Liver cancer is one of the most common cancers with
an increasing death rate. Approximately 0.56 million new cases are
reported annually (1). Approximately
50% of patients during treatment with chemotherapy develop
metastasis, thus reducing their survival rate (2). Difficulties in chemotherapeutic
techniques have led to other treatment options such as the use of
nutraceuticals and natural therapies for combating this diseased
with prior knowledge about their mechanisms of action (3). Thus, an emerging theme in cancer
biology is targeting genes and/or proteins that can link to the
progression of cancer and factors that directly or indirectly
affect the proliferation and metastasis (4).
Natural herbs have gained attraction in the current
era due to their roles in controlling cancer growth by targeting
oncogenes and proteins that are altered in cancers. Isolated
monomer polymethoxyflavones (PMFs) have shown a broad spectrum of
anti-cancer activities by inhibiting cell proliferation and cancer
prevention (5). Sinensetin (SIN) is
one such polymethoxyflavone present in citrus fruits that has
notable anti-inflammatory and anti-tumor activities (6). Although the anti-cancer effect of SIN
in cancer cells has been studied recently, gene expression changes
and molecular mechanisms associated with its anti-cancer activities
remain largely unknown. Transcription profile using RNA-sequencing
(RNA-seq) technology can help us understand its mechanism of action
(7). Transcriptome analysis is a
technology using bioinformatics and data-mining tools to analyze
changed target genes and understand the mechanism of action of a
drug after treatment with an in vitro model (8). The major way to explore the molecular
mechanism involved in the anti-cancer effect of flavonoids is by
determining gene activities and functions using transcriptome
analysis (9). Genomic findings
through a sequencing approach provide features of genomes due to
external influences such as a drug treatment. Differential gene
expression under treated conditions of a disease can provide us a
better understanding of factors in a particular state modified by a
candidate drug (10). Furthermore,
gene expression obtained from transcriptome data can lead to the
discovery of novel key genes associated with the related pathway
(11). The advent of next-generation
sequencing (NGS) data provides a detailed cancer profile that can
explain the relationship and connection of genes involved in a
disease. In addition, the analysis of differential patterns of
genes can help us understand biological processes, cellular
components, and interacting pathway network related to cancer
pathogenesis for each one (12).
Furthermore, bioinformatics analysis of differential gene profiles
is an attractive strategy to identify novel therapeutic biomarkers
for treating a disease (13). In
this regard, taking an integrated approach to identify mRNA targets
using next-generation sequencing data can reveal specific
biomarkers for cancer types.
Developed almost a decade ago, RNA-seq is a potent
tool for understanding genomic functions. Differentially expressed
genes (DEG) remains the primary application of RNA-seq (14). DEGs are genes whose expression levels
are significantly altered between two or more conditions such as
before and after treatment with a drug. In this regard, the concept
of hub genes is gaining interest. Hub genes are highly
interconnected genes in a protein-protein interaction (PPI)
network. Their associated biological process gene ontology terms
and pathways might improve our understanding of their roles in
carcinogenicity (15).
In the current study, we performed Illumina
NovaSeq6000 sequencing for SIN-treated and -untreated HepG2 liver
cancer cells and studied differential gene expression patterns.
Highly interconnected genes (hub) genes among these were studied
extensively for their roles in cancer. The expression of these hub
genes was further validated by qRT-PCR.
A human liver cancer cell line (HepG2) authenticated
using short tandem repeat (STR) profiling was obtained from the
Korean Cell Line Bank (Seoul, Korea). HepG2 cells were cultured in
DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100
U/ml penicillin, and 100 µg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C with 5% CO2.
HepG2 cells were seeded into 6-well plates and
treated with 100 µM of SIN for 48 h at 37°C. After 48 h treatment,
total RNAs were extracted using TRIzol. The concentration of RNA
was determined using a spectrophotometer. Isolated total RNA was
then subjected to sequencing to obtain expression data.
Sequencing was performed by TheragenEtex
(Gyeonggi-do) with the following described protocol. RNA-seq
libraries were constructed using a TruSeq Stranded mRNA Sample Prep
Kit (Illumina, Inc.) for 150-bp paired-end sequencing.
Poly-A-containing mRNA molecules were purified and fragmented from
2 µg of DNase-treated total RNA using oligo(dT) magnetic beads.
Following the purification step, mRNAs were fragmented and used for
synthesis of single-stranded cDNAs by means of random hexamer
priming. With the constructed single-stranded cDNAs as templates,
second strand cDNA synthesis was carried out to prepare
double-stranded cDNAs. These cDNAs were then amplified with a
sequential process of end-pair repair, addition of A-tail, and
adapter ligation using polymerase chain reaction (PCR). The quality
of constructed cDNA libraries wase evaluated with an Agilent 2100
BioAnalyzer (Agilent Technologies, Inc.) and quantified with a KAPA
library quantification kit (Kapa Biosystems) according to each
manufacturer's protocol. These products were then purified and
enriched with cluster amplification using PCR to obtain the final
complementary DNA library for high-throughput paired-end (2×150 bp)
DNA sequencing using an Illumina NovaSeq6000 (Illumina, Inc.).
After filtering out low quality reads, TopHat was
used to map quality-filtered reads to a reference genome (hg38)
(16). We measured gene expression
levels with Cufflinks v2.1.1 using the Ensembl human gene
annotation database. We performed differential expression analysis
using cuffdiff (17). To improve the
accuracy of measurement, we applied frag bias and multi-read
correct options for both cufflinks and cuffdiff. All other options
were set to default values. DEGs were identified and filtered with
the following criteria: false discovery rate <0.05 and
|log2 FC| >1 (18).
To identify hub genes among DEGs, a PPI network of
43 DEGs was constructed using STRING (https://string-db.org/) with a ‘minimum required
interaction score’ set to medium confidence (0.400) and ‘maximum
number of interactors to show’ set to query proteins for both 1st
and 2nd shells (19). The PPI
network was imported in cytoscape using CytoHubba's Maximal Clique
Centrality (MCC) scoring method to identify top ten hub genes
incorporated in STRING and to discover significantly enriched
biological process gene ontology (GO) terms and KEGG pathways
(20). Top ten biological process GO
terms with the lowest false discovery rate were analyzed using
REVIGO (21). Additionally, we
performed an extensive literature survey for these genes to uncover
their roles in cancer.
To validate hub genes differentially expressed based
on transcriptome analysis, we studied mRNA expression levels of
these hub genes by quantitative real-time polymerase chain reaction
(qRT-PCR). HepG2 cells were seeded into 6-well plates and treated
with 100 µM of SIN for 48 h at 37°C. Total RNA was isolated and its
concentration was determined using a spectrophotometer. Total RNA
(1 µg) was converted to cDNA using an iScript™ cDNA synthesis kit
(Bio-Rad Laboratories, Inc.). qRT-PCR was then performed using cDNA
and AccuPower® 2X Greenstar™ qPCR Master (Bioneer). qPCR
primers used in this study are listed in Table I. All data were analyzed using
Bio-Rad CFX Manager Version 3.1. We measured relative
quantification using the 2-ΔΔCq method. mRNA expression
levels of target genes were normalized against those of
β-actin.
