Introduction
During the treatment of head and neck cancer (HNC),
chemotherapy plays an important role, in addition to surgery and
radiotherapy. However, the response rate to current drugs is not
sufficient. To overcome this, the development of new, more
effective anti-cancer agents and proper preclinical animal models
to recapitulate patient disease is required. Rodent models have
been conventionally used for translational cancer research, which
ranges from the biological understanding of HNC to the evaluation
of pharmacokinetics. Cell line-derived xenograft (CDX) models have
been established by injecting cell lines, which were generated
using tumour cells isolated from patients with HNC, subcutaneously
into immunodeficient mice, and these are widely used for in
vivo experiments. However, even though CDX models can suggest
effective novel drug candidates, a significant number of these
drugs fail in clinical trials, especially those for solid tumours
(1). This limited predictive power
is attributed to the CDX models' inability to capture the diverse
heterogeneity of human malignancies as well as their differences
from the actual patient tumours (2).
To overcome the limitations of CDX models, patient-derived
xenograft (PDX) models have been introduced to reflect the original
patient tumours (2). PDX models have
been established by transplanting tumour specimens directly into
immunodeficient mice, and these retain the tumour heterogeneity
observed in primary tumour specimens. In previous reports, PDX
models of HNC were shown to recapitulate the histology of the
original tumour and generate stable gene expression patterns
(3); however, the therapeutic
responses of HNC PDXs relative to those of the corresponding
patient tumours have not been sufficiently evaluated. Here, we
aimed to confirm that PDX models of HNC accurately replicate
clinical outcomes for patients. To investigate the change in
biomarker expression by administration of a target drug in a PDX
model, we evaluated the expression of adenosine
triphosphate-binding cassette (ABC) transporters, a group of
membrane transporters that translocate different molecules through
the cellular membrane, mainly to the extracellular space, using ATP
as an energy source (4). The
expression levels of ABC transporters on exposure to anti-HNC drugs
have been evaluated in vivo using HNC cell lines (5). Moreover, previous studies showed that
ABC transporters are involved in intrinsic and acquired drug
resistance and are associated with worse prognosis for HNC
(6). Thus, in this study, we also
assessed the relationship between ABC transporter expression and
chemosensitivity in PDXs that reflect original patient tumours.
Materials and methods
Patient samples
Eighteen resected HNC tumour specimens obtained at
the Division of Otolaryngology and Head and Neck Surgery at
Kanazawa University were implanted for the establishment of PDXs.
TNM classification of the patients was compliant with the UICC TNM
classification, 8th edition (7).
This study complied with the Declaration of Helsinki and was
approved by the investigational Review Board of Kanazawa University
(no. 2015-125). All patients included in this study provided
written informed consent.
Establishment and passage of
patient-derived xenografts
Non-obese diabetic severe combined immunodeficient
(NOD-SCID) mice (Charles river laboratories Japan, INC., Kanagawa,
Japan) were used to implant tumour fragments from patients (F0
generation). Five tumour pieces (1–2 mm in diameter) were suspended
in Matrigel and subcutaneously transplanted into NOD-SCID mice
within 24 h of tumour excision. Tumour fragments were implanted
into ~4 mice on the basis of patient tumour volume. Additional
tissue samples were stored at −80°C with Cell Reserver One (Nacalai
Tesque) and DMSO for further experiments. About 2–3 months
post-transplantation, engrafted tumours of approximately 1
cm3 corresponding to F1 generations were surgically
excised and small fragments were retransplanted into another
NOD-SCID mouse. Xenograft tumours larger than 1 cm3 were
not observed in this study. Tumours were passaged no more than five
times. Tumour collection date [X(year)/(month)/(day)], time to
harvest (days), and last passage are shown in Tables SI, SII, and SIII. All animal procedures were approved
by the Ethical Committee of the Laboratory for the Animal
Experiments, Graduate School of Medical Science, Kanazawa
University (permit number: AP-173861) and were performed in
compliance with the guidelines of this committee. A flow rate of
30% chamber volume displaced/min with CO2 was used for
euthanizing the animals that had completed the experiment.
Hematoxylin and eosin (H&E)
staining of primary tumours and xenografts
To compare xenografts to the original specimens,
tissues from patient tumours and PDXs were formalin-fixed
immediately after collection, paraffin-embedded, and stained with
H&E according to standard protocols.
Short tandem repeat (STR)
profiling
The profiles of 10 core STR markers (TH01, D21S11,
D5S818, D13S317, D7S820, D16S539, CSF1PO, AMEL, vWA, and TPOX) were
examined to determine the relatedness of patient tumours to a
series of their PDXs (BEX Co., Ltd.). All frozen tissue specimens
were used in this experiment.
Immunohistochemistry (IHC)
The original patient tumours and PDXs were embedded
in paraffin and used for the immunohistochemical analysis of the
expression of Ki-67, epidermal growth factor receptor (EGFR), p53,
multiple drug resistance-1 (MDR-1), and multidrug
resistance-associated protein-2 (MRP-2). MDR-1 and MRP-2 are ABC
transporters that were reported to be prognostic markers for HNC
(6). Three-micrometer-thick sections
were prepared from each block of tissues embedded in paraffin.
Deparaffinized sections were treated with 3% hydrogen peroxide for
10 min to inactivate endogenous peroxidase activity. The sections
were then incubated with a protein blocker (Dako) for 20 min and
incubated at 4°C overnight with anti-Ki-67 (rabbit monoclonal,
ab16667, RRID:AB_302459, 1:200, Abcam), anti-EGFR monoclonal
(rabbit monoclonal, ab40815, RRID:AB_732110, 1:250, Abcam),
anti-p53 monoclonal (rabbit monoclonal, ab33889, RRID:AB_776988,
1:200, Abcam), anti-MDR-1 polyclonal (rabbit polyclonal, bs-0563R,
RRID:AB_10856233, 1:100, BIOSS Inc., Boston, USA), and anti-MRP-2
polyclonal (rabbit polyclonal, bs-1092R, RRID:AB_10856413, 1:100,
BIOSS Inc) primary antibodies. The sections were then washed three
times with phosphate-buffered saline (PBS, pH 7.2). After washing,
the sections were exposed to Envision + System-HRP Labelled Polymer
Anti-Rabbit secondary antibody (Dako) for 60 min. The reaction
products were developed by immersing the sections in a
3,3′-diaminobenzidine tetrahydrochloride solution. Sections were
counterstained with hematoxylin. The Ki-67 index is calculated as
the percentage of positive cells per 1,000 counts of the total
cells.
Chemosensitivity testing
F2 generation PDXs obtained from NOD-SCID mice were
engrafted into BALB/c-nu/nu mice (Charles River Laboratories,
Inc.), and small fragments were retransplanted into new
BALB/c-nu/nu mice for drug administration tests. When tumours were
palpable, F3 generation PDX tumour-bearing BALB/c-nu/nu mice were
randomized to treatment or control groups consisting of six mice
each. BALB/c-nu/nu mice were chosen because passaged tumours would
continue to grow in less immunocompromised mouse strains and to
ensure the comparability of results because the appropriate doses
were previously assessed in this strain by our group. PDX
tumour-bearing BALB/c-nu/nu mice were treated for two consecutive
weeks with weekly paclitaxel (20 mg/kg; Nippon Kayaku Co., Ltd.),
weekly cisplatin (2.5 mg/kg; Nippon Kayaku), or weekly PBS (as a
control) intravenous injections. Two-dimensional tumour
measurements were performed with a sliding calliper once weekly.
