Introduction
Glioma is a common malignant tumor of the central
nervous system and prognosis is extremely poor even after years of
treatment (1). Glioblastoma (GBM) as
the most malignant type of glioma is classified as grade IV
according to the 2016 WHO classification criteria (2). Numerous patients undergo total
resection followed by adjuvant chemoradiotherapy, which is the
current standard treatment for GBM, but the overall survival and
quality of life have been disappointing (3). Currently, GBM has ~12,000 newly
diagnosed cases annually in the United States (4). The Central Brain Tumor Registry of the
United States recently described 1- and 5-year overall survival
rates of 40.2 and 5.6%, respectively for GBM from 2000–2015
(5). Abnormal proliferation of cells
is an important feature of GBM and inhibiting the proliferation of
GBM cells could suppress tumor growth and improve the survival of
patients (6). GBM proliferation is
associated with a number of molecular pathways, including the
neurogenic locus notch homolog protein 1 (Notch1) signaling pathway
(7).
The Notch1 signaling pathway participates in cell
proliferation, metastasis, embryo development and tissue generation
(8). Previous studies have found
that the Notch signaling pathway is abnormally activated in gliomas
(9,10) and that hyperactivation of Notch1 can
promote differentiation and vascularization of malignant glioma
stem cells, thereby accelerating tumor invasion and metastasis
(11). The most common target genes
in the classic notch signaling pathway are members of the HES
family of transcription repressors (12). Hairy enhancer of split 1
(Hes1) and hairy enhancer of split 5 (Hes5)
are important target genes downstream of the Notch1 signaling
pathway, and they have been reported to be associated with GBM
proliferation (13,14). Recently, more research revealed that
the Notch signaling pathway was closely associated with abnormal
proliferation of GBM cells, but the regulatory mechanism behind
this remains unclear (15,16). Hence, an improved understanding of
Notch pathway components, such as the Notch intracellular domain
(NICD) is an important step towards understanding the exact
function of Notch and the development of GBM targeted therapy.
In further studies investigating the Notch1
signaling pathway, a molecule called the Notch intracellular domain
(NICD) was identified that has attracted the attention of
researchers. The intracellular fragment of Notch1 can be cleaved by
γ-secretase to release NICD, the activated form of Notch1 (17). NICD then is transferred into the
nucleus where it can act as a transcriptional activation factor
(18). The expression of NICD in
glioma can be influenced by hypoxia (19). Chronic hypoxia (48 h) can
continuously enhance the expression of hypoxia inducible factor-2 α
which can activate the Notch signaling pathway (20,21). The
upregulation of NICD induces the activation of Notch1, which
promotes GBM invasion and migration by promoting β-catenin and
NF-κB signaling (22). The role of
NICD in the abnormal proliferation of GBM remains unclear.
The aim of the present study was to explore whether
the expression of NICD in GBM is different from that in normal
brain tissue, and to better understand the role and regulatory
mechanism of NICD in GBM. The present study revealed the function
of NICD in GBM proliferation and its role in the Notch1 signaling
pathway which may provide a new molecular tool for the diagnosis
and treatment of GBM.
Materials and methods
Antibodies and reagents
Primary antibodies used were as follows: i)
Anti-NICD [1:1,000 for western blotting and 1:200 for
immunohistochemistry (IHC); cat. no. 2421; Cell Signaling
Technology, Inc.]; ii) anti-Hes5 (1:1,000 for western blotting;
cat. no. sc-13859; Santa Cruz Biotechnology, Inc.); iii)
anti-phosphorylated (p)-Histone H3 (1:1,000 for western blotting;
cat. no. sc-8656; Santa Cruz Biotechnology, Inc.); iv) anti-Histone
H3 (1:1,000 for western blotting; cat. no. GB13102-1; Wuhan
Servicebio Technology, Co., Ltd); v) anti-Notch1 (1:2,000 for WB;
cat. no. ab27526; Abcam); and vi) anti-GAPDH (1:2,000 for western
blotting; cat. no. 5174; Cell Signaling Technology, Inc.).
Secondary antibodies used were as follows: i) Alex Fluor 680/790
(1:10,000 for western blotting; cat. no. ANT091; Antgene); ii)
HRP-labeled (1:100 for IHC; cat. no. GB23303; Wuhan Servicebio
Technology, Co., Ltd). The antibodies used in the BrdU assay were
anti-BrdU (1:200; cat. no. 552598; Becton and Dickinson and
Company) and Alexa Fluor 594 (1:100; cat. no. A11032; Invitrogen;
Thermo Fisher Scientific, Inc.). Compound E (cat. no. AG-CR1-0081;
Adipogen Life Sciences, Inc.) was used as an inhibitor of NICD at
an intervention concentration of 1 µM which was in accordance with
the manufacturer's instructions.
Tissue samples
Human control brain tissues and GBM tissues were
acquired from the Department of Neurosurgery, Renmin Hospital of
Wuhan University (Wuhan, China). GBM tissues were sampled during
resection surgeries and stored at −80°C. A total of 19
pathologically diagnosed GBM samples were enrolled in this study.
