Introduction
Women in whom high-grade cervical intraepithelial
neoplasia (CIN) has been identified and treated require long-term
follow-up compared to the general population (1), because of their increased risk for
disease recurrence (2). However,
evidence-based guidelines to optimize post-therapeutic screening
are still needed (3).
Because of the well-established role of high-risk
human papilloma virus (HPV) in the etiology of cervical cancer, HPV
testing is now accepted to be used to assess recurrence risk after
high-grade CIN treatment. Indispensible insights are gleaned
thereby (2,4,5).
Besides clinician-collected samples, women
themselves can collect vaginal and/or urine samples for HPV
testing. With concerted efforts to maximize self-sampling
reliability (6), using validated
polymerase chain reaction (PCR)-based assays (7), accuracy is reportedly similar from
self-collected compared to clinician-collected samples (8). With these developments, HPV testing
from self-collected samples is becoming a viable, cost-effective
cervical-screening option (9).
In Ref. (10) we
recently examined the views on self-sampling for HPV among 479
women treated for high-grade CIN. The vast majority of these women
considered self-sampling to be easily implementable, and could
envision themselves performing self-sampling at home before their
next gynecologic examination. We concluded that insofar as HPV
self-sampling was as diagnostically accurate as clinician-collected
samples for this high-risk cohort, the former could become an
integral part of the post-therapeutic screening armamentarium.
This possibility becomes especially timely given the
present COVID-19 pandemic. In other words, these women at elevated
risk for cervical cancer could eventually perform at least a part
of the needed screening outside the clinic setting. In a broader
framework, given that long-term follow-up is essential for this
cohort, whether or not HPV self-sampling is a viable option for
these women becomes a critical issue to be examined.
The present study addresses this question, examining
how well positive HPV findings from self-sampled vaginal and urine
specimens, compared to clinician-collected cervical samples,
correctly identify women from this cohort with recurrent high-grade
CIN. We also compare how well HPV findings from self-collected
vaginal and urine samples versus clinician-collected cervical
samples identify women from this cohort with abnormal versus normal
cytology at follow-up. The latter outcome variable currently
impacts directly upon decision-making: Namely, whether the patient
will be triaged for further intensive follow-up or whether she will
be returned to the routine screening program.
Materials and methods
Study design, population and
setting
This study includes patients first-time treated by
conization for histologically-confirmed CIN2+ or
adenocarcinoma-in-situ (AIS) at Stockholm Hospitals: Karolinska
University, Danderyd or South General, from 10/2014-1/2017. The
Research Coordinator, Ellinor Östensson, contacted these patients
shortly after treatment, to arrange 1st follow-up at Karolinska
University Hospital. This 1st follow-up visit was targeted to be at
approximately six months post-treatment. With determined efforts to
schedule a convenient time, all 532 patients attended follow-up
#1.
Upon arrival for follow-up #1, each woman met with
the Research Coordinator (EÖ), who explained the study procedures:
Self-collection of samples for HPV testing; questionnaire [results,
including detailed demographic analysis (10,11)];
gynecologic examination with colposcopy and cervical sampling as
clinical follow-up. The stated study aim was cervical cancer
prevention. Assurance was given of confidentiality and freedom to
withdraw any time without adverse consequences. Informed consent
was signed with the options: Agreement or decline to participate.
All but one patient agreed. Karolinska Ethics Committee approved
the study protocol (2006/1273-31, 2014/2034-3). Thus, the total
number of patients in the present study is 531.
Self-collected samples at follow-up
#1
The participants gave urine samples and carried-out
vaginal self-sampling (VSS) in the care-site restroom. Verbal
instructions were given for collecting initial urine stream in a
plain cup and for VSS with a kit (Qvintip-Aprovix-AB), plus written
description for kit use. The patients were instructed to collect
urine before VSS. Both samples were given to EÖ for handling.
Aprovix AB, Uppsala, Sweden provided Qvintip devices for
self-collection of vaginal material. Abbott provided sample kits
for HPV analyses performed at Fürst Medical Laboratory, Oslo,
Norway. Aprovix and (Abbott had no influence on study design,
statistical analyses, or article writing).
Colposcopy, clinician-collected
cervical samples at follow-up #1
The patients met the gynecologist (Dr Andersson or
Dr Mints), who performed colposcopy and cervical sampling.
Colposcopy-directed punch biopsies were taken from visible lesions,
when present. Histologic grading of biopsies was performed at
Karolinska University Hospital, following standard procedures,
according to CIN classification (12). Samples were taken from the ectocervix
using plastic spatulas and from the endocervix with cervical
brushes, and transferred into PreservCyt liquid-based cytology
(LBC) vials according to European guidelines (13).
Routine follow-up tests, further
patient management
The LBC was performed at the Cytology Department,
Karolinska University Hospital, according to the Bethesda system
(14). The HPV DNA testing was
completed on-site with the hospital's standard: Cobas 4800 HPV
(Roche Diagnostics). Cobas HPV and LBC results from follow-up #1
informed subsequent management: Women with positive Cobas HPV
and/or cytological abnormalities were referred for follow-up #2,
which entailed the same standard protocol as follow-up #1,
according to national guidelines and was most often scheduled at
about one year after follow-up #1. Women with negative HPV Cobas
findings and cytology negative for intraepithelial lesions or
malignancy (NILM) returned to routine triennial screening, as per
national guidelines. When a recurrent lesion was found, the patient
was sent for follow-up treatment. Depending on the clinical
evaluation and other considerations, treatment entailed re-excision
or simple total hysterectomy.
Handling of triplet samples for
comparative HPV testing
Within 1 h of collection, urine samples were
vortexed for 15–20 sec prior to transferring a 2.5 ml aliquot to a
Cervi-Collect transport tube (Abbott-Molecular), containing
transport medium. The tubes were labeled with a unique identifier,
mixed with transport medium by vortexing for 15–20 sec prior to
storage at −10°C or colder up to 1 month before shipment. Urine
samples packed in plastic bags were put in polystyrene boxes with
dry ice for cold-chain maintenance (−78.5°C) during air-transport.
The VSS were air dried for ~3–5 min before the Qvintip device
brush-heads were placed into barcoded capped tubes. The VSS were
stored at room temperature for maximum 1 month before shipment. For
clinician-collected samples, LBC vials were vortexed for 15–20 sec
followed by immediate transfer of a 2 ml aliquot into a test tube
labeled with a unique identifier. The aliquots were stored at room
temperature for maximum 1 month before shipment. All matched
triplet samples (urine, VSS, clinician-collected) were
air-transported from Karolinska University Hospital to the testing
laboratory: Fürst Medical Laboratory, Oslo.
Comparative HPV testing
At Fürst Laboratory, the triplet samples were
analyzed for the presence of HPV-DNA with the RealTime High-Risk
HPV PCR assay (hereafter termed ‘Abbott’), as per manufacturer
instructions. These results were for comparative purposes only and
not considered for patient management.
