Introduction
Lung cancer is a common malignant tumor and a
leading cause of cancer-associated mortality worldwide (1). Non-small cell lung cancer (NSCLC) is
the most common type of lung cancer worldwide, accounting for 80%
of all lung cancer cases (2).
Furthermore, 60% of patients with NSCLC are diagnosed with
advanced-stage tumors (1). At
present, patients with advanced or metastatic NSCLC are usually
treated with platinum-based chemotherapy (3). Due to adverse effects of chemotherapy,
such as neutropenia, stomatitis, mucositis, diarrhea, emesis and
chemoresistance, patients with advanced NSCLC become less sensitive
to chemotherapy (4,5). It is therefore essential to improve the
specificity of platinum-based chemotherapy and decrease its side
effects in order to improve its efficiency. Numerous natural
extracts, such as glycyrrhizin, 18β-glycyrrhetinic acid and glabrin
A and B, have demonstrated extensive biological activity and low
toxicity in animal models of NSCLC and might therefore be
considered as potential adjuvant drugs for the treatment of NSCLC
(6).
Emodin (1,3,8-trihydroxy-6-methyl-anthraquinone) is
a natural anthraquinone derivative extracted from the roots of
Chinese rhubarb and other plants, such as buckthorn and cassia tora
(7,8). Emodin displays a variety of
pharmacological and biological functions, including some
anti-inflammatory, antibacterial and chemoprophylactic effects
(9–11). In addition, previous studies have
demonstrated that emodin exhibits some anticancer effects in
breast, pancreatic and cervical cancers by inhibiting cancer cell
proliferation and increasing cancer cell apoptosis and
chemosensitization (12–14). Other studies have reported that
emodin can reverse the chemoresistance in certain types of cancer,
including leukemia, NSCLC and gallbladder cancer (15–17).
Although certain studies have reported the effect of emodin on
NSCLC chemosensitivity toward paclitaxel (17), the effect of emodin on NSCLC
chemosensitivity toward other chemical drugs and the underlying
mechanisms remain unclear.
Multidrug resistance proteins are the most important
factors that cause chemoresistance, which leads to a decrease in
chemotherapy efficacy and survival rate of patients with cervical,
liver, breast and lung cancers (18). Members of the ATP binding cassette
(ABC) family are associated with multidrug resistance (MDR), and
include P-glycoprotein (Pgp), multidrug resistance-associated
protein 1 (MRP1) and MRP2 (19–21). MDR
often occurs during the treatment of NSCLC, which leads most
patients to eventually relapse or to the disease to progress
(22). Therefore, determining
adjuvant drugs that could inhibit the expression of multidrug
resistance protein may improve NSCLC sensitivity to
chemotherapy.
The present study investigated the effect of emodin
on the chemosensitivity of A549 and H460 cells and on the
expression of Pgp and MRP1, which are key proteins involved in
MDR.
Materials and methods
Cell culture
The NSCLC cell lines A549 and H460 were purchased
from the American Type Culture Collection. Cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Biological
Industries), 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco;
Thermo Fisher Scientific, Inc.) and placed at 37°C in a humidified
atmosphere with 5% CO2. Trypsin (0.25%; Gibco; Thermo
Fisher Scientific, Inc.) was used to passage cells once they
reached 70–90% confluence.
Cell proliferation assay
Once cells reached 70–90% confluence, they were
harvested and seeded into 96-well plates at the density of 2,500
cells/well and cultured for 12–24 h at 37°C. Subsequently, cells
were treated with emodin (0, 1, 2.5, 5, 10, 20, 50, 100, 200 and
300 µM) (17) and/or cisplatin (0,
1, 2, 4, 6, 8, 10, 15, 20 and 30 µM) (23) for 48 h, the blank control (0 µM) was
treated with equal amounts of vehicle (DMSO). Cell Counting Kit-8
(CCK-8; 10 µl) reagent (Nanjing KeyGen Biotech Co., Ltd.) was added
to each well and incubated for 2–4 h at 37°C. Absorbance was read
at 450 nm using a Multilabel Plate Reader (Monobind, Inc.)
(24).
Western blotting
Cells were lysed with RIPA buffer (50 mM Tris/HCl,
150 mM NaCl, 1% (v/v) Nonidet P40 (NP40), 0.5% sodium deoxycholate,
0.1% SDS and protease inhibitor; pH 7.4) on ice and samples were
centrifuged at 10,000 × g for 5 min at 4°C. Protein concentration
was measured using the BCA protein assay kit (Thermo Fisher
Scientific, Inc.). Proteins were mixed with 5X loading buffer (0.5
M Tris-HCl pH 6.8, 2% SDS, 0.05% bromphenol-blue, 20%
2-mercaptoethanol and 10% glycerol) and boiled for 5 min. Proteins
were separated via SDS-PAGE (10% gel) as previously described and
transferred onto PVDF membranes (25). After blocking for 2 h in TBST
containing 5% non-fat milk, membranes were incubated with primary
antibodies against Pgp (1:1,000; Sigma-Aldrich; Merck KGaA; cat.
no. P7965), MRP1 (1:1,000; Cell Signaling Technology, Inc.; cat.
no. 72202) and GAPDH (1:5,000; ProteinTech Group, Inc.; cat. no.
60004-1-Ig) for overnight at 4°C. Membranes were then incubated
with the secondary antibodies, HRP-conjugated anti-rabbit IgG
(1:5,000; ProteinTech Group, Inc.; cat. no. 51832-2) or
HRP-conjugated anti-mouse IgG (1:5,000; ProteinTech Group, Inc.;
cat. no. 51866-5) for 2 h at 37°C. The signal on the membrane was
detected using enhanced chemiluminescence detection kit (Thermo
Fisher Scientific, Inc.). Relative expression levels were
normalized to endogenous control GAPDH using ImageJ software
(version 1.32; National Institutes of Health).
Cell apoptosis assay
Following treatment with emodin and/or cisplatin for
48 h, cells were harvested and 4×105 cells were
double-stained with 5 µl Annexin V-FITC and PI solution for 10 min
at room temperature (Absin Technologies, Inc.; cat. no. abs50001).