All the clinical samples were collected between December 2012 and
September 2014. The mean ages were 47 and 35 years for GBM and
controls, respectively. The average weight of the sample was
generally ~3 g of which 0.5 g was used for western blotting and the
rest was used for immunohistochemistry and other related
experiments. Control brain tissues used in the present study were
normal tissues collected from 11 non-tumor patients during
emergency surgeries of traumatic brain injury. Patients in the
present study had the following inclusion criteria: i) confirmed
histological diagnosis of GBM; and ii) performed surgical
resection. The exclusion criteria for patients were: i) History of
multiple tumors or severe cerebral vascular or cardiovascular
diseases (heart stent surgery or admission to the acute ward due to
cardiovascular disease within the last 6 months), severe vascular
lesions, tuberculosis and extensive acute inflammation (including,
but not limited to acute meningitis and vasculitis) within the last
3 months; and ii) participation in a clinical trial within the last
3 months. The histological diagnosis of glioma was confirmed by the
pathologists of the Department of Pathology, Renmin Hospital of
Wuhan University (Wuhan, China). All tumor samples were subjected
to pathological examination and related molecular testing
[Methylation of O6-methylguanine-DNA methyltransferase (MGMT)],
1p19q, and isotrate dehydrogenase (IDH)1/IDH2], which were all
defined according to the 2016 WHO classification (1). All clinical information for the
patients is presented in Table SI.
The procurement and use of tissue in the present study were
approved by the Institutional Ethics Committee of the Faculty of
Medicine, Renmin Hospital of Wuhan University [approval number,
2012LKSZ (010) H]. Written informed consent for use of tissue was
obtained from all patients in the present study.
Cell culture
U87 (glioblastoma cell line of unknown origin;
CL-0238) was purchased from the Cell Bank of Type Culture
Collection of the Chinese Academy of Sciences. The U87 cell line
was STR authenticated. Cells were cultured in DMEM high glucose
medium (cat. no. GNM12800; Jinuo Biomedical Technology Co., Ltd.)
supplemented with 10% fetal bovine serum (FBS) (cat. no. 04-001-1A;
Biological Industries) and 100 U/ml penicillin and 100 µg/ml
streptomycin. Cells were incubated in humidified air with 5%
CO2 at 37°C.
Plasmid construction and
transfection
A Flag-NICD and Flag-control plasmid was provided by
Dr Chundong Yu (Xiamen University, Xiamen, China) and its
construction has been described in a previous study by Lin et
al (23). An siRNA (siNotch1)
and a non-targeting control siRNA (siCtrl) were purchase from
Thermo Scientific Inc. The sequences used were as follows:
siRNA-Notch1, sense 5′-GCAACCUGCAGUGUAAUAATT-3′ and antisense
5′-UUAUUACACUGCAGGUUGCTT-3′; siRNA-non-targeting control, sense
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense
5′-ACGUGACACGUUCGGAGAATT-3′. U87 cells were seeded in 6-well plates
at a density of about 5×105 cells/well and cultured
overnight at 37°C. Plasmids 2 µg per well were transfected by
Lipofectamine 3000® transfection reagent (Thermo Fisher
Scientific, Inc.), and siRNA 200 pM per well was transfected by
Lipofectamine RNAiMax reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Plasmids and siRNA
were incubated with Lipofectamine 3000® transfection
reagent for 15 min at room temperature before transfection and U87
cells were transfected for 48 h prior to performing subsequent
experiments.
Western blotting
U87 cell samples and brain tissues were lysed on ice
in RIPA buffer (50 mm Tris, 150 mm NaCl, 0.5% EDTA and 0.5% NP-40)
with PMSF protease inhibitors (Beyotime Institute of Biotechnology)
and an inhibitor cocktail (Roche Diagnostics). The concentration of
total proteins was detected using a bicinchoninic acid (BCA) kit
(Biosharp Life Sciences). Protein was loaded and separated by 10%
SDS-PAGE and then transferred onto PVDF membranes (cat. no. 88520;
Thermo Fisher Scientific, Inc.). In total ~20 µg/well of protein
was added. After blocking with 5% skimmed milk for 30 min at room
temperature, membranes were incubated with primary antibodies
overnight and with secondary antibodies for 1 h. The samples were
visualized with a LI-COR Odyssey Infrared Imaging System (LI-COR
Biosciences). GAPDH was used as a loading control.
Immunohistochemical staining
Human brain tissues were fixed in 4%
paraformaldehyde at room temperature for 48 h and embedded in
paraffin and cut into sections. The thickness of sections used for
immunohistochemical staining was 6-µm and these sections were cut
using a paraffin section machine (cat. no. HM340E; Thermo Fisher
Scientific, Inc.). After paraffin sections were deparaffinized at
65°C for 2 h, hydrated with 100/95/75% ethanol for 10 min each,
antigen recovery was performed by exposing sections to 0.01 mol/l
citrate buffer (pH 6.0) at 100°C for 20 min. Endogenous peroxidase
was removed by treatment with 3% H2O2.
Samples were blocked with 1% BSA (Amresco, LLC) at room temperature
for 1 h and were then incubated with primary antibodies at 37°C
overnight. Following 3 washes with PBS, the sections were incubated
with secondary antibody for 1 h at room temperature. DAB (Wuhan
Servicebio Technology, Co., Ltd.) was used for dyeing and the
nuclei were stained by hematoxylin at room temperature for 30 sec.
Images were captured with an Olympus BX51 fluorescence microscope
(Olympus Corporation).
Reverse-transcription quantitative
(RT-q) PCR
Brain tissues were ground with a Hybrid Mill (cat.
no. MM400; RETSCH GmbH) for RNA extraction. RNA was isolated using
an RNA isolation kit (Takara Bio, Inc.) and cDNA was synthesized
from 1 µg of total RNA using a PrimeScript™ RT reagent kit (Takara
Bio, Inc.) at 37°C for 5 min, 42°C for 30 min and then 85°C for 5
sec. RT-qPCR experiments were performed using a
SYBR®Premix Ex Taq™ Kit (Takara Bio, Inc.) in a CFX
Connect system (Bio-Rad Laboratories, Inc.). The RT-PCR
thermocycling conditions were as follows: 95°C for 30 sec for
initial denaturation, 95°C for 5 sec followed by 40 cycles of 60°C
for 40 sec for PCR reaction stage and the dissociation was set by
CFX Connect system by default. Gene expression levels were
determined by the 2−ΔΔCq threshold cycle method
(24). The forward and reverse PCR
primer sequences were as follows: Hes5, forward,
5′GGAATTCCAATGGCCCCCAGCACTGTG-3′ and reverse,
5′-GGGTACCCCACGGCCACAGTGCTGG-3′ and GAPDH, forward,
5′-GAGTCAACGGATTTGGTCGT-3′ and reverse, 5′-TTGATTTTGGAGGGATCTCG-3′.
