Introduction
Squamous cell carcinoma (SCC) of the tongue is a
common oral malignancy affecting patients worldwide (1–3). Lymph
node metastasis of the neck is an important prognostic factor for
this disease (4, 5). In our previous study of early SCC of the
tongue, post-operative lymph node relapse was also identified as an
important prognostic factor (6).
In recent years, novel immunotherapies have received
much attention. However, the mechanisms involved in their
anticancer effects are still not fully understood. In addition, it
has been suggested that chemokines may also influence mechanisms
within the tumor microenvironment (7), which is composed of many immune and
inflammatory cells, including macrophages. M2-like macrophages, in
particular, are major constituent cells and an important source of
chemokines (8).
A pivotal chemokine, CCL22 is mainly expressed by
macrophages and dendritic cells (9),
while its receptor, chemokine C-C motif receptor 4 (CCR4), is
mainly expressed by T cells (10).
With regard to the tumor microenvironment, CCL22 is expressed
mainly by M2-like tumor-associated macrophages (TAMs) (6). CCR4 is also known as a marker of
regulatory T cells (Tregs) and Th2 cells (11,12).
CCL22 attracts Th2 cells via CCR4 increasing Th2 cytokines [e.g.,
interleukin (IL)-4], which leads to the increased expression of
CCL22 in M2-like macrophages (12,13).
Cytotoxic T cells (CTLs) are anti-tumor immune cells that are also
found in the tumor microenvironment (14). The expression of CCL22 was suggested
to be involved in the suppression of CTLs in tongue SCCs via the
expression of Tregs (6).
Several studies have revealed that vascular
endothelial growth factor (VEGF)-C leads to lymphangiogenesis and
the induction of lymph node metastasis (15,16).
CD163, one of the representative markers of M2-like macrophages
(17), is involved in the expression
of VEGF-C in oral SCC (18). Since
CD163 is expressed in the same macrophages as CCL22 (6,19), it
follows that whether CCL22 expression is involved in VEGF-C
expression and lymphangiogenesis should be investigated. In
addition, several head and neck SCCs express CCR4, which, together
with CCL22, allow carcinoma cells to migrate to lymph nodes
(20).
In the diagnosis of tongue SCC, it should be noted
that characterizing an invasive pattern is useful for a prognosis
and to predict lymph node metastasis (6,21). The
worst pattern of invasion (WPOI) is one such example. The WPOI,
originally described in 2005, sets out various grades: type 1
refers to a pushing border; type 2 is a finger-like growth, type 3
refers to large separate islands with more than 15 cells per
island; type 4 represents small tumor islands with 15 cells or
fewer per island; and type 5 represents a satellite of the tumor,
≥1 mm from the main tumor or the next closest satellite (22). The WPOI mode of invasion was found to
significantly correlate and affect the prognosis of OSCC (23).
More recently, the depth of invasion (DOI) has also
attracted attention as a prognostic factor (24) and is associated with lymph node
metastasis (25). According to the
new 8th TNM classification, DOI determines pathological or clinical
T staging (26).
In the current study, the relationship between the
expression of CCL22 in the tumor microenvironment and lymph node
relapse in patients with tongue SCC, with reference to WPOI and
DOI, was investigated.
Materials and methods
Patient cohort and study design
We examined tumor sections from 110 patients with
tongue SCC who underwent primary surgery at a hospital at the
University of Occupational and Environmental Health, Kitakyushu,
Japan, between January 1997 and August 2017. Patients in this study
had the following background: Tumor size ≤40 mm; depth of invasion
≤10 mm; no metastasis in any node; surgical margins >5 mm; and
had not undertaken neoadjuvant therapy prior to surgery. Lymph node
metastasis was monitored using imaging analyses (ultrasonography,
computed tomography, or magnetic resonance imaging) in 2- to
6-month intervals post-surgery and verified by pathological
diagnosis. Lymph node relapse-free survival (LNFS) and overall
survival (OS), as the endpoints of analysis (September 2019), were
defined as the time from surgery until lymph node relapse or death
had occurred, respectively, or until the last follow-up visit.
Patient samples (females, 32; males, 78) aged 32–89
years [mean ± standard deviation (SD), 64.6±13.3 years] were
obtained at diagnosis.
Our study was approved by the Research Ethics
Committee of the University of Occupational and Environmental
Health (permission nos. H29-212 and H24-6) and was performed in
accordance with the guidelines of the Declaration of Helsinki.
Histopathological staining and
classification of tongue SCC, WPOI, and histological grade
After fixation of resected tumor tissues in 10%
formalin and embedding in paraffin, sections (thickness, 3 µm)
underwent hematoxylin and eosin (H&E) staining.
Histological classification was based on the
American Joint Committee on Cancer Staging Manual, 8th edition
(27). The WPOI classification
system was used to assess the pattern of tumor invasion in
patients. Tumor differentiation was divided into three groups:
well-differentiated (I), moderately differentiated (II), and poorly
differentiated (III; Table I).
 | Table I.Clinicopathological data of 110
patients with tongue SCC divided in WPOI-1 (n=19), WPOI-2 (n=24),
WPOI-3 (n=24), WPOI-4 (n=29) and WPOI-5 (n=14). |
Table I.
Clinicopathological data of 110
patients with tongue SCC divided in WPOI-1 (n=19), WPOI-2 (n=24),
WPOI-3 (n=24), WPOI-4 (n=29) and WPOI-5 (n=14).
| Parameters | WPOI-1 | WPOI-2 | WPOI-3 | WPOI-4 | WPOI-5 | Total |
|---|
| Age, n (%) |
| ≥75
years | 6 (31.6) | 6 (25.0) | 7 (29.2) | 11 (37.9) | 2 (14.3) | 32 (29.1) |
| <75
years | 13 (68.4) | 18 (75.0) | 17 (70.8) | 18 (62.1) | 12 (85.7) | 78 (70.9) |
| Sex, n (%) |
|
Male | 13 (68.4) | 19 (79.2) | 17 (70.8) | 20 (69.0) | 9 (64.3) | 78 (69.9) |
|
Female | 6 (31.6) | 5 (20.8) | 7 (29.2) | 9 (31.0) | 5 (35.7) | 32 (29.1) |
| Alcohol use, n
(%) |
|
Yes | 12 (63.2) | 14 (58.3) | 13 (54.2) | 18 (62.1) | 9 (64.3) | 66 (60.0) |
| No | 7 (36.8) | 10 (41.7) | 11 (45.8) | 11 (37.9) | 5 (35.7) | 44 (40.0) |
| Smoking, n (%) |
|
Yes | 15 (78.9) | 19 (79.2) | 20 (83.3) | 22 (75.9) | 9 (64.3) | 85 (77.3) |
| No | 4 (21.1) | 5 (20.8) | 4 (16.7) | 7 (24.1) | 5 (35.7) | 25 (22.7) |
| Grade, n (%) |
| I | 15 (78.9) | 18 (75.0) | 14 (58.3) | 16 (55.2) | 3 (21.4) | 66 (60.0) |
| II | 4 (21.1) | 6 (25.0) | 9 (37.5) | 10 (34.5) | 4 (28.6) | 33 (30.0) |
|
III | 0 (0.0) | 0 (0.0) | 1 (4.2) | 3 (10.3) | 7 (50.0) | 11 (10.0) |
| DOI, n (%) |
| ≥5
mm | 0 (0.0) | 0 (0.0) | 4 (16.7) | 13 (44.8) | 9 (64.3) | 26 (23.6) |
| <5
mm | 19 (100.0) | 24 (100.0) | 20 (83.3) | 16 (55.2) | 5 (35.7) | 84 (76.4) |
| Mean
DOI, mm | 0.816 | 1.621 | 3.142 | 4.590 | 6.679 | 3.240 |
| Lymphatic vessel
invasion, n (%) |
|
Yes | 0 (0.0) | 3 (12.5) | 12 (50.0) | 18 (62.1) | 14 (100.0) | 47 (42.7) |
| No | 19 (100.0) | 21 (87.5) | 12 (50.0) | 11 (37.9) | 0 (0.0) | 63 (57.3) |
| Lymph node relapse,
n (%) |
|
Yes | 1 (5.3) | 1 (4.2) | 3 (12.5) | 5 (17.2) | 8 (57.1) | 18 (16.4) |
| No | 18 (94.7) | 23 (95.8) | 21 (87.5) | 24 (82.8) | 6 (42.9) | 92 (83.6) |
| Death due to tongue
SCC, n (%) |
|
Yes | 1 (5.3) | 2 (8.3) | 2 (8.3) | 4 (13.8) | 10 (71.4) | 19 (17.3) |
| No | 18 (94.7) | 22 (91.7) | 22 (91.7) | 25 (86.2) | 4 (28.6) | 91 (82.7) |
Immunohistochemical studies
An automated immunostainer, Histostainer 36A
(Nichirei Biosciences Inc.), was used to process sections that had
been fixed in 10% formalin and immersed in paraffin in accordance
with the manufacturer's protocol. Antibodies used were: mouse
monoclonal anti-human CD68 (clone Kp-1, 1:100) and CD8 (clone
C8/144B, 1:100), both from Dako Japan; and rabbit polyclonal
antibody to CCL22 (1:100) and goat polyclonal antibody to CCR4
(1:300), both from Abcam. Mouse monoclonal anti-human VEGF-C (clone
MM0006-2E65, 1:50) was obtained from Novus Biologicals. Mouse
monoclonal anti-human antibody to D2-40 (clone D2-40, 1:1;
Nichirei) was used to examine lymphatic vessels.