Individual tumour volumes were calculated using the formula: V
(mm3) = 1/2×axb2 (where ‘a’ was the longest
tumour diameter and ‘b’ was the shortest tumour diameter). We could
test drug response of P-2, P-3 and P-5 in vivo, but not of
P-1 and P-4, because we only had frozen PDX specimens of P-1 and
P-4 and could not make PDX models for this examination from these
samples.
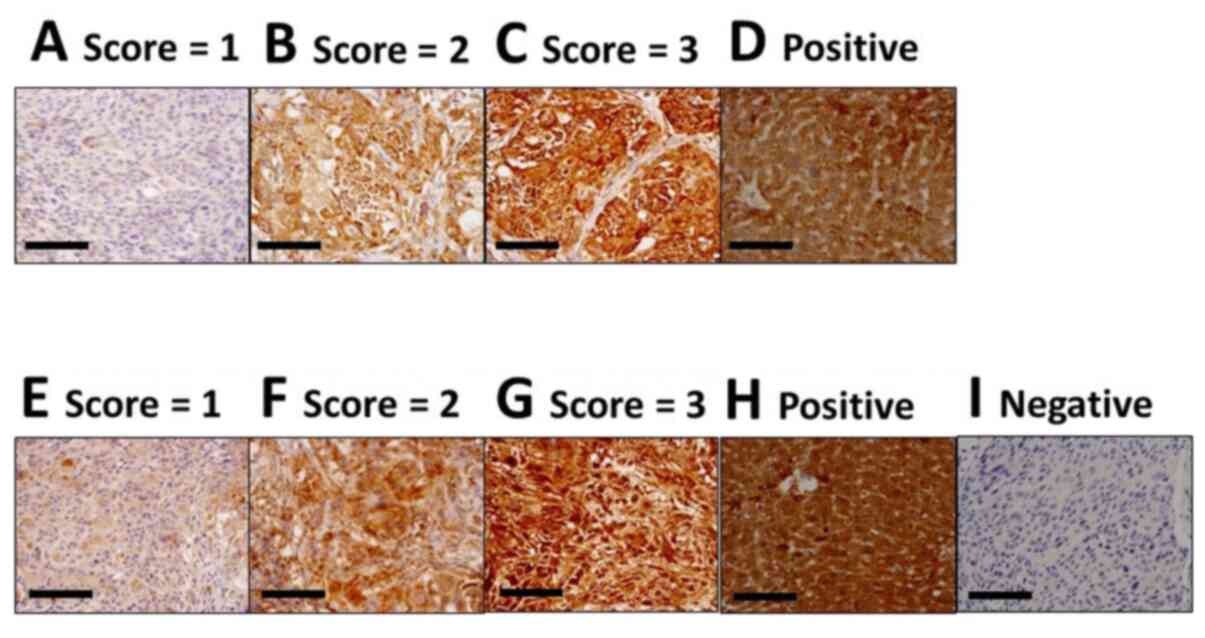
Scoring of ABC transporter protein
expression
The sections were observed using the ECLIPSE Ni
upright microscope (Nikon). Positive ratio scores were based on the
percentage of positive cells in a high-power field, as follows:
Score 0 (0–10%); score 1 (11–25%); score 2 (26–50%); score 3
(51–75%); and score 4 (76–100%). The investigator also ranked the
expression intensity from 0 (no expression) to 3 (very strong
expression). The positive ratio score and expression intensity
score were multiplied, and each tumour score was then calculated as
the mean of five high-power fields. For F3′ (F3 after drug
treatment), the final scores were averaged from six mice.
Consequently, the lowest score was 0, and the highest score was 12.
Tissues slides from healthy liver sections were used as positive
controls. We could examine the expression of ABC transporters
expression in P-2, P-3 and P-5, not P-1 and P-4, as mentioned
above.
Statistical analysis
All analyses were performed with SPSS statistical
software (Version 23; RRID:SCR_002865, IBM Corp.). Inter-relations
between engraftment rates and patient characteristics were examined
by performing Fisher's exact tests or unpaired Student's t-tests.
The in vivo effects of paclitaxel or cisplatin vs. the
control were evaluated using a mixed two-way analysis of variance
with Bonferroni's test as a post hoc test to compare the tumour
volumes. The protein expression scores of MDR-1 and MRP-2 in F0,
F3, and F3′ (F3 at the end of chemotherapy) groups were evaluated
by performing a Kruskal-Wallis test, using Mann-Whitney tests and a
Bonferroni correction as a post hoc test [F3 or F3′ (treated with
PBS, paclitaxel, and cisplatin) vs. F0]. A P-value of <0.05 was
considered statistically significant.
Results
Establishment of PDXs and patient
characteristics
The primary lesions of tumours are shown in Table I. Oral cancers comprised six cases
and were the most common. Of all 18 resected HNC tumours that were
implanted into NOD-SCID mice for PDX establishment, five (28%)
engrafted and could be passaged. The characteristics of patients
are shown in Table II. PDX
establishment was significantly associated with surgical
margin-positive cases, but not with sex, smoking status,
pre-treatment, histologic type (squamous cell carcinoma or other),
Ki-67 index, TNM, staging, or recurrence at 6 months after surgery.
