Introduction
Colorectal cancer (CRC) is one of the most common
types of malignant cancer of the gastrointestinal tract and was
ranked as the fourth leading cause of cancer-associated deaths
worldwide in 2019 (1,2). Liver metastasis often develops in
patients with CRC. Only 15–20% of patients with CRC with liver
metastases are considered resectable, resulting in low survival
rates (3). In China, CRC is the
fourth most common type of cancer and fifth leading cause of
cancer-related deaths, and a calculated 370,000 newly-diagnosed
patients and 191,000 deaths occurred in 2015 (4). Moreover, the incidence and mortality
rates have continuously increased over the last decades (1). Therefore, elucidating the molecular
mechanisms underlying the progression and metastasis of CRC and
identifying new biomarkers for the treatment of CRC is
essential.
In mammals, the Hippo signaling pathway is involved
in tissue repair, cell regeneration, proliferation and apoptosis.
Impaired Hippo signaling contributes to the development and
progression of cancer, including CRC (5–8).
Yes-associated protein (YAP) is a major downstream effector in the
Hippo signaling pathway (5). Through
a kinase cascade, the Hippo signaling pathway results in the
phosphorylation of YAP and inactivates it by cytoplasmic retention
or proteasomal degradation (5). This
prevents YAP translocation to the nucleus, where it normally
functions as a transcriptional co-activator by interacting with TEA
domain family members 1–4 and other transcription factors (5). The YAP gene has been proposed as
a candidate oncogene, which may be closely associated with the
initiation and progression of several types of cancer, including
pancreatic cancer (9), breast cancer
(10), hepatocellular carcinoma
(11), esophageal cancer (12), cervical cancer (13), ovarian cancer (14) and gastric cancer (15,16).
Recently, several studies have focused on the role of YAP in CRC.
These studies demonstrated that YAP was overexpressed in CRC
tissue, and promoted the proliferation, migration and
epithelial-to-mesenchymal transition (EMT) of CRC cells (17–20).
However, the underlying mechanism remains unclear and requires
further study.
In the present study, the mRNA and protein
expression levels of YAP in human CRC tissue and adjacent normal
tissue were examined using public datasets and western blot
analysis, and their potential role in the proliferation and
migration of CRC cells was investigated in vitro. Moreover,
the correlation of YAP with EMT-related genes was studied and
confirmed using western blot analysis. In vitro studies
further illustrated that YAP functioned as a regulator of glucose
transporter 3 (Glut3)/AMP-activated protein kinase (AMPK) in order
to mediate the proliferation and migration of CRC cells. Thus, YAP
may serve as a candidate for the development of new strategies for
CRC treatment.
Materials and methods
Clinical data and CRC tissue
samples
Clinical data were obtained from public databases,
including Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/gds/; accession nos.
GSE74602 and GSE44076), Oncomine [Kaiser (21), Hong (22) and Sabates-Bellver (23) datasets; https://www.oncomine.org/], Human Protein Atlas
(http://www.proteinatlas.org/), Gene
Expressing Profiling Interactive Analysis [GEPIA; colon
adenocarcinoma (COAD) and rectum adenocarcinoma (READ); http://gepia.cancer-pku.cn/].
Additionally, 18 pairs of colorectal adenocarcinoma
and adjacent normal mucosal tissue samples (5 cm from tumor edge)
were collected from patients who had not received radiotherapy and
chemotherapy prior to surgery between January 2016 and January 2017
at the Department of General Surgery, The First Affiliated Hospital
of Soochow University (Suzhou, China). The clinical specimens were
histologically confirmed in the pathology department. The
clinicopathological features of these patients are shown in
Table I. Ethics approval was granted
by The Ethics Committee of The First Affiliated Hospital of Soochow
University. In addition, the study was conducted in compliance with
the Declaration of Helsinki. Written informed consent was obtained
from all patients.
 | Table I.Association between YAP expression
and clinicopathological parameters in patients with colorectal
cancer. |
Table I.
Association between YAP expression
and clinicopathological parameters in patients with colorectal
cancer.
|
|
| YAP expression, n
(%) |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
parameters | Total, n (%) | Low, n=9 | High, n=9 | P-value |
|---|
| Age, years |
|
|
| 0.319 |
|
<65 | 8 (44.4) | 3 (33.3) | 5 (55.6) |
|
|
≥65 | 10 (55.6) | 6 (66.7) | 4 (44.4) |
|
| Sex |
|
|
| 0.500 |
|
Male | 13 (72.2) | 6 (66.7) | 7 (77.8) |
|
|
Female | 5 (27.8) | 3 (33.3) | 2 (22.2) |
|
| Tumor size, cm |
|
|
| 0.500 |
|
<5 | 9 (50.0) | 5 (55.6) | 4 (44.4) |
|
| ≥5 | 9 (50.0) | 4 (44.4) | 5 (55.6) |
|
| Lymph node
metastasis |
|
|
| 0.167 |
| No | 11 (61.1) | 7 (77.8) | 4 (44.4) |
|
|
Yes | 7 (38.9) | 2 (22.2) | 5 (55.6) |
|
| TNM stage |
|
|
| 0.167 |
|
I/II | 11 (61.1) | 7 (77.8) | 4 (44.4) |
|
|
III/IV | 7 (38.9) | 2 (22.2) | 5 (55.6) |
|
Cell lines
The human CRC cell lines (HCT116, CCL244, CaCo2,
SW480, LoVo and RKO) were purchased from the Cell Bank of Type
Culture Collection of Chinese Academy of Sciences. The cell
cultures were routinely maintained in RPMI-1640 medium (HyClone;
Cytiva) or DMEM (Hyclone; Cytiva) containing 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin G sodium and 100
µg/ml streptomycin sulfate (Gibco; Thermo Fisher Scientific, Inc.)
at 37°C in a humidified atmosphere containing 5%
CO2.
Protein extraction and western
blotting
CRC tissues or cells were lysed in RIPA lysis buffer
(Sigma-Aldrich; Merck KGaA). Protein concentration was quantified
using a BCA Protein Assay kit (Pierce; Thermo Fisher Scientific,
Inc.). Following protein separation (10 µg/lane) via 8% SDS-PAGE,
proteins were transferred onto polyvinylidene difluoride membranes
(Bio-Rad Laboratories, Inc.) and were then blocked in 5% skimmed
milk for 1 h at room temperature. Subsequently, the membranes were
incubated with primary antibodies overnight at 4°C and then with
HRP-conjugated secondary antibodies (1:5,000; cat. nos. SA00001-2
or SA00001-1; ProteinTech Group, Inc.) for 1 h at room temperature.
Next, the proteins were visualized using a chemiluminescence kit
(EMD Millipore) and signals were quantified using ImageJ software
(v1.46; National Institutes of Health). The antibodies used in this
study included anti-YAP (1:1,000; cat. no. 14074; Cell Signaling
Technology, Inc.), anti-E-cadherin (1:1,000; cat. no. 3195; Cell
Signaling Technology, Inc.), anti-vimentin (1:1,000; cat. no. 5741;
Cell Signaling Technology, Inc.), anti-Glut3 (1:1,000; cat. no.
