Introduction
Uterine cervical carcinoma (UCC) is one of the most
common types of malignant cancer in women, representing the fourth
most frequent malignancy worldwide (1). In 2018, ~570,000 new cases of UCC were
identified, and up to 311,000 associated deaths were recorded
worldwide (2). Epidemiological data
have demonstrated that the incidence and mortality rates of UCC
vary across different regions, with more cases in Sub-Saharan
Africa and South-Eastern Asia, and less cases in North America,
Australia, New Zealand and Western Asia (2). Although it has been reported that UCC
incidence and mortality rates have decreased in several regions of
the world over the last few decades (3), UCC remains a serious health issue in
China, with an estimated 106,430 new cases and 47,739 mortalities
in 2018 (3).
Ubiquitin-specific peptidase (USP)18, also known as
ubiquitin-specific protease 43 (UBP43), is a member of the USP
family and is involved in deubiquitinating activity, thereby
resulting in stabilization of substrates (4). It is well known that USPs are the
largest sub-family of deubiquitinase enzymes, and exert biological
roles through their cysteine endopeptidase activity (5). The USP family consists of >100
members, which predominantly differ in amino acid sequence and
protein size, but are characterized by several highly homologous
sequences around the essential domains important for their
catalytic activity (6,7). USP18 was originally identified from
acute myelogenous leukemia 1- RUNX1 partner transcriptional
co-repressor 1 knock-in mice and was characterized by Liu et
al (8). Previous studies have
demonstrated that USP18 expression is present in multiple types of
tissues, including liver, lung, spleen, thymus, bone marrow and
adipose tissues (8,9), and is also expressed in different types
of cells, such as macrophages, lymphocytes and hematopoietic cells
(10,11). The function of USP18 has
predominantly been associated with the regulation of cell
proliferation, cell differentiation, stress, inflammatory reaction
and immune response (9–11). Additionally, it has been suggested
that USP18 serves a vital role in regulating T-cell activation and
T helper 17 cell differentiation through its ability to remove the
transforming growth factor β-activated kinase 1-TAK1-binding
protein 1 complex (12).
Several studies have suggested that USP18 may be
involved in tumor biology (10,13).
USP18 is overexpressed in several types of human cancer including
glioblastoma, hepatocellular carcinoma, bladder cancer and breast
cancer, and its high expression is associated with a poor prognosis
in patients with glioblastoma and bladder cancer (14–17).
Furthermore, some studies have demonstrated that USP18 is important
for the malignant behaviors of tumor cells, including cellular
proliferation, migration, apoptosis and epithelial-to-mesenchymal
transition (EMT) (14,17–19).
Conversely, it has been demonstrated that USP18 may suppress
tumorigenesis due to its involvement in the antitumor immune
response (20).
Although the biological functions and clinical
presentation of USP18 have been well characterized in several types
of human cancer including glioblastoma, breast cancer and melanoma
(14,17,19,20), its
underlying molecular mechanisms in UCC remain unclear. Thus, the
present study aimed to investigate USP18 expression in a cervix
tissue microarray, and determine its potential role and molecular
mechanism in UCC malignant phenotypes.
Materials and methods
Cell line, cell culture and cell
transfection
Human UCC HeLa cells were purchased from the
American Type Culture Collection and maintained in RPMI-1640 medium
supplemented with 10% fetal bovine serum, 100 U/ml penicillin and
100 mg/ml streptomycin (all Gibco; Thermo Fisher Scientific, Inc.)
at 37°C with 5% CO2 in a humidified incubator.
To generate USP18-deficient HeLa cells, an RNA
interference silencing strategy was used to design and construct a
lentivirus vector carrying small interfering (si)RNA sequences
targeting USP18 by GeneCopoeia, Inc. The lentiviruses containing
USP18-siRNA vector and scrambled negative control vector (mock)
were synthesized by GeneCopoeia, Inc (cat. nos. HSH117922-LVRU6GP-c
and CSHCTR001-3-LVRU6GP, respectively). The sequences targeting
USP18 were 5′-CCAACATTAATTCCATATGAA-3′, and the scrambled sequences
of 5′-ACGCGTATTCGTTTACTGT-3′ were used as negative control.
Following infection with the vector-carrying lentiviruses,
according to the manufacturer's protocol, cells were subsequently
treated with 2 ng/ml puromycin (Gibco; Thermo Fisher Scientific,
Inc.) and transfection efficiency was observed under a fluorescence
microscope (magnification, ×200). Western blot analysis was
subsequently performed to detect USP18 protein expression.
Immunohistochemistry (IHC)
A commercial cervix tissue microarray containing 15
squamous cell carcinoma tissues, five adenosquamous carcinoma
tissues, 20 adenocarcinoma tissues, 20 cervical intraepithelial
lesions tissues, 14 cervicitis tissues, four unpaired
para-cancerous tissues and two cervical canal tissues (cat. no.
F801301; Bioaitech Co., Ltd.) was used to detect USP18 expression.
The characteristics of the tissues are listed in Table I.
 | Table I.Patient characteristics of tissues in
cervix tissue microarray. |
Table I.
Patient characteristics of tissues in
cervix tissue microarray.
|
Characteristics | Cancer tissues,
n=40 | Non-cancer tissues,
n=40 |
|---|
| Age, years |
|
|
|
Range | 26–63 | 32–66 |
|
Median | 48.68 | 48.20 |
| Stage, n |
|
|
| I | 29 |
|
| II | 9 |
|
|
III | 2 |
|
| Grade, n |
|
|
| 1 | 10 |
|
| 2 | 15 |
|
| 3 | 10 |
|
| Lymph node
metastasis, n |
|
|
|
Yes | 4 |
|
| No | 34 |
|
The microarray was processed routinely via
deparaffinization, rehydration, endogenous peroxidase quenching and
antigen retrieval, and subsequently blocked with 20% normal goat
serum (Wuhan Boster Biological Technology, Ltd.) for 30 min at 37°C
to remove the background from staining. The microarray was
incubated with a mouse antibody against human USP18 (1:50; cat. no.
sc-374064; Santa Cruz Biotechnology, Inc.) overnight at 4°C.
Following the primary antibody incubation, the microarray was
incubated for 35 min at 37°C using the SABC kit (cat. no. SA1021;
Wuhan Boster Biological Technology, Ltd.). DAB and hematoxylin were
used for visualization and nuclear counterstaining at room
temperature for 30 and 5 sec, respectively. The USP18 protein
expression profiles were estimated based on the staining intensity
and the percentage of positive cells using a fluorescence
microscope (magnification, ×200). The staining intensity was ranked
as follows: 1, weak; 2, moderate; 3, intensive; and 4, super
intensive. The percentage of positive cells was scored as follows:
1, <25%; 2, 26–50%; 3, 51–75%; and 4, >75%. The total score
for each sample was the sum of the two parameters, as previously
described (21).