When surgical margin-positive cases were retrospectively evaluated
from the pathological report, extranodal infiltration was
identified in three PDX-failed cases and in two PDX-established
cases; excision margin positive primary lesions were identified in
two PDX-failed cases and in three PDX-established cases. The
positivity of infiltration features (e.g. vein, lymphatic, and
perineural invasion) was also determined according to the
pathological report. Because 3 of 5 PDX-succeeded cases did not
have any information regarding infiltration patterns, the sample
number included in our study was too small to perform any
statistical analysis. Moreover, all five patients from whom PDX
tumours were developed were treated with concurrent cisplatin
radiation therapy (CCRT) after surgery. The detailed patient
profiles accompanying the five models (P-1, P-2, P-3, P-4, and P-5)
are shown in Table III. P-1 and
P-2 were cisplatin-non-responder PDX models according to the
definition of recurrence within 6 months post-CCRT. In contrast,
P-3, P-4, and P-5 were cisplatin-responder PDX models according to
the definition of recurrence 6 months or later or complete
remission for more than 6 months post-CCRT. Further, the H&E
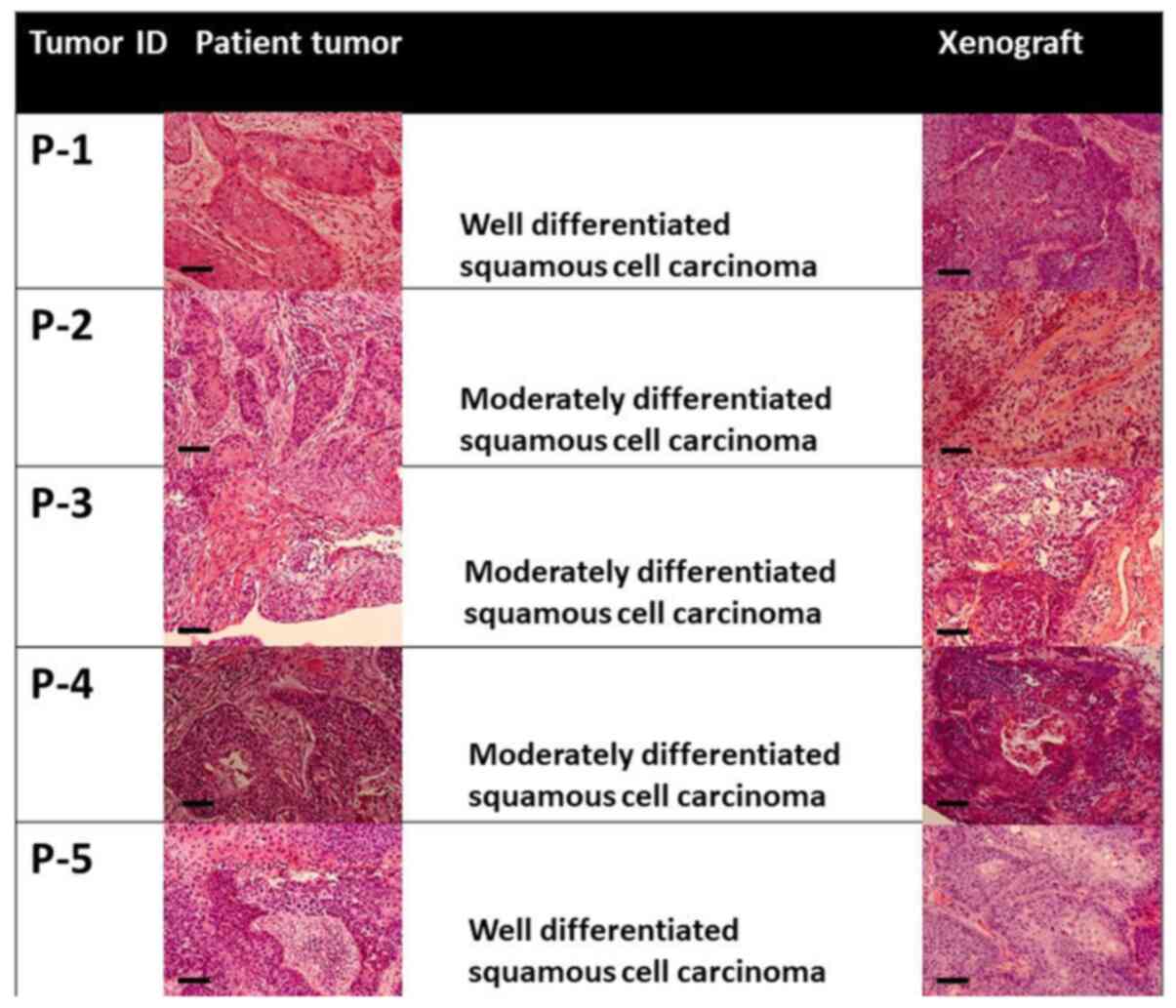
staining of primary tumours and xenografts is shown in Fig. 1. The tissue type and degree of
differentiation were consistent between primary tumours and
xenografts. The histopathologic features of human HNC tumours were
also retained in mice. The Ki-67 index was passed down from all
patient samples to the PDXs (Table
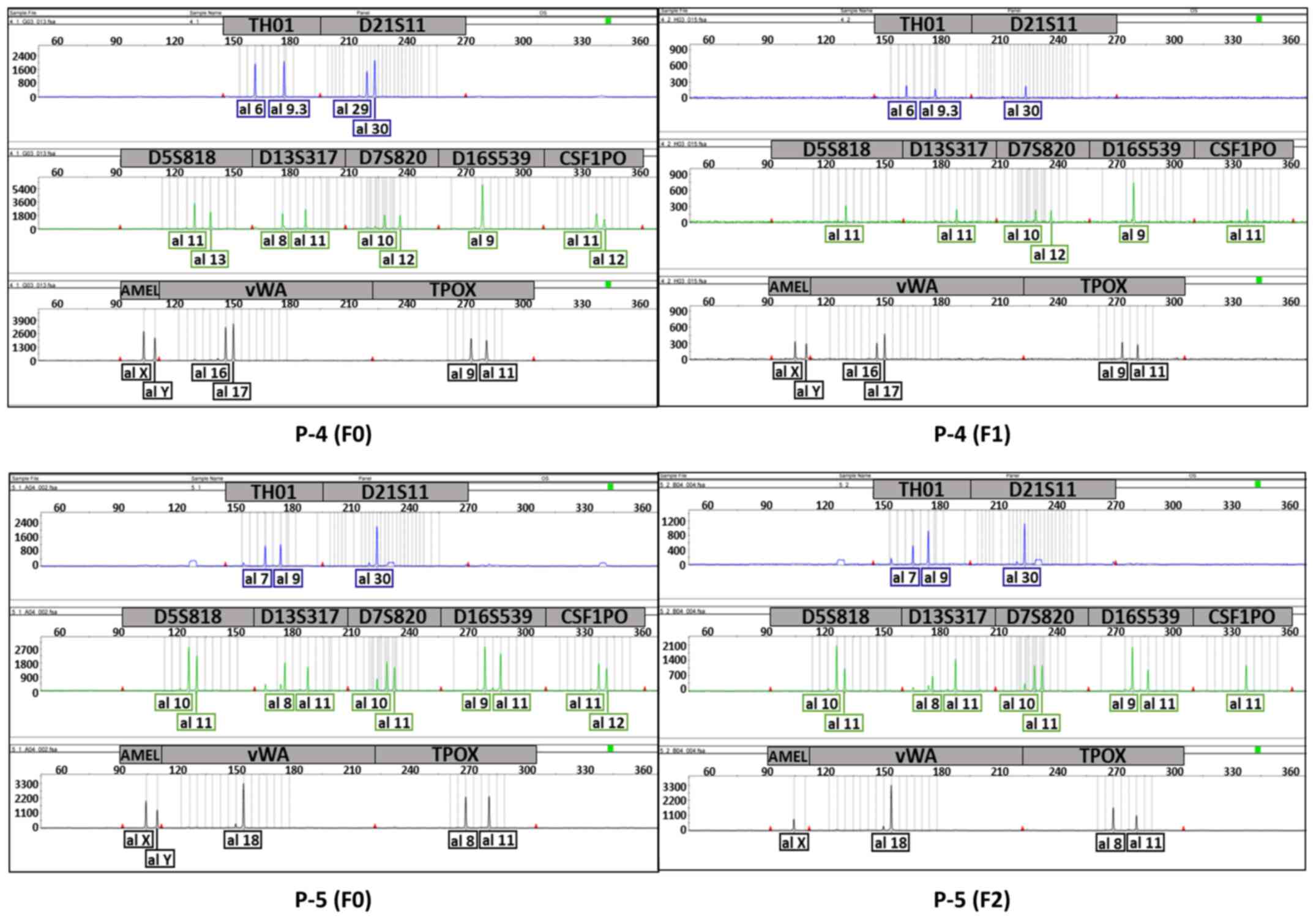
SIV). We confirmed the uniqueness between the patient tumours
and each PDX (P-1, P-3, P-4, and P-5) or two generations of PDXs
(P-2) using STR profiling (Figs. 2
and 3; Table IV). Except P-1, which had an
evaluation value (EV) of 0.53 [EV = (number of coincidental peaks)
× 2 /(total number of peaks in tissue A + total number of peaks in
tissue B)], all sample pairs showed an EV of 0.8 or higher,
indicating that the tissues were almost identical (8).