Apoptotic cells were subsequently analyzed using a CytoFlex flow
cytometer (Beckman Coulter, Inc.) the apoptotic rate was determined
using CytExpert 2.3 software (Beckman Coulter, Inc.) (26).
Immunocytochemical analysis of γ-H2A.X
Foci
Double-stranded DNA breaks (DSBs) induce serine
phosphorylation of histone H2A.X, producing γ-H2A.X foci that are
then recognized by DNA damage response pathway proteins. γ-H2A.X
foci are hallmark of DSBs and are markedly enhanced in irradiated
cells (27). Following cell
treatment with emodin and/or cisplatin for 48 h, cells were fixed
with 4% paraformaldehyde for 20 min, permeabilized with 0.3% Triton
X-100 for 5 min at room temperature and blocked with 5% bovine
serum albumin (Sigma-Aldrich; Merck KGaA) for 30 min at 37°C.
Subsequently, cells were incubated with antibodies against
phospho-histone H2A.X (Ser139; 1:400; Cell Signaling Technology,
Inc.; cat. no. 9718) overnight at 4°C. Cells were then incubated
with CL488-conjugated anti-rabbit IgG (1:200; ProteinTech Group,
Inc.; cat. no. SA00013-2) antibody for 1 h at 37°C and were washed
three times with PBS. Cells were eventually stained with DAPI
(1:1,000; Sigma-Aldrich; Merck KGaA; cat. no. D9542) for 3 min at
room temperature and washed with PBS three times. Cells were imaged
using a fluorescence microscope (magnification, ×20; Leica
Microsystems GmbH).
Fluorescence microscopy to analyze
intracellular rhodamine 123 accumulation
A549 and H460 cells were cultured for 12–24 h and
treated with 0, 1, 2.5, 5, 10 and 20 µM emodin for 12 h at 37°C.
Cells were harvested, resuspended in fresh medium, and stained with
5 µM rhodamine 123 (Sigma-Aldrich; Merck KGaA) for 30 min at 37°C.
Cells were washed three times with PBS, and drug accumulation
levels were determined by fluorescence microscopy (Leica
Microsystems GmbH) (28). The
fluorescence intensity was determined using ImageJ software
(version 1.32; National Institutes of Health).
Statistical analysis
Statistical analyses were performed using IBM SPSS
Statistics 22 (SPSS, Inc.) and GraphPad Prism 5 (GraphPad Software,
Inc.) software. All data are expressed as the mean ± standard of
three independent experiments. Student's t-test was used to
