Introduction
Chondrosarcomas are heterogeneous, mostly
slow-growing, primary malignancies of the bone that are
characterized by hyaline cartilaginous neoplastic tissue formation
(1,2). Surgery remains the gold standard,
although macroscopically complete resection is often impeded by the
presence of adjacent critical structures. Therefore, adjuvant or
definitive radiotherapy is frequently recommended for inoperable
cases or following incomplete resection (3–8).
Particle radiotherapy with protons and carbon ions
has emerged as a novel therapy option and has been demonstrated to
be beneficial in the treatment of chondrosarcomas. The physical and
biological characteristics of particles provide the possibility to
deliver higher doses to larger target volumes while adhering to the
tolerance doses of normal tissue structures surrounding the tumor
(9–11). However, these complex technologies
are only available at a limited number of particle radiation
centers worldwide; thus, patient access is highly restricted.
Therefore, it is crucial to optimize widely available photon
therapy treatment despite lower therapeutic success. Specific focus
should be drawn to combinatorial approaches with
chemotherapeutics.
Previous studies have demonstrated a synergistic
effect of the proteasome inhibitor bortezomib and ionizing
radiation (IR) on the viability of various types of cancer,
including oral (12), cervical
(13) and colorectal (14) cancer, as well as esophageal squamous
cell carcinoma (15), and suggested
a radiosensitizing effect of bortezomib. The proteasome pathway
serves an essential role in the regulation of a variety of cellular
processes associated with the proliferation and survival of tumor
cells (12–15). Our previous study demonstrated that
bortezomib exhibits antitumor activity against chondrosarcoma cells
(16).
Cell-based assays are the key tool to assess the
potential efficacy of new treatment options in cancer research.
Cellular heterogeneity, morphology and the interactions of cells
grown in three-dimensional (3D) cultures are similar to the in
vivo microenvironment (17). 3D
spheroids are formed from cells in a variety of stages, including
proliferating, quiescent, hypoxic, apoptotic and necrotic cells
(18). The outer spheroid layer,
which is exposed the most to the medium, is mostly comprised of
proliferating cells (19). Thus, the
cellular processes of these cells mimic those occurring in
vivo (20). Therefore, the
present study used the 3D spheroid model for the experiments.
This aim of the present study was to determine the
potential radiosensitizing effects of concomitant bortezomib
treatment in human chondrosarcoma 3D spheroid cultures and to
elucidate the underlying cellular processes.
Materials and methods
3D spheroid cultures
Chondrosarcoma SW1353 cells (ATCC®
HTB-94™; LGC Standards, Ltd.) were cultured in Dulbecco's modified
Eagle's medium (DMEM-F12) supplemented with 10% fetal bovine serum
(FBS), 1% L-glutamine, 1% penicillin/streptomycin and 0.25 µg
amphotericin B (all Gibco; Thermo Fisher Scientific, Inc.). Human
healthy chondrocytes (HCs) (cat. no. 402-05a; Cell Applications,
Inc.) were cultured in DMEM-F12 supplemented with 10% FBS, 1%
L-glutamine, 1% penicillin/streptomycin, 0.25 µg amphotericin B,
TGFβ3 and FGFβ (both 1 ng/ml; Sigma-Aldrich; Merck KGaA). The
SW1353 cell line was verified by short tandem repeat analysis
utilizing a PowerPlex™ 16 System kit (Promega Corporation). The
primary chondrocytes were used between passages 2 and 4. Regular
testing for mycoplasma infection was conducted by PCR. Cells were
incubated at 37°C in a humidified atmosphere with 5% CO2
and passaged by trypsinization upon reaching confluence. 3D
spheroids were generated by the hanging drop method. A total of
5,000 cells/spheroid mixed with 1.2% Methocel A4M (Sigma-Aldrich;
Merck KGaA) dissolved in the culture medium were placed on the
cover of a petri dish and incubated upside-down for 48 h at 37°C in
a humidified atmosphere with 5% CO2. Subsequently, the
spheroids were transferred to appropriate culture plates. To avoid
adhesion of the spheroid cultures, 1% poly(2-hydroxyethyl
methacrylate)-coated plates were used.