Abbott is a clinically-validated, qualitative,
multiplex real-time PCR test which detects HPV16, HPV18, plus 12
other high-risk HPV (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66,
68) reported as a pooled signal. The assay detects a sequence of
endogenous human β-globin as sample validity control for
cell adequacy, sample extraction, and amplification efficiency in
each reaction. Signal strength for HPV types and for
β-globin gene was expressed as cycle numbers (CN): The
number of PCR cycles in which a positive signal is observed. High
viral load corresponds to low CN values and vice-verse; 32 was the
cut-off between positive results and noise (negative signal). CN
were reported by the assay software and recorded by the testing
lab. Tests with negative signal for HPV and β-globin were
excluded.
Statistical analysis
Univariate data analysis was performed, with
attention to HPV findings: Any HPV, HPV16, HPV18 or other HPV with
side-by-side comparisons of the methods by which these were
assessed. Pearson χ2 tests (or Fisher's if any expected
cell was <5) were used to assess the relation between biopsy or
cytology vis-à-vis HPV results from each of the four methods.
Biopsy results were dichotomized: (CIN2+ or AIS) vs. (normal
findings or CIN1). Cytology results were dichotomized as abnormal
versus NILM, negative for intraepithelial lesions or malignancy.
Biopsy and cytology results at each follow-up were assessed in
relation to the HPV test results taken at follow-up #1.
Additionally, biopsy and cytology results at follow-up #2 were
analyzed vis-à-vis HPV results from clinician-taken samples at
follow-up #2. Fisher's and Pearson χ2 tests were
employed, respectively, to evaluate the relation between biopsy and
cytology results at follow-up versus HPV16 and/or HPV18 positivity,
as assessed from Abbott clinician-collected samples, VSS and urine
samples. Sensitivity and specificity were computed with 95%
confidence intervals (CI). Negative predictive values (NPV) and
positive predictive values (PPV) were also computed. Using logistic
regression, odds ratios (OR) and 95% CI were computed for
dichotomized clinical outcomes. Each method for assessing HPV
results was the independent variable, with age as a covariate.
Concordance between methods for HPV sampling was assessed using
Cohen's kappa statistic with 95% CI.
Results
Univariate data and protocol by which
the patients were triaged
Altogether, 531 were patients included in the study.
Tables I–III summarize the univariate data. At the
time of treatment, the mean age was 34 years, with most patients
between age 21–50; four were 20 or younger and twenty-seven were
over age 50. The majority of the patients had completed university
education and over 70% were gainfully employed. More detailed
demographic information about the patients can be found in Ref.
(11).
 | Table I.Univariate findings for
semi-continuous data. |
Table I.
Univariate findings for
semi-continuous data.
| Variable | No. | Mean | Minimum | Maximum | SD |
|---|
| Age at time of
treatment, years | 531 | 34 | 16 | 66 | 9 |
| Days from treatment
to 1st follow-up | 531 | 184 | 37 | 447 | 40 |
| Days from 1st to
2nd follow-up | 113 | 389 | 9 | 1,291 | 229 |
 | Table III.HPV results. |
Table III.
HPV results.
| A, Follow-up 1 |
|---|
|
|---|
| HPV results | Clinician sampled:
Cobas-4800, n | Clinician sampled:
Abbott, n | Self-sampled:
Vaginal, n | Self-sampled:
Urine, na |
|---|
| Positive for any
high-risk type | 86 | 100 | 139 | 85 |
| Negative for any
high-risk type | 445 | 431 | 392 | 402 |
| HPV16 positive |
| 18 | 24 | 16 |
| HPV16 negative |
| 502 | 494 | 462 |
| CN ≥32 |
| 11 | 13 | 9 |
| HPV18 positive |
| 9 | 7 | 5 |
| HPV18 negative |
| 517 | 517 | 476 |
| CN ≥32 |
| 5 | 7 | 6 |
| HPV Other
positive |
| 77 | 117 | 71 |
| HPV Other
negative |
| 423 | 355 | 358 |
| CN ≥32 |
| 31 | 59 | 58 |
|
| B, Follow-up
2b |
|
| HPV
results | Clinician
sampled: Cobas-4800, n | Clinician
sampled: Abbott, n | Self-sampled:
Vaginal, n | Self-sampled:
Urine, na |
|
| Positive for any
high-risk type | 47 |
|
|
|
| Negative for any
high-risk type | 52 |
|
|
|
The histology in the excised cone was CIN2 in 133
patients (25%), CIN3 in 370 patients (69.7%), CIN3/AIS in fifteen
patients (2.8%) and AIS in thirteen patients (2.5%). Most patients
came to follow-up #1 within eight months.
Table IIA shows that
at follow-up #1, recurrent CIN2+ (CIN3 in all cases) was found in
four of thirteen patients who underwent biopsy (30.8%). Thus, at
1st follow-up the diagnosed recurrence rate among the 531 patients
was 0.8%. At follow-up #2, biopsy was performed in twenty patients,
including the four who had recurrent CIN2+ at follow-up
#1. Nine more patients were found to have recurrence on biopsy:
Seven with CIN2+ and two with AIS among those sixteen
patients (56.3%) who underwent biopsy at follow-up #2, excluding
the four patients with CIN3 who underwent repeat biopsy at
follow-up #2. The newly diagnosed recurrence rate among the
remaining 109 patients who came to 2nd follow-up was thus 8.3%.
 | Table II.Univariate findings for biopsy and
cytology results. |
Table II.
Univariate findings for biopsy and
cytology results.
| A, Biopsy
results |
|---|
|
|---|
| Variable | Follow-up 1, n | Follow-up 1, % | Follow-up 2, n | Follow-up 2, % |
|---|
| Within normal
limits | 6 | 46 | 2 | 13 |
| CIN1 | 3 | 23 | 5 | 31 |
|
CIN2+ | 4 | 31 | 7a | 44 |
| AIS | 0 | 0 | 2 | 13 |
|
| B, Cytology
results (via LBC) |
|
|
Variable | Follow-up 1,
n | Follow-up 1,
% | Follow-up 2,
nb | Follow-up 2,
% |
|
| NILM | 454 | 86 | 75 | 68 |
| ASC-US | 28 | 5 | 6 | 6 |
| AGC | 5 | 1 | 2 | 2 |
| LSIL | 27 | 5 | 14 | 13 |
| ASC-H | 1 | 0 | 0 | 0 |
| HSIL | 16 | 3 | 12 | 11 |
| AIS | 0 | 0 | 1 | 1 |
On Table IIB, for
follow-up #1, seventy-seven patients had abnormal cytology,
eighty-six patients had HPV positive findings according to the
standard clinician-taken COBAS analysis and thirty-seven patients
had both positive HPV via COBAS and abnormal cytology. Thus,
altogether, 126 patients were referred to 2nd follow-up, 113 of
whom attended. Most patients had NILM on cytology at follow-up #2.
One patient had AIS and ~11% had high-grade squamous
intraepithelial lesions (HSIL).
Table III further
reveals that testing for any high-risk HPV at follow-up #1 yielded
all valid results for clinician-collected samples and VSS; 44 urine
samples were ‘invalid’ due to absent HPV and β-globin. For
HPV16 or HPV18, there were more omitted results due to CN ≥32 for
VSS than for clinician-collected samples. For HPV16 VSS showed the
highest positivity rate (4.6%), whereas for HPV18,
clinician-samples had the highest positivity rate (1.7%). Overall,
VSS yielded the largest number of positive results for HPV16 and
for other high-risk HPV.