Five areas were randomly selected and
CCL22−, CCR4−, CD8−, and
VEGF-C-positive cells were counted using a microscope
(magnification, ×400); data was expressed as per mm2
surface area. Two independent pathologists, blinded to patients'
backgrounds or their prognosis, evaluated all immunochemical and
histological slides.
Cell proliferation assays were also performed, as
described in our previous study, by using anti-Ki67 (mouse
monoclonal, clone MIB-1; 1:100; Dako) (6).
Agreement among observers was excellent (agreement
>95%) for all antibodies and according to an interclass
correlation coefficient. In the case of a rare disagreement, a
third, departmental board-certified pathologist calculated a
consensus score.
Immunofluorescence assays
Thin sections (4 µm) were used for
immunofluorescence assays and anti-CCL22, CCR4 and VEGF-C
antibodies were used in experiments as described above. In
addition, we used rabbit polyclonal anti-human VEGF-C (1:100; Santa
Cruz Biotechnology, Inc.), and rabbit polyclonal anti-human signal
transducer and activator of a transcription 6 (STAT6; phospho Y641)
(1:100; Abcam). Secondary antibodies used were:
rhodamine-conjugated donkey anti-rabbit IgG (1:200; Merck) and goat
anti-mouse IgG (H+L; 1:200; Merck); and FITC-conjugated goat
anti-rabbit IgG (H+L; 1:200; Merck) and donkey anti-goat IgG
(1:200; Merck). A nuclear stain, 4′,6-diamidino-2-phenylindole
(DAPI; GeneTex), was used on sections, which were then mounted. An
ECLIPSE E600 inverted fluorescence microscope (Nikon) was used to
examine slides, and images captured and analyzed with LuminaVision
software (v. 2.2.2; Mitani Corporation).
Quantification of
lymphangiogenesis
Using anti-D2-40, the density of lymphatic vessels
(LVD) was measured as previously described (28). LVD was evaluated at the periphery,
within 2 mm of the tumor, and next to the invasive front. Five
areas that showed many lymphatic vessels were chosen using light
microscopy at a ×40 magnification. All stained vessels in each area
at a ×200 magnification were counted and the data was expressed as
per mm2. The mean number of lymphatic vessels was
calculated and expressed as LVD in a blinded manner (described
above).
Cell culture
A human macrophage cell line (human CD14+
monocytes from peripheral blood, single donor; C-12909, http://www.promocell.com/product/human-cd14-monocytes-hmocd14-pb/)
from PromoCell GmbH (Heidelberg, Germany) was cultured at a density
of 1×106 cells in Monocyte Attachment Medium (PromoCell
GmbH). Cells were cultured in M1-Macrophage Generation Medium DXF
so that they could differentiate into M1- and M2-like macrophages
as in our previous study (19). In
addition, these cells were treated with 1, 10 or 20 ng/ml of
interleukin-4 (IL-4; R&D Systems) for 24 h. Human monocytic
(THP-1) cells were obtained from the American Type Culture
Collection and maintained in RPMI-1640 containing 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.), 1%
penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.),
and 2 mmol/l of glutamine (Gibco; Thermo Fisher Scientific,
Inc.).
Real-time polymerase chain
reaction
For quantitative real-time polymerase chain
reactions (qRT-PCR), a TaqMan assay and a 7700 Sequence Detection
System (Applied Biosystems; Thermo Fisher Scientific, Inc.) were
used as in our previous study (19).
A pre-made primer/probe set (Applied Biosystems Japan, Ltd.)
containing primers and fluorogenic probes for CCL22 and
VEGFC was used. Messenger RNA expression was normalized to
that of 18s ribosomal RNA in each sample.
Luciferase reporter assay
The CCL22 gene was cloned from an upstream
gene region using information on chromosome 16q13, and a luciferase
assay was performed as described in our previous study (29). Genomic DNA was extracted from
monocytes isolated from 15 ml whole blood donated from four healthy
volunteer donors. A human CCL22 promoter region spanning
between −966 and +32 bp from the transcription start site was then
generated by PCR. A pGEM-T Easy vector was used to subclone the PCR
product. Serial 5′-deletion constructs (−518/+32, −491/+32,
−281/+32) were generated by PCR and digestion with appropriate
restriction enzymes. Luciferase reporter genes that included a
mutation in the STAT6 site of the CCL22 promoter were
generated by site-directed mutagenesis (Stratagene) using the
following primers: 5′-ATGTGGACAGCACGAGAAGCCCCAGAT-3′ for
STAT6 mutation, where the underlined nucleotides represented
the mutated site.
Twenty micrograms of promoterless pGL3-basic vector
or CCL22 promoter luciferase constructs were used to transfect
THP-1 monocytic cells treated with 20 ng/ml
12-O-tetradecanoylphorbol-13-acetate as in our previous study
(29). A pSV-beta-galactosidase
control plasmid (Promega Corporation) was used as an internal
control. A luminometer (Bio-Orbit Oy) was used to measure
luminescence.
Secretion and regulation of CCL22 in
macrophages
CCL22 protein expression via IL-4 (R&D Systems),
with or without AS1517499 (Axon Medchem BV) as a selective STAT6
inhibitor, in human macrophage cell line (C-12909; PromoCell GmbH)
culture supernatants was measured using an enzyme-linked
immunosorbent assay (ELISA) kit (R&D Systems) as in a previous
study (29).
Statistical analysis
Statistical analyses of data between the groups were
performed using one-way ANOVA followed by the Tukey-Kramer post-hoc
comparison test. Survival curves were plotted according to a
Kaplan-Meier method and compared using a log-rank test. Odds ratios
(ORs) and corresponding 95% confidence intervals were calculated
from logistic regression models. Pearson's correlation coefficient
was used to assess the association between lymphangiogenesis and
other factors.
Two-sidedP-values were used
A P-value ≤0.05 was considered statistically
significant. EZR software (Saitama Medical Center, Jichi Medical
University, Saitama, Japan) was used for all analyses. EZR software
is a graphical user interface for R, a modified version of R
commander (version 1.6–3; The R Foundation for Statistical
Computing, Vienna, Austria, version 2.13.0) with statistical
functions used in biostatistics (30).
The optimal cut-off values for lymph node relapse
(maximizing the sum of sensitivity and specificity) were also
analyzed by EZR software using receiver operating characteristic
(ROC) curve analysis.
Results
Clinical and pathological
characteristics of 110 patients with tongue SCC
Table I shows
clinicopathological features of the 110 patients of this study
based on a WPOI classification system. The patients were
categorized as follows, and the various parameters were compared:
WPOI-1, 19 (17.3%); WPOI-2, 24 (21.8%); WPOI-3, 24 (21.8%); WPOI-4,
29 (26.4%); and WPOI-5, 14 (12.7%).
There was a high correlation between lymphatic
invasion and WPOI classification (WPOI-1 vs. WPOI-2 vs. WPOI-3 vs.
