Introduction
Liver cancer has become one of the most malignant
cancers worldwide (1), with
hepatocellular carcinoma (HCC) accounting for ~80% cases of all
cases (2). Due to the high rate of
recurrence or intrahepatic metastasis after curative resection, the
overall prognosis of patients with HCC remains poor despite marked
improvements in surgical techniques and perioperative management
(2–4). The overall 5-year survival rate of HCC
is still <20% (5); thus, an
improved understanding of the molecular mechanisms underlying HCC
metastasis will help prevent HCC recurrence and metastasis.
Epithelial-mesenchymal transition (EMT) is a crucial
event in tumour metastasis, where epithelial cell layers lose
polarity and cell-cell contact, which results in dramatic
remodelling of the cytoskeleton (6).
The main characteristic of EMT is loss of E-cadherin expression,
which is associated with tumour invasiveness, metastasis and poor
prognosis (7–9). The activation of various ligands, such
as vascular endothelial growth factor (VEGF), epidermal growth
factor (EGF) and transforming growth factor-β (TGF-β) can induce
the expression of several EMT-associated transcription factors,
including zinc finger e-box binding homeobox (ZEB) 1, snail, slug
and Twist (10). A previous study
has demonstrated positive correlations between EMT-associated
transcription factors and poor clinical outcomes in cancer, such as
lung cancer, breast cancer, melanoma and HCC (10).
GATA3, a member of the GATA family (11,12),
serves a crucial role in T-cell proliferation and differentiation
(13). In addition, numerous studies
have demonstrated that GATA3 serves different roles in different
cancers, for example, GATA3 serves as a tumour activator in soft
tissue sarcomas, endometrial carcinomas and neuroblastomas
(14–16). In addition, GATA3 suppresses cell
proliferation, migration and invasion in osteosarcoma (17). Furthermore, a recent study
demonstrated that zinc finger protein 503 (ZNF503) accelerates HCC
cell aggressiveness by downregulating GATA3 expression via
microRNA-495, suggesting that GATA3 may serve as a tumour
suppressor in HCC (18). However,
the detailed function of GATA3 in HCC remains unclear.
The aim of present study was to explore whether
GATA3 serves as a tumour suppressor to inhibit HCC development and
further investigate whether GATA3 may be a molecular therapy target
in HCC.
Materials and methods
Tumour samples
A total of 162 HCC tissues and adjacent non-tumour
tissues (resected 1–2 cm from the malignant tumor) were obtained
from the Affiliated Hospital of Shaoxing University from July 2000
to May 2018. These patients with gastric cancer included 92 males
and 70 females aged between 23–78 years, with a mean age of 42.3
years. Tumour tissue (TT) and adjacent non-tumour tissue (ANT) were
resected by surgical excision. No prior treatments (including
chemotherapy or radiotherapy) were conducted before liver resection
surgery. Pathological staging was determined according to the
seventh edition of the tumour node metastasis (TNM) classification
of the International Union Against Cancer (19). All tissue samples were confirmed
using histopathological evaluation and stored at −80°C until
further use. The tissue samples were used in accordance with the
policies of the institutional review board at the Affiliated
Hospital of Shaoxing University, China. The study was approved the
by the review board of the Affiliated Hospital of Shaoxing
University and written informed consent was obtained from all
patients.
Cell culture
HCC cell lines, including MHCC97-H and Hep3B were
obtained from the Institute of Biochemistry and Cell Biology
(Chinese Academy of Sciences). All cells were cultured in DMEM
medium (Thermo Fisher Scientific, Inc.) supplemented with 100 U/ml
penicillin-streptomycin mixture (Beyotime Institute of
Biotechnology) and 10% FBS (Sigma-Aldrich: Merck KGaA) at 37°C with
5% CO2.
Cell transfection
Transfections were performed using
Lipofectamine® 2000 or Lipofectamine® RNAiMAX
Reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. FLAG-GATA3 (pcDNA3.1) and
FLAG-slug (pcDNA3.1) plasmids were obtained from Vigene Bioscience
Inc. The sequences of small interfering (si)RNAs were as follows:
Scramble siRNA (SCR): 5′-UUCUCCGAACGUGUCACGU-3′; siGATA3#1:
5′-AAACUAGGUCUGAUAUUCAUU-3′; siGATA3#2:
5′-CUUUAUUGCAUCUGGGUAGUU-3′. For overexpression of GATA3 or/and
slug, cells were transfected with 2.5 µg FLAG-GATA3 and/or 2.5 µg
FLAG-slug using Lipofectamine® 2000. For inhibition of
GATA3, cells were transfected with 40 nM siRNAs using
Lipofectamine® RNAiMAX Reagent. After transfection for
48 h, cells were collected.
RNA extraction and quantitative
reverse transcription quantitative (RT-q)PCR
Total RNA was extracted from tissue samples or cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. A
total of 2 µg RNA was used to synthesize cDNA using a PrimeScript
Reverse Transcriptase kit (Takara Biotechnology Co., Ltd.). The
reverse transcription protocol was as follows: initial denaturation
at 37°C for 15 min and then 85°C for 5 sec. The samples were stored
at 4°C. Subsequently, RT-qPCR was performed by SYBRGreen (Takara
Bio, Inc.) in the Applied Biosystems 7500 Sequence Detection system
(Thermo Fisher Scientific Inc.). The PCR cycles were performed by
initial denaturation at 95°C for 15 min, followed by 40 cycles at
95°C for 20 sec, 57°C for 35 sec and elongation at 72°C for 2 min.
Relative mRNA levels were normalized against that of GAPDH, and the
levels of the transcripts were quantified using the
2−ΔΔCq method (20). The
sequences of primers as follows: GATA3 forward,
5′-GTTGTGCTCGGAGGGTTTCT-3′, reverse, 5′-GCACGCTGGTAGCTCATACA-3′;
Slug forward, 5′-ATCACTGTGTGGACTACCGC-3′, reverse,
5′-TCACTCGCCCCAAAGATGAG-3′; Snail forward,
5′-GTTTACCTTCCAGCAGCCCT-3′, reverse, 5′-TCCCAGATGAGCATTGGCAG-3′;
ZEB1 forward, 5′-GATGACCTGCCAACAGACCA-3′, reverse:
5′-CTGTGTCATCCTCCCAGCAG-3′; and GAPDH forward,
5′-GAAAGCCTGCCGGTGACTAA-3′, reverse, 5′-AGGAAAAGCATCACCCGGAG-3′.
Each experiment was performed at least three times.
Western blotting
Cell lysates were prepared using RIPA lysis buffer
(Pierce; Thermo Fisher Scientific, Inc.) with a protease inhibitor
cocktail (Roche Applied Science) and protein concentration was
measured using a bicinchoninic acid kit (Beyotime Institute of
Biotechnology) according to manufacturer's protocol. Subsequently,
40 µg protein was separated on 12% SDS-PAGE and then transferred
onto a PVDF membrane (EMD Millipore). After blocking with 5%
non-fat milk in TBST for 1 h at room temperature, membranes were
incubated with the indicated primary antibodies at 4°C overnight.
