carcinoma with a Gleason score ≤7
(OR, 1.97; 95% CI 1.09–3.86; P=0.029); however, a more significant
association was observed in patients with Gleason score >7 (OR,
2.66; 95% CI 1.27–5.64; P=0.005). The rs3020449 G allele was
significantly associated with development of carcinoma with a
Gleason score >7 (OR, 1.53; 95% CI 1.09–2.13; P=0.01) (Table III).
 | Table III.Association between the ESR2
genotypes and alleles and Gleason score in prostate cancer
patients. |
Table III.
Association between the ESR2
genotypes and alleles and Gleason score in prostate cancer
patients.
|
| Gleason score
≤7 | Gleason score
>7 |
|---|
|
|
|
|
|---|
| Genotype | n | OR (95% CI) | P-value | n | OR (95% CI) | P-value |
|---|
| ESR2
rs3020449 |
|
|
|
|
|
|
|
Codominant model |
|
|
|
|
|
|
|
AA | 100 | 1.00 (ref.) |
| 43 | 1.00 (ref.) |
|
|
AG | 120 | 1.00
(0.65–1.53) | 1.00 | 64 | 1.24
(0.74–2.10) | 0.45 |
|
GG | 50 | 1.97
(1.04–3.84) | 0.029a | 29 | 2.66
(1.27–5.64) | 0.005a |
|
Dominant model |
|
|
|
|
|
|
|
AA | 100 | 1.00 (ref.) |
| 43 |
|
|
|
AG + GG | 170 | 1.17
(0.78–1.75) | 0.43 | 93 | 1.49
(0.91–2.44) | 0.10 |
|
Recessive model |
|
|
|
|
|
|
|
AA + AG | 220 | 1.00 (ref.) |
| 107 | 1.00 (ref.) |
|
|
GG | 50 | 1.97
(1.09–3.68) | 0.02a | 29 | 2.35
(1.20–4.67) | 0.007a |
|
Allele |
|
|
|
|
|
|
|
A | 320 | 1.00 (ref.) |
| 150 | 1.00 (ref.) |
|
|
G | 220 | 1.29
(0.97–1.71) | 0.07 | 122 | 1.53
(1.09–2.13) | 0.01a |
| ESR2
rs1256049 |
|
|
|
|
|
|
|
Codominant model |
|
|
|
|
|
|
|
GG | 242 | 1.00 (ref.) |
| 122 | 1.00 (ref.) |
|
|
GA | 28 | 1.06
(0.55–2.12) | 0.88 | 11 | 0.83
(0.34–1.94) | 0.70 |
|
AA | 0 | NA | NA | 3 | NA | NA |
|
Dominant model |
|
|
|
|
|
|
|
GG | 242 | 1.00 (ref.) |
| 122 | 1.00 (ref.) |
|
|
GA + AA | 28 | 1.06
(0.55–2.12) | 0.88 | 14 | 1.05
(0.47–2.35) | 1.00 |
|
Allele |
|
|
|
|
|
|
|
G | 512 | 1.00 (ref.) |
| 255 | 1.00 (ref.) |
|
|
A | 28 | 1.06
(0.56–2.07) | 0.88 | 17 | 1.30
(0.61–2.72) | 0.49 |
| ESR2
rs4986938 |
|
|
|
|
|
|
|
Codominant model |
|
|
|
|
|
|
|
GG | 118 | 1.00 (ref.) |
| 66 | 1.00 (ref.) |
|
|
GA | 125 | 1.21
(0.80–1.83) | 0.36 | 54 | 0.93
(0.57–1.54) | 0.81 |
|
AA | 26 | 1.02
(0.51–2.08) | 1.00 | 13 | 0.91
(0.38–2.11) | 0.85 |
|
Dominant model |
|
|
|
|
|
|
|
GG | 118 | 1.00 (ref.) |
| 66 | 1.00 (ref.) |
|
|
GA + AA | 151 | 1.17
(0.79–1.74) | 0.44 | 67 | 0.93
(0.58–1.49) | 0.82 |
|
Recessive model |
|
|
|
|
|
|
|
GG + GA | 243 | 1.00 (ref.) |
| 120 | 1.00 (ref.) |
|
|
AA | 26 | 0.93
(0.48–1.84) | 0.87 | 13 | 0.94
(0.41–2.10) | 1.00 |
|
Allele |
|
|
|
|
|
|
|
G | 361 | 1.00 (ref.) |
| 186 | 1.00 (ref.) |
|
|
A | 177 | 1.08
(0.80–1.45) | 0.61 | 80 | 0.94
(0.66–1.35) | 0.79 |
After stratification of the patients with prostate
cancer according to pathological T stage, a significant association
between the rs3020449 GG genotype (OR, 2.28; 95% CI 1.10–4.76;
P=0.02), as well as with the G allele (OR, 1.39; 95% CI 1.00–1.93;
P=0.04) and the development of carcinoma with pT3/pT4 was detected.
In the group of patients with pT1/pT2, there was no significant
association with increased risk. The results are summarized in
Table IV. The rs3020449 GG genotype
was significantly associated with a higher risk of prostate cancer
development in both groups of patients with PSA <10 ng/ml (OR,
2.24; 95% CI 1.19–4.26; P=0.01), as well as with PSA≥10 ng/ml (OR,
2.10; 95% CI 1.12–4.00; P=0.02) (Table
V). There was no association between the rs4986938 and
rs1256049 variants and Gleason score, pathological T stage and PSA
levels in patients with prostate cancer (Tables III–V).
 | Table IV.Distribution of the ESR2
genotypes and alleles in patients stratified according to the
pathological stage. |
Table IV.
