Introduction
18F-fluorodeoxyglucose-positron emission
tomography/computed tomography (18F-FDG-PET/CT), as a
whole-body scan, is powerful for cancer staging.
18F-FDG-PET/CT has the ability to detect early malignant
lesions characterized by an increased rate of glycolysis (1). 18F-FDG-PET/CT is also useful
when identifying local recurrence or metastases when tumor marker
levels are elevated after the completion of treatment. However, a
region of novel FDG uptake, which usually reflects recurrence or
metastases from the known tumor, can also indicate a secondary
primary cancer. Because of the nature of patient management and
early cancers that require radical treatment, the detection of
secondary primary cancer is an important prognostic factor
(2).
Secondary primary cancer could occur months or years
after the diagnosis and treatment of the original primary cancer
(3). Patients who had experienced
cancer could be at increased risk of developing secondary primary
cancer (3). Even if the progress of
the original primary cancer was good, secondary primary cancer
might narrow the choice of treatment methods and result in poor
prognosis. In particular, pancreatic cancer has a very poor
prognosis and progresses quickly (4). The prognosis of pancreatic cancer has
not improved despite extensive research and advances in imaging
modalities. There are several reasons for the difficulties in the
early diagnosis of pancreatic cancer, including the absence of
early-stage biomarkers, anatomical location in the retroperitoneum
allowing invasion of the surrounding organs and blood vessels, and
non-specific symptoms (5).
18F-FDG-PET has already been used to
diagnose pancreatic cancer (6–11).
However, to our knowledge, no study has examined the frequency of
new pancreatic FDG uptake and proportion of secondary primary
pancreatic cancers after finding unexpected pancreatic FDG uptake
on follow-up PET/CT in patients with cancer other than pancreatic
cancers. Therefore, this study aimed to evaluate the breakdown of
unexpected pancreatic FDG uptake and the proportion of secondary
primary pancreatic cancer on follow-up PET/CT in patients with
cancer.
Materials and methods
Ethic statement
The present study was approved by the Ethical Review
Board of Kochi Medical School (Nankoku, Japan). Due to the
retrospective nature of the present study, written informed consent
was waived.
Patients
The participants consisted of 4,473 consecutive
patients who underwent 18F-FDGPET/CT to exclude
metastatic cancers or local recurrence between January 2015 and
March 2019 at Kochi Medical School. Of the 4,473 patients, 225 with
a past history of pancreatic cancer were excluded. The remaining
4,248 patients who underwent previous imaging scans including
18F-FDG-PET/CT for the initial cancer staging or
follow-up were included in this study. None of them had pancreatic
diseases, neither primary cancer nor others.
Imaging protocol
PET/CT was performed 50 min after an intravenous
injection of 3.5 MBq/kg FDG via an already secured peripheral
venous catheter. Images from the head to the mid-thigh were
acquired in three-dimensional (3D) mode at 2 min per bed position
in the supine position using a PET/CT scanner (Discovery ST Elite,
GE Healthcare, Waukesha, WI, USA) with a 16-slice Light-Speed CT
component. All participants fasted for 6 h before undergoing
PET/CT, and all participants had fasting blood glucose levels of
<150 mg/dl before the injection. Non-contrast-enhanced CT images
(140 kVp, 100–200 mAs) were acquired in the helical mode using a
3.75-mm slice thickness. The acquired data were reconstructed using
a standard vendor-provided ordered subset expectation maximization
algorithm (VUE point plus). CT, PET, and PET/CT images were all
reviewed.
Imaging analysis
FDG uptake in the pancreatic area was
retrospectively and blindly evaluated in 4,248 patients. Positive
FDG uptake was defined as a maximum standardized uptake value
(SUVmax) ≥2.5. In the presence of pancreatic FDG uptake,
FDG uptake distribution and localization in the pancreas and the
site of abnormal FDG uptake excluding the pancreas were evaluated.
Two radiologists evaluated the results by reaching a consensus.
Final diagnosis
The final diagnosis was determined pathologically.
Patients who were not diagnosed pathologically were diagnosed
clinically using the follow-up imaging or clinical data.
Results
Patient clinical data
FDG uptake in the pancreatic area was detected in
14/4,248 patients [0.3%; 12 men, two women; mean age, 75 years
(range: 49–88 years)]. Table I lists
the patient characteristics, 18F-FDG-PET/CT findings,
diagnostic procedures, and final diagnoses. Two of the 14 patients
already had double cancer (esophageal and oropharyngeal cancer;
rectal and bladder cancer). Pancreatic abnormalities in 14 patients
included five cases of pancreatic metastases (36%), four cases of
secondary primary pancreatic cancer (29%), two cases of lymph node
metastases (14%), one case of malignant lymphoma (7%), one case of
autoimmune pancreatitis (7%), and one case of pseudolesion (7%).
