Introduction
Hematopoietic stem cell transplantation (HSCT) has
been identified as a potential curative treatment for leukemia
(1,2). However, previous studies have
demonstrated that patients who develop chronic graft-versus-host
disease (cGVHD) following HSCT have an increased risk of developing
solid tumors, such as squamous cell carcinoma (SCC) (3–5); In a
large cohort of HSCT recipients, the oral cavity is one of the most
common SCC sites, accounting for 15% of all solid cancers (6,7). And
cGVHD is a significant risk factor independently associated with
the development of secondary carcinoma (8,9). This is
primarily considered to occur due to the immunosuppressive
therapeutic regimen, which generally includes cyclosporine,
tacrolimus and corticosteroids (10). The present case report described a
patient with multiorgan cGVHD, who developed quadruple SCC in the
oral cavity, esophagus and skin following the prolonged exposure to
combined immunosuppressant drug therapies. The main purpose of the
present study was to determine its pathogenesis, and to determine
whether p53, EGFR, KRAS, BRAF genes and immune status are related
to tumors after HSCT, and to provide novel methods for the
diagnosis and treatment of similar cases. In conclusion, the
presence of microsatellite instability after HSCT indicated the
instability of the genome, and might be the basis for other genetic
changes. The P53 mutation might be an important promoter of
multiple cancers after transplantation in the reported patient;
high expression of EGFR protein increased the activity of MAPK
signal transduction pathway and promoted the occurrence of tumors
to a certain extent. Additionally, the long-term use of
immunosuppressive agents after transplantation might promote the
occurrence of tumors. Furthermore, advances in treatment and
supportive care have translated into steady improvements in
survival after HSCT (5). With larger
numbers of long-term survivors, quantifying the late effects and
related complications of transplantation has been a research
priority.
Case report
Patient history
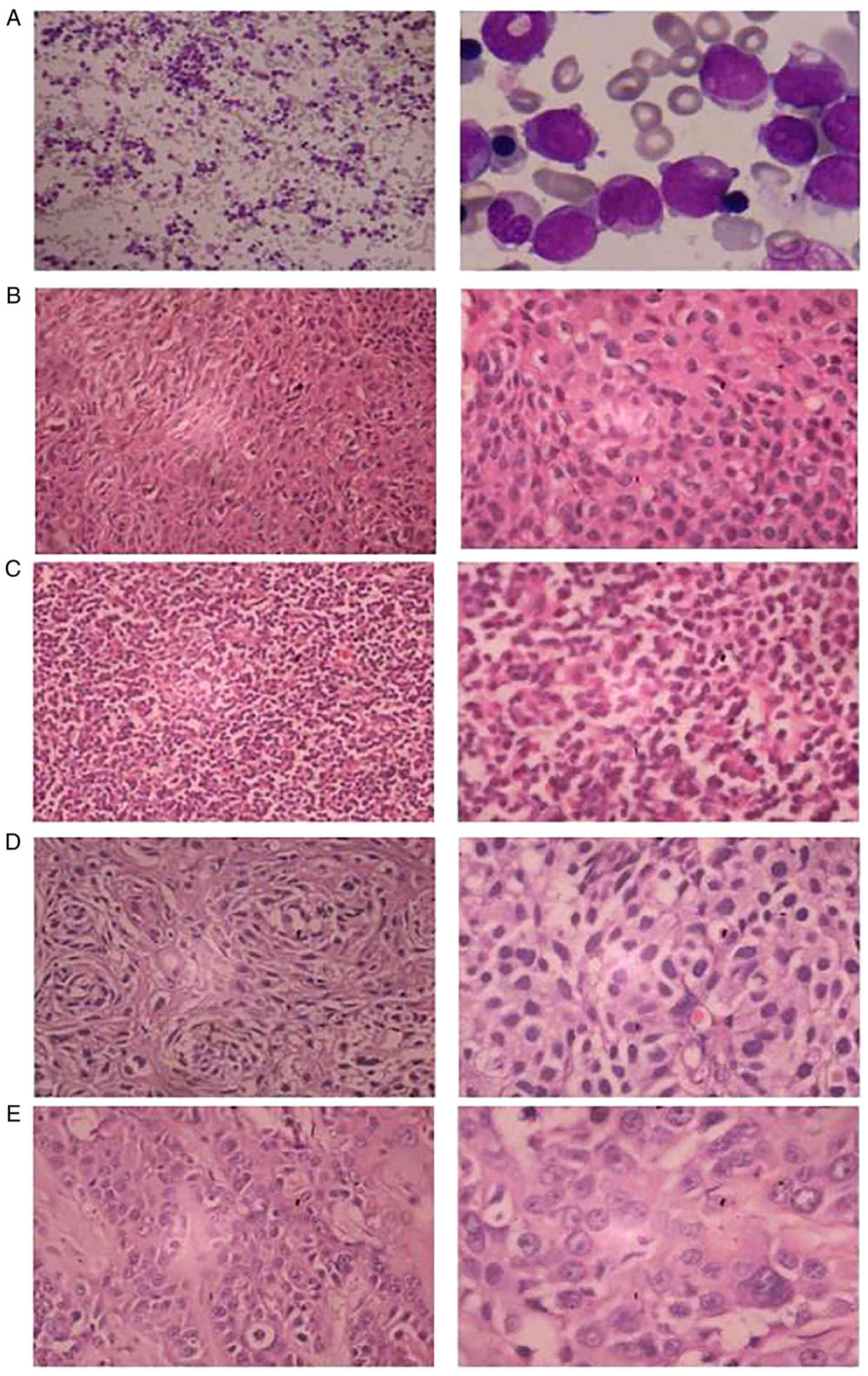
A 41-year-old male presented in September 1998 with
a history of fever for 10 days, who was subsequently diagnosed with
acute nonlymphocytic leukemia (M2a type) by bone marrow
examination using Wright's and Giemsa staining (Fig. 1A). This staining was performed by
incubation of samples with 100% methanol for 1–2 min, followed by
incubation with the Wright stain for 4 min, working buffer for a
further 4 min and working Giemsa stain for 4 min. The samples were
then washed in distilled water and observed under a light optical
microscope. The patient was treated with chemotherapy at regular
intervals, which achieved a complete remission that lasted ~2.5
years. The chemotherapy regimens included daunorubicin and
cytarabine (‘DA’), omacetaxine mepesuccinate, cytarabine and
daunorubicin (‘HAD’), omacetaxine mepesuccinate, cytarabine and
vumon (‘HA+Vm26′), daunorubicin, cytarabine and epirubicin (‘DAE’),
omacetaxine mepesuccinate, oncovin, cytarabine and prednisone
(‘HOAP’). The patient relapsed in August 2000, and then underwent
an allogeneic peripheral blood (allo)-HSCT in Beijing from a fully
human leukocyte antigen-matched male sibling. The myeloablative
conditioning regimen consisted of total body irradiation, high dose
cyclophosphamide and granulocyte colony-stimulating factor.