Distribution of the ESR2
genotypes and alleles in patients stratified according to the
pathological stage.
|
| pT1/pT2 | pT3/pT4 |
|---|
|
|
|
|
|---|
| Genotype | n | OR (95% CI) | P-value | n | OR (95% CI) | P-value |
|---|
| ESR2
rs3020449 |
|
|
|
|
|
|
|
Codominant model |
|
|
|
|
|
|
|
AA | 35 | 1.00 (ref.) |
| 52 | 1.00 (ref.) |
|
|
AG | 37 | 0.88
(0.49–1.59) | 0.67 | 66 | 1.06
(0.64–1.75) | 0.90 |
|
GG | 16 | 1.80
(0.77–4.20) | 0.16 | 30 | 2.28
(1.10–4.76) | 0.02a |
|
Dominant model |
|
|
|
|
|
|
|
AA | 35 | 1.00 (ref.) |
| 52 | 1.00 (ref.) |
|
|
AG + GG | 53 | 1.04
(0.60–1.81) | 0.90 | 96 | 1.27
(0.79–2.04) | 0.31 |
|
Recessive model |
|
|
|
|
|
|
|
AA + AG | 72 | 1.00 (ref.) |
| 118 | 1.00 (ref.) |
|
|
GG | 16 | 1.93
(0.87–4.21) | 0.08 | 30 | 2.21
(1.14–4.36) | 0.01a |
|
Allele |
|
|
|
|
|
|
|
A | 107 | 1.00 (ref.) |
| 170 | 1.00 (ref.) |
|
|
G | 69 | 1.21
(0.82–1.78) | 0.34 | 126 | 1.39
(1.00–1.93) | 0.04a |
| ESR2
rs1256049 |
|
|
|
|
|
|
|
Codominant model |
|
|
|
|
|
|
|
GG | 82 | 1.00 (ref.) |
| 133 | 1.00 (ref.) |
|
|
GA | 7 | 0.79
(0.27–2.08) | 0.66 | 15 | 1.04
(0.47–2.28) | 1.00 |
|
AA | 0 | NA | NA | 3 | NA | NA |
|
Dominant model |
|
|
|
|
|
|
|
GG | 82 | 1.00 (ref.) |
| 133 | 1.00 (ref.) |
|
|
GA + AA | 7 | 0.79
(0.27–2.08) | 0.66 | 18 | 1.25
(0.59–2.65) | 0.60 |
|
Allele |
|
|
|
|
|
|
|
G | 171 | 1.00 (ref.) |
| 281 | 1.00 (ref.) |
|
|
A | 7 | 0.79
(0.28–2.05) | 0.83 | 21 | 1.45
(0.72–2.95) | 0.32 |
| ESR2
rs4986938 |
|
|
|
|
|
|
|
Codominant model |
|
|
|
|
|
|
|
GG | 40 | 1.00 (ref.) |
| 74 | 1.00 (ref.) |
|
|
GA | 42 | 1.20
(0.68–2.11) | 0.59 | 57 | 0.83
(0.51–1.36) | 0.48 |
|
AA | 7 | 0.81
(0.27–2.22) | 0.82 | 16 | 1.00
(0.45–2.22) | 1.00 |
|
Dominant model |
|
|
|
|
|
|
|
GG | 40 | 1.00 (ref.) |
| 74 | 1.00 (ref.) |
|
|
GA + AA | 49 | 1.12
(0.66–1.93) | 0.70 | 73 | 0.90
(0.57–1.42) | 0.66 |
|
Recessive model |
|
|
|
|
|
|
|
GG + GA | 82 | 1.00 (ref.) |
| 131 | 1.00 (ref.) |
|
|
AA | 7 | 0.74
(0.25–1.94) | 0.66 | 16 | 1.06
(0.49–2.27) | 1.00 |
|
Allele |
|
|
|
|
|
|
|
G | 122 | 1.00 (ref.) |
| 205 | 1.00 (ref.) |
|
|
A | 56 | 1.01
(0.67–1.51) | 1.00 | 89 | 0.96
(0.68–1.35) | 0.80 |
 | Table V.Distribution of the ESR2
genotypes and alleles in patients stratified according to the PSA
levels. |
Table V.
Distribution of the ESR2
genotypes and alleles in patients stratified according to the PSA
levels.