The breakdown of the original lesions in the five cancers that
metastasized to the pancreas are as follows: Two lung cancer cases,
one renal cell carcinoma, one malignant melanoma and one laryngeal
cancer. The primary cancer in the four patients with secondary
primary pancreatic cancer included one rectal, one cecal, one
vaginal, and one bile duct cancer.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
|
|
|
|
18F-FDG-PET/CT |
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Patient | Age, years | Sex | Primary | FDG uptake |
SUVmax | Localization | Period from last
examination (months) | Diagnostic
procedure | Final diagnosis |
|---|
| 1 | 65 | M | Rectal ca. | Solitary | 5.1 | Tail | 4 | EUS-FNA | Pancreatic ca. |
| 2 | 80 | F | Malignant
melanoma | Solitary | 5.1 | Body | 6 | EUS-FNA | Pancreatic
metastasis |
| 3 | 68 | M | Esophageal ca.,
oropharynx ca. | Solitary | 10.0 | Head | 17 | Subsequent CT | Lymph node
metastasis |
| 4 | 76 | M | Lung ca. | Solitary | 6.9 | Body | 15 | EUS-FNA | Pancreatic
metastasis |
| 5 | 80 | M | Malignant
lymphoma | Multiple | 12.5 | Head, Body,
Tail | 29 | EUS-FNA | Malignant
lymphoma |
| 6 | 82 | F | Vaginal ca. | Solitary | 5.7 | Head | 8 | EUS-FNA | Pancreatic ca. |
| 7 | 49 | M | Laryngeal ca. | Solitary | 7.1 | Body | 6 | Subsequent CT | Pancreatic
metastasis |
| 8 | 79 | M | Esophageal ca. | Solitary | 5.1 | Body | 11 | Subsequent CT | Pseudolesion |
| 9 | 83 | M | Renal cell ca. | Multiple | 3.8 | Head, Body | 7 | EUS-FNA | Pancreatic
metastasis |
| 10 | 73 | M | Rectal ca., bladder
ca. | Solitary | 5.1 | Head | 5 | Subsequent CT | Autoimmune
pancreatitis |
| 11 | 72 | M | Lung ca. | Solitary | 6.1 | Body | 3 | EUS-FNA | Pancreatic
metastasis |
| 12 | 85 | M | Unknown primary
ca. | Solitary | 15.1 | Head | 3 | Subsequent CT | Lymph node
metastasis |
| 13 | 88 | M | Cecal ca. | Solitary | 5.8 | Head | 8 | EUS-FNA | Pancreatic ca. |
| 14 | 72 | M | Bile duct ca. | Solitary | 2.6 | Tail | 8 | EUS-FNA | Pancreatic ca. |
18F-FDG-PET/CT results
The mean SUVmax of pancreatic FDG uptake
in the four proven secondary primary pancreatic cancers was 4.8
(range: 2.6–5.8). Three of the four secondary primary pancreatic
cancers in patients were advanced cancer (stage III: One patient,
stage IV: Two patients), and one of the three had obvious FDG
uptake in the liver that was considered to reflect metastasis. One
patient with early-stage secondary primary pancreatic cancer had
the lowest SUVmax among the 14 patients. The mean
SUVmax of pancreatic FDG uptake in the five patients
with pancreatic metastases was 5.8 (range: 3.8–7.1). Four of the
five patients with pancreatic metastasis had FDG uptake of multiple
lesions, expect for the pancreas. The SUVmax of the
pancreatic abnormal FDG uptake was higher than 10.0 in three
patients with two lymph node metastases and one malignant
lymphoma.
Of the five patients that had metastasis to the
pancreas, four patients had a solitary FDG uptake in the pancreas
body, and the remaining patient (renal cell carcinoma) had multiple
FDG uptake. Two of four patients with secondary primary pancreatic
cancer had a solitary FDG uptake in the pancreatic head, and two
patients had a solitary FDG uptake in the pancreatic tail. All
patients with lymph node metastases had a solitary FDG uptake in
the pancreatic head. The patient with pancreatic infiltration in
lymphoma had multiple FDG uptake throughout the pancreas.
After PET/CT, all 14 patients underwent
contrast-enhanced CT (CE-CT), which revealed abnormalities in the
pancreas, except for lymph node metastasis in two patients and
pseudolesion in one. The interval from the previous imaging to the
follow-up 18F-FDG-PET/CT where pancreatic abnormality
was found was <8 months (1:4, 3:8 months) in all four patients
with secondary primary pancreatic cancer.