evaluate differences between two groups. Differences between
multiple groups were analyzed using two-way ANOVA followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
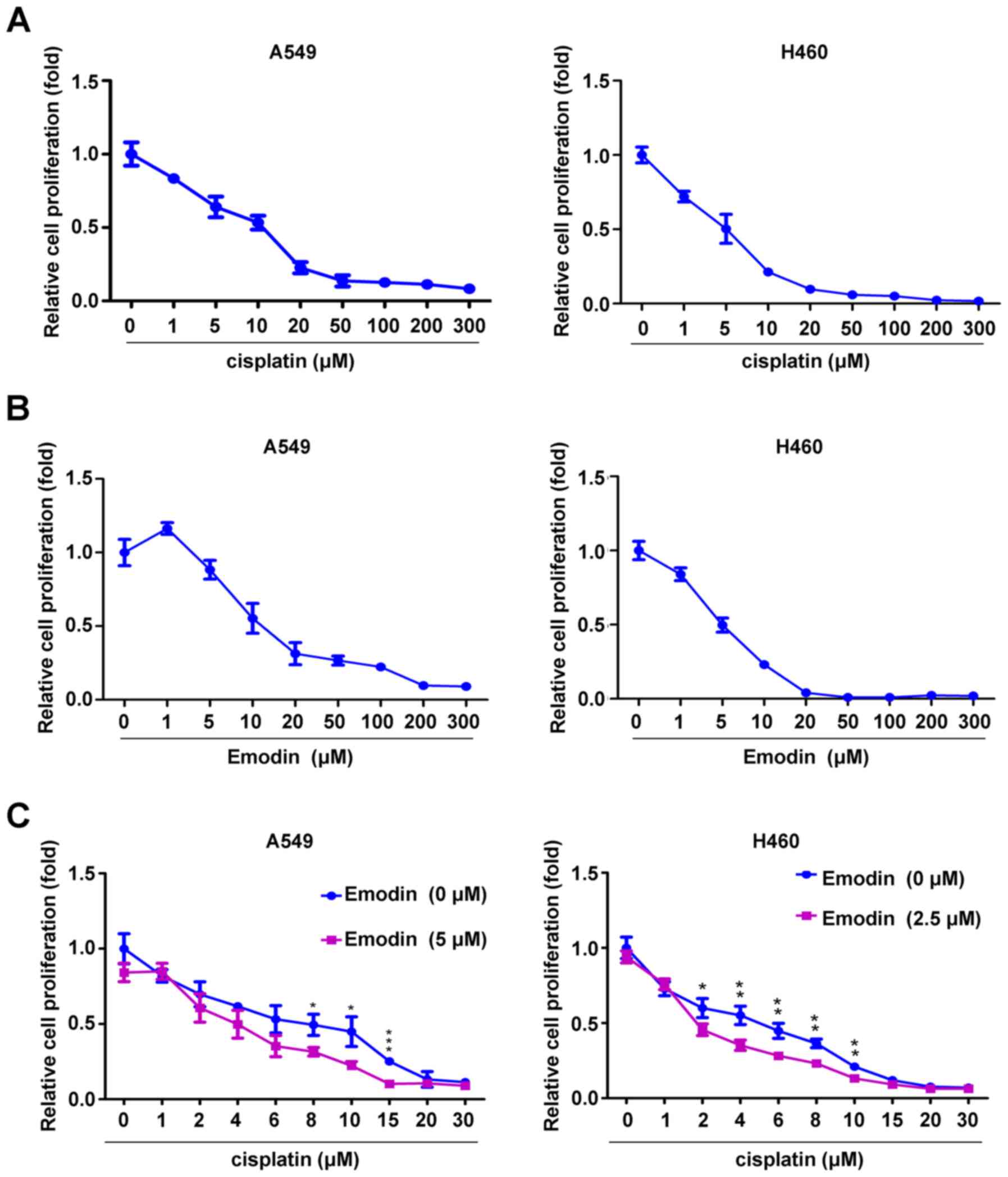
Emodin and cisplatin inhibit A549 and
H460 cell proliferation
CCK-8 assay was used to evaluate the effect of
emodin and/or cisplatin on the proliferation of A549 and H460
cells. The results indicated that cisplatin and emodin at
concentrations ranging from 0 to 300 µM inhibited A549 and H460
cell proliferation in a dose-dependent manner. Notably, low dose
emodin (1 µM) slightly enhanced the proliferation of A549 cells,
but not of H460 cells (Fig. 1A and
B). The IC50 of cisplatin and emodin for A549 cells (29) was 5.25 and 13.65 µM, respectively,
whereas the IC50 of cisplatin and emodin for H460 cells
was 4.83 and 5.17 µM, respectively. To investigate whether emodin
could be used as a cosensitizer for cisplatin, a low dose of emodin
(A549 cells, 5 µM; H460 cells, 2.5 µM) was selected to determine
its effect on cisplatin sensitization. Compared with cisplatin
treatment alone, treatment with 5 µM emodin significantly enhanced
the anti-proliferative effect of 8, 10 and 15 µM cisplatin on A549
cells, whereas 2.5 µM emodin significantly enhanced the
anti-proliferative effect of 2, 4, 6, 8 and 10 µM cisplatin on H460
cells (Fig. 1C). These results
indicated that emodin and cisplatin may synergistically inhibit the
proliferation of A549 and H460 cells.
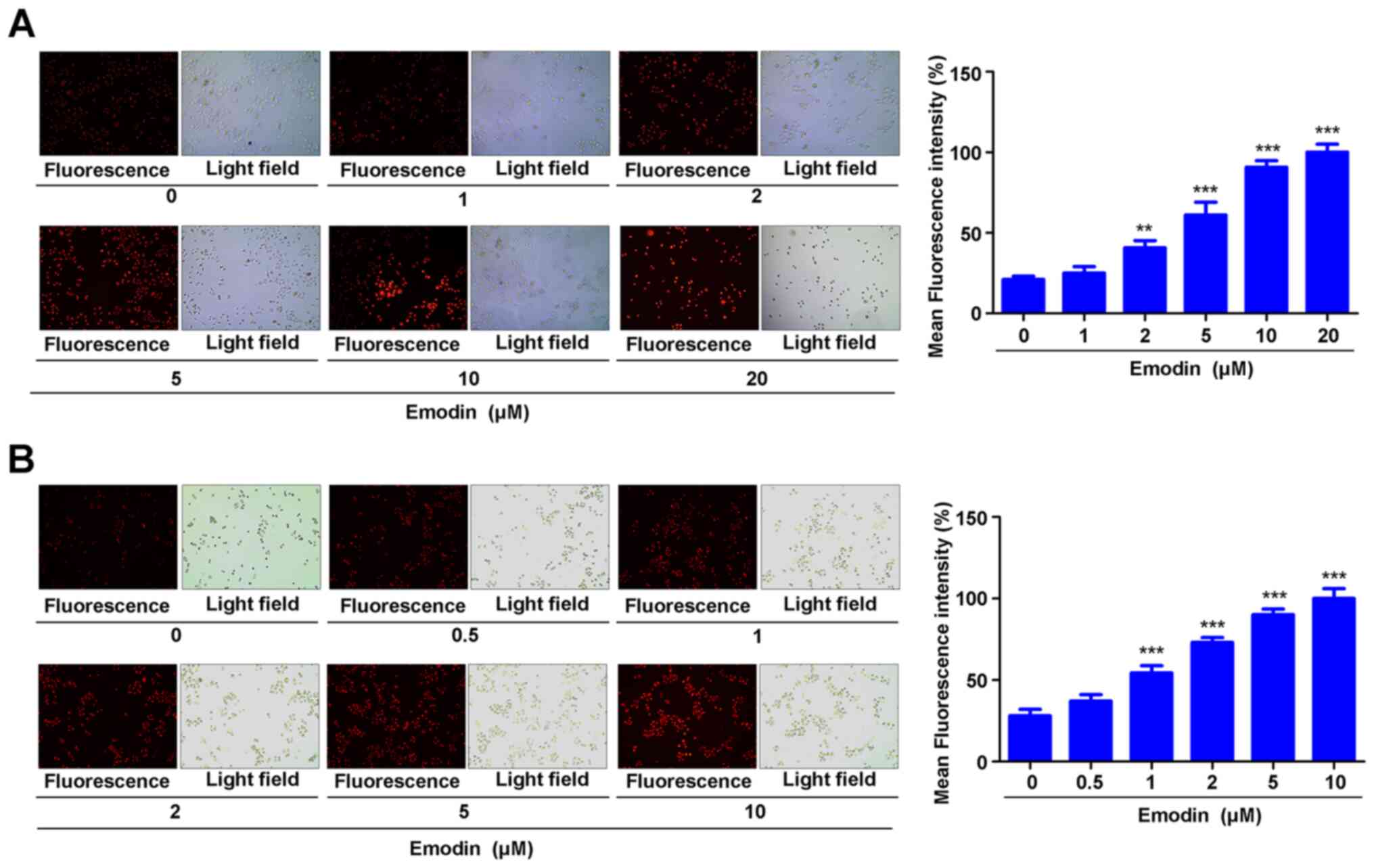
Emodin inhibits drug efflux in A549
and H460 cells
In order to investigate the effect of emodin on the
chemosensitivity of A549 and H460 cells toward cisplatin, a drug
efflux experiment was performed. Briefly, A549 and H460 cells were
treated with various concentrations of emodin and stained with
rhodamine 123, and immunofluorescence was used to detect the
intracellular accumulation of rhodamine 123. The results
demonstrated that 2, 5, 10 and 20 µM emodin significantly enhanced
the accumulation of rhodamine 123 in A549 cells (Fig. 2A). Emodin (1, 2, 5 and 10 µM) also
significantly enhanced rhodamine 123 accumulation in H460 cells
(Fig. 2B). These data indicated that
emodin may inhibit the efflux of drugs from A549 and H460
cells.
