Introduction
Glioblastoma multiforme (GBM) is the most common and
most lethal type of primary malignant brain tumor (1). Initially, GBM responds favorably to
intensive multimodal treatment comprising surgical resection
combined with radiation and chemotherapy; however, patients often
experience rapid recurrence, due to the highly chemoresistant
tumors (2,3), with a poor prognosis (4) and a median survival time of <15
months (5). Temozolomide (TMZ) is an
alkylating agent that is currently used as a first-line
chemotherapeutic agent against GBM (6–8),
although chemoresistance to TMZ has been identified as a major
cause of pretreatment failure. Therefore, an improved understanding
of the mechanisms through which GBM obtains resistance to TMZ may
aid the development of improved treatment methods. Multiple studies
have been conducted to determine the mechanisms underlying TMZ
resistance, the majority of which focus on O6-methylguanine-DNA
methyltransferase (MGMT), which mediates TMZ-induced cytotoxicity.
However, MGMT alone does not fully account for the chemoresistance
of GBM to TMZ (9–11). This has prompted the investigation of
other genes implicated in TMZ resistance. On the basis of our
previous work (12), the present
study focused on the involvement of Forkhead box protein O3a
(FoxO3a)/β-catenin in TMZ resistance of GBM.
β-catenin, a member of the catenin protein family,
is a subunit of the cadherin protein complex that serves as a
fundamental component of the Wnt signaling pathway (13,14).
Numerous studies have demonstrated that β-catenin is implicated in
GBM development and progression. For example, β-catenin has been
positively correlated with the grade of glial neoplasms (15,16) and
has been identified as a marker of poor prognosis in patients with
glial neoplasms (17). Additionally,
the nuclear accumulation of β-catenin has been associated with a
poorer cancer prognosis compared with β-catenin localization at the
cell membrane (18–20). β-catenin not only serves an important
role in cancer, but is also implicated in chemoresistance. Nuclear
β-catenin mediates the continuous activation of the Wnt/β-catenin
pathway, and confers doxorubicin resistance on neuroblastoma
(5). β-catenin activation by
glycogen synthase kinase-3 inhibitor induces chemoresistance to
interferon-α/5-fluorouracil combination therapy in hepatocellular
carcinoma (21). More importantly,
the downregulation of β-catenin in melanoma cell lines
significantly increased the effectiveness of TMZ, cisplatin and
doxorubicin, and the inhibition of β-catenin chemosensitized
resistant GBM cells (5).
FoxO3a, a Forkhead box O (FoxO) protein of the
Forkhead family, plays a key role in the regulation of cellular
differentiation, proliferation and survival (22). Although FoxO3a has been defined as a
ubiquitous tumor suppressor, as it induces apoptosis (23), emerging evidence indicates that
FoxO3a is strongly associated with poor clinical outcome in
specific types of cancer (24,25). For
example, FoxO3a enhanced cellular proliferation and invasiveness in
specific glioma cell types (26),
and has been closely implicated in multidrug resistance in a
limited number of cancers (27,28). In
agreement with these findings, one of our previous studies
indicated that FoxO3a depletion may sensitize glioma cells to TMZ,
along with the inhibition of β-catenin nuclear entry (12). However, whether re-expression of
FoxO3a reverses the TMZ-mediated impairment in cell viability, and
how FoxO3a regulates the nuclear entry of β-catenin, remain to be
elucidated. The aim of the present study was to thoroughly
investigate the specific roles of FoxO3a and β-catenin in glioma
cell TMZ resistance and elucidate the possible underlying
mechanisms.
Materials and methods
Cell lines and culture
The U251 glioma cell line was obtained from the Cell
Bank of the Chinese Academy of Sciences (Shanghai, China), and U87
cells (cat. no. HTB-14; glioblastoma of unknown origin) were
obtained from the American Type Culture Collection. Both cell lines
were cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.) containing 2 mM glutamine, 10% fetal calf
serum, 100 U/ml penicillin and 100 µg/ml streptomycin (all from
Sigma-Aldrich; Merck KGaA), and maintained at 37°C in a 5%
CO2 incubator.
Generation of TMZ-resistant GBM
cells
To generate TMZ-resistant colonies, parental U251
and U87 cells were exposed to TMZ for 3 weeks. Briefly, the cell
lines we initially cultured in six-well plates and allowed to
adhere overnight at 37°C. Treatment with 400 µM TMZ was repeated
every 24 h for 5 consecutive days, and the cells were then exposed
to fresh TMZ every 3 days for a total of 3 weeks. At the end of the
treatment period, a small population of cells had survived and
propagated. The surviving colonies were selected and established as
TMZ-resistant U251 (U251-TR) and U87 (U87-TR) cell lines.
Establishment of stable cell
lines
DNA oligos encoding human FoxO3a short hairpin
(sh)RNA (5′-GCATGTTCAATGGGAGCTTGGA-3′) were designed using BLOCK-iT
RNAi Designer (Invitrogen; Thermo Fisher Scientific, Inc.), and
synthesized and cloned into the pHY-LV-KD1.1 vector (HanYin
Biotech) to generate pHY-FoxO3a-KD2, as previously described
(26). A vector expressing shRNA
against an irrelevant sequence (5′-TGGTTTACATGTCGACTAA-3′) was used
as the negative control (shRNA-NC). Full-length human FoxO3a cDNA
was purchased from Open Biosystems (Horizon Discovery Ltd.) and
sub-cloned into the PHY-LV-OE1.6 vector (HanYin Biotech). The
construct expressing FoxO3a was designated pHY-FoxO3a-OE. The
Trans-Lentiviral Packaging System and the Vira Power Lentiviral
Expression System (Invitrogen; Thermo Fisher Scientific, Inc.) were
used to produce shRNA and overexpression lentiviruses,
respectively. In addition, shRNAs targeting matrix metallopeptidase
(MMP)9 (MMP9-sh1, 5′-CGGCAATGCTGATGGGAAA-3′; MMP9-sh2,
5′-CTTCCAGTACCGAGAGAAA-3′; and MMP9-sh3, 5′-GGCAGCTGGCAGAGGAATA-3′)
and β-catenin (5′-GCATAACCTTTCCCATCATCG-3′) were constructed as
aforementioned. These constructs were co-transfected with packaging
plasmids into 293T cells using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions; the viral particles were harvested 48
h later. Upon lentivirus-mediated transduction, stable cell lines
were generated via puromycin selection (2 µg/ml; Sigma-Aldrich;
Merck KGaA).