The primary antibodies used were as follows: GATA3 (1:1,000; cat.
no. ab199428; Abcam); E-cadherin (1:1,000; cat. no. 14472; CST
Biological Reagents Co., Ltd.); N-cadherin (1:1,000; cat. no.
13116; CST Biological Reagents Co. Ltd.); and Slug (1:1,000; cat.
no. ab51772; Abcam). The membranes were then washed with TBST three
times and incubated with horse radish peroxide-conjugated goat
anti-rabbit IgG (1:2,000; cat. no. ab6721; Abcam) or goat
anti-mouse IgG (1:4,000; cat. no. ab6789; Abcam) secondary
antibodies for 1 h at room temperature. The specific bands were
visualized using the enhanced chemiluminescence (ECL; Merck KGaA)
according to manufacturer's protocol. β-actin (1:5,000; cat. no.
ab8226; Abcam) was used as the internal control. Each experiment
was performed at least three times. Western blotting densitometry
was analysed using Image J software (v1.48; National Institutes of
Health).
Cell Counting Kit-8 (CCK-8) assay
CCK-8 (Dojindo Molecular Technologies, Inc.) was
utilized to determine the effect of GATA3 on cell proliferation
according to the manufacturer's instructions. Briefly, Hep3B and
MHCC97-H cells were transfected with FLAG-GATA3 or GATA3 siRNA.
After transfection for 48 h, ~2×103 cells were seeded in
96-well plates with 200 µl DMEM at 37°C with 5% CO2.
After 24 and 48 h of incubation, 20 µl CCK-8 reagent was added to
each well and cultured for 2 h. Finally, the absorbance at 450 nm
measured using a microplate reader (Bio-Rad laboratories, Inc.).
Each experiment was performed at least three times.
Colony formation assay
To evaluate the effect of GATA3 on cell
proliferation in HCC, colony formation assay was performed. In
brief, 5×103 transfected Hep3B and MHCC97-H cells were
plated into 6-well plate. After incubation at 37°C with 5%
CO2 for 2 weeks, cells were fixed in 4% paraformaldehyde
at room temperature for 10 min and stained with 0.5% crystal violet
at room temperature for 10 min. Finally, the colonies were counted.
Each experiment was performed at least three times.
Wound healing assay
GATA3 was overexpressed or knocked down in Hep3B and
MHCC97-H cells. After transfection for 48 h, 5×106 cells
were grown in 6-well plates. After cell density reached 100%
confluency, the monolayer was scratched with a 20 µl sterile
plastic pipette tip. After washing with PBS three times, cells were
cultured in serum-free DMEM at 37°C with 5% CO2 for 48
h. Then, wound closure was imaged under a light microscope
(magnification, ×40) and the rate of wound closure was measured
using Image J software version 1.2 (National Institutes of Health).
Relative distance of cell migration = [wound closure (0 h) - wound
closure (48 h)]/wound closure (0 h) ×100. Each experiment was
performed at least three times.
Transwell assay
A transwell invasion assay was performed to
determine the effect of GATA3 on the capacity of cell invasion. The
upper chamber was precoated with BioCoat Matrigel (BD Biosciences)
and PBS (1:8) at 37°C with 5% CO2 for 30 min. GATA3 was
overexpressed or knocked down in MHCC97-H cells. After transfection
for 48 h, 2×105 cells were placed into upper chamber
containing 400 μl serum-free DMEM and 500 µl DMEM medium containing
10% FBS was added into the lower chamber. After incubation at 37°C
with 5% CO2 for 24 h, cells were stained with 0.5%
crystal violet for 5 min at room temperature and non-invading cells
on the upper chamber surface were removed using a cotton swab.
Finally, invading cells were imaged under a light microscope
(magnification, ×40). Each experiment was performed at least three
times.
Enzyme-linked immunosorbent assay
(ELISA)
MHCC97-H cells were collected and plated into 6-well
culture plates at a density of 4×105 cells/well. Cell
supernatants in serum-free medium were homogenized and harvested 72
h later and centrifuged at 1,000 × g for 30 min. The levels of MMP2
and MMP9 were subsequently determined using commercially available
ELISA kits (cat. nos. E0100Hu and E0553Hu; Wuhan USCN Business Co.,
Ltd.) according to the manufacturer's instructions.
Chromatin immunoprecipitation (ChIP)
and quantitative (q) ChIP assay
ChIP and qChIP analyses were performed using an
EZ-ChIP kit (EMD Millipore) according to the manufacturer's
instructions. Briefly, Hep3B and MHCC97-H cells were cultured to
80–100% confluence and the chromatin was cross-linked by 1%
formaldehyde at 37°C for 10 min. Subsequently, cross-linked
chromatin was sonicated (20 kHz; amplitude, 40%; 30 cycles, 1 sec
on and 1 sec off) at 4°C to generate 200–1,000 bp fragments. Next,
4 µg of anti-GATA3 (cat. no. ab199428; Abcam) or anti-IgG antibody
(cat. no. ab171870; Abcam) were used to immunoprecipitate chromatin
fragments at 4°C overnight. IgG antibody was used as the control.
The protein-DNA complexes were incubated with protein A Sepharose
beads (Thermo Fisher Scientific, Inc.), and eluted in 1% SDS/0.1 M
NaHCO3. The protein-DNA cross-link was reversed by heating at 65°C
for 6 h. The DNA was purified using QIAEX II Gel Extraction kit
(Qiagen GmbH) according to manufacturer's instructions. After
purifying the antibody-interact DNA, RT-qPCR was conducted to
analyze the precipitated chromatin DNA, as aforementioned. The
primer sequences were as follows: Slug forward,
5′-TCCGGTGGTTCCAAATGACA-3′; and reverse,
5′-TCCGGTGGTTCCAAATGACA-3′. The qPCR conditions were as follows: 5
min at 98°C, denaturation at 98°C for 30 sec, annealing at 56°C for
30 sec and extension at 72°C for 20 sec, performed for 32
cycles.
Luciferase reporter assay
The sequence of the slug promoter region was
amplified from human genomic DNA. The sequence was then cloned into
pGL3-basic luciferase reporter vector (Promega Corporation). A
total of 2 µg pGL3-slug, Renilla and GATA3 or 50 nM GATA3 siRNA
were co-transfected into Hep3B and MHCC97-H cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After transfection for 24 h, the relative
luciferase activity was measured using Dual-Luciferase Reporter
Assay System (Promega Corporation) according to the manufacturer's
instructions. Renilla activity was used as the internal control.
The relative luciferase activity was normalized with Renilla
luciferase activity. Each experiment was performed at least three
times.