Following the HSCT, cyclosporin A was prescribed to prevent GVHD
prophylactically for 1 year. However, upon terminating the
treatment regimen, the patient developed extensive multiorgan
cGVHD, including a skin rash, stomatitis, diarrhea, Sjögren's
syndrome and nephrotic syndrome, which was subsequently controlled
by the combined treatment of a glucocorticoid and mycophenolate
mofetil for 1.5 years. In 2004, the patient suffered condyloma
acuminate, which did not recur following laser treatment
The patient was subsequently diagnosed with
middle-differentiation squamous carcinoma of the right upper lip
(Fig. 1B), poor-differentiation
squamous carcinoma of the bottom left gum (Fig. 1C), middle-differentiation squamous
carcinoma in the lower part of the esophagus (Fig. 1D) and high- and
middle-differentiation squamous carcinoma of the left submaxillary
gland (Fig. 1E) using H&E
staining in the Second Xiangya Hospital of Central South University
in July 2005, January 2007, October 2008 and October 2009,
respectively. H&E staining was performed on 4-µm-thick tissue
sections. Specimens were stained with hematoxylin for 5 min and 1%
eosin for 1 min, and observed under a light optical microscope
(magnifications, ×200 and ×400).
Each squamous carcinoma was treated with surgery and
no metastasis was identified during the lymphadenectomy. The
patient died in December 2009 due to postoperative pulmonary
infection and malnutrition.
The patient was described as healthy beforehand,
with no significant family history, and no history of smoking, or
alcohol or betel nut use.
Methods
The specimens used in the following method were all
from the patient who was diagnosed with quadruple SCC following
allogeneic HSCT for leukemia. Cell-free DNA was extracted from the
blood plasma of the peripheral blood of the patient in 2009 (since
tumor cells in the peripheral blood are easier to lyse compared
with normal blood cells, cell-free DNA from tumor cells are shed
from the blood). Tissue samples were routinely fixed in 10% (v/v)
neutral formalin, and embedded in paraffin at a temperature of
60°C. The paraffin-embedded sections were then cut into
3–4-µm-thick sections, mounted on slides and then deparaffinized.
To block endogenous peroxidase activity, slides were treated for 5
min at room temperature with 3% H2O2, diluted
in distilled water in the case of protease digestion. And the
classical phenol-chloroform method was used to extract tissue DNA
(11). Subsequently, PCR was
performed to amplify the p53, EGFR, KRAS and BRAF genes, in
addition to five microsatellite accepted loci, including D2S123,
D5S346, D17S250, BAT25 and BAT26. The thermocycling conditions
utilized are presented in Tables SI
and SII. Denaturing
high-performance liquid chromatography (DHPLC) was used to screen
for mutations in the PCR amplification products of the p53, EGFR,
KRAS and BRAF genes present in the blood plasma of the patient's
peripheral blood (Table SIII).
DHPLC was performed using the Transgenomic Wave Nucleic Acid
Fragment Analysis System with a DNASep column (Transgenomic, Inc.).
Triethylammonium acetate at 0.05% acetonitrile in 0.1 M (TEAA;
eluent A) and 25% acetonitrile in 0.1 M TEAA (eluent B) were
comprised in the mobile phases. Using pericancerous tissues as a
comparison, after surgery to remove the carcinoma of the gum, an
ideal column temperature of 50°C was used to analyze the presence
of microsatellite instability (MSI) in the patient's tumor tissue
in five accepted loci (D2S123, D5S346, D17S250, BAT25 and BAT26).
Then, the ABI 3730 instrument (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used to sequence the p53, EGFR, KRAS and BRAF
gene amplification products from the paraffin-embedded tumor
tissues following the four operations. Immunohistochemistry was
also used to analyze the expression levels of p53 and EGFR in the
tumor cells of the tissue samples. Immunohistochemistry was
performed by adding 3% hydrogen peroxide solution to the tissue
sections for 15 min at room temperature in the dark to eliminate
the endogenous peroxidase activity of the tissue. Then, mouse
anti-human p53 monoclonal antibody (1:50; cat. no. MAB-0674; Fuzhou
Maixin Biotech Co., Ltd.), and mouse anti-human EGFR monoclonal
antibody (1:50; cat. no. MAB-0196; Fuzhou Maixin Biotech Co., Ltd.)
were used as primary antibodies and incubated in a humidified
chamber at 4°C overnight. The used antibody dilution followed the
guidance provided in the kit instructions. On the following day,
tissue samples were incubated with the secondary biotin-labeled
goat anti-rabbit antibody (UltraSensitive SP; cat. no. KIT-9709;
Fuzhou Maixin Biotech Co., Ltd.), at room temperature for 15 min.
Brown-yellow particles in the cell indicated positive cell
staining. Each slide was counted in 5 high-power fields randomly
selected under a light optical microscope (magnification, ×400),
and the proportion of positive cells stained was counted as
follows: i) ≤25%, (-); ii) 25–50%, (+); iii) 50–75%, (++); and iv)
>75%, (+++). Finally, a FACSCalibur™ flow cytometer (FACS101; BD
Biosciences) was used to analyze the lymphocyte subsets present in
the patient's peripheral blood to roughly estimate the patient's
immune state. Analysis was performed with the CellQuest software
(BD Biosciences).
Results
Genetic mutations identified in the
tumor cells of the peripheral blood
For the tumor suppressor gene P53 exon E5, the
sample peak shape was different from the control peak shape at
64.5°C; at 65.5°C, the peak shape was not easy to judge due to
insufficient injection volume, and the sample exon E5 was
considered to be a suspicious mutation. No mutations were found in
EGFR, BRAF and KRAS genes (Table
I).
 | Table I.Gene mutations from cell-free tumor
cells of the blood detected by denaturing high-performance liquid
chromatography. |
Table I.
Gene mutations from cell-free tumor
cells of the blood detected by denaturing high-performance liquid
chromatography.
| A, EGFR |
|---|
|
|---|
| Exon | Detection
result | Interpretation
result |
|---|
| E18 | Peak shape of the
sample is same as that of the control | No mutations |
| E19 | Peak shape of the
sample is same as that of the control | No mutations |
| E20 | Sample
amplification failed |
|
| E21 | Peak shape of the
sample is same as that of the control | No mutations |
|
| B, BRAF |
|
| E11 | Peak shape of the
sample is same as that of the control | No mutations |
| E15 | Peak shape of the
sample is same as that of the control | No mutations |
|
| C, KRAS |
|
| E2 | Peak shape of the
sample is same as that of the control | No mutations |
| E3 | Peak shape of the
sample is same as that of the control | No mutations |
|
| D, p53 |
|
| E5 | 64.5°C, peak shape
of the sample differs from that of the control; 65.5°C, it is hard
to judge due to insufficient sampling volume; other two
temperatures, peak shape of the sample is same as that of the
control | Suspicious
mutations |
| E6 | Peak shape of the
sample is same as that of the control | No mutations |
| E7 | Sample
amplification failed |
|
| E8 | Peak shape of the
sample is same as that of the control | No mutations |
Sequencing results of amplified gene
products in the tumor tissues
There was a C-A mutation in the EGFR gene E20 of
gingival cancer tissue. The amino acid coded before and after the
mutation was the same as isoleucine, and E21 had a G-A mutation,
which encodes valine before and after the mutation. None of the
mutations caused any changes in gene function. No abnormality was
found in the sequencing results of BRAF and KRAS (Table II).
 | Table II.Gene sequencing result in tumor
tissues. |
Table II.