Cell viability assay
Cell viability was determined using the Cell
Counting Kit 8 (CCK-8) assay. Cells were seeded into 96-well plates
at a density of 3×103 cells/well. After overnight
incubation at 37°C, the cells were transduced with lentivirus and
then treated with various concentrations of TMZ (range, 200–2,000
µM) for 1 to 5 days. After a 2-h incubation with 10 µl CCK-8
solution (Dojindo Molecular Technologies, Inc.), cell viability was
assessed at OD450 nm using a microplate reader (BioTek Instruments,
Inc.). The survival rate of untreated cells was set at 100% and
used to calculate the half-maximal inhibitory concentration
(IC50). Each experiment was conducted in triplicate.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was extracted from the TMZ-resistant and
GBM parental cell lines using RNeasy Plus Mini Kit (Qiagen, Inc.),
according to the manufacturer's protocol. cDNA was prepared with 1
µg total RNA from each sample using SuperScript® VILO™
cDNA Master Mix (Thermo Fisher Scientific, Inc.); 6 ng cDNA was
then used for qPCR analysis in a final reaction volume of 20 µl.
Samples were analyzed in triplicate, and statistical analysis was
performed using ANOVA. The qPCR conditions included initial
denaturation at 95°C for 5 min, denaturation at 95°C for 15 sec
annealing and extension at 60°C for 1 min with 40 cycles.
The following primers were used: FoxO3a forward,
5′-AAGCCAGCTACCTTCTCTTCCA-3′ and reverse,
5′-GTGGCAAGTCAGTCCGAACTGA-3′; GAPDH forward,
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse,
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′; and β-catenin forward,
5′-CCTCCAGGTGACAGCAATCAG-3′ and reverse,
5′-GCCCTCTCAGCAACTCTACAG-3′.
Western blotting
RIPA lysis buffer (cat. no. P0013C; Beyotime
Institute of Biotechnology) was used to extract total cellular
protein, and the BCA kit (cat. no. P0009; Beyotime Institute of
Biotechnology) was used to determine the protein concentration. The
proteins in the SW20 and LOVO cell lysates were separated by 10%
SDS-page with 50 µg total protein loaded per lane. The proteins
were then transferred to nitrocellulose membranes and the membranes
were incubated with the appropriate primary antibodies. The primary
antibodies were the following: MMP9 (cat. no. 3852; Cell Signaling
Technology, Inc., dilution 1:1,000), β-actin (cat. no. ab8227;
Abcam, dilution 1:1,000), β-catenin (cat. no. 8480; Cell Signaling
Technology, Inc., dilution 1:1,000) and Histone H3 (cat. no.
ab1791; Abcam, dilution 1:1,000). The secondary antibody was goat
anti-rabbit (cat. no. ab150077; Abcam, dilution 1:1,000). The
primary antibodies were incubated overnight at 4°C and the
secondary antibody was incubated for 1 h at room temperature. The
BeyoECL Plus kit (cat. no. P0018S, Beyotime Institute of
Biotechnology) was used for the chromogenic protein bands with
Beckman Coulter Immunoassay System (UniCel DxI 800; Beckman
Coulter). Nuclear and cytoplasmic fractions of the total protein
were separated using NE-PER Nuclear and Cytoplasmic Extraction
Reagent (Thermo Fisher Scientific, Inc.) and then subjected to
western blotting.
Dataset collection and analysis
Survival analysis was performed with Kaplan-Meier