Normalizations across samples were performed using the geometric
average of the constitutive gene expression of GAPDH.
BrdU assay
Cell samples were treated with BrdU reagent (cat.
no. 00-0103; Invitrogen; Thermo Fisher Scientific, Inc.) for 30 min
at 37°C. Prior to harvesting the cells, 200 µl of 1X BrdU/well was
added. Next, the cells were fixed in 4% paraformaldehyde for 10 min
at room temperature, lysed by treatment with Triton X-100 for 5 min
at room temperature and blocked in BSA for 1 h at room temperature.
The sample was incubated with a primary antibody (anti-BrdU) at 4°C
overnight and a secondary antibody (Alexa Fluor 594) at room
temperature for 1 h. Nuclei were stained with
diamidino-phenyl-indole (DAPI) (cat. no. ANT046; Wuhan Antejie
Biotechnology Co., Ltd.). Fluorescence images of BrdU were
visualized with an Olympus BX51 microscope (Olympus Corporation),
which is a confocal microscope. The number of BrdU-positive cells
was quantified using ImageJ software (v.1.48; National Institutes
of Health).
Statistical analysis
Statistical analyses performed using SPSS 20.0
software (IBM Corp.). The data are presented as the mean ± standard
deviation of three independent repeats. Differences between two
groups were analyzed using an unpaired Student's t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
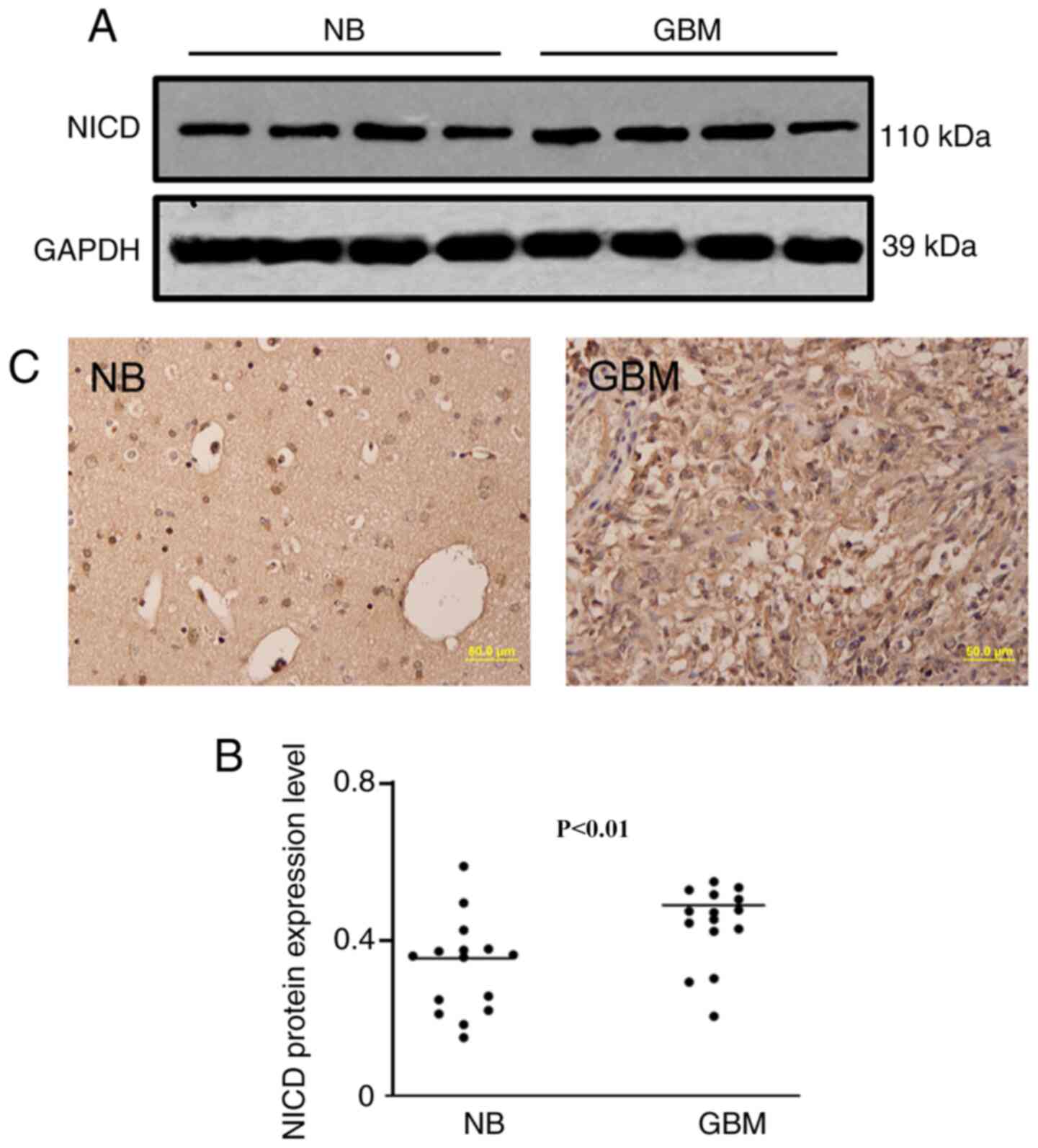
NICD is upregulated in human GBM
tissues compared with normal brain tissues
First, to explore the clinical value of NICD, its
expression level was detected in 11 human normal brain samples and
19 human GBM samples. Western blotting demonstrated that the NICD
protein level was significantly increased in human GBM tissues
compared with normal brain tissues (Fig.