Gene sequencing result in tumor
tissues.
| A,
Cheilocarcinoma |
|---|
|
|---|
| Gene | Exon | Detection
result | Interpretation
result |
|---|
| EGFR | E18 | Normal |
|
|
| E19 | Normal |
|
|
| E20 | Normal |
|
|
| E21 | Normal |
|
| BRAF | E11 | Normal |
|
|
| E15 | Normal |
|
| KRAS | E2 | Normal |
|
|
| E3 | Low sequencing
quality |
|
| p53 | E5 | C-A
(serine-termination) |
|
|
| E6 | Normal |
|
|
| E7 | G-A (mutation of
intron) | Mutation of
intron |
|
| E8 | A-T being
suspicious (low mutation proportion; glutamic acid-valine) |
|
|
| B, Gingival
carcinoma |
|
| EGFR | E18 | Normal |
|
|
| E19 | Normal |
|
|
| E20 | C-A
(isoleucine-isoleucine) |
|
|
| E21 | G-A
(valine-valine) |
|
| BRAF | E11 | Normal |
|
|
| E15 | Normal |
|
| KRAS | E2 | Normal |
|
|
| E3 | Low sequencing
quality |
|
| p53 | E5 | G-A
(leucine-leucine) |
|
|
|
| A-G
(histidine-arginine) |
|
|
| E6 | Normal |
|
|
| E7 | Low sequencing
quality, suspicious C-A |
|
|
| E8 | A-T being
suspicious (low mutation proportion; glutamic acid-valine) |
|
|
| C, Esophageal
carcinoma |
|
| EGFR | E18 | Normal |
|
|
| E19 | Normal |
|
|
| E20 | Normal |
|
|
| E21 | Normal |
|
| BRAF | E11 | Not yet
amplify |
|
|
| E15 | Normal |
|
| KRAS | E2 | Normal |
|
|
| E3 | Low sequencing
quality |
|
| p53 | E5 | G-A
(leucine-leucine) |
|
|
| E6 | Normal |
|
|
| E7 | G-A | Glutamic acid
altered to terminator codon |
|
| E8 | Normal |
|
|
| D, Submaxillary
carcinoma |
|
| EGFR | E18 | Normal |
|
|
| E19 | Normal |
|
|
| E20 | Normal |
|
|
| E21 | Normal |
|
| BRAF | E11 | Normal |
|
|
| E15 | Normal |
|
| KRAS | E2 | Low sequencing
quality |
|
|
| E3 | Low sequencing
quality |
|
| p53 | E5 | A-G
(histidine-arginine) |
|
|
| E6 | Normal |
|
|
| E7 | Normal |
|
|
| E8 | Normal |
|
For the sequencing of the P53 gene in
cheilocarcinoma, it was found that there was a C-A mutation in E5,
which converted the original codon encoding serine into a stop
codon. A G-A mutation in the non-coding region of E7 of P53, which
is an intron mutation, was observed. In the P53 gene E8 there was a
suspicious A-T mutation in the coding region, and the coding amino
acid changed from glutamic acid to valine. In gingival carcinoma
tissue G-A and A-G mutations were detected in E5 of P53. The
formerly coded amino acids were unchanged and both were leucine,
whereas the latter changed from coding histidine to arginine. There
was a suspected non-coding region C-A mutation at E7. The
proportion of mutations in the coding region was low at E8, and A-T
mutations were suspected, the encoded amino acid was changed from
glutamic acid to valine. Sequencing of the P53 gene in esophageal
carcinoma tissue showed that E5 had G-A mutations, the encoded
amino acids before and after the mutation were unchanged, and all
were leucine. E7 had G-A mutations, which meant that the codon GAA
changed to AAA, that is, glutamate changed to a stop codon.
Sequencing of E5 in the P53 gene in submaxillary carcinoma tissue
revealed an A-G mutation, and the encoded amino acid changed from
histidine to arginine (Table
II).
Protein expression levels of p53 and
EGFR in the tumor tissues
The immunohistochemical results were divided into
three grades according to the number of positive cells and the
positive intensity. The expression levels of P53 protein in the
tissues of cheilocarcinoma, gingival carcinoma and esophageal
carcinoma were (++), (+++) and (+++), respectively. The expression
levels of EGFR in cheilocarcinoma, gingival carcinoma, esophageal
carcinoma and carcinoma of submaxillary were (+~++), (+), (++) and
(++), respectively. Therefore, the expression levels of EGFR and
P53 protein in the four tumor tissues were different (Table III).
 | Table III.Protein expression level of p53 and
EGFR in tumor tissues. |
Table III.
Protein expression level of p53 and
EGFR in tumor tissues.
| Tumor tissue
(paraffin-embedded tumor tissue) | Protein
expression | Detection
result |
|---|
|
Cheilocarcinoma | EGFR | (+~++) |
|
| p53 | (++) |
| Gingival
carcinoma | EGFR | (+) |
|
| p53 | (+++) |
| Esophageal
carcinoma | EGFR | (++) |
|
| p53 | (+++) |
| Submaxillary
carcinoma | EGFR | (++) |
|
| p53 | Off |
Presence of MSI in the tumor
tissues
Using pericancerous tissues of gingival carcinoma as
a reference, the size of the gene segments in the cheilocarcinoma
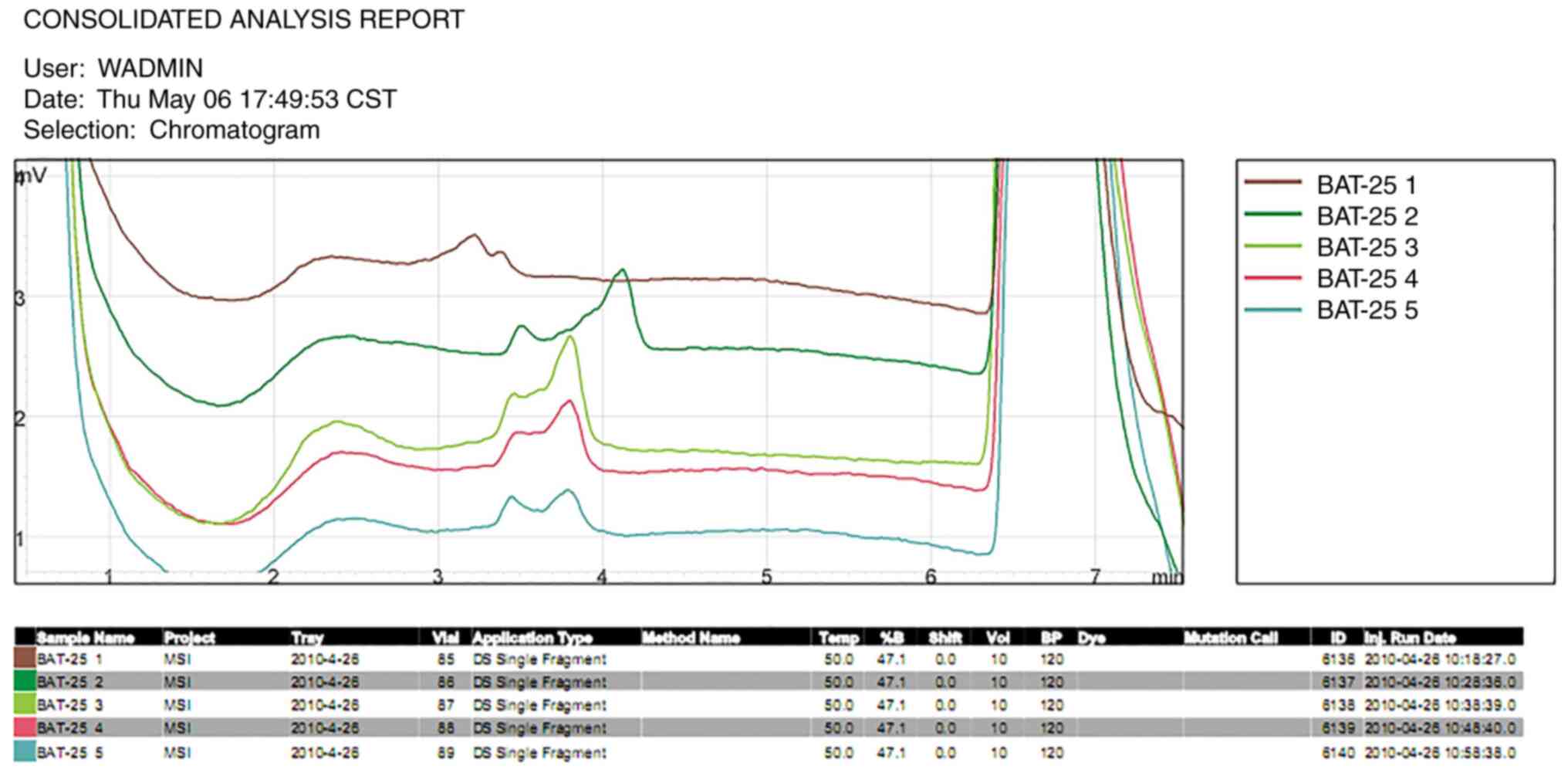
and esophageal carcinoma tissues at the BAT25 (Fig. 2) locus were reported to be altered.