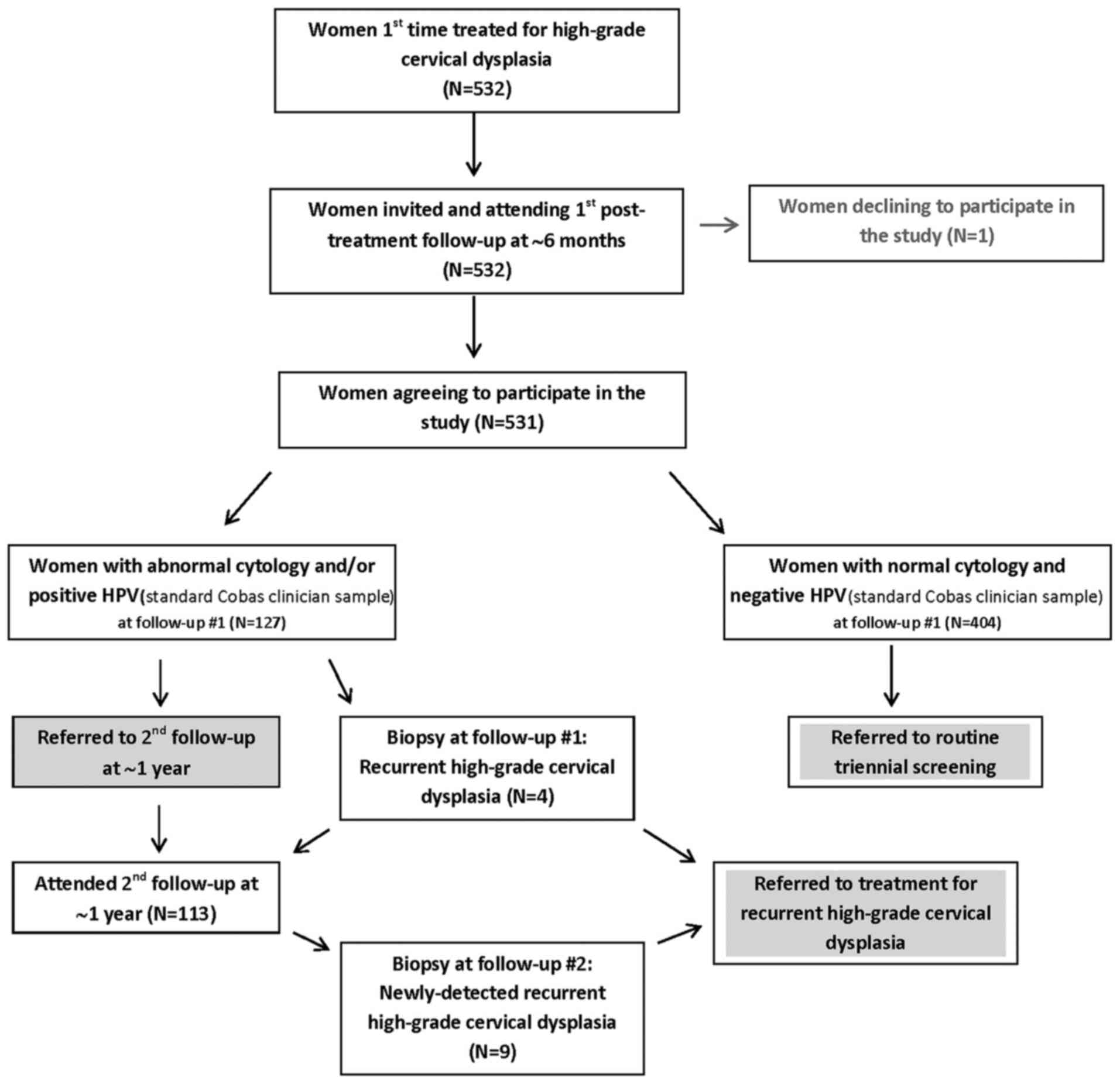
Fig. 1 summarizes the
protocol according to which the patients were triaged. Numerical
information is provided therein concerning the various
outcomes.
HPV findings in relation to the biopsy
results
Table IV presents
the predictive value of HPV findings vis-à-vis biopsy results. All
4 methods revealed positive HPV findings in the four patients with
recurrent CIN2+ at follow-up #1. Two patients showed
positive HPV16 and/or HPV18 results with clinician-sampling and
VSS. For all the patients who underwent biopsy, the HPV16 and 18
results were complete for clinician-sampling and VSS. However, for
the urine self-samples, at follow-up #1 the results for HPV16
and/or HPV18 were missing due to CN >32 for one patient with
recurrent CIN2+ and at follow-up #2 for two patients:
One with recurrent CIN2+ and one with AIS.
 | Table IV.Predictive value of HPV findings
vis-à-vis biopsy results. |
Table IV.
Predictive value of HPV findings
vis-à-vis biopsy results.
| A, HPV vs. biopsy
results at follow-up #1 |
|---|
|
|---|
| HPV results | Normal, n | CIN1, n | P-value | CIN2+, n | AIS, n | Sensitivity, % (95%
CI) | Specificity, % (95%
CI) | NPV, % | PPV, % |
|---|
| Clinician-Sampled:
Cobas-4800 |
|
|
|
|
| 100 (40–100) | 67 (30–93) | 100 | 57 |
|
Positive for any high-risk
type | 1 | 2 |
| 4 | 0 |
|
|
|
|
|
Negative for any high-risk
type | 5 | 1 | ≤0.09 | 0 | 0 |
|
|
|
|
| Clinician-Sampled:
Abbott |
|
|
|
|
| 100 (40–100) | 67 (30–93) | 100 | 57 |
|
Positive for any high-risk
type | 1 | 2 |
| 4 | 0 |
|
|
|
|
|
Negative for any high-risk
type | 5 | 1 | ≤0.09 | 0 | 0 |
|
|
|
|
| HPV16 and/or
18 |
|
|
|
|
| 50 (7–93) | 67 (30–93) | 75 | 40 |
| HPV16
and/or 18 positive | 1 | 2 |
| 2 | 0 |
|
|
|
|
| HPV16
and HPV18 negative | 5 | 1 | NS | 2 | 0 |
|
|
|
|
| Self-sampled:
Vaginal |
|
|
|
|
| 100 (40–100) | 67 (30–93) | 100 | 57 |
|
Positive for any high-risk
type | 2 | 1 | ≤0.09 | 4 | 0 |
|
|
|
|
|
Negative for any high-risk
type | 4 | 2 |
| 0 | 0 |
|
|
|
|
| HPV16 and/or
18 |
|
|
|
|
| 50 (7–93) | 78 (40–97) | 78 | 50 |
| HPV16
and/or 18 positive | 1 | 1 |
| 2 | 0 |
|
|
|
|
| HPV16
and HPV18 negative | 5 | 2 | NS | 2 | 0 |
|
|
|
|
| Self-Sampled:
Urine |
|
|
|
|
| 100 (40–100) | 67 (30–93) | 100 | 57 |
|
Positive for any high-risk
type | 2 | 1 |
| 4 | 0 |
|
|
|
|
|
Negative for any high-risk
type | 4 | 2 | ≤0.09 | 0 | 0 |
|
|
|
|
| HPV16 and/or
18a |
|
|
|
|
| 33 (1–91) | 78 (40–97) | 78 | 33 |
| HPV16
and/or 18 positive | 1 | 1 |
| 1 | 0 |
|
|
|
|
| HPV16
and HPV18 negative | 5 | 2 | NS | 2 | 0 |
|
|
|
|
|
| B, HPV results
at follow-up #1 vs. biopsy results at follow-up #2b |
|
| HPV
results | Normal,
n | CIN1, n | P-value | CIN2+,
n | AIS, n | Sensitivity, %
(95% CI) | Specificity, %
(95% CI) | NPV, % | PPV, % |
|
| Clinician-Sampled:
Cobas-4800 |
|
|
|
|
| 89 (52–100) | 43 (10–82) | 75 | 67 |
|
Positive for any high-risk
type | 0 | 4 |
| 7 | 1 |
|
|
|
|
|
Negative for any high-risk
type | 2 | 1 | NS | 0 | 1 |
|
|
|
|
| Clinician-Sampled:
Abbott |
|
|
|
|
| 100 (66–100) | 43 (10–82) | 100 | 69 |
|
Positive for any high-risk
type | 0 | 4 | ≤0.09 | 7 | 2 |
|
|
|
|
|
Negative for any high-risk
type | 2 | 1 |
| 0 | 0 |
|
|
|
|
| HPV16 and/or
18 |
|
|
|
|
| 56 (21–86) | 100 (59–100) | 64 | 100 |