20403-1-AP; ProteinTech Group, Inc.), anti-phosphorylated (p)-AMPK
Thr172 (1:1,000; cat. no. 2535; Cell Signaling Technology, Inc.),
anti-AMPK (1:1,000; cat. no. 2532; Cell Signaling Technology, Inc.)
and anti-GAPDH (1:5,000; cat. no. AG019; Beyotime Institute of
Biotechnology).
Plasmid and small interfering RNA
(siRNA) transfection
The siRNA against YAP (specific target sequence,
5′-GGUCAGAGAUACUUCUUAAAU-3′), siRNA against Glut3 (specific target
sequence, 5′-UAGCCAAAUUGGAAAGAGCTT-3′) and their scrambled siRNA
(target sequence, 5′-UUCUCCGAACGUGUCACGUTT-3′) were synthesized by
Shanghai GenePharma Co., Ltd. SW480 and HCT116 cells were
transfected with the aforementioned siRNAs at a final concentration
of 20 nM using Lipofectamine™ RNAiMax (Invitrogen; Thermo Fisher
Scientific, Inc.) at 4°C according to the manufacturer's
protocol. After 48–72 h of transfection, the cells were used for
subsequent experiments. Plasmids encoding the human YAP protein and
the empty pcDNA3.1 vector were purchased from Shanghai GenePharma
Co., Ltd. Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to transfect HCT116 cells according to
the manufacturer's instructions and as described previously
(16). After 48–72 h of cell
transfection, the cells were used for subsequent experiments.
Colony formation assay
In brief, ~1×103 transfected SW480 or
HCT116 cells were seeded in 6-well plates and cultured for 7–10
days, then fixed with 4% paraformaldehyde (Beyotime Institute of
Biotechnology) at room temperature for 30 min and stained with 0.1%
crystal violet (Beyotime Institute of Biotechnology) at room
temperature for 15 min. The number of colonies containing >100
cells was determined using a light microscope (magnification, ×40;
Nikon Corporation).
Cell migration assay
Cell migration was assessed using Transwell™
chambers (pore size, 8.0 µm; Corning, Inc.). Briefly, 200 µl
serum-free DMEM containing 5×104 transfected SW480 or
HCT116 cells was seeded onto the filters in 24-well chambers and
750 µl medium containing 10% FBS was placed in the lower chambers
as a chemoattractant. After 24 h at 4°C, the filters were fixed
with 4% paraformaldehyde (Beyotime Institute of Biotechnology) at
room temperature for 30 min and stained with 0.1% crystal violet
solution (Beyotime Institute of Biotechnology) at room temperature
for 15 min. Stained cells were visualized in three randomly
selected fields using a light microscope (magnification, ×200). The
detailed procedure has been described previously (24).
ATP measurement
Relative ATP levels were measured using an enhanced
ATP assay kit (cat. no. S0027; Beyotime Institute of Biotechnology)
according to the manufacturer's instructions. Total ATP levels were
calculated from the luminescence signals and were normalized by the
protein concentrations.
Statistical analysis
Continuous data are presented as the mean ± SEM,
whereas categorical variables are presented as numbers and
frequencies. Two-tailed paired or unpaired Student's t-tests were
used for the comparison of two groups. One-way ANOVA followed by
Tukey's post hoc test were used for multi-group comparisons.
Fisher's exact test was used to analyze the relationships between
YAP expression and clinicopathological characteristics. Spearman's
correlation was used to analyze the association between the
expression levels of two genes. P<0.05 was considered to
indicate a statistically significant difference.
Results
YAP is upregulated in human CRC tissue
samples
In order to determine the function of YAP in CRC,
its mRNA expression was analyzed using public GEO datasets. The
results showed that YAP mRNA expression levels were
significantly upregulated in human CRC tumor samples compared with
in adjacent normal tissue samples (Fig.
1A).
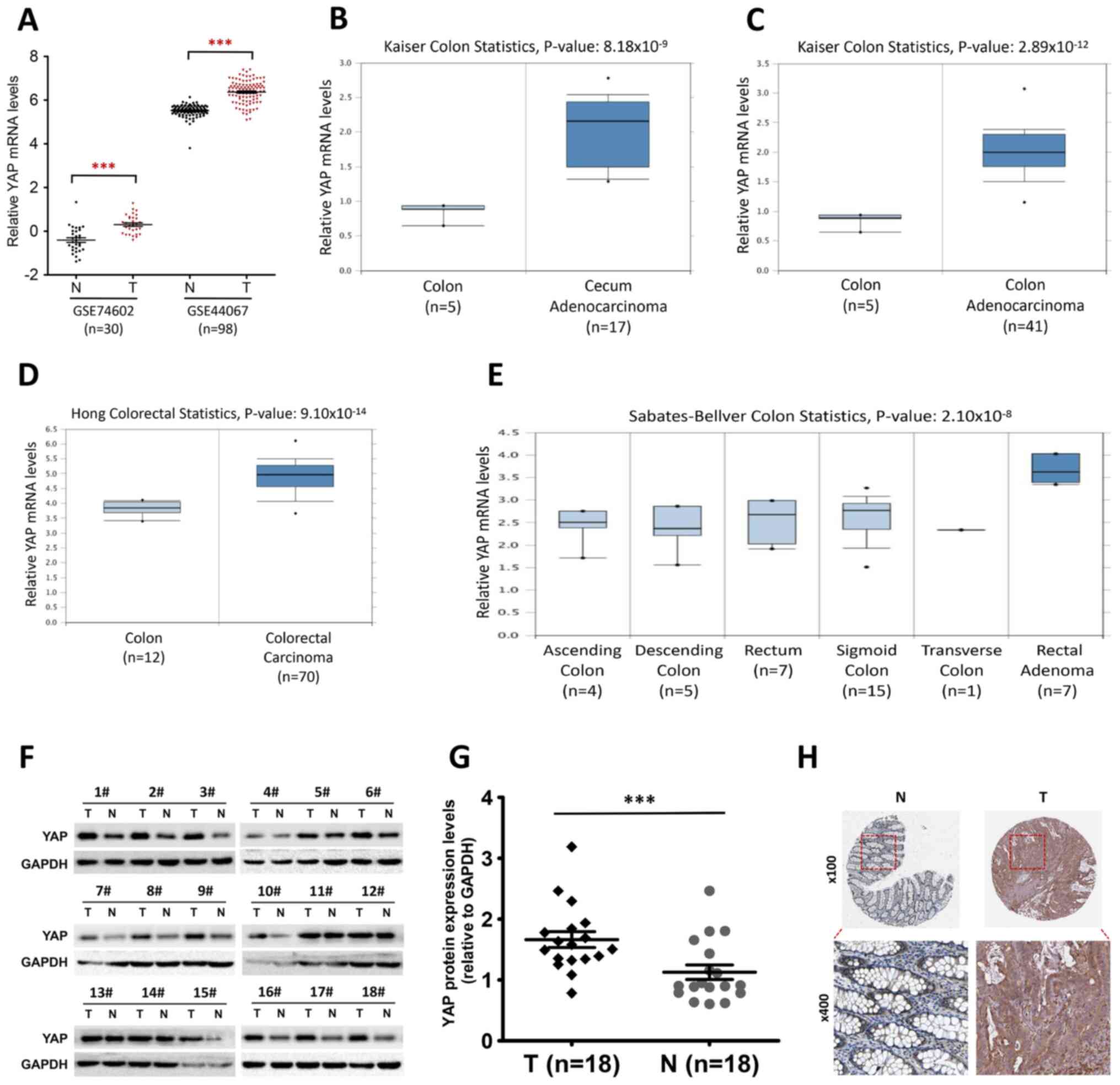 | Figure 1.YAP is upregulated in human CRC. (A)
Column dot plots indicating a difference in YAP mRNA
expression between human CRC tissue and normal tissue based on two
public microarray datasets (accession nos. GSE74602 and GSE44067).