Cell proliferation assay
The Cell Counting Kit-8 (CCK-8; Wuhan Boster
Biological Technology, Ltd.) assay was performed to assess the
proliferation of HeLa cells following USP18-knockdown. Briefly,
mock- and USP18-siRNA-transfected HeLa cells were seeded into
96-well plates at a density of 1.5×103 cells/well in a
final volume of 100 µl RPMI-1640 medium supplemented with or
without 80 µΜ of the ERK1/2 blocker PD98059 (Sigma-Aldrich; Merck
KGaA). Following incubation at 37°C for 24, 48 or 72 h, 10 µl CCK-8
reagent was added to each well and incubated at 37°C for 1 h. Cell
proliferation was subsequently analyzed at a wavelength of 450 nm,
using a microplate reader (Omega Bio-Tek, Inc.). The inhibitory
role of PD98059 on the proliferation of HeLa cells was estimated as
follows: Inhibition rate (%)=(treatment with 0 µM PD98059-treatment
with 80 µM PD98059)/treatment with 0 µM PD98059 ×100.
Clonogenic ability assay
Mock- and USP18-siRNA-transfected HeLa cells were
seeded into 24-well plates at a density of 5×102
cells/well in 500 µl RPMI-1640 medium supplemented with 10% fetal
bovine serum. Cells were cultured at 37°C and the medium was
replaced every 2–3 days. Following incubation for 10 days, the
medium was removed and cells were stained with 0.01% crystal violet
for 15 min at room temperature. A minimum of 3 mm diameter or more
was considered as a colony. Images of cell colonies were captured
using an imaging system (Tanon Science & Technology Co., Ltd.)
and counted under a fluorescence microscope (magnification,
×50).
Cell migration assay
The migratory ability of HeLa cells was assessed
using 24-well Transwell chambers with polycarbonate filter of 8-µm
pore size. A total of 2×104 mock- and
USP18-siRNA-transfected HeLa cells were plated in the upper
chambers of Transwell plates in 100 µl serum-free RPMI-1640 medium.
A total of 600 µl RPMI-1640 medium containing 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with or without 80 µΜ
of the ERK1/2 blocker PD98059 was plated in the lower chambers.
Following incubation for 24 h at 37°C, cells in the upper chambers
were removed using a cotton swab, while the migratory cells were
fixed and stained using a solution of crystal violet in ethanol for
15 min at room temperature. Stained cells were counted using a
fluorescence microscope (magnification, ×200). The inhibitory role
of PD98059 on the migration of HeLa cells was estimated as follows:
Inhibition rate (%)=(treatment with 0 µM PD98059-treatment with 80
µM PD98059)/treatment with 0 µM PD98059 ×100.
Wound healing assay
Mock- and USP18-siRNA-transfected HeLa cells were
seeded into 6-well plates at a density of 2×106
cells/well in 1 ml RPMI-1640 medium supplemented with 10% fetal
bovine serum. After incubation until confluent, the culture medium
was replaced with serum-free medium, and a cell-free wound zone was
created by scraping the monolayer with a sterile pipette tip. The
images of the wounds were captured and the numbers of migrating
cells were counted at 0 and 24 h after wounding using a
fluorescence microscope by eye (magnification, ×100).
Western blotting
Untransfected parental, mock- and
USP18-siRNA-transfected HeLa cells were harvested and lysed using a
commercial RIPA buffer kit (cat. no. P0013C; Beyotime Institute of
Biotechnology) supplemented with phenylmethylsulfonyl fluoride.
Protein concentrations were determined using the bicinchoninic acid
protein assay kit (cat. no. P0010; Beyotime Institute of
Biotechnology) and 40 µg protein/lane was subjected to 12%
SDS-PAGE. The separated proteins were subsequently transferred onto
polyvinylidene difluoride membranes and blocked with 5% skimmed
milk in PBS for 60 min at 37°C. The membranes were incubated with
primary antibodies against USP18 (1:1,000; cat. no. DF7968;
Affinity Biosciences), Bcl-2 (1:1,000; cat. no. AF6139; Affinity
Biosciences), STAT3 (1:1,000; cat. no. CY5165; Shanghai Abways
Biotechnology Co., Ltd.), ERK (1:1,000; cat. no. CY5487; Shanghai
Abways Biotechnology Co., Ltd.), phosphorylated (p)-ERK (1:1,000;
cat. no. CY5277; Shanghai Abways Biotechnology Co., Ltd.) and
β-actin (1:5,000; cat. no. AB0011; Shanghai Abways Biotechnology
Co., Ltd.) overnight at 4°C. Following the primary antibody
incubation, membranes were incubated with HRP-coupled goat
anti-rabbit and anti-mouse secondary antibodies (1:10,000; cat.
nos. BA1054 and BA1050, respectively; Wuhan Boster Biological
Technology, Ltd.). Protein blots were visualized using ECL reagent
(Thermo Fisher Scientific, Inc.). The protein expression levels of
USP18, Bcl-2 and STAT3 were normalized to β-actin, whereas p-ERK
expression was normalized to total ERK expression using ImageJ
software (version 1.45 s; National Institutes of Health).
Statistical analysis
Statistical analysis was performed using SPSS 22.0
software (IBM Corp.). All experiments were performed in triplicate
and data are presented as the mean ± SD. Comparisons between two
groups were analyzed using unpaired Student's t-test (if the
variance was homogeneous) or Cochran and Cox separate variance
estimation t-test (if the variance was not homogeneous).