| HPV16
and/or 18 positive | 0 | 0 | <0.05 | 3 | 2 |
|
|
|
|
| HPV 16
and 18 negative | 2 | 5 |
| 4 | 0 |
|
|
|
|
| Self-Sampled:
Vaginal |
|
|
|
|
| 78 (40–97) | 43 (10–82) | 60 | 64 |
|
Positive for any high-risk
type | 0 | 4 | NS | 7 | 0 |
|
|
|
|
|
Negative for any high-risk
type | 2 | 1 |
| 0 | 2 |
|
|
|
|
| HPV16 and/or
18 |
|
|
|
|
| 44 (14–79) | 100 (59–100) | 58 | 100 |
| HPV16
and/or 18 positive | 0 | 0 |
| 4 | 0 |
|
|
|
|
| HPV 16
and 18 negative | 2 | 5 | ≤0.09 | 3 | 2 |
|
|
|
|
| Self-Sampled:
Urine |
|
|
|
|
| 56 (21–86) | 71 (29–96) | 56 | 71 |
|
Positive for any high-risk
type | 0 | 2 |
| 5 | 0 |
|
|
|
|
|
Negative for any high-risk
type | 2 | 3 | NS | 2 | 2 |
|
|
|
|
| HPV16 and/or
18c |
|
|
|
|
| 43 (10–82) | 100 (59–100) | 64 | 100 |
| HPV16
and/or 18 positive | 0 | 0 |
| 3 | 0 |
|
|
|
|
| HPV 16
and 18 negative | 2 | 5 | NS | 3 | 1 |
|
|
|
|
|
| C, HPV results
at follow-up #2 vs. biopsy results at follow-up #2 |
|
| HPV
results | Normal,
n | CIN1, n | P-value | CIN2+,
n | AIS, n | Sensitivity, %
(95% CI) | Specificity, %
(95% CI) | NPV, % | PPV, % |
|
| Clinician-Sampled:
Cobas-4800d |
|
|
|
|
| 100 (63–100) | 33 (4–78) | 100 | 67 |
|
Positive for any high-risk
type | 0 | 4 |
| 6 | 2 |
|
|
|
|
|
Negative for any high-risk
type | 1 | 1 | NS | 0 | 0 |
|
|
|
|
Both clinician-sampling methods and VSS, all taken
at follow-up #1, revealed HPV positive findings in the seven
patients with newly-detected CIN2+ at follow-up #2. From
urine self-samples, HPV positivity was seen in five of those
patients. Only Abbott clinician-taken samples were HPV positive for
both patients with AIS at follow-up #2; in both cases HPV18 was
also positive.
As noted at the end of Table IV, eight of the nine patients with
high-grade cervical dysplasia on biopsy at follow-up #2 showed HPV
positivity via the standard assessment with Cobas clinician
sampling taken at follow-up #2. The HPV data were missing for the
ninth patient with recurrent high-grade CIN detected on biopsy at
follow-up #2. With clinician sampling using the Abbott assay, all
nine cases with high-grade cervical dysplasia on biopsy showed HPV
positivity at follow-up #1. Thus, it appears that these
biopsy-diagnosed recurrent cases at follow-up #2 had persistent HPV
positivity.
HPV findings in relation to the
cytology results
Table V shows the HPV
findings in relation to abnormal cytology versus NILM. At follow-up
#1, overall HPV positivity from VSS was most sensitive in
predicting abnormal cytology. Further analysis revealed positive
HPV findings from VSS in twelve patients with HSIL, whereas eleven
patients with HSIL had positive HPV findings on clinician-taken and
urine samples. Positivity for HPV16 and/or HPV18 showed low
sensitivity for predicting abnormal cytology at follow-up#1, but
very high NPV and specificity with all three methods. At follow-up
#2, twenty-eight patients with abnormal cytology had HPV positive
findings with VSS and both clinician-samples from follow-up #1.
With missing data for urine samples, there were fewer cases of
positive HPV associated with abnormal cytology at both
follow-ups.
 | Table V.Predictive value of HPV findings
vis-à-vis cytology results. |
Table V.
Predictive value of HPV findings
vis-à-vis cytology results.