|
| PSA <10
ng/ml | PSA ≥10 ng/ml |
|---|
|
|
|
|
|---|
| Genotype | n | OR (95% CI) | P-value | n | OR (95% CI) | P-value |
|---|
| ESR2
rs3020449 |
|
|
|
|
|
|
|
Codominant model |
|
|
|
|
|
|
|
AA | 74 | 1.00 (ref.) |
| 77 | 1.00 (ref.) |
|
|
AG | 91 | 1.02
(0.66–1.58) | 0.91 | 104 | 1.13
(0.73–1.72) | 0.59 |
|
GG | 42 | 2.24
(1.19–4.26) | 0.01a | 41 | 2.10
(1.12–4.00) | 0.02a |
|
Dominant model |
|
|
|
|
|
|
|
AA | 74 | 1.00 (ref.) |
| 77 | 1.00 (ref.) |
|
|
AG + GG | 133 | 1.24
(0.82–1.90) | 0.31 | 145 | 1.30
(0.86–1.94) | 0.21 |
|
Recessive model |
|
|
|
|
|
|
|
AA + AG | 165 | 1.00 (ref.) |
| 181 | 1.00 (ref.) |
|
|
GG | 42 | 2.21
(1.24–4.03) | 0.007a | 41 | 1.97
(1.10–3.58 | 0.02a |
|
Allele |
|
|
|
|
|
|
|
A | 239 | 1.00 (ref.) |
| 258 | 1.00 (ref.) |
|
|
G | 175 | 1.37
(1.03–1.84) | 0.03a | 186 | 1.35
(1.02–1.80) | 0.04a |
| ESR2
rs1256049 |
|
|
|
|
|
|
|
Codominant model |
|
|
|
|
|
|
|
GG | 186 | 1.00 (ref.) |
| 198 | 1.00 (ref.) |
|
|
GA | 21 | 1.04
(0.53–2.05) | 0.91 | 21 | 0.98
(0.50–1.92) | 0.95 |
|
AA | 0 | NA | NA | 3 | NA | NA |
|
Dominant model |
|
|
|
|
|
|
|
GG | 186 | 1.00 (ref.) |
| 198 | 1.00 (ref.) |
|
|
GA + AA | 21 | 1.04
(0.53–2.05) | 0.91 | 24 | 1.12
(0.59–2.16) | 0.74 |
|
Allele |
|
|
|
|
|
|
|
G | 393 | 1.00 (ref.) |
| 420 | 1.00 (ref.) |
|
|
A | 21 | 1.04
(0.54–2.01) | 0.91 | 24 | 1.11
(0.59–2.11) | 0.75 |
| ESR2
rs4986938 |
|
|
|
|
|
|
|
Codominant model |
|
|
|
|
|
|