Final diagnosis
Four of five pancreatic metastases, four secondary
primary pancreatic cancers, and one malignant lymphoma were
pathologically proven following endoscopic ultrasound-guided fine
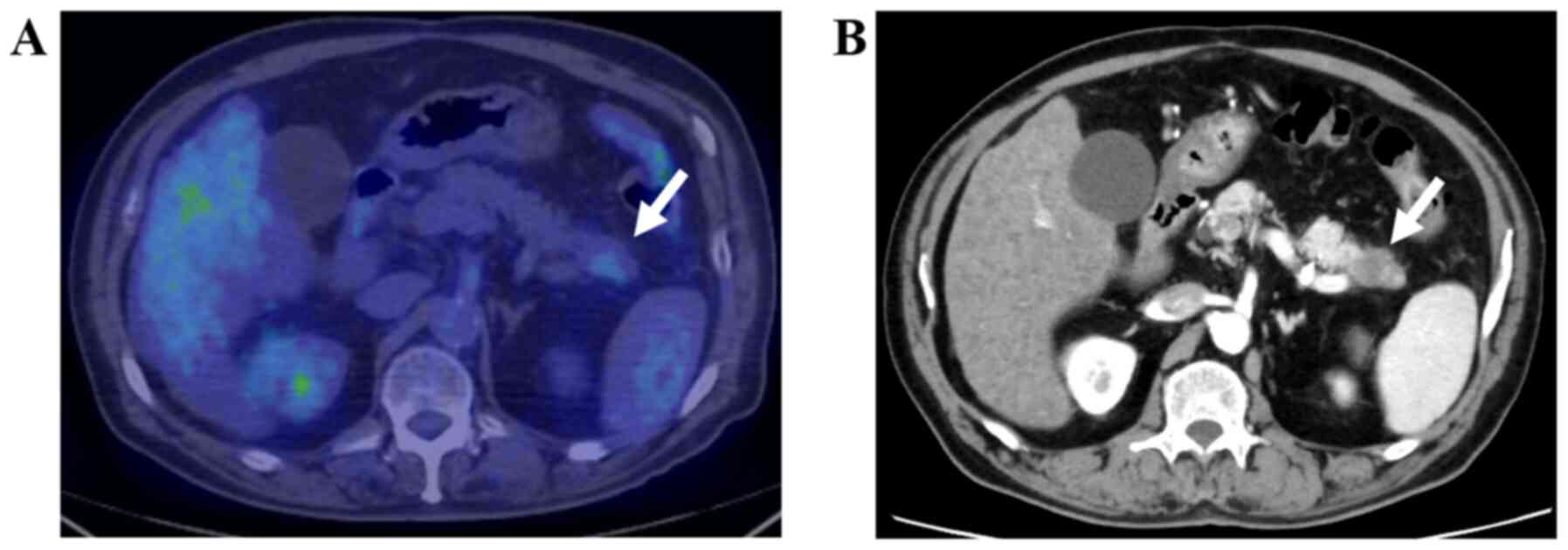
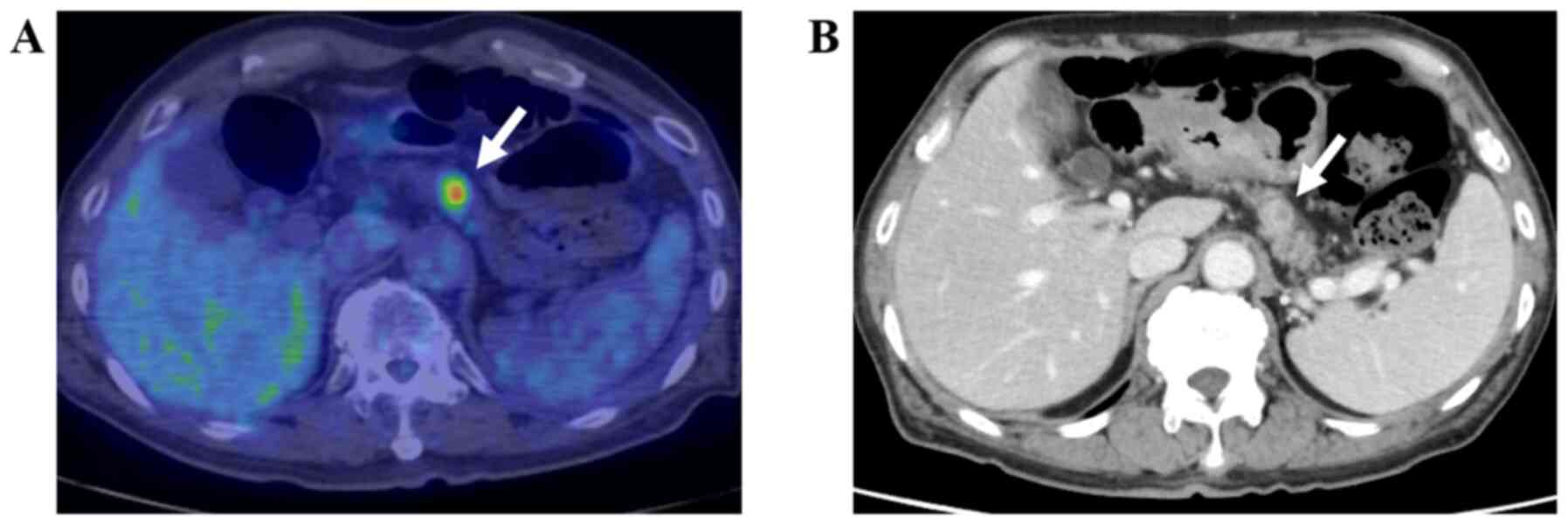
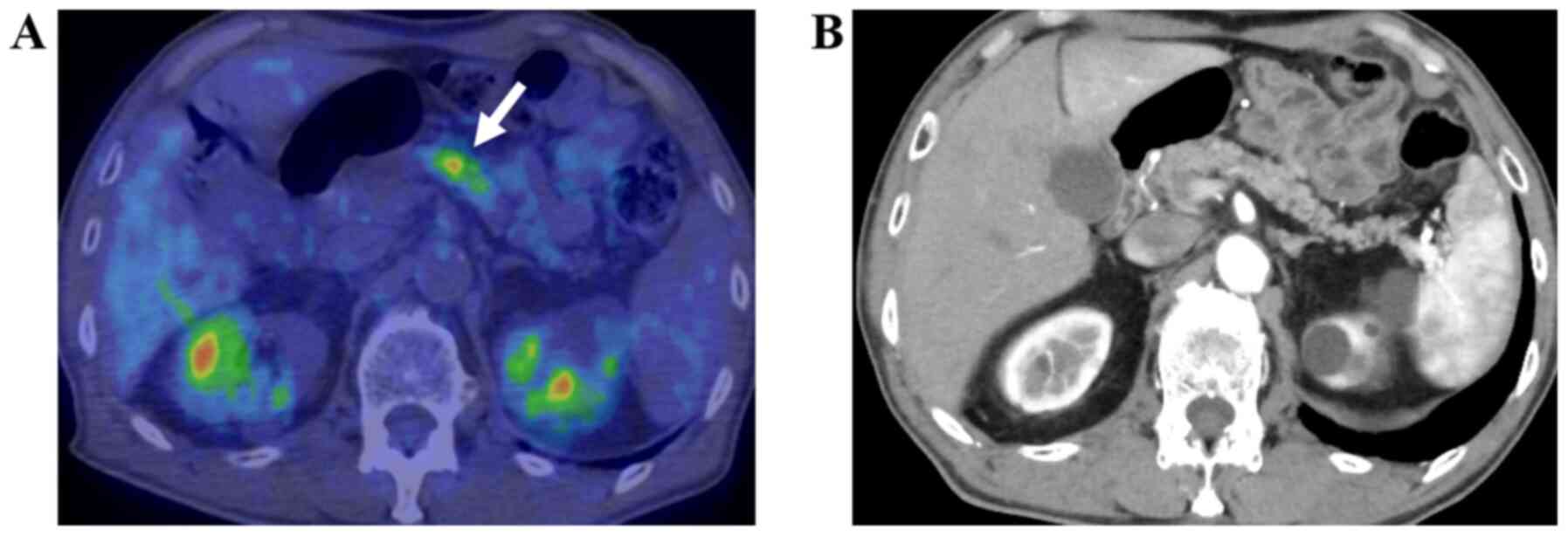
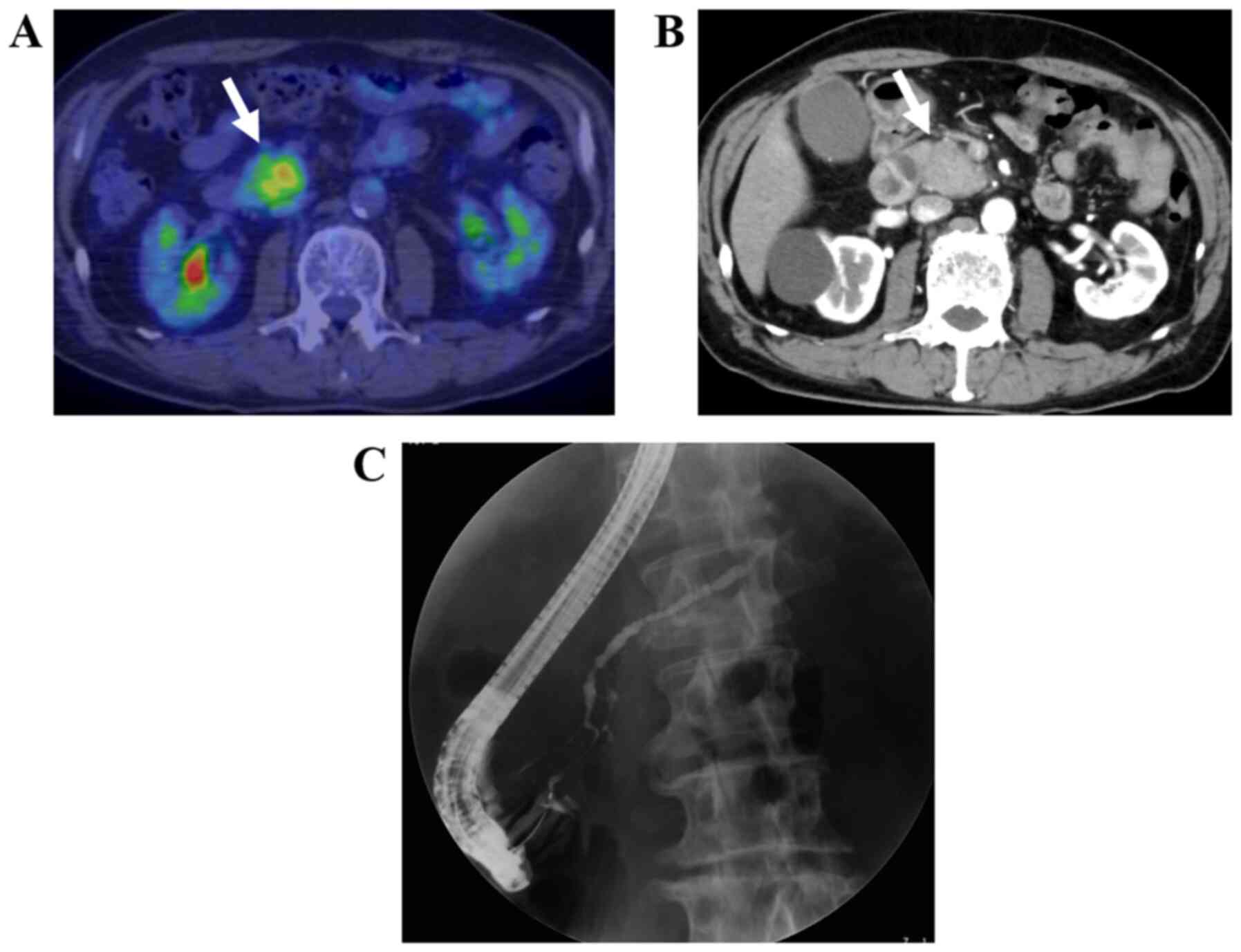
needle aspiration (EUS-FNA) (Figs.
1–4). The remaining five, i.e.,
one pancreatic metastasis, two lymph node metastases, one
autoimmune pancreatitis, and one pseudolesion, were diagnosed
clinically (Figs. 5 and 6).
Discussion
This study aimed to evaluate the breakdown of
unexpected pancreatic FDG uptake on follow-up PET/CT in patients
with cancer. The majority of patients with unexpected pancreatic
FDG uptake on follow-up PET/CT exhibited malignancies. Furthermore,
~30% of the malignancies detected in patients with pancreatic FDG
uptake were secondary primary pancreatic cancers.
Pancreatic ductal adenocarcinoma, which is by far
the most common in various pancreatic neoplasms, is the worst
prognosis of all solid cancers (4).
It has been reported that 5-year survival rates for patients with
completely resected patients with pancreatic ductal adenocarcinoma
are only up to 18.8% (4). Most