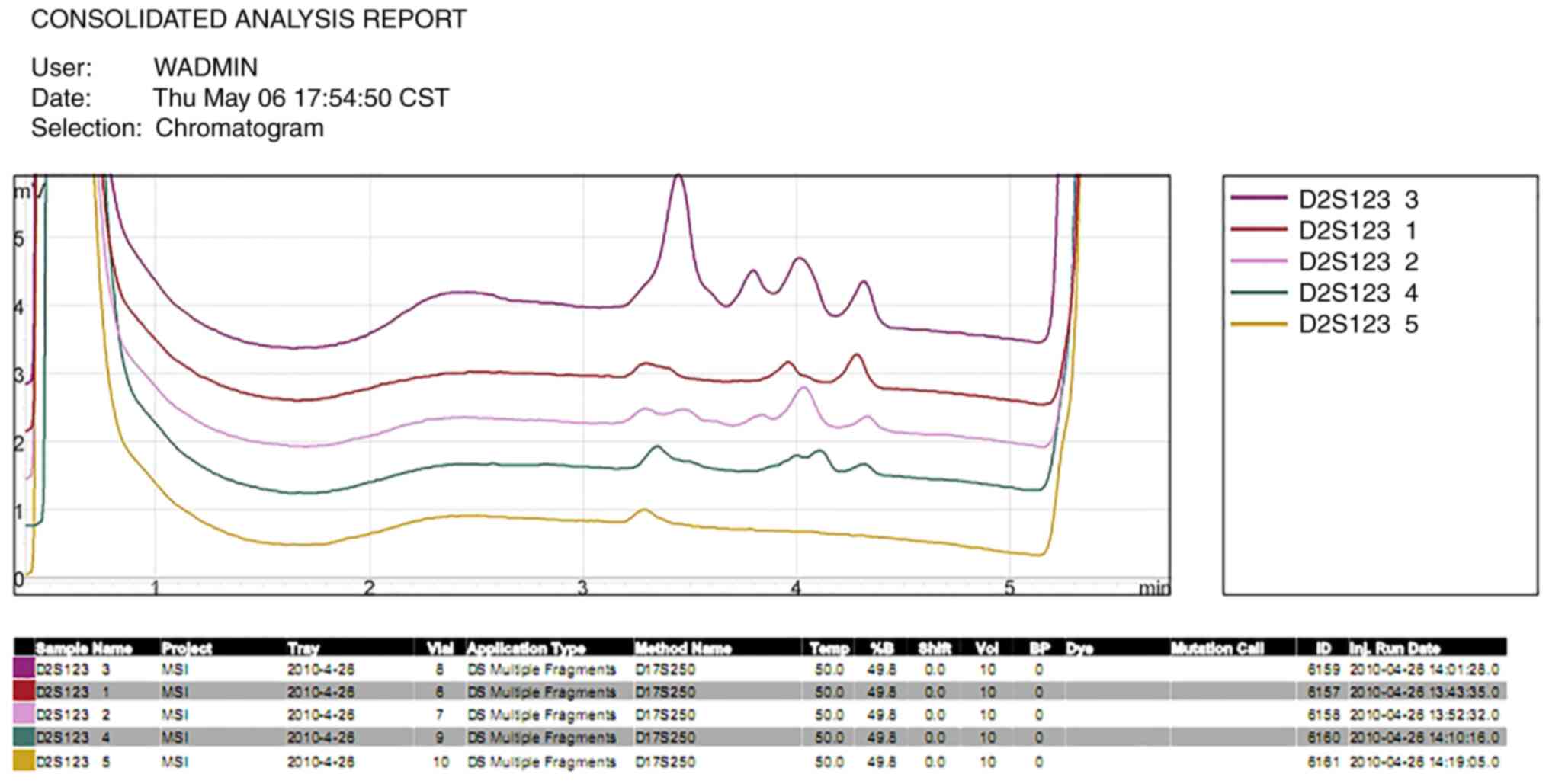
However, the genes in the control group at the D2S123 locus
(Fig. 3) were not amplified.