|
GG | 87 | 1.00 (ref.) |
| 107 | 1.00 (ref.) |
|
|
GA | 99 | 1.30
(0.85–1.98) | 0.22 | 90 | 0.96
(0.63–1.46) | 0.85 |
|
AA | 21 | 1.12
(0.56–2.25) | 0.75 | 21 | 0.91
0.46–1.82) | 0.78 |
|
Dominant model |
|
|
|
|
|
|
|
GG | 87 | 1.00 (ref.) |
| 107 | 1.00 (ref.) |
|
|
GA + AA | 120 | 1.26
(0.85–1.89) | 0.25 | 111 | 0.95
(0.64–1.41) | 0.80 |
|
Recessive model |
|
|
|
|
|
|
|
GG + GA | 186 | 1.00 (ref.) |
| 197 | 1.00 (ref.) |
|
|
AA | 21 | 0.98
(0.51–1.91) | 0.95 | 21 | 0.93
(0.48–1.80) | 0.82 |
|
Allele |
|
|
|
|
|
|
|
G | 273 | 1.00 (ref.) |
| 304 | 1.00 (ref.) |
|
|
A | 141 | 1.14
(0.84–1.53) | 0.41 | 132 | 0.96
(0.71–1.29) | 0.77 |
Expression levels of ESR2 mRNA
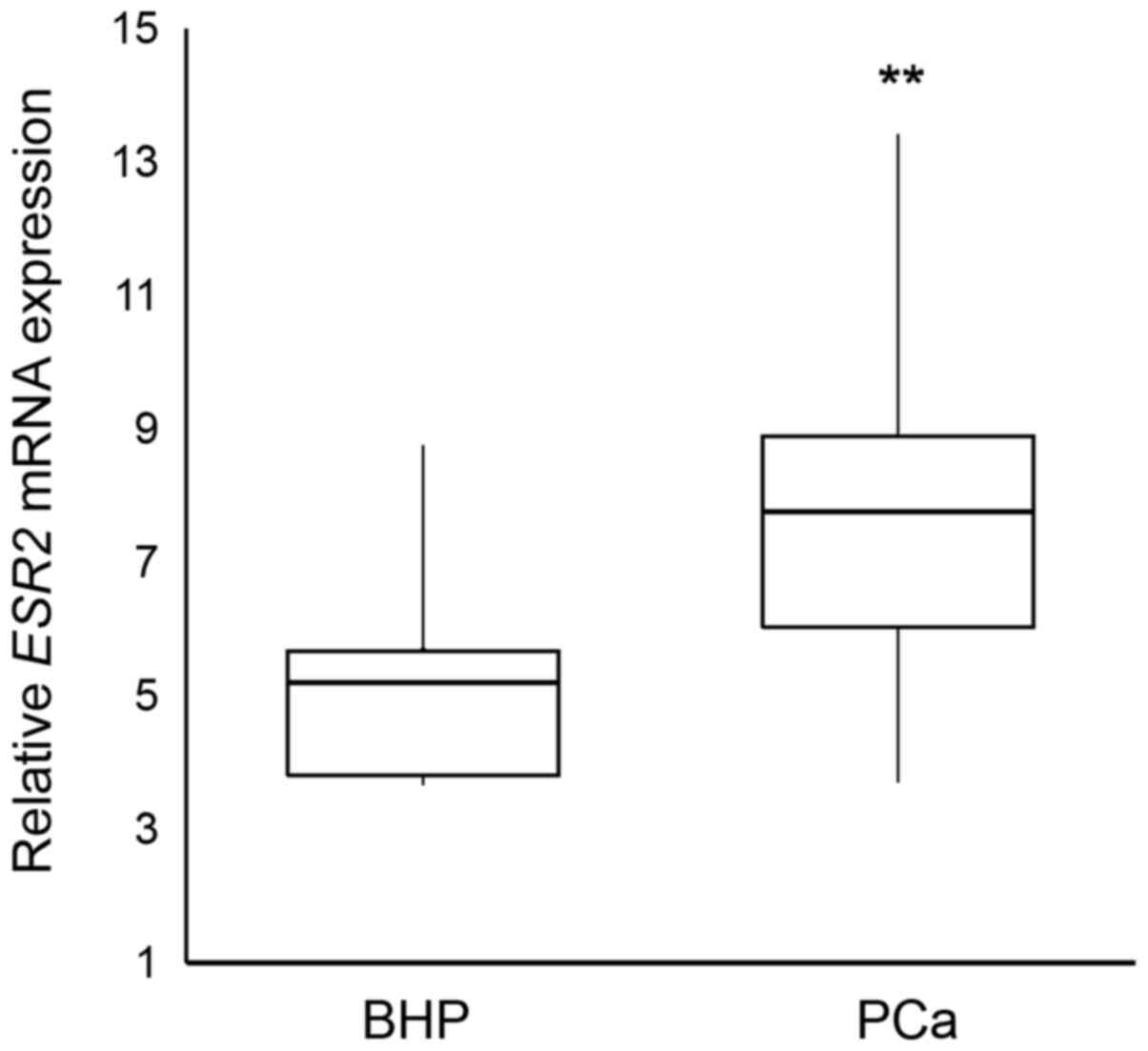
The relative ESR2 mRNA expression levels were
found to be significantly higher in BHP tissues compared with that
in prostate cancer tissues (P=0.002) (Fig. 2). It was found that ESR2 mRNA
expression levels were 5.47-fold higher in BHP tissues compared
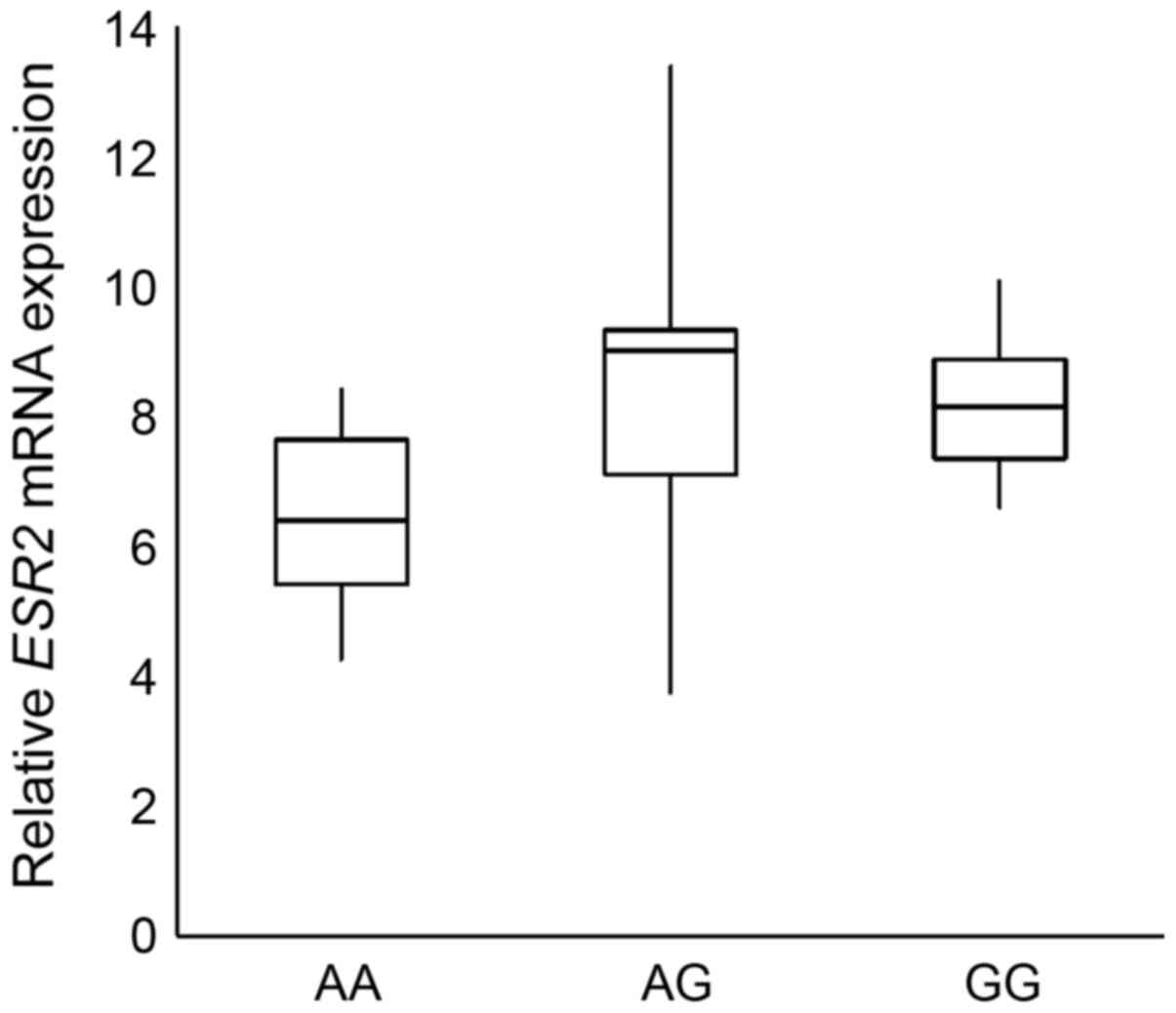
with that in prostate cancer tissues. Analysis of relative
ESR2 mRNA expression levels in patients with prostate cancer
with different rs3020449 genotypes revealed that the rs3020449 GG
genotype had 3.38-fold lower ESR2 expression levels compared
with that in patients with the AA genotype (P=0.04) (Fig. 3); however the result was not
statistically significant after Bonferroni correction for multiple
comparisons.