Statistical analysis
All data were represented as the mean ± SD. The
association between GATA3 expression and clinicopathological
features of HCC was analysed by χ2 test. Student's
t-test was used to compare the statistical differences between two
groups and one-way ANOVA followed by Tukey's post-hoc test was used
to compare the statistical differences between multiple groups. The
overall survival of patients with HCC with high or low level of
GATA3 was estimated using the Kaplan-Meier method. All data were
analysed by SPSS 18.0 software (SPSS, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
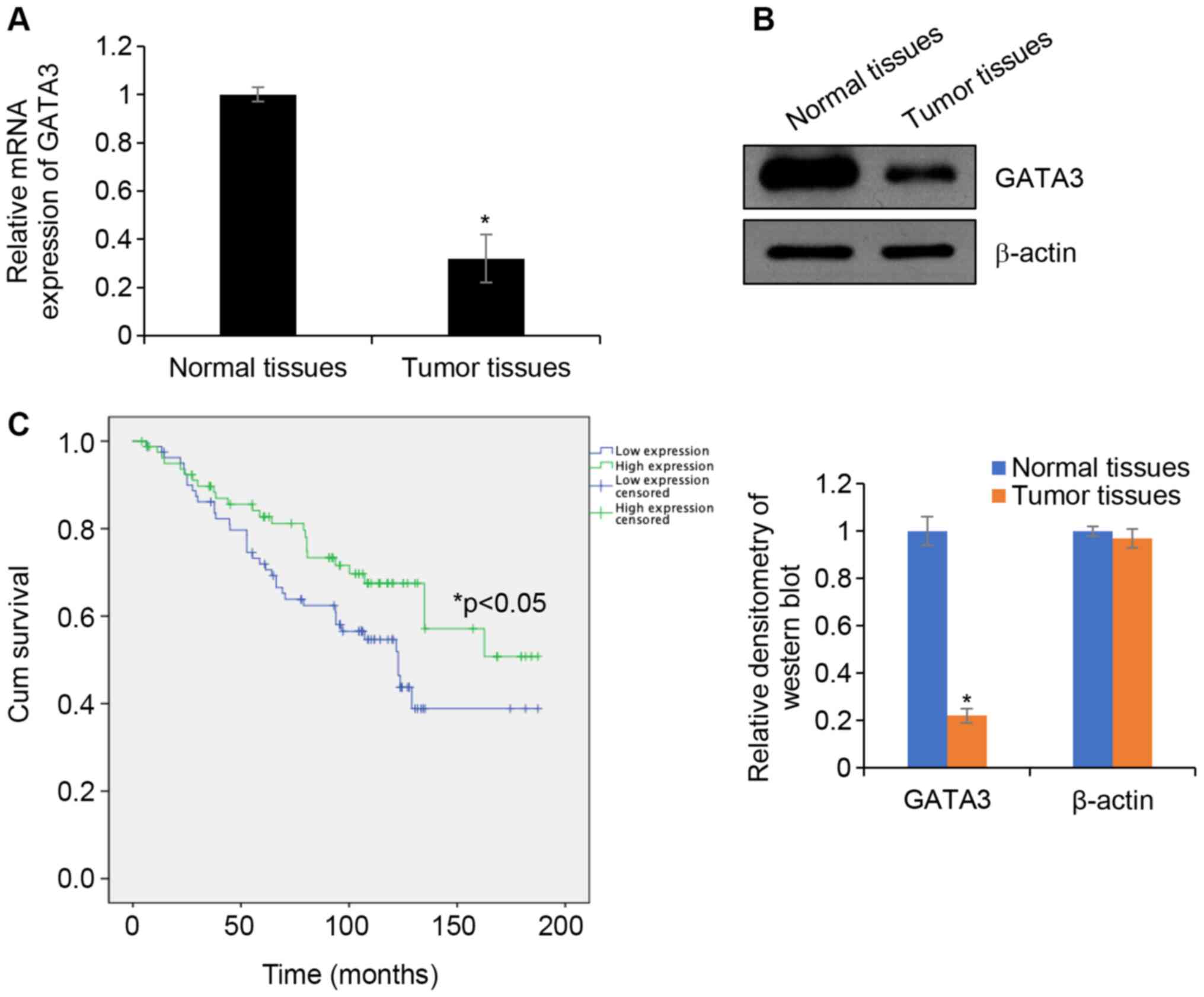
GATA3 is downregulated in HCC
To investigate whether GATA3 serves as a tumour
suppressor in HCC, the expression of GATA3 in HCC tissues was
determined. HCC tissue samples and adjacent normal tissues samples
were collected and RT-qPCR was performed to determine GTAT3 mRNA
levels. The results demonstrated that the expression of GATA3 mRNA
in HCC tissues was significantly downregulated in TT compared with
that in ANT (Fig. 1A). In addition,
western blotting analyses demonstrated that the GATA3 protein
expression in HCC tissues was lower in TT compared with that in ANT
(Fig. 1B). To further determine the
roles of GATA3 in HCC, the association of GATA3 and the
clinicopathological features of HCC were analysed. GATA3 expression
was negatively associated with tumour size, pathological grade and
lymph node metastasis, but no significant association was observed
between GATA3 and other factors, such as age and sex (Table I). Additionally, the survival curve
demonstrated that patients with high expression of GATA3 had an
improved prognosis compared with patients exhibiting low GATA3
expression (Fig. 1C). Collectively,
the results indicated that GATA3 serves a key function in HCC.
 | Table I.Clinicopathological variables in 162
patients with hepatocellular carcinoma |
Table I.