1A and B). Immunohistochemical staining also demonstrated an
increased expression level of NICD in GBM compared with normal
brain tissue (Fig. 1C). Both results
demonstrated an obvious increase in expression of NICD in human GBM
compared with healthy brain tissue.
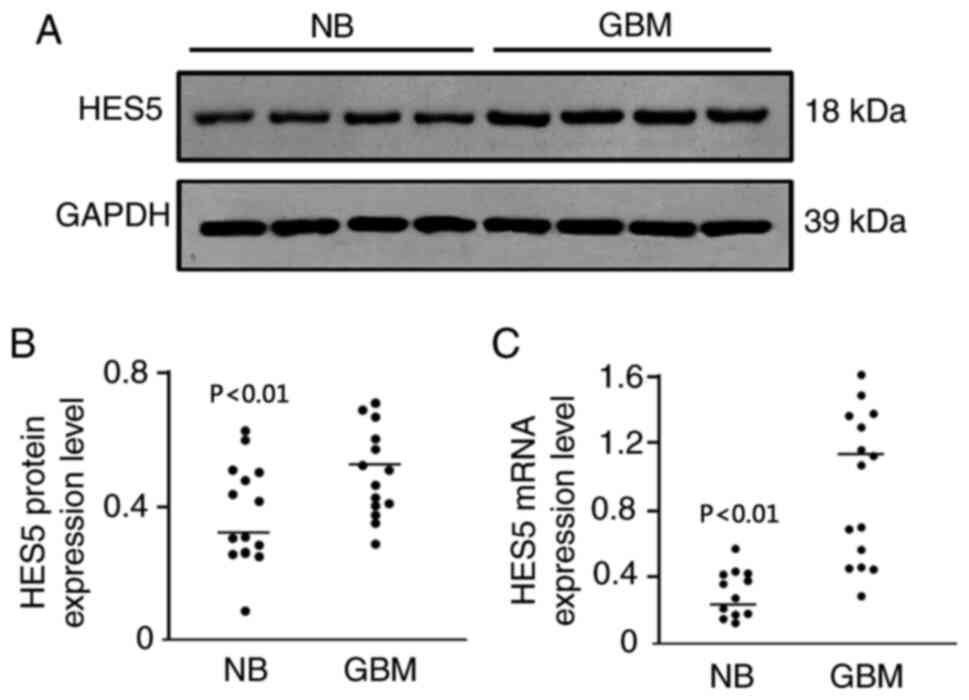
The Notch1 signaling pathway is
activated in GBM tissues
To investigate the activation of the Notch1
signaling pathway in GBM, the expression of Hes5 was investigated
as it is an important target gene of Notch1 signaling (25). The findings demonstrated that Hes5
protein levels were significantly upregulated in human GBM tissues
compared with normal brain tissues (Fig.
2A and B). In addition, Hes5 mRNA expression was also increased
in human GBM tissues compared with normal brain tissues (Fig. 2C). Both results indicated that the
Notch1 signaling pathway was activated in human GBM.
NICD promotes the proliferation of U87
cells
NICD protein was overexpressed by transfection of
U87 cells with a Flag-NICD plasmid (Fig.
3A) and the change in cellular proliferation induced by NICD
was assessed. The level of histone H3 phosphorylation reflects cell
proliferation (26). An increase in
the phosphorylation level of histone H3 in the NICD group compared
to the control group was observed by western blotting (Fig. 3A). Compared with the control group,
the group overexpressing NICD exhibited a significant increase in
the number of U87 cells as seen in the proliferation curve
(Fig. 3B), and BrdU assays
demonstrated that the proportion of BrdU-positive U87 cells
increased (Fig. 3C and D). These
data demonstrated that NICD induced the proliferation of U87 cells
in vitro.