All data were analyzed using GraphPad Prism version
5.0 (GraphPad Software). Results are expressed as means ± SEM. They
were evaluated using the Student's t-test. P<0.05 was considered
to indicate statistical significance.
In our previous study on SIN treatment in HepG2
cells, we found that SIN could induce HCC cell death in
vitro (22). RNA-seq and
Cuffdiff identified 43 DEGs (Table
II) between SIN-untreated and SIN-treated HepG2 cells based on
the selection criteria (a false discovery rate <0.05 and
|log2 FC| >1). Out of these 43 DEG's, 39 were
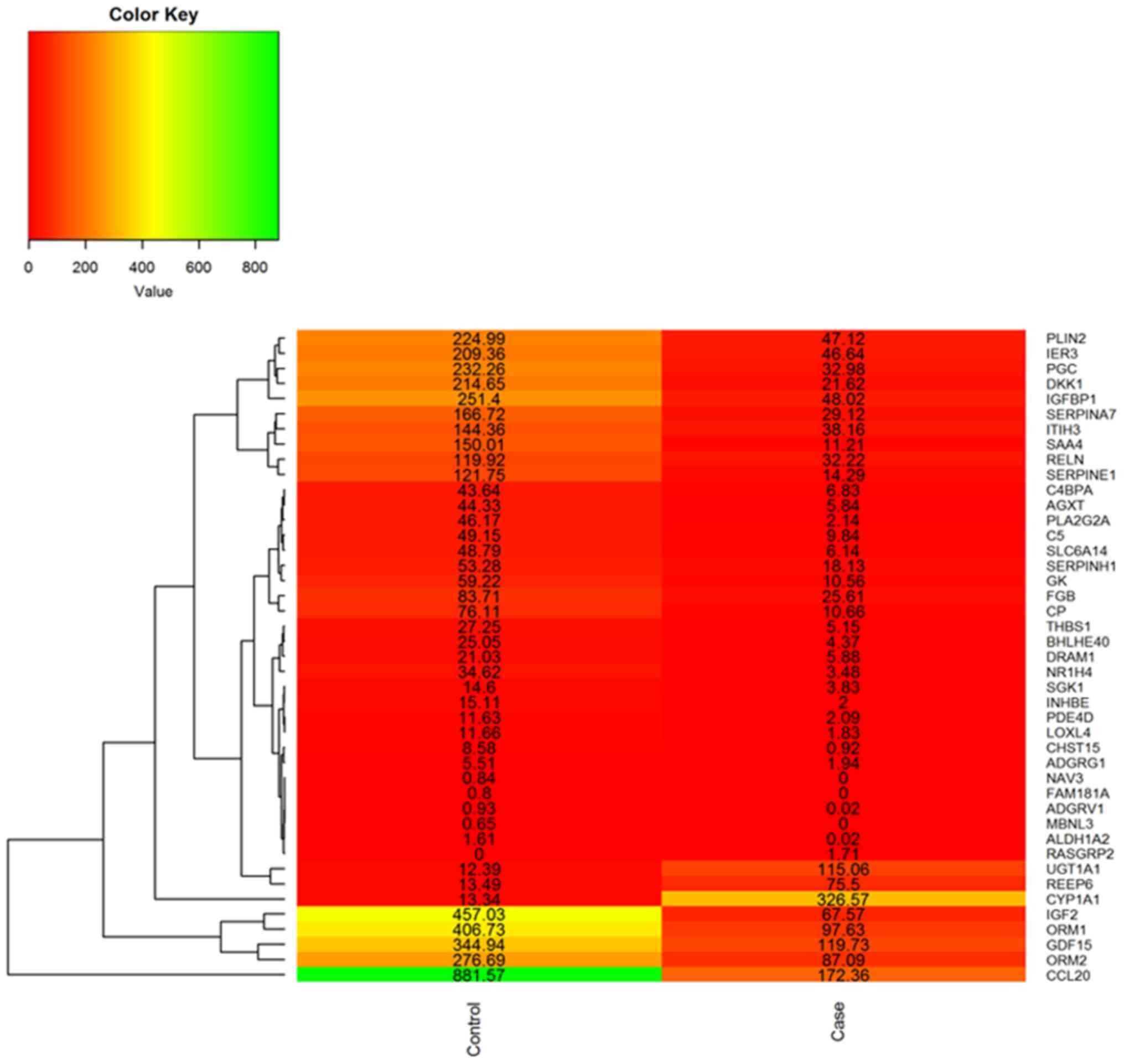
downregulated and 4 were upregulated. These DEGs were plotted using
the heatmap.2 function from R's gplot package. Fragments per
kilobase of transcript per million (FPKM) values per genes in
SIN-untreated (control) and SIN-treated (case) HepG2 cells were
used for heatmap generation (Fig.
1).
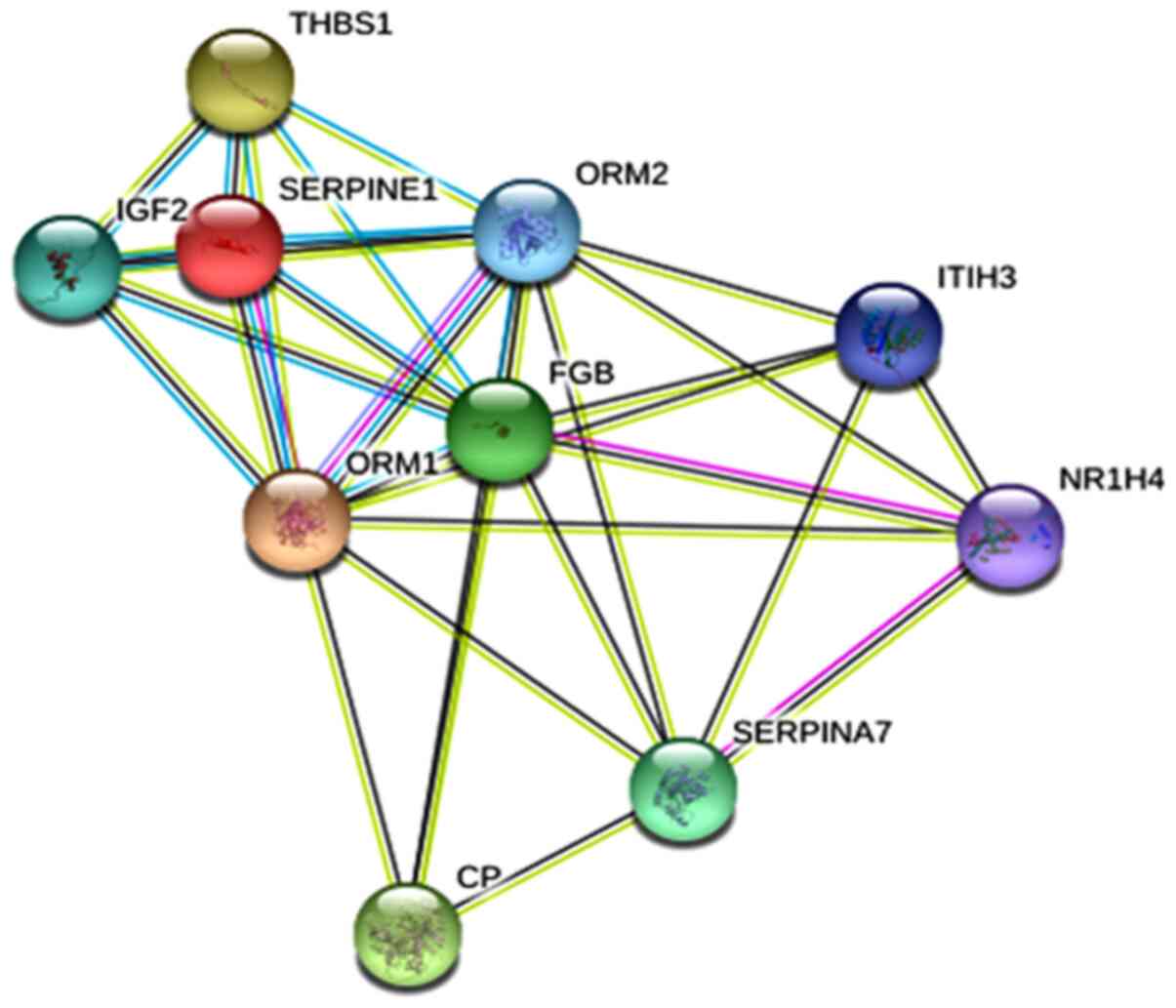
Hub genes are highly interconnected genes that might
be involved in important cancer-related biological processes and
functions. Fig. 2 shows the
protein-protein interaction network of the ten hub genes identified
by Cytohubba's MCC tool.