| A, HPV vs. cytology
results (both at follow-up #1) |
|---|
|
|---|
| HPV results | NILM, n | P-value | Abnormal, n | Sensitivity, % (95%
CI) | Specificity, % (95%
CI) | NPV, % | PPV, % |
|---|
|
Clinician-Sampled:Cobas-4800 |
|
|
| 48 (37–60) | 89 (86–92) | 91 | 43 |
|
Positive for any high-risk
type | 49 | <0.001 | 37 |
|
|
|
|
|
Negative for any high-risk
type | 405 |
| 40 |
|
|
|
|
| Clinician-Sampled:
Abbott |
|
|
| 49 (38–61) | 86 (83–89) | 91 | 38 |
|
Positive for any high-risk
type | 62 | <0.001 | 38 |
|
|
|
|
|
Negative for any high-risk
type | 392 |
| 39 |
|
|
|
|
| HPV 16 and/or
18a |
|
|
| 14 (7–24) | 96 (94–98) | 87 | 41 |
| HPV16
and/or 18 positive | 16 | <0.001 | 11 |
|
|
|
|
| HPV16
and 18 negative | 423 |
| 66 |
|
|
|
|
| Self-Sampled:
Vaginal |
|
|
| 51 (39–62) | 78 (74–82) | 90 | 28 |
|
Positive for any high-risk
type | 100 |
| 39 |
|
|
|
|
|
Negative for any high-risk
type | 354 | <0.001 | 38 |
|
|
|
|
| HPV 16 and/or
18b |
|
|
| 12 (6–21) | 95 (92–97) | 86 | 41 |
| HPV16
and/or 18 positive | 22 | <0.05 | 9 |
|
|
|
|
| HPV16
and 18 negative | 413 |
| 67 |
|
|
|
|
| Self-Sampled:
Urinec |
|
|
| 43 (31–55) | 87 (83–90) | 90 | 37 |
|
Positive for any high-risk
type | 54 | <0.001 | 31 |
|
|
|
|
|
Negative for any high-risk
type | 360 |
| 42 |
|
|
|
|
| HPV 16 and/or
18d |
|
|
| 13 (6–23) | 97 (95–99) | 87 | 43 |
| HPV16
and/or 18 positive | 12 | <0.001 | 9 |
|
|
|
|
| HPV16
and 18 negative | 392 |
| 60 |
|
|
|
|
|
| B, HPV at
follow-up #1 vs. cytology results at follow-up #2 |
|
| HPV
results | NILM, n | P-value | Abnormal,
n | Sensitivity, %
(95% CI) | Specificity, %
(95% CI) | NPV, % | PPV, % |
|
| Clinician-Sampled:
Cobas-4800 |
|
|
| 80 (63–92) | 36 (25–48) | 79 | 37 |
|
Positive for any high-risk
type | 48 | NS | 28 |
|
|
|
|
|
Negative for any high-risk
type | 27 |
| 7 |
|
|
|
|
| Clinician-Sampled:
Abbott |
|
|
| 80 (63–92) | 40 (29–52) | 81 | 38 |
|
Positive for any high-risk
type | 45 | <0.05 | 28 |
|
|
|
|
|
Negative for any high-risk
type | 30 |
| 7 |
|
|
|
|
| HPV 16 and/or
18e |
|
|
| 23 (10–40) | 85 (75–92) | 70 | 42 |
| HPV16
and/or 18 positive | 11 | NS | 8 |
|
|
|
|
| HPV16
and 18 negative | 63 |
| 27 |
|
|
|
|
| Self-Sampled:
Vaginal |
|
|
| 80 (63–92) | 36 (25–48) | 79 | 37 |
|
Positive for any high-risk
type | 48 | NS | 28 |
|
|
|
|
|
Negative for any high-risk
type | 27 |
| 7 |
|
|
|
|
| HPV 16 and/or
18e |
|
|
| 20 (8–37) | 85 (75–92) | 69 | 39 |
| HPV16
and/or 18 positive | 11 | NS | 7 |
|
|
|
|
| HPV16
and 18 negative | 63 |
| 28 |
|
|
|
|
| Self-Sampled:
Urinef |
|
|
| 68 (50–83) | 58 (45–69) | 79 | 43 |
|
Positive for any high-risk
type | 30 | <0.05 | 23 |
|
|
|
|
|
Negative for any high-risk
type | 41 |
| 11 |
|
|
|
|
| HPV 16 and/or
18g |
|
|
| 16 (6–34) | 88 (78–95) | 69 | 39 |
| HPV16
and/or 18 positive | 8 | NS | 5 |
|
|
|
|
| HPV16
and 18 negative | 59 |
| 26 |
|
|
|
|
|
| C, HPV results
at follow-up #2 vs. cytology results at follow-up #2 |
|
| HPV
results | NILM, n | P-value | Abnormal,
n | Sensitivity, %
(95% CI) | Specificity, %
(95% CI) | NPV, % | PPV, % |
|
|
Clinician-sampled:Cobas-4800h |
|
|
| 84 (66–95) | 68 (55–79) | 90 | 57 |
|
Positive for any high-risk
type | 20 | <0.001 | 26 |
|
|
|
|
|
Negative for any high-risk
type | 43 |
| 5 |
|
|
|
|
Table VI presents
the significant logistic regression models predicting abnormal
cytology at follow-up #1. All four methods yielded significant
age-adjusted ORs. None of the methods generated significant
age-adjusted models for predicting cytology at follow-up #2. The
small number of biopsies precluded multivariate analysis.
 | Table VI.Clinician-sampled and self-sampled
HPV for predicting abnormal cytology at follow-up#1 assessed via
multiple logistic regression models with adjustment for age. |
Table VI.
Clinician-sampled and self-sampled
HPV for predicting abnormal cytology at follow-up#1 assessed via
multiple logistic regression models with adjustment for age.
| Model
χ2 | Variable | OR | −95% CI | +95% CI |
|---|
| 54.6a (n=531) | Cobas
Clinician-sample HPV positive | 7.62a | 4.44 | 13.10 |
|
| Age | 0.98 | 0.96 | 1.00 |
| 46.6a (n=531) | Abbott
Clinician-sample HPV positive | 6.13a | 3.63 | 10.40 |
|
| Age | 0.98 | 0.96 | 1.00 |
| 27.7a (n=531) | Vaginal Self-sample
HPV positive | 3.71a | 2.24 | 6.13 |
|
| Age | 0.98 | 0.95 | 1.00 |
| 32.0a (n=487)b | Urine Self-sample
HPV positive | 4.84a | 2.80 | 8.39 |
|
| Age | 0.99 | 0.96 | 1.00 |
Concordance between the methods for
assessing HPV
The highest Cohen's kappa was for the two
clinician-sampling methods (Table
VII). Agreement was substantial between VSS and
clinician-sampling methods, and moderate for urine sampling versus
clinician-sampling or VSS. For valid HPV16 there was close
agreement for each pair. Agreement was substantial between
clinician and VSS for HPV18. Concordance between clinician and
urine sampling was fair for HPV18; VSS versus urine sampling
agreement was moderate. Concordance was substantial for the three
methods assessing other HPV.
 | Table VII.Pairwise concordance between methods
for HPV assessment using Cohen's kappa. |
Table VII.
Pairwise concordance between methods
for HPV assessment using Cohen's kappa.
| Variable | Abbott
clinician | Vaginal
self-sample | Urine
self-sample |
|---|
| Overall HPV |
| Cobas
clinician | 0.83
(0.77–0.89) | 0.63
(0.55–0.71) | 0.53
(0.42–0.64)a |
| Abbott
clinician |
| 0.68
(0.60–0.76) | 0.58
(0.48–0.68)a |
| Vaginal
self-sample |
|
| 0.60
(0.51–0.69)a |
| HPV16 |
| Abbott
clinician |
| 0.89
(0.77–1.00)b | 0.85
(0.70–1.00)c |
| Vaginal
self-sample |
|
| 0.83
(0.69–0.97)c |
| HPV18 |
| Abbott
clinician |
| 0.71
(0.43–0.99)d | 0.36
(0.00–0.81)e |
| Vaginal
self-sample |
|
| 0.60
(0.15–1.00)f |
| Other HPV |
| Abbott
clinician |
| 0.79
(0.71–0.87)g | 0.73
(0.63–0.83)h |
| Vaginal
self-sample |
|
| 0.78
(0.70–0.86)i |
Discussion
The present results indicate that VSS is as
sensitive as clinician-collected samples for predicting recurrent
high-grade pathohistologic results on biopsy and cytologic
abnormalities among women treated for high-grade CIN, unless the
pathology is glandular. Urine self-sampling yielded slightly poorer
sensitivity compared to VSS.
Our results for VSS cohere with the literature
concerning the value of post-therapeutic HPV testing from
clinician-collected samples for predicting subsequent outcome among
patients treated for high-grade CIN (2,5,15–19).