plots, and The Cancer Genome Atlas (TCGA) data were analyzed using
R version 3.6.3, along with the ‘edgr’ package (https://bioconductor.org/packages/release/bioc/html/edgeR.html).
Statistical analysis
Graphically presented data represent the mean ± SD
of three independent experiments. The differences among groups were
determined by one-way ANOVA with Tukey's HSD as post hoc test, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
FoxO3a and β-catenin confer TMZ
resistance on glioma cells
Initially, TMZ-resistant cell lines (U87-TR and
U251-TR) were developed from two parental tumor cell lines
(designated as TMZ-sensitive), both of which are typical glioma
cell lines (U87 and U251). The IC50 values 205 and 733
µm were selected for subsequent experiments using U87 and U87-TR
cells, and 260.1 and 817.6 µm for those with U251 and U251-TR
cells, as presented in a previous study (12). In order to investigate the functional
contributions of FoxO3a and β-catenin to glioma cell TMZ
resistance, a combination of knockdown and overexpression analyses
were conducted using both TMZ-sensitive cells and their
corresponding resistant counterparts. Specifically, cells stably
overexpressing FoxO3a or β-catenin (mediated by lentiviral
infection) were generated from the sensitive cell lines, while
stably silenced FoxO3a or β-catenin cells were generated from the
resistant cell lines; ectopic overexpression of FoxO3a was
performed in the context of FoxO3a knockdown or β-catenin
overexpression. These overexpression and silenced cell lines were
then treated with relevant concentrations of TMZ for 5 consecutive
days. Cell viability was assessed daily using the CCK-8 assay. As
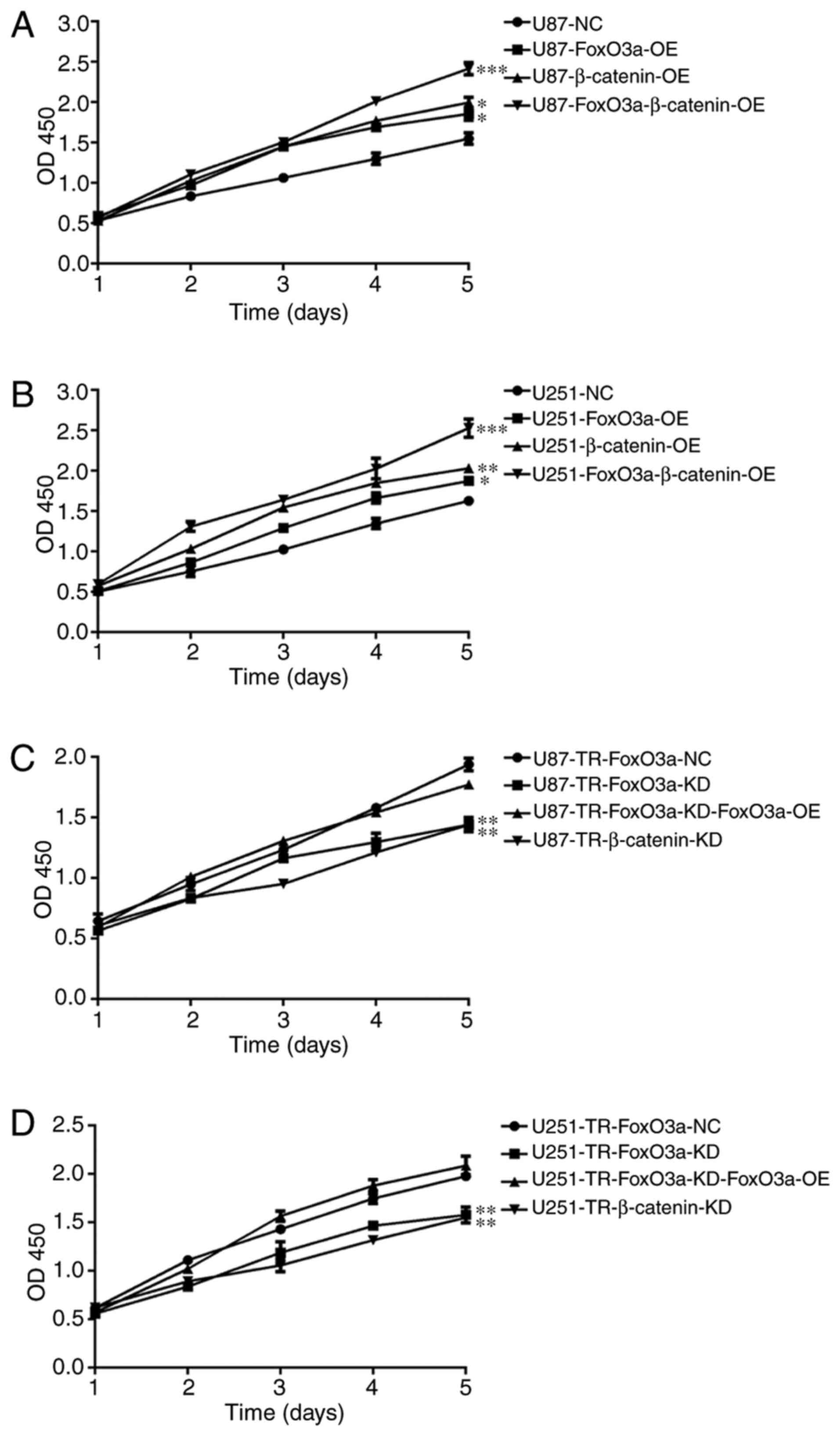
shown in Fig. 1A, the overexpression
of FoxO3a markedly increased U87 cell proliferation when compared
with U87-NC (U87 negative control). A similar trend was observed in
U251 cells (Fig. 1B). It appears
that either FoxO3a or β-catenin overexpression alone resulted in a
comparable increase in U87 cell proliferation (Fig. 1A). As predicted, this trend was also
observed in U251 cells (Fig. 1B).
Notably, co-expression of both FoxO3a and β-catenin led to the
highest increase in proliferation (Fig.
1A and B). Next, the effects of overexpression and knockdown
were assessed in TMZ-resistant cells. Since our previous work
demonstrated that FoxO3a and β-catenin were upregulated in
resistant cell lines (12) relative
to their sensitive counterparts, the roles of FoxO3a and β-catenin
in TMZ resistance were explored in the context of the relevant gene
knockdown in the present study. As shown in Fig. 1C, knocking down FoxO3a or β-catenin
in U87-TR cells significantly decreased cellular proliferation,
which became more apparent over time. Consistent with the
observations in U87-TR cells, FoxO3a or β-catenin knockdown in
U251-TR cells also resulted in a marked decrease in proliferation
(Fig. 1D). While the depletion of
either FoxO3a or β-catenin resulted in a notable impairment in cell
viability, we hypothesized that the re-expression of FoxO3a in
knockdown cells may restore TMZ resistance capability. As shown in
Fig. 1C and D, the cell viability
levels were comparable between the control and FoxO3a-knockdown