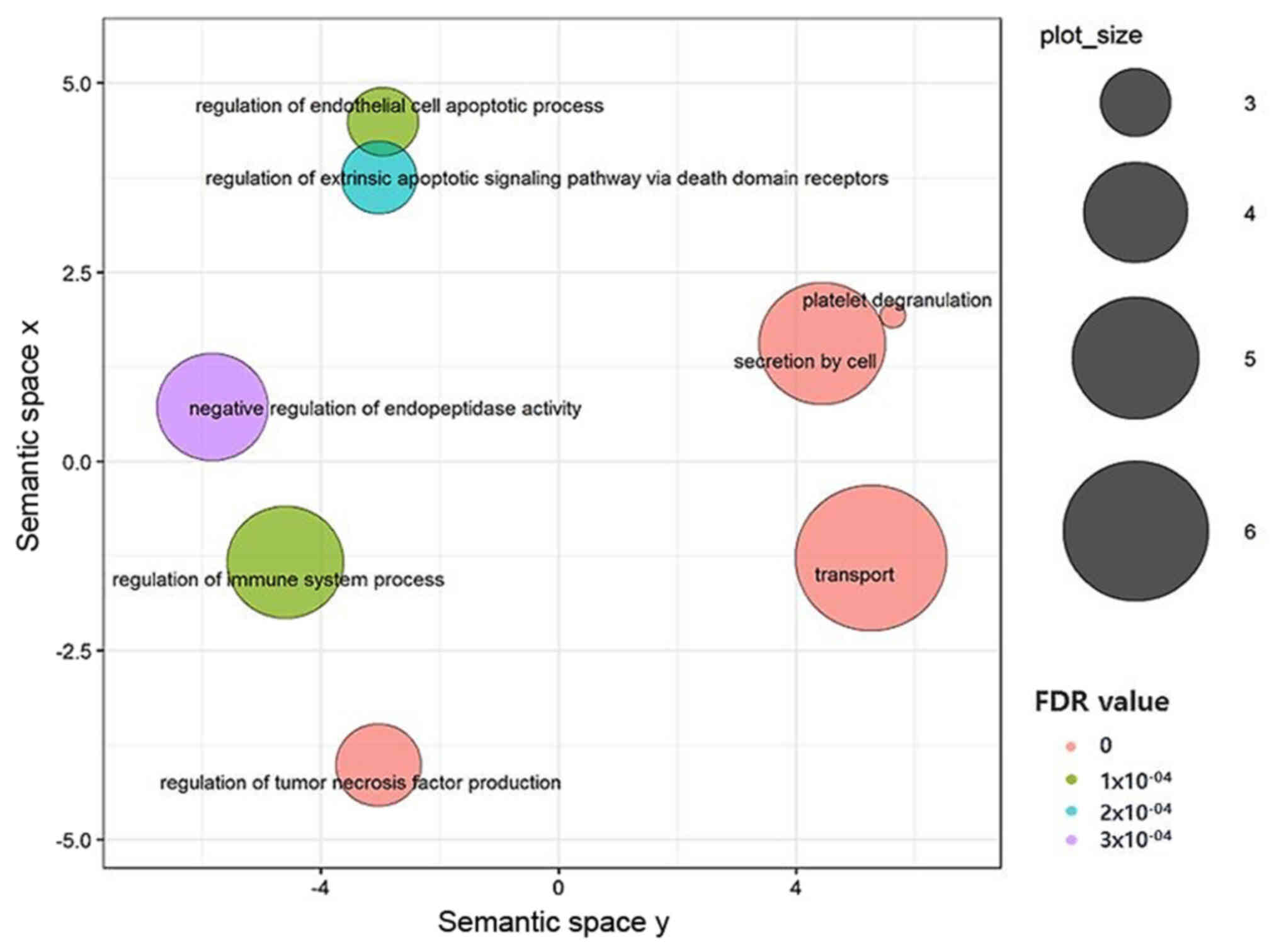
When these hub genes were subjected to STRING and
enrichment analysis to identify significant GO terms and KEGG
pathways, a list of 101 biological process GO terms and three
significant pathways were retrieved. For the ease of analysis and
visualization, we did a REVIGO analysis for top ten biological
process GO terms showing the lowest FDR values (23). REVIGO indicated that these genes were
mainly involved in immune-system responses, regulation of tumor
necrosis factor production, regulation of the apoptotic process,
regulation of protein metabolism, and transport and secretion
processes as shown in the REVIGO scatter plot (Fig. 3). Additionally, KEGG pathway analysis
revealed that SERPINE1 and THBS1 modified wild-type human p53
(TP53), complement, coagulation cascade, and proteoglycans in
cancer pathways.
All our hub genes were downregulated. Therefore, we
did a comprehensive literature search to figure out roles of these
upregulated hub genes in cancer.