Discussion
Prostate cancer is a heterogenous disease, with
numerous factors contributing to its development and progression.
The prostate is a hormone-dependent tissue, and estrogens are the
targets of research. The aim of the present study was to evaluate
the association between three ESR2 polymorphisms and the
increased risk of prostate cancer, and to determine the relative
ESR2 mRNA expression levels in hyperplastic and malignant
prostate tissues. There was only a limited number of tissue
samples; however, significantly higher relative ESR2 mRNA
expression levels were found in BHP tissues compared with that in
prostate cancer tissues. Several publications have reported the
decrease or loss of ERβ protein expression during prostate cancer
progression using immunohistochemical staining (32,33).
Latil et al (34) also
reported a decrease in ERβ mRNA expression levels in the majority
of prostate tumors compared to that in normal tissue. Pasquali
et al (35) described that
the loss of the ERβ protein may promote cell proliferation and
possibly carcinogenesis. On the other hand, some reports also
suggest a negative role of ERβ protein expression levels in
prostate cancer prognosis (36,37).
Grindstad et al (38) found
that the ERβ protein expression levels were associated with reduced
time to biochemical failure. Opposing observations obtained by
different research groups might be partially explained by the
existence of different ERβ isoforms. The wild-type ERβ1 inhibits
proliferation, has tumor-suppressing effects and is lost during
prostate cancer progression. Its splice variant, ERβ2, increases
proliferation, therefore is oncogenic and is expressed in advanced
prostate cancer (39).
One of the factors with the potential to affect the
expression levels of the ESR2 gene are single nucleotide
polymorphisms. In the present study, there was no association
between the rs4986938 and rs1256049 polymorphisms and the risk of
prostate cancer development and progression. The majority of
published studies have also not confirmed an association between
the rs4986938 and rs1256049 polymorphisms in the Caucasian
population (40–44) or in mixed populations (45–47) and
prostate cancer risk. On contrary, there are some studies that have
described a significant association between the rs4986938 and
rs1256049 polymorphisms and increased risk of prostate cancer
development in Iranian (48) and
Caucasian populations (49). On the
other hand, a Japanese study discovered that both the rs4986938 and
rs1256049 polymorphisms were significantly associated with a
decreased risk of prostate cancer (24). As a result of the conflicting results
of the published studies, Li et al (25) conducted a meta-analysis, in which
they found no evidence of an association between the rs4986938
polymorphism and prostate cancer risk, while a meta-analysis into
the association between the rs1256049 and prostate cancer revealed
a significant association in the Caucasian population, but not in
the overall population (19).
The promoter region of the ESR2 gene is
complex and has not been fully described; however, it is
hypothesized that polymorphisms in this region could affect the
binding of enhancers or repressors to regulate gene transcription
(50). To the best of our knowledge,
this is the first study evaluating the association between the
rs3020449 polymorphism and the risk of prostate cancer. A
significant association between the rs3020449 polymorphism and a
higher risk of prostate cancer development and progression was
found. The functional impact of this polymorphism is unknown.
Decrease in ESR2 mRNA expression levels were found in
patients with prostate cancer and the rs3020449 GG genotype
compared with that in the AA genotype; however, results were not
statistically significant after Bonferroni correction. The
potential limitation of presented study is the lack of survival
analysis.
There are a limited number of studies that have
analyzed the rs3020449 polymorphism with other diseases. Lattrich
et al (27) found that
rs3020449 was not associated with the development of endometrial
cancer. The polymorphism was also found to be associated with the
progression of ovarian cancer, as it was more frequent in patients
with FIGO staged III + IV (28). On
the other hand, it was not found to be associated with uterine
fibroids (51). With respect to
breast cancer, some studies have found no association of rs3020449
(52,53). On the other hand, Dai et al
(29) described an association
between rs3020449 and increased risk of breast cancer, as well as
with tumor size and histological grade.
There are no published studies revealing an
association between the rs3020449 polymorphism and prostate cancer
risk; however, there are studies describing an association between
other promoter polymorphisms in ESR2 and prostate cancer.
The National Cancer Institute's Breast and Prostate Cancer Cohort
Consortium study reported an overall increased risk in prostate
cancer and advanced stage with the rs3020450 (45). This polymorphism was found to be in
complete linkage disequilibrium with rs2987983, which authors of a
Swedish study found to be associated with prostate cancer risk and
suggested that the genetic variation in the promoter region of
ESR2 may play a part in the etiology of prostate cancer
(54). Holt et al (42) reported an association between the
rs1952586 polymorphism and the risk for higher Gleason score
tumors.