cells, as well as those overexpressing FoxO3a, indicating that the
FoxO3a knockdown-induced reduction in cell viability was reversed
by overexpressing FoxO3a. These results (Figs. 1 and S1) demonstrated that FoxO3a and β-catenin
serve key roles in the TMZ-resistant phenotype of glioma cells.
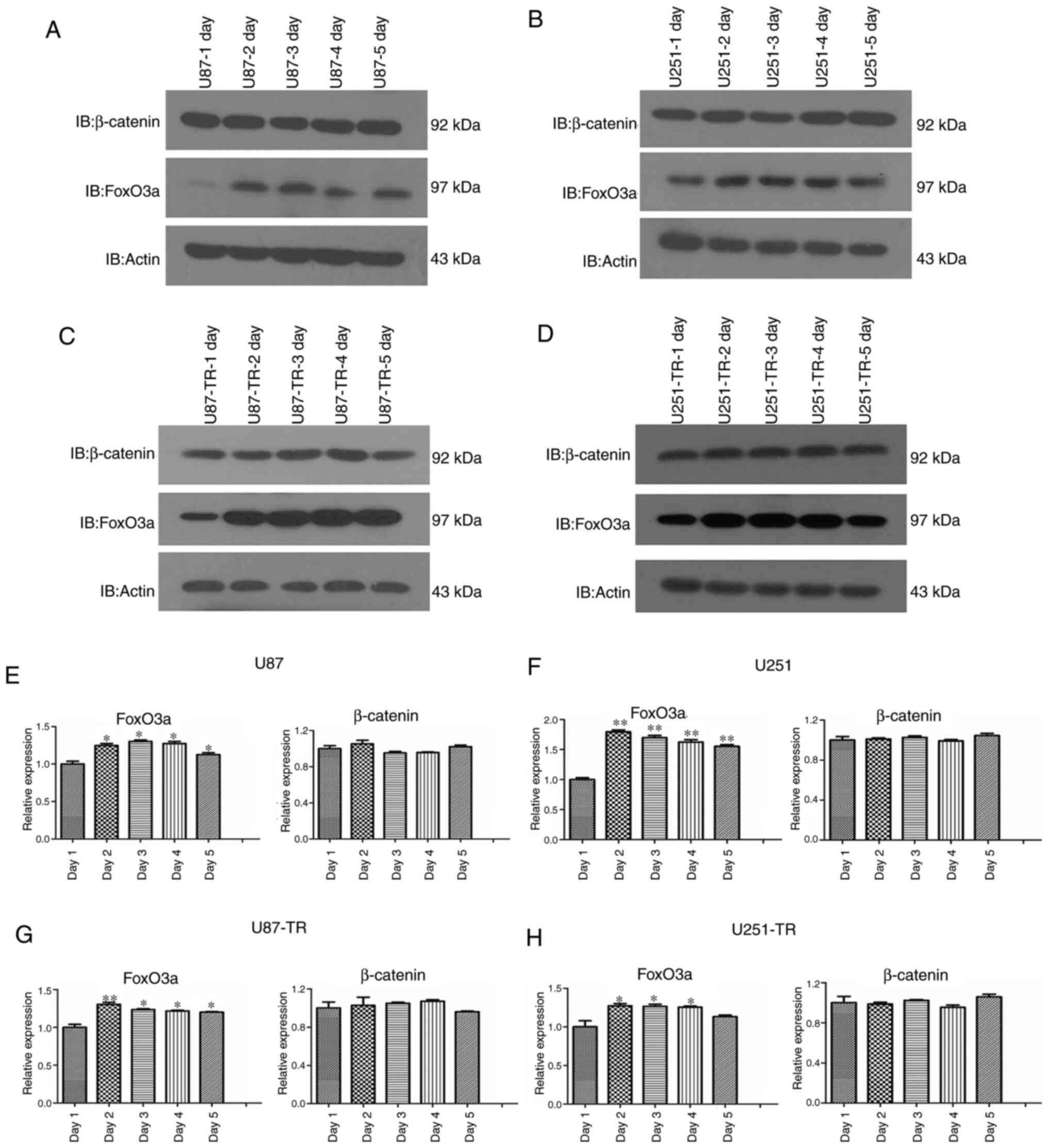
Expression levels of FoxO3a, but not
β-catenin, in glioma cells are altered over time
Although our previous study revealed that elevated
FoxO3a and β-catenin protein expression is associated with glioma
cell TMZ resistance (12), the
timing and underlying mechanisms were not specified. A key question
is whether the levels of FoxO3a and β-catenin protein are altered
over time; as our previous work merely reflected an endpoint
observation, a dynamic change during this process may have been
overlooked. In order to answer this question, both TMZ-sensitive
and -resistant cell lines were treated with TMZ for 5 consecutive
days, and then assessed from days 1–5. Following the appropriate
treatments, the protein lysate was subjected to western blot
analysis. In sensitive glioma cells, treatment with TMZ increased
the levels of FoxO3a protein up to day 2, which remained largely
unchanged between days 2 and 5. However, β-catenin protein
expression remained unaltered over the entire TMZ treatment period
(Fig. 2A and B). FoxO3a protein
levels in the resistant glioma cells were also significantly
increased after 2 days of TMZ treatment, and remained largely
unchanged thereafter. By contrast, there were no significant
differences in β-catenin protein levels over the course of TMZ
treatment. Intriguingly, the change in FoxO3a protein level
appeared to be more preserved in the resistant cell lines, as it
increased sharply on day 2, but there were no apparent changes in
expression thereafter (Fig. 2C and
D). Conversely, a modest decrease in Foxo3a was observed in U87
cells by day 4, and this trend was partially reversed by day 5. A
similar decrease in Foxo3a expression was observed in U251 cells on
day 5 (Fig. 2A and B). Next, changes
at the mRNA level were compared with those observed at the protein
level. As shown in Fig. 2E-H, FoxO3a
mRNA levels were markedly increased in both the sensitive and
resistant cell lines, while no significant change in β-catenin mRNA
levels was observed, which is consistent with the corresponding
protein expression observations.
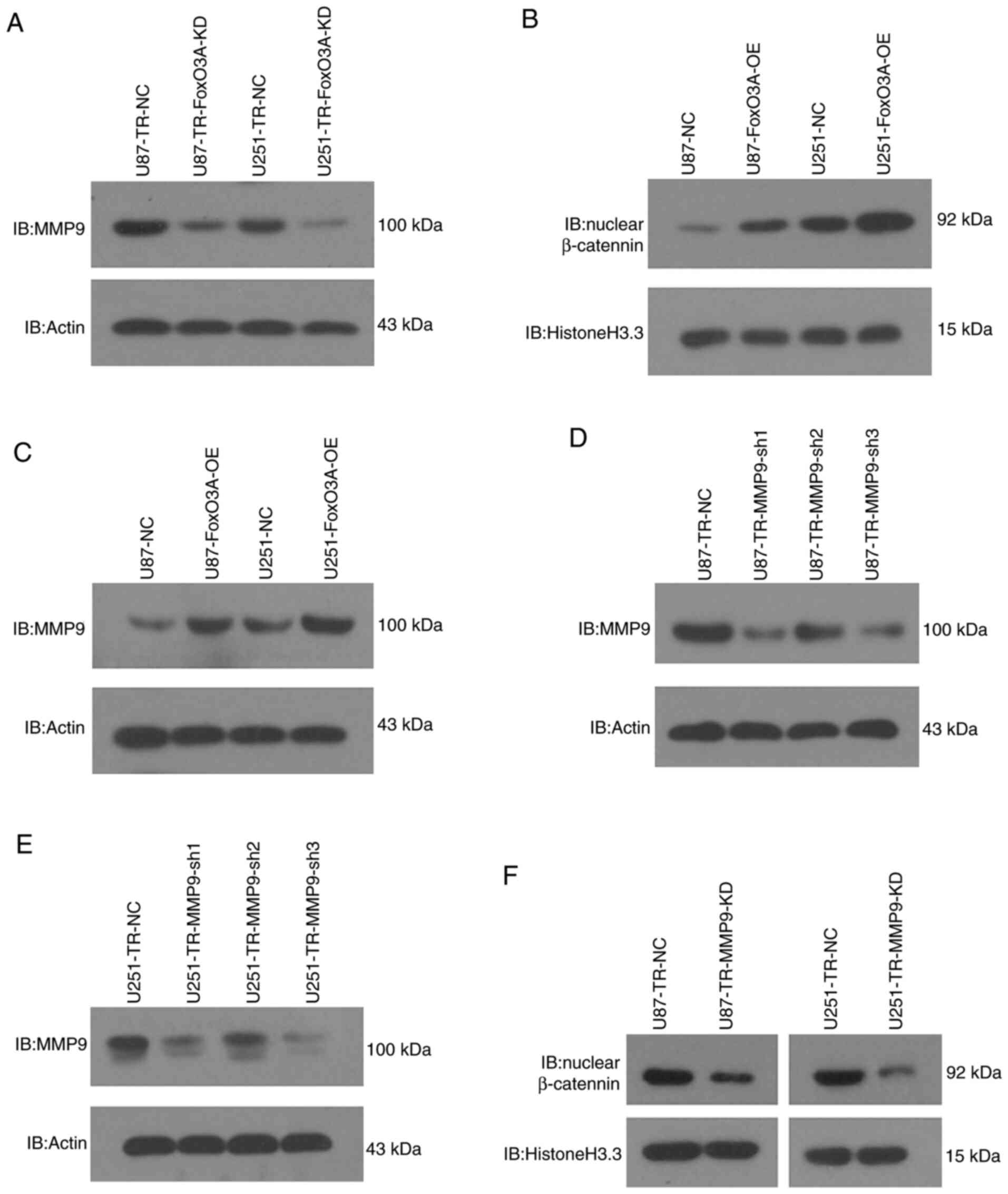
FoxO3a regulates β-catenin nuclear
accumulation by modulating MMP9 expression
In our previous study, the depletion of FoxO3a was
found to reduce the nuclear localization of β-catenin with no
discernable effect on its overall level (12), suggesting that FoxO3a may lead to
β-catenin nuclear accumulation. However, demonstrating this remains
a challenge, as the underlying molecular mechanisms are yet to be
elucidated. Nevertheless, the mechanism by which FoxO3a promotes
β-catenin nuclear accumulation was investigated in the present
study. FoxO3a knockdown was found to attenuate MMP9 expression and
inhibit glioma cell invasiveness (26), and the invasive phenotype regularly
coincides with β-catenin nuclear accumulation. Moreover, another