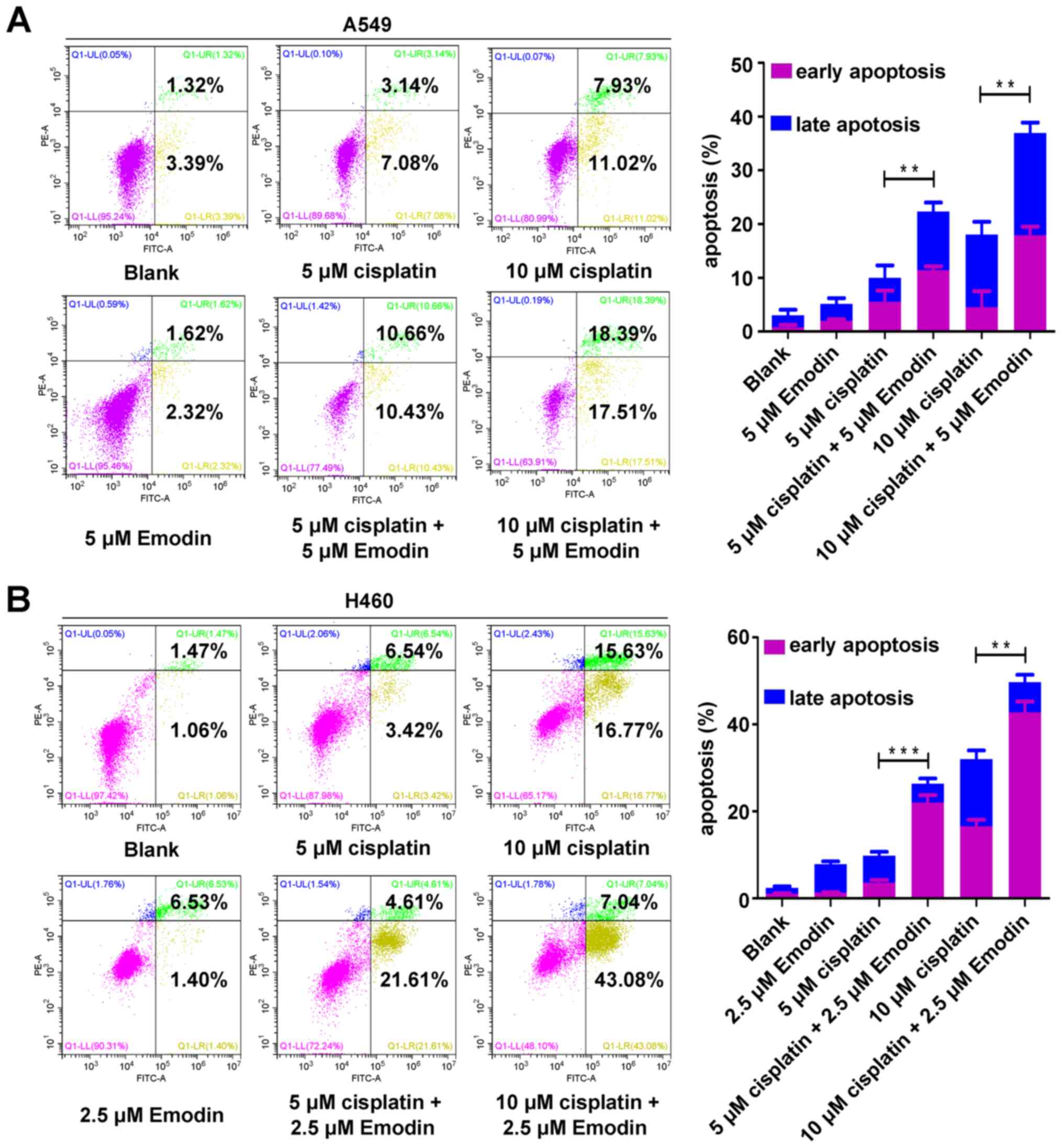
Emodin enhances cisplatin-induced
apoptosis in A549 and H460 cells
To investigate the effect of emodin on the
chemosensitivity of A549 and H460 cells, apoptosis experiments were
conducted. A549 and H460 cells were treated with emodin and/or
cisplatin, and the apoptotic rate was detected by flow cytometry.
The results demonstrated that 5 µM emodin did not induce A549 cell
apoptosis, but significantly enhanced A549 cell apoptosis induced
by 5 and 10 µM cisplatin (Fig. 3A).
Similarly, 2.5 µM emodin did not induce H460 cell apoptosis, but
significantly enhanced the apoptosis of H460 cells induced by 5 and
10 µM cisplatin (Fig. 3B). These
results suggested that emodin may enhance cisplatin-induced
apoptosis in A549 and H460 cells.