WPOI-4 vs. WPOI-5; 0.0 vs. 12.5 vs. 50.0 vs. 62.1% vs. 100%,
respectively). WPOI-1 and −2 patients with lymph node relapse were
2/43 (4.7%) of the patient cohort. In comparison, 16/67 patients
(23.9%) with WPOI-3, −4, and −5 showed lymph node relapse (Table I). The occurrence of lymph node
relapse was significant in WPOI-3, −4, and −5 cases (P=0.008;
Fisher's exact test) suggesting WPOI-3, −4, and −5 lesions were
prone to lymph node relapse. In terms of lymph node relapse rates,
WPOI was classified into two distinct groups: WPOI-1 and −2
(WPOI-low); and WPOI-3, −4, and −5 (WPOI-high).
Impacts of WPOI classification for
prognosis
The 5-year LNFS rates in patients in WPOI-low vs.
WPOI-high groups were 88.4% vs. 65.7%, respectively; the difference
was statistically significant (P=0.001; Fig. 1A). In addition, the 5-year OS rates
in patients in WPOI-low vs. WPOI-high groups were 93.6 vs. 76.1%,
respectively; the difference was statistically significant
(P=0.006) (data not shown).
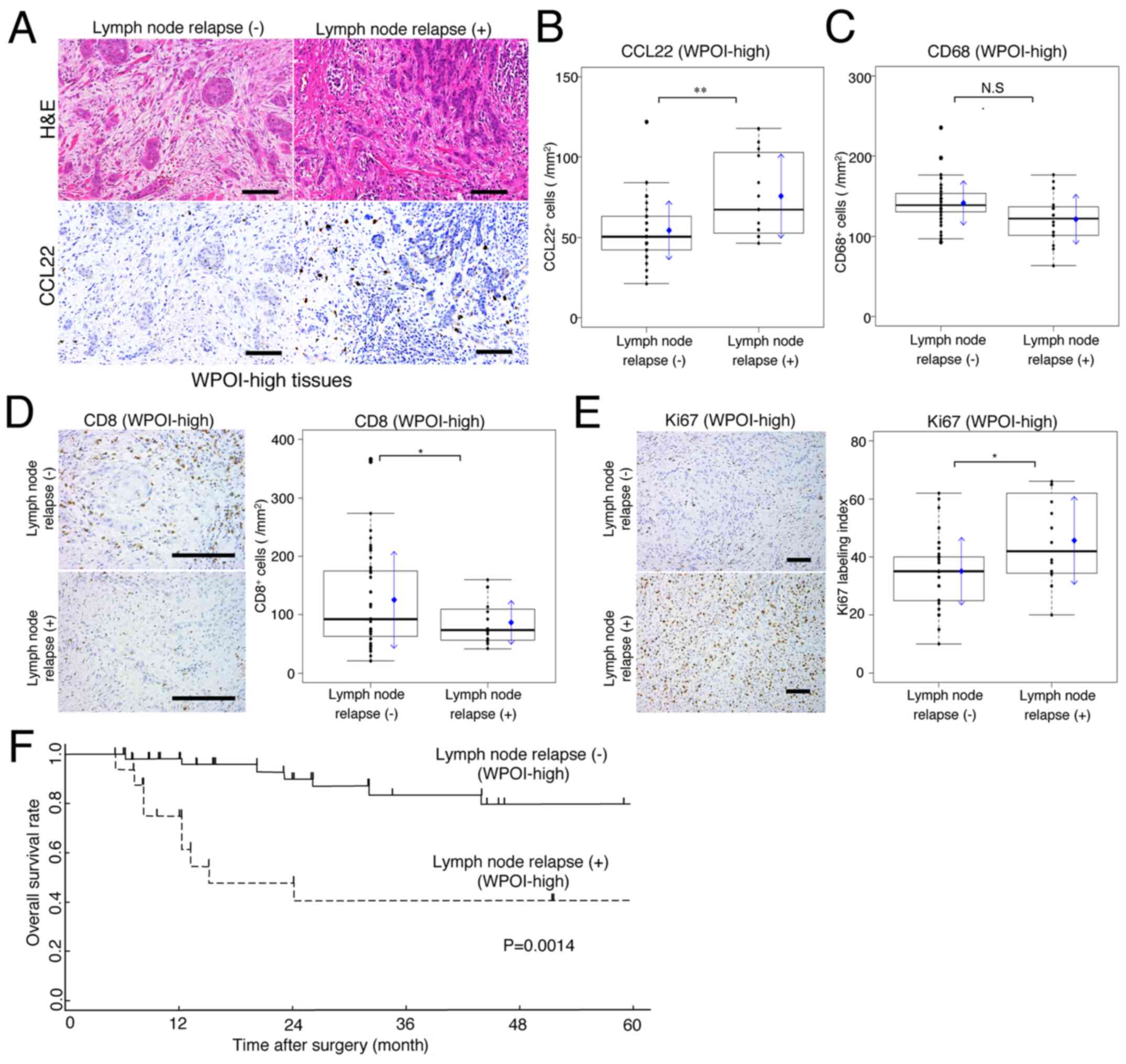
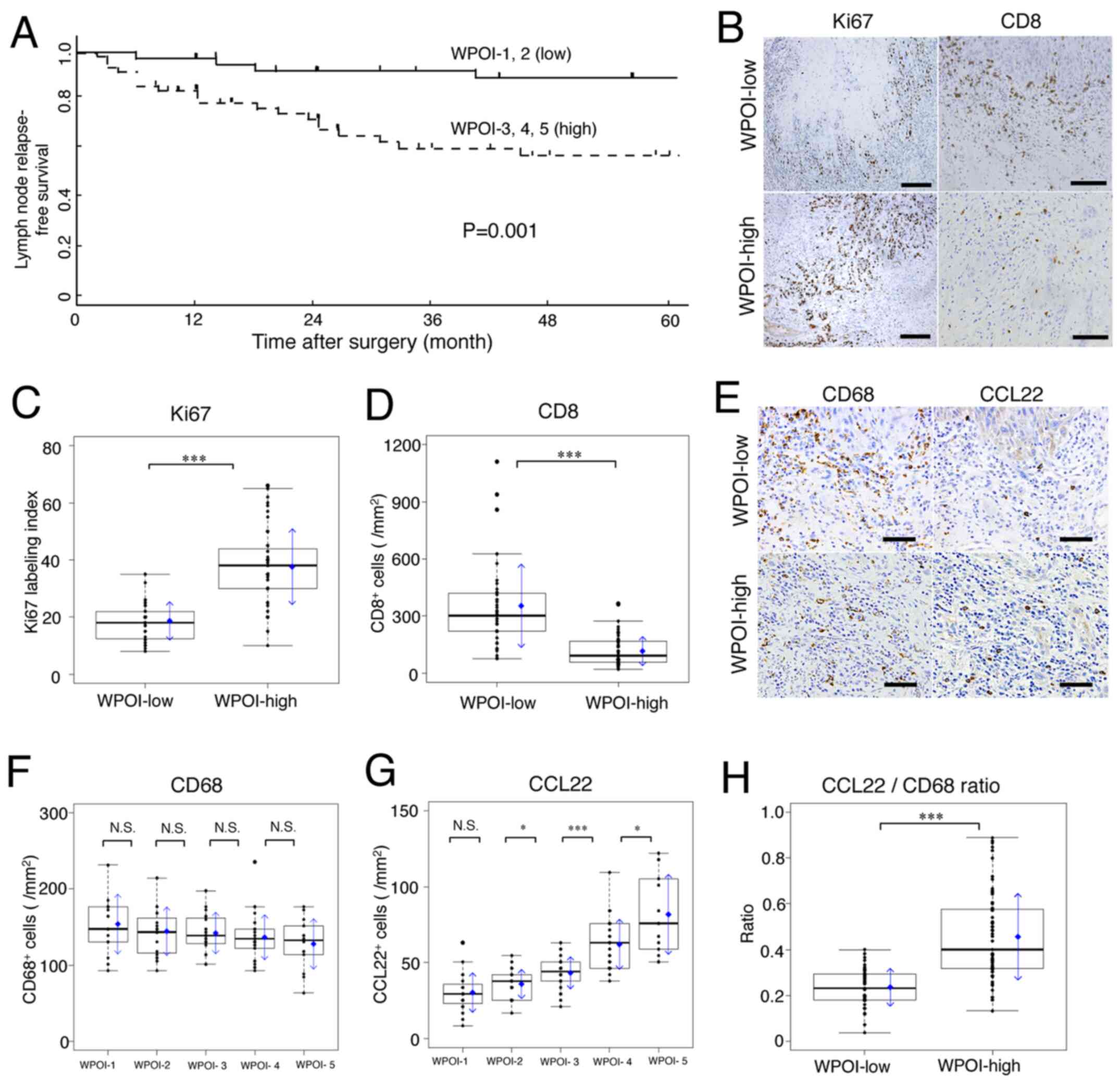 | Figure 1.Lymph node relapse-free survival
curves for patients with tongue SCC, and association of Ki67, CD8,
CD68 and CCL22 with WPOI. WPOI-1 to −5 were classified into two
categories based on the frequency of lymph node relapse: WPOI-1 and
−2 were considered as WPOI-low, and WPOI-3, −4 and −5 were
considered as WPOI-high. (A) Kaplan-Meier lymph node relapse-free
survival curves for 110 patients with tongue SCC based on WPOI-low
and -high status. (B) Ki67 (scale bar, 200 µm) and CD8 (scale bar,
100 µm) expression in WPOI-low and -high groups by
immunohistochemical staining. (C) Ki67 expression was significantly
increased and (D) CD8+ cells were significantly
decreased in the WPOI-high compared with in the WPOI-low group. (E)
CD68 and CCL22 expression in WPOI-low and -high groups by
immunohistochemical staining (scale bar, 50 µm). (F)
CD68+ cells were not significantly different across WPOI
classifications, but mean values tended to be inversely
proportional to WPOI. (G) CCL22+ cells increased
proportionally to WPOI classification. (H) Comparison of the ratio
between CCL22+ cells to CD68+ cells between
WPOI-low and -high groups. In box plots, the boxes display the
median and interquartile range of the data, and the whiskers
display the 10th and 90th percentiles. Blue diamonds and blue
arrows indicate the mean ± SD. *P<0.05; ***P<0.001. N.S., not
significant; SCC, squamous cell carcinoma; WPOI, worst pattern of
invasion; CCL22, C-C motif chemokine ligand 22. |
Ki67 (a marker of tumor proliferation) was expressed
only in the margin of the tumor infiltrating region in WPOI-low and
WPOI-high cases; it was randomly expressed in tumor cells of the
whole infiltrate (Fig. 1B; Ki67).