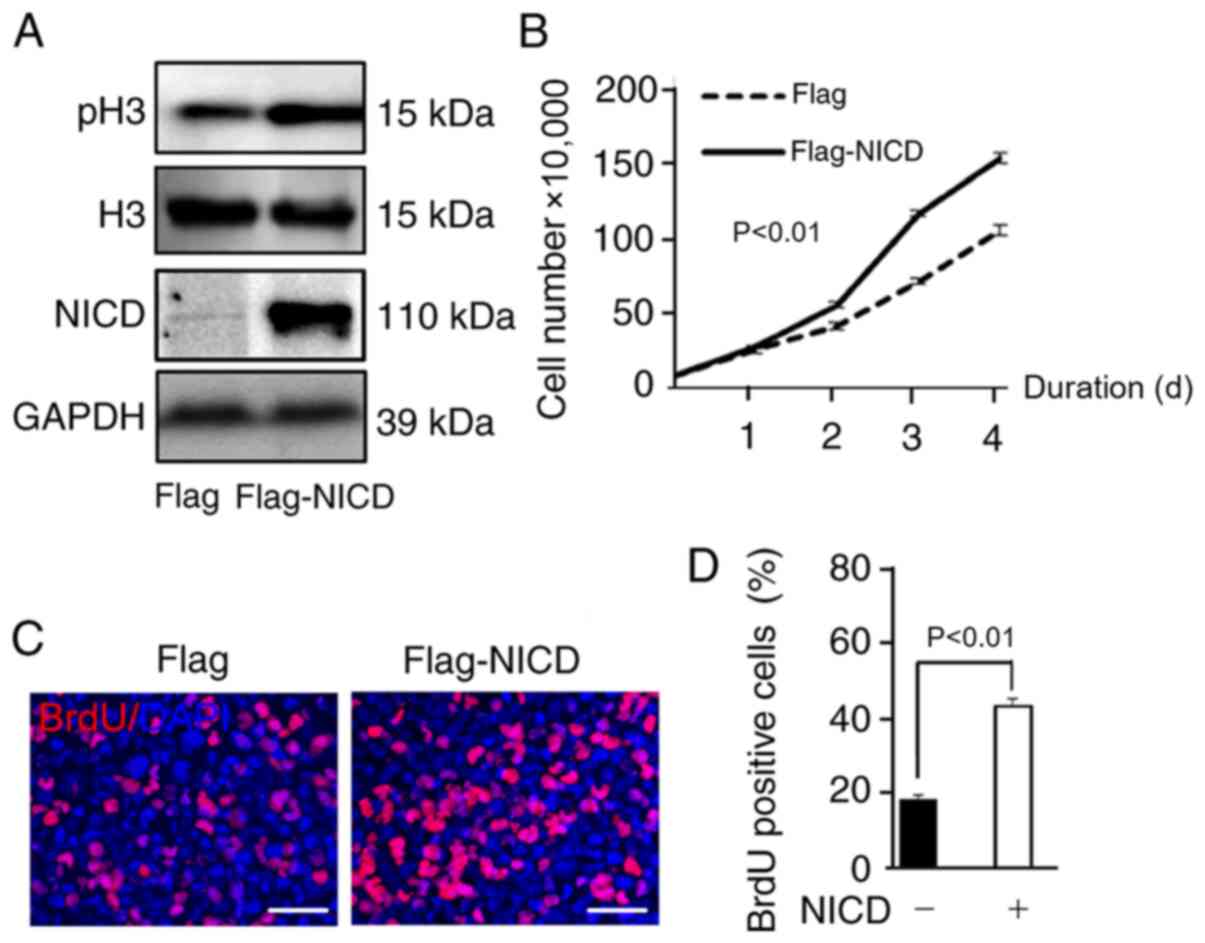 | Figure 3.NICD promotes the proliferation of
glioblastoma cells. (A) NICD was upregulated by the transfection of
Flag-NICD plasmid and overexpressed NICD increased the protein
level of pH3. (B) Cell growth was promoted in NICD group compared
with control group as can be seen from the proliferation curve
(P<0.01), and the data of each time point in panel B represent
the average of three measurements (P1=0.0082; P2<0.0001;
P3<0.0001; and P4=0.0003. p1, p2, p3, p4 represent the p-value
of the time points of the first, second, third and fourth day,
respectively). (C and D) BrdU assay demonstrated that the
proportion of BrdU-positive cells was increased in NICD group
compared with control group (P<0.01). Scale bar, 50 µm. NICD,
Notch intracellular domain; pH3, phosphohistone-H3. |
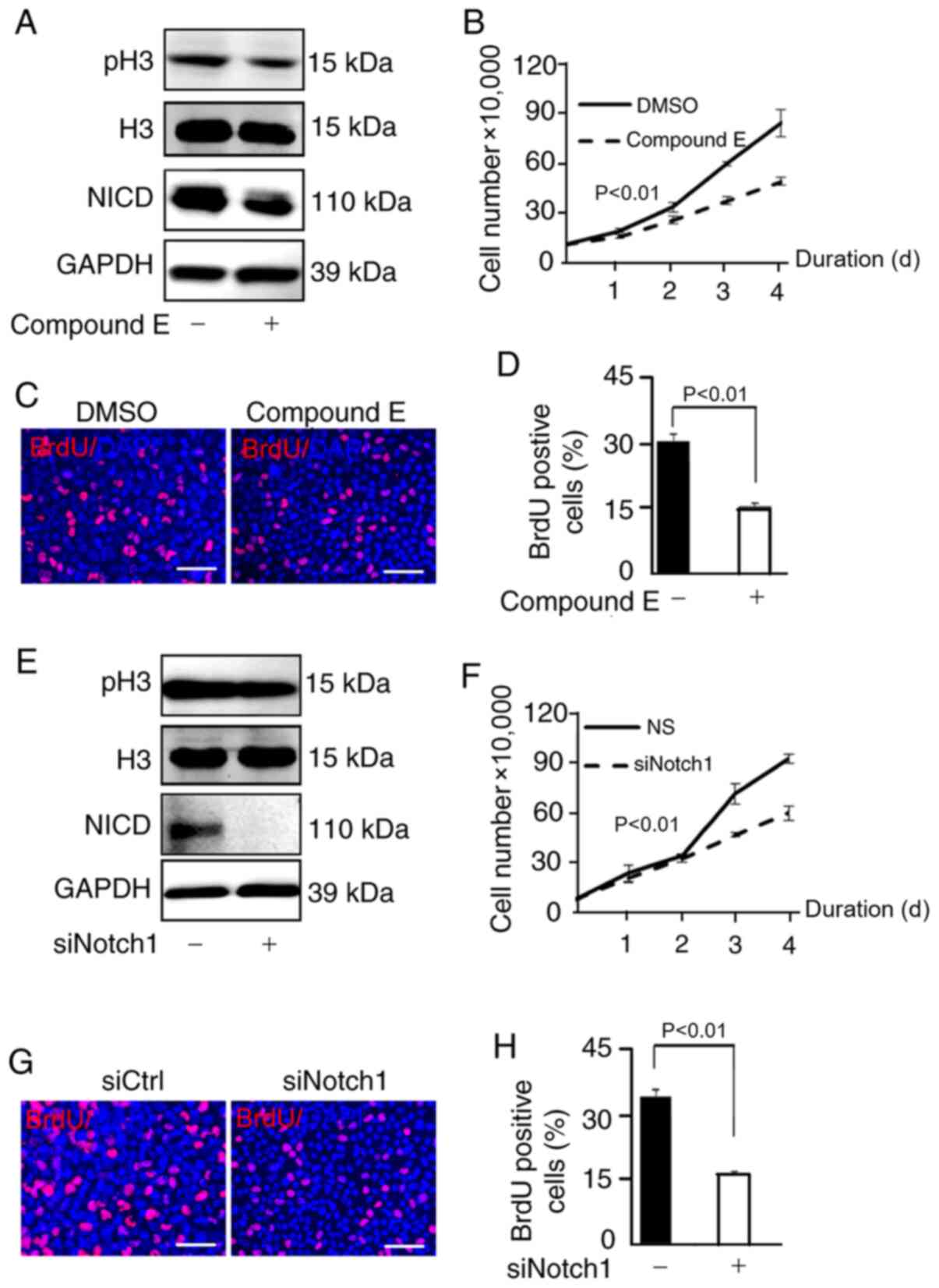
Downregulation of NICD suppresses the
proliferation of U87 cells
To further confirm the relationship between NICD and
U87 cell proliferation, U87 cells were observed when NICD was
downregulated. Compound E is an inhibitor of γ-secretase and thus
has an inhibitory effect on NICD (27). The expression of NICD and the level
of H3 phosphorylation was clearly downregulated after cells were
treated with compound E (Fig. 4A).