patients with pancreatic cancer remain asymptomatic until the
disease develops to an advanced stage (12). Prognosis could depend on degree of
the cancer progression. Therefore, early diagnosis of pancreatic
cancer is essential for improved prognosis. Early pancreatic
cancers have histopathologic features such as less fibrosis and
more remnant pancreatic tissue, which appear isoattenuating in
pancreatic phase of dynamic CT (13). The frequency of visually
isoattenuating pancreatic adenocarcinomas based on every phase of
dynamic CT among pathologically proven pancreatic cancers was 5.4%,
particularly 27% of small pancreatic cancers measuring ≤20 mm has
been reported to appear isoattenuating in any phase (14). Although few reports have described
the utility of FDG-PET in early pancreatic cancer, FDG uptake had
been reportedly observed in 60% of patients with stage I pancreatic
cancer (5). In other words,
approximately one-half of stage I pancreatic cancers could not be
detected with FDG-PET. Therefore, FDG-PET is not suitable for the
detection of early pancreatic cancer due to the spatial resolution
limit. Three of the four patients had advanced cancer, two of whom
had stage IV cancer.
18F-FDG-PET/CT, as a whole-body scan, is
a powerful tool for oncology imaging. In this study, we evaluated
the proportion of secondary primary pancreatic cancer in unexpected
pancreatic FDG uptake on follow-up PET/CT in patients with cancer.
The majority of patients with unexpected pancreatic FDG uptake on
follow-up PET/CT had malignancies; furthermore, ~30% of the
malignancies detected in patients with pancreatic FDG uptake were
secondary primary pancreatic cancers. The difference between FDG
uptake in secondary primary pancreatic cancers and those in
pancreatic metastases remains unclear. Therefore, distinguishing
secondary primary pancreatic cancers from pancreatic metastases is
difficult. According to a previous report, differentiation of
secondary primary pancreatic cancers and pancreatic metastases
based on their SUVmax is difficult (15). Even if the FDG uptake considered to
be metastasis with multiple lesions as well as pancreas is
recognized, diagnosing pancreatic metastases remains uncertain
because pancreatic cancer is advanced quickly. In this study,
patients with multiple foci of FDG uptake in the pancreas exhibited
pancreatic metastasis from renal cell carcinoma and malignant
lymphoma. A diagnosis of pancreatic cancer could be excluded in
patients with multiple foci of FDG uptake in the pancreas.