In summary, the rs3020449 polymorphism in the
ESR2 gene markedly contributed to a higher prostate cancer
risk in the Slovak population. Analysis of this polymorphism could
also provide information regarding the prognosis of the disease, as
it was significantly associated with the development of high-grade
carcinomas (Gleason score >7) and tumors with pT3/pT4. The
significance of the presented study underlines the fact that the
rs3020449 was not found to be in linkage disequilibrium with
polymorphisms previously studied with prostate cancer (26). Therefore, it is not likely that the
association found in the present study was due to linkage of
rs3020449 with previously reported polymorphisms. The functional
impact of this polymorphism on the ESR2 gene is still
unknown. Analysis of relative ESR2 mRNA expression levels
revealed that patients with the rs3020449 GG genotype had tendency
to have lower ESR2 expression levels compared with those
with the AA genotype. There might also be considerable differences
in the genotype frequencies between populations, therefore the
results are valid for Slovak and related populations; however,
confirmation is required for populations with a different ethnic
origin.
Acknowledgements
Not applicable.
Funding
This publication is the result of the project
implementation: ‘Center Of Excellence For Research In Personalized
Therapy’, ITMS: 26220120053 and VEGA grants (grant nos. 1/0172/18
and 1/0271/19) and APVV-15-0181.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
JJ, MKS, HD, MH, DE, JK and DD contributed to the
study conception and design. Material preparation and data
collection were performed by JK, DE and MH. Analysis was performed
by JJ, MKS and HD. The first draft of the manuscript was written by
JJ and all authors commented on previous versions of the
manuscript. JJ, DD and MKS confirmed the authenticity of all raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Comenius University in Bratislava, Jessenius Faculty
of Medicine in Martin, Slovakia (approval number: EK 43/2018) and
was performed in accordance with ethical standards as laid down in
the 1964 Declaration of Helsinki and its later amendments. Informed
consent was obtained from all individual participants included in
the study.
Patient consent to publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vrdoljak E, Wojtukiewicz MZ, Pienkowski T,
Bodoky G, Berzinec P, Finek J, Todorović V, Borojević N and
Croitoru A; South eastern European Research Oncology Group, :
Cancer epidemiology in central, south and eastern European
countries. Croat Med J. 52:478–487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gann PH: Risk factors for prostate cancer.
Rev Urol. 4 (Suppl 5):S3–S10. 2002.PubMed/NCBI
|
|
3
|
Carruba G: Estrogens and mechanisms of
prostate cancer progression. Ann NY Acad Sci. 1089:201–217. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rohrmann S, Platz EA, Selvin E, Shiels MS,
Joshu CE, Menke A, Feinleib M, Basaria S, Rifai N, Dobs AS, et al:
The prevalence of low sex steroid hormone concentrations in men in
the third national health and nutrition examination survey (NHANES
III). Clin Endocrinol (Oxf). 75:232–239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bonkhoff H: Estrogen receptor signaling in
prostate cancer: Implications for carcinogenesis and tumor
progression. Prostate. 78:2–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yao S, Till C, Kristal AR, Goodman PJ,
Hsing AW, Tangen CM, Platz EA, Stanczyk FZ, Reichardt JK, Tang L,
et al: Serum estrogen levels and prostate cancer risk in the
prostate cancer prevention trial: A nested case-control study.
Cancer Causes Control. 22:1121–1131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsai CJ, Cohn BA, Cirillo PM, Feldman D,
Stanczyk FZ and Whittemore AS: Sex steroid hormones in young
manhood and the risk of subsequent prostate cancer: A longitudinal
study in African-Americans and caucasians (United States). Cancer
Causes Control. 17:1237–1244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barba M, Yang L, Schünemann HJ, Sperati F,
Grioni S, Stranges S, Westerlind KC, Blandino G, Gallucci M, Lauria
R, et al: Urinary estrogen metabolites and prostate cancer: A
case-control study and meta-analysis. J Exp Clin Cancer Res.
28:1352009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parker MG: Structure and function of
estrogen receptors. Vitam Horm. 51:267–287. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Levin ER and Hammes SR: Nuclear receptors
outside the nucleus: Extranuclear signaling by steroid receptors.
Nat Rev Mol Cell Biol. 17:783–797. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bonkhoff H and Berges R: The evolving role
of oestrogens and their receptors in the development and
progression of prostate cancer. Eur Urol. 55:533–542. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng J, Lee EJ, Madison LD and Lazennec
G: Expression of estrogen receptor beta in prostate carcinoma cells
inhibits invasion and proliferation and triggers apoptosis. FEBS
Lett. 566:169–72. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ellem SJ and Risbridger GP: The dual,
opposing roles of estrogen in the prostate. Ann NY Acad Sci.
1155:174–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Omoto Y and Iwase H: Clinical significance
of estrogen receptor β in breast and prostate cancer from
biological aspects. Cancer Sci. 106:337–343. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Enmark E, Pelto-Huikko M, Grandien K,
Lagercrantz S, Lagercrantz J, Fried G, Nordenskjöld M and
Gustafsson JA: Human estrogen receptor beta-gene structure,
chromosomal localization, and expression pattern. J Clin Endocrinol
Metab. 82:4258–4265. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Christoforou P, Christopoulos PF and
Koutsilieris M: The role of estrogen receptor β in prostate cancer.