Consequently, the number of U87 cells was significantly decreased
compared with the control group (DMSO) as shown in the
proliferation curve (Fig. 4B), and
the proportion of BrdU-positive U87 cells was significantly reduced
compared with the control group (DMSO) in the BrdU assay (Fig. 4C and D). In addition, Brdu postive
cells in the control group (NICD-) (Fig.
3D) were less compared with the control group (compound E-)
(Fig. 4D) as vector transfection in
the NICD- group had a certain inhibiting effect on cells while 3 µm
DMSO treatment in the compound E- group had almost no toxic effect.
In addition, w U87 cells were transfected with siNotch1 to suppress
the activity of the Notch1 signaling pathway; the results
demonstrated that the NICD level and the level of H3
phosphorylation were reduced compared with the control group
(siNotch1-) (Fig. 4E) and the
proliferation of U87 cells was inhibited as well compared with the
control group (siCtrl) (Fig. 4F-H).
In conclusion, NICD was inhibited by compound E and knockdown of
Notch1 also reduced the expression of NICD and suppressed the
proliferation of U87 cells.
Discussion
The Notch1 signaling pathway has been reported to
associate with various cancers, such as lung cancer and gastric
cancer and participate in several neoplastic biological behaviors
including drug resistance and apoptosis (28,29).
Although some studies have demonstrated that Notch1 is related to
the abnormal proliferation of GBM cells (30–33), the
specific regulatory mechanism of this remains unclear. As
significant effectors downstream of the Notch1 signaling pathway,
Hes1 and Hes5 have recently received increasing attention (25). Hes1 is expressed at low levels in the
human brain, and several studies have detected uninduced Hes1
expression in GBM tissues (34,35). In
comparison, the Hes5 expression level was relatively high (34), however, the expression of Hes5 in
human GBM is still controversial and remains to be solved. Hence,
the present study investigated the level of Hes5 fixed GBM samples.
Interestingly, the expression of Hes5 was higher at both the
protein and mRNA levels in the GBM samples compared with normal
control brain samples, indicating an activated state of the Notch1
signaling pathway in GBM tissues which has not been previously
reported to the best of our knowledge. According to the existing
literature, HES5 serves an important role in the regulation of
mammalian neuronal differentiation and the maintenance of neural
stem cells and can actively regulate the self-renewal of neuronal
stem cells (13). Based on the
findings of the present study, it may be speculated that HES5 in
GBM may have a significant impact on the occurrence and development
of GBM.
NICD is the activated form of Notch1 and it serves
an important role in the Notch1 signaling pathway (36). Notably, most studies of NICD explored
is effect on cell differentiation (19), invasion (22) and apoptosis in glioma (37). It is generally accepted that the
growth, metastasis, and invasion of tumors are inseparable from the
proliferation of tumor cells (38),
however, it was still unclear whether NICD could regulate cell
proliferation in GBM. Hence, the present study explored the
connection between NICD and proliferation to fill in the gaps in
the knowledge regarding GBM.
In the present study, the expression of NICD in
human GBM tissues which has not been thoroughly explored in
previous research was explored and the findings demonstrated an
increase in NICD protein level in GBM tissues compared with normal
brain tissues. To determine whether NICD could regulate cell
proliferation in GBM, NICD was overexpressed in vitro using
the plasmid Flag-NICD. Phosphorylated histone H3 levels, a key
marker of cell proliferation have been demonstrated to be
upregulated by induced cell growth (39). The findings of the present study
showed demonstrated that phosphorylated histone H3 levels were
increased and cell growth was promoted following the overexpression
of NICD. In conclusion, in the present study NICD was activated in
GBM tissues and promoted GBM proliferation in vitro.
In addition, to explore whether NICD regulates cell
proliferation through the Notch1 signaling pathway, compound E and
siNotch1 were used to suppress the activation of NICD and Notch1,
respectively. Compound E, a selective inhibitor of γ-secretase that
has the ability to suppress the expression of NICD (40) was observed to decrease NICD protein
levels and phosphorylated histone H3 levels as well as to repress
the growth of U87 cells in the present study. In addition, it has
been reported that overexpression of NICD in PC12 cells can promote
cell apoptosis and inhibit cell proliferation by regulating S phase
arrest (41). Similar results were
obtained in the present study where NICD was inhibited and cell
proliferation suppressed when Notch1 was knocked down by siNotch1.