Comparisons among multiple groups were analyzed using one-way ANOVA
followed by Student-Newman-Keuls post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
USP18 expression is downregulated in
UCC tissues
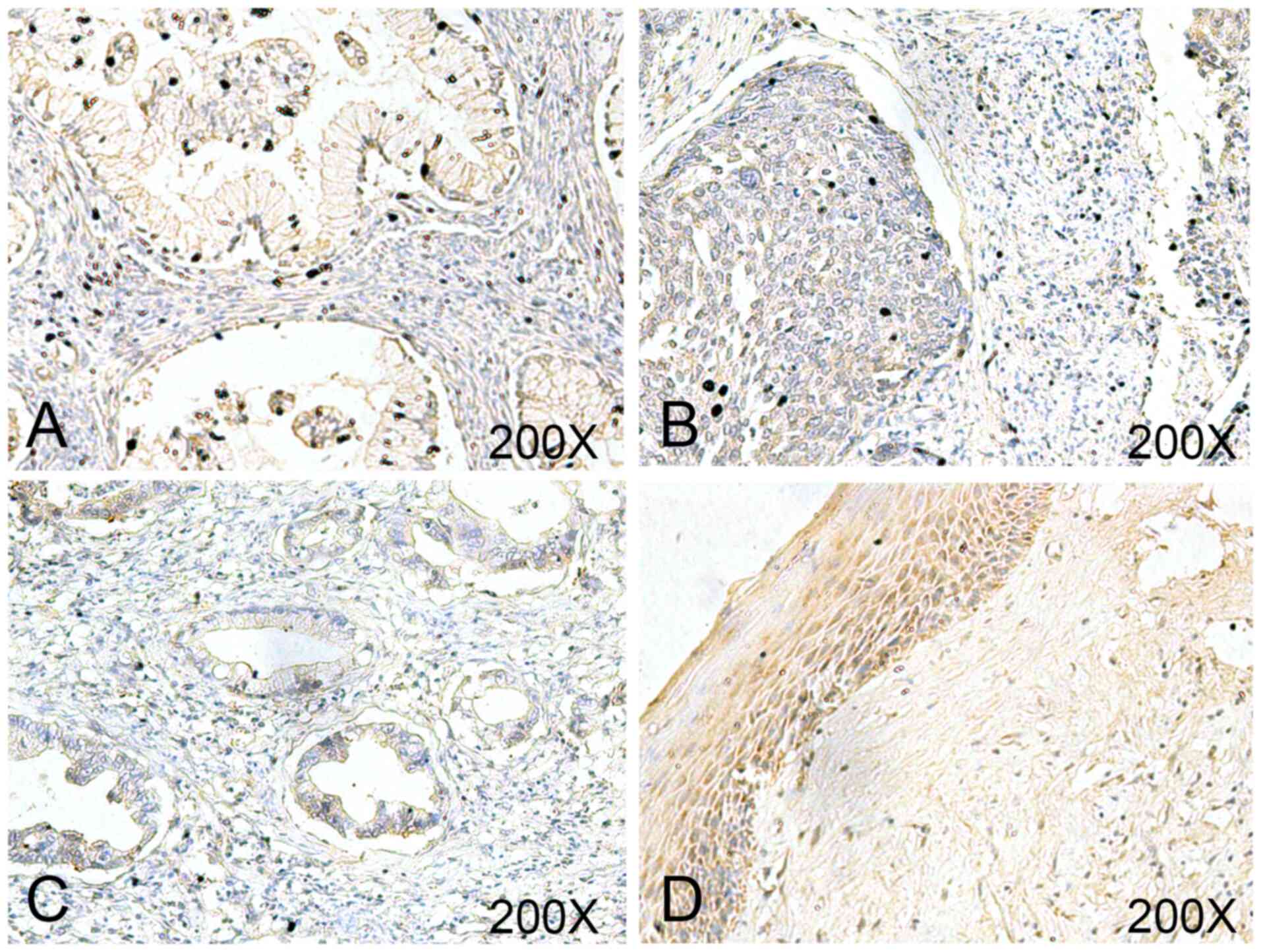
To evaluate the clinical significance of USP18,
USP18 expression was determined via IHC analysis in a commercial
cervix tissue microarray (Fig. 1).
The final score demonstrated that USP18 was expressed at
significantly lower levels in UCC tissues compared with in normal
tissues (Table II). However, there
was no significant difference in USP18 expression between different
stages, grades, age and lymph node metastasis (Table II).
 | Table II.Ubiquitin-specific peptidase 18
expression in cervix tissue microarray (mean ± S). |
Table II.
Ubiquitin-specific peptidase 18
expression in cervix tissue microarray (mean ± S).
|
Characteristics | USP18 level | t | P-value |
|---|
| Tissues |
|
|
|
|
Non-cancer | 5.25±1.532 | 2.1750 | 0.0327 |
|
Cancer | 4.50±1.553 |
|
|
| Stage |
|
|
|
| I | 4.62±1.568 | 0.7945 | 0.4318 |
|
II–III | 4.18±1.537 |
|