study uncovered a novel molecular event in which MMP9 allows
β-catenin to enter the nucleus (29). Based on these findings, it was
hypothesized that FoxO3a may induce β-catenin nuclear accumulation
by regulating MMP9 expression in glioma cells. As shown in Fig. 3A-C, FoxO3a knockdown significantly
reduced the protein levels of MMP9, while overexpressing FoxO3a in
TMZ-sensitive cells enhanced the nuclear accumulation of β-catenin,
concomitant with an increase in MMP9. In addition, our previous
work indicated that depleting FoxO3a resulted in a marked reduction
in the protein levels of nuclear β-catenin, with no change in its
overall level (12). Furthermore,
lentivirus-mediated MMP9 knockdown was conducted based on the data
presented in Fig. 3D and E, and was
found to markedly reduced the levels of nuclear β-catenin (Fig. 3F). These results suggest that FoxO3a
regulates β-catenin nuclear accumulation by modulating MMP9
expression.
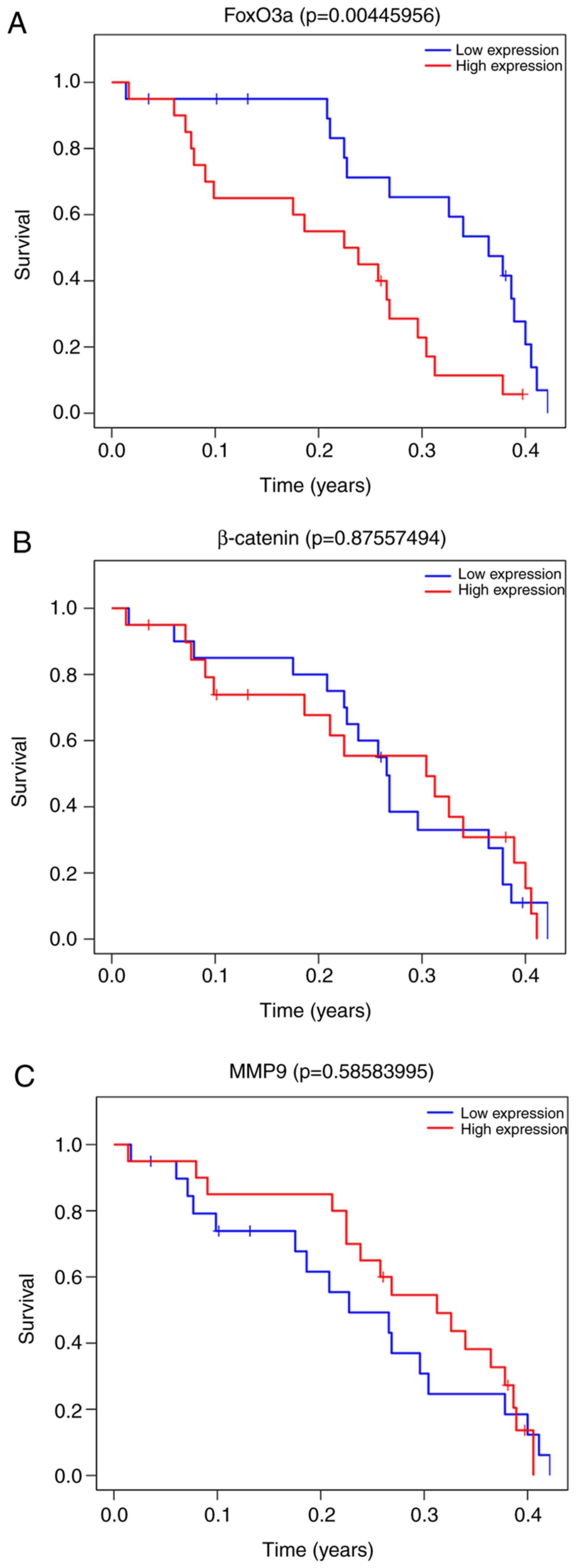
FoxO3a and MMP9 expression are
clinically relevant in GBM
The coordinated actions of FoxO3a, MMP9 and
β-catenin in cancer cells have already been determined. Therefore,
the association between the expression of those three gene and
patient survival was assessed. The relevant patient data (mRNA
expression and clinicopathological characteristics) were collected
from TCGA and preliminarily processed, followed by division into
groups according to the stratified median cutoff values. Briefly,
the data from 160 cases were collected, of which 104 were male and
56 were female. The age of the patients ranged between 21 and 89
years, with a median age of 59.5 years. There were no significant
differences between any of the three genes between the patient
groups, which were divided by a primary median survival time (536
days; data not shown). The patients were then further divided into
4 groups based on median cutoff values (median 1=361 days, and
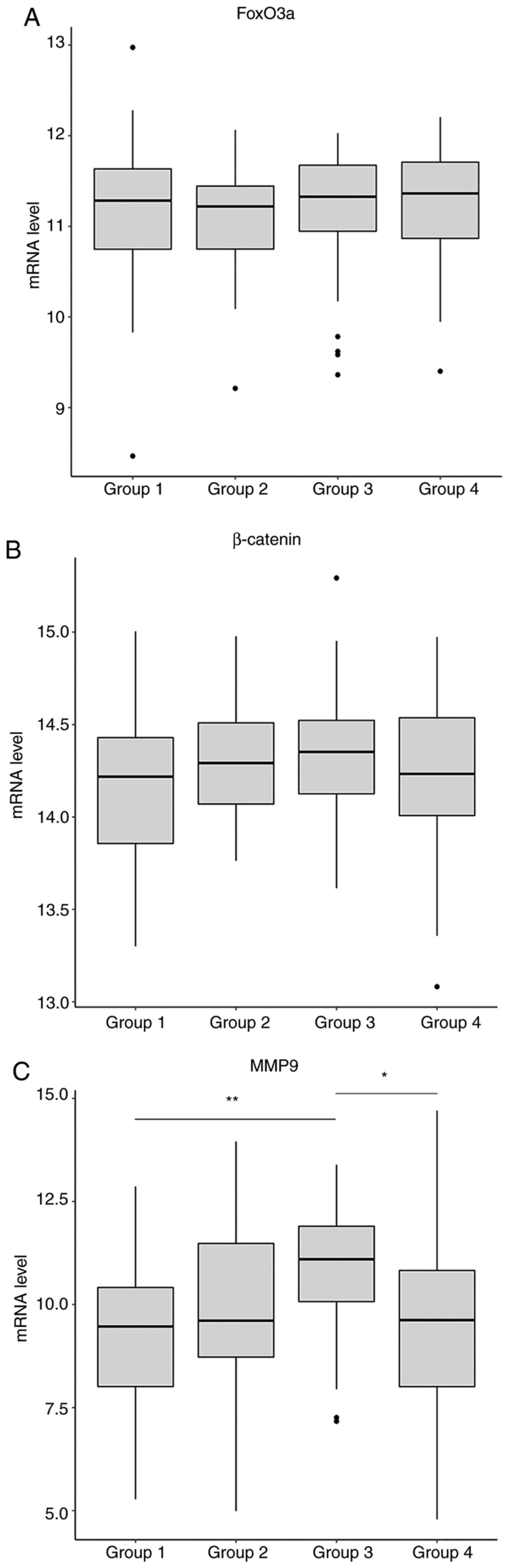
median 2=154.5 days). In the group with the poorest survival values
(Group 4), patients with high levels of FoxO3a expression exhibited
shorter survival times compared with those with low FoxO3a levels
(Figs. 4A and 5A). This trend was not observed in any of
the other groups (Groups 1, 2 and 3), suggesting that low
expression levels of FoxO3a play a protective role in the more
severe cases. However, similar results were not obtained in
association with MMP9 in that group (Group 4; Fig. 4C), although the MMP9 expression
levels between the groups were significantly different, where they
progressively increased until the third group, and then declined in
the poorest survival group (Fig.
5C). As predicted, there was no association between β-catenin
level and patient survival, regardless of the associated grouping
(Figs. 4B and 5B). These results suggest that the in
vitro findings of the present study are consistent with those
in clinical samples. However, the findings regarding the changes in
the mRNA levels of MMP9 and FoxO3a do not entirely coincide with
those at the protein level, which may be attributed to differences
in post-translational modification.
Discussion
A number of studies have revealed that FoxO3a