Higher positivity rates of VSS compared to clinician-taken samples
for overall HPV and HPV16 found herein, were also reported in
Reference (20).
Positive HPV findings have been shown to powerfully
predict high-grade cervical lesions among patients with glandular
pathology (21,22). Positive HPV18 is strongly associated
with cervical adenocarcinoma risk, especially in its more
aggressive form (23,24). In our cohort, it was only HPV18 which
was less frequently detected with VSS compared to
clinician-collected samples.
To our knowledge, there is only one other study
evaluating self-sampling versus clinician-collected samples as
follow-up among patients treated for high-grade CIN (25). In Reference (25) fifty-two of 103 treated patients
(50.4%) participated in tri-monthly urine self-collection and
cervical scrapings. All three cases of CIN2+ detected
during one-year follow-up showed repeated positive HPV findings on
self-sampled urine and cervical scrapings. All pre-treatment and
recurrent findings were squamous in Reference (25).
A recent investigation (26) comparing histologic findings and
triple HPV results in women undergoing colposcopy revealed that
urine-based HPV testing was somewhat less sensitive in identifying
women with high-grade CIN, compared to VSS and provider-sampled HPV
results, similarly to our study. Our samples were from the initial
urine stream, thought to contain highest concentrations of
diagnostically relevant components (27) and to be more accurate for detecting
cervical HPV than mid-stream or end-stream samples (28). Timing of collection may also impact
the amount of viral DNA in the sample, since more HPV DNA could be
present with an increased interval between two urinations because
more excreted mucus and debris from the genital organs can
accumulate (4). Thus, there should
be sufficient time between urine collection and previous urination
(29). We specifically instructed
the participants to collect urine samples before VSS to avoid
interfering with the material from the cervicovaginal tract.
However, the urine samples were not collected first-void in the
morning, but randomly during the day. We paid careful attention to
storage conditions and preparation of urine samples, in light of
their importance for HPV DNA detection (30). Nevertheless, 44 of the 531 urine
samples were invalid due to absence of high-risk HPV and
β-globin, whereas all VSS and clinician-collected samples
were valid.
Assessment of HPV16 and/or HPV18 was nearly always
associated with higher specificity than overall HPV findings. This
is particularly notable for VSS for which there was the largest
number, 100, of overall HPV positive findings associated with
normal cytology at follow-up #1, whereas only twenty-two patients
with normal cytology showed positive HPV16 and/or HPV18 findings on
VSS. The importance of assessing HPV16 and 18 was underscored in
the review of patterns of HPV infection after treatment of
high-grade CIN (3).
The present findings indicate that VSS could be a
viable option for follow-up of women treated for high-grade CIN, if
the pathology is squamous. In considering the self-sampling option,
the advantages and disadvantages need to be presented to the
patient. Namely, the chances of false positive findings are a bit
higher with VSS than with clinician-sampling, such that repeated
self-sampling might be needed. This is reflected in a much higher
percentage of positive ‘other HPV’ with VSS, compared to
clinician-sampling. Notably, among women below age 30, positive
findings only for other HPV may not impact substantially upon risk
of future high-grade CIN (31).
Also, for HPV16, 18 and other HPV, but not for overall HPV results,
there was a somewhat larger chance of a missing result with VSS
(due to above-threshold CN values). Thus, women who would be
comfortable and willing to repeat home self-sampling could choose
the VSS option.
Based on these results, our recommendation would be
against self-sampling for patients with glandular pathology. This
recommendation is based on the two patients with recurrence in whom
VSS did not yield a positive result, both of whom had glandular
pathology. In both these patients, clinician-sampling revealed
HPV18 positivity, which, as noted, appears to be a particularly
important risk indicator (23,24). To
more completely address this cautious clinical recommendation,
further research is needed vis-à-vis self-sampling for follow-up
among treated patients with glandular pathology, with special
attention to HPV18. Longer follow-up and repeated HPV assessments
are also needed in future studies comparing self-sampling and
clinician-sampling among patients treated for high-grade CIN.
Practically complete data were available for this
cohort of patients followed-up post-conization treatment for
CIN2+ or AIS. To our knowledge, this is the largest,
most complete follow-up study in which HPV results were compared
for two different clinician-sampling methods and two different
self-sampling methods. Besides biopsy data, complete cytology data
were available for all 531 patients. Inclusion of this latter
outcome-variable provides further insight into the post-therapeutic
clinical status of these patients. However, follow-up thereafter is
limited; only 21% attended follow-up #2. Of 127 patients referred
to follow-up #2 for abnormal cytology and/or HPV positive findings
from standard COBAS clinician samples, 113 patients attended. The
404 patients with normal cytology and negative HPV findings from
standard-COBAS clinician-samples returned to routine screening,
without follow-up within this study. The latter includes fifty-nine
patients with positive HPV findings on VSS and/or Abbott
clinician-sampling. Another limitation of the study may have been
reliance upon colposcopically-visible lesions for biopsy. This
could have underestimated the actual number of recurrences, since
biopsies taken from colposcopically negative sites may also
identify patients with high-grade cervical dysplasia (32).
All participants performed self-sampling in the
clinic restroom. Questionnaire data were available from 479 of 531
patients concerning their readiness to perform self-sampling at
home and whether self-sampling was easy to carry-out. These
statements were endorsed, respectively, by 74 and 86% of the 479
women. In a study using the same questionnaire (33), forty-one long-term screening
non-attenders performed VSS at home with positive HPV results, for
which they subsequently underwent gynecologic examination. All
forty-one patients endorsed both statements regarding
self-sampling; 95% cited comfort as a reason for performing
self-sampling. In contrast, only 14% of the women in the present
study cited comfort as a reason for performing self-sampling.
Indeed, the home-setting is more comfortable for carrying-out VSS.
Home self-sampling is also practical and cost-effective for
repeated assessment. On the other hand, the quality of home
self-sampling might not be as high as self-sampling performed in
the clinic immediately after specific instructions are directly
given. This underscores the need to provide very clear written
instructions, and that health professionals are easily accessible
to answer any queries that arise when performing self-sampling at
home.
The home self-sampling option could be particularly
favorable as an alternative to clinic visits in face of the current
COVID19-pandemic, plus being convenient and cost-effective
(9). Self-collection of samples for
HPV testing is becoming an increasingly accepted, and even
preferred cervical screening option for many women (34–42).
The present findings concerning urine self-sampling
cohere substantially with the literature. In home-based settings
for collecting first-void urine (27), urine self-sampling may also hold
promise for follow-up after treatment for high-grade CIN.
These considerations reflect more personalized
approaches for women at elevated risk of recurrent high-grade CIN.
Embodied therein is empowerment, whereby women would be
well-informed about available options, actively participating in
decision-making regarding cervical screening. Such a strategy has
been successful in other cervical screening contexts (43,44) and
is likely to enhance fuller participation in the needed long-term
follow-up for these women at increased cervical cancer risk.