|
| Grade |
|
|
|
| 1 | 5.00±1.155 | 1.0299 | 0.3105 |
|
2–3 | 4.40±1.683 |
|
|
| Age, years |
|
|
|
|
≤50 | 4.35±1.522 | 0.8511 | 0.4001 |
|
≥50 | 4.79±1.626 |
|
|
| Lymph node
metastasis |
|
|
|
| No | 4.62±1.615 | 1.0452 | 0.3029 |
|
Yes | 3.75±0.957 |
|
|
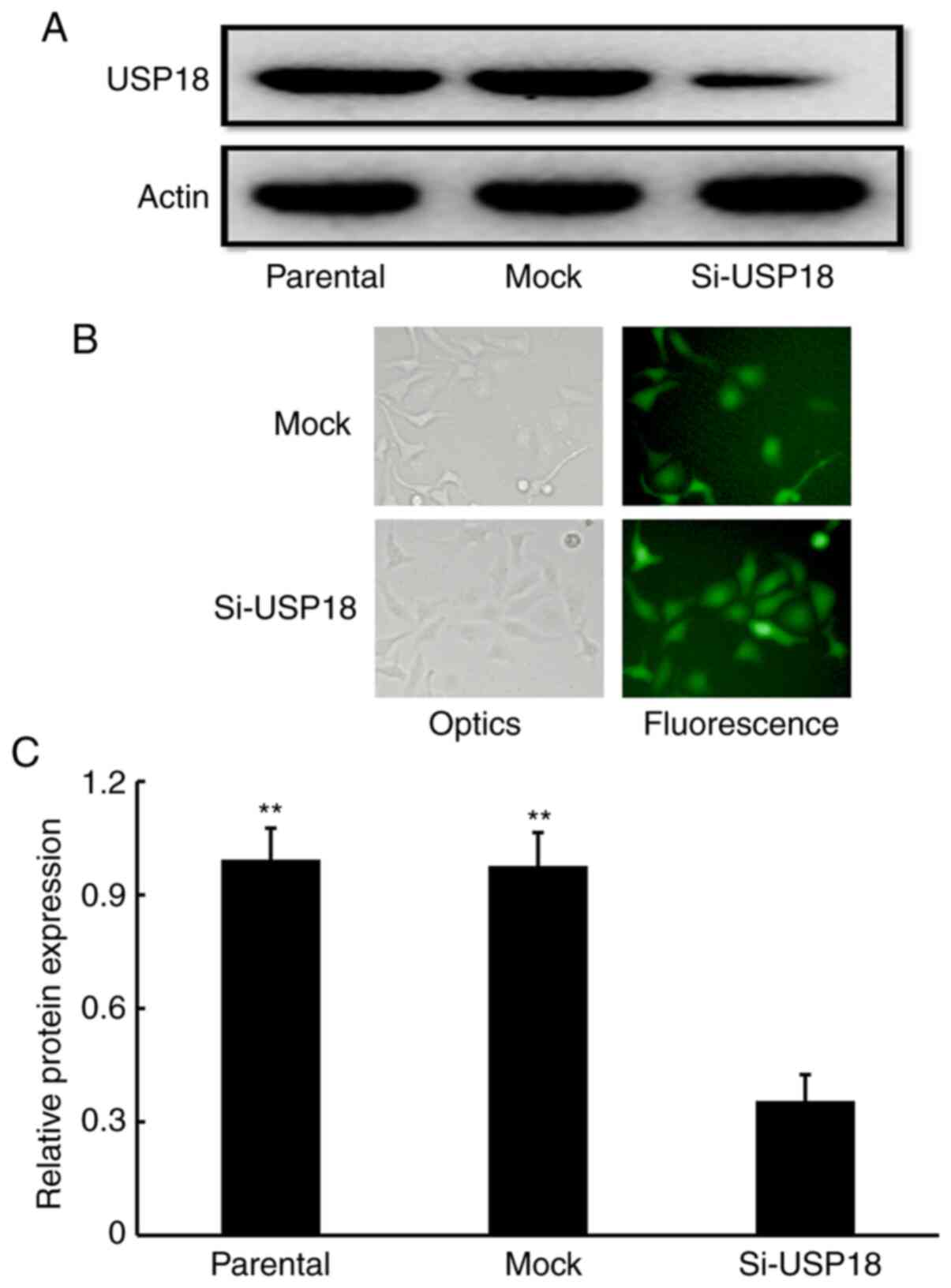
USP18 is expressed in HeLa cells
To assess the potential involvement of USP18 in the
malignant phenotypes of HeLa cells, USP18 protein expression was
detected. Western blot analysis confirmed that USP18 protein was
expressed in untransfected parental HeLa cells (Fig. 2A). Following transfection with
lentiviruses, fluorescent imaging demonstrated that siRNA- and
mock-transfected cells expressed high-intensity screening
fluorescence, suggesting that the cells were effectively infected
by lentiviruses (Fig. 2B).
Subsequent western blotting showed that USP18 protein expression
was significantly decreased in siRNA-transfected HeLa cells
compared with untransfected parental and mock-transfected HeLa
cells (Fig. 2A and C), which
indicated that USP18-deficient HeLa cells were successfully