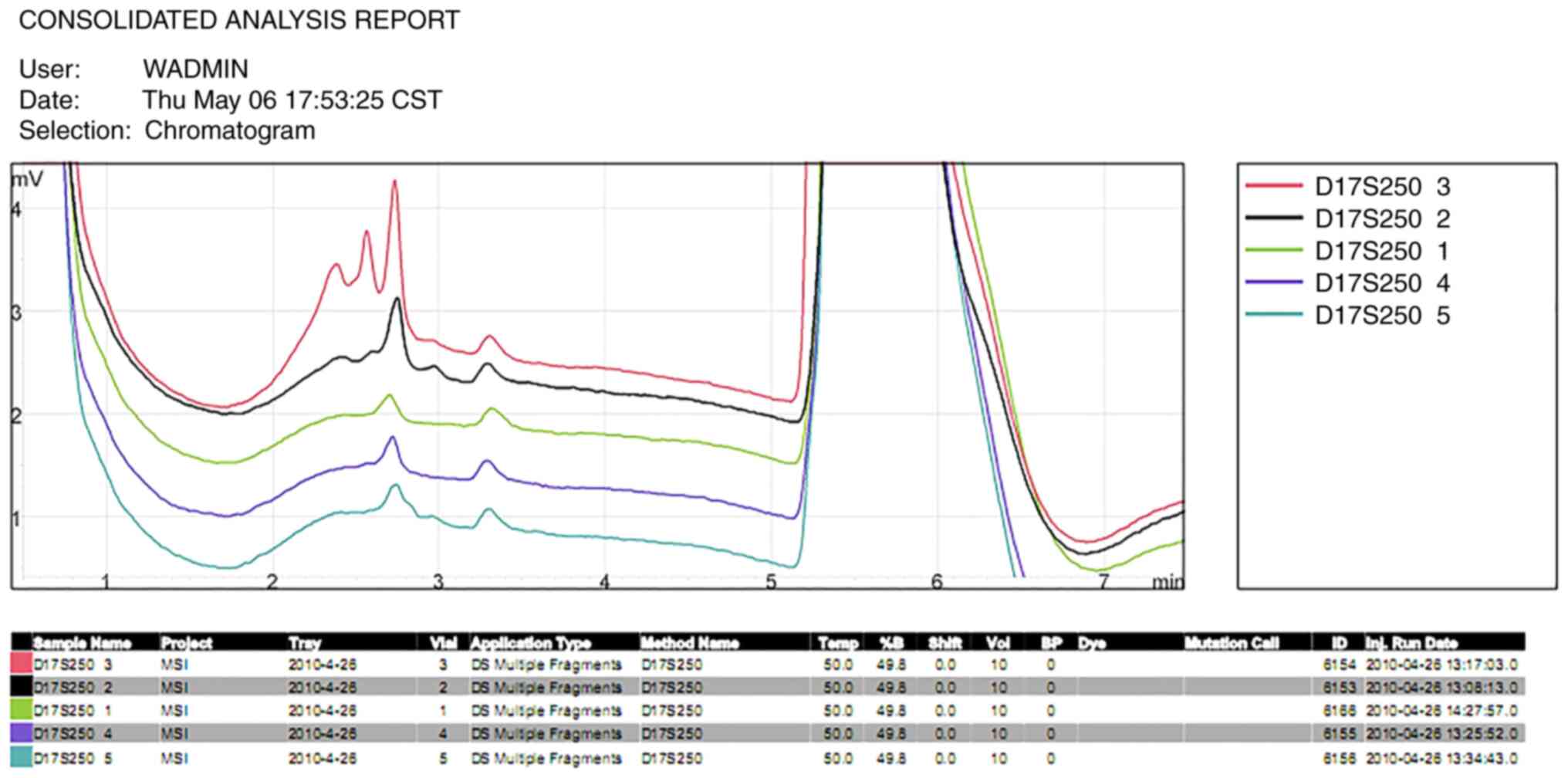
Notably, the length of every gene segment in the submaxillary gland
carcinoma tissue differed from that of the other tumor tissues, the
length of the microsatellite gene segments in the submaxillary
gland carcinoma tissues were altered at the D17S250 locus (Fig. 4) and no significant differences were
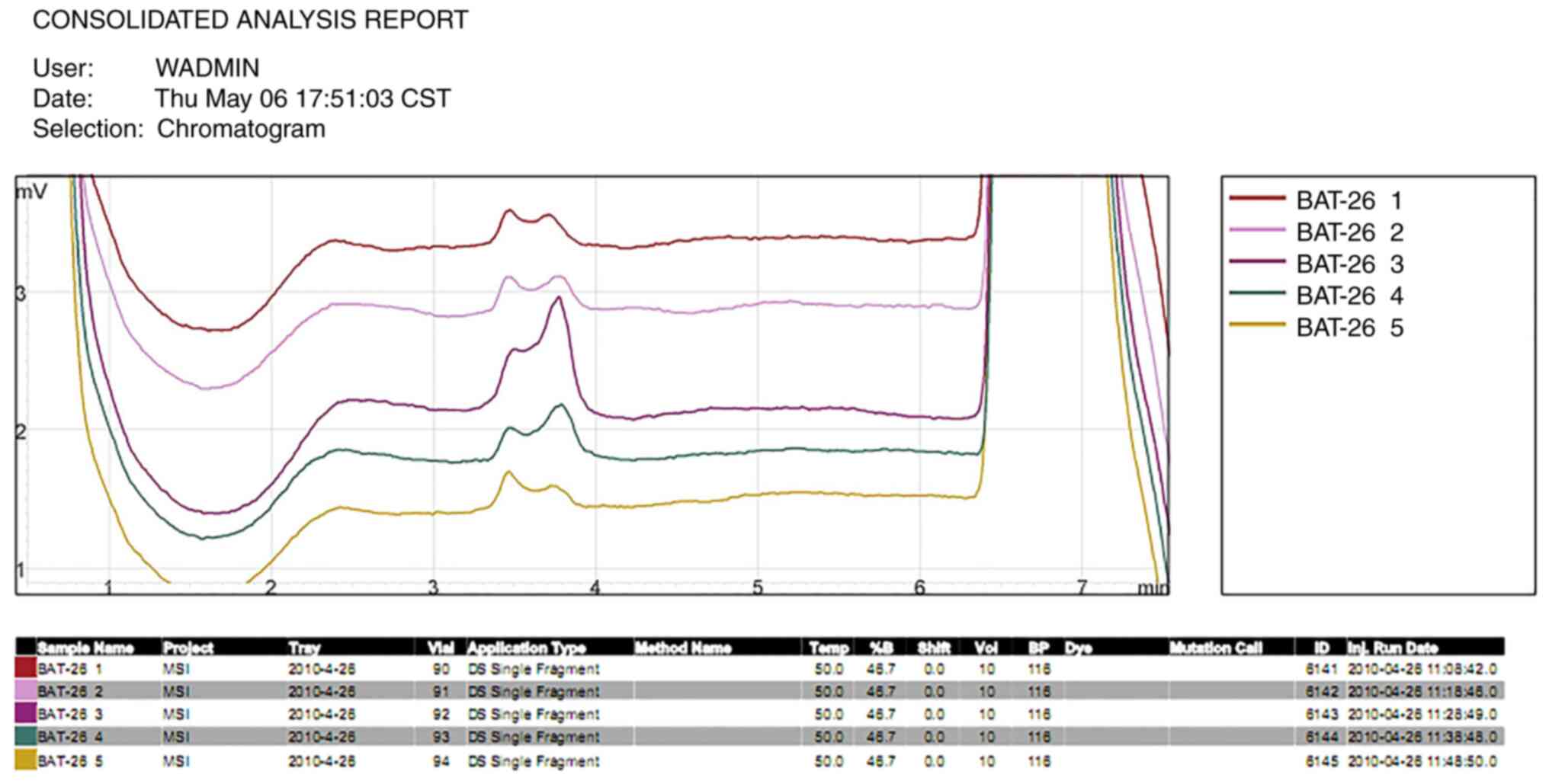
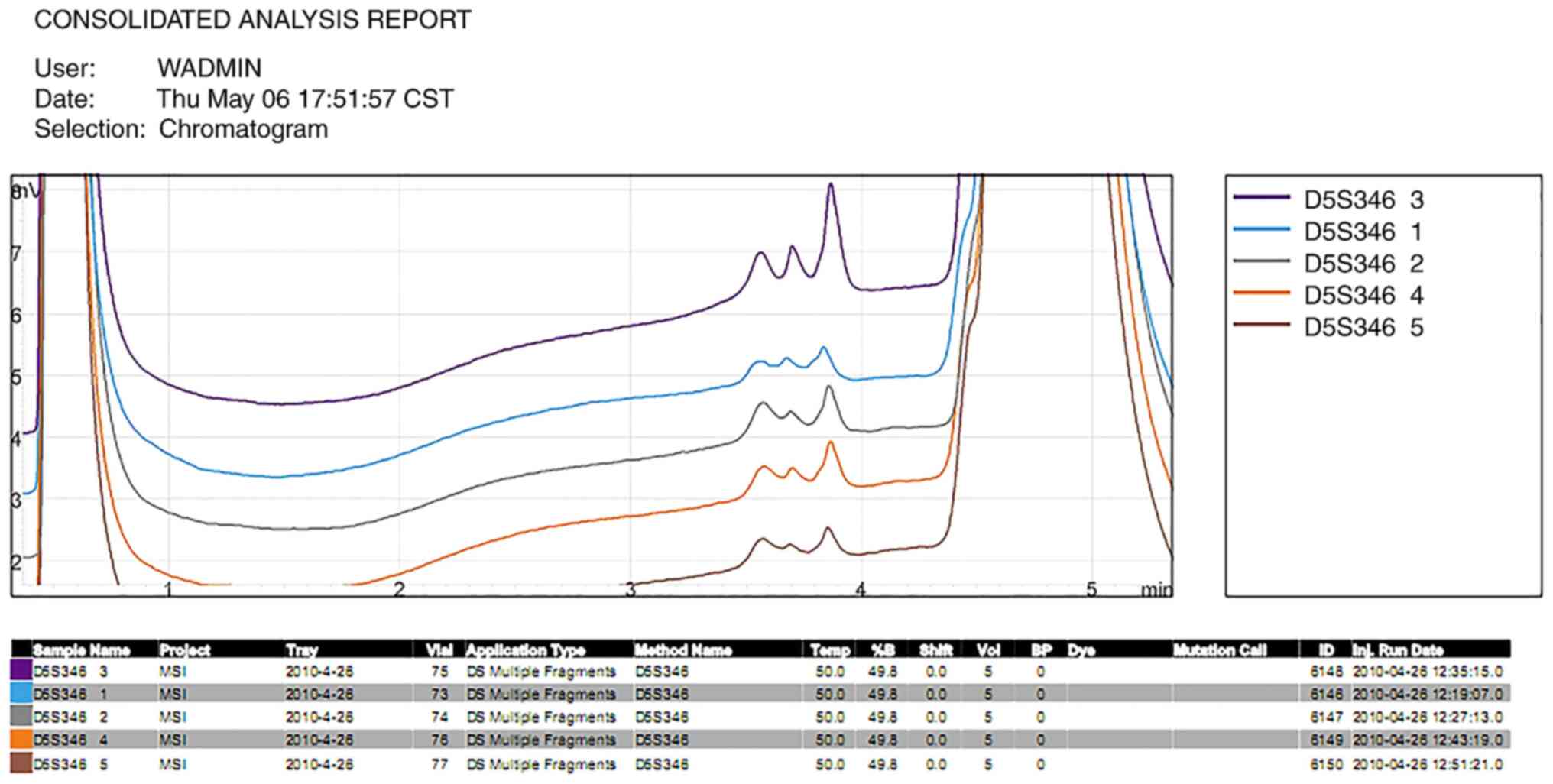
identified in the length of the DNA segment at the BAT26 (Fig. 5) and D5S346 (Fig. 6) loci in the four tumor tissues.
MSI was identified in cheilocarcinoma tissues at the
BAT25 locus, in esophageal carcinoma tissues at the BAT25 locus and
in submaxillary gland carcinoma tissues at the D2S123 and D17S250
loci; however, no abnormalities were identified in the gingival
carcinoma tissues at any of the five microsatellite loci.
Identification of lymphocyte subsets
in the peripheral blood
The lymphocyte subsets present in the peripheral
blood were analyzed using flow cytometry. However, due to the
present study being retrospective, the patient's peripheral blood
was only extracted following the final operation. The results
revealed the following ratio of T cells: i) CD3+ T
cells, 56% (55–84%); ii) CD8+ T cells, 29% (13–41%);
iii) CD4+ T cells, 26% (31–60%); and iv)
CD4+/CD8+ ratio, 0.9 (1.4–2.5), thus the
ratio was decreased.
Discussion
There has been a number of reported cases (12,13) of
multiple primary carcinomas (MPCs) in recent years. For example,
Fukuda et al (14) studied
p53 mutations in relation to MPCs in a case of four separate oral
cancers. However, to the best of our knowledge, the present case
study was the first case involving quadruple SCC following HSCT, of
which the presence of multiorgan cGVHD and the prolonged exposure
to immunosuppressive therapies were factors suggested to be related
to the development of SCC.
Currently, according to Warren's diagnostic
criterion of MPCs in 1932 (15), the
following criteria can be used to diagnose a patient with rare
MPCs: i) the four squamous carcinomas are of different degrees and
pathomorphologies; ii) the MPCs are located at different sites and
not adjacent; and iii) the onset is during an early stage, where
there is less opportunity for metastasis. The risk factors for MPCs
in patients with head and neck cancer include an older age, being
male, and smoking and alcohol consumption (16). In addition, it has been reported that
MPCs most frequently occurred in the keratinized epithelium, such
as the gingiva and hard palate (17), which is consistent with the findings
in the present case study. However, the patient of the present
study had no history of smoking, or alcohol or betel nut use, which
suggested that other risk factors may be involved. Thus, close
attention was paid to the underlying mechanism.
Allogeneic HSCT is a potential curative option for
numerous hematological malignancies. However, patients undergoing