Clinicopathological variables in 162
patients with hepatocellular carcinoma
|
|
| GATA3 expression |
|
|---|
|
|
|
|
|
|---|
| Variables | No. (n=162) | Low (n=111) | High (n=51) | P-value |
|---|
| Age, years |
|
|
|
0.527 |
| ≥40 | 83 | 55 | 28 |
|
|
<40 | 79 | 56 | 23 |
|
| Sex |
|
|
|
0.503 |
|
Male | 92 | 65 | 27 |
|
|
Female | 70 | 46 | 24 |
|
| Tumour size,
cm |
|
|
|
0.009a |
| Large
(≥2) | 94 | 72 | 22 |
|
| Small
(<2) | 68 | 39 | 29 |
|
| Pathological
grade |
|
|
|
0.047a |
|
I–II | 83 | 51 | 32 |
|
|
III–IV | 79 | 60 | 19 |
|
| Lymph node
metastasis |
|
|
|
0.049a |
|
Yes | 82 | 62 | 20 |
|
| No | 80 | 49 | 31 |
|
| Slug
expression |
|
|
|
<0.001a |
|
High | 77 | 63 | 14 |
|
|
Low | 85 | 48 | 37 |
|
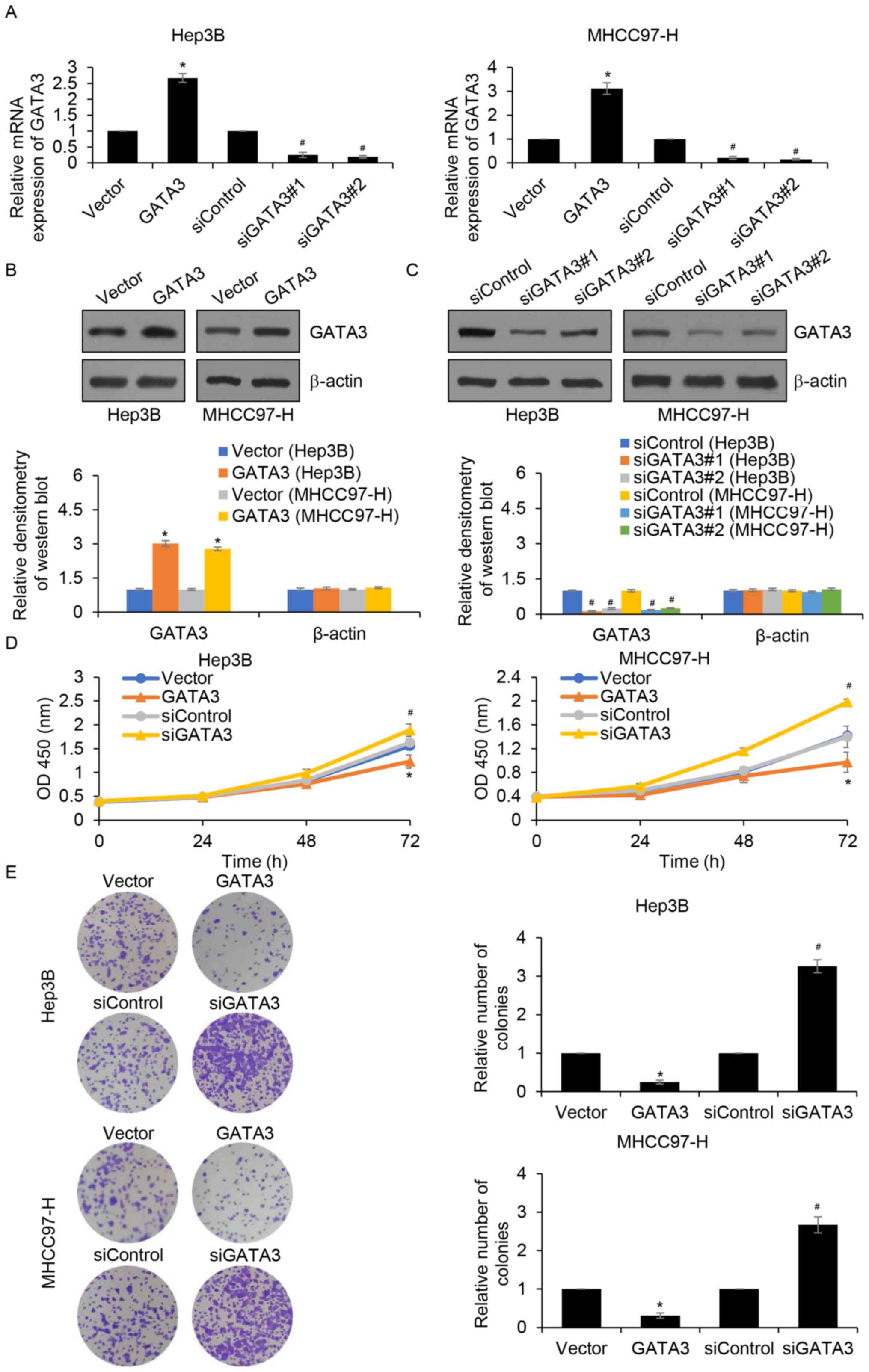
GATA3 suppresses HCC cell
proliferation
Since GATA3 expression was associated with tumour
size, it was hypothesized that GATA3 may suppress cell
proliferation in HCC. To verify this hypothesis, GATA3 was
overexpressed or knocked down in Hep3B and MHCC97-H cells and the
expression of GATA3 was established by RT-qPCR (Fig. 2A) and western blotting (Fig. 2B and C). The results demonstrated
that GATA3 mRNA and protein levels were significantly increased in
Hep3B and MHCC97-H cells transfected with FLAG-GATA3 compared with
those of the vector group. In addition, GATA3 mRNA and protein
levels were significantly decreased following siGATA3 transfection
compared with those of the siControl group. CCK-8 and colony
formation assays were subsequently performed to detect the effects
of GATA3 on cell proliferation. The results of the CCK-8 assay
demonstrated that, when compared with the control groups, GATA3
overexpression significantly suppressed cell proliferation, whereas
inhibition of GATA3 significantly increased cell proliferation
(Fig. 2D). Additionally, the colony
formation assay demonstrated that GATA3 overexpression
significantly reduced the number of colonies compared with the
control groups, whereas inhibition of GATA3 significantly increased
the number of colonies, suggesting that GATA3 may suppress cell
proliferation in HCC (Fig. 2E).