inhibits tumor development and exerts cytostatic and cytotoxic
effects, indicative of its tumor-suppressive function (23). However, emerging evidence suggests a
correlation between the metastatic functions of FoxO3a and poor
cancer prognosis (24,25) which promotes cellular invasiveness,
and further supports the oncogenic role of FoxO3a. Moreover,
radioresistant glioma cells expressed increased levels of FoxO3a,
which, together with nuclear β-catenin, conferred chemoresistance
on colon cancer (30). In agreement
with these findings, our previous study demonstrated that
glioma-resistant cells exhibit high levels of FoxO3a and β-catenin,
and that FoxO3a induces β-catenin nuclear accumulation, although
the exact mechanisms are yet to be specified (12). For example, when FoxO3a protein
levels increase, it is unclear whether the nuclear protein levels
of β-catenin are altered over time, or whether these findings
simply represent an endpoint observation. To investigate these
questions, cell lines with stable overexpression and knockdown of
specific target genes were generated, and a combined knockdown and
overexpression strategy was employed. An increase in FoxO3a protein
level was present on day 2, while no change was observed in the
overall protein level of β-catenin. It is conceivable that the
increased protein levels FoxO3a were attributed to a corresponding
rise in mRNA level in response to TMZ treatment. As predicted,
FoxO3a mRNA levels were also increased on day 2, and remained
unchanged between days 2 and 5, whereas the β-catenin mRNA level
remained unchanged over time. In addition, our previous study
(12) merely showed that depleting
FoxO3a sensitized glioma cells to TMZ treatment, as well as
decreased the protein levels of nuclear β-catenin. However, it did
not fully investigate the following possibilities: i) Whether the
overexpression of FoxO3a, β-catenin, or both, augments glioma cell
resistance to TMZ; and ii) Whether the FoxO3a knockdown-induced
attenuation of the TMZ-resistant phenotype is reversed by simply
re-introducing FoxO3a. To address these issues, a series of
experiments were performed on the established stable cell lines.
The results demonstrated that overexpressing FoxO3a or β-catenin
alone augmented glioma cell resistance to TMZ, and that the
co-overexpression of these two genes imparted the highest degree of
TMZ resistance. Despite this, co-overexpression of FoxO3a and
β-catenin appears not to produce a synergistic effect, as the level
of TMZ resistance driven by co-overexpression was comparable to
that induced by FoxO3a or β-catenin alone. Additionally, knocking
down either FoxO3a or β-catenin decreased TMZ resistance, while
re-introducing FoxO3a (in the context of FoxO3a depletion) restored
the TMZ-resistant phenotype, suggesting that FoxO3a is required to
maintain TMZ resistance in GBM cells. FoxO3a, together with
β-catenin, potentially underlies the TMZ-resistant phenotype of
glioma cells. Taken together, these findings support those of our
previous study, thus improving our understanding of the roles of
FoxO3a and β-catenin in glioma cell TMZ resistance. However, how
FoxO3a promotes β-catenin accumulation in the nucleus has yet to be
fully elucidated.