(B and C) Relative YAP mRNA expression in (B) normal colon
and cecum adenocarcinoma tissue or (C) normal colon and colon
adenocarcinoma tissue in the Oncomine dataset by Kaiser et
al (21). (D) Relative
YAP mRNA expression in normal colon or colorectal carcinoma
tissue in the Oncomine dataset by Hong et al (22). (E) Relative YAP mRNA
expression in normal ascending colon, descending colon, rectum,
sigmoid colon, transverse colon or rectal adenoma tissue in the
Oncomine dataset by Sabates-Bellver et al (23). (F) Western blot analysis of YAP
protein expression levels in 18 pairs of CRC tumor and normal
adjacent tissue samples. (G) Semi-quantitative analysis of YAP
expression levels (relative to GAPDH) in 18 pairs of CRC tumor and
normal adjacent tissue samples. ***P<0.001, two-tailed paired
Student's t-test. (H) YAP expression in human colorectal tumor and
normal tissue. Images were taken from the Human Protein Atlas
online database (magnification, ×100 and ×400) and are available
from https://www.proteinatlas.org/ENSG00000137693-YAP1/tissue/colon#img
and https://www.proteinatlas.org/ENSG00000137693-YAP1/pathology/colorectal+cancer#img.
CRC, colorectal cancer; GEPIA, Gene Expression Profiling
Interactive Analysis; N, normal adjacent tissue; T, tumor tissue;
YAP, Yes-associated protein. |
The transcriptional levels of the YAP gene
were also measured in public datasets from the Oncomine database.
In the Kaiser (21) dataset
(Fig. 1B and C), YAP mRNA
expression levels were higher in cecum adenocarcinoma (n=17) and
colon adenocarcinoma (n=41) compared with in normal colon tissue
samples (n=5) (P=8.18×10−9 and P=2.89×10−12,
respectively). Moreover, in the Hong (22) Oncomine dataset (Fig. 1D), the mRNA expression levels of
YAP in colorectal carcinoma tissue (n=70) were higher than
in normal colon (n=12; P=9.10×10−14). In addition,
YAP mRNA expression levels in rectal adenoma tissue (n=7)
were higher than those in normal colorectal tissue in the
Sabates-Bellver (23) Oncomine
dataset (Fig. 1E;
P=2.10×10−8). However, no significant differences were
observed in the expression of the YAP gene between normal
ascending colon, descending colon, sigmoid colon, transverse colon
and normal rectum samples (Fig.
1E).
Consistent with the public datasets, YAP protein
expression levels in clinical samples were significantly higher in
CRC tissue than in normal tissue (Fig.
1F and G; n=18 in each group). The relationship between YAP
expression levels and the clinicopathological parameters of
patients with CRC was then evaluated. Notably, there were no
significant associations between YAP and age, sex, tumor size,
lymph node metastasis or TNM stage (Table I). In order to further determine the
clinical relevance of YAP, the expression of the YAP protein in
histological specimens from the Human Protein Atlas program was
analyzed. The results indicated a weak expression of YAP in normal
colorectal tissue but a strong expression in CRC tissue (Fig. 1H). Collectively, these results
suggested that YAP expression was upregulated in human CRC tissue
both at the mRNA and the protein levels, regardless of the tumor
location.
YAP expression in the CRC cell
lines
The expression of the YAP protein was examined in
six CRC cell lines (HCT116, CCL244, CaCo2, SW480, LoVo and RKO)
using western blot analysis. The results indicated that the YAP
protein was expressed at high levels in the SW480 and CaCo2 cell
lines but at relatively lower levels in HCT116 and RKO cells
(Fig. 2A). Therefore, the expression
of YAP was silenced in SW480 cells (since they displayed high
endogenous expression of YAP) and overexpressed in HCT116 cells
(due to their low endogenous expression of YAP protein). The
efficiency of YAP gene knockdown and overexpression was
confirmed using western blot analysis. As shown in Fig. 2B, YAP protein expression was
significantly decreased in SW480 cells transfected with
YAP-specific siRNA compared with that in cells transfected with
siRNA NC. Conversely, YAP protein expression was significantly
increased in HCT116 cells transfected with a plasmid encoding the
human YAP protein compared with a control vector (Fig. 2C).