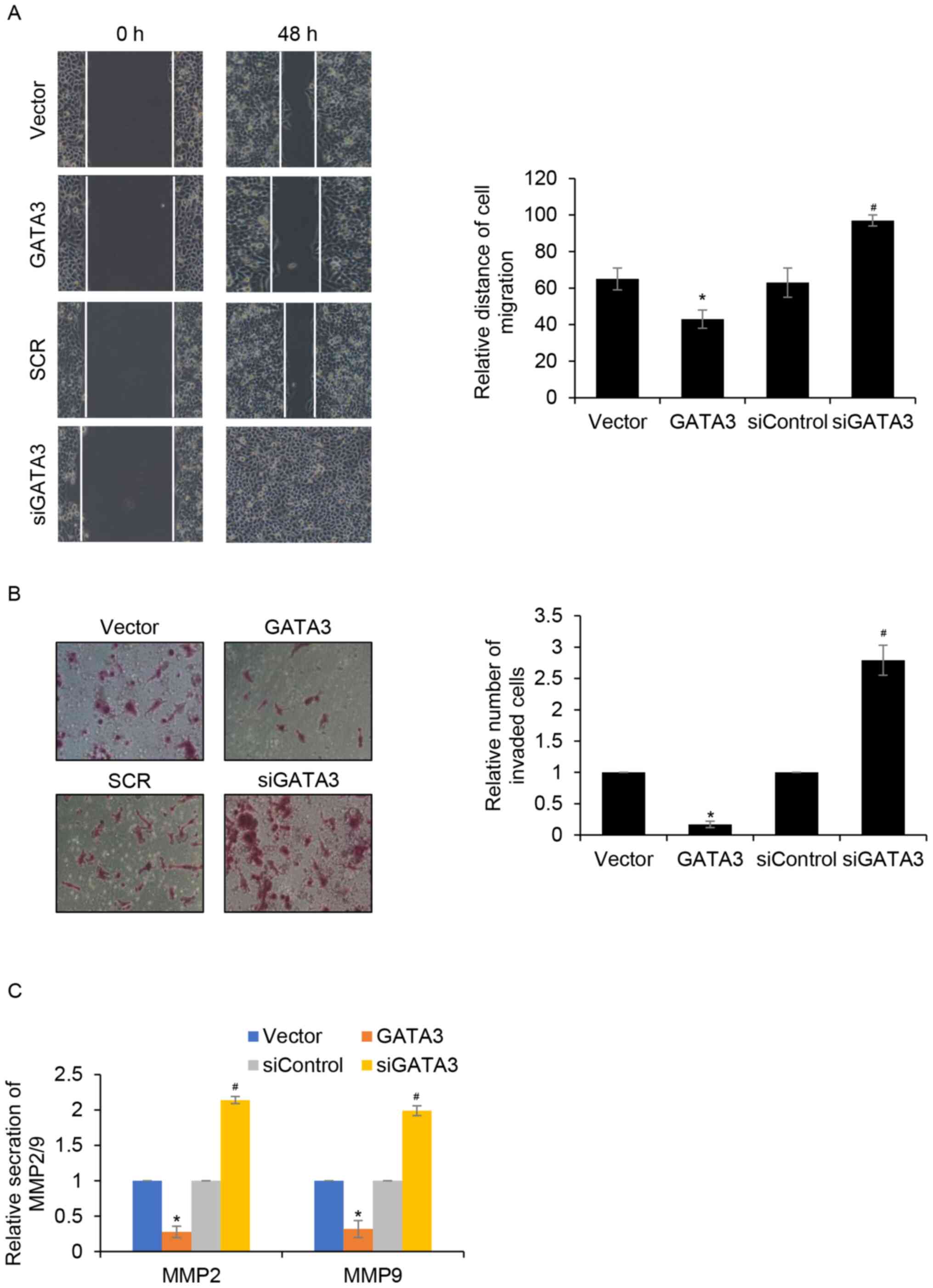
GATA3 inhibits cell migration and
invasion in HCC in vitro
The effects of GATA3 on cell migration and invasion
in MHCC97-H cells was investigated. The results of wound healing
analysis demonstrated that MHCC97-H cell migration was
significantly reduced when GATA3 was overexpressed compared with
the control group. Furthermore, MHCC97-H cell migration was
significantly increased when GATA3 was knocked down compared with
the siControl group (Fig. 3A).
Transwell invasion analysis demonstrated that GATA3 overexpression
significantly suppressed the invasion of MHCC97-H cells compared
with the control group. Additionally, inhibition of GATA3 promoted
MHCC97-H cell invasion compared with that of the siControl group
(Fig. 3B). Metalloproteinase (MMP)2
and MMP9 have been reported to be strongly associated with tumour
aggressiveness and poor prognosis in multiple types of cancer, such
as lung adenocarcinoma and tongue carcinoma (21,22).
Thus, whether GATA3 promotes HCC cell migration and invasion
through regulation of MMP2 or MMP9 was explored. The effect of
GATA3 on MMP2 and MMP9 secretion was determined using ELISA assay.
The results demonstrated that GATA3 inhibition significantly
increased MMP2 and MMP9 secretion compared with the siControl group
(Fig. 3C). By contrast, GATA3
overexpression significantly suppressed MMP2 and MMP9 secretion
compared with the same group. These data suggested that GATA3 may
suppress HCC cell migration and invasion by inhibiting MMP2 or MMP9
secretion.
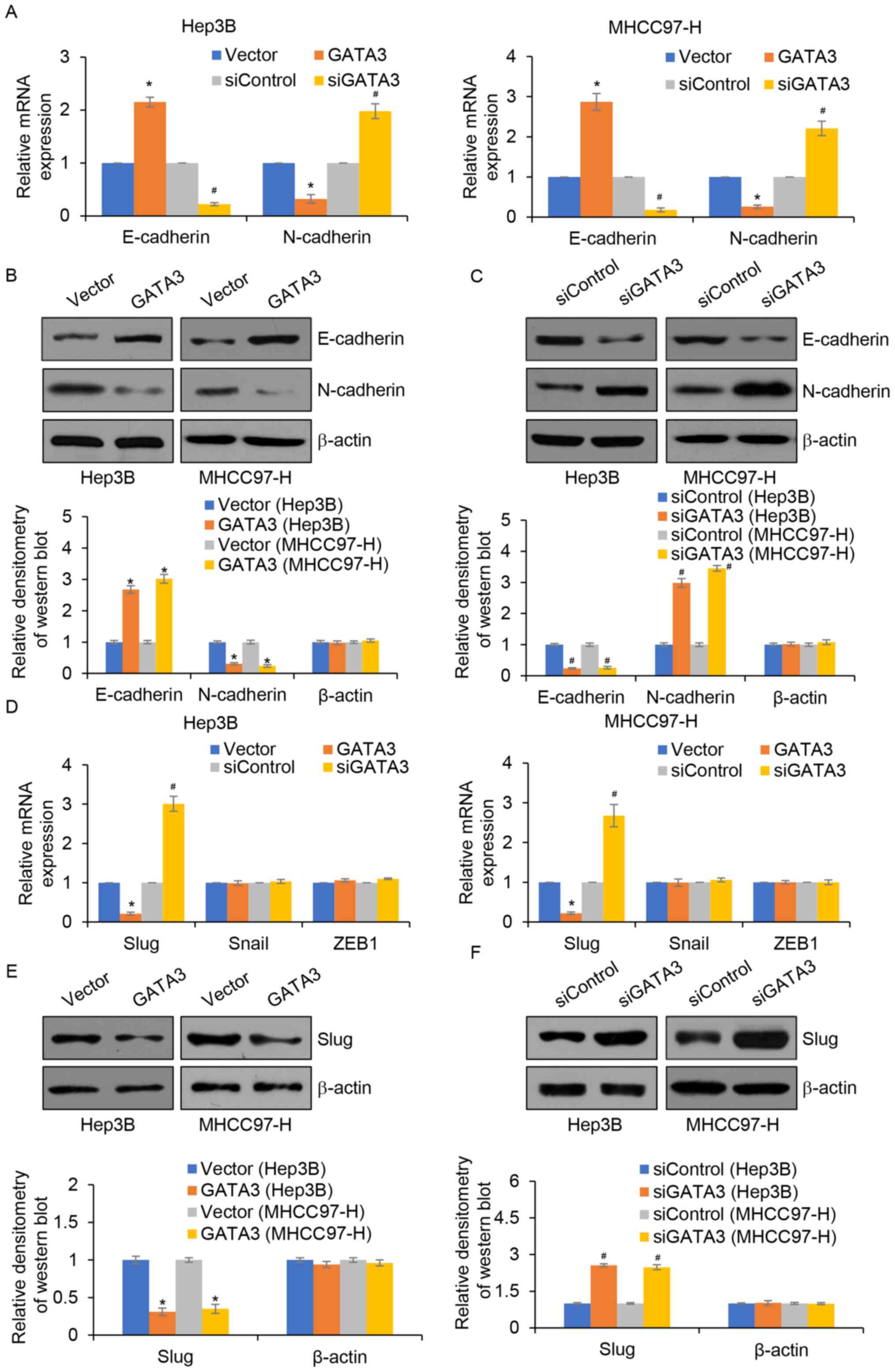
GATA3 inhibits EMT in HCC by
regulating Slug
EMT is a complex process associated with metastasis
(23); thus, whether GATA3
suppresses migration and invasion through the regulation of EMT in
HCC was explored. The results demonstrated that GATA3
overexpression significantly increased the mRNA and protein
expression of E-cadherin, an epithetical marker. However, the mRNA
and protein expression of N-cadherin, a mesenchymal marker was
significantly reduced compared with the control group (Fig. 4A and B). By contrast, inhibition of
GATA3 significantly reduced the mRNA and protein expression levels
of E-cadherin, but significantly increased the mRNA and protein
expression levels of N-cadherin and vimentin compared with those of
the control group (Fig. 4A and C).