SERPINE1 and SERPINA7 belong to the human SERPIN
gene family, which gets its name from its originally identified
function of serine proteinase inhibition. However, many of its
members also act as chaperones involved in storage, transport, and
other roles (29–31). SERPINE1 encodes for a serine
proteinase inhibitor. It can inhibit tissue plasminogen activator
(tPA) and urokinase (uPA). High levels of SERPINE1 have been
associated with low prognosis rate and survival of lung, breast,
oral, stomach, and ovarian carcinoma patients (32–34). In
addition, reducing the level of SERPINE1 can decrease cell
migration in thyroid cancer (35).
In relation to HCC, higher levels of SERPINE1 and increased
SERPINE1 proteins associated with invasiveness, metastasis, and
prognosis in patients with liver cancer have been observed
(36,37). SERPINA7 encodes thyroxine-binding
globulin (TBG), a human thyroid hormone protein. SERPINA7 has been
found to be upregulated in colorectal cancer patients (38). One study on 30 patients with primary
liver cancer has found that 22 of them have higher TBG
concentrations. Additionally, in 28 out of 31 patients with liver
metastasis, TBG concentration is higher than normal (39).
Fibrinogen beta chain (FGB). Fibrinogen is a
glycopeptide synthesized by hepatocytes. It is formed by connection
of two trimers with each trimer containing a fibrinogen alpha chain
that is encoded by the FGA gene, along with the fibrinogen beta
chain or gamma chains encoded by FGB or FGG gene, respectively.
Increased fibrinogen activity can affect tumor cell growth,
progression, and metastasis (40).
Moreover, colorectal cancer growth is reduced in
fibrinogen-deficient mice (41). The
FGB gene is also upregulated in lung carcinomas and breast cancer
(42,43). Although we could not find a direct
link between upregulation of FGB and HCC, in vitro studies
have shown that FGG (another gene involved in fibrinogen formation)
upregulation can promote the migration and invasion of HCC whereas
knockdown of FGG can significantly inhibit phenotypes (44).
The orosomucoid gene family contains two polymorphic
genes (ORM1 and ORM2) primarily secreted by the liver, although
they are also abundant in the plasma. They encode for acute-phase
proteins that are expressed during stressful conditions such as
tissue injury, infections, and inflammations (45). It has been reported that ORM genes
are over-expressed in breast cancer, bladder cancer, lung cancer,
cholangiocarcinoma (bile duct cancer), colorectal cancer, and HCC
(46–50). However, the mechanism of how
orosomucoid genes affect cancer cells remains unclear. Of
particular interest, knockdown of ORM1 can result in decreased
proliferation of HCC cells (46).
NR1H4, also known as farnesoid X receptor (FXR), is
mainly expressed in the liver, kidney, intestine, and adrenal
gland. It is a member of the nuclear receptor superfamily. It is
activated upon binding to bile acid for regulating bile acid
homeostasis (51–53). In vitro studies have revealed
that the expression of FXR is significantly increased in
non-small-cell lung carcinoma (NSCLC), resulting in cell
proliferation. Knockdown of NR1H4 can inhibit cell proliferation
and xenograft growth in nude mice models. A delay in the G1/S
transition of cell cycle has also been reported after knockdown of
NR1H4 in NSCLC (54). The expression
of FXR in esophageal carcinoma is shown to be highly associated
with increased tumor size and lymph-node metastasis, whereas
deletion of this gene can suppress tumor-cell growth in both in
vitro and in vivo studies (55). Some evidence has highlighted the role
of FXR in liver carcinogenesis. How FXR promotes cell proliferation
has been elucidated in a HepG2 cell line among others by
suppressing the expression of p16/INK4a. Downregulation of FXR also
shows proliferation-inhibitory effects (56).
THBS1, a member of the thrombospondins family of
proteins, is an important component of the extracellular matrix
(57). Upregulation of THBS1 can
increase the invasion and migration of gastric cancer cells,
prostate cancer, gliomas, pancreatic cancer, and ovarian cancer
(58–62). High expression level of THBS1 is
associated with invasiveness and progression of hepatocellular
carcinoma cells as well as poor prognosis (63). THBS1 expression is also observed in
stromal cells surrounding the cancer (64). A study by Lee et al has
highlighted the role of THBS1 in HCC tumor progression because
suppression of THBS1 can mediate CD47 signaling and decrease the
growth of cancerous liver cells (65).
CP is an enzyme involved in ferroxidase activity,
copper transport, amine oxidase activity, and superoxide dismutase
activity (66). In breast cancer,
elevated CP levels are associated with metastasis and higher
chances of recurrences (67). CP
levels are also increased in patients with pancreatic ductal
adenocarcinoma, ovarian cancer, bile duct cancer, and cervical
cancer (68–71).
Inter-alpha-trypsin inhibitors are a family of
serine protease inhibitors that are formed by the combination of
one light chain and one or two heavy chain proteins. Structurally,
two or more heavy chains are covalently linked to hyaluronic acid
(HA) which forms the major portion of the cellular matrix (72). Upregulation of ITIH3 expression has
been observed in lung cancer and gastric cancer (73–75). The
present transcriptome data showed that ITIH3 was downregulated in
SIN-treated HepG2 cells than in untreated HepG2 cells. Literature
evidence accentuates the role of these hub genes in cancer
development when they are expressed at higher than normal
levels.