FDG uptake in the lymph nodes or neighboring organs
might be confused with that in the pancreas. In fact, in this
study, two lymph node metastases and physiological small intestine
uptake were initially identified as pancreatic uptake. Lymph node
metastasis was identified in two patients because CE-CT revealed
enhanced nodes with smooth margins near the pancreas. A
pseudolesion was identified in one patient because CE-CT revealed
physiological uptake in a portion of the small intestine near the
pancreas. When PET/non-CE-CT reveals abnormal FDG uptake in the
pancreas, CE-CT may help exclude false-positive FDG uptake around
the pancreas. Autoimmune pancreatitis was clinically diagnosed
based on capsule-like rim enhancement on CE-CT, the IgG4 level,
narrowing of the main pancreatic duct according to endoscopic
retrograde cholangiopancreatography (ERCP), and the efficacy of
steroid therapy. Extrapancreatic lesions were not noted in the
patient diagnosed with autoimmune pancreatitis.
It may be difficult to narrow the diagnosis of
pancreatic lesions on PET/non-CE-CT in patients with subtle or
limited uptake in the pancreatic lesion. SUVmax ≥1.3 has
been used to distinguish malignant from benign Intraductal
Papillary Mucinous Neoplasms (IPMNs) in the pancreas (16). Low FDG uptake might cause
overestimation. In general, SUVmax of 2.5 has been used
as a cutoff value for diagnosing malignancies with
18F-FDG-PET (17,18). Therefore, we used the positive FDG
uptake of SUVmax ≥2.5. In this study, one patient with
early-stage secondary primary pancreatic cancer had FDG uptake with
SUVmax of 2.6. The remaining 13 patients including three
advanced secondary pancreatic cancer had FDG uptake with
SUVmax ≥3.8. FDG uptake in secondary primary pancreatic
cancer in early stage could be lower than that in advanced stage.
However, FDG uptake could rise with the progression of cancer in a
short period. Early detection of secondary primary pancreatic
cancer by follow-up PET/CT might be difficult because pancreatic
cancer proceeds rapidly.
Some pancreatic cancers are related with inherited
predisposition; therefore, three groups have an increased risk of
developing pancreatic cancer: i) Hereditary pancreatitis; ii)
inherited cancer syndromes including hereditary breast-ovarian
cancer, Peutz-Jeghers syndrome, hereditary nonpolyposis colorectal
carcinoma, familial adenomatous polyposis, and familial atypical
multiple mole melanoma; iii) familial pancreatic cancer (19,20).
These three groups (hereditary pancreatitis, inherited cancer
syndromes, and familial pancreatic cancer) are considered to be
high risk for pancreatic cancer. In particular, pancreatic FDG
uptake should be carefully considered in patients with a history of
primary breast and/or ovarian cancer. In this study, the primary
cancer in two of the three patients with secondary primary
pancreatic cancer was colon cancer. However, the genetic background
of the two patients was unknown.
A search of the literature performed by the current
authors indicates that no previous studies have examined incidental
FDG uptake in the pancreas, but studies have reported incidental
FDG uptake in the thyroid, the breasts, the colon, and the prostate
(1,2,21,22). A
study that examined incidental FDG uptake in the thyroid noted FDG
uptake in the thyroid in 3.8% of patients with no history of
thyroid disease (21). Diffuse
uptake was noted in 1.8% of those patients and focal uptake was
noted in 2.0%. Of the patients in whom focal uptake was noted and
who underwent fine-needle aspiration or surgery, a malignancy was
noted in 63.6% (21). In a study
that examined incidental FDG uptake in the breasts, focal FDG
uptake was noted in the breasts of 0.82% of female patients with a
type of cancer other than breast cancer (1). Of those patients who were followed up,
a malignancy was noted in 57% (1).
In a study that examined incidental FDG uptake in the colon, focal
uptake was noted in the colon in 0.94% of patients (22). Of those patients who underwent a
colonoscopy, malignancy was noted in 43.4% and precancerous lesions
were noted in 26.1% (22). In a
study that examined incidental FDG uptake in the prostate, uptake
in the prostate was noted in 1.3% of patients (2). Of those patients who were followed up,
malignancy was noted in 12.7% (2).
Recently, the occurrence of second malignant
neoplasms has been reportedly influenced by a myriad of factors,
including the late effects of cancer therapy (such as chemotherapy
and radiotherapy), shared etiological factors with the primary
cancer (such as tobacco use, excessive alcohol intake, and
obesity), genetic predisposition, environmental determinants, host
effects, and combinations of factors (3). 18F-FDG-PET/CT in patients
with a past cancer history should be evaluated in detail,
considering the possibility of secondary primary cancers. This
study noted unexpected pancreatic FDG uptake in 0.3% of patients
with cancer. This frequency is presumably higher than the frequency
with which pancreatic FDG uptake is noted on PET/CT in people with
no history of cancer, though this has yet to be reported in
studies.