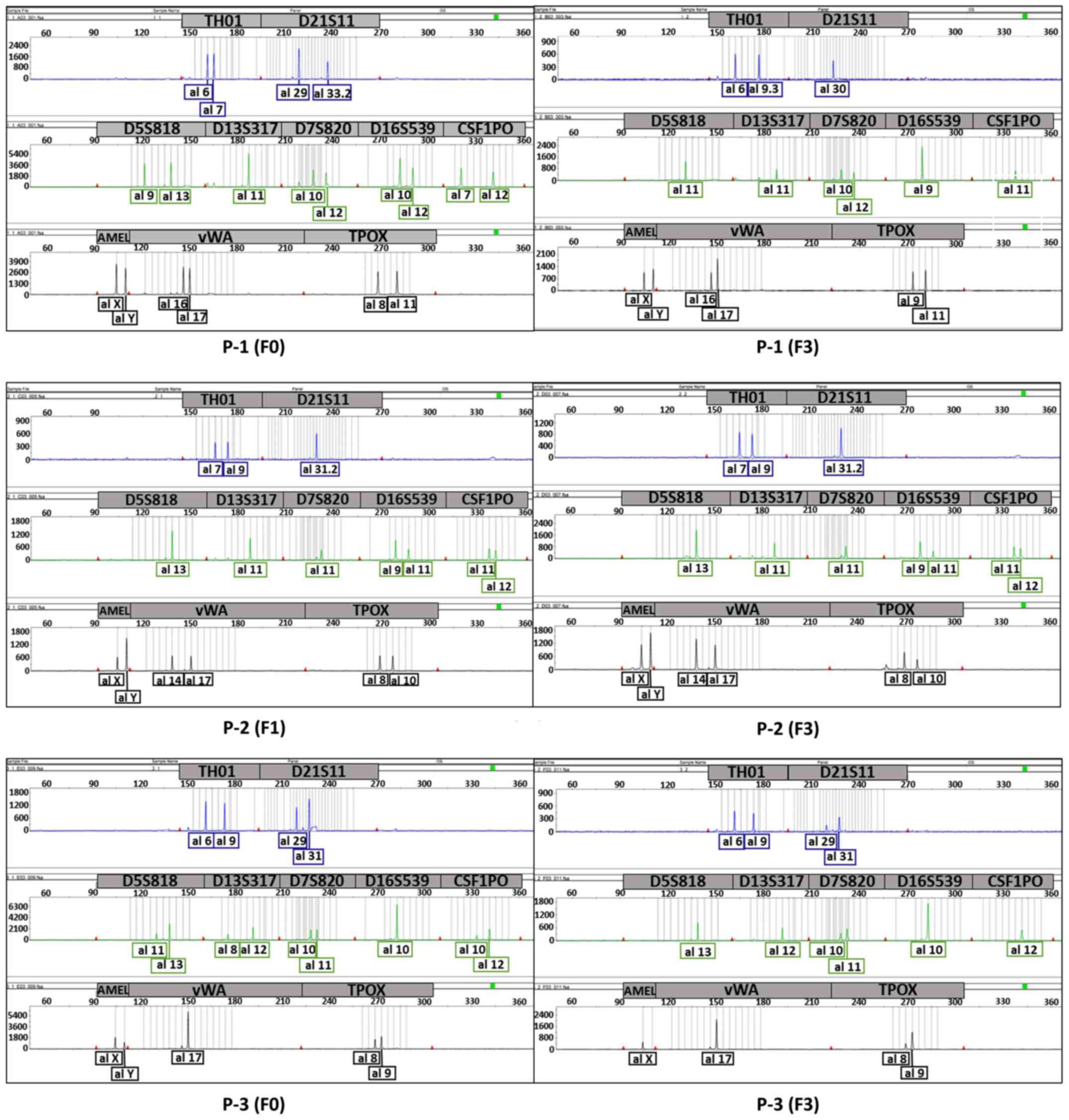 | Figure 2.Electropherogram showing the unique
profile of each tumour to compare patient tumours (F0) and their
PDXs (P-1 and P-3) or different generations of each PDX to another
(P-2). The top panel (blue) shows the graphs for TH01 and D21S11,
the middle panel (green) shows the graphs for D5S818, D13S317,
D7S820, D16S539 and CSF1PO, and the bottom panel (black) shows the
graphs for AMEL, vWA and TPOX. P-1, F3 generation (3 passages);
P-2, F1 and F3 generation; P-3, F3 generation; PDX, patient-derived
xenograft. |
 | Table I.Primary tumour lesions. |
Table I.
Primary tumour lesions.
| Primary lesion | Patients, n
(n=18) |
|---|
| Oral cancer | 6 |
|
Tongue | 4 |
| Floor of
mouth | 1 |
| Gums | 1 |
| Hypopharynx | 3 |
| Oropharynx | 2 |
| Larynx | 2 |
| Salivary gland | 2 |
| Paranasal Sinus | 1 |
| Nasopharynx | 1 |
| Thyroid (papillary
carcinoma and squamous cell carcinoma) | 1 |
 | Table II.Patient characteristics. |
Table II.
Patient characteristics.
| Characteristics | XG (n=5) | No XG (n=13) | Total (n=18) | P-value |
|---|
| Age, years (mean;
range) | 58.4 (43–69) | 65.9 (53–84) |
| 0.096 |
| Sex, n |
|
|
| 0.522 |
| Male | 5 | 10 | 15 |
|
|
Female | 0 | 3 | 3 |
|
| Smoking status,
n |
|
|
| 0.533 |
|
Yes | 3 | 11 | 14 |
|
| No | 2 | 2 | 4 |
|
| Origin, n |
|
|
| 0.583 |
|
Primary | 3 | 10 | 13 |
|
| Lymph
node | 2 | 3 | 5 |
|
| Histology, n |
|
|
| 0.278 |
|
SCC | 5 | 9 | 14 |
|
|
Other | 0 | 4 | 4 |
|
| Surgical margin,
n |
|
|
| 0.036 |
|
Positive | 5 | 5 | 10 |
|
|
Negative | 0 | 8 | 8 |
|
| Ki-67 index, %
(mean; range) | 7 (5–10) | 5 (0–25) |
| 0.178 |
| Postsurgical
treatment, n |
|
|
| 0.002 |
|
CDDP+RT | 5 | 2 | 7 |
|
| Only
RT | 0 | 3 | 3 |
|
|
TS-1 | 0 | 2 | 2 |
|
| No
treatment | 0 | 6 | 6 |
|
| Primary/recurrent,
n |
|
|
| 0.583 |
|
Primary | 3 | 10 | 13 |
|
|
Recurrent | 2 | 3 | 5 |
|
| T, n |
|
|
| 0.596 |
|
T0-2 | 1 | 6 | 7 |
|
|
T3-4 | 4 | 7 | 11 |
|
| N, n |
|
|
| >0.999 |
| N0 | 2 | 7 | 9 |
|
|
N1-3 | 3 | 6 | 9 |
|
| M, n |
|
|
| >0.999 |
| M0 | 5 | 13 | 18 |
|
| M1 | 0 | 0 | 0 |
|
| Stage, n |
|
|
| >0.999 |
|
I–II | 0 | 2 | 2 |
|
|
III–IV | 5 | 11 | 16 |
|
| Recurrence at 6
months after surgery, n |
|
|
| >0.999 |
|
Yes | 2 | 4 | 6 |
|
| No | 3 | 8 | 11 |
|
|
Othera | 0 | 1 | 1 |
|
 | Table III.Detailed profiles of patients in the
five models. |
Table III.