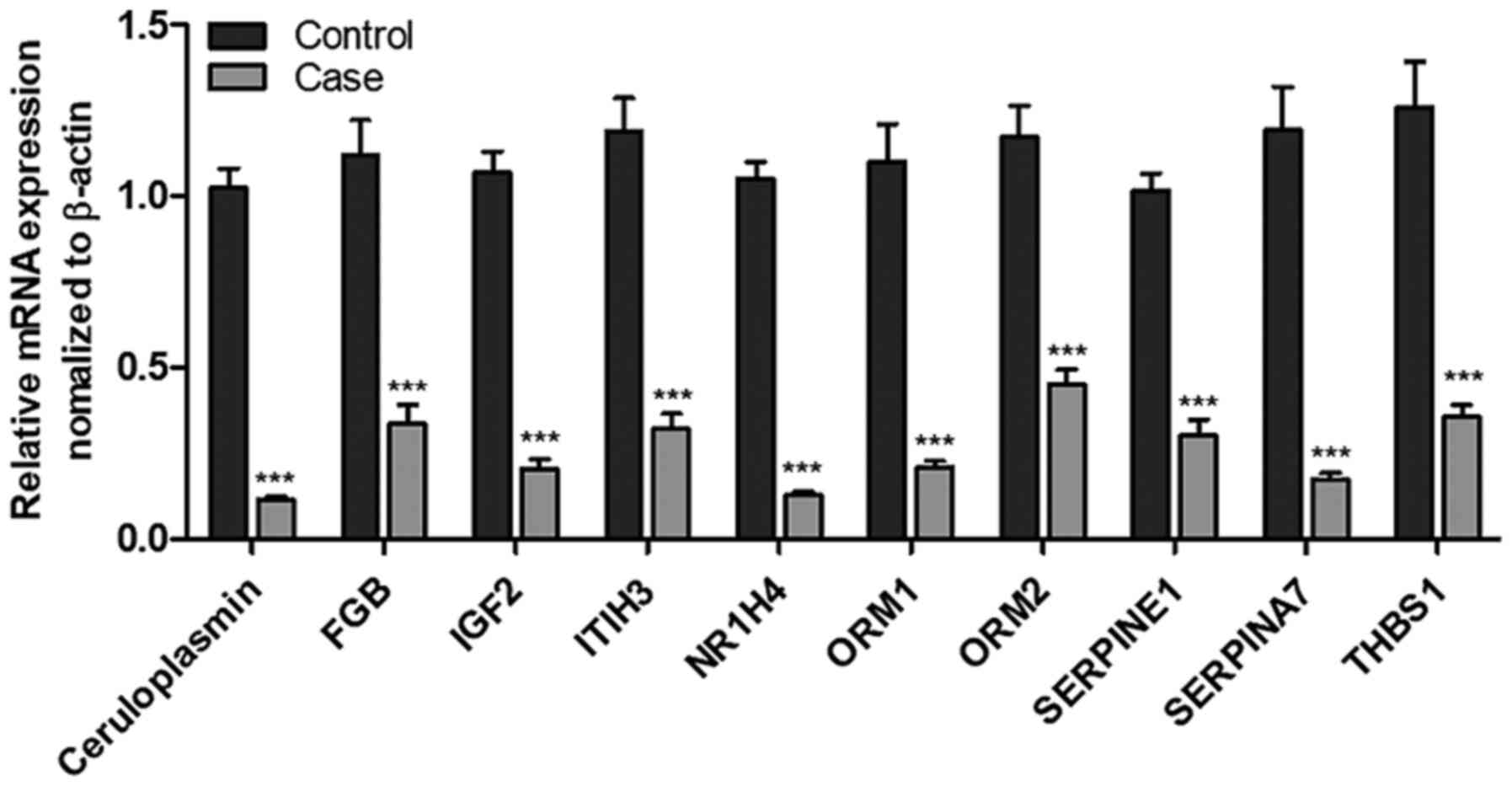
Differently expressed, highly integrated hub genes
(CP, FGB, IGF2, ITIH3, NRIH4, ORM1, ORM2, SERPINE1, SERPINA7 and
THBS1) were quantified for mRNA expression by Qrt-PCR. In agreement
with transcriptome analysis data, qRT-PCR analysis results
validated the downregulation of these hub genes in SIN-treated
cells than in untreated HepG2 cells (Fig. 4). These data further confirm the role
of SIN in regulating the expression of hub genes in HepG2
cells.
A transcriptome of an organism describes a small
proportion of its genetic codes that can be transcribed into RNA
molecules. Post-transcriptional processing of RNA plays a crucial
role in terms of producing more variant forms of mRNA (76). Thus, it is clear that studying the
whole transcriptome and understanding their modifications can
provide an extensive knowledge for developing novel strategies to
control diseases. Transcriptome profiling has gained extensive
attention in cancer research because it enables disease condition
analysis and treatment outcomes. The analysis of RNA-seq data to
obtain gene expression and transcriptional changes in cancer
supports moral outcomes and treatment options (77). Genes and signaling pathways involved
in cancer and treatment outcomes have been identified by microarray
and RNA-seq approaches. With the advent of second- and
third-generation sequencing technologies, RNA-seq is a significant
method owing to its low false-positive results and increased
reproducibility rate compared to microarrays (78). It also gives an accurate expression
change of transcripts and their isoforms that can help us discover
novel transcripts. Transcriptome profiling via RNA-seq has
discovered many genes that are potentially involved in the
anti-cancer effect of natural compounds like flavonoids. A recent
report on integrated whole-transcriptome profiling of genes in
HCT-116 cancer cells by quercetin treatment has revealed pathways
that can regulate cancer progression (79).
Sinensetin (SIN), a polymethoxyflavone present in
the citrus family, can inhibit several cancers by regulating
oxidative stress of cells (80). Our
previous study has displayed an autophagy-mediated anti-cancer
potential of SIN in liver cancer cells. In the current research, we
performed RNA-seq analysis for SIN-treated and SIN-untreated liver
cancer cells (HepG2) to identify critical genes associated with the
anti-cancer potential of SIN.
In the current study, a total of 43 differentially
expressed genes were identified between SIN-treated and untreated
samples in HepG2 cells. Interestingly, most (39/43) of these genes
were downregulated while only four were upregulated by treatment
with SIN. With the help of STRING and Cytohubba, we identified ten
hub genes from the DEG list. Enrichment analysis indicated that
these hub genes were mainly involved in immune-system responses and
regulation of tumor necrosis factor production, apoptosis, and
protein metabolism. As presented in detail in the results section,
we did an extensive literature survey on these identified hub
genes, highlighting their roles in tumor growth, tumor
invasiveness, poor prognosis, and recurrence in various cancers
upon upregulation of their expression. RNA-seq analysis of HepG2
cells treated with SIN showed downregulation of these hub genes.
Literature analysis sheds light on how downregulation of these hub
genes might mediate anti-cancer processes. qRT-PCR data confirmed
that expression levels of these hub genes were consistent with
RNA-seq data. Hub genes CP, FGB, IGF2, ITIH3, NRIH4, ORM1, ORM2,
SERPINE1, SERPINA7 and THBS1 are highly expressed in several
cancers, including liver, lung, pancreatic, and cervical cancers.
Significant downregulation of these genes upon SIN treatment showed
its prominent capacity in suppressing these genes in HepG2 cells.
The confirmation of expression data revealed that these genes could
emerge as attractive therapeutic targets in the treatment of liver
cancer. Furthermore, RNA-seq and relative expression data
strengthened the argument that SIN is a strong anti-cancer agent in
HCC.
In conclusion, ιn the current study, transcriptome
analysis of SIN-treated HepG2 cells by next generation sequencing
supported its anti-cancer effect. Analysis of DEGs provided a
strong insight on the involvement of hub genes related to cancer
progression. Results of this study indicate that SIN might induce
HCC cell death by regulating the expression of these genes. The
objective of this study was to identify the related gene on SIN
anti-cancer effect using transcriptome analysis. This paper
highlights the necessity for further studies to support anti-cancer
effect of those genes.
Not applicable.
The present study was supported by grants from the
National Research Foundation funded by the Ministry of Science and
ICT, Republic of Korea (grant nos. 2012M3A9B8019303 and
2020R1A2B5B01001807).
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
GSK and KWL conceived, designed and performed the
experiments. SMK, SR and AMK organized focus group discussion,
collected and analyzed all study data, and prepared the final
manuscript. SMK, PV, SEH and HHK performed some experiments and
revised the final manuscript. GSK and SMK confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Li J, Fu Y, Hu X and Xiong Y: Psoralidin
inhibits the proliferation of human liver cancer cells by
triggering cell cycle arrest, apoptosis and autophagy and inhibits
tumor growth in vivo. J BUON. 24:1950–1955. 2019.PubMed/NCBI
|
|
2
|
Sakin A, Sahin S, Atci MM, Yasar N,
Geredeli C, Aribal S, Alemdar A, Karataş F and Cihan S: Factors
affecting survival in patients with isolated liver-metastatic
colorectal cancer treated with local ablative or surgical
treatments for liver metastasis. J BUON. 24:1801–1808.