Scattered CD8-positive cells (cytotoxic T lymphocytes; CTLs) were
found in the tumor microenvironment and only a few were found in
WPOI-high compared to WPOI-low tissues (Fig. 1B; CD8). WPOI-high tissues had
significantly more Ki67-positive tumor cells than WPOI-low tissues
(P<0.001; Fig. 1C). In contrast,
CD8-positive cells were significantly decreased in WPOI-high
compared to WPOI-low lesions (P<0.001; Fig. 1D).
In short, the WPOI classification correlated well
with the growth potential of the tumor and decreased cellular
immunity.
Macrophage expression in tumor
microenvironment and relationship with WPOI and lymph node
relapse
The number of CD68-positive macrophages per area did
not significantly differ with WPOI classification, but the mean
value tended to be inversely proportional to WPOI classification
(Fig. 1E-CD68 and F). In contrast,
the numbers of CCL22-positive macrophages per area did not differ
in WPOI-1 vs. −2, but increased proportionally from WPOI-2 to −5
(Fig. 1E-CCL22 and G). Fig. 1H shows the CCL22/CD68 ratio (CCL22
ratio). WPOI-high SCC had a significantly high CCL22 ratio (vs.
WPOI-low; P<0.001). These results suggest that the CCL22 ratio
may be an indicator of WPOI classification.
Implications of expression of CCL22
and lymph node relapse for prognosis
Fig. 2A shows H&E
and CCL22 stains for the same grade of WPOI-high cases, with or
without lymph node relapse, during the observation period.
CCL22-positive macrophage expression was significantly higher in
lymph node relapse cases (Fig. 2B).
In comparison, CD68 expression showed a slight low mean and median
value in patients with lymph node relapse, but this was not
significantly different compared to those without lymph node
relapse (Fig. 2C). In cases of lymph
node relapse, the expression of CD8 positive cells was
significantly lower, whereas the expression of Ki67 was
significantly higher (Fig. 2D and
E).
For WPOI-high patients, the relationship between
lymph node relapse and prognosis was examined. As a result, a
difference in the 5-year OS rates between patients, with and
without lymph node relapse, was statistically significant
(P=0.0014; Fig. 2F).
Relationship between LVD and CCL22
expression via WPOI and DOI
Lymphatic vessels with D2-40 immunohistological
staining are shown in Fig. 3A. A
significant relationship between increased LVD and WPOI was
observed (WPOI-low vs. -high; P<0.001; Fig. 3B). When LVD was compared in WPOI-high
cases, with or without lymph node relapse, it was found to be
significantly increased in the lymph node relapse group
(P<0.001; Fig. 3C).
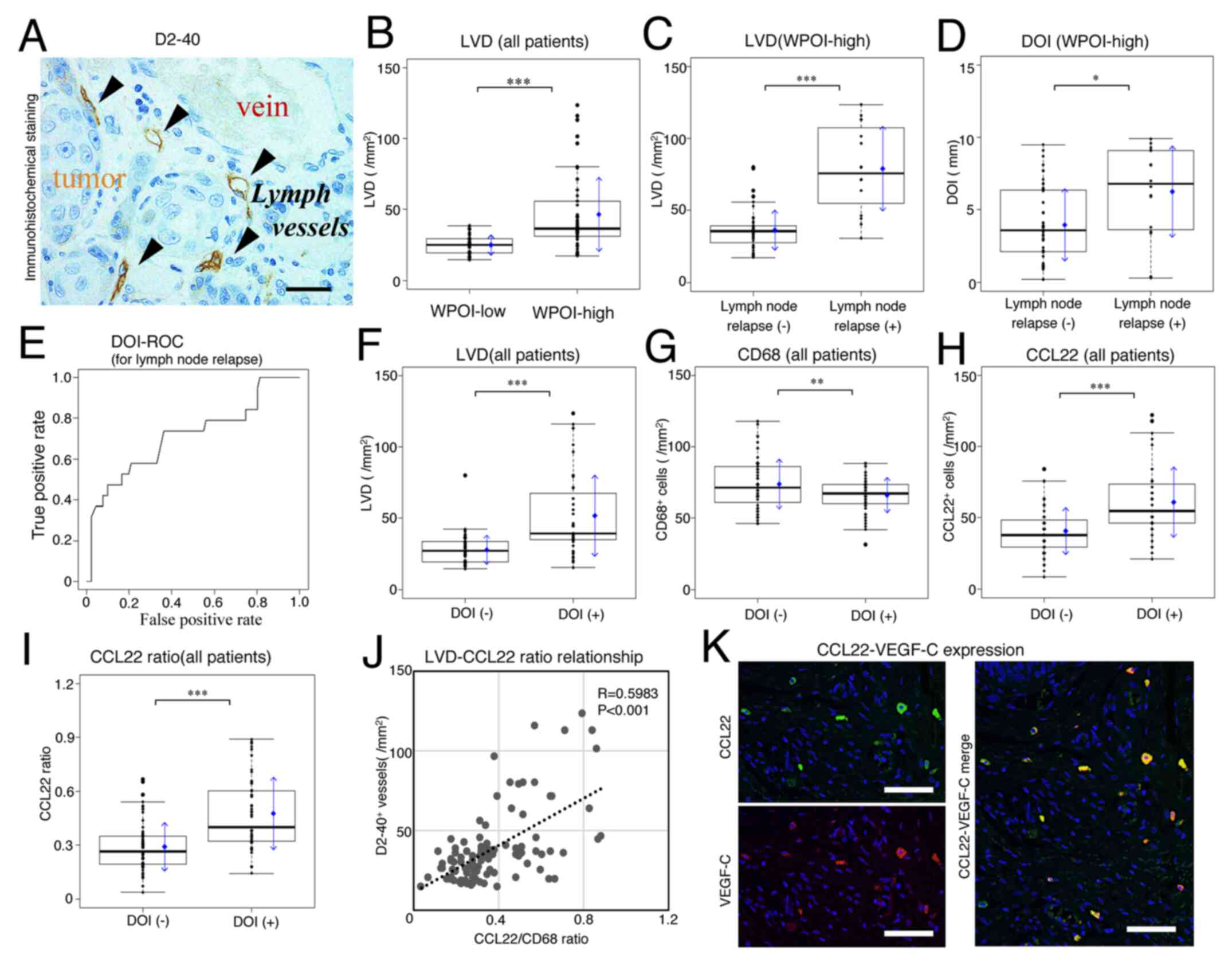 | Figure 3.Comparison of LVD and DOI by WPOI
grade, lymph node relapse and CCL22 expression. (A) Vessels with
positive D2-40 immunohistochemical staining, as well as a vein and
tumor tissue (scale bar, 10 µm). Arrowheads indicate lymph vessels.