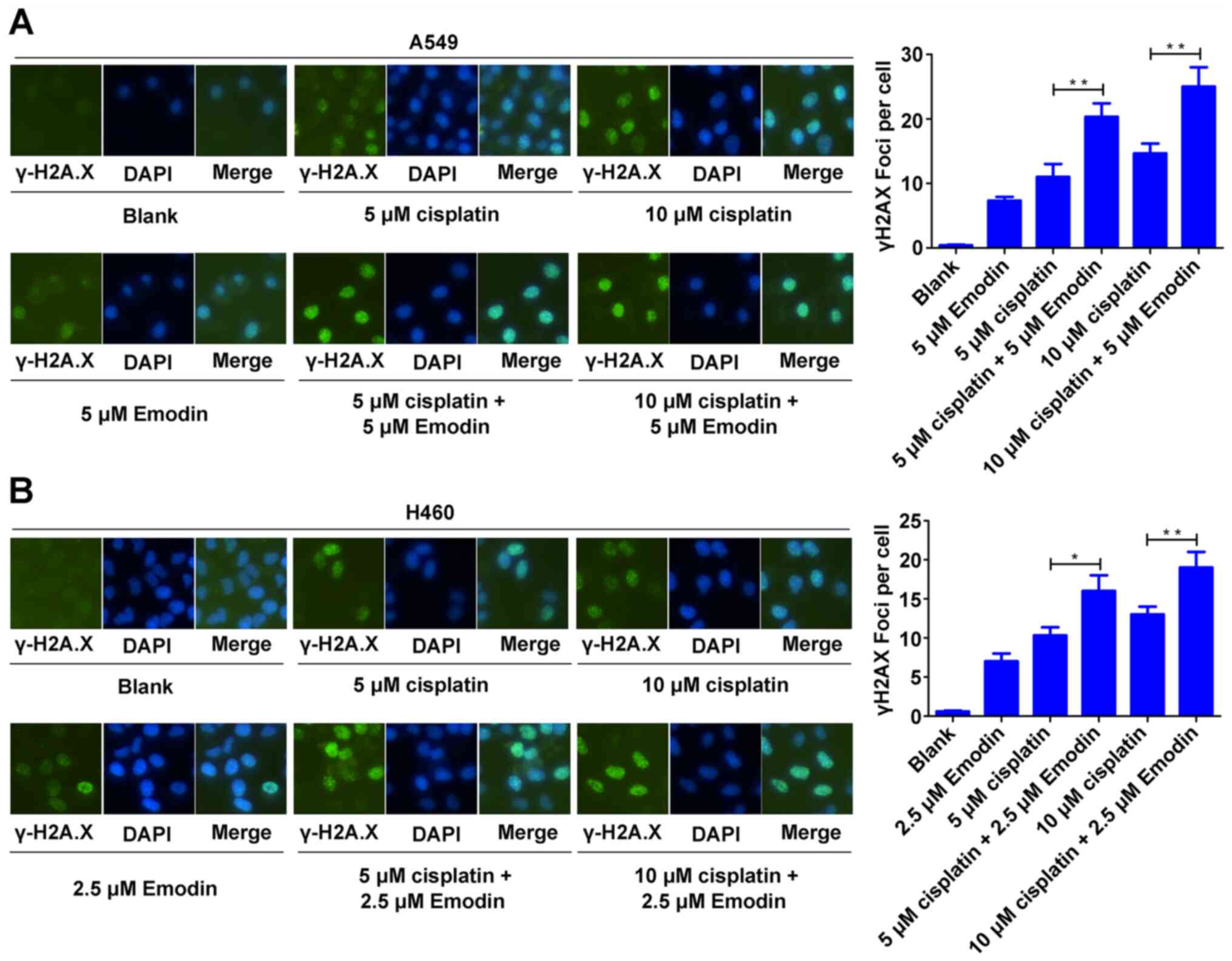
Emodin enhances cisplatin-induced DNA
damage in A549 and H460 cells
Cisplatin mainly kills tumor cells by inducing DNA
damage (30). The effect of emodin
on the chemosensitivity of A549 and H460 cells toward DNA damage
was therefore determined through immunocytochemical analysis of
γ-H2A.X foci. The results demonstrated that 5 µM emodin did not
induce γ-H2A.X foci formation in A549 cells but significantly
enhanced γ-H2A.X foci formation in A549 cells induced by 5 and 10
µM cisplatin (Fig. 4A). Similarly,
2.5 µM emodin did not induce γ-H2A.X foci formation in H460 cells
but significantly enhanced 5 and 10 µM cisplatin-induced γ-H2A.X
foci formation in H460 cells (Fig.
4B). These data indicated that emodin may increase
cisplatin-induced DNA damage in A549 and H460 cells.
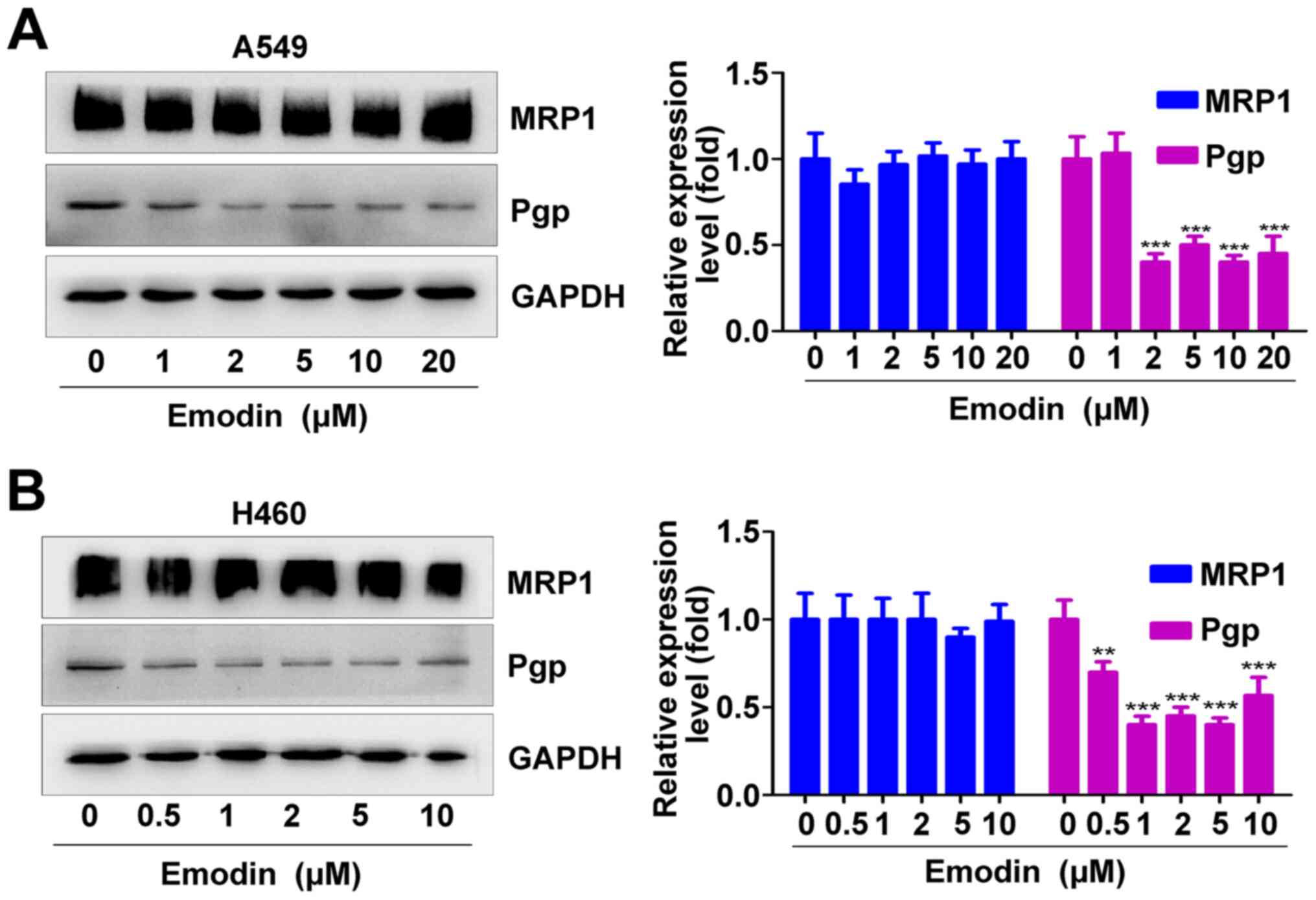
Emodin decreases Pgp expression in
A549 and H460 cells
In order to investigate the molecular mechanism by
which emodin enhances cisplatin sensitivity in A549 and H460 cells,
the effect of different concentrations of emodin on the expression
of Pgp and MRP1 were analyzed. The results from western blotting
demonstrated that 2, 5, 10 and 20 µM emodin significantly decreased
the expression of Pgp in A549 cells but did not affect the
expression of MRP1 (Fig. 5A). Emodin
(0.5, 1, 2, 5 and 10 µM) significantly inhibited the expression of
Pgp in H460 cells but did not affect the expression of MRP1
(Fig. 5B). These results suggested
that emodin may inhibit MDR related protein-Pgp expression.
Discussion
Cisplatin is a common chemotherapy drug used in the
treatment of various types of cancer; however, cisplatin also
exhibits serious adverse effects, particularly nephrotoxicity and
oxidative injury (23,31). Previous studies have reported that
the therapeutic efficacy of emodin combined with chemical drugs is
higher than that of chemical drugs alone, and that combination
treatment also results in fewer adverse effects (7,15,32). For
example, emodin can enhance the therapeutic effect of gemcitabine
on pancreatic cancer without other toxic effects (33). Other studies have demonstrated that
emodin can increase the antitumor effect of gemcitabine even when
gemcitabine is administered at a low dose (33,34).
Compared with treatment with cisplatin, carboplatin or oxaliplatin
alone, cotreatment of emodin with cisplatin, carboplatin or
oxaliplatin effectively enhances the chemosensitivity of the
gallbladder cancer cell line SGC996 via glutathione depletion and
MRP1 downregulation both in vitro and in vivo
(34,35). Therefore, the combination of emodin