Mol Med. 20:427–434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu X, Leav I, Leung YK, Wu M, Liu Q, Gao
Y, McNeal JE and Ho SM: Dynamic regulation of estrogen
receptor-beta expression by DNA methylation during prostate cancer
development and metastasis. Am J Pathol. 164:2003–2012. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rosenkranz K, Hinney A, Ziegler A, Hermann
H, Fichter M, Mayer H, Siegfried W, Young JK, Remschmidt H and
Hebebrand J: Systematic mutation screening of the estrogen receptor
beta gene in probands of different weight extremes: Identification
of several genetic variants. J Clin Endocrinol Metab. 12:4524–4527.
1998. View Article : Google Scholar
|
|
19
|
Fu C, Dong WQ, Wang A and Qiu G: The
influence of ESR1 rs9340799 and ESR2 rs1256049 polymorphisms on
prostate cancer risk. Tumour Biol. 35:8319–8328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Putnik M, Zhao C, Gustafsson JA and
Dahlman-Wright K: Effects of two common polymorphisms in the
3′untranslated regions of estrogen receptor beta on mRNA stability
and translatability. BMC Genet. 10:552009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barrett LW, Fletcher S and Wilton SD:
Regulation of eukaryotic gene expression by the untranslated gene
regions and other non-coding elements. Cell Mol Life Sci.
69:3613–2634. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu KD, Rao NY, Chen AX, Fan L, Yang C and
Shao ZM: A systematic review of the relationship between
polymorphic sites in the estrogen receptor-beta (ESR2) gene and
breast cancer risk. Breast Cancer Res Treat. 126:37–45. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rezende LM, Marson FA, Lima CS and
Bertuzzo CS: Variants of estrogen receptor alpha and beta genes
modify the severity of sporadic breast cancer. Gene. 608:73–78.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu X, Yamano Y, Takahashi H, Koda M,
Fujiwara Y, Hisada A, Miyazaki W and Katoh T: Associations between
estrogen receptor genetic polymorphisms, smoking status, and
prostate cancer risk: A case-control study in Japanese men. Environ
Health Prev Med. 20:332–337. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Z, Yang X, Zhang R, Zhang D, Li B,
Zhang D, Li Q and Xiong Y: No association between estrogen
receptor-Β Rs4986938 and cancer risk: A systematic review and
meta-analysis. Iran J Public Health. 48:784–795. 2019.PubMed/NCBI
|
|
26
|
Bharathi C, Anupama D, Pratibha N and
Venkateshwari A: Impact of genetic variants in estrogen receptor-β
gene in the etiology of uterine leiomyomas. J Reprod Infertil.
20:151–160. 2019.PubMed/NCBI
|
|
27
|
Lattrich C, Häring J, Schüler S,
Skrzypczak M, Ortmann O and Treeck O: Polymorphisms in the promoter
region of estrogen receptor β gene in endometrial cancer. Arch
Gynecol Obstet. 289:631–635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schüler S, Lattrich C, Skrzypczak M, Fehm
T, Ortmann O and Treeck O: Polymorphisms in the promoter region of
ESR2 gene and susceptibility to ovarian cancer. Gene. 546:283–287.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dai Z, Tian T, Wang M, Yang T, Li H, Lin
S, Hao Q, Xu P, Deng Y, Zhou L, et al: Genetic polymorphisms of
estrogen receptor genes are associated with breast cancer
susceptibility in Chinese women. Cancer Cell Int. 19:112019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ye S, Dhillon S, Ke X, Collins AR and Day
IN: An efficient procedure for genotyping single nucleotide
polymorphisms. Nucleic Acids Res. 29:E88. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-(Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Horvath LG, Henshall SM, Lee CS, Head DR,
Quinn DI, Makela S, Delprado W, Golovsky D, Brenner PC, O'Neill G,
et al: Frequent loss of estrogen receptor-beta expression in
prostate cancer. Cancer Res. 61:5331–5335. 2001.PubMed/NCBI
|
|
33
|
Gabal SM, Habib FM, Helmy DO and Ibrahim
MF: Expression of estrogen receptor-B (ER-B) in benign and
malignant prostatic epithelial cells and its correlation with the
clinico-pathological features. J Egypt Natl Canc Inst. 19:239–248.
2007.PubMed/NCBI
|
|
34
|
Latil A, Bieche I, Vidaud D, Lidereau R,
Berthon P, Cussenot O and Vidaud M: Evaluation of androgen,
estrogen (ER alpha and ER beta), progesterone receptor expression
inhuman prostate cancer by real-time quantitative reverse
transcription-polymerase chain reaction assays. Cancer Res.
61:1919–1926. 2001.PubMed/NCBI
|
|
35
|
Pasquali D, Staibano S, Prezioso D, Franco
R, Esposito D, Notaro A, De Rosa G, Bellastella A and Sinisi AA:
Estrogen receptor beta expression in human prostate tissue. Mol
Cell Endocrinol. 178:47–50. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Leung YK, Lam HM, Wu S, Song D, Levin L,
Cheng L, Wu CL and Ho SM: Estrogen receptor beta2 and beta5 are
associated with poor prognosis in prostate cancer, and promote
cancer cell migration and invasion. Endocr Relat Cancer.