Detailed profiles of patients in the
five models.
| Tumour ID | Site of tumour
origin | Age, years | Sex |
Primary/recurrent | TNM, Stage | Histology | Progress after
CCRT |
|---|
| P-1 | Tongue | 43 | Male | Recurrent | T4aN0M0, Stage
IVA | SCC | Neck and armpit
lymph node metastases appeared at 1 month |
| P-2 | Hypopharynx
(LN) | 68 | Male | Primary | T2T2bM0, Stage
IVA | SCC | Bone metastasis
appeared at 2 months |
| P-3 | Hypopharynx
(LN) | 57 | Male | Primary | T3N3M0, Stage
IVB | SCC | CR at 14
months |
| P-4 | Laryngeal | 55 | Male | Recurrent | T4aN2bM0, Stage
IVA | SCC | Lung metastasis
appeared at 11 months |
| P-5 | Paranasal
sinus | 69 | Male | Primary | T4bN0M0, Stage
IVB | SCC | CR at 6 months |
 | Table IV.STR profiling of tumour samples. |
Table IV.
STR profiling of tumour samples.
| Tumour samples | TH01 | D21S11 | D5S818 | D13S317 | D7S820 | D16S539 | CSF1PO | AMEL | vWA | TPOX | EV |
|---|
| P-1 (F0) | 6,7 | 29,33.2 | 9,13 | 11 | 10,12 | 10,12 | 7,12 | X, Y | 16,17 | 8,11 | 0.53 |
| P-1 (F3) | 6,9.3 | 30 | 11 | 11 | 10,12 | 9 | 11 | X, Y | 16,17 | 9,11 |
|
| P-2 (F1) | 7,9 | 31.2 | 13 | 11 | 11 | 9,11 | 11,12 | X, Y | 14,17 | 8,10 | 1 |
| P-2 (F3) | 7,9 | 31.2 | 13 | 11 | 11 | 9,11 | 11,12 | X, Y | 14,17 | 8,10 |
|
| P-3 (F0) | 6,9 | 29,31 | 11,13 | 8,12 | 10,11 | 10 | 10,12 | X, Y | 17 | 8,9 | 0.88 |
| P-3 (F3) | 6,9 | 29,31 | 13 | 12 | 10,11 | 10 | 12 | X | 17 | 8,9 |
|
| P-4 (F0) | 6,9.3 | 29,30 | 11,13 | 8,11 | 10,12 | 9 | 11,12 | X, Y | 16,17 | 9,11 | 0.88 |
| P-4 (F1) | 6,9.3 | 30 | 11 | 11 | 10,12 | 9 | 11 | X, Y | 16,17 | 9,11 |
|
| P-5 (F0) | 7,9 | 30 | 10,11 | 8,11 | 10,11 | 9,11 | 11,12 | X, Y | 18 | 8,11 | 0.94 |
| P-5 (F2) | 7,9 | 30 | 10,11 | 8,11 | 10,11 | 9,11 | 11 | X | 18 | 8,11 |
|
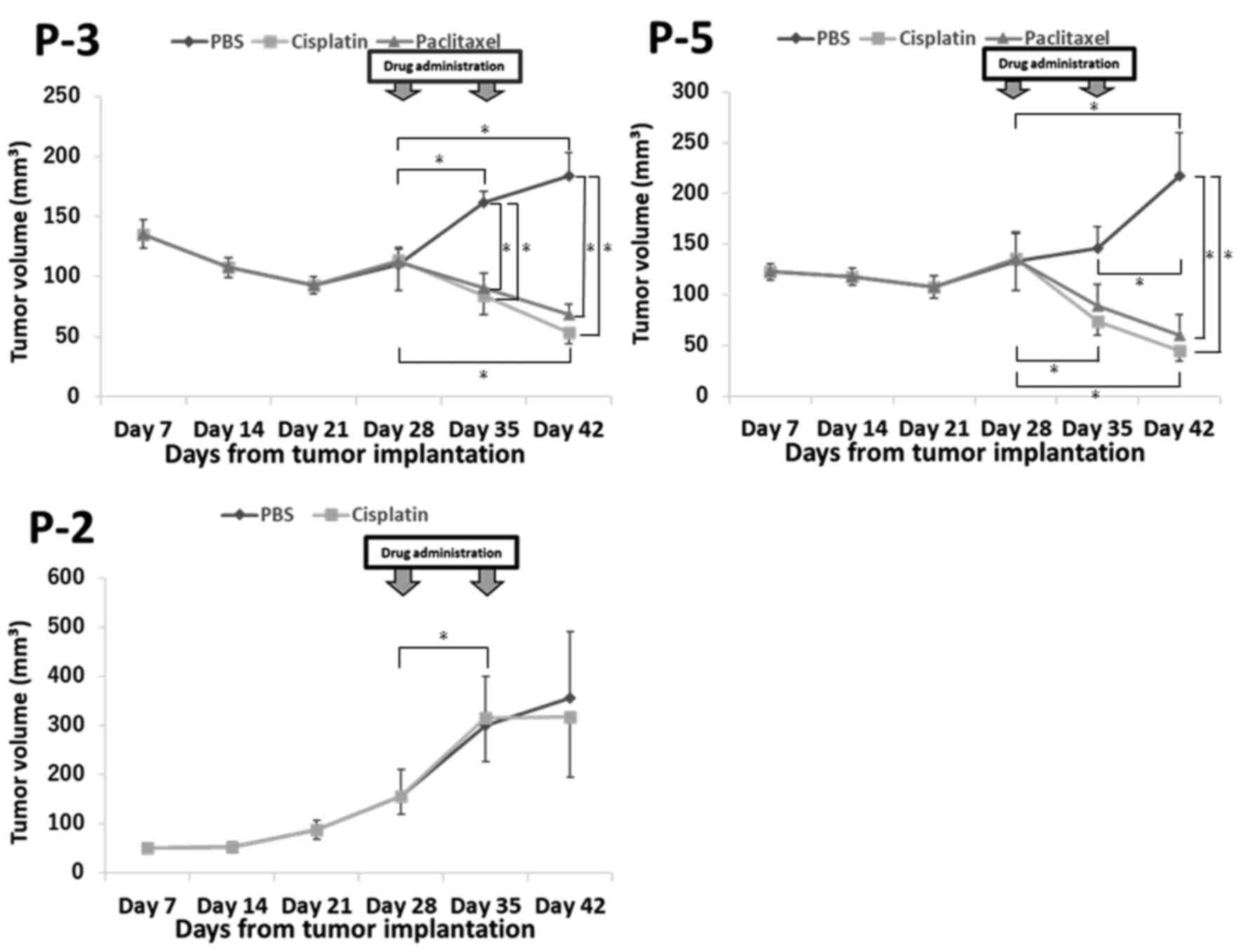
Chemosensitivity testing
To compare chemotherapy efficacies between patients
and PDX models, chemosensitivity testing was performed using three
models (P-2, P-3, and P-5). For P-2, bone metastasis appeared 2
months post-CCRT, and thus, this case was considered a
cisplatin-non-responder. For P-3 and P-5, complete remission
continued for 6 months post-CCRT; therefore, these cases were
considered cisplatin-responders. The tumour growth curves for these
models are shown in Fig. 4. For P-3,
cisplatin and paclitaxel treatment resulted in significant
decreases in the tumour sizes at days 35 and 42 compared with those
in the control (PBS). For P-5, cisplatin and paclitaxel treatment
resulted in significant decreases in the tumour sizes at day 42
compared with those in the control (PBS). In contrast, for P-2, a
cisplatin non-responder HNC PDX model, there were no significant
differences in tumour size between the PBS and cisplatin-treated
groups. For P-3 and P-5, the tumour size decreased significantly in
the cisplatin group, increased significantly in the PBS group, and
was not significantly different in the paclitaxel group based on
day 28. On the contrary, for P-2, the tumour size was significantly
increased in the cisplatin and PBS groups based on day 28.