derived from traditional Chinese medicine and cisplatin may
therefore represent a potential method to decrease the toxicity of
cisplatin toward normal cells and increase its toxicity toward
tumor cells.
In the present study, the effect of emodin and
cisplatin on A549 and H460 cells behavior was evaluated. The
results demonstrated that emodin significantly enhanced the
antiproliferative, antidrug efflux, pro-apoptotic and DNA-damaging
effects in combination with cisplatin in vitro. These data
suggested that emodin may enhance cisplatin-induced antitumor
activity in A549 and H460 cells in a dose-dependent manner.
Previous studies have reported that 5 µM emodin slightly promotes
the proliferation of bladder cancer cells, although there was no
significant difference (15,32). In addition, emodin significantly
decreases the antitumor effect of tamoxifen in HER2+
breast cancer cells (36). Emodin
may likely show different levels of antitumor activity depending on
the type of tumor and the antitumor activity of different
concentrations of emodin could be different in the same tumor. In
the present study, different concentrations of emodin had different
effects on the proliferation of A549 cells. Emodin at 1 µM had a
slight promoting effect on the proliferation of A549 cells, while
emodin at >5 µM significantly inhibited the proliferation of
A549 cells. Different concentrations of emodin had a certain
inhibitory effect on H460 cell proliferation. Therefore, emodin
exerted an anti-tumor effect in a concentration-dependent manner in
NSCLC.
MDR is an important defense mechanism of tumor cells
against chemical drugs (37).
However, multiple factors are associated with MDR, including the
efflux pump mechanism of drug-resistant proteins [Pgp, MRP and
lipoprotein receptor-related protein-1 (LRP1)], the decrease in DNA
topoisomerase activity, and the abnormal DNA repair (38). In particular, the drug protein pump
mediated by Pgp, MRP and other drug resistance-related proteins,
such as BCRP and LRP1, is the main mechanism by which tumors
develop MDR (27). Previous studies
have demonstrated that emodin and cisplatin alone or in combination
can significantly decrease the expression of Pgp and MRP1 in
bladder cancer cells (32,35,39).
Furthermore, emodin and doxorubicin significantly decrease the
expression of Pgp and MRP1 in colon cancer cells (39). In the present study, the effect of
emodin on the expression of Pgp and MRP1 in A549 and H460 cells was
investigated. The results demonstrated that emodin enhanced the
sensitivity of NSCLC cells toward cisplatin by decreasing the
expression of Pgp but not of MRP.