established.
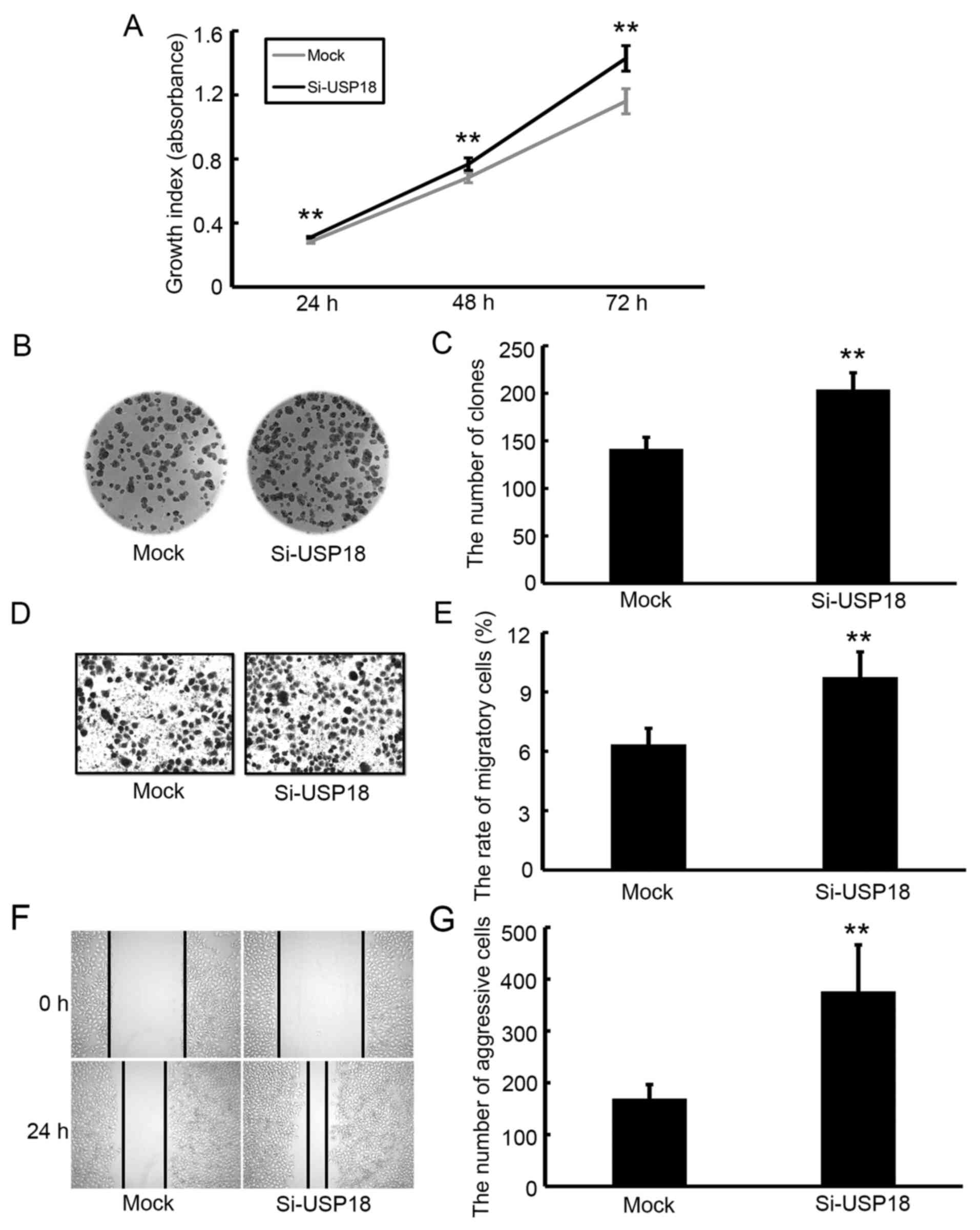
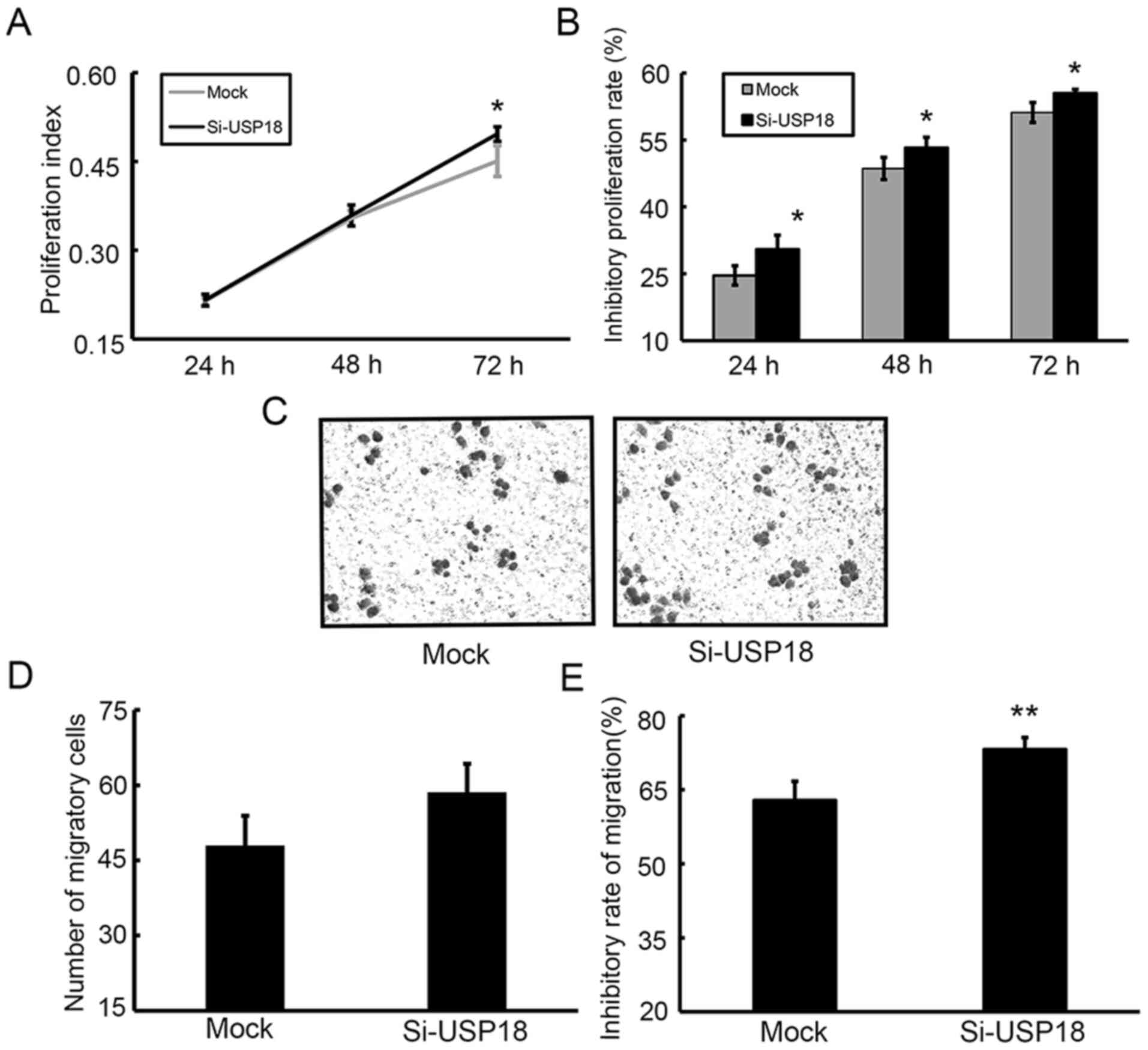
Downregulation of USP18 promotes the
proliferation, colony formation, migration and aggressiveness of
HeLa cells
The CCK-8 assay was performed to assess the effect
of USP18 on the malignant behavior of HeLa cells. Following
incubation for 24, 48 and 72 h, the results demonstrated that the
proliferative ability of siRNA-transfected HeLa cells was
significantly increased compared with mock-transfected HeLa cells
(Fig. 3A). The results of the colony
formation, migration and wound healing assays demonstrated that
silencing of USP18 in HeLa cells significantly increased their
colony forming (Fig. 3B and C),
migratory (Fig. 3D and E) and
aggressive abilities (Fig. 3F and
G).
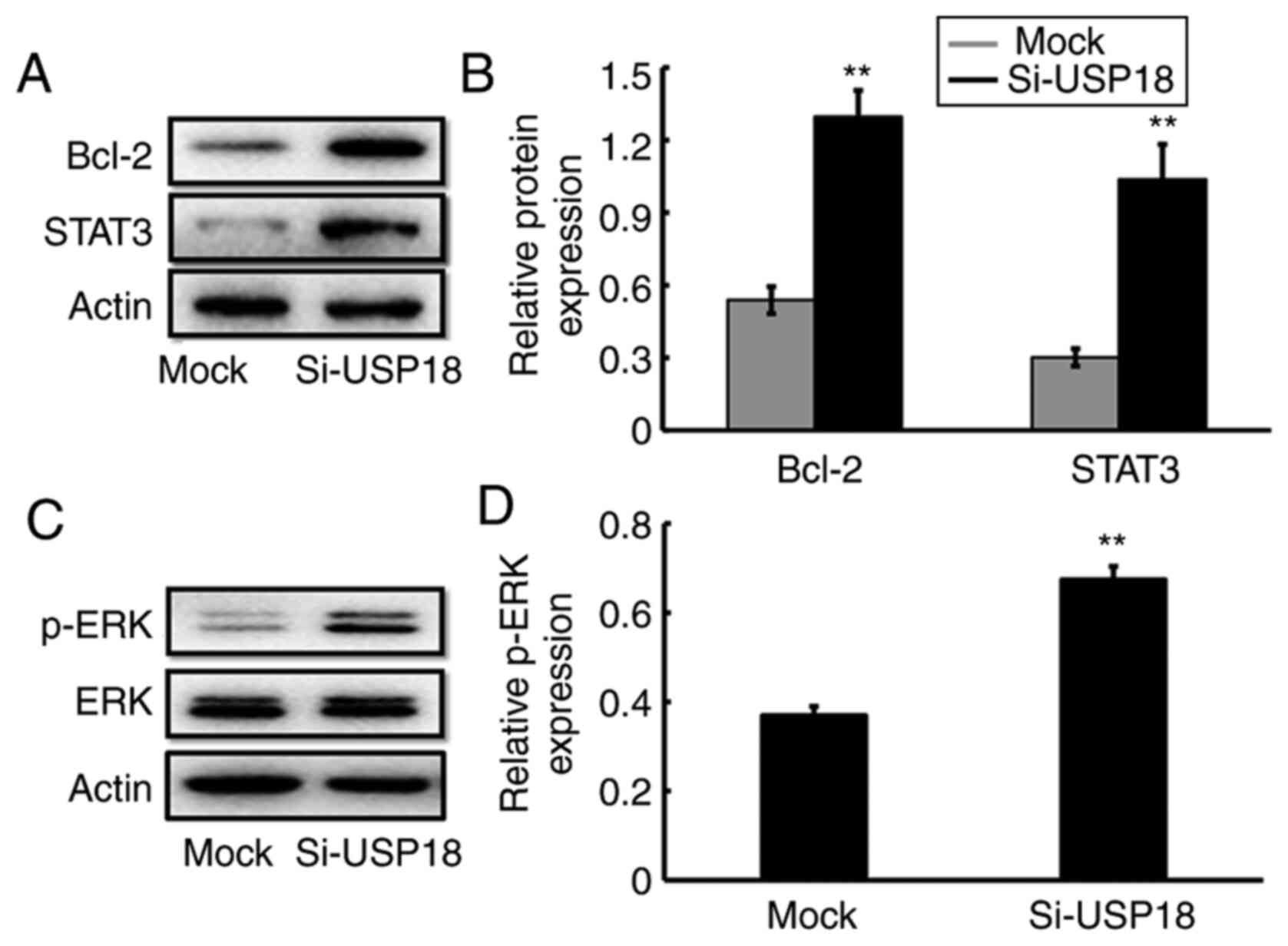
USP18-knockdown alters ERK-signaling
pathway-specific protein expression
The potential molecular mechanism by which USP18
regulates the malignant process of HeLa cells was assessed via
western blotting. The results demonstrated that the protein
expression levels of Bcl-2, STAT3 (Fig.