Pancreatic metastases are uncommon and account for
2–5% of all pancreatic malignant tumors (23,24).
However, the prevalence of pancreatic metastases has been reported
to range from 1.6 to 11% by studying a large number of autopsies
(25). The original lesions of the
five cancers that metastasized to the pancreas were as follows: Two
lung cancers, one renal cell carcinoma, one malignant melanoma, and
one laryngeal cancer. The majority of metastatic pancreatic cancers
had FDG uptake in multiple regions, including the pancreas. The
most common primary tumors that more frequently have pancreatic
metastases are renal cell carcinoma, lung cancer, breast cancer,
and colorectal carcinoma, followed by malignant melanoma and
leiomyosarcoma (25,26). In this study, the most common primary
malignancy was lung cancer. Pancreatic metastases are less common;
however, pancreatic metastases are considered to be common in the
terminal stage especially in performing autopsy. Regardless of
whether the underlying cause is pancreatic metastases or secondary
primary pancreatic cancer, pancreatic FDG uptake could suggest
advanced cancer when found on follow-up PET/CT in patients with
cancer. However, differentiation of pancreatic metastases and
secondary primary pancreatic cancer could be important in
determining the treatment strategy.
This study has some limitations. First, this was a
retrospective study, and the sample size was relatively small.
Second, the aim of the current study was to examine unexpected
pancreatic FDG uptake on PET/CT in patients with past history of
cancer. This study was unable to examine the effectiveness of
subsequent treatment in or the prognosis of these patients. Third,
the interval from the previous imaging to the follow-up
18F-FDG-PET/CT varied. Fourth, all patients in this
study were not diagnosed pathologically. Therefore, some early
secondary primary pancreatic cancers with FDG uptake lower than
SUVmax <2.5 might be overlooked.
In conclusion, the majority of patients with
unexpected pancreatic FDG uptake on follow-up PET/CT exhibited
malignancies; furthermore, ~30% of the malignancies detected in
patients with pancreatic FDG uptake were secondary primary
pancreatic cancers. In unexpected pancreatic FDG uptake on
follow-up PET/CT, primary cancer should be considered as well as
metastatic tumors.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HI and YM designed the study and wrote the initial
draft of the manuscript. MN, KM, and SK contributed to data
collection and interpretation. NH and NA performed the imaging
examinations. TK, KU and TY participated in the design of the study
and provided guidance. HI and YM confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was performed in accordance with the
Declaration of Helsinki and was approved by Ethical Review Board of
Kochi Medical School (Kochi, Japan). Due to the retrospective
nature of the present study, written informed consent was waived. A
statement explaining that individuals who did not want to
participate in the study could request to opt-out was posted on the
bulletin board at Kochi Medical School.
Patient consent for publication
The patients in our study provided consent for the
publication of any associated data and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Litmanovich D, Gourevich K, Israel O and
Gallimidi Z: Unexpected foci of 18F-FDG uptake in the breast
detected by PET/CT: Incidence and clinical significance. Eur J Nucl
Med Mol Imaging. 36:1558–1564. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sahin E, Elboga U, Kalender E, Basıbuyuk
M, Demir HD and Celen YZ: Clinical significance of incidental FDG
uptake in the prostate gland detected by PET/CT. Int J Clin Exp
Med. 8:10577–10585. 2015.PubMed/NCBI
|
|
3
|
Travis LB, Demark Wahnefried W, Allan JM,
Wood ME and Ng AK: Aetiology, genetics, and prevention of secondary
neoplasms in adult cancer survivors. Nat Rev Clin Oncol.
10:289–301. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Egawa S, Toma H, Ohigashi H, Okusaka T,
Nakao A, Hatori T, Maguchi H, Yanagisawa A and Tanaka M: Japan
pancreatic cancer registry; 30th year anniversary: Japan pancreas
society. Pancreas. 41:985–492. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kanno A, Masamune A, Hanada K, Maguchi H,
Simizu Y, Ueki T, Hasebe O, Ohtsuka T, Nakamura M, Takenaka M, et
al: Multicenter study of early pancreatic cancer in Japan.