Notably, a previous study demonstrated that GATA3 regulates the
expression of slug in osteosarcoma (17). Thus, whether GATA3 regulated slug
expression in HCC was determined. The results demonstrated that
compared with the controls, GATA3 overexpression significantly
decreased the expression of slug, whereas inhibition of GATA3
significantly increased slug levels (Fig. 4D, E and F).
Slug is a direct target of GATA3 in
HCC cells
To further determine whether GATA3 inhibits EMT in
HCC through the regulation of slug expression, slug levels were
detected in HCC specimens. The results demonstrated that slug
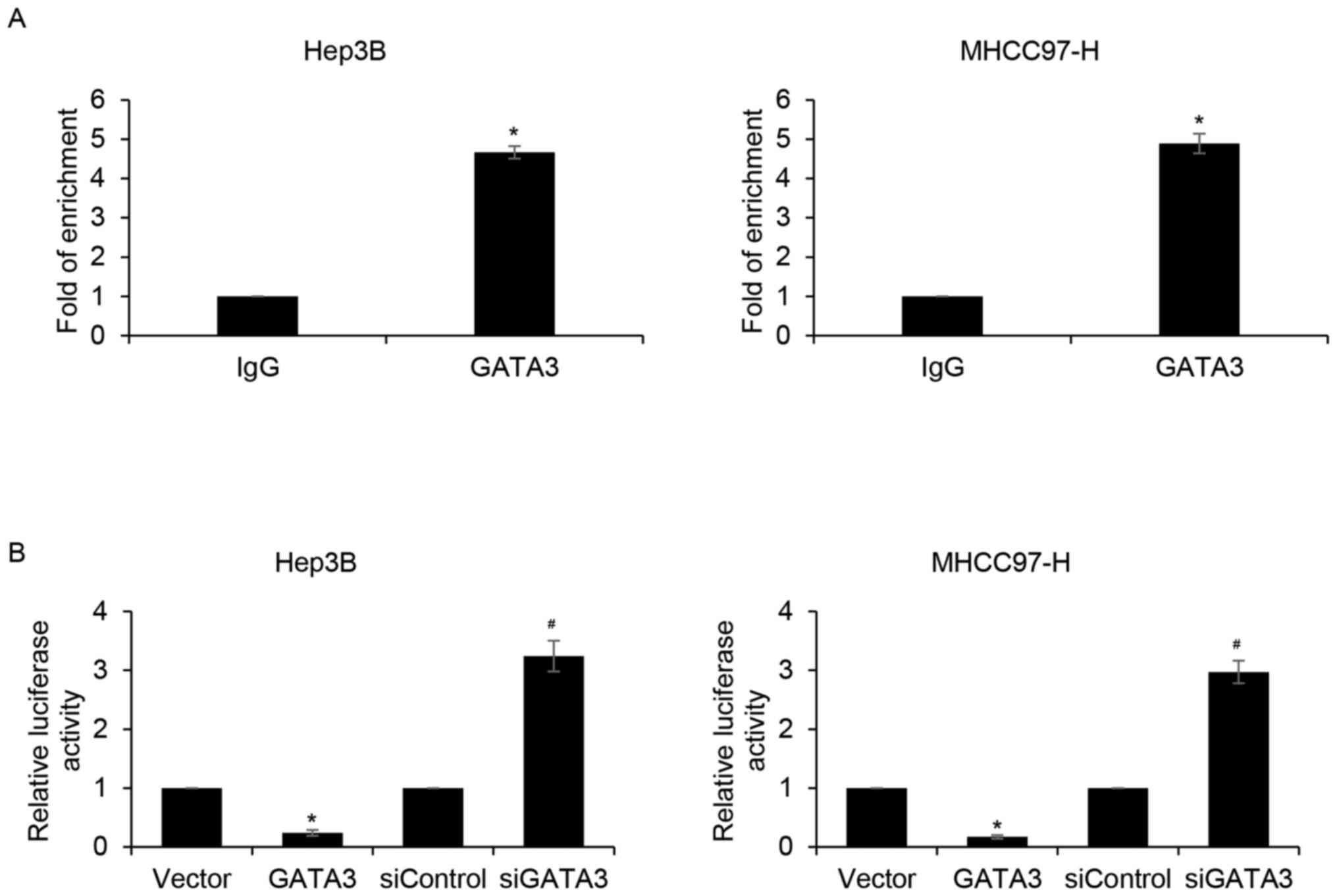
expression was negatively associated with GATA3 (Table I). GATA3 is a transcription factor;
thus, to further determine whether GATA3 directly binds to the
promoter region of slug, qChIP and dual luciferase reporter assays
were performed. The results demonstrated that the relative
enrichment of GATA3 in the promoter region of slug was
significantly increased compared with that of the IgG group
(Fig. 5A). In addition, the relative
luciferase activity of Hep3B and MHCC97-H cells were significantly
reduced in GATA3-overexpression cells compared with that of the
vector group (Fig. 5B). By contrast,
inhibition of GATA3 significantly increased relative luciferase
activity compared the siControl group (Fig. 5B). The results suggested that GATA3
may transcriptionally inhibit slug expression in HCC cells.