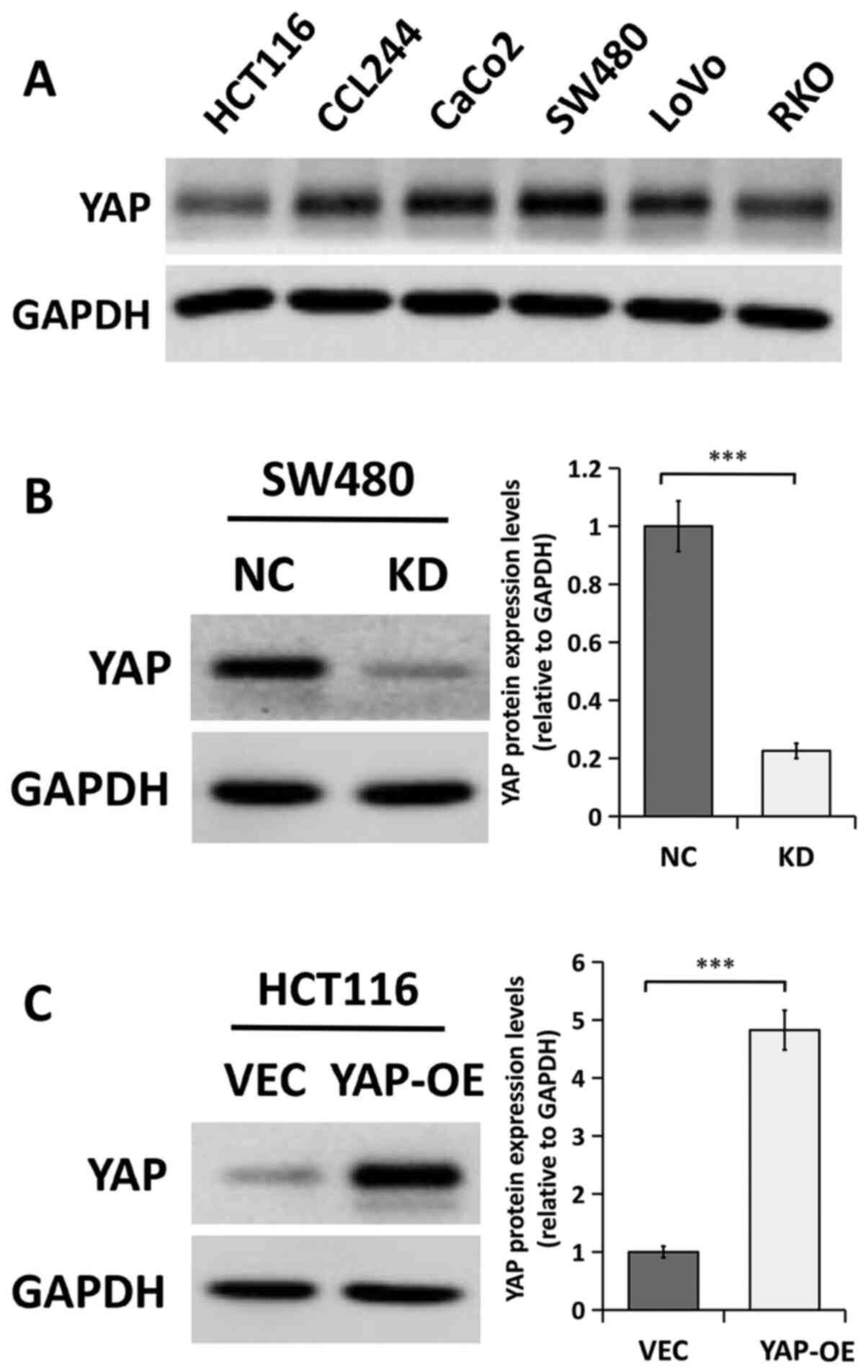 | Figure 2.YAP protein expression levels in
human CRC cells. (A) Western blot analysis of YAP protein levels in
six CRC cell lines (HCT116, CCL244, CaCo2, SW480, LoVo and RKO)
performed in triplicate. (B) Western blot analysis of YAP protein
expression in SW480 cells transfected with NC or KD. The bands were
semi-quantified and the results are presented as the mean ± SEM.
n=3. (C) Western blot analysis of YAP in HCT116 cells transfected
with VEC or YAP-OE. The bands were semi-quantified and the results
are presented as the mean ± SEM. n=3. ***P<0.001, two-tailed
unpaired Student's t-test. CRC, colorectal cancer; KD, YAP siRNA
knockdown; NC, siRNA negative control; OE, overexpression; VEC,
empty vector; YAP, Yes-associated protein; siRNA, small interfering
RNA. |
YAP enhances the proliferation and
migration of CRC cells in vitro
In order to determine whether YAP played an
oncogenic role in CRC, functional experiments were performed to
evaluate the effects of YAP on the proliferation and migration of
CRC cells in vitro. Colony formation assays demonstrated
that YAP silencing inhibited the ability of SW480 cells to form
colonies (Fig. 3A). By contrast, the
proliferation of HCT116 cells was significantly enhanced following
the overexpression of YAP (Fig.
3B).
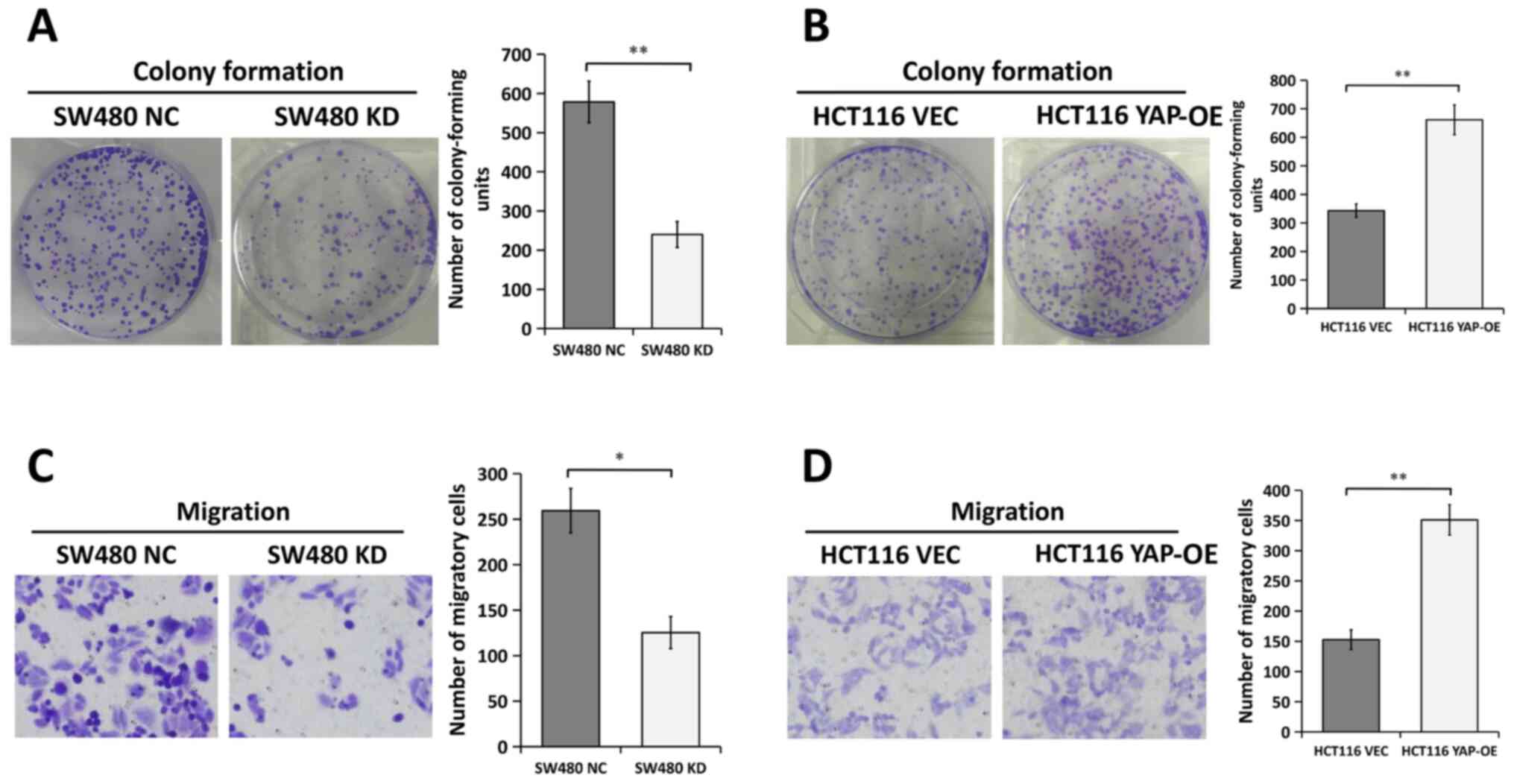 | Figure 3.Effects of YAP on CRC cell
proliferation and migration in vitro. Colony formation
assays were performed in (A) SW480 cells transfected with NC or KD
and (B) HCT116 cells transfected with VEC or YAP-OE. Representative
images of the wells are presented (left). The number of
colony-forming units (right) is presented as the mean ± SEM. n=3.