2019.PubMed/NCBI
|
|
3
|
Zhang X, Lv J, Luo H, Liu Z, Xu C, Zhou D,
Tang L, Zhang Z, Liu J, Xiao M, et al: Nucleostemin promotes
hepatocellular carcinoma by regulating the function of STAT3. Exp
Cell Res. 387:1117482020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu L, Wang R, Ziegelbauer J, Wu WW, Shen
RF, Juhl H, Zhang Y, Pelosof L and Rosenberg AS: Transcriptome
analysis of human colorectal cancer biopsies reveals extensive
expression correlations among genes related to cell proliferation,
lipid metabolism, immune response and collagen catabolism.
Oncotarget. 8:74703–74719. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu Y, Lv X, Yang G, Zhan J, Li M, Long T,
Ho CT and Li S: Simultaneous separation of six pure
polymethoxyflavones from sweet orange peel extract by high
performance counter current chromatography. Food Chem. 292:160–165.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kang SI, Shin HS and Kim SJ: Sinensetin
enhances adipogenesis and lipolysis by increasing cyclic adenosine
monophosphate levels in 3T3-L1 adipocytes. Biol Pharm Bull.
38:552–558. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu T, Zhang H and Qi H: Transcriptome
profiling analysis reveals biomarkers in colon cancer samples of
various differentiation. Oncol Lett. 16:48–54. 2018.PubMed/NCBI
|
|
8
|
Luo J, Dai X, Hu H, Chen J, Zhao L, Yang
C, Sun J, Zhang L, Wang Q, Xu S, et al: Fluzoparib increases
radiation sensitivity of non-small cell lung cancer (NSCLC) cells
without BRCA1/2 mutation, a novel PARP1 inhibitor undergoing
clinical trials. J Cancer Res Clin Oncol. 146:721–737. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin KH, Huang MY, Cheng WC, Wang SC, Fang
SH, Tu HP, Su CC, Hung YL, Liu PL, Chen CS, et al: RNA-seq
transcriptome analysis of breast cancer cell lines under shikonin
treatment. Sci Rep. 8:26722018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang D, Zhao L, Wang D, Liu J, Yu X, Wei Y
and Ouyang Z: Transcriptome analysis and identification of key
genes involved in 1-deoxynojirimycin biosynthesis of mulberry
(Morus alba L.). PeerJ. 6:e54432018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Kang Z, Lv D, Zhang X, Liao Y, Li
Y, Liu R, Li P, Tong M, Tian J, et al: Longitudinal whole-genome
sequencing reveals the evolution of MPAL. Cancer Genet. 240:59–65.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Niemira M, Collin F, Szalkowska A, Bielska
A, Chwialkowska K, Reszec J, Niklinski J, Kwasniewski M and
Kretowski A: Molecular signature of subtypes of non-small-cell lung
cancer by large-scale transcriptional profiling: Identification of
key modules and genes by Weighted Gene Co-Expression Network
Analysis (WGCNA). Cancers (Basel). 12:E372019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Z, Zhang Z, Zhang C and Xu Y:
Identification of potential pathogenic biomarkers in clear cell
renal cell carcinoma. Oncol Lett. 15:8491–8499. 2018.PubMed/NCBI
|
|
14
|
Stark R, Grzelak M and Hadfield J: RNA
sequencing: The teenage years. Nat Rev Genet. 20:631–656. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Gu HY, Zhu J, Niu YM, Zhang C and
Guo GL: Identification of hub genes and key pathways associated
with bipolar disorder based on weighted gene co-expression Network
Analysis. Front Physiol. 10:10812019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trapnell C, Pachter L and Salzberg SL:
TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics.
25:1105–1111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Trapnell C, Williams BA, Pertea G,
Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ and Pachter
L: Transcript assembly and quantification by RNA-Seq reveals
unannotated transcripts and isoform switching during cell
differentiation. Nat Biotechnol. 28:511–515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Benjamini Y, Drai D, Elmer G, Kafkafi N
and Golani I: Controlling the false discovery rate in behavior
genetics research. Behav Brain Res. 125:279–284. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Szklarczyk D, Gable AL, Lyon D, Junge A,
Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork
P, et al: STRING v11: Protein-protein association networks with
increased coverage, supporting functional discovery in genome-wide
experimental datasets. Nucleic Acids Res. 47:D607–D613. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Supek F, Bošnjak M, Škunca N and Šmuc T:
REVIGO summarizes and visualizes long lists of gene ontology terms.
PLoS One. 6:e218002011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim SM, Ha SE, Ho JL, Rampogu S, Vetrivel
P, Kim HH, Saralamma VVG, Lee KW and Kim GS: Sinensetin Induces
Autophagic Cell Death through p53-Related AMPK/mTOR Signaling in
Hepatocellular Carcinoma HepG2 Cells. Nutrients. 12:24622020.
View Article : Google Scholar
|
|
23
|
Xiong H, Guo H, Xie Y, Zhao L, Gu J, Zhao
S, Li J and Liu L: RNAseq analysis reveals pathways and candidate
genes associated with salinity tolerance in a spaceflight-induced
wheat mutant. Sci Rep. 7:27312017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livingstone C: IGF2 and cancer. Endocr
Relat Cancer. 20:R321–R339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Macaulay VM: Insulin-like growth factors
and cancer. Br J Cancer. 65:311–320. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guerra FK, Eijan AM, Puricelli L, Alonso
DF, Bal de Kier Joffé E, Kornblihgtt AR, Charreau EH and Elizalde
PV: Varying patterns of expression of insulin-like growth factors I
and II and their receptors in murine mammary adenocarcinomas of
different metastasizing ability. Int J Cancer. 65:812–820. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin SB, Hsieh SH, Hsu HL, Lai MY, Kan LS
and Au LC: Antisense oligodeoxynucleotides of IGF-II selectively
inhibit growth of human hepatoma cells overproducing IGF-II. J
Biochem. 122:717–722. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martinez-Quetglas I, Pinyol R, Dauch D,
Torrecilla S, Tovar V, Moeini A, Alsinet C, Portela A,
Rodriguez-Carunchio L, Solé M, et al: IGF2 Is up-regulated by
epigenetic mechanisms in hepatocellular carcinomas and is an
actionable oncogene product in experimental models.
Gastroenterology. 151:1192–1205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Andreasen PA, Kjøller L, Christensen L and
Duffy MJ: The urokinase-type plasminogen activator system in cancer
metastasis: A review. Int J Cancer. 72:1–22. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hundsdorfer B, Zeilhofer HF, Bock KP,
Dettmar P, Schmitt M and Horch HH: The prognostic importance of
urinase type plasminogen activators (uPA) and plasminogen activator
inhibitors (PAI-1) in the primary resection of oral squamous cell
carcinoma. Mund Kiefer Gesichtschir. 8:173–179. 2004.(In German).