4A and B) and p-ERK (Fig. 4C and
D) were significantly increased following USP18-knockdown in
HeLa cells, suggesting the involvement of ERK signaling in the
malignant phenotypes of UCC cells.
USP18 silencing-induced malignant
behaviors of HeLa cells depend on ERK signaling
To further clarify whether the roles of USP18
silencing on HeLa cells were mediated by ERK signaling, the ERK1/2
blocker PD98059 was used. The results demonstrated that there were
no significant differences in cell proliferation at 24 or 48 h
(Fig. 5A) and cell migration at 24 h
(Fig. 5C and D) between siRNA- and
mock-transfected cells following treatment with PD98059. Although
the proliferative rate of siRNA-transfected cells was significantly
higher compared with that of mock-transfected cells after 72 h of
exposure to PD98059 (P<0.05; Fig.
5A), the difference between the two groups was slightly lower
compared with that between the two groups treated without PD98059
(Fig. 3A). Following treatment with
PD98059, the inhibition rates exerted by PD98059 on the
proliferation (Fig. 5B) and
migration (Fig. 5E) of
siRNA-transfected HeLa cells were significantly higher compared
with those in mock-transfected HeLa cells. Overall, the present
results suggested that USP18 silencing-induced malignant responses
in HeLa cells depend on the activation of the ERK signaling
pathway.
Discussion
USP18 is a major isopeptidase, which was initially
identified based on its role to efficiently deconjugate
interferon-stimulated gene 15 (ISG15), a two-domain ubiquitin-like
protein, from ISGylation (9). In
addition to ISG15, USP18 is highly induced by type I and III
interferons, and it has been proposed that USP18 is a vital blocker
of the type I interferons signaling pathway (22). Furthermore, a study demonstrated that
type III interferons may induce USP18 production (23). In the presence of USP18, type III
interferons acquire higher properties to weaken type I
interferons-mediated actions by repressing JAK-STAT signaling
(23).
Increasing evidence has suggested that USP18 is
implicated in a variety of physiological and pathological processes
in different tissues and cells, including cell development, viral
infection, viral replication and antibacterial response (10,24,25).
However, a vast expansion in the understanding of USP18 expression
and its association with tumor biology has occurred. It has been
reported that USP18 is frequently overexpressed in different types
of cancer, including breast cancer, bladder cancer and
hepatocellular carcinoma, and its overexpression is positively
associated with several pathological tumor characteristics
(15–17). For example, a recent study indicated
that USP18 methylation is predominantly downregulated, whereas its
expression is upregulated in breast cancer, which is positively
associated with increasing TNM stage, worse disease-free survival
rate and HER2+ patients, but negatively associated with
apoptosis (17). Accordingly, it has
been suggested that USP18 may be used as a predictive marker for
poor prognosis in muscle invasive bladder cancer, since high USP18
expression is a significant risk factor for cancer-specific death,
and decreased USP18 expression is markedly associated with longer
cancer-specific survival (16).
USP18 has also been the focus of investigations evaluating its
functions in tumorigenesis. USP18 silencing in a mouse model for
breast cancer exhibited a significant decrease in tumor growth, and
USP18-deficiency in breast cancer MCF-7 cells in vitro
triggered an increase in the induction of apoptosis (18,19). In
addition, downregulation of USP18 expression in glioblastoma cells
may protect against tumor cell invasion and migration by repressing
EMT (14), an essential event for
cancer metastasis, by which tumor cells obtain increased motility
and invasiveness. Gain-of-function assays in vitro have
demonstrated that overexpression of USP18 has an important role in
regulating tumor progression due to its contribution in enhancing
breast cancer tumor cell proliferation, colony formation and cell
cycle progression (17). However,
studies have also revealed that USP18 may exert an opposing role in
the control of cancer development. For example, ectopic USP18
expression in B16 melanoma cancer cells may suppress tumorigenesis,
restraining cancer cell-mediated inhibition of T-cell proliferation
and activation, thus facilitating cancer cells to specific immune
responses (20). In human
leiomyosarcoma, downregulation of USP18 is associated with a poor
clinical outcome, and USP18-deficient mice exhibited an enhanced
ability to develop these sarcomas (26).
Although a recent study demonstrated that USP18 is a
critical regulator for the tumorigenicity of cervical cancer CaSki
and SiHa cells (27), the results of
the present study demonstrated that USP18 expression was
downregulated in UCC compared with in normal tissues. USP18
expression was knocked down in HeLa cells, and the malignant
behaviors in cells, including proliferation, colony formation and
migration, were enhanced. Mechanistically, p-ERK expression was
significantly upregulated following USP18-knockdown in HeLa cells.