The present study did have some limitations. First,
the effect of emodin on the mRNA expression of Pgp and MRP1 in A549
and H460 cells, and the effect of emodin on the expression of Pgp
and MRP1 in combination with cisplatin, were not investigated.
These topics need to be investigated in future. Secondly, the
present study did not investigate the effect of emodin on the
chemotherapy sensitivity of NSCLC cells in xenograft animal models.
Although emodin/cisplatin administration has been found to have no
significant effect on body weight and histological findings in
treated mice (tissue structure, cell morphology and vascular
distribution) (32), this does not
imply that emodin is not toxic. Future work will perform
pharmacodynamic, acute toxicity, long-term toxicity and
irritability tests in order to verify the safety of emodin. To the
best of our knowledge, there is currently no clinical study on the
combination of cisplatin and emodin. Clinical trials and long-term
follow-up are needed to fully assess the toxicity of combination
therapy in the future.
In summary, the present study demonstrated that
emodin could increase the sensitivity of A549 and H460 cells to
cisplatin by downregulating Pgp expression. The results suggested
that emodin may be considered as an effective sensitizer for
cisplatin-based chemotherapy in patients with NSCLC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81802997, 81602391
and 81502666); the Students' Innovation and Entrepreneurship
Training Program of Hubei University of Medicine (grant no.
S201910929006), the Foundation for Free Exploration of Hubei
University of Medicine (grant no. FDFR201802); the Natural Science
Foundation of Hubei Province of China (grant nos. 2019CFA034,
2017CFB167, 2018CFB405 and 2017CFB456) and the Scientific and
Technological Project of Shiyan City of Hubei Province (grant no.
19Y40).
Availability of data and materials
Not applicable.
Authors' contributions
XZD and QK designed the present study. SP, JW and CL
performed all the experiments, analyzed the data and prepared the
figures. XD and ZX were responsible for the initial manuscript and
interpretation of data. JJC was involved in drafting the
manuscript, analysis and interpretation of data. All authors read
and approved the final version.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Romaszko AM and Doboszyńska A: Multiple
primary lung cancer: A literature review. Adv Clin Exp Med.
27:725–730. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheema PK, Rothenstein J, Melosky B, Brade
A and Hirsh V: Perspectives on treatment advances for stage III
locally advanced unresectable non-small-cell lung cancer. Curr
Oncol. 26:37–42. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Driesen P, Lambrechts M, Kraaij K,
Soldatenkova V, Chouaki N and Colinet B: A phase II single-arm
study of induction chemotherapy with cisplatin and gemcitabine
followed by concurrent cisplatin and gemcitabine with thoracic
radiation for unresectable locally advanced non-small cell lung
cancer. Ther Adv Med Oncol. 5:159–168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baldini E, Tibaldi C and Delli Paoli C:
Chemo-radiotherapy integration in unresectable locally advanced
non-small-cell lung cancer: A review. Clin Transl Oncol.
22:1681–1686. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hauner K, Maisch P and Retz M: Side
effects of chemotherapy. Urologe A. 56:472–479. 2017.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pastorino G, Cornara L, Soares S,
Rodrigues F and Oliveira MBPP: Liquorice (Glycyrrhiza
glabra): A phytochemical and pharmacological review. Phytother
Res. 32:2323–2339. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei WT, Lin SZ, Liu DL and Wang ZH: The
distinct mechanisms of the antitumor activity of emodin in
different types of cancer (Review). Oncol Rep. 30:2555–2562. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Shan C, Wu Z, Yu H, Yang A and Tan
B: Emodin alleviated pulmonary inflammation in rats with
LPS-induced acute lung injury through inhibiting the
mTOR/HIF-1α/VEGF signaling pathway. Inflamm Res. 69:365–373. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu C, Zhang J, Liu J, Li Z, Liu Z, Luo Y,
Xu Q, Wang M, Zhang G, Wang F, et al: Proteomic analysis reveals
the protective effects of emodin on severe acute pancreatitis
induced lung injury by inhibiting neutrophil proteases activity. J
Proteomics. 220:1037602020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma W, Liu C, Li J, Hao M, Ji Y and Zeng X:
The effects of aloe emodin-mediated antimicrobial photodynamic
therapy on drug-sensitive and resistant Candida albicans. Photochem
Photobiol Sci. 19:485–494. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen Q, Li KT, Tian S, Yu TH, Yu LH, Lin
HD and Bai DQ: Photodynamic therapy mediated by aloe-emodin
inhibited angiogenesis and cell metastasis through activating MAPK
signaling pathway on HUVECs. Technol Cancer Res Treat.
17:15330338187855122018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zu C, Qin G, Yang C, Liu N, He A, Zhang M
and Zheng X: Low dose Emodin induces tumor senescence for boosting
breast cancer chemotherapy via silencing NRARP. Biochem Biophys Res
Commun. 505:973–978. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu M, Tang W, Liu JJ, Gong XQ, Kong L, Yao
XM, Jing M, Cai FY, Li XT and Ju RJ: Combination of targeted
daunorubicin liposomes and targeted emodin liposomes for treatment
of invasive breast cancer. J Drug Target. 28:245–258. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Akev N, Candoken E and Erdem Kuruca S:
Comparative study on the anticancer drug potential of a lectin
purified from aloe vera and aloe-emodin. Asian Pac J Cancer Prev.
21:99–106. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang W, Sun Y, Li X, Li H, Chen Y, Tian Y,
Yi J and Wang J: Emodin potentiates the anticancer effect of
cisplatin on gallbladder cancer cells through the generation of
reactive oxygen species and the inhibition of survivin expression.
Oncol Rep. 26:1143–1148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Y, Gan D, Huang Q, Luo X, Lin D and
Hu J: Emodin and Its combination with cytarabine induce apoptosis
in resistant acute myeloid leukemia cells in vitro and in vivo.