PubMed/NCBI
|
|
31
|
Annecke K, Schmitt M, Euler U, Zerm M,
Paepke D, Paepke S, von Minckwitz G, Thomssen C and Harbeck N: uPA
and PAI-1 in breast cancer: Review of their clinical utility and
current validation in the prospective NNBC-3 trial. Adv Clin Chem.
45:31–45. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Harbeck N, Schmitt M, Paepke S, Allgayer H
and Kates RE: Tumor-associated proteolytic factors uPA and PAI-1:
Critical appraisal of their clinical relevance in breast cancer and
their integration into decision-support algorithms. Crit Rev Clin
Lab Sci. 44:179–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vairaktaris E, Yapijakis C, Psyrri A,
Spyridonidou S, Yannopoulos A, Lazaris A, Vassiliou S, Ferekidis E,
Vylliotis A, Nkenke E, et al: Loss of tumour suppressor p16
expression in initial stages of oral oncogenesis. Anticancer Res.
27:979–984. 2007.PubMed/NCBI
|
|
34
|
Li L, Zhu Z, Zhao Y, Zhang Q, Wu X, Miao
B, Cao J and Fei S: FN1, SPARC, and SERPINE1 are highly expressed
and significantly related to a poor prognosis of gastric
adenocarcinoma revealed by microarray and bioinformatics. Sci Rep.
9:78272019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu XM, Jaskula-Sztul R, Georgen MR,
Aburjania Z, Somnay YR, Leverson G, Sippel RS, Lloyd RV, Johnson BP
and Chen H: Notch1 signaling regulates the aggressiveness of
differentiated thyroid cancer and inhibits SERPINE1 expression.
Clin Cancer Res. 22:3582–3592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng Q, Tang ZY, Xue Q, Shi DR, Song HY
and Tang HB: Invasion and metastasis of hepatocellular carcinoma in
relation to urokinase-type plasminogen activator, its receptor and
inhibitor. J Cancer Res Clin Oncol. 126:641–646. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Divella R, Mazzocca A, Gadaleta C, Simone
G, Paradiso A, Quaranta M and Daniele A: Influence of plasminogen
activator inhibitor-1 (SERPINE1) 4G/5G polymorphism on circulating
SERPINE-1 antigen expression in HCC associated with viral
infection. Cancer Genomics Proteomics. 9:193–198. 2012.PubMed/NCBI
|
|
38
|
Holm M, Saraswat M, Joenväärä S, Ristimäki
A, Haglund C and Renkonen R: Colorectal cancer patients with
different C-reactive protein levels and 5-year survival times can
be differentiated with quantitative serum proteomics. PLoS One.
13:e01953542018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Berger HR, Creech MK, Hannoush Z, Watanabe
Y, Kargi A and Weiss RE: A novel mutation causing complete thyroid
binding globulin deficiency (Tbg-Cd Mia) in a male with coexisting
graves disease. AACE Clin Case Rep. 3:e134–e139. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Falanga A, Marchetti M and Vignoli A:
Coagulation and cancer: Biological and clinical aspects. J Thromb
Haemost. 11:223–233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Adams GN, Rosenfeldt L, Frederick M,
Miller W, Waltz D, Kombrinck K, McElhinney KE, Flick MJ, Monia BP,
Revenko AS, et al: Colon cancer growth and dissemination relies
upon thrombin, stromal PAR-1, and fibrinogen. Cancer Res.
75:4235–4243. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ciereszko A, Dietrich MA, Słowińska M,
Nynca J, Ciborowski M, Kisluk J, Michalska-Falkowska A, Reszec J,
Sierko E and Nikliński J: Identification of protein changes in the
blood plasma of lung cancer patients subjected to chemotherapy
using a 2D-DIGE approach. PLoS One. 14:e02238402019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dowling P, Palmerini V, Henry M, Meleady
P, Lynch V, Ballot J, Gullo G, Crown J, Moriarty M and Clynes M:
Transferrin-bound proteins as potential biomarkers for advanced
breast cancer patients. BBA Clin. 2:24–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang X, Wang F, Huang Y, Ke K, Zhao B,
Chen L, Liao N, Wang L, Li Q, Liu X, et al: FGG promotes migration
and invasion in hepatocellular carcinoma cells through activating
epithelial to mesenchymal transition. Cancer Manag Res.
11:1653–1665. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee YS, Choi JW, Hwang I, Lee JW, Lee JH,
Kim AY, Huh JY, Koh YJ, Koh GY, Son HJ, et al: Adipocytokine
orosomucoid integrates inflammatory and metabolic signals to
preserve energy homeostasis by resolving immoderate inflammation. J
Biol Chem. 285:22174–22185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li F, Yu Z, Chen P, Lin G, Li T, Hou L, Du
Y and Tan W: The increased excretion of urinary orosomucoid 1 as a
useful biomarker for bladder cancer. Am J Cancer Res. 6:331–340.
2016.PubMed/NCBI
|
|
47
|
Ayyub A, Saleem M, Fatima I, Tariq A,
Hashmi N and Musharraf SG: Glycosylated alpha-1-acid glycoprotein 1
as a potential lung cancer serum biomarker. Int J Biochem Cell
Biol. 70:68–75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rucksaken R, Charoensuk L, Pinlaor P,
Pairojkul C, Khuntikeo N and Pinlaor S: Plasma orosomucoid 2 as a
potential risk marker of cholangiocarcinoma. Cancer Biomark.
18:27–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Falleti E, Pirisi M, Fabris C, Bortolotti
N, Soardo G, Toniutto P, Gonano F and Bartoli E: Increase of serum
alpha 1-acid glycoprotein despite the decline of liver synthetic
function in cirrhotics with hepatocellular carcinoma. Eur J Clin
Chem Clin Biochem. 31:407–411. 1993.PubMed/NCBI
|
|
50
|
Alexander H, Stegner AL, Wagner-Mann C, Du
Bois GC, Alexander S and Sauter ER: Proteomic analysis to identify
breast cancer biomarkers in nipple aspirate fluid. Clin Cancer Res.
10:7500–7510. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Forman BM, Goode E, Chen J, Oro AE,
Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW,
et al: Identification of a nuclear receptor that is activated by
farnesol metabolites. Cell. 81:687–693. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sinal CJ, Tohkin M, Miyata M, Ward JM,
Lambert G and Gonzalez FJ: Targeted disruption of the nuclear
receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell.
102:731–744. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lu TT, Makishima M, Repa JJ, Schoonjans K,
Kerr TA, Auwerx J and Mangelsdorf DJ: Molecular basis for feedback
regulation of bile acid synthesis by nuclear receptors. Mol Cell.