Evaluation of ABC transporter response
to chemotherapy
To evaluate the expression of ABC transporters,
MDR-1 and MRP-2 levels were compared between tumours from
drug-treated mice and patient samples (F0). The expression
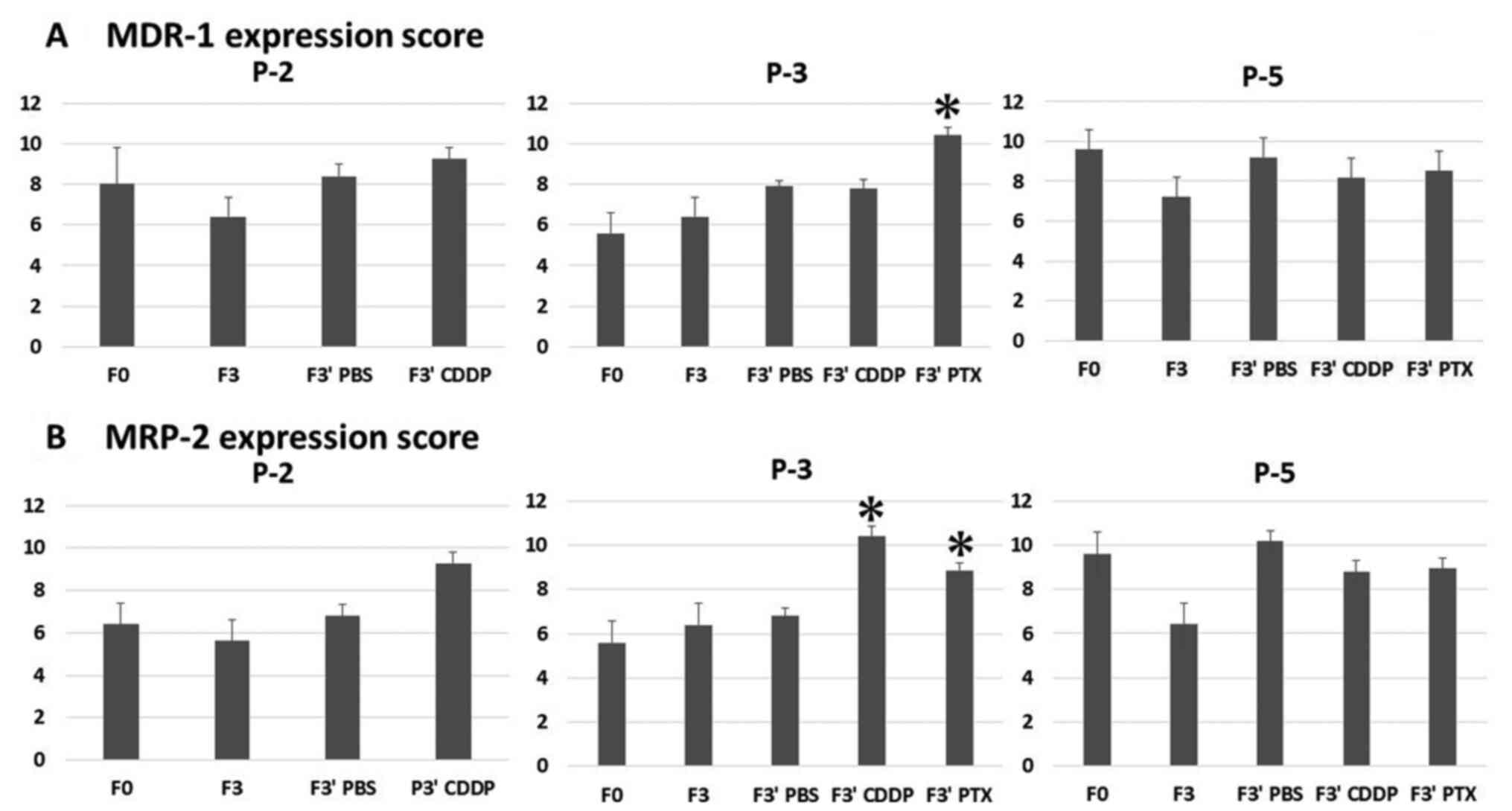
intensity scores are shown in Fig.
5. Averages of the final scores are shown in Fig. 6. In P-3, the protein expression
scores of MDR-1 were 5.6 for F0, 7.8 for F3′ (treated with
cisplatin), and 10.4 for F3′ (treated with paclitaxel), and the
scores for MRP-2 changed from 5.6 for F0 to 10.4 for F3′ (treated
with cisplatin) and 8.85 for F3′ (treated with paclitaxel). The
scores after drug administration, except for those of MDR-1 in F3′
(treated by cisplatin) samples, significantly increased compared
with those in F0 samples for P-3. For P-5 and P-2, the protein
expression scores in F3′ (treated by paclitaxel and cisplatin)
samples were not significantly different from those in F0 samples.
In all three models, the MDR-1 and MRP-2 expression scores in F3
and F3′ (treated by PBS) samples were not significantly changed
compared with those in F0 samples.
Discussion
In this study, we established PDXs from the tumours
of patients with HNC. H&E staining, Ki-67 index, and STR
profiling demonstrated the consistency between the patient tumours
and xenografts. We showed the genomic stability of PDXs (P-2, P-3,
P-4, and P-5) and identified genotypic changes through serial
propagation (P-1). Further, the protein expression patterns of EGFR
and p53 were passed down from all patient samples to the PDXs
(Fig. S1). Although the sample size
of this study was relatively small, our PDX models may be capable
of recapitulating the complexity of HNC malignancy remarkably well.