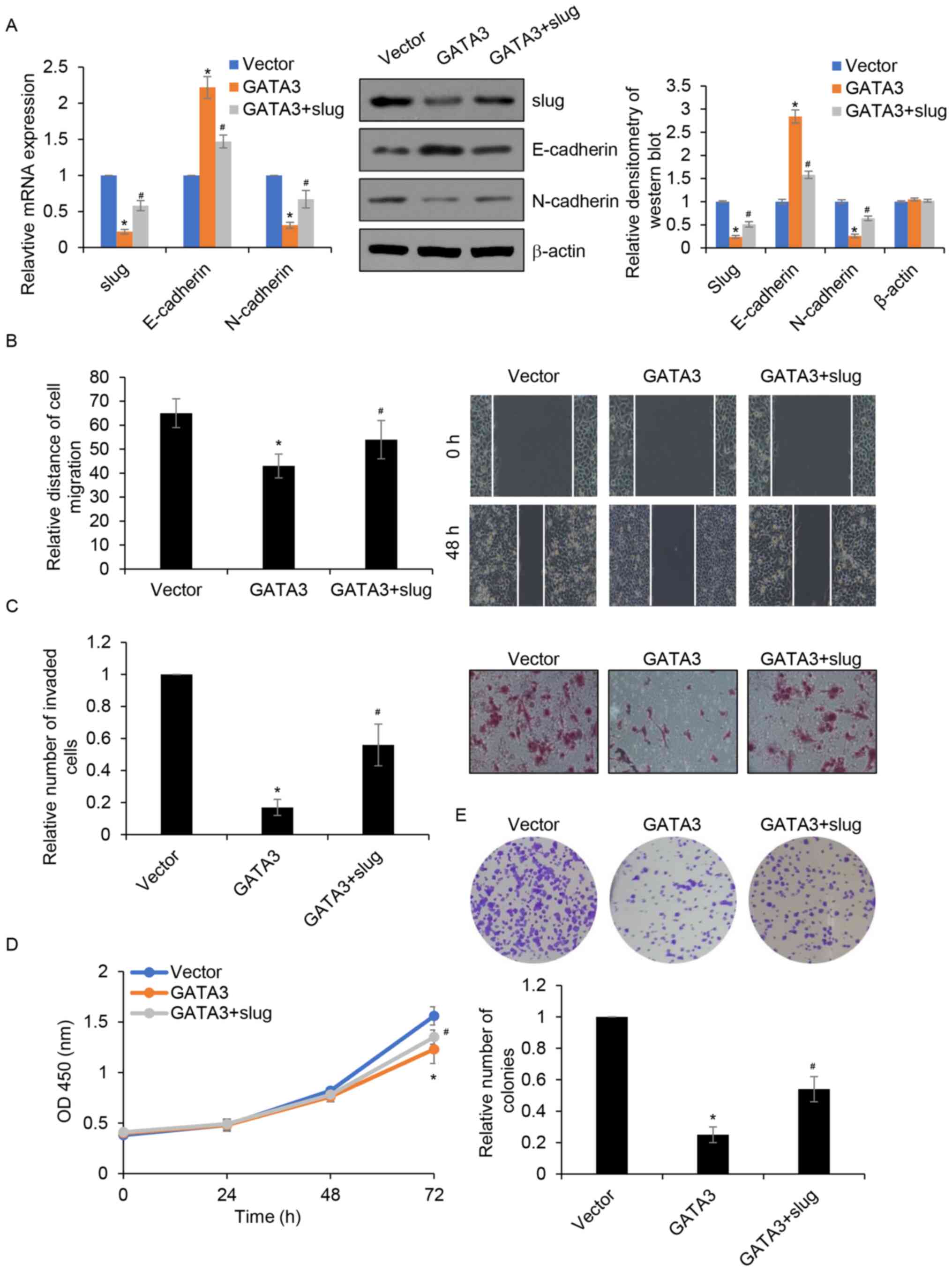
Overexpression of slug partially
attenuates GATA3 inhibited migration, invasion and
proliferation
Slug serves as a downstream target of GATA3; thus,
whether the roles of GATA3 in HCC occurred through the
transcriptional regulation of slug was investigated. GATA3 and slug
was simultaneously overexpressed in MHCC97-H cells and the
expression of slug was determined by RT-qPCR and western blotting.
The results demonstrated that compared with those the control
group, the mRNA and protein levels of slug were significantly
reduced in the GATA3 group and that this was partially restored in
the GATA3+ slug (Fig. 6A). To
further confirm whether GATA3 regulated cell migration and invasion
by transcriptionally inhibiting slug expression, wound healing and
Transwell invasion assays were performed. The results demonstrated
that overexpression of slug partially rescued the GATA3
overexpression-induced suppression of MHCC97-H cell migration and
invasion (Fig. 6B and C).
Furthermore, CCK-8 and colony formation assays demonstrated that
overexpression of slug in GATA3-overexpressed MHCC97-H cells
partially reversed the dampening effect of GATA3 overexpression on
cell proliferation (Fig. 6D and E).
Together, the results suggested that slug acts as a downstream
effector of GATA3 in the regulation of migration, invasion and
proliferation of HCC cells.
Discussion
The function of GATA3 varies in different cancers.
For example, previous studies have demonstrated that GATA3 is a
poor prognostic marker in soft tissue sarcomas, endometrial
carcinomas and neuroblastomas (14–16,24).
However, GATA3 has also been demonstrated to suppress cell
proliferation, migration and invasion in osteosarcoma (17). In breast cancer cells, GATA3
interacts with the G9A/NuRD (MTA3) complex to suppress tumour
growth factor (TGF) β1, ZEB2 and other EMT-related genes (25). By contrast, GATA3 facilitates the
cell cycle by activating the transcription of cyclin D1 in
luminal-type breast cancer cells (26). However, the detailed mechanisms
underlying the distinct roles of GATA3 in HCC remains unclear.
The present study suggested that GATA3 may act as a
tumour suppressor in HCC. The results of the present study
demonstrated that GATA3 expression is downregulated in HCC tissue
species and cell lines and that the expression of GATA3 is
associated with tumour size, lymph node metastasis, TNM stage and
prognosis. Additionally, the present study demonstrated that GATA3
overexpression inhibited cell proliferation, migration and invasion
in HCC. In addition, GATA3 overexpression suppressed EMT by
transcriptionally regulating slug expression.
Several gain of function and loss of function
analyses were performed to identify the functions of GATA3 in HCC.
Colony formation and CCK-8 assays revealed that overexpression of
GATA3 suppressed cell proliferation in HCC. In addition, wound
healing assay and Transwell invasion assays demonstrated that
overexpression of GATA3 suppressed cell migration and invasion in
HCC. EMT serves key roles in cancer metastasis (27). In corroboration with previous studies
(17,28), the present study demonstrated that
GATA3 suppressed EMT in HCC cells by increasing the expression of
E-cadherin and decreasing the expression of N-cadherin and
vimentin. In addition, qChIP and dual luciferase reporter assays
demonstrated that GATA3 transcriptionally regulated slug, thereby
inhibiting EMT in HCC.
A previous study revealed that GATA3 may serve as a
tumour suppressor in HCC (18).
However, Guan et al (29)
identified that hepatitis B virus-regulated GATA3 helps HCC cells
escape from natural killer cell surveillance by regulating major
histocompatibility complex class I polypeptide-related sequence B,
ligands of the NKG2D receptor. These contradictory findings imply
that the roles of GATA3 in HCC is complex and that the detailed
mechanism of GATA3 in HCC needs to be further investigated.