Migration assays were conducted in (C) SW480 cells transfected with
NC or KD and (D) HCT116 cells transfected with VEC or YAP-OE.
Representative images are presented (left). Magnification, ×200.
The number of migratory cells (right) is presented as the mean ±
SEM. n=3. *P<0.05, **P<0.01, two-tailed unpaired Student's
t-test. CRC, colorectal cancer; KD, YAP siRNA knockdown; NC, siRNA
negative control; OE, overexpression; VEC, empty vector; YAP,
Yes-associated protein; siRNA, small interfering RNA. |
Transwell assays were conducted to measure the
migratory ability of CRC cells. The results suggested that the
migration of SW480 cells was significantly impaired following
transfection with YAP-specific siRNA compared with NC siRNA
(Fig. 3C). However, the migration of
HCT116 cells transfected with the YAP overexpression plasmid
doubled compared with cells transfected with the empty vector
(Fig. 3D). These results indicated
that YAP served an oncogenic role in CRC cells.
Expression of YAP is associated with
the EMT
Considering that YAP promotes the migration of CRC
cells and that EMT is an early event in the metastasis of cancer
(25,26), the regulation of EMT progression by
YAP was examined. The correlation between YAP mRNA
expression levels and EMT-related gene expression was analyzed in
CRC specimens from GEPIA datasets. The results showed that
YAP mRNA expression levels were positively correlated with
those of EMT-related genes, including vimentin (Fig. 4A; ρ=0.37; P=4.2×10−13),
cadherin-2 (also known as N-cadherin; Fig. 4B; ρ=0.35; P=8.4×10−12),
Snail family transcriptional repressor 1 (SNAI1; Fig. 4C; ρ=0.36, P=7×10−13),
SNAI2 (also known as Slug; Fig.
4D; ρ=0.4; P=1.3×10−15), zinc finger E-box binding
homeobox (ZEB1; Fig. 4E;
ρ=0.51; P=4.2×10−26) and ZEB2 (Fig. 4F; ρ=0.43; P=2.5×10−18).
Furthermore, the expression levels of the epithelial marker
E-cadherin and mesenchymal marker vimentin were detected using
western blot analysis. As shown in Fig.
4G, YAP silencing significantly increased the expression of
E-cadherin and decreased that of vimentin. By contrast, YAP
overexpression had the opposite effect on the expression of these
EMT markers (Fig. 4H). These results
indicated that YAP played a role in the regulation of EMT in CRC
cells.
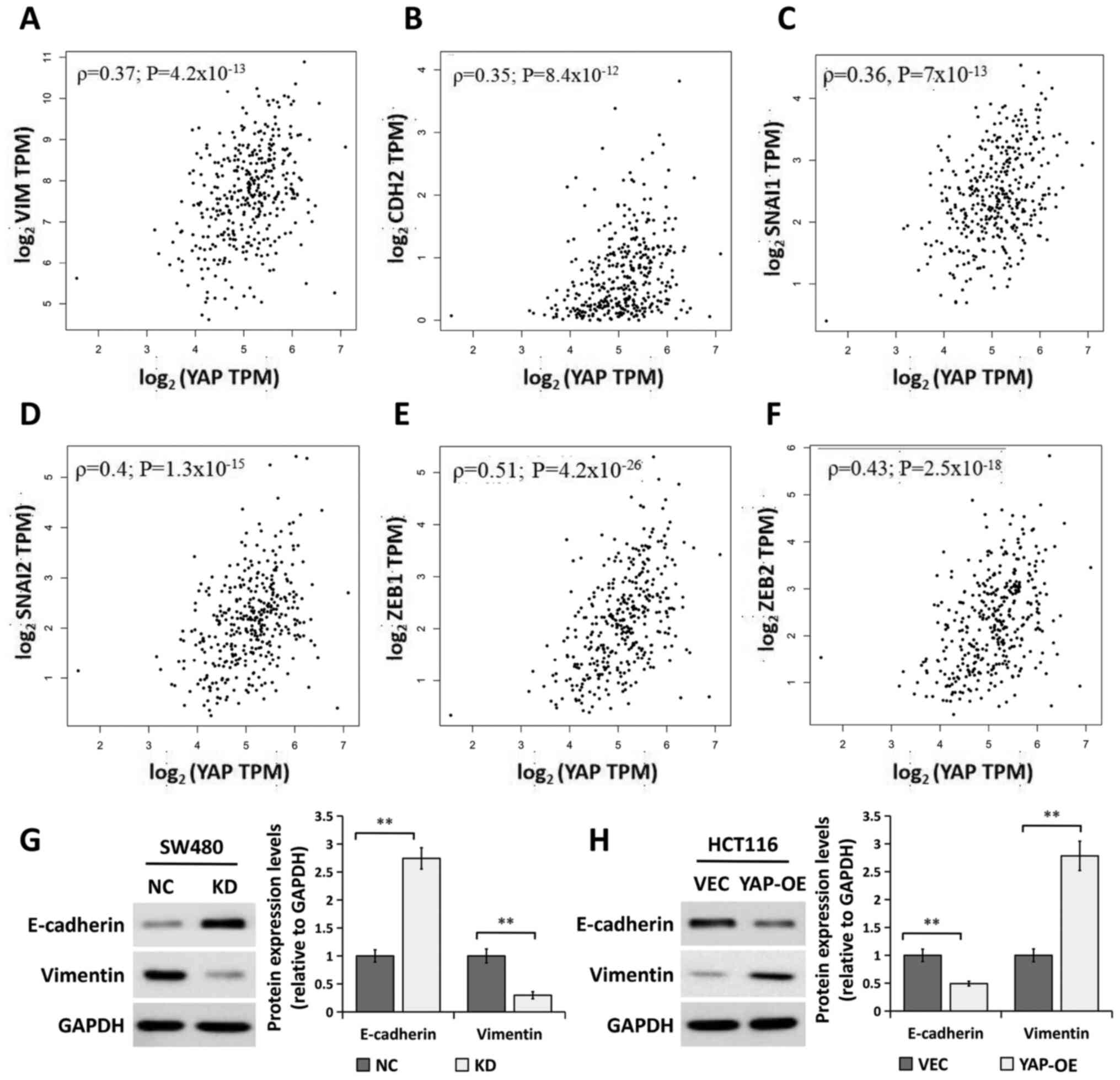 | Figure 4.YAP is associated with EMT. (A)
Correlation analysis of YAP1 and VIM gene expression
levels in patients with CRC using GEPIA datasets (COAD and READ).