allografts are at an increased risk of developing a second solid
cancer, in which the overall survival varies depending on the type
of secondary cancer (5,18,19). A
previous study (20) demonstrated
that being male was associated with the development of cutaneous
and oral SCC following HSCT. Furthermore, the age at
transplantation, exposure to radiation as part of the conditioning
regimen and chronic GVHD were identified as important risk factors
for invasive solid cancers (18,21).
Specifically, GVHD is a major complication of allogeneic HSCT,
which occurs as a result of complex immunological and inflammatory
interactions, and it has been demonstrated to be responsible for
significant morbidity and mortality rates (22,23). In
addition, taking long-term immunosuppressants was discovered to be
a major risk factor, especially in combination with other drugs,
such as azathioprine, cyclosporin ciclosporin and hormones
(24,25). For instance, the patient in the
present study was a male who took ciclosporin regularly for 1 year
following HSCT, after which GVHD occurred soon after drug
withdrawal. The patient continued to receive immunization therapy
for half a year with a glucocorticoid and 1-year immunization
therapy with mycophenolate. In addition, the peripheral blood
lymphocyte subsets present in the patient were investigated; the
results revealed that the ratio of CD4+/CD8+
T cells was markedly decreased, which indicated that the patient
was under a state of immunosuppression. It was previously reported
that immunosuppressive conditions led to infections and the
reproduction of the tumor virus, which eventually developed into
malignant tumors related to said virus (26,27). The
patient suffered condyloma acuminate, which is an HPV infection. In
addition, inflammatory and epithelial cells release reactive oxygen
and nitrogen species that are capable of causing DNA damage, which
has been reported to serve important roles in cancer development
(28). Therefore, the patient who
underwent HSCT and developed GVHD in the present study was at an
increased risk of developing a secondary cancer. Nevertheless, the
mechanism by which this occurred remains unclear.
Moreover, genomic alterations in the mucosal
epithelium have been frequently reported following allogeneic HSCT,
especially among tissues affected by GVHD; this occurrence is
considered to contribute to the development of secondary
malignancies (29). In these
circumstances, MSI has been widely identified in the genome of the
patient's tumor tissue, where it was suggested to underlie the
basic pathology of the tumorigenic process. Furthermore, under
inflammatory conditions, inflammatory and epithelial cells release
reactive oxygen and nitrogen species, which are capable of causing
DNA damage (28). This, it was
hypothesized that the chronic stimulation of inflammation and GVHD,
in addition to the imperfect DNA repair mechanism, may be
associated with the presence of MSI in tissues following
transplantation.