Cell Physiol Biochem. 48:2061–2073. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen S, Zhang Z and Zhang J: Emodin
enhances antitumor effect of paclitaxel on human non-small-cell
lung cancer cells in vitro and in vivo. Drug Des Devel Ther.
13:1145–1153. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Holford J, Beale PJ, Boxall FE, Sharp SY
and Kelland LR: Mechanisms of drug resistance to the platinum
complex ZD0473 in ovarian cancer cell lines. Eur J Cancer.
36:1984–1990. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi B, Xu FF, Xiang CP, Jia R, Yan CH, Ma
SQ, Wang N, Wang AJ and Fan P: Effect of sodium butyrate on ABC
transporters in lung cancer A549 and colorectal cancer HCT116
cells. Oncol Lett. 20:1482020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian J, Hu J, Liu G, Yin H, Chen M, Miao
P, Bai P and Yin J: Altered Gene expression of ABC transporters,
nuclear receptors and oxidative stress signaling in zebrafish
embryos exposed to CdTe quantum dots. Environ Pollut. 244:588–599.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sauerborn Klobucar R, Zaja R, Franjević D,
Brozović A and Smital T: Presence of ecotoxicologically relevant
Pgp and MRP transcripts and proteins in Cyprinid fish. Arh Hig Rada
Toksikol. 61:175–182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou J, Li Z, Li J, Gao B and Song W:
Chemotherapy resistance molecular mechanism in small cell lung
cancer. Curr Mol Med. 19:157–163. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Y, Lei L and Liu Y: Propofol
improves sensitivity of lung cancer cells to cisplatin and its
mechanism. Med Sci Monit. 26:e9197862020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deng X, Hu Y, Ding Q, Han R, Guo Q, Qin J,
Li J, Xiao R, Tian S, Hu W, et al: PEG10 plays a crucial role in
human lung cancer proliferation, progression, prognosis and
metastasis. Oncol Rep. 32:2159–2167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deng X, Tu Z, Xiong M, Tembo K, Zhou L,
Liu P, Pan S, Xiong J, Yang X, Leng J, et al: Wnt5a and CCL25
promote adult T-cell acute lymphoblastic leukemia cell migration,
invasion and metastasis. Oncotarget. 8:39033–39047. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong X, Luo Z, Wang Y, Meng L, Duan Q, Qiu
L, Peng F and Shen L: Altered O-glycosylation is associated with
inherent radioresistance and malignancy of human laryngeal
carcinoma. Exp Cell Res. 362:302–310. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao L, Tsutsui T and Miwa N: The
lipophilic vitamin C derivative, 6-o-palmitoylascorbate, protects
human lymphocytes, preferentially over ascorbate, against
X-ray-induced DNA damage, lipid peroxidation, and protein
carbonylation. Mol Cell Biochem. 394:247–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dong W, Liao ZG, Zhao GW, Guan XJ, Zhang
J, Liang XL and Yang M: Reversal effect of oxypeucedanin on
P-glycoprotein-mediated drug transport. Molecules. 23:18412018.
View Article : Google Scholar
|
|
29
|
Hurtado M, Sankpal UT, Chhabra J, Brown
DT, Maram R, Patel R, Gurung RK, Simecka J, Holder AA and Basha R:
Copper-tolfenamic acid: Evaluation of stability and anti-cancer
activity. Invest New Drugs. 37:27–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Y, Adebali O, Wu G, Selby CP, Chiou
YY, Rashid N, Hu J, Hogenesch JB and Sancar A: Cisplatin-DNA adduct
repair of transcribed genes is controlled by two circadian programs
in mouse tissues. Proc Natl Acad Sci USA. 115:E4777–E4785. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shah N, Liu Z, Tallman RM, Mohammad A,
Sprowls SA, Saralkar PA, Vickers SD, Pinti MV, Gao W and Lockman
PR: Drug resistance occurred in a newly characterized preclinical
model of lung cancer brain metastasis. BMC Cancer. 20:2922020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li X, Wang H, Wang J, Chen Y, Yin X, Shi
G, Li H, Hu Z and Liang X: Emodin enhances cisplatin-induced
cytotoxicity in human bladder cancer cells through ROS elevation
and MRP1 downregulation. BMC Cancer. 16:5782016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu A, Chen H, Tong H, Ye S, Qiu M, Wang
Z, Tan W, Liu J and Lin S: Emodin potentiates the antitumor effects
of gemcitabine in pancreatic cancer cells via inhibition of nuclear
factor-κB. Mol Med Rep. 4:221–227. 2011.PubMed/NCBI
|
|
34
|
Lin SZ, Wei WT, Chen H, Chen KJ, Tong HF,
Wang ZH, Ni ZL, Liu HB, Guo HC and Liu DL: Antitumor activity of
emodin against pancreatic cancer depends on its dual role:
Promotion of apoptosis and suppression of angiogenesis. PLoS One.
7:e421462012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang W, Sun YP, Huang XZ, He M, Chen YY,
Shi GY, Li H, Yi J and Wang J: Emodin enhances sensitivity of
gallbladder cancer cells to platinum drugs via glutathion depletion
and MRP1 downregulation. Biochem Pharmacol. 79:1134–1140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tseng HS, Wang YF, Tzeng YM, Chen DR, Liao
YF, Chiu HY and Hsieh WT: Aloe-emodin enhances tamoxifen
cytotoxicity by suppressing Ras/ERK and PI3K/mTOR in breast cancer
cells. Am J Chin Med. 45:337–350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang X, Lei T and Zhang M: Expression and
functions of formyl peptide receptor 1 in drug-resistant bladder
cancer. Technol Cancer Res Treat. 17:15330346187694132018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee CH: Reversing agents for ATP-binding
cassette drug transporters. Methods Mol Biol. 596:325–340. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Iyer VV, Priya PY and Kangeyavelu J:
Effects of increased accumulation of doxorubicin due to emodin on
efflux transporter and LRP1 expression in lung adenocarcinoma and
colorectal carcinoma cells. Mol Cell Biochem. 449:91–104. 2018.
View Article : Google Scholar : PubMed/NCBI
|