In summary, the current study suggested that GATA3
may act as a tumour suppressor in HCC. Mechanistically, GATA3
directly regulated slug expression to suppress the EMT process,
thereby inhibiting HCC cell migration and invasion. In addition,
GATA3 also reduced cell proliferation. Thus, the current study
provides a strong rationale for GATA3 as a therapeutic target in
HCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ and XF conceived and designed the study. ZZ, XF,
JZ and GX performed the experiments. XF, GX and JZ wrote the
manuscript. ZZ, XF, GX and JZ reviewed and edited the manuscript.
All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Shaoxing University
(approval no. LK1999006). All patients provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruix J, Gores GJ and Mazzaferro V:
Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut.
63:844–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torres HA, Vauthey JN, Economides MP,
Mahale P and Kaseb A: Hepatocellular carcinoma recurrence after
treatment with direct-acting antivirals: First, do no harm by
withdrawing treatment. J Hepatol. 65:862–864. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye
QH, Wang L, Zhou J, Qiu SJ, Li Y, et al: A decade's studies on
metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol.
130:187–196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chapman BC, Paniccia A, Hosokawa PW,
Henderson WG, Overbey DM, Messersmith W, McCarter MD, Gleisner A,
Edil BH, Schulick RD, et al: Impact of Facility Type and Surgical
Volume on 10-Year Survival in Patients Undergoing Hepatic Resection
for Hepatocellular Carcinoma. J Am Coll Surg. 224:362–372. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hirohashi S and Kanai Y: Cell adhesion
system and human cancer morphogenesis. Cancer Sci. 94:575–581.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sugimachi K, Taguchi K, Aishima S, Tanaka
S, Shimada M, Kajiyama K, Sugimachi K and Tsuneyoshi M: Altered
expression of beta-catenin without genetic mutation in intrahepatic
cholangiocarcinoma. Mod Pathol. 14:900–905. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rhim AD, Mirek ET, Aiello NM, Maitra A,
Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK,
Vonderheide RH, et al: EMT and dissemination precede pancreatic
tumor formation. Cell. 148:349–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ho IC and Pai SY: GATA-3 - not just for
Th2 cells anymore. Cell Mol Immunol. 4:15–29. 2007.PubMed/NCBI
|
|
12
|
Simon MC: Gotta have GATA. Nat Genet.
11:9–11. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hosoya T, Maillard I and Engel JD: From
the cradle to the grave: Activities of GATA-3 throughout T-cell
development and differentiation. Immunol Rev. 238:110–125. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haraguchi T, Miyoshi H, Hiraoka K,
Yokoyama S, Ishibashi Y, Hashiguchi T, Matsuda K, Hamada T, Okawa
T, Shiba N, et al: GATA3 Expression Is a Poor Prognostic Factor in
Soft Tissue Sarcomas. PLoS One. 11:e01565242016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Engelsen IB, Stefansson IM, Akslen LA and
Salvesen HB: GATA3 expression in estrogen receptor alpha-negative
endometrial carcinomas identifies aggressive tumours with high
proliferation and poor patient survival. Am J Obstet Gynecol.
199:543.e1–7. 2008. View Article : Google Scholar
|
|
16
|
Peng H, Ke XX, Hu R, Yang L, Cui H and Wei
Y: Essential role of GATA3 in regulation of differentiation and
cell proliferation in SK-N-SH neuroblastoma cells. Mol Med Rep.
11:881–886. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma L, Xue W and Ma X: GATA3 is
downregulated in osteosarcoma and facilitates EMT as well as
migration through regulation of slug. OncoTargets Ther.
11:7579–7589. 2018. View Article : Google Scholar
|
|
18
|
Yin G, Liu Z, Wang Y, Sun L, Wang L, Yao
B, Liu R, Chen T, Niu Y and Liu Q: ZNF503 accelerates
aggressiveness of hepatocellular carcinoma cells by down-regulation
of GATA3 expression and regulated by microRNA-495. Am J Transl Res.
11:3426–3437. 2019.PubMed/NCBI
|
|
19
|
Zheng Y, Wang DD, Wang W, Pan K, Huang CY,
Li YF, Wang QJ, Yuan SQ, Jiang SS, Qiu HB, et al: Reduced
expression of uroplakin 1A is associated with the poor prognosis of
gastric adenocarcinoma patients. PLoS One. 9:e930732014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cai X, Zhu H and Li Y: PKCζ, MMP-2 and
MMP-9 expression in lung adenocarcinoma and association with a
metastatic phenotype. Mol Med Rep. 16:8301–8306. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao Y, Zhou FL, Li WP, Wang J and Wang
LJ: Slit2 Robo1 signaling promotes the adhesion, invasion and
migration of tongue carcinoma cells via upregulating matrix
metalloproteinases 2 and 9, and downregulating E cadherin. Mol Med
Rep. 14:1901–1906. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liao TT and Yang MH: Revisiting
epithelial-mesenchymal transition in cancer metastasis: The
connection between epithelial plasticity and stemness. Mol Oncol.
11:792–804. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mehra R, Varambally S, Ding L, Shen R,
Sabel MS, Ghosh D, Chinnaiyan AM and Kleer CG: Identification of
GATA3 as a breast cancer prognostic marker by global gene
expression meta-analysis. Cancer Res. 65:11259–11264. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Si W, Huang W, Zheng Y, Yang Y, Liu X,
Shan L, Zhou X, Wang Y, Su D, Gao J, et al: Dysfunction of the
reciprocal feedback loop between GATA3- and ZEB2-nucleated
repression programs contributes to breast cancer metastasis. Cancer
Cell. 27:822–836. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shan L, Li X, Liu L, Ding X, Wang Q, Zheng
Y, Duan Y, Xuan C, Wang Y, Yang F, et al: GATA3 cooperates with
PARP1 to regulate CCND1 transcription through modulating histone H1
incorporation. Oncogene. 33:3205–3216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takai M, Terai Y, Kawaguchi H, Ashihara K,
Fujiwara S, Tanaka T, Tsunetoh S, Tanaka Y, Sasaki H, Kanemura M,
et al: The EMT (epithelial-mesenchymal-transition)-related protein
expression indicates the metastatic status and prognosis in
patients with ovarian cancer. J Ovarian Res. 7:762014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei S, Zhong L, Wang X and Zhang W: Low
expression of GATA3 promotes cell proliferation and metastasis in
gastric cancer. Cancer Manag Res. 9:769–780. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guan Y, Li W, Hou Z, Han Q, Lan P, Zhang
J, Tian Z and Zhang C: HBV suppresses expression of MICA/B on
hepatoma cells through up-regulation of transcription factors GATA2
and GATA3 to escape from NK cell surveillance. Oncotarget.
7:56107–56119. 2016. View Article : Google Scholar : PubMed/NCBI
|