(B) Correlation analysis of YAP1 and CDH2 gene
expression levels using CRC datasets from GEPIA. (C) Correlation
analysis between YAP1 and SNAI1 gene expression in
patients with CRC using GEPIA datasets. (D) Correlation analysis
between YAP1 and SNAI2 gene expression in patients
with CRC using GEPIA datasets. (E) Correlation analysis between
YAP1 and ZEB1 gene expression in patients with CRC
using GEPIA datasets. (F) Correlation analysis between YAP1
and ZEB2 gene expression in patients with CRC using GEPIA
datasets. Western blot analysis of E-cadherin and vimentin protein
expression in (G) SW480 cells transfected with NC or KD and (H)
HCT116 transfected with VEC or YAP-OE. GAPDH was used as a loading
control. The bands were semi-quantified and the results are
presented as the mean ± SEM (right). n=3. **P<0.01, two-tailed
unpaired Student's t-test. CRC, colorectal cancer, CDH2,
N-cadherin; GEPIA, Gene Expression Profiling Interactive Analysis;
SNAI1/2, Snail family transcriptional repressor 1/2;
ZEB1/2, zinc finger E-box binding homeobox 1/2; TPM,
transcripts per million; KD, YAP siRNA knockdown; NC, siRNA
negative control; OE, overexpression; VEC, empty vector; YAP,
Yes-associated protein; siRNA, small interfering RNA; COAD, colon
adenocarcinoma; READ, rectum adenocarcinoma. |
YAP regulates the Glut3/AMPK pathway
in CRC cells
It has previously been reported that YAP
transcriptionally regulates the SLC2A3 gene (encoding the
Glut3 protein) and that the expression levels of these two
molecules are positively correlated in CRC tissue (27,28). To
confirm this, the correlation between the mRNA expression levels of
YAP and SLC2A3 in CRC specimens was examined using
GEPIA datasets. A positive correlation was observed between the
mRNA expression of YAP and that of SLC2A3 (Fig. 5A; ρ=0.3; P=7.4×10−9).
Furthermore, the results from western blot analysis indicated that
YAP silencing significantly decreased the expression of Glut3
(Fig. 5B). By contrast, YAP
overexpression significantly increased Glut3 expression levels
(Fig. 5C). Moreover, the regulation
of Glut3 by YAP was also evidenced by the fact that YAP silencing
increased the levels of p-AMPK and decreased cellular ATP levels
(Fig. 5B and D). Notably, YAP
overexpression had the opposite effects on the levels of p-AMPK and
ATP (Fig. 5C and D).
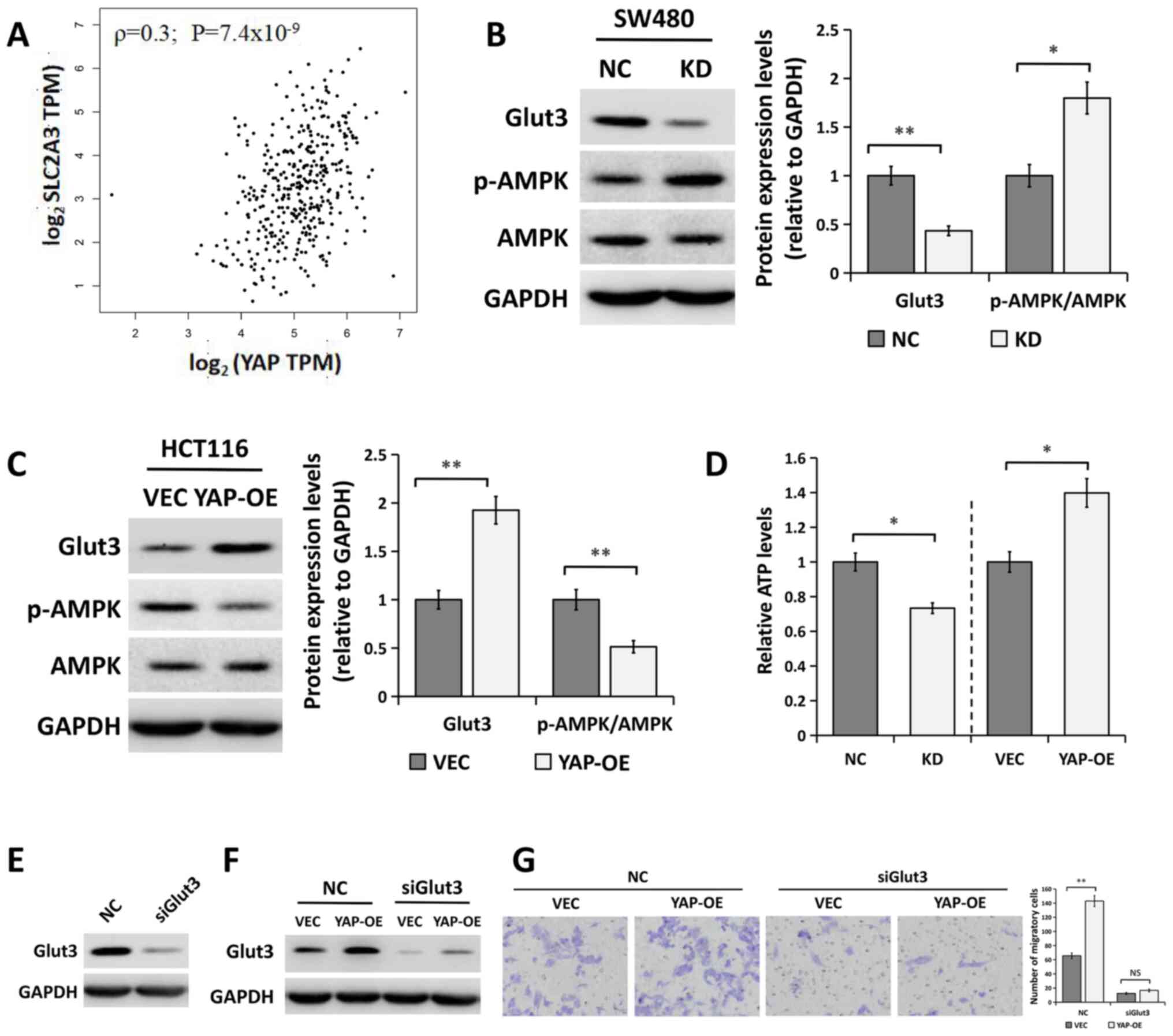 | Figure 5.YAP regulates the Glut3/AMPK pathway
in CRC cells. (A) Correlation analysis between YAP1 and
SLC2A3 (Glut3) gene expression levels in patients with CRC
using GEPIA datasets. Western blot analysis of Glut3, p-AMPK and
AMPK in (B) SW480 cells transfected NC or KD and (C) HCT116 cells
transfected with VEC or YAP-OE. GAPDH was used as a loading
control. The bands were semi-quantified and the results are
presented as the mean ± SEM (right). n=3. (D) Relative ATP levels
in SW480 cells transfected with NC or KD and HCT116 cells
transfected with VEC or YAP-OE. (E) Western blot analysis of Glut3
protein expression in HCT116 cells transfected with siGlut3 or NC.