Hence, in the present study a reduced level of NICD suppressed the
proliferation of GBM cells and NICD regulated proliferation through
the Notch1 signaling pathway.
GBM is still the most malignant type of glioma, and
it exhibits abnormal proliferation and a high mortality rate
according to the statistical analysis of glioma cases in US between
2010 and 2014 (4). Early diagnosis
and precision treatments have great significance for providing an
improved prognosis for patients with GBM (42). The findings of the present study
emphasized the significance of NICD in the Notch1 signaling pathway
regulation of the proliferation of GBM. Hence, NICD may be a
potential diagnostic marker and therapeutic target for GBM.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BL and QC designed the research. YW and QS performed
the experiments. RG, HL, FY and YX analyzed the data and wrote the
manuscript. YQ and HJ participated in data analysis and revised the
article for important intellectual content. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
All tissue specimens used in this study were
approved by the Institutional Ethics Committee of the Faculty of
Medicine, Renmin Hospital of Wuhan University (approval no.
2012LKSZ (010) H) (Wuhan, China). Written informed consent was
obtained from all the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reni M, Mazza E, Zanon S, Gatta G and
Vecht CJ: Central nervous system gliomas. Crit Rev Oncol Hematol.
113:213–234. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Le Rhun E, Rhun EL, Taillibert S and
Chamberlain MC: The future of high-grade glioma: Where we are and
where are we going. Surg Neurol Int. 6 (Suppl 1):S9–S44. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ostrom QT, Gittleman H, Liao P,
Vecchione-Koval T, Wolinsky Y, Kruchko C and Barnholtz-Sloan JS:
CBTRUS Statistical Report: Primary brain and other central nervous
system tumors diagnosed in the United States in 2010–2014. Neuro
Oncol. 19 (Suppl_5):v1–v88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ostrom QT, Gittleman H, Truitt G, Boscia
A, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical report:
Primary brain and other central nervous system tumors diagnosed in
the United States in 2011–2015. Neuro Oncol. 20 (Suppl_4):iv1–iv86.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han D, Yu T, Dong N, Wang B, Sun F and
Jiang D: Napabucasin, a novel STAT3 inhibitor suppresses
proliferation, invasion and stemness of glioblastoma cells. J Exp
Clin Cancer Res. 38:2892019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ji Y, Sun Q, Zhang J and Hu H: MiR-615
inhibits cell proliferation, migration and invasion by targeting
EGFR in human glioblastoma. Biochem Biophys Res Commun.
499:719–726. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gil-García B and Baladrón V: The complex
role of NOTCH receptors and their ligands in the development of
hepatoblastoma, cholangiocarcinoma and hepatocellular carcinoma.
Biol Cell. 108:29–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stockhausen MT, Kristoffersen K and
Poulsen HS: The functional role of Notch signaling in human
gliomas. Neuro Oncol. 12:199–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lino MM, Merlo A and Boulay JL: Notch
signaling in glioblastoma: A developmental drug target? BMC Med.
8:722010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guichet PO, Guelfi S, Teigell M, Hoppe L,
Bakalara N, Bauchet L, Duffau H, Lamszus K, Rothhut B and Hugnot
JP: Notch1 stimulation induces a vascularization switch with
pericyte-like cell differentiation of glioblastoma stem cells. Stem
Cells. 33:21–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aster JC, Pear WS and Blacklow SC: The
varied roles of Notch in cancer. Annu Rev Pathol. 12:245–275. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohtsuka T, Ishibashi M, Gradwohl G,
Nakanishi S, Guillemot F and Kageyama R: Hes1 and Hes5 as notch
effectors in mammalian neuronal differentiation. EMBO J.
18:2196–2207. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang YM, Chen SX, Dai QF, Jiang ST, Chen
AL, Tang CZ and Zhang YQ: Effect of acupuncture on the notch
signaling pathway in rats with brain injury. Chin J Integr Med.
24:537–544. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Purow BW, Haque RM, Noel MW, Su Q, Burdick
MJ, Lee J, Sundaresan T, Pastorino S, Park JK, Mikolaenko I, et al:
Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1,
is critical for glioma cell survival and proliferation. Cancer Res.
65:2353–2363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu GW, Wu L, Kuang W, Chen Y, Zhu XG, Guo
H and Lang HL: Knockdown of linc-OIP5 inhibits proliferation and
migration of glioma cells through down-regulation of YAP-NOTCH
signaling pathway. Gene. 610:24–31. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu S, Chen Q, Lin T, Hong W, Wu W, Wu M,
Du X and Jin R: The function of Notch1 intracellular domain in the
differentiation of gastric cancer. Oncol Lett. 15:6171–6178.
2018.PubMed/NCBI
|
|
18
|
Tamura K, Taniguchi Y, Minoguchi S, Sakai
T, Tun T, Furukawa T and Honjo T: Physical interaction between a
novel domain of the receptor Notch and the transcription factor
RBP-J kappa/Su(H). Curr Biol. 5:1416–1423. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu YY, Fu LA, Li SZ, Chen Y, Li JC, Han J,
Liang L, Li L, Ji CC, Zheng MH and Han H: Hif-1α and Hif-2α
differentially regulate Notch signaling through competitive
interaction with the intracellular domain of Notch receptors in
glioma stem cells. Cancer Lett. 349:67–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Renfrow JJ, Soike MH, Debinski W,
Ramkissoon SH, Mott RT, Frenkel MB, Sarkaria JN, Lesser GJ and
Strowd RE: Hypoxia-inducible factor 2α: A novel target in gliomas.