In cancers other than HNC, histologic and immunohistochemical
analyses have already revealed a high degree of pathologic
similarity (9). Of the 18 tumour
samples obtained from surgical specimens, five (28%) PDX models
were established. The rate of PDX establishment was significantly
associated with the presence of positive surgical margins and CCRT
after surgery. Previous data regarding the engraftment rate of HNC
PDXs suggest variability, from 17 to 85% (3,10,11). The
success rates of PDX are influenced by several factors (2). First, the patient tumour
characteristics might be related to the success rate. With regard
to clinical parameters, PDX engraftment was previously found to be
associated with poor disease free and overall survival (12) and an increased risk of metastasis
(13). Histologically,
lymphovascular invasion (14) and
perineural invasion (15) are
associated with increased PDX formation. Prince et al
(16) reported that engraftment bias
may occur because of the stem cell-like property (CD44+
cells). Theoretically, tumours with massive invasion have more
frequent positive surgical margins. In this study, the positivity
of infiltration features (e.g., vein, lymphatic, and perineural
invasion) was determined based on the pathological records. We
undertook statistical analysis for the case based on comments from
the pathologists. However, more than half of the succeeded cases
did not have any information regarding invasion patterns. It was
thus inappropriate to perform statistical analysis for the entire
cohort. Although we cannot mention the direct relationship between
the positive surgical margins and PDX engraftment in this study
alone, we suggest that patient tumour characteristics such as
invasive ability or stem-cell like property around the tumour could
be considered as factors that influence the success rates of PDX.
Second, the degree of immunodeficiency in mice might influence the
success rate. NOD-SCID mice do not contain functional T and B
cells; however, NK cells are somewhat functional. In contrast,
NOD/Scid/IL2Rγnull (NSG) mice do not have functional T, B, or NK
cells (2). Therefore, NSG mice have
recently become preferred, compared with other mouse strains, for
the development of PDXs. Third, the location of implantation might
affect the success rate (2). We
implanted primary tumours subcutaneously, the most common site of
implantation; however, implantation in the renal capsule has been
found to maintain the original tumour stroma and the equivalent
host stroma, making this approach more likely to succeed (2).
Although not yet investigated in HNC, correlations
have been observed in clinical outcomes between drug responses in
PDXs and other organs of patients with cancer (12,17). Our
study also showed an association between the anti-tumour effect of
cisplatin in three PDX models (P-2, P-3, and P-5) and that observed
in corresponding patients. PDX tumour sizes in P-3 and P-5 models,
for which a positive response to CCRT was noted in corresponding
patients, were ultimately reduced to 29% (P-3) and 21% (P-5) of
respective PBS-treated tumour sizes upon cisplatin treatment. In
contrast, PDX tumour sizes in the P-2 model, derived from a tumour
refractory to CCRT, showed no differences between PBS and cisplatin
treatment groups. We considered the following two reasons for the
rapid drug response in P-3 and P-5 PDX models. First, the rapid
tumour reduction rate in PDX models reflected the chemosensitivity
of the original tumour that was categorized as platinum-sensitive.
In many clinical studies on recurrent/metastatic HNC, patients with
recurrence after platinum-containing therapy within 6 months were
platinum resistant and those after 6 months were platinum
sensitive. This classification based on ovarian cancer studies
(18,19) was used in this study. Because the
correlation of drug responses between the original tumours and PDXs
in various cancers has been reported, similar platinum sensitivity
was observed in this study. In a clinical study on taxane,
regardless of previous platinum sensitivity in recurrent/metastatic
patients with HNC, approximately half of the patients responded to
docetaxel plus cetuximab combination treatment (20). Similarly, platinum-sensitive P-3 and
P-5 PDXs were sensitive to taxane in this study. Second, the
characteristic drug reaction rate in PDX models may affect the
rapid drug response. Based on previous studies (12,17), the
drug responses in PDX models were relatively rapid and were
observed about 2 weeks after drug administration where the
corresponding patients were clinical responders. This rapid tumour
size reduction may thus be a feature of drug responsiveness in PDX
model. Despite limited sample size, these results suggest that drug
responses in HNC PDXs reflect tumour response to the candidate
drugs and that PDXs could be utilized for drug screening.
Because decrease of drug absorption and increase of
drug efflux are common mechanisms of drug resistance, we focused on
ABC transporters, which transport a large variety of drugs and
mediate drug resistance. In this study, the expression of ABC
transporters in three PDX models did not change even upon passage.
However, the expression was increased by paclitaxel and cisplatin
treatments in the P-3 PDX model, whereas the expression scores did
not significantly change in P-2 and P-5 PDX models. The baselines
of MDR-1 and MRP-2 expressions seems to be higher in P-5 tissue
than in P-2 and P-3 tissues. Because P-5 patients and PDXs were
cisplatin responders and had higher expression of two ABC
transporters, not only MDR-1/MRP-2 but also other ABC transporters
might be involved in pharmacokinetics (4). In addition, the evasion of tumour cell
apoptosis, other than drug efflux, may have an effect. Although the
number of observations was limited and the association between
cisplatin resistance and overexpression of ABC transporters was not
determined, PDXs can be used in animal models to observe changes in
target biomarker expression on drug administration. For example,
ABC transporter-expressing PDXs might be used as an in vivo
model to verify the effect of ABC transporter blocker.
Based on previous reports, we suggest three
applications for PDX models in cancer research. First, they are a
promising option for drug screening and biomarker development. The
relationship between drug efficacy and molecular characteristics
could be easily studied using PDX models, and previously, excellent
biomarkers have been discovered for melanoma (21). Second, co-clinical trials could be
performed. Co-clinical trials denote clinical trials that are
conducted in parallel with PDX model experimentation. This type of
trial provides a more useful platform than conventional trials to
investigate biomarkers of drug response and resistance and can also
be used to advance new drug development and clinical introduction
(2). Third, these models could be
used for precision medicine. Oncology research has evolved on the
basis of the improved understanding of cancer genotypes and
phenotypes, which has led to a new era of precision medicine. The
patient could thus be treated with an appropriate drug that elicits
the best response in corresponding PDXs (2). The increased use of PDXs will
accelerate HNC research to investigate biomarkers and responses to
new drugs.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Japan Society for
the Promotion of Science KAKENHI (grant no. 17K16899) and a
Research Grant from the Cancer Research Institute of Kanazawa
University (grant no. 02-44).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HM was involved in drafting the manuscript or
revising it critically for important intellectual content. HM and
KE made substantial contributions to conception and design,
acquisition of data, and analysis and interpretation of data. HM
and KE confirm the authenticity of all the raw data. YK aided in
collecting patient samples and worked on the preparation of PDX
models along with AN. MMK performed immunostaining. KI, TU, YN, SK
and NW were involved in patient sample collection and contributed
to analysis and interpretation of data. NG and TY contributed to
the conception and design of the present study. TY worked on the
final version of the manuscript. All authors discussed the results
and commented on the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study complied with the Declaration of
Helsinki and was approved by the Investigational Review Board of
Kanazawa University (approval no. 2015-125; Kanazawa, Japan). All
patients included in the present study provided written informed
consent. Furthermore, all animal procedures were approved by the
Ethical Committee of the Laboratory for the Animal Experiments,
Graduate School of Medical Science, Kanazawa University (permit no.