(F) Western blot analysis of Glut3 expression in HCT116 cells
co-transfected with siGlut3 or NC together with YAP-OE or VEC. (G)
Migration ability of HCT116 cells in HCT116 cells co-transfected
with siGlut3 or NC together with YAP-OE or VEC. Representative
images are presented (left). Magnification, ×200. The number of
migratory cells (right) is presented as the mean ± SEM. n=3.
*P<0.05; **P<0.01; NS, not significant, two-tailed unpaired
Student's t-test. AMPK, AMP-activated protein kinase; CRC,
colorectal cancer; Glut3, glucose transporter 3; KD, YAP siRNA
knockdown; NC, siRNA negative control; p-; phosphorylated; OE,
overexpression; SLC2A3, solute carrier family 2 member 3; VEC,
empty vector; YAP, Yes-associated protein; siRNA, small interfering
RNA. |
In order to further explore whether Glut3 was
involved in the regulation of YAP during cell migration,
Glut3-specific siRNA (siGlut3) was used to silence the expression
of Glut3 in YAP-overexpressing HCT116 cells. The results of western
blot analysis showed that Glut3 protein expression levels were
markedly decreased in cells transfected with siRNA compared with
the control group (Fig. 5E). As
shown in Fig. 5F, YAP overexpression
increased Glut3 expression compared with the control group, which
was then decreased in HCT116 cells following Glut3 siRNA treatment.
Moreover, migration assays suggested that the overexpression of YAP
significantly enhanced the migration ability of cells transfected
with a control siRNA, but this effect was inhibited following
co-transfection with siGlut3 (Fig.
5G).
Discussion
Several lines of evidence have suggested that the
Hippo signaling pathway has a tumor-suppressive role and that
impaired Hippo signaling contributes to the development and
progression of cancer, including CRC (5–8). YAP, as
a major downstream effector in the Hippo signaling pathway, has
been identified as an oncogene that is frequently overexpressed in
several common human cancer types (9–16). The
upregulation of YAP has been reported to promote the proliferation,
migration and chemoresistance of tumor cells in multiple types of
cancer, including pancreatic cancer (9), breast cancer (10), hepatocellular carcinoma (11), esophageal cancer (12), cervical cancer (13), ovarian cancer (14) and gastric cancer (15,16).
Although previous studies focusing on the role of YAP in CRC have
shown that YAP is expressed at high levels in CRC tissue, and
promotes the proliferation and migration of CRC cells (17–20), the
underlying mechanism is not fully understood and remains to be
examined. Using public databases and western blot analysis of
clinical samples, the present study demonstrated that both the mRNA
and protein expression levels of YAP were significantly upregulated
in human CRC tissue compared with in normal tissue. Moreover, in
vitro data showed that YAP not only regulated the proliferative
ability of CRC cells but also enhanced their migration, indicating
that the upregulation of YAP contributed to the proliferation and
migration of CRC cells after malignant transformation.
The induction of EMT has been proposed to play a
critical role in tumor metastasis and stemness of cancer cells, and
is involved in tumor initiation (20,25,26). EMT
is triggered by several transcription factors, including
zinc-finger binding transcription factors, such as Snail1 and −2
(also known as Slug), and ZEB1 and −2 (25,29). The
progression of EMT is accompanied by the upregulation of
mesenchymal markers, including vimentin and N-cadherin, and the
downregulation of epithelial markers, such as E-cadherin (25,29). In
the present study, the expression of the YAP gene was
positively correlated with the EMT markers VIM and
CDH2, as well as the EMT-inducing transcription factors
SNAI1, SNAI2, ZEB1 and ZEB2 in CRC specimens from
public datasets. Furthermore, it was demonstrated that YAP
silencing caused an increase in the protein expression of the
epithelial marker E-cadherin and a decrease in that of the
mesenchymal marker vimentin in CRC cells. However, the
overexpression of YAP resulted in the opposite effect. These
findings indicated that YAP might serve a crucial role in the
regulation of EMT.
Previous studies have demonstrated that YAP
expression may be positively correlated with Glut3 and could
regulate its transcription in CRC tissue (27,28). The
present study confirmed the positive correlation between the
expression levels of the YAP and SLC2A3 genes in CRC
specimens from public datasets. Furthermore, it was demonstrated
that YAP promoted the expression of the Glut3 protein, leading to
an increase in ATP levels, which may lead to decreased AMPK
phosphorylation. Furthermore, a recent study indicated that Glut3
had a greater impact on the proliferation of CRC cells than Glut1
under glucose-limiting stress conditions, and that high Glut3
expression markedly increased the sensitivity of CRC cells to
treatment with vitamin C (30),
highlighting the therapeutic potential of targeting Glut3 in the
treatment of CRC.
There are certain limitations in the present study.
Firstly, the expression of YAP protein was measured using western
blot analysis in a small number of CRC tissue samples. More samples
should be examined and the correlation between the expression of
YAP and clinicopathological parameters of patients with CRC should
be assessed. Secondly, the expression of Glut3 or AMPK activity was
not detected in CRC tissue. Thirdly, the effect of YAP on CRC
tumorigenesis and metastasis was not examined in vivo in the
present study, and studies involving subcutaneous xenograft models
should be further performed in the future.
In conclusion, the present study demonstrated the
upregulation of YAP in CRC tissue, and emphasized the regulatory
role of YAP in the proliferation and migration of CRC cells.
Moreover, a potential mechanism was revealed through which YAP
could induce proliferation and migration of CRC cells and EMT
progression via Glut3/AMPK signaling. These findings may provide
insight into novel approaches for the treatment of CRC involving
YAP targeting.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Project of
National Science Foundation of Jiangsu Province of China (grant no.
BK20161225) and the Project of Science and Technology Plan of
Suzhou of China (grant no. SYS2019050).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request. Additionally, the datasets generated and/or analyzed
during the current study are available from public databases,
including Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/gds/; accession nos.
GSE74602 and GSE44076), Oncomine [Kaiser (21), Hong (22) and Sabates-Bellver (23) datasets; https://www.oncomine.org/], Human Protein Atlas
(http://www.proteinatlas.org/), Gene
Expressing Profiling Interactive Analysis [colon adenocarcinoma
(COAD) and rectum adenocarcinoma (READ) datasets; http://gepia.cancer-pku.cn/].