6:507–515. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
You W, Chen B, Liu X, Xue S, Qin H and
Jiang H: Farnesoid X receptor, a novel proto-oncogene in non-small
cell lung cancer, promotes tumor growth via directly
transactivating CCND1. Sci Rep. 7:5912017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Guan B, Li H, Yang Z, Hoque A and Xu X:
Inhibition of farnesoid X receptor controls esophageal cancer cell
growth in vitro and in nude mouse xenografts. Cancer.
119:1321–1329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Guo F, Xu Z, Zhang Y, Jiang P, Huang G,
Chen S, Lyu X, Zheng P, Zhao X, Zeng Y, et al: FXR induces SOCS3
and suppresses hepatocellular carcinoma. Oncotarget. 6:34606–34616.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Adams JC and Lawler J: The
thrombospondins. Int J Biochem Cell Biol. 36:961–968. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Huang T, Wang L, Liu D, Li P, Xiong H,
Zhuang L, Sun L, Yuan X and Qiu H: FGF7/FGFR2 signal promotes
invasion and migration in human gastric cancer through upregulation
of thrombospondin-1. Int J Oncol. 50:1501–1512. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Firlej V, Mathieu JR, Gilbert C, Lemonnier
L, Nakhlé J, Gallou-Kabani C, Guarmit B, Morin A, Prevarskaya N,
Delongchamps NB, et al: Thrombospondin-1 triggers cell migration
and development of advanced prostate tumors. Cancer Res.
71:7649–7658. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Perez-Janices N, Blanco-Luquin I, Tuñón
MT, Barba-Ramos E, Ibáñez B, Zazpe-Cenoz I, Martinez-Aguillo M,
Hernandez B, Martínez-Lopez E, Fernández AF, et al: EPB41L3, TSP-1
and RASSF2 as new clinically relevant prognostic biomarkers in
diffuse gliomas. Oncotarget. 6:368–380. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Nie S, Lo A, Wu J, Zhu J, Tan Z, Simeone
DM, Anderson MA, Shedden KA, Ruffin MT and Lubman DM: Glycoprotein
biomarker panel for pancreatic cancer discovered by quantitative
proteomics analysis. J Proteome Res. 13:1873–1884. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lyu T, Jia N, Wang J, Yan X, Yu Y, Lu Z,
Bast RC Jr, Hua K and Feng W: Expression and epigenetic regulation
of angiogenesis-related factors during dormancy and recurrent
growth of ovarian carcinoma. Epigenetics. 8:1330–1346. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Poon RT, Chung KK, Cheung ST, Lau CP, Tong
SW, Leung KL, Yu WC, Tuszynski GP and Fan ST: Clinical significance
of thrombospondin 1 expression in hepatocellular carcinoma. Clin
Cancer Res. 10:4150–4157. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hayashi K, Kurohiji T and Shirouzu K:
Localization of thrombospondin in hepatocellular carcinoma.
Hepatology. 25:569–574. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lee TK, Cheung VC, Lu P, Lau EY, Ma S,
Tang KH, Tong M, Lo J and Ng IO: Blockade of CD47-mediated
cathepsin S/protease-activated receptor 2 signaling provides a
therapeutic target for hepatocellular carcinoma. Hepatology.
60:179–191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Tórsdóttir G, Gudmundsson G, Kristinsson
J, Snaedal J and Jóhannesson T: Ceruloplasmin and superoxide
dismutase (SOD1) in heterozygotes for Wilson disease: A case
control study. Neuropsychiatr Dis Treat. 5:55–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Schapira DV and Schapira M: Use of
ceruloplasmin levels to monitor response to therapy and predict
recurrence of breast cancer. Breast Cancer Res Treat. 3:221–224.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Brandi J, Dalla Pozza E, Dando I, Biondani
G, Robotti E, Jenkins R, Elliott V, Park K, Marengo E, Costello E,
et al: Secretome protein signature of human pancreatic cancer
stem-like cells. J Proteomics. 136:1–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Han IW, Jang JY, Kwon W, Park T, Kim Y,
Lee KB and Kim SW: Ceruloplasmin as a prognostic marker in patients
with bile duct cancer. Oncotarget. 8:29028–29037. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Upadhya S, Upadhya S and Prabhu KS: Serum
glycoconjugates and ceruloplasmin in cancer of uterine cervix.
Indian J Clin Biochem. 17:20–24. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Arumanayagam M, Wong FW, Rogers M and
Swaminathan R: Serum ceruloplasmin, plasma copper concentration and
copper to ceruloplasmin ratio in cervical carcinoma. Gynecol Obstet
Invest. 35:175–178. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Huang L, Yoneda M and Kimata K: A
serum-derived hyaluronan-associated protein (SHAP) is the heavy
chain of the inter alpha-trypsin inhibitor. J Biol Chem.
268:26725–26730. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chatterji B and Borlak J: A 2-DE MALDI-TOF
study to identify disease regulated serum proteins in lung cancer
of c-myc transgenic mice. Proteomics. 9:1044–1056. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Heo SH, Lee SJ, Ryoo HM, Park JY and Cho
JY: Identification of putative serum glycoprotein biomarkers for
human lung adenocarcinoma by multilectin affinity chromatography
and LC-MS/MS. Proteomics. 7:4292–4302. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chong PK, Lee H, Zhou J, Liu SC, Loh MC,
Wang TT, Chan SP, Smoot DT, Ashktorab H, So JB, et al: ITIH3 is a
potential biomarker for early detection of gastric cancer. J
Proteome Res. 9:3671–3679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Pertea M: The human transcriptome: An
unfinished story. Genes (Basel). 3:344–360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cajigas-Du Ross CK, Martinez SR,
Woods-Burnham L, Durán AM, Roy S, Basu A, Ramirez JA,
Ortiz-Hernández GL, Ríos-Colón L, Chirshev E, et al: RNA sequencing
reveals upregulation of a transcriptomic program associated with
stemness in metastatic prostate cancer cells selected for taxane
resistance. Oncotarget. 9:30363–30384. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhao S, Fung-Leung WP, Bittner A, Ngo K
and Liu X: Comparison of RNA-Seq and microarray in transcriptome
profiling of activated T cells. PLoS One. 9:e786442014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang Z, Li B, Xu P and Yang B: Integrated
whole transcriptome profiling and bioinformatics analysis for
revealing regulatory pathways associated with quercetin-induced
apoptosis in HCT-116 cells. Front Pharmacol. 10:7982019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Xiong YJ, Deng ZB, Liu JN, Qiu JJ, Guo L,
Feng PP, Sui JR, Chen DP and Guo HS: Enhancement of epithelial cell
autophagy induced by sinensetin alleviates epithelial barrier
dysfunction in colitis. Pharmacol Res. 148:1044612019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Forni C, Facchiano F, Bartoli M, Pieretti
S, Facchiano A, D'Arcangelo D, Norelli S, Valle G, Nisini R,
Beninati S, et al: Beneficial role of phytochemicals on oxidative
stress and age-related diseases. BioMed Res Int. 2019:87482532019.
View Article : Google Scholar : PubMed/NCBI
|