Future Med Chem. 10:2227–2236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patel SA and Simon MC: Biology of
hypoxia-inducible factor-2alpha in development and disease. Cell
Death Differ. 15:628–634. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang X, Chen T, Zhang J, Mao Q, Li S,
Xiong W, Qiu Y, Xie Q and Ge J: Notch1 promotes glioma cell
migration and invasion by stimulating β-catenin and NF-κB signaling
via AKT activation. Cancer Sci. 103:181–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin X, Liu B, Yang X, Yue X, Diao L, Wang
J and Chang J: Genetic deletion of Rnd3 results in aqueductal
stenosis leading to hydrocephalus through up-regulation of Notch
signaling. Proc Natl Acad Sci USA. 110:8236–8241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ronchi CL, Sbiera S, Altieri B, Steinhauer
S, Wild V, Bekteshi M, Kroiss M, Fassnacht M and Allolio B: Notch1
pathway in adrenocortical carcinomas: Correlations with clinical
outcome. Endocr Relat Cancer. 22:531–543. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Elmaci İ, Altinoz MA, Sari R and Bolukbasi
FH: Phosphorylated histone H3 (PHH3) as a novel cell proliferation
marker and prognosticator for meningeal tumors: A short review.
Appl Immunohistochem Mol Morphol. 26:627–631. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang T, Arslanova D, Gu Y, Augelli-Szafran
C and Xia W: Quantification of gamma-secretase modulation
differentiates inhibitor compound selectivity between two
substrates Notch and amyloid precursor protein. Mol Brain.
1:152008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jun HT, Stevens J and Kaplan-Lefko P: Top
NOTCH targets: Notch signaling in cancer. Drug Development
Research. 69:319–328. 2010. View Article : Google Scholar
|
|
29
|
Pancewicz-Wojtkiewicz J: Epidermal growth
factor receptor and notch signaling in non-small-cell lung cancer.
Cancer Med. 5:3572–3578. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feng HB, Wang J, Jiang HR, Mei X, Zhao YY,
Chen FR, Qu Y, Sai K, Guo CC, Yang QY, et al: β-elemene selectively
inhibits the proliferation of glioma stem-like cells through the
downregulation of Notch1. Stem Cells Transl Med. 6:830–839. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang BQ, Yang B, Yang HC, Wang JY, Hu S,
Gao YS and Bu XY: MicroRNA-499a decelerates glioma cell
proliferation while accelerating apoptosis through the suppression
of Notch1 and the MAPK signaling pathway. Brain Res Bull.
142:96–106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hai L, Zhang C, Li T, Zhou X, Liu B, Li S,
Zhu M, Lin Y, Yu S, Zhang K, et al: Notch1 is a prognostic factor
that is distinctly activated in the classical and proneural subtype
of glioblastoma and that promotes glioma cell survival via the
NF-κB(p65) pathway. Cell Death Dis. 9:1582018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Q, Zheng JM, Chen JK, Yan XL, Chen HM,
Nong WX and Huang HQ: Histone deacetylase 5 promotes the
proliferation of glioma cells by upregulation of Notch 1. Mol Med
Rep. 10:2045–2050. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheung HC, Corley LJ, Fuller GN,
McCutcheon IE and Cote GJ: Polypyrimidine tract binding protein and
Notch1 are independently re-expressed in glioma. Mod Pathol.
19:1034–1041. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Saito N, Fu J, Zheng S, Yao J, Wang S, Liu
DD, Yuan Y, Sulman EP, Lang FF, Colman H, et al: A high Notch
pathway activation predicts response to γ secretase inhibitors in
proneural subtype of glioma tumor-initiating cells. Stem Cells.
32:301–312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu B, Lin X, Yang X, Dong H, Yue X,
Andrade KC, Guo Z, Yang J, Wu L, Zhu X, et al: Downregulation of
RND3/RhoE in glioblastoma patients promotes tumorigenesis through
augmentation of notch transcriptional complex activity. Cancer Med.
4:1404–1416. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Wang H, Ge H and Yang Z: AG-1031
induced autophagic cell death and apoptosis in C6 glioma cells
associated with Notch-1 signaling pathway. J Cell Biochem.
119:5893–5903. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xie Q, Mittal S and Berens ME: Targeting
adaptive glioblastoma: An overview of proliferation and invasion.
Neuro Oncol. 16:1575–1584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Villani V, Mahadevan KK, Ligorio M,
Fernández-Del Castillo C, Ting DT, Sabbatino F, Zhang I, Vangel M,
Ferrone S, Warshaw AL, et al: Phosphorylated histone H3 (PHH3) is a
superior proliferation marker for prognosis of pancreatic
neuroendocrine tumors. Ann Surg Oncol. 23:609–617. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Arumugam TV, Cheng YL, Choi Y, Choi YH,
Yang S, Yun YK, Park JS, Yang DK, Thundyil J, Gelderblom M, et al:
Evidence that gamma-secretase-mediated Notch signaling induces
neuronal cell death via the nuclear factor-kappaB-Bcl-2-interacting
mediator of cell death pathway in ischemic stroke. Mol Pharmacol.
80:23–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li B, Duan P, Han X, Yan W and Xing Y:
NICD inhibits cell proliferation and promotes apoptosis and
autophagy in PC12 cells. Mol Med Rep. 16:2755–2760. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Alexander BM and Cloughesy TF: Adult
glioblastoma. J Clin Oncol. 35:2402–2409. 2017. View Article : Google Scholar : PubMed/NCBI
|