Previous studies have reported that several signaling pathways,
such as the PTEN/AKT (28), AKT/Skp2
(16) and JAK/STAT (29) signaling pathways, are implicated in
USP18 associated-biological roles. To the best of our knowledge,
the present study was the first to illustrate an involvement of ERK
signaling in the function of USP18 in tumorigenesis. ERKs are a
family of protein-serine/threonine kinases, which serve vital roles
in the control of diverse cell functions, such as cell
differentiation, proliferation and survival, by phosphorylating
several substrates including transcription factors, protein kinases
and phosphatases (30). Increasing
evidence has suggested that amplification or activation of ERK
signaling frequently occurs in several malignant tumors, such as
gastric adenocarcinoma and lung cancer (31,32),
which results in increased cell proliferation, promotion of cell
cycle progression and repressed apoptosis of tumor cells (33). Given that the ERK signaling pathway
participates in several aspects of tumorigenesis by regulating the
expression of its downstream signaling molecules, such as NF-κB
(34), Bcl-2 (35) and STAT3 (36), the present study assessed whether the
expression levels of these genes were affected following
USP18-knockdown. The results demonstrated that Bcl-2 and STAT3
expression was upregulated, whereas NF-κB expression remained
unchanged (data not shown) following USP18-knockdown in HeLa cells.
Bcl-2 is a novel gene encoding a unique apoptosis inhibitor that
efficiently suppresses apoptosis induced by the p53 tumor
suppressor protein (37). STAT3 is
an important proto-oncogene essential for modulating the transition
from the G1 to S phase of the cell cycle (38), and most cancer cases arise due to
proliferating cells losing control of cell cycle regulation, in
which loss of the G1/S-phase transition checkpoint is a
major cause of cancer (39).
In conclusion, the results of the presents study
demonstrated that USP18 expression was downregulated in UCC
tissues, and USP18-knockdown facilitated tumor cell proliferation
and migration by affecting the expression levels of genes
associated with the ERK signaling pathway. Overall, the current
results provide a novel mechanism for USP18-deficiency, which may
serve a crucial role in UCC progression in an ERK-dependent
manner.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81272854), Key
Projects of Natural Science Foundation of Heilongjiang Province
(grant no. ZD2019H008), Excellent Innovation Team Construction
Project of Basic Scientific Research Business Fee of Provincial
Colleges and Universities in Heilongjiang Province (grant no.
2019-KYYWF-1334), Double First-class Discipline Construction
Project in Heilongjiang Province (grant name. northern medicine and
functional food), Young innovative talents training project of
regular undergraduate colleges and universities in Heilongjiang
Province (grant no. UNPYSCT-2020054), Youth Academic Backbone
Support Program for Institution of Common Higher Education in
Heilongjiang Province (grant no. 1252G059), Personnel Training
Project of Basic Scientific Research Business Expenses of
Department of Education in Heilongjiang Province (grant no.
2019-KYYWF-1338), Science and Innovation Team Foundation of Jiamusi
University (grant no. cxtd-2016-03) and Biology Team Project of
Jiamusi University (grant no. jdxktd-2019003).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AP and YL participated in cell experiments and
drafted the manuscript. JG and PZ participated in statistical
analyses. YH, LS and JRW performed immunohistochemistry assay. CZ,
YC, and QR participated in cell transfection and cell experiments.
SL, SF and TZ performed western blot analyses. AP, YL and JTW
confirm the authenticity of all the raw data. WW and JTW designed
the study and performed the revision of the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The medical ethics committee at Jiamusi University
(Jiamusi, China) approved all procedures performed in the present
study involving animals and human participants, which were in
accordance with ethical standards, and all patients provided
written informed consent prior to participation in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Van Hede D, Langers I, Delvenne P and
Jacobs N: Origin and immunoescape of uterine cervical cancer.
Presse Med. 43:e413–e421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Colombet M,
Soerjomataram I, Siegel RL, Torre A and Jemal A: Global cancer
statistics 2018: GLOBOCAN estimates of incidence and mortality
worldwide for 36 cancers in 185 countries. CA Cancer J Clin.
68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arbyn M, Weiderpass E, Bruni L, de Sanjosé
S, Saraiya M, Ferlay J and Bray F: Estimates of incidence and
mortality of cervical cancer in 2018: A worldwide analysis. Lancet
Glob Health. 8:e191–e203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oikonomaki M, Bady P and Hegi ME:
Ubiquitin specific peptidase 15 (USP15) suppresses glioblastoma
cell growth via stabilization of HECTD1 E3 ligase attenuating WNT
pathway activity. Oncotarget. 8:110490–110502. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baker RT, Wang XW, Woollatt E, White JA
and Sutherland GR: Identification, functional characterization, and
chromosomal localization of USP15, a novel human ubiquitin-specific
protease related to the UNP oncoprotein, and a systematic
nomenclature for human ubiquitin-specific proteases. Genomics.
59:264–274. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilkinson KD: Regulation of
ubiquitin-dependent processes by deubiquitinating enzymes. FASEBJ.
11:1245–1256. 1997. View Article : Google Scholar
|
|
7
|
Chung CH and Baek SH: Deubiquitinating
enzymes: Their diversity and emerging roles. Biochem Biophys Res
Commun. 266:633–640. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu LQ, Ilaria R Jr, Kingsley PD, Iwama A,
van Etten RA, Palis J and Zhang DE: A novel ubiquitin-specific
protease, UBP43, cloned from leukemia fusion protein
AML1-ETO-expressing mice, functions in hematopoietic cell
differentiation. Mol Cell Biol. 19:3029–3038. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Malakhov MP, Malakhova OA, Kim KI, Ritchie
KJ and Zhang DE: UBP43 (USP18) specifically removes ISG 15 from
conjugated proteins. J Biol Chem. 277:9976–9981. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Honke N, Shaabani N, Zhang DE, Hardt C and
Lang KS: Multiple functions of USP18. Cell Death Dis. 7:e24442016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Friedrich SK, Schmitz R, Bergerhausen M,
Lang J, Cham LB, Duhan V, Häussinger D, Hardt C, Addo M, Prinz M,
et al: Usp18 expression in CD169+ macrophages is
important for strong immune response after vaccination with
VSV-EBOV. Vaccines (Basel). 8:1422020. View Article : Google Scholar
|
|
12
|
Liu X, Li H, Zhong B, Blonska M,
Gorjestani S, Yan M, Tian Q, Zhang DE, Lin X and Dong C: USP18
inhibits NF-κB and NFAT activation during Th17 differentiation by
deubiquitinating the TAK1-TAB1 complex. J Exp Med. 210:1575–1590.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dziamałek-Macioszczyk P, Haraźna J and
Stompór T: Versatility of USP18 in physiology and pathophysiology.
Acta Biochim Pol. 66:389–392. 2019.PubMed/NCBI
|
|
14
|
Cai X, Feng S, Zhang J, Qiu W, Qian M and
Wang Y: USP18 deubiquitinates and stabilizes Twist1 to promote
epithelial-mesenchymal transition in glioblastoma cells. Am J
Cancer Res. 10:1156–1169. 2020.PubMed/NCBI
|
|
15
|
Tong HV, Hoan NX, Binh MT, Quyen DT, Meyer
CG, Hang DT, Hang DT, Son HA, Van Luong H, Thuan ND, et al:
Upregulation of Enzymes involved in ISGylation and Ubiquitination
in patients with hepatocellular carcinoma. Int J Med Sci.