Pancreatology. 18:61–67. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zimny M, Bares R, Fass J, Adam G,
Cremerius U, Dohmen B, Klever P, Sabri O, Schumpelick V and Buell
U: Fluorine-18 fluorodeoxyglucose positron emission tomography in
the differential diagnosis of pancreatic carcinoma: A report of 106
cases. Eur J Nucl Med. 24:678–682. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rose DM, Delbeke D, Beauchamp RD, Chapman
WC, Sandler MP, Sharp KW, Richards WO, Wright JK, Frexes ME, Pinson
CW and Leach SD: 18Fluorodeoxyglucose-positron emission tomography
in the management of patients with suspected pancreatic cancer. Ann
Surg. 229:729–738. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Imdahl A, Nitzsche E, Krautmann F, Högerle
S, Boos S, Einert A, Sontheimer J and Farthmann EH: Evaluation of
positron emission tomography with 2-(18F)fluoro-2-deoxy-D-glucose
for the differentiation of chronic pancreatitis and pancreatic
cancer. Br J Surg. 86:194–199. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakamoto Y, Higashi T, Sakahara H, Tamaki
N, Kogire M, Doi R, Hosotani R, Imamura M and Konishi J: Delayed
(18)F-fluoro-2-deoxy-D-glucose positron emission tomography scan
for differentiation between malignant and benign lesions in the
pancreas. Cancer. 89:2547–2554. 2000. View Article : Google Scholar : PubMed/NCBIPubMed/NCBI
|
|
10
|
Seo S, Doi R, Machimoto T, Kami K, Masui
T, Hatano E, Ogawa K, Higashi T and Uemoto S: Contribution of
18F-fluorodeoxyglucose positron emission tomography to the
diagnosis of early pancreatic carcinoma. J Hepatobiliary Pancreat
Surg. 15:634–639. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rijkers AP, Valkema R, Duivenvoorden HJ
and van Eijck CH: Usefulness of F-18-fluorodeoxyglucose positron
emission tomography to confirm suspected pancreatic cancer: A
meta-analysis. Eur J Surg Oncol. 40:794–804. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kamisawa T, Wood LD, Itoi T and Takaori K:
Pancreatic cancer. Lancet. 388:73–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoon SH, Lee JM, Cho JY, Lee KB, Kim JE,
Moon SK, Kim SJ, Baek JH, Kim SH, Kim SH, et al: Small (≤20 mm)
pancreatic adenocarcinomas: Analysis of enhancement patterns and
secondary signs with multiphasic multidetector CT. Radiology.
259:442–452. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JH, Park SH, Yu ES, Kim MH, Kim J,
Byun JH, Lee SS, Hwang HJ, Hwang JY, Lee SS and Lee MG: Visually
isoattenuating pancreatic adenocarcinoma at dynamic-enhanced CT:
Frequency, clinical and pathologic characteristics, and diagnosis
at imaging examinations. Radiology. 257:87–96. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu S, Zhang J, Zuo C, Cheng C, Liu Q and
Sun G: (18)F-FDG-PET/CT findings in pancreatic metastasis. Radiol
Med. 10:887–898. 2015. View Article : Google Scholar
|
|
16
|
Yamashita YI, Okabe H, Hayashi H, Imai K,
Nakagawa S, Nakao Y, Yusa T, Itoyama R, Yama T, Umesaki N, et al:
Usefulness of 18-FDG PET/CT in detecting malignancy in intraductal
papillary mucinous neoplasms of the pancreas. Anticancer Res.
39:2493–2499. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
AI-Sugair A and Coleman RE: Applications
of PET in lung cancer. Semin Nucl Med. 28:303–319. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hashimoto Y, Tsujikawa T, Kondo C, Maki M,
Momose M, Nagai A, Ohnuki T, Nishikawa T and Kusakabe K: Accuracy
of PET for diagnosis of solid pulmonary lesions with 18F-FDG uptake
below the standardized uptake value of 2.5. J Nucl Med. 47:426–431.
2006.
|
|
19
|
Frendrich V, Langer P and Bartsch DK:
Familial pancreatic cancer-status quo. Int J Colorectal Dis.
29:139–145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liede A, Karlan BY and Narod SA: Cancer
risks for male carriers of germline mutations in BRCA1 or BRCA2: A
review of the literature. J Clin Oncol. 22:735–742. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen W, Parsons M, Torigian DA, Zhuang H
and Alavi A: Evaluation of thyroid FDG uptake incidentally
identified on FDG-PET/CT imaging. Nucl Med Commun. 30:240–244.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Servente L, Gigirey V, García Fontes M and
Alonso O: Incidental focal colonic uptake in studies
18F-FDG PET/CT. Rev Esp Med Nucl Imagen Mol. 37:15–19.
2018.(In English, Spanish). PubMed/NCBI
|
|
23
|
Ascenti G, Visalli C, Genitori A, Certo A,
Pitrone A and Mazziotti S: Multiple hypervascular pancreatic
metastases from renal cell carcinoma: Dynamic MR and spiral CT in
three cases. Clin Imaging. 28:349–352. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kassabian A, Stein J, Jabbour N, Parsa K,
Skinner D, Parekh D, Cosenza C and Selby R: Renal cell carcinoma
metastatic to the pancreas: A single-institution series and review
of the literature. Urology. 56:211–215. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Crippa S, Angelini C, Mussi C, Bonardi C,
Romano F, Sartori P, Uggeri F and Bovo G: Surgical treatment of
metastatic tumors to the pancreas: A single center experience and
review of the literature. World J Surg. 30:1536–1542. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsitouridis I, Diamantopoulou A,
Michaelides M, Arvanity M and Papaioannou S: Pancreatic metastases:
CT and MRI findings. Diagn Interv Radiol. 16:45–51. 2010.PubMed/NCBI
|