(B) LVD expression was compared between WPOI-1 and −2 (low) and
WPOI-3, −4 and −5 (high) lesions. Comparisons of (C) LVD and (D)
DOI in patients with lymph node relapse in the WPOI-high group. (E)
ROC curve for DOI and lymph node relapse (3.4 mm was defined as the
cut-off value). Comparisons between the DOI-negative (<3.4 mm)
and -positive (≥3.4 mm) groups of (F) LVD, (G) CD68+ and
(H) CCL22+ macrophages. (I) Association between the
CCL22+/CD68+ macrophage ratio (CCL22 ratio) and DOI. (J)
Correlation between the CCL22 ratio and LVD. (K) Immunofluorescence
micrographs of CCL22 (green) and VEGF-C (red) expression on
macrophages in the tumor microenvironment (scale bar, 100 µm). In
box plots, the boxes display the median and interquartile range of
the data, and the whiskers display the 10th and 90th percentiles.
Blue diamonds and blue arrows indicate the mean ± SD. *P<0.05;
**P<0.01; ***P<0.001. N.S., not significant; DOI, depth of
invasion; LVD, density of lymphatic vessels; ROC, receiver
operating characteristic; VEGF, vascular endothelial growth factor;
WPOI, worst pattern of invasion; CCL22, C-C motif chemokine ligand
22. |
Fig. 3D shows the
relationship between DOI and lymph node relapse in WPOI-high SCC.
DOI was significantly higher in patients with lymph node relapse
(without vs. with lymph node relapse; mean, 3.96 vs. 6.26 mm,
P=0.0145). Therefore, not only WPOI but also DOI seems to be
related to LVD.
Fig. 3E shows the ROC
curve of DOI for lymph node relapse [area under the curve (AUC);
0.7524]. DOI could be thus classified into two (<3.4 mm; n=63;
and ≥3.4 mm; n=47) expression groups using the Youden index from
the ROC curve (sensitivity, 0.8333; specificity, 0.6522). When
comparing between WPOI-low and -high for DOI cut-off values (n=5
and n=42), the positive rate of DOI was significantly higher in the
WPOI-high group (Fisher's exact test; P<0.001). Additionally,
this cut-off value was significantly correlated with LVDs in all
patients (Fig. 3F). The expression
of CD68 and CCL22 in TAMs showed opposing trends when comparing
negative and positive DOI (Fig. 3G and
H). As a result, the CCL22-positive/CD68-positive macrophage
ratio (CCL22 ratio) was most useful as an index for macrophages
with a DOI cut-off value for lymph node relapse (Fig. 3I).
A correlation between LVD and the number of
CCL22-positive macrophages or the CCL22 ratio was examined. The
increase in the LVD was proportional to the expression of both the
number of CCL22-positive macrophages/mm2 (R=0.4400;
P<0.001) and CCL22 ratio (R=0.5983; P<0.001; Fig. 3J), showing a significantly positive
correlation.
These results suggest that both WPOI and DOI may be
involved in CCL22-mediated lymph node metastasis. Additionally, a
reason for the correlation between CCL22 and LVD was thought to be
that CCL22-positive macrophages expressed VEGF-C (Fig. 3K).
Implications of CCL22 ratio for lymph
node relapse in patients with WPOI-high SCC
WPOI-high patients had a higher lymph node relapse
rate. We therefore examined the predictors for lymph node relapse
in this patient group.
We were able to divide the patients into two groups
according to the CCL22 ratio as follows: low (<0.38; n=72) and
high (≥0.38; n=38) based on ROC curve analysis for lymph node
relapse. The AUCs for the number of CCL22/mm2 and CCL22
ratios for lymph node relapse according to the ROC curve were
0.7811 vs. 0.8210. The AUC was higher for the CCL22 ratio. In
patients with high WPOI, predictor variables including grade, DOI,
CCL22 ratio, and majority parameters (age, alcohol use, sex, and
smoking) were analyzed in a logistic regression model with lymph
node recurrence as the dependent variable. Table II shows adjusted OR and p values.
The lymph node relapse in patients with WPOI-high was significantly
associated with a high CCL22 ratio (P=0.0225) but not with the
other variables by univariate and multivariate logistic regression
model analyses.
 | Table II.Univariate and multivariate logistic
regression model used to identify independent predictors for lymph
node relapse in WPOI-3, −4 and −5 groups. |
Table II.
Univariate and multivariate logistic
regression model used to identify independent predictors for lymph
node relapse in WPOI-3, −4 and −5 groups.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Parameters | N (%) | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Age, years |
|
≥75 | 20 (29.9) | 0.729 | 0.182–2.474 | 0.628 | – | – | – |
|
<75 | 47 (70.1) |
|
|
|
|
|
|
| Sex |
|
Female | 21 (31.3) | 1.500 | 0.445–5.995 | 0.532 | – | – | – |
|
Male | 46 (68.7) |
|
|
|
|
|
|
| Alcohol use |
|
Yes | 40 (59.7) | 1.167 | 0.373–3.889 | 0.794 | – | – | – |
| No | 27 (40.3) |
|
|
|
|
|
|
| Smoking |
|
Yes | 51 (76.1) | 0.407 | 0.119–1.430 | 0.150 | – | – | – |
| No | 16 (23.9) |
|
|
|
|
|
|
| Grade |
|
III | 11 (16.4) | 9.139 | 2.298–41.605 | 0.002 | 3.408 | 0.751–17.575 | 0.120 |
| I or
II | 56 (83.6) |
|
|
|
|
|
|
| DOI, mm |
|
≥3.4 | 42(62.7) |
5.750 |
1.414–39.059 |
0.030 | 4.251 | 0.871–32.042 | 0.100 |
|
<3.4 | 25(37.3) |
|
|
|
|
|
|
| CCL22/CD68
ratio |
|
≥0.38 | 35 (52.2) | 21.429 | 3.885–402.398 | 0.004 | 12.736 | 2.018–250.014 | 0.023 |
|
<0.38 | 32 (47.8) |
|
|
|
|
|
|
Relationship of CCL22 and VEGF-C
expression via CCR4 expression
In WPOI-high lesions, the expression of
CCR4-positive cells was found to be significantly higher than in
WPOI-low lesions (Fig. 4A). In
addition, more CCR4-positive cells were observed
immunohistologically in patients with lymph node relapse in the
same lesion as in Fig. 2A (Fig. 4B). Among WPOI-high cases,
CCR4-positive cells were significantly more prevalent in lymph node
relapse cases (Fig. 4B) and were
found around VEGF-C positive macrophages (Fig. 4C). The aggregation of CCR4-positive
cells was proportional to the expression of VEGF-C-positive cells,
with a significant correlation noted (R=0.6532; P<0.001;
Fig. 4D).