In conclusion, for patients with squamous cell
pathology, post-therapeutic follow-up based on HPV analysis from
self-collected vaginal samples appears to be as sensitive as HPV
analysis from clinician-collected cervical samples for predicting
outcome. Based on a very small number of patients with the far less
common glandular pathology, the present study suggests that vaginal
self-sampling is not adequately sensitive, such that HPV analysis
should be based on clinician-collected cervical samples when
assessing risk of recurrence. The vast majority of patients treated
for high-grade cervical intraepithelial neoplasia have squamous
pathology. For these patients, vaginal self-sampling for HPV
analysis may well be a viable option that can maximize
participation in the needed long-term follow-up for these women at
increased cervical cancer risk.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Swedish
Cancer Foundation (grant no. 110544, CAN2011/471), Karolinska
Institute (Cancer Strategic Grants; grant no. 5888/05-722), Swedish
Research Council (grant no. 521-2008-2899), Stockholm County
Council (grant nos. 20130097 and 20160155) and Gustaf V Jubilee
Fund (grant nos. 154022 and 151202).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EÖ participated in the design and conception of the
study, was responsible for identifying and recruiting all the
participants, met with all the participants, gave the instructions
for self-sampling, prepared the self-samples for transport,
assessed the authenticity of the raw data, prepared the data set
for analysis, collected the related literature, participated in
drafting the manuscript and revised the manuscript. KB performed
the statistical analysis, collected the related literature, and
wrote and revised the manuscript. TR contributed to the
interpretation of the HPV data and revised the manuscript. MM
participated in the design and conception of the study, performed
colposcopy, cervical sampling and punch biopsies of visible
lesions, and revised the manuscript. SA conceived and designed the
study, performed colposcopy, cervical sampling and punch biopsies
of visible lesions, assessed the authenticity of the raw data,
reviewed the data, collected the related literature, and revised
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Karolinska Ethics Committee approved the study
protocol (2006/1273-31, 2014/2034-3). Informed consent by each
patient was signed with options: Agreement or decline to
participate.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AIS
|
adenocarcinoma-in-situ
|
|
CI
|
confidence interval
|
|
CIN
|
cervical intraepithelial neoplasia
|
|
CN
|
cycle numbers
|
|
HPV
|
high-risk human papillomavirus
|
|
HSIL
|
high-grade
squamous-intraepithelial-lesions
|
|
LBC
|
liquid based cytology
|
|
NILM
|
negative for intraepithelial lesions
or malignancy
|
|
NPV
|
negative predictive value
|
|
PCR
|
polymerase chain reaction
|
|
PPV
|
positive predictive value
|
|
OR
|
odds ratio
|
|
VSS
|
vaginal self-sampling
|
References
|
1
|
Ebisch RMF, Rutten DWF, IntHout J,
Melchers WJG, Massuger LFAG, Bulten J, Bekkers RLM and Siebers AG:
Long-lasting increased risk of human papillomavirus-related
carcinomas and premalignancies after cervical intraepithelial
neoplasia grade 3: A population-based cohort study. J Clin Oncol.
35:2542–2550. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kocken M, Helmerhorst TJ, Berkhof J,
Louwers JA, Nobbenhuis MA, Bais AG, Hogewoning CJ, Zaal A,
Verheijen RH, Snijders PJ and Meijer CJ: Risk of recurrent
high-grade cervical intraepithelial neoplasia after successful
treatment: A long-term multi-cohort study. Lancet Oncol.
12:441–450. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hoffman SR, Le T, Lockhart A, Sanusi A,
Dal Santo L, Davis M, McKinney DA, Brown M, Poole C, Willame C and
Smith JS: Patterns of persistent HPV infection after treatment for
cervical intraepithelial neoplasia (CIN): A systematic review. Int
J Cancer. 141:8–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arbyn M, Ronco G, Anttila A, Meijer CJ,
Poljak M, Ogilvie G, Koliopoulos G, Naucler P, Sankaranarayanan R
and Peto J: Evidence regarding human papillomavirus testing in
secondary prevention of cervical cancer. Vaccine. 30 (Suppl
5):F88–F99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruhn LV, Andersen SJ and Hariri J:
HPV-testing versus HPV-cytology co-testing to predict the outcome
after conization. Acta Obstet Gynecol Scand. 97:758–765. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arbyn M, Verdoodt F, Snijders PJ, Verhoef
VM, Suonio E, Dillner L, Minozzi S, Bellisario C, Banzi R, Zhao F,
et al: Accuracy of human papillomavirus testing on self-collected
versus clinician-collected samples: A meta-analysis. Lancet Oncol.
15:172–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arbyn M, Snijders PJ, Meijer CJ, Berkhof
J, Cuschieri K, Kocjan BJ and Poljak M: Which high-risk HPV assays
fulfill criteria for use in primary cervical cancer screening? Clin
Microbiol Infect. 21:817–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jentschke M, Chen K, Arbyn M, Hertel B,
Noskowicz M, Soergel P and Hillemans P: Direct comparison of two
vaginal self-sampling devices for the detection of human
papillomavirus infections. J Clin Virol. 82:46–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Östensson E, Hellström AC, Hellman K,
Gustavsson I, Gyllensten U, Wilander E, Zethraeus N and Andersson
S: Projected cost-effectiveness of repeat HPV testing using
self-collected vaginal samples in the Swedish cervical cancer
screening program. Acta Obstet Gynecol Scand. 92:830–840. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andersson S, Belkić K, Mints M and
Östensson E: Is self-sampling to test for high-risk papillomavirus
an acceptable option among women who have been treated for
high-grade cervical intraepithelial neoplasia? PLoS One.
13:e01990382018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Andersson S, Belkić K, Safer Demirbüker
SS, Mints M and Östensson E: Perceived cervical cancer risk among
women treated for high-grade cervical intraepithelial neoplasia:
The importance of specific knowledge. PLoS One. 12:e1901562017.
View Article : Google Scholar
|
|
12
|
Richart RM: Cervical intraepithelial
neoplasia. Pathol Annu. 8:301–328. 1973.PubMed/NCBI
|
|
13
|
Jordan J, Martin-Hirsch P, Arbyn M,
Schenck U, Baldauf JJ, Da Silva D, Anttila A, Nieminen P and
Prendiville W: European guidelines for clinical management of
abnormal cervical cytology, part-2. Cytopathology. 20:5–16. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Solomon D, Davey D, Kurman R, Moriarty A,
O'Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T Jr, et
al: The 2001 Bethesda system: Terminology for reporting results of
cervical cytology. JAMA. 287:2114–2119. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brismar S, Johansson B, Borjesson M, Arbyn
M and Andersson S: Follow-up after treatment of cervical
intraepithelial neoplasia by human papillomavirus genotyping. Am J
Obstet Gynecol. 201:17.e1–8. 2009. View Article : Google Scholar
|
|
16
|
Kocken M, Uijterwaal MH, de Vries AL,
Berkhof J, Ket JC, Helmerhorst TJ and Meijer CJ: High-risk human
papillomavirus testing versus cytology in predicting post-treatment
disease in women treated for high-grade cervical disease: A
systematic review and meta-analysis. Gynecol Oncol. 125:500–507.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Persson M, Brismar Wendel S, Ljungblad L,
Johansson B, Weiderpass E and Andersson S: High-risk human
papillomavirus E6/E7 mRNA and L1 DNA as markers of
residual/recurrent cervical intraepithelial neoplasia. Oncol Rep.