17:347–353. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim YH, Kim WT, Jeong P, Ha YS, Kang HW,
Yun SJ, Moon SK, Choi YH, Kim IY and Kim WJ: Novel combination
markers for predicting survival in patients with muscle invasive
bladder cancer: USP18 and DGCR2. J Korean Med Sci. 29:351–356.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tan Y, Zhou G, Wang X, Chen W and Gao H:
USP18 promotes breast cancer growth by upregulating EGFR and
activating the AKT/Skp2 pathway. Int J Oncol. 53:371–383.
2018.PubMed/NCBI
|
|
18
|
Burkart C, Arimoto K, Tang T, Cong X, Xiao
N, Liu YC, Kotenko SV, Ellies LG and Zhang DE: Usp18 deficient
mammary epithelial cells create an antitumour environment driven by
hypersensitivity to IFN-λ and elevated secretion of Cxcl10. EMBO
Mol Med. 5:1035–1050. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Potu H, Sgorbissa A and Brancolini C:
Identification of USP18 as an important regulator of the
susceptibility to IFN-alpha and drug-induced apoptosis. Cancer Res.
70:655–665. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hong B, Li H, Lu Y, Zhang M, Zheng Y, Qian
J and Yi Q: USP18 is crucial for IFN-γ-mediated inhibition of B16
melanoma tumorigenesis and antitumor immunity. Mol Cancer.
13:1322014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu S, Shang H, Cui L, Zhang Z, Zhang Y, Li
Y, Wu J, Li RK and Xie J: Targeted blockade of interleukin-8
abrogates its promotion of cervical cancer growth and metastasis.
Mol Cell Biochem. 375:69–79. 2013.PubMed/NCBI
|
|
22
|
François-Newton V, Magno de Freitas
Almeida G, Payelle-Brogard B, Monneron D, Pichard-Garcia L, Piehler
J, Pellegrini S and Uzé G: USP18-Based negative feedback control is
induced by type I and type III interferons and specifically
inactivates interferon a response. PLoS One. 6:e222002011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan W, Xie S, Zhao X, Li N, Chang C, Li L,
Yu G, Chi X, Pan Y, Niu J, et al: IFN-λ4 desensitizes the response
to IFN-α treatment in chronic hepatitis C through long-term
induction of USP18. J Gen Virol. 97:2210–2220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dagenais-Lussier X, Loucif H, Cadorel H,
Blumberger J, Isnard S, Bego MG, Cohen ÉA, Routy JP and van
Grevenynghe J; Montreal Primary Infection Study Group, : USP18 is a
significant driver of memory CD4 T-cell reduced viability caused by
type I IFN signaling during primary HIV-1 infection. PLoS Pathog.
15:e10080602019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang JA and Jeon YJ: Emerging roles of
USP18: From biology to pathophysiology. Int J Mol Sci. 21:68252020.
View Article : Google Scholar
|
|
26
|
Chinyengetere F, Sekula DJ, Lu Y, Giustini
AJ, Sanglikar A, Kawakami M, Ma T, Burkett SS, Eisenberg BL, Wells
WA, et al: Mice null for the deubiquitinase USP18 spontaneously
develop leiomyosarcomas. BMC Cancer. 15:8862015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Diao W, Guo Q, Zhu C, Song Y, Feng H, Cao
Y, Du M and Chen H: USP18 promotes cell proliferation and
suppressed apoptosis in cervical cancer cells via activating AKT
signaling pathway. BMC Cancer. 20:7412020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mustachio LM, Kawakami M, Lu Y,
Rodriguez-Canales J, Mino B, Behrens C, Wistuba I, Bota-Rabassedas
N, Yu J, Lee JJ, et al: The ISG15-specific protease USP18 regulates
stability of PTEN. Oncotarget. 8:3–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gu T, Lu L, An C, Zhang Y, Wu X, Xu Q and
Chen G: Negative regulation of the RLR-mediated IFN signaling
pathway by duck ubiquitin-specific protease 18 (USP18). J Cell
Physiol. 234:3995–4004. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoon S and Seger R: The extracellular
signal-regulated kinase: Multiple substrates regulate diverse
cellular functions. Growth Factors. 24:21–44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bang YJ, Kwon JH, Kang SH, Kim JW and Yang
YC: Increased MAPK activity and MKP-1 overexpression in human
gastric adenocarcinoma. Biochem Biophys Res Commun. 250:43–47.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang Q, Wu J, Zheng F, Hann SS and Chen Y:
Emodin increases expression of insulin-like growth factor binding
protein 1 through activation of MEK/ERK/AMPKα and interaction of
PPAR γ and Sp1 in lung cancer. Cell Physiol Biochem. 41:339–357.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y and
Hu LL: ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med.
193:1997–2007. 2020.
|
|
34
|
Lin CW, Shen SC, Chien CC, Yang LY, Shia
LT and Chen YC: 12-O-tetradecanoylphorbol-13-acetate-induced
invasion/migration of glioblastoma cells through activating
PKCalpha/ERK/NF-kappaB-dependent MMP-9 expression. J Cell Physiol.
225:472–481. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang T, Xu F, Sheng Y, Zhang W and Chen Y:
A targeted proteomics approach to the quantitative analysis of
ERK/Bcl-2-mediated anti-apoptosis and multi-drug resistance in
breast cancer. Anal Bioanal Chem. 408:7491–7503. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mu X, Shi W, Xu Y, Xu C, Zhao T, Geng B,
Yang J, Pan J, Hu S, Zhang C, et al: Tumor-derived lactate induces
M2 macrophage polarization via the activation of the ERK/STAT3
signaling pathway in breast cancer. Cell Cycle. 17:428–438. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miyake H, Hanada N, Nakamura H, Kagawa S,
Fujiwara T, Hara I, Eto H, Gohji K, Arakawa S, Kamidono S and Saya
H: Overexpression of Bcl-2 in bladder cancer cells inhibits
apoptosis induced by cisplatin and adenoviral-mediated p53 gene
transfer. Oncogene. 16:933–943. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Luo J, Yan R, He X and He J: Constitutive
activation of STAT3 and cyclin D1 overexpression contribute to
proliferation, migration and invasion in gastric cancer cells. Am J
Transl Res. 9:5671–5677. 2107.
|
|
39
|
Ragkousi K and Gibson MC: Epithelial
integrity and cell division: Concerted cell cycle control. Cell
Cycle. 17:399–400. 2018. View Article : Google Scholar : PubMed/NCBI
|