17:675–689. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zellweger T, Stürm S, Rey S, Zlobec I,
Gsponer JR, Rentsch CA, Terracciano LM, Bachmann A, Bubendorf L and
Ruiz C: Estrogen receptor β expression and androgen receptor
phosphorylation correlate with a poor clinical outcome in
hormone-naive prostate cancer and are elevated in
castration-resistant disease. Endocr Relat Cancer. 20:403–413.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Grindstad T, Skjefstad K, Andersen S, Ness
N, Nordby Y, Al-Saad S, Fismen S, Donnem T, Khanehkenari MR, Busund
LT, et al: Estrogen receptors α and β and aromatase as independent
predictors for prostate cancer outcome. Sci Rep. 6:331142016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dey P, Jonsson P, Hartman J, Williams C,
Ström A and Gustafsson JÅ: Estrogen receptors β1 and β2 have
opposing roles in regulating proliferation and bone metastasis
genes in the prostate cancer cell line PC3. Mol Endocrinol.
26:1991–2003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cunningham JM, Hebbring SJ, McDonnell SK,
Cicek MS, Christensen GB, Wang L, Jacobsen SJ, Cerhan JR, Blute ML,
Schaid DJ and Thibodeau SN: Evaluation of genetic variations in the
androgen and estrogen metabolic pathways as risk factors for
sporadic and familial prostate cancer. Cancer Epidemiol Biomarkers
Prev. 16:969–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nicolaiew N, Cancel-Tassin G, Azzouzi AR,
Le Grand B, Mangin P, Cormier L, Fournier G, Giordanella JP,
Pouchard M, Escary JL, et al: Association between estrogen and
androgen receptor genes and prostate cancer risk. Eur J Endocrinol.
160:101–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Holt SK, Kwon EM, Fu R, Kolb S, Feng Z,
Ostrander EA and Stanford JL: Association of variants in
estrogen-related pathway genes with prostate cancer risk. Prostate.
73:1–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Robles-Fernandez I, Martinez-Gonzalez LJ,
Pascual-Geler M, Cozar JM, Puche-Sanz I, Serrano MJ, Lorente JA and
Alvarez-Cubero MJ: Association between polymorphisms in sex
hormones synthesis and metabolism and prostate cancer
aggressiveness. PLoS One. 12:e01854472017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tang L, Platek ME, Yao S, Till C, Goodman
PJ, Tangen CM, Wu Y, Platz EA, Neuhouser ML, Stanczyk FZ, et al:
Associations between polymorphisms in genes related to estrogen
metabolism and function and prostate cancer risk: Results from the
prostate cancer prevention trial. Carcinogenesis. 39:125–133. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen YC, Kraft P, Bretsky P, Ketkar S,
Hunter DJ, Albanes D, Altshuler D, Andriole G, Berg CD, Boeing H,
et al: Sequence variants of estrogen receptor beta and risk of
prostate cancer in the national cancer institute breast and
prostate cancer cohort consortium. Cancer Epidemiol Biomarkers
Prev. 16:1973–1981. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chae YK, Huang HY, Strickland P, Hoffman
SC and Helzlsouer K: Genetic polymorphisms of estrogen receptors
alpha and beta and the risk of developing prostate cancer. PLoS
One. 4:e65232009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sun T, Lee GS, Werner L, Pomerantz M, Oh
WK, Kantoff PW and Freedman ML: Inherited variations in AR, ESR1,
and ESR2 genes are not associated with prostate cancer
aggressiveness or with efficacy of androgen deprivation therapy.
Cancer Epidemiol Biomarkers Prev. 19:1871–1878. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Safarinejad MR and Safarinejad S, Shafiei
N and Safarinejad S: Estrogen receptors alpha (rs2234693 and
rs9340799), and beta (rs4986938 and rs1256049) genes polymorphism
in prostate cancer: Evidence for association with risk and
histopathological tumor characteristics in Iranian men. Mol
Carcinog. 51:E104–E117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Balistreri CR, Caruso C, Carruba G, Miceli
V and Candore G: Genotyping of sex hormone-related pathways in
benign and malignant human prostate tissues: Data of a preliminary
study. OMICS. 15:369–374. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hoogendoorn B, Coleman SL, Guy CA, Smith
K, Bowen T, Buckland PR and O'Donovan MC: Functional analysis of
human promoter polymorphisms. Hum Mol Genet. 12:2249–2254. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fischer C, Juhasz-Boess I, Lattrich C,
Ortmann O and Treeck O: Estrogen receptor beta gene polymorphisms
and susceptibility to uterine fibroids. Gynecol Endocrinol. 26:4–9.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Treeck O, Elemenler E, Kriener C, Horn F,
Springwald A, Hartmann A and Ortmann O: Polymorphisms in the
promoter region of ESR2 gene and breast cancer susceptibility. J
Steroid Biochem Mol Biol. 114:207–211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen L, Liang Y, Qiu J, Zhang L, Chen X,
Luo X and Jiang J: Significance of rs1271572 in the estrogen
receptor beta gene promoter and its correlation with breast cancer
in a southwestern Chinese population. J Biomed Sci. 20:322013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Thellenberg-Karlsson C, Lindström S,
Malmer B, Wiklund F, Augustsson-Bälter K, Adami HO, Stattin P,
Nilsson M, Dahlman-Wright K, Gustafsson JA and Grönberg H: Estrogen
receptor beta polymorphism is associated with prostate cancer risk.
Clin Cancer Res. 12:1936–1941. 2006. View Article : Google Scholar : PubMed/NCBI
|