28:346–352. 2012.PubMed/NCBI
|
|
18
|
Asciutto KC, Henic E, Darlin L, Forslund O
and Borgfeldt C: Follow up with HPV test and cytology as test of
cure, 6 months after conization, is reliable. Acta Obstet Gynecol
Scand. 95:1251–1257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garutti P, Borghi C, Bedoni C, Bonaccorsi
G, Greco P, Tognon M and Martini F: HPV-based strategy in follow-up
of patients treated for high-grade cervical intra-epithelial
neoplasia: 5-year results in a public health surveillance setting.
Eur J Obstet Gynecol Reprod Biol. 210:236–241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saville M, Hawkes D, Keung M, Ip E,
Silvers J, Sultana F, Malloy MJ, Velentzis LS, Canfel LK, Wrede CD
and Brotherton J: Analytical performance of HPV assays on vaginal
self-collected vs. practitioner-collected cervical samples. J Clin
Virol. 127:1043752020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Norman I, Hjerpe A and Dillner J: Risk of
high-grade lesions after atypical glandular cells in cervical
screening: A population-based cohort study. BMJ Open.
7:e0170702017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Patadji S, Li Z, Pradhan D and Zhao C:
Significance of high-risk HPV detection in women with atypical
glandular cells on pap testing: Analysis of 1857 cases from an
academic institution. Cancer Cytopathol. 125:205–211. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Andersson S, Larson B, Hjerpe A,
Silfverswärd C, Sällström J, Wilander E and Rylander E:
Adenocarcinoma of the uterine cervix: The presence of human
papillomavirus and the method of detection. Acta Obstet Gynecol
Scand. 82:960–965. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dahlström LA, Ylitalo N, Sundström K,
Palmgren J, Ploner A, Eloranta S, Sanjeevi CB, Andersson S, Rohan
T, Dillner J, et al: Prospective study of human papillomavirus and
risk of cervical adenocarcinoma. Int J Cancer. 127:1923–1930. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fambrini M, Penna C, Pieralli A, Bussani
C, Fallani MG, Andersson KL, Scarselli G and Marchionni M: PCR
detection rates of high risk human papillomavirus DNA in paired
self-collected urine and cervical scrapes after laser CO2
conization for high-grade cervical intraepithelial neoplasia.
Gynecol Oncol. 109:59–64. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rohner E, Rahangdale L, Sanusi B, Knittel
AK, Vaughan L, Chesko K, Faherty B, Tulenko SE, Schmitt JW, Romocki
LS, et al: Test accuracy of human papillomavirus in urine for
detection of cervical intraepithelial neoplasia. J Clin Microbiol.
58:e01443–19. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vorsters A, Van Damme P and Clifford G:
Urine testing for HPV: Rationale for using first void. BMJ.
349:g62522014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pathak N, Dodds J, Zamora J and Khan K:
Accuracy of urinary human papillomavirus testing for presence of
cervical HPV: Systematic review and meta-analysis. BMJ.
349:g52642014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pattyn J, Van Keer S, Biesmans S, Ieven M,
Vanderborght C, Beyers K, Vankerckhoven V, Bruyndonckx R, Van Damme
P and Vorsters A: Human papillomavirus detection in urine: Effect
of a first-void urine collection device and timing of collection. J
Virol Methods. 264:23–30. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vorsters A, Micalessi I, Bilcke J, Ieven
M, Bogers J and Van Damme P: Detection of human papillomavirus DNA
in urine. A review of the literature. Eur J Clin Microbiol Infect
Dis. 31:627–640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fröberg M, Östensson E, Belkić K, Oštrbenk
A, Poljak M, Mints M, Arbyn M and Andersson S: Impact of the human
papillomavirus status on development of high-grade cervical
intraepithelial neoplasia in women negative for intraepithelial
lesions or malignancy at baseline: A 9-year Swedish nested
case-control follow-up study. Cancer. 125:239–248. 2019.PubMed/NCBI
|
|
32
|
Baasland I, Hagen B, Vogt C, Valla M and
Romundstad PR: Colposcopy and additive diagnostic value of biopsies
from colposcopy-negative areas to detect cervical dysplasia. Acta
Obstet Gynecol Scand. 95:1258–1263. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Andersson S, Belkić K, Mints M and
Östensson E: Acceptance of self-sampling among long-term cervical
screening non-attenders with HPV positive results: Promising
opportunity for specific cancer education. J Cancer Educ.
36:126–133. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Galbraith KV, Gilkey MB, Smith JS, Richman
AR, Barclay L and Brewer NT: Perceptions of mailed HPV self-testing
among women at higher risk for cervical cancer. J Community Health.
39:849–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Crofts V, Flahault E, Tebeu PM, Untiet S,
Fosso GK, Boulvain M, Vassilakos P and Petignat P: Education
efforts may contribute to wider acceptance of human papillomavirus
self-sampling. Int J Womens Health. 7:149–154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Arrossi S, Ramos S, Straw C, Thouyaret L
and Orellana L: HPV testing: A mixed-method approach to understand
why women prefer self-collection in a middle-income country. BMC
Public Health. 16:8322016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma'som M, Bhoo-Pathy N, Nasir NH,
Bellinson J, Subramaniam S, Ma Y, Yap SH, Goh PP, Gravitt P and Woo
YL: Attitudes and factors affecting acceptability of
self-administered cervicovaginal sampling for human papillomavirus
(HPV) genotyping as an alternative to Pap testing among multiethnic
Malaysian women. BMJ Open. 6:e0110222016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vahabi M and Lofters A: Muslim immigrant
women's views on cervical cancer screening and HPV self-sampling in
Ontario, Canada. BMC Public Health. 16:8682016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Racey CS and Gesink DC: Barriers and
facilitators to cervical cancer screening among women in rural
Ontario, Canada: The role of self-collected HPV testing. J Rural
Health. 32:136–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
de Melo Kuil L, Lorenzi AT, Stein MD,
Resende JCP, Antoniazzi M, Longatto-Filho A, Scapulatempo-Neto C
and Fregnani JHTG: The role of self-collection by vaginal lavage
for the detection of HPV and high-grade intraepithelial neoplasia.
Acta Cytol. 61:425–433. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mao C, Kulasingam SL, Whitham HK, Hawes
SE, Lin J and Kiviat NB: Clinician and patient acceptability of
self-collected human papillomavirus testing for cervical cancer
screening. J Womens Health (Larchmt). 26:609–615. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bergengren L, Kaliff M, Larsson GL,
Karlsson MG and Helenius G: Comparison between professional
sampling and self-sampling for HPV-based cervical cancer screening
among postmenopausal women. Int J Gynecol Obstet. 142:359–364.
2018. View Article : Google Scholar
|
|
43
|
Kahn JA, Slap GB, Bernstein DI, Kollar LM,
Tissot AM, Hillard PA and Rosenthal SL: Psychological, behavioral,
and interpersonal impact of human papillomavirus and pap test
results. J Womens Health (Larchmt). 14:650–659. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Luszczynska A, Durawa AB, Scholz U and
Knoll N: Empowerment beliefs and intention to uptake cervical
cancer screening: Three psychosocial mediating mechanisms. Women
Health. 52:162–181. 2012. View Article : Google Scholar : PubMed/NCBI
|