An increasing number of studies have highlighted the
role of mutant p53 proteins in altering the cancer cell secretome
and modifying the tumor microenvironment (30–32).
Therefore, mutations in both the p53 gene and protein in tumor
tissues are likely explanations for the development of the
patient's secondary solid tumor. Since the publication of Knudson's
two-hit hypothesis in 1993 (33), it
is common knowledge that cancer is caused by the accumulation of
mutations in the DNA of cells. The term loss of heterozygosity
(LOH) has gathered increasing attention for its role in
malignancies, and its accumulation in different tumor suppressor
genes suggests the association of LOH with cancer (34). In the p53 gene, exon 4 site has been
discovered to have 66% LOH in heterozygous individuals. From these
results, it was concluded that the inactivation of the p53 gene is
related to the progression of oral cancer (35). In fact, the inactivation of p53 is
considered a common step in the progression of numerous types of
human cancer. Yamasaki et al (36) reported a rare case of SCC in the
tongue dorsum in a 69-year-old man with a history of multiple
cancers, including esophageal cancer, gastric cancer and renal cell
carcinoma. Germline TP53 mutations were also discovered to be risk
factors for uncontrolled cell proliferation (36). In addition, p53 expression levels
were identified as an independent risk factor for early oral
squamous cell carcinoma with dysplastic surgical margins (37), thus additional therapeutics and close
follow-ups are required for these patients. However, to the best of
our knowledge, no previous studies have reported the association
between p53 mutations and HSCT; it was hypothesized that the high
presence of MSI following allogeneic HSCT may inactivate tumor
suppressor genes. Due to the universal presence of p53 gene
mutations in the majority of types of tumor, it cannot be excluded
that such mutations may be caused by non-transplantation factors;
however, it also cannot be denied that HSCT itself, and the
administration of immunosuppressive therapy before and after HSCT
may promote the development of the tumor to a certain degree.
In addition, it was previously demonstrated that p53
interacted with EGFR to promote the activation of the
EGFR/ERK/MMP-2 signaling pathway (38). The MAPK pathway is known to be
closely associated with malignant tumors of the epithelium, and
EGFR, KRAS and BRAF genes are all major effector molecules of the
pathway. In fact, upregulated expression levels of EGFR have been
detected in numerous types of tumor tissues (39). In the present study, no mutations
were identified in cell-free tumor cells of the peripheral blood,
or within the EGFR, KRAS and BRAF genes in the tumor tissue
following the operation; however, immunohistochemistry analysis
revealed that the EGFR protein expression levels in the tumor
tissues were upregulated, which may be related to the amplification
of the EGFR gene or the dysregulation of the MAPK signaling
pathway. Although the results of the present study are unable to
confirm that the overexpression of EGFR was a pathogenic factor for
the patient, it has been suggested that the development of
secondary tumors following HSCT are likely to overexpress EGFR,
which may be sensitive to target-specific drug treatments.
The findings of the present and previous studies
suggested that a frequent and thorough oral and skin examination
should be recommended for all long-term HSCT survivors, with
increased attention provided to those individuals who develop cGVHD
and those on long-term cumulative immunosuppressant therapies. The
primary therapeutic option for MPCs should be surgical removal, as
the prognosis is improved compared with metastatic or recurrent
carcinoma (40,41). In addition, to decrease the number of
deaths related to surgery, such as postoperative pneumonia or
sudden death, nutritional support should also be provided following
the operation.
In conclusion, the long-term use of
immunosuppressants and chronic inflammation caused by HPV infection
following HSCT may lead to DNA damage. The presence of MSI
following HSCT indicated the instability of the genome, which
provided reasons for the mutations found within the p53 gene, and
suggested a major mechanism for the development of secondary
multiple tumors following HSCT. Meanwhile, the identified
upregulated protein expression levels of EGFR may increase the
activity of the MAPK signaling transduction pathway, which may in
turn, promote oncogenesis to a certain extent.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CH participated in the analysis, data interpretation
and experimental design. XW performed the majority of the
experiments. YP analyzed the data and wrote the manuscript. LS
assisted with the experiments. FW designed the experiments and
analyzed the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Second Xiangya Hospital of Central South
University (approval no. 030).
Patient consent for publication
Written informed consent was obtained from the
patient for publication of any accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Copelan EA: Hematopoietic stem-cell
transplantation. N Engl J Med. 354:1813–1826. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kanakry CG, Fuchs EJ and Luznik L: Modern
approaches to HLA-haploidentical blood or marrow transplantation.
Nat Rev Clin Oncol. 13:1322016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Majhail NS, Brazauskas R, Rizzo JD,
Sobecks RM, Wang Z, Horowitz MM, Bolwell B, Wingard JR and Socie G:
Secondary solid cancers after allogeneic hematopoietic cell
transplantation using busulfan-cyclophosphamide conditioning.
Blood. 117:316–322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Majhail NS: Old and new cancers after
hematopoietic-cell transplantation. Hematology Am Soc Hematol Educ
Program. 142–149. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ehrhardt MJ, Brazauskas R, He W, Rizzo JD
and Shaw BE: Survival of patients who develop solid tumors
following hematopoietic stem cell transplantation. Bone Marrow
Transplant. 51:83–88. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rizzo JD, Curtis RE, Socié G, Sobocinski
KA, Gilbert E, Landgren O, Travis LB, Travis WD, Flowers ME,
Friedman DL, et al: Solid cancers after allogeneic hematopoietic
cell transplantation. Blood. 113:1175–1183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inamoto Y, Shah NN, Savani BN, Shaw BE,
Abraham AA, Ahmed IA, Akpek G, Atsuta Y, Baker KS, Basak GW, et al:
Secondary solid cancer screening following hematopoietic cell
transplantation. Bone Marrow Transplant. 50:1013–1023. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen MH, Chang PM, Li WY, Hsiao LT, Hong
YC, Liu CY, Gau JP, Liu JH, Chen PM, Chiou TJ and Tzeng CH: High
incidence of oral squamous cell carcinoma independent of HPV
infection after allogeneic hematopoietic SCT in Taiwan. Bone Marrow
Transplant. 46:567–572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Themeli M, Petrikkos L, Waterhouse M,
Bertz H, Lagadinou E, Zoumbos N, Finke J and Spyridonidis A:
Alloreactive microenvironment after human hematopoietic cell
transplantation induces genomic alterations in epithelium through
an ROS-mediated mechanism: In vivo and in vitro study and
implications to secondary neoplasia. Leukemia. 24:536–543. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, Yu J and Wei W: Advance in
targeted immunotherapy for graft-versus-host disease. Front
Immunol. 9:10872018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Santella RM: Approaches to DNA/RNA
Extraction and whole genome amplification. Cancer Epidemiol
Biomarkers Prev. 15:1585–1587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Obata T, Nakamura M, Mizumoto Y, Matsumoto
T, Takakura M and Fujiwara H: Synchronous endometrioid
adenocarcinomas in the uterine cervix and corpus. J Obstet Gynaecol
Res. 42:1390–1394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Adeyanju MA and Ilori AA: Multiple primary
tumors. Niger J Clin Pract. 20:1346–1349. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fukuda M, Nakatsuka T, Kusama K and
Sakashita H: Patient with multiple primary carcinomas including 4
separate oral cancers: Study of p53 mutations and their
implications for management. J Oral Maxillofac Surg. 64:1672–1679.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Warren S: Multiple primary malignant
tumors. A survey of the literature and a statistical study. Am J
cancer. 16:1358–1414. 1932.