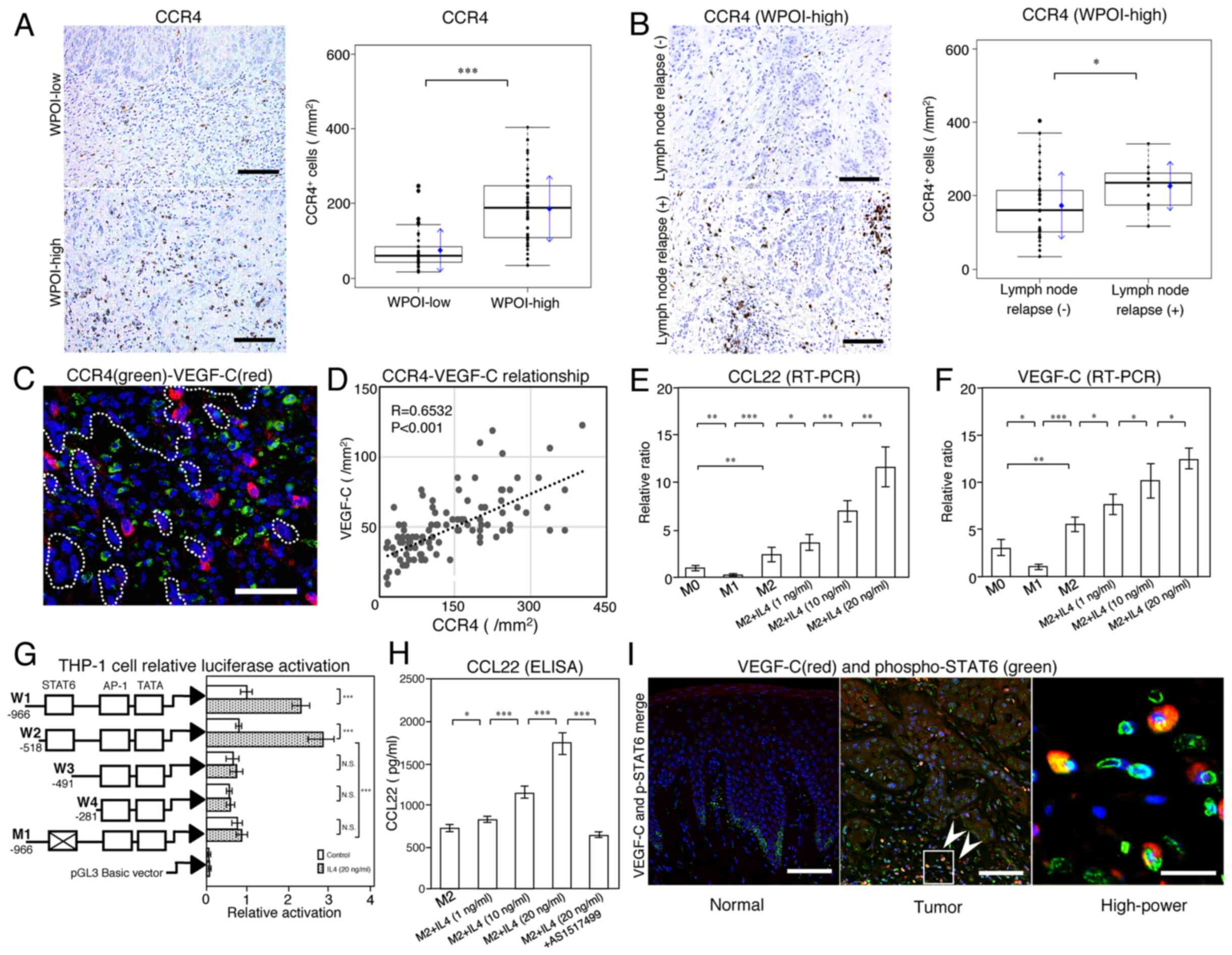 | Figure 4.Association between CCL22 and CCR4
via the IL-4/STAT6 signaling pathway. Comparisons of CCR4
expression in (A) WPOI-low and -high lesions, and (B) WPOI-high
tissues with or without lymph node relapse (scale bar, 100 µm). In
box plots, the boxes display the median and interquartile range of
the data, and the whiskers display the 10th and 90th percentiles.
Blue diamonds and blue arrows indicate the mean ± SD. (C)
Localization of VEGF-C+ and CCR4+ cells.
VEGF-C+ cells appeared surrounding the invasive tumor
and CCR4+ cells were observed within their vicinity. The
dotted line outlines the boundary of the tumor and invading tumor
cells. The blue DAPI stain indicates cellular nuclei (scale bar, 50
µm). (D) Correlation between CCR4 and VEGF-C expression. Macrophage
subtypes and the effect of IL-4 stimulation on (E) CCL22 and (F)
VEGF-C expression were examined using human macrophages. (G)
Luciferase assay in THP-1 cells with 5′-serial deletion constructs
and point mutations, and responses to 20 ng/ml IL-4. The luciferase
activity of each sample was normalized to β-galactosidase activity.
The ‘X’ in the box indicates a mutation. (H) CCL22 levels in
M2-like macrophages in response to IL-4 (1, 10 and 20 ng/ml) and
AS1517499 (200 nM) in cell culture supernatants measured by ELISA.
(I) Activation of IL-4/STAT6 and VEGF-C expression in a case of
WPOI-4 with lymph node relapse. The image labeled ‘Normal’ shows
normal tissue away from the tumor, while the image labeled ‘Tumor’
shows a deep infiltration site (scale bar, 200 µm). The
‘High-power’ image shows a high-power magnification image of the
square indicated by arrowheads in the ‘Tumor’ image (scale bar, 20
µm). Phospho-STAT6 (green) was expressed in the nucleus and VEGF-C
(red) was expressed in the cytoplasm. The blue DAPI stain indicates
cellular nuclei. The data are expressed as the mean ± SD of
triplicates from three independent experiments. *P<0.05;
**P<0.01; ***P<0.001. N.S., not significant; IL-4,
interleukin-4; p/phospho-STAT6, phosphorylated signal transducer
and activator of transcription 6; VEGF, vascular endothelial growth
factor; WPOI, worst pattern of invasion; CCL22, C-C motif chemokine
ligand 22. |
Thus, the function of CCR4-positive cells was also
important as a factor in the correlation between CCL22 and
VEGF-C.
Regulation of CCL22 and VEGF-C
expression via IL-4/STAT6
In vitro, CCL22 expression was significantly
higher in M2-like compared to M1-like macrophages, as was VEGF-C
expression; in M2-like macrophages, the expression of both was
increased by IL-4 in a dose-dependent manner (Fig. 4E and F). The regulation of VEGF-C
expression in macrophages is known to be via an IL-4/STAT6
signaling pathway (31); CCL22 was
also examined in this study. We found that W1 and W2 promotors,
including a STAT6 motif, had significantly higher luciferase
activity after IL-4 stimulation. However, there was no significant
difference in the luciferase activities of W3 and W4 promoters
without a STAT6 motif after IL-4 stimulation. When a STAT6 mutation
was inserted into W1 (M1), the luciferase activity significantly
decreased (Fig. 4G) and no longer
responded to IL-4 stimulation. STAT6 activation also affected the
production of CCL22 protein via IL-4 (Fig. 4H). Our results supported the
conclusion that IL-4 induces CCL22 expression via an
STAT6-dependent pathway. Fig. 4I
shows STAT6 activated-cells (green) and VEGF-C-positive macrophages
(red). VEGF-C-positive cells accompanied STAT6 activation. In
addition, STAT6 activation of almost all carcinoma cells was
observed in the deeply invaded part of the tumor. However, in
normal tissue around the tumor, STAT6 activation was observed only
in basal cells (Fig. 4I normal).
Thus, STAT6 activation was considered to be an
important factor in lymph node metastasis of tongue SCC.
Discussion
Immunosuppressive and anti-inflammatory molecules
linked to TAMs are associated with a poor cancer prognosis
(32,33). Interactions between cells in the
tumor microenvironment are critical for tumor progression and are
involved in invasion and metastasis. Certainly, macrophages are
also involved in the interaction (34). In this study, we focused on
lymphangiogenesis through cell-cell interactions in which M2-like
TAMs attract Th2 cells in the tumor microenvironment.
In patients with tongue SCC, having a WPOI-high
grade classification generally led to a poor prognosis (23). The WPOI classification was divided
into two groups according to the lymph node relapse ratio. A
WPOI-high group, involving invasive tumor islands, showed a
significant decrease in CD8 and increase in Ki67, and a
significantly poor prognosis. The expression of CCL22 in the tumor
microenvironment also correlated with decreased CD8 and increased
Ki67 in our previous study (6). The
molecular biological effects of CCL22 include several factors that
influence tumor prognosis. However, few studies have investigated
the correlation between CCL22 expression and lymph node metastasis.
Lymph node relapse and lymphatic vessel invasion are important
prognostic determinants of OSCC. However, venous invasion did not
correlate with prognosis in patients with early tongue SCC
(6). Lymphangiogenesis is one of the
causes of lymph node metastasis via lymph node invasion and leads
to a poor prognosis (35).