MMP9 (also known as 92 kDa type IV collagenase, 92
kDa gelatinase and gelatinase B) is a key member of the MMP family
involved in the degradation of the extracellular matrix (ECM). MMP9
is implicated in the metastasis and invasion of a variety of cancer
types, and acts as a key effector of epithelial-to-mesenchymal
transition (EMT). Of note, several recent reports have documented
an EMT-like phenotype of glioma cells with increased TMZ resistance
capacity (31), suggesting that, in
addition to their role in EMT, certain essential EMT genes may also
be involved in glioma cell resistance to TMZ. Our previous work
(12,26) has demonstrated that FoxO3a promotes
glioma cell invasion by upregulating MMP9 protein levels, and that
FoxO3a participates in glioma cell TMZ resistance. Furthermore,
Dwivedi et al (29) found
that MMP9 promotes the entry of β-catenin into the nucleus.
Therefore, we hypothesized that FoxO3a may induce β-catenin nuclear
accumulation by regulating MMP9 expression, thereby augmenting
glioma cell resistance to TMZ. Indeed, FoxO3a knockdown
significantly reduced the protein expression level of MMP9, whereas
overexpression of FoxO3a enhanced the nuclear accumulation of
β-catenin. Moreover, the results of our previous study indicated
that depleting FoxO3a results in a marked reduction in the protein
levels of nuclear β-catenin, but does not alter its overall level
(12). Furthermore, FoxO3a depletion
has previously been reported to reduce MMP9 protein expression
(26). These findings suggest that
FoxO3a alone is capable of regulating β-catenin localization and
MMP9 expression, but do not conclusively demonstrate that MMP9
plays a critical role in the regulation of nuclear β-catenin
accumulation. Therefore, in the present study, the effects of MMP9
knockdown on the nuclear accumulation of β-catenin were
investigated. Notably, nuclear β-catenin accumulation was markedly
reduced following MMP9 knockdown. These data confirm the previous
observations and, together with our previous work, suggest that
FoxO3a induces nuclear β-catenin accumulation primarily by
regulating MMP9 expression.
To the best of our knowledge, the results of the
present study represent a striking and novel example that links the
FoxO3a, MMP9 and β-catenin genes to glioma cell TMZ resistance, and
highlight a novel functional role of MMP9 in TMZ resistance. Such a
mechanism appears plausible, as the roles of different genes are
often context-dependent (such as the cell type and associated
stimulus). Although MMP9 is considered to be an effector of
cellular invasiveness and ECM remodeling, an increasing number of
studies have indicated alternative roles for MMP9. For example,
inhibiting MMP9 prior to the use of cisplatin (which is used to
treat ovarian cancer) can reduce cisplatin resistance (32). Moreover, MMP9 augmented tumor cell
resistance to natural killer cell-mediated cytotoxicity by cleaving
intercellular adhesion molecule 1 (33). In brief, the results of the present
study, as well as those of other researchers, support the view that
the functions of a particular gene are context-dependent, which may
inspire different methods for investigating MMP9.
Another aim of the present study was to investigate
the association between FoxO3a, MMP9 and β-catenin in clinical GBM
samples. High levels of FoxO3a mRNA appeared to be associated with
the poorest survival outcome, and the difference in MMP9 levels
among the stratified groups partly followed suit. Since the
localization, but not the expression, of β-catenin plays a key role
in glioma, no correlation was expected between overall β-catenin
mRNA levels and patient survival. As predicted, no significant
difference was observed across the patient groups. Although not as
solid as expected, these clinical findings remain relevant to the
associated in vitro findings.
In conclusion, the results of the present study
highlight the FoxO3a/MMP9/β-catenin network as a novel regulatory
mechanism in glioma cells, which promotes glioma cell resistance to
TMZ. However, the more specific molecular signaling events will be
investigated in future studies. These findings provide novel
insights into the mechanisms of TMZ resistance in glioma cells.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81660502).
Availability of materials and data
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DS wrote the manuscript and conducted data analysis.
SY and XZ performed the experiments. SL, LW and JC collected and
analyzed the data and fixed the syntax errors. CQ and KX
contributed to the study design and revised the manuscript for
important intellectual content. KX and CQ confirm the authenticity
of all the raw data presented. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gautam M, Singh S, Aggarwal M, Sharma MK,
Dang S and Gabrani R: Glioblastoma multiforme; drug resistance
& combination therapy. Front Anti Cancer Drug Discovery Volume.
10:1112019. View Article : Google Scholar
|
|
2
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF,
de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. New Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clarke J, Butowski N and Chang S: Recent
advances in therapy for glioblastoma. Arch Neurol. 67:279–283.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Y, Chen L, Bao Z, Li S, You G, Yan W,
Shi Z, Liu Y, Yang P, Zhang W, et al: Inhibition of STAT3 reverses
alkylator resistance through modulation of the AKT and β-catenin
signaling pathways. Oncol Rep. 26:1173–1180. 2011.PubMed/NCBI
|
|
5
|
Sinnberg T, Menzel M, Ewerth D, Sauer B,
Schwarz M, Schaller M, Garbe C and Schittek B: β-Catenin signaling
increases during melanoma progression and promotes tumor cell
survival and chemoresistance. PLoS One. 6:e234292011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mrugala MM and Chamberlain MC: Mechanisms
of disease: Temozolomide and glioblastoma-look to the future. Nat
Clin Pract Oncol. 5:476–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Agnihotri S, Gajadhar AS, Ternamian C,
Gorlia T, Diefes KL, Mischel PS, Kelly J, McGown G, Thorncroft M,
Carlson BL, et al: Alkylpurine-DNA-N-Glycosylase confers resistance
to temozolomide in xenograft models of glioblastoma multiforme and
is associated with poor survival in patients. J Clin Invest.