AP-173861; Kanazawa, Japan) and were performed in compliance with
the guidelines of this committee.
Patient consent for publication
All patients included in the present study provided
written informed consent for the publication of any associated data
and accompanying images at the point of recruitment to the
trial.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HNC
|
head and neck cancer
|
|
PDX
|
patient-derived xenograft
|
|
CDX
|
cell line-derived xenograft
|
|
ABC transporter
|
adenosine triphosphate-binding
cassette transporter
|
|
NOD-SCID mouse
|
non-obese diabetic severe combined
immunodeficient mouse
|
|
MDR-1
|
multiple drug resistance-1
|
|
MRP-2
|
multidrug resistance-associated
protein-2
|
|
CCRT
|
concurrent cisplatin-radiation
therapy
|
References
|
1
|
DiMasi JA, Reichert JM, Feldman L and
Malins A: Clinical approval success rates for investigational
cancer drugs. Clin Pharmacol Ther. 94:329–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jung J, Seol HS and Chang S: The
generation and application of patient-derived xenograft model for
cancer research. Cancer Res Treat. 50:1–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peng S, Creighton CJ, Zhang Y, Sen B,
Mazumdar T, Myers JN, Lai SY, Woolfson A, Lorenzi MV, Bell D, et
al: Tumor grafts derived from patients with head and neck squamous
carcinoma authentically maintain the molecular and histologic
characteristics of human cancers. J Transl Med. 11:1982013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vrana D, Hlavac V, Brynychova V,
Vaclavikova R, Neoral C, Vrba J, Aujesky R, Matzenauer M, Melichar
B and Soucek P: ABC transporters and their role in the neoadjuvant
treatment of esophageal cancer. Int J Mol Sci. 19:8682018.
View Article : Google Scholar
|
|
5
|
Theile D, Gal Z, Warta R, Rigalli JP,
Lahrmann B, Grabe N, Herold Mende C, Dyckhoff G and Weiss J:
Antiproliferative efficacies but minor drug transporter inducing
effects of paclitaxel, cisplatin, or 5-fluorourcil in a murine
xenograft model for head and neck squamous cell carcinoma. Cancer
Biol Ther. 15:436–442. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Warta R, Theile D, Mogler C, Herpel E,
Grabe N, Lahrmann B, Plinkert PK, Herolde-Mende C, Weiss J and
Dyckhoff G: Association of drug transporter expression with
mortality and progression-free survival in stage IV head and neck
squamous cell carcinoma. PLos One. 9:e1089082014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brierley JD, Gospodarowicz MK and
Wittekind C: UICC TNM Classification of Malignant Tumours. 8th
edition. Wiley Blackwell; New York, NY: 2017
|
|
8
|
Tanabe H, Takada Y, Minegishi D, Kurematsu
M, Masui T and Mizusawa H: Cell line individualization by STR
multiplex system in the cell bank found cross-contamination between
ECV304 and EJ-1/T24. Tiss Cult Res Commun. 18:329–338. 1999.
|
|
9
|
Cho YB, Hong HK, Choi YL, Oh E, Joo KM,
Jin J, Nam DH, Ko YH and Lee WY: Colorectal cancer patient-derived
xenografted tumors maintain characteristic features of the original
tumors. J Surg Res. 187:502–509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klinghammmer K, Raguse JD, Plath T, Albers
AE, Joehrens K, Zakameh A, Brzezicha B, Wulf-Goldenberg A, Keilholz
U, Hoffmann J and Fichtner I: A comprehensively characterized large
panel of head and neck cancer patient-derived xenografts identifies
the mTOR inhibitor everolimus as potential new treatment option.
Int J Cancer. 136:2940–2948. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kimple RJ, Harari PM, Torres AD, Yang RZ,
Soriano BJ, Yu M, Armstrong EA, Blitzer GC, Smith MA, Lorenz LD, et
al: Development and characterization of HPV-positive and
HPV-negative head and neck squamous cell carcinoma tumorgrafts.
Clin Cancer Res. 19:855–864. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stewart EL, Mascaux C, Pham NA, Sakashita
S, Sykes J, Kim L, Yanagawa N, Allo G, Ishizawa K, Wang D, et al:
Clinical utility of patient-derived xenografts to determine
biomarkers of prognosis and map resistance pathways in EGFR-mutant
lung adenocarcinoma. J Clin Oncol. 33:2472–2480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garrido-Laguna I, Uson M, Rajeshkumar NV,
Tan AC, de Oliveira E, Karikari C, Villaroel MC, Salomon A, Taylor
G, Sharma R, et al: Tumor engraftment in nude mice and enrichment
in stroma-related gene pathways predicts poor survival and
resistance to gemcitabine in patients with pancreatic cancer. Clin
Cancer Res. 17:5793–5800. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pergolini I, Morales-Oyarvide V,
Mino-Kenudson M, Honselmann KC, Rosenbaum MW, Nahar S, Kem M,
Ferrone CR, Lillemoe KD, Bardeesy N, et al: Tumor engraftment in
patient-derived xenografts of pancreatic ductal adenocarcinoma is
associated with adverse clinicopathological features and poor
survival. PLoS One. 12:e01828552017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Facompre ND, Sahu V, Montone KT, Harmeyer
KM, Nakagawa H, Rustgi AK, Weinstein GS, Gimotty PA and Basu D:
Barriers to generating PDX models of HPV-related head and neck
cancer. Laryngoscope. 127:2777–2783. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prince ME, Sivanandan R, Kaczorowski A,
Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF and Ailles
LE: Identification of a subpopulation of cells with cancer stem
cell properties in head and neck squamous cell carcinoma. Proc Natl
Acad Sci USA. 104:973–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu J, Qin B, Moyer AM, Sinnwell JP,
Thompson KJ, Copland JA III, Marlow LA, Miller JL, Yin P, Gao B, et
al: Establishing and characterizing patient-derived xenografts
using pre-chemotherapy percutaneous biopsy and post-chemotherapy
surgical samples from a prospective neoadjuvant breast cancer
study. Breast Cancer Res. 19:1302017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Markman M, Rothman R, Hakes T, Reichman B,
Hoskins W, Rubin S, Jones W, Almadrones L and Lewis JL Jr:
Second-line platinum therapy in patients with ovarian cancer
previously treated with cisplatin. J Clin Oncol. 9:389–393. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harries M and Gore M: Part II:
chemotherapy for epithelial ovarian cancer-treatment of recurrent
disease. Lancet Oncol. 3:537–545. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Knoedler M, Gauler TC, Gruenwald V,
Matzdorff A, Schroeder M, Dietz A, Jordan WO, Arnold D, Hennemann
B, Hofele C, et al: Phase II study of cetuximab in combination with
docetaxel in patients with recurrent and/or metastatic squamous
cell carcinoma of the head and neck after platinum-containing
therapy: A multicenter study of the Arbeitsgemeinschaft
Internistische Onkologie. Oncology. 84:284–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Das Thakur M, Salangsang F, Landman AS,
Sellers WR, Pryer NK, Levesque MP, Dummer R, McMahon M and Stuart
DD: Modelling vemurafenib resistance in melanoma reveals a strategy
to forestall drug resistance. Nature. 494:251–255. 2013. View Article : Google Scholar : PubMed/NCBI
|