Compared with WPOI-low, the WPOI-high group showed
increased expression of CCL22 and LVD. WPOI-high with lymph node
relapse also showed a further increase in expression of CCL22, LVD,
and DOI. When the correlation between the CCL22 ratio and LVD was
examined, a positive and significant correlation was found. In this
study, the cut-off value of DOI for lymph node recurrence was
defined as 3.4 mm, which was also consistent with the cut-off DOI
value for positive sentinel lymph node metastasis in OSCC (25). In future, the DOI cut-off value is
expected to be a reference value for lymph node metastasis in early
OSCC.
IL-4 is a typical Th2 cytokine that differentiates T
lymphocytes predominantly into Th2 cells, and also differentiates
macrophages into M2-like macrophages (19). M2-like macrophages produced CCL22 and
VEGF-C, and the response was enhanced by IL-4. An IL-4 dominant
environment also allows the migration of macrophages into the local
environment and, consequently, TAMs differentiate into M2-like
macrophages (36,37). Moreover, lymphatic vessels
proliferate when the cytokine balance is predominantly Th2
(38). IL-4, in cooperation with
tumor cells and macrophages, has various roles in the tumor
microenvironment (36).
Interestingly, the proliferation of lymphatic endothelial cells is
known to be suppressed by Th2 cytokines (39), supporting the notion that the effect
of IL-4 on lymphangiogenesis in the tumor microenvironment depends
on cell-cell interactions via the M2-like differentiation of TAMs.
Macrophage-mediated cell-cell interaction is thought to be
important, as seen in lymphangiogenesis due to increased VEGF-C in
a Th2-dominant environment in other diseases (38,40). The
tumor microenvironment contains many immune or inflammatory cells,
such as M1-like macrophages and Th1 cells. The proportion of
M2-like macrophages, but not the total number of CD68-positive
macrophages, is associated with the presence of lymph node relapse.
Therefore, the balance of each type of inflammatory cell may affect
the extent of tumor progression via cell-cell interactions
(41).
Macrophage CCL22 expression is also dependent on the
IL-4/STAT6 signaling pathway, which is generally known as a pathway
for the differentiation of T cells into Th2 as shown in animal
experimental models (42). In this
study, the activation of the IL-4/STAT6 signaling pathway in TAMs
led to the expression of CCL22 and VEGF-C in the tumor
microenvironment of tongue SCC via a Th2-predominant environment.
The activation of this pathway may play a critical role in the
tumor progression response via CCL22 expression in the tumor
microenvironment. However, a quantitative correlation was not found
between the number of positive lymph vessels for VEGF receptor-3
(VEGFR3), a VEGF-C receptor, and lymph node relapse (data not
shown). Since the expression of VEGFR3 has been demonstrated in
many tumor and immune cells, activation of signal transduction
pathways, in addition to VEGFR3-expressing cell types, may be
important for lymph node relapse (43). Further examination of various
quantitative and qualitative parameters of VEGFR3 is considered
necessary in future.
In conclusion, WPOI and DOI were revealed to be
useful parameters for lymph node relapse in patients with tongue
SCC. It is suggested that CCL22 contributes to the role of M2-like
differentiated TAMs in prognosis and lymph node relapse via
IL-4/STAT6 and VEGF. The IL-4/STAT6 signaling pathway may be a new
molecular target for tongue SCC, as shown in other cancers
(44).
Acknowledgements
The authors would like to thank Professor Sato
Hiroaki (Department of Forensic Medicine, School of Medicine,
University of Occupational and Environmental Health, Kitakyushu,
Japan) for the useful discussion with respect to the writing of the
manuscript.
Funding
The present study was financially supported by a
grant (grant no. H28-031202) from the University of Occupational
and Environmental Health (Kitakyushu, Japan).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SK and TN designed the experiments and confirm the
authenticity of all the raw data. SK and HN collected the patient
samples. SK, HN and UN performed the experiments. SK, HN, UN and TN
analyzed the data and wrote the manuscript. All authors revised,
edited, read and approved the final manuscript.
Ethics approval and consent to
participate
Ethics approval for the present study was obtained
from the Ethics Committee of the University of Occupational and
Environmental Health (Kitakyushu, Japan). Considering the
retrospective nature of the protocol for patients with tongue
squamous cell carcinoma, which involved using only already existing
medical data and specimens that were previously anonymized with no
impact on patient care, no specific written informed consent was
required by the Ethics Committee. However, blood donors provided
written informed consent. The study was performed in accordance
with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TAM
|
tumor-associated macrophage
|
|
CCR4
|
chemokine C-C motif receptor 4
|
|
SCC
|
squamous cell carcinoma
|
|
VEGF
|
vascular endothelial growth factor
|
|
IL-4
|
interleukin-4
|
|
STAT6
|
signal transducer and activator of
transcription 6
|
|
CTL
|
cytotoxic T cells
|
|
LNFS
|
lymph node relapse-free survival
|
|
OS
|
overall survival
|
|
CCL22
|
C-C motif chemokine 22
|
|
WPOI
|
worst pattern of invasion
|
|
DOI
|
depth of invasion
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ariyoshi Y, Shimahara M, Omura K, Yamamoto
E, Mizuki H, Chiba H, Imai Y, Fujita S, Shinohara M and Seto K;
Japanese Society of Oral and Maxillofacial Surgeons, 2002, :
Epidemiological study of malignant tumors in the oral and
maxillofacial region: Survey of member institutions of the Japanese
Society of Oral and Maxillofacial Surgeons, 2002. Int J Clin Oncol.
13:220–228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dünne AA, Müller HH, Eisele DW, Kessel K,
Moll R and Werner JA: Meta-analysis of the prognostic significance
of perinodal spread in head and neck squamous cell carcinomas
(HNSCC) patients. Eur J Cancer. 42:1863–1868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Afzali P and Ward BB: Management of the
neck in oral squamous cell carcinoma: Background, classification,
and current philosophy. Oral Maxillofac Surg Clin North Am.
31:69–84. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kimura S, Nanbu U, Noguchi H, Harada Y,
Kumamoto K, Sasaguri Y and Nakayama T: Macrophage CCL22 expression
in the tumor microenvironment and implications for survival in
patients with squamous cell carcinoma of the tongue. J Oral Pathol
Med. 48:677–685. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaesler S, Wölbing F, Kempf WE, Skabytska
Y, Köberle M, Volz T, Sinnberg T, Amaral T, Möckel S, Yazdi A, et
al: Targeting tumor-resident mast cells for effective anti-melanoma
immune responses. JCI Insight. 4:e1250572019. View Article : Google Scholar
|
|
8
|
Messex JK, Byrd CJ and Liou GY: Signaling
of macrophages that contours the tumor microenvironment for
promoting cancer development. Cells. 9:e9192020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Godiska R, Chantry D, Raport CJ, Sozzani
S, Allavena P, Leviten D, Mantovani A and Gray PW: Human
macrophage-derived chemokine (MDC), a novel chemoattractant for
monocytes, monocyte-derived dendritic cells, and natural killer
cells. J Exp Med. 185:1595–1604. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Imai T, Chantry D, Raport CJ, Wood CL,
Nishimura M, Godiska R, Yoshie O and Gray PW: Macrophage-derived
chemokine is a functional ligand for the CC chemokine receptor 4. J
Biol Chem. 273:1764–1768. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Curiel TJ, Coukos G, Zou L, Alvarez X,
Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L,
Burow M, et al: Specific recruitment of regulatory T cells in
ovarian carcinoma fosters immune privilege and predicts reduced
survival. Nat Med. 10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Imai T, Nagira M, Takagi S, Kakizaki M,
Nishimura M, Wang J, Gray PW, Matsushima K and Yoshie O: Selective
recruitment of CCR4-bearing Th2 cells toward antigen-presenting
cells by the CC chemokines thymus and activation-regulated
chemokine and macrophage-derived chemokine. Int Immunol. 11:81–88.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Biedermann T, Schwärzler C,
Lametschwandtner G, Thoma G, Carballido-Perrig N, Kund J, de Vries
JE, Rot A and Carballido JM: Targeting CLA/E-selectin interactions
prevents CCR4-mediated recruitment of human Th2 memory cells to
human skin in vivo. Eur J Immunol. 32:3171–3180. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Franciszkiewicz K, Boissonnas A, Boutet M,
Combadière C and Mami-Chouaib F: Role of chemokines and chemokine
receptors in shaping the effector phase of the antitumor immune
response. Cancer Res. 72:6325–6332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zwaans BM and Bielenberg DR: Potential
therapeutic strategies for lymphatic metastasis. Microvasc Res.