|
|
16
|
Hosokawa S, Takahashi G, Okamura J, Imai
A, Mochizuki D, Takizawa Y, Yamatodani T, Misawa K and Mineta H:
Risk and prognostic factors for multiple primary carcinomas in
patients with head and neck cancer. Jpn J Clin Oncol. 48:124–129.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li YD, Ma X, Han YL and Peng LW: Clinical
features of multiple primary carcinomas of the oral cavity. Exp
Ther Med. 13:634–638. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Danylesko I and Shimoni A: Second
malignancies after hematopoietic stem cell transplantation. Curr
Treat Options Oncol. 19:92018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vajdic CM, Mayson E, Dodds AJ, O'Brien T,
Wilcox L, Nivison-Smith I, Le Marsney R, Daniels B and Ashton LJ;
CAST study investigators, : Second cancer risk and late mortality
in adult australians receiving allogeneic hematopoietic stem cell
transplantation: A population-based cohort study. Biol Blood Marrow
Transplant. 22:949–956. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rizzo JD, Curtis RE, Socie G, Sobocinski
KA, Gilbert E, Landgren O, Travis LB, Travis WD, Flowers ME,
Friedman DL, et al: Solid cancers after allogeneic hematopoietic
cell transplantation. Blood. 113:1175–1183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morton LM, Saber W, Baker KS, Barrett AJ,
Bhatia S, Engels EA, Gadalla SM, Kleiner DE, Pavletic S and Burns
LJ: National institutes of health hematopoietic cell
transplantation late effects initiative: The subsequent neoplasms
working group report. Biol Blood Marrow Transplant. 23:367–378.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weng X, Xing Y and Cheng B: Multiple and
recurrent squamous cell carcinoma of the oral cavity after
graft-versus-host disease. J Oral Maxillofac Surg. 75:1899–1905.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Taskinen M, Ryhanen S and Vettenranta K:
Graft-versus-host disease in stem cell transplantation. Duodecim.
133:251–258. 2017.PubMed/NCBI
|
|
24
|
Chien SH, Liu CJ, Hong YC, Teng CJ, Hu YW,
Shen CC, Ku FC, Chen SC, Yeh CM, Chiou TJ, et al: Use of
azathioprine for graft-vs-host disease is the major risk for
development of secondary malignancies after haematopoietic stem
cell transplantation: A nationwide population-based study. Br J
Cancer. 112:177–184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Curtis RE, Metayer C, Rizzo JD, Socié G,
Sobocinski KA, Flowers ME, Travis WD, Travis LB, Horowitz MM and
Deeg HJ: Impact of chronic GVHD therapy on the development of
squamous-cell cancers after hematopoietic stem-cell
transplantation: An international case-control study. Blood.
105:3802–3811. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang HA, Armenian SH and Dellinger TH:
Secondary neoplasms of the female lower genital tract after
hematopoietic cell transplantation. J Natl Compr Canc Netw.
16:211–218. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shanis D, Anandi P, Grant C, Bachi A, Vyas
N, Merideth MA, Pophali PA, Koklanaris E, Ito S, Savani BN, et al:
Risks factors and timing of genital human papillomavirus (HPV)
infection in female stem cell transplant survivors: a longitudinal
study. Bone Marrow Transplant. 53:78–83. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kawanishi S, Ohnishi S, Ma N, Hiraku Y and
Murata M: Crosstalk between DNA damage and inflammation in the
multiple steps of carcinogenesis. Int J Mol Sci. 18:18082017.
View Article : Google Scholar
|
|
29
|
Akiyama M, Yamaoka M, Ohyama W, Yokoi K,
Ashizuka S, Aizawa D, Ikegami M, Suzuki H, Ozaki K, Ida H and Yuza
Y: Genetic profile and microsatellite instability in a case of
secondary esophageal squamous cell carcinoma 12 years after
allogeneic hematopoietic stem cell transplantation for aplastic
anemia. J Pediatr Hematol Oncol. 42:302–306. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cordani M, Pacchiana R, Butera G, D'Orazi
G, Scarpa A and Donadelli M: Mutant p53 proteins alter cancer cell
secretome and tumour microenvironment: Involvement in cancer
invasion and metastasis. Cancer Lett. 376:303–309. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo G and Cui Y: New perspective on
targeting the tumor suppressor p53 pathway in the tumor
microenvironment to enhance the efficacy of immunotherapy. J
Immunother Cancer. 3:92015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lujambio A, Akkari L, Simon J, Grace D,
Tschaharganeh DF, Bolden JE, Zhao Z, Thapar V, Joyce JA,
Krizhanovsky V and Lowe SW: Non-cell-autonomous tumor suppression
by p53. Cell. 153:449–460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Knudson AG: Antioncogenes and human
cancer. Proc Natl Acad Sci USA. 90:10914–10921. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jain K, Mohapatra T, Das P, Misra MC,
Gupta SD, Ghosh M, Kabra M, Bansal VK, Kumar S, Sreenivas V and
Garg PK: Sequential occurrence of preneoplastic lesions and
accumulation of loss of heterozygosity in patients with gallbladder
stones suggest causal association with gallbladder cancer. Ann
Surg. 260:1073–1080. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang MF, Chang YC, Liao PS, Huang TH,
Tsay CH and Chou MY: Loss of heterozygosity of p53 gene of oral
cancer detected by exfoliative cytology. Oral Oncol. 35:296–301.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamasaki S, Tani R, Sakurai S, Toratani S
and Okamoto T: Oral squamous cell carcinoma of the tongue dorsum
with multiple cancer-associated mutations in the TP53 gene. Oral
Oncol. 109:1047742020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang XH, Ding L, Fu Y, Chen S, Zhang L,
Zhang XX, Huang XF, Lu ZY, Ni YH and Hu QG: p53-positive expression
in dysplastic surgical margins is a predictor of tumor recurrence
in patients with early oral squamous cell carcinoma. Cancer Manag
Res. 11:1465–1472. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gao J, Wang Y, Yang J, Zhang W, Meng K,
Sun Y, Li Y and He QY: RNF128 promotes invasion and metastasis via
the EGFR/MAPK/MMP-2 pathway in esophageal squamous cell carcinoma.
Cancers (Basel). 11:8402019. View Article : Google Scholar
|
|
39
|
Lin X, Wen G, Wang S, Lu H, Li C and Wang
X: Expression and role of EGFR, cyclin D1 and KRAS in
laryngocarcinoma tissues. Exp Ther Med. 17:782–790. 2019.PubMed/NCBI
|
|
40
|
Mukerji AN and Wolf A: Synchronous
esophageal and lung cancer. Thorac Surg Clin. 28:97–104. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dai L, Yang HL, Yan WP, Liang Z, Xiong HC,
Kang XZ, Yang YB, Fu H, Fan MY and Chen KN: The equivalent efficacy
of multiple operations for multiple primary lung cancer and a
single operation for single primary lung cancer. J Thorac Dis.
8:855–861. 2016. View Article : Google Scholar : PubMed/NCBI
|