Authors' contributions
LJ, JZ and QX conducted the research, analyzed the
data and wrote the manuscript. BW, YY, LS, XW, DZ, LG and SS
contributed to data collection and analysis. XZ designed the study
and supervised the preparation of the manuscript. LJ and JZ confirm
the authenticity of the data. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
This study was approved by The Biomedical Research
Ethics Committee of The First Affiliated Hospital of Soochow
University. The experiments performed using human tissue were in
compliance with the Declaration of Helsinki. Written informed
consent was obtained from all patients in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lintoiu-Ursut B, Tulin A and Constantinoiu
S: Recurrence after hepatic resection in colorectal cancer liver
metastasis-review article. J Med Life. 8:12–14. 2015.PubMed/NCBI
|
|
4
|
Liu S, Zheng R, Zhang M, Zhang S, Sun X
and Chen W: Incidence and mortality of colorectal cancer in China,
2011. Chin J Cancer Res. 27:22–28. 2015.PubMed/NCBI
|
|
5
|
Moroishi T, Hansen CG and Guan KL: The
emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 15:73–79.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao B, Tumaneng K and Guan KL: The Hippo
pathway in organ size control, tissue regeneration and stem cell
self-renewal. Nat Cell Biol. 13:877–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wierzbicki PM and Rybarczyk A: The Hippo
pathway in colorectal cancer. Folia Histochem Cytobiol. 53:105–119.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou Q, Bauden M, Andersson R, Hu D,
Marko-Varga G, Xu J, Sasor A, Dai H, Pawłowski K, Said Hilmersson
K, et al: YAP1 is an independent prognostic marker in pancreatic
cancer and associated with extracellular matrix remodeling. J
Transl Med. 18:772020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Z, Qiu N, Yin J, Zhang J, Liu H, Guo
W, Liu M, Liu T, Chen D, Luo K, et al: SRGN crosstalks with YAP to
maintain chemoresistance and stemness in breast cancer cells by
modulating HDAC2 expression. Theranostics. 10:4290–4307. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou Y, Wang Y, Zhou W, Chen T, Wu Q,
Chutturghoon VK, Lin B, Geng L, Yang Z, Zhou L and Zheng S: YAP
promotes multi-drug resistance and inhibits autophagy-related cell
death in hepatocellular carcinoma via the RAC1-ROS-mTOR pathway.
Cancer Cell Int. 19:1792019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qu Y, Zhang L, Wang J, Chen P, Jia Y, Wang
C, Yang W, Wen Z, Song Q, Tan B and Cheng Y: Yes-associated protein
(YAP) predicts poor prognosis and regulates progression of
esophageal squamous cell cancer through epithelial-mesenchymal
transition. Exp Ther Med. 18:2993–3001. 2019.PubMed/NCBI
|
|
13
|
He C, Mao D, Hua G, Lv X, Chen X,
Angeletti PC, Dong J, Remmenga SW, Rodabaugh KJ, Zhou J, et al: The
Hippo/YAP pathway interacts with EGFR signaling and HPV
oncoproteins to regulate cervical cancer progression. EMBO Mol Med.
7:1426–1449. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei X, Jia Y, Lou H, Ma J, Huang Q, Meng
Y, Sun C, Yang Z, Li X, Xu S, et al: Targeting YAP suppresses
ovarian cancer progression through regulation of the PI3K/Akt/mTOR
pathway. Oncol Rep. 42:2768–2776. 2019.PubMed/NCBI
|
|
15
|
Kang W, Tong JH, Chan AW, Lee TL, Lung RW,
Leung PP, So KK, Wu K, Fan D, Yu J, et al: Yes-associated protein 1
exhibits oncogenic property in gastric cancer and its nuclear
accumulation associates with poor prognosis. Clin Cancer Res.
17:2130–2139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu T, Sun L and Zhu X: Yes-associated
protein enhances proliferation and attenuates sensitivity to
cisplatin in human gastric cancer cells. Biomed Pharmacother.
105:1269–1275. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Avruch J, Zhou D and Bardeesy N: YAP
oncogene overexpression supercharges colon cancer proliferation.
Cell Cycle. 11:1090–1096. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun Z, Ou C, Liu J, Chen C, Zhou Q, Yang
S, Li G, Wang G, Song J, Li Z, et al: YAP1-induced MALAT1 promotes
epithelial-mesenchymal transition and angiogenesis by sponging
miR-126-5p in colorectal cancer. Oncogene. 38:2627–2644. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen C, Zhu D, Zhang H, Han C, Xue G, Zhu
T, Luo J and Kong L: YAP-dependent ubiquitination and degradation
of β-catenin mediates inhibition of Wnt signalling induced by
Physalin F in colorectal cancer. Cell Death Dis. 9:5912018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ling HH, Kuo CC, Lin BX, Huang YH and Lin
CW: Elevation of YAP promotes the epithelial-mesenchymal transition
and tumor aggressiveness in colorectal cancer. Exp Cell Res.
350:218–225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaiser S, Park YK, Franklin JL, Halberg
RB, Yu M, Jessen WJ, Freudenberg J, Chen X, Haigis K, Jegga AG, et
al: Transcriptional recapitulation and subversion of embryonic
colon development by mouse colon tumor models and human colon
cancer. Genome Biol. 8:R1312007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hong Y, Downey T, Eu KW, Koh PK and Cheah
PY: A ‘metastasis-prone’ signature for early-stage mismatch-repair
proficient sporadic colorectal cancer patients and its implications
for possible therapeutics. Clin Exp Metastasis. 27:83–90. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sabates-Bellver J, Van der Flier LG, de
Palo M, Cattaneo E, Maake C, Rehrauer H, Laczko E, Kurowski MA,
Bujnicki JM, Menigatti M, et al: Transcriptome profile of human
colorectal adenomas. Mol Cancer Res. 5:1263–1275. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun L, Lu T, Tian K, Zhou D, Yuan J, Wang
X, Zhu Z, Wan D, Yao Y, Zhu X and He S: Alpha-enolase promotes
gastric cancer cell proliferation and metastasis via regulating AKT
signaling pathway. Eur J Pharmacol. 845:8–15. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tania M, Khan MA and Fu J: Epithelial to
mesenchymal transition inducing transcription factors and
metastatic cancer. Tumour Biol. 35:7335–7342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang W, Xiao ZD, Li X, Aziz KE, Gan B,
Johnson RL and Chen J: AMPK modulates Hippo pathway activity to
regulate energy homeostasis. Nat Cell Biol. 17:490–499. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kuo CC, Ling HH, Chiang MC, Chung CH, Lee
WY, Chu CY, Wu YC, Chen CH, Lai YW, Tsai IL, et al: Metastatic
colorectal cancer rewrites metabolic program through a
Glut3-YAP-dependent signaling circuit. Theranostics. 9:2526–2540.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peinado H, Portillo F and Cano A:
Transcriptional regulation of cadherins during development and
carcinogenesis. Int J Dev Biol. 48:365–375. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dai W, Xu Y, Mo S, Li Q, Yu J, Wang R, Ma
Y, Ni Y, Xiang W, Han L, et al: GLUT3 induced by AMPK/CREB1 axis is
key for withstanding energy stress and augments the efficacy of
current colorectal cancer therapies. Signal Transduct Target Ther.
5:1772020. View Article : Google Scholar : PubMed/NCBI
|



