74:145–158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moussai D, Mitsui H, Pettersen JS, Pierson
KC, Shah KR, Suárez-Fariñas M, Cardinale IR, Bluth MJ, Krueger JG,
Carucci JA, et al: The human cutaneous squamous cell carcinoma
microenvironment is characterized by increased lymphatic density
and enhanced expression of macrophage-derived VEGF-C. J Invest
Dermato. 131:229–236. 2011. View Article : Google Scholar
|
|
17
|
Nakagawa T, Ohnishi K, Kosaki Y, Saito Y,
Horlad H, Fujiwara Y, Takeya M and Komohara Y: Optimum
immunohistochemical procedures for analysis of macrophages in human
and mouse formalin fixed paraffin-embedded tissue samples. J Clin
Exp Hematop. 57:31–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamagata Y, Tomioka H, Sakamoto K, Sato K,
Harada H, Ikeda T and Kayamori K: CD163-positive macrophages within
the tumor stroma are associated with lymphangiogenesis and lymph
node metastasis in oral squamous cell carcinoma. J Oral Maxillofac
Surg. 75:2144–2153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kimura S, Noguchi H, Nanbu U, Wang KY,
Sasaguri Y and Nakayama T: Relationship between CCL22 expression by
vascular smooth muscle cells and macrophage histamine receptors in
atherosclerosis. J Atheroscler Thromb. 25:1240–1254. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsujikawa T, Yaguchi T, Ohmura G, Ohta S,
Kobayashi A, Kawamura N, Fujita T, Nakano H, Shimada T, Takahashi
T, et al: Autocrine and paracrine loops between cancer cells and
macrophages promote lymph node metastasis via CCR4/CCL22 in head
and neck squamous cell carcinoma. Int J Cancer. 132:2755–2766.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Almangush A, Bello IO, Keski-Säntti H,
Mäkinen LK, Kauppila JH, Pukkila M, Hagström J, Laranne J, Tommola
S, Nieminen O, et al: Depth of invasion, tumor budding, and worst
pattern of invasion: Prognostic indicators in early-stage oral
tongue cancer. Head Neck. 36:811–818. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brandwein-Gensler M, Teixeira MS, Lewis
CM, Lee B, Rolnitzky L, Hille JJ, Genden E, Urken ML and Wang BY:
Oral squamous cell carcinoma: Histologic risk assessment, but not
margin status, is strongly predictive of local disease-free and
overall survival. Am J Surg Pathol. 29:167–178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shimizu S, Miyazaki A, Sonoda T, Koike K,
Ogi K, Kobayashi JI, Kaneko T, Igarashi T, Ueda M, Dehari H, et al:
Tumor budding is an independent prognostic marker in early stage
oral squamous cell carcinoma: With special reference to the mode of
invasion and worst pattern of invasion. PLoS One. 13:e01954512018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Caldeira PC, Soto AML, de Aguiar MCF and
Martins CC: Tumor depth of invasion and prognosis of early-stage
oral squamous cell carcinoma: A meta-analysis. Oral Dis.
26:1357–1365. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
den Toom IJ, Janssen LM, van Es RJJ,
Karagozoglu KH, de Keizer B, van Weert S, Willems SM, Bloemena E,
Leemans CR and de Bree R: Depth of invasion in patients with early
stage oral cancer staged by sentinel node biopsy. Head Neck.
41:2100–2106. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Amin MB, Edge SB, Greene FL, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC Cancer Staging Manual. 8th edition.
Springer; New York, NY: 2017, View Article : Google Scholar
|
|
27
|
Lydiatt WM, Patel SG, O'Sullivan B,
Brandwein MS, Ridge JA, Migliacci JC, Loomis AM and Shah JP: Head
and Neck cancers-major changes in the American Joint Committee on
cancer eighth edition cancer staging manual. CA Cancer J Clin.
67:122–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Audet N, Beasley NJ, MacMillan C, Jackson
DG, Gullane PJ and Kamel-Reid S: Lymphatic vessel density, nodal
metastases, and prognosis in patients with head and neck cancer.
Arch Otolaryngol Head Neck Surg. 131:1065–1070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kimura S, Tanimoto A, Wang KY, Shimajiri
S, Guo X, Tasaki T, Yamada S and Sasaguri Y: Expression of
macrophage-derived chemokine (CCL22) in atherosclerosis and
regulation by histamine via the H2 receptor. Pathol Int.
62:675–683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
D'Alessio S, Correale C, Tacconi C,
Gandelli A, Pietrogrande G, Vetrano S, Genua M, Arena V, Spinelli
A, Peyrin-Biroulet L, et al: VEGF-C-dependent stimulation of
lymphatic function ameliorates experimental inflammatory bowel
disease. J Clin Invest. 124:3863–3878. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Komohara Y, Fujiwara Y, Ohnishi K and
Takeya M: Tumor-associated macrophages: Potential therapeutic
targets for anti-cancer therapy. Adv Drug Deliv Rev. 99:180–185.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miyasato Y, Takashima Y, Takeya H, Yano H,
Hayano A, Nakagawa T, Makino K, Takeya M, Yamanaka R and Komohara
Y: The expression of PD-1 ligands and IDO1 by macrophage/microglia
in primary central nervous system lymphoma. J Clin Exp Hematop.
58:95–101. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Komohara Y and Takeya M: CAFs and TAMs:
Maestros of the tumour microenvironment. J Pathol. 241:313–315.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Alitalo K, Tammela T and Petrova TV:
Lymphangiogenesis in development and human disease. Nature.
438:946–953. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shi VY, Bao L and Chan LS:
Inflammation-driven dermal lymphangiogenesis in atopic dermatitis
is associated with CD11b+ macrophage recruitment and
VEGF-C up-regulation in the IL-4-transgenic mouse model.
Microcirculation. 19:567–579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Roca H, Varsos ZS, Sud S, Craig MJ, Ying C
and Pienta KJ: CCL2 and interleukin-6 promote survival of human
CD11b+ peripheral blood mononuclear cells and induce
M2-type macrophage polarization. J Biol Chem. 284:34342–34354.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang B, Yao G, Zhang Y and Gao J, Yang B,
Rao Z and Gao J and Gao J: M2-polarized tumor-associated
macrophages are associated with poor prognoses resulting from
accelerated lymphangiogenesis in lung adenocarcinoma. Clinics (São
Paulo). 66:1879–1886. 2011. View Article : Google Scholar
|
|
39
|
Savetsky IL, Ghanta S, Gardenier JC,
Torrisi JS, García Nores GD, Hespe GE, Nitti MD, Kataru RP and
Mehrara BJ: Th2 cytokines inhibit lymphangiogenesis. PLoS One.
10:e01269082015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang B, Wang J, Gao J, Guo Y, Chen X,
Wang B, Gao J, Rao Z and Chen Z: Alternatively activated RAW264.7
macrophages enhance tumor lymphangiogenesis in mouse lung
adenocarcinoma. J Cell Biochem. 107:134–143. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hinshaw DC and Shevde LA: The tumor
microenvironment innately modulates cancer progression. Cancer Res.
79:4557–4566. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fulkerson PC, Zimmermann N, Hassman LM,
Finkelman FD and Rothenberg ME: Pulmonary chemokine expression is
coordinately regulated by STAT1, STAT6, and IFN-gamma. J Immunol.
173:7565–7574. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hsu MC, Pan MR and Hung WC: Two birds, one
stone: Double hits on tumor growth and lymphangiogenesis by
targeting vascular endothelial growth factor receptor 3. Cells.
8:e2702019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Binnemars-Postma K, Bansal R, Storm G and
Prakash J: Targeting the Stat6 pathway in tumor-associated
macrophages reduces tumor growth and metastatic niche formation in
breast cancer. FASEB J. 32:969–978. 2018. View Article : Google Scholar : PubMed/NCBI
|