122:253–266. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Motomura K, Natsume A and Wakabayashi T:
Intravenous administration of temozolomide as a useful alternative
over oral treatment with temozolomide capsules in patients with
gliomas. J Neuroncol. 106:209–211. 2012. View Article : Google Scholar
|
|
9
|
Cahill DP, Levine KK, Betensky RA, Codd
PJ, Romany CA, Reavie LB, Batchelor TT, Futreal PA, Stratton MR,
Curry WT, et al: Loss of the mismatch repair protein MSH6 in human
glioblastomas is associated with tumor progression during
temozolomide treatment. Clin Cancer Res. 13:2038–2045. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yip S, Miao J, Cahill DP, Iafrate AJ,
Aldape K, Nutt CL and Louis DN: MSH6 mutations arise in
glioblastomas during temozolomide therapy and mediate temozolomide
resistance. Clin Cancer Res. 15:4622–4629. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Avgeropoulos NG and Batchelor TT: New
treatment strategies for malignant gliomas. Oncologist. 4:209–224.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu K, Zhang Z, Pei H, Wang H, Li L and Xia
Q: FoxO3a induces temozolomide resistance in glioblastoma cells via
the regulation of β-catenin nuclear accumulation. Oncol Rep.
37:2391–2397. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peifer M, Rauskolb C, Williams M,
Riggleman B and Wieschaus E: The segment polarity gene armadillo
interacts with the wingless signaling pathway in both embryonic and
adult pattern formation. Development. 111:1029–1043.
1991.PubMed/NCBI
|
|
14
|
Noordermeer J, Klingensmith J, Perrimon N
and Nusse R: Dishevelled and armadillo act in the wingless
signalling pathway in drosophila. Nature. 367:80–83. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Z, Chen H, Chen Y and Cheng X:
Significance of beta-catenin and Cyclin D1 express in glioma. Xi
Bao Yu Fen Zi Mian Yi Xue Za Zhi. 25:1010–1012. 2009.(In Chinese).
PubMed/NCBI
|
|
16
|
Liu X, Wang L, Zhao S, Ji X, Luo Y and
Ling F: β-Catenin overexpression in malignant glioma and its role
in proliferation and apoptosis in glioblastma cells. Med Oncol.
28:608–614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu C, Tu Y, Sun X, Jiang J, Jin X, Bo X,
Li Z, Bian A, Wang X, Liu D, et al: Wnt/Beta-Catenin pathway in
human glioma: Expression pattern and clinical/prognostic
correlations. Clin Exp Med. 11:105–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pukkila M, Virtaniemi J, Kumpulainen E,
Pirinen RT, Johansson RT, Valtonen HJ, Juhola MT and Kosma VM:
Nuclear beta catenin expression is related to unfavourable outcome
in oropharyngeal and hypopharyngeal squamous cell carcinoma. J Clin
Pathol. 54:42–47. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Elzagheid A, Buhmeida A, Korkeila E,
Collan Y, Syrjänen K and Pyrhönen S: Nuclear beta-catenin
expression as a prognostic factor in advanced colorectal carcinoma.
World J Gastroenterol. 14:3866–3871. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang CL, Liu D, Ishikawa S, Nakashima T,
Nakashima N, Yokomise H, Kadota K and Ueno M: Wnt1 overexpression
promotes tumour progression in non-small cell lung cancer. Eur J
Cancer. 44:2680–2688. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Noda T, Nagano H, Takemasa I, Yoshioka S,
Murakami M, Wada H, Kobayashi S, Marubashi S, Takeda Y, Dono K, et
al: Activation of wnt/beta-catenin signalling pathway induces
chemoresistance to interferon-alpha/5-fluorouracil combination
therapy for hepatocellular carcinoma. Br J Cancer. 100:1647–1658.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Accili D and Arden KC: FoxOs at the
crossroads of cellular metabolism, differentiation, and
transformation. Cell. 117:421–426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Myatt SS and Lam EWF: The emerging roles
of forkhead box (Fox) proteins in cancer. Nat Rev Cancer.
7:847–859. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen J, Gomes AR, Monteiro LJ, Wong SY, Wu
LH, Ng TT, Karadedou CT, Millour J, Ip YC, Cheung YN, et al:
Constitutively nuclear FOXO3a localization predicts poor survival
and promotes akt phosphorylation in breast cancer. PLoS One.
5:e122932010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Storz P, Döppler H, Copland JA, Simpson KJ
and Toker A: FOXO3a promotes tumor cell invasion through the
induction of matrix metalloproteinases. Mol Cell Biol.
29:4906–4917. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu K, Pei H, Zhang Z, Dong S, Fu RJ, Wang
WM and Wang H: FoxO3a mediates glioma cell invasion by regulating
MMP9 expression. Oncol Rep. 36:3044–3050. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun J, Wei H, Yi J, Chen J and Tian B:
Human umbilical cord mesenchymal stem cells restore imatinib and
doxorubicin sensitivity in drug-resistant chronic myeloid leukemia
cells. Int J Clin Exp Med. 11:2142–2147. 2018.
|
|
28
|
Aldonza MBD, Hong JY and Lee SK:
Paclitaxel-Resistant cancer cell-derived secretomes elicit
ABCB1-associated docetaxel cross-resistance and escape from
apoptosis through FOXO3a-driven glycolytic regulation. Exp Mol Med.
49:e2862017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dwivedi A, Slater SC and George SJ: MMP-9
and-12 cause N-cadherin shedding and thereby beta-catenin
signalling and vascular smooth muscle cell proliferation.
Cardiovasc Res. 81:178–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tenbaum SP, Ordóñez-Morán P, Puig I,
Chicote I, Arqués O, Landolfi S, Fernández Y, Herance JR, Gispert
JD, Mendizabal L, et al: β-Catenin confers resistance to PI3K and
AKT inhibitors and subverts FOXO3a to promote metastasis in colon
cancer. Nat Med. 18:8922012. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kahlert U, Nikkhah G and Maciaczyk J:
Epithelial-To-Mesenchymal (-like) transition as a relevant
molecular event in malignant gliomas. Cancer Lett. 331:131–138.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Laios A, Mohamed BM, Kelly L, Flavin R,
Finn S, McEvoy L, Gallagher M, Martin C, Sheils O, Ring M, et al:
Pre-Treatment of platinum resistant ovarian cancer cells with an
MMP-9/MMP-2 inhibitor prior to cisplatin enhances cytotoxicity as
determined by high content screening. Int J Mol Sci. 14:2085–2103.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fiore E, Fusco C, Romero P and Stamenkovic
I: Matrix metalloproteinase 9 (MMP-9/gelatinase B) proteolytically
cleaves ICAM-1 and participates in tumor cell resistance to natural
killer cell-mediated cytotoxicity. Oncogene. 21:5213–5223. 2002.
View Article : Google Scholar : PubMed/NCBI
|