Hematoxylin and eosin stain
For histological and immunohistochemical evaluation,
the chondrosarcoma 3D spheroids were fixed for 30 min with 10%
formalin (Carl Roth GmbH) at room temperature and embedded in
paraffin. For morphological evaluation, staining with hematoxylin
and eosin was performed. Briefly, following a 15-min incubation in
xylene (Merck KGaA) and a descending ethanol series (100, 90, 70
and 50%; 3 min each), the sections were stained with hematoxylin
and eosin for 2 min each at room temperature. The embedding of the
samples was performed with n-buthyl-acetate and
Entellan® (both Merck KGaA).
X-ray irradiation
X-ray irradiation was performed at the Division of
Biomedical Research, Medical University Graz (Graz, Austria) with
an RS-2000 biological irradiator (RadSource Technologies, Inc.)
equipped with a 3-mm Al/0.3-mm Cu filter and a current of 25 mA to
provide 160 kV X-rays at a dose rate of 2.108 Gy/min.
Cell viability assay
For the dose-response relationship, 100 spheroids
each of HC and SW1353 were pretreated with the IC50
concentration of 5 nM bortezomib (Selleck Chemicals) for 24 h. Cell
viability was determined with the CellTiter-Glo® 3D Cell
Viability assay (Promega Corporation) at 3 and 48 h
post-administration of 0 (control) to 20 Gy IR. Background
reference values were derived from the culture media. Absorbance
was measured with a LUMIstar™ microplate luminometer (BMG Labtech
GmbH), and the mean ± SD was calculated (HC, n=3; SW1353, n=7;
performed in biological quadruplicates).
Cell cycle analysis
Following pretreatment with 5 nM bortezomib and
treatment with 0, 5 or 20 Gy IR, spheroid cultures were harvested
at 3 and 48 h. A single-cell suspension was prepared by
trypsinization, and cells were fixed with 70% ice-cold ethanol for
10 min at 4°C. Prior to flow cytometry, the cell pellet was
resuspended in PI-staining buffer (50 µl/ml PI, 100 µl/ml RNAse A,
0.1% Triton X-100 and 0.1% sodium citrate) and incubated for 20 min
at room temperature. Cell cycle distribution was measured with a
CytoFlexLX flow cytometer (Beckman Coulter, Inc.) and analyzed
using ModFit LT software Version 4.1.7 (Verity software house).
Annexin V/PI apoptosis assay
To detect translocated phosphatidylserine, the
eBioscience™ FITC Annexin V Apoptosis Detection kit (Thermo Fisher
Scientific, Inc.) was used 48 h after treatment with IR and/or
bortezomib according to the manufacturer's instructions. Flow
cytometry analysis was performed with a CytoFlexLX flow cytometer,
and a total of 10,000 events were collected. Cells were identified
in the side and forward scatter with a linear scale. Fluorescence
signals were presented with a logarithmic scale. Compensation was
performed by single Annexin and PI measurements, and analyzed by
FCS3 express software Version 7 (De Novo Software, LLC). The
graphical representations of Annexin−/PI−
(viable cells), Annexin+/PI− (early apoptotic
cells), Annexin+/PI+ (late apoptotic cells)
and Annexin−/PI+ (necrotic cells) were
plotted as stacked bars (n=3).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was isolated following bortezomib
pretreatment and 48 h post-5 Gy IR from spheroid cultures using the
RNeasy Mini kit (Qiagen GmbH) and DNase-I treatment according to
the manufacturer's instructions. A total of 1 µg RNA was
reverse-transcribed with an iScript cDNA Synthesis kit, (Bio-Rad
Laboratories, Inc.) using a blend of oligo(dT) and random hexamer
primers. Amplification was performed with the SsoAdvanced Universal
SYBR®-Green Supermix (Bio-Rad Laboratories, Inc.) and
measured by the CFX96 Touch PCR instrument (BioRad Laboratories,
Inc.). Each qPCR run comprised a standard three-step PCR
temperature protocol (95°C for 30 sec, followed by 40 cycles of
95°C for 10 sec and 60°C for 20 sec), followed by a melting curve
protocol to confirm a single gene-specific peak and to detect
primer dimerization (65–95°C). Relative quantification of
expression levels was performed using the ΔΔCq method based on the
geometric mean of the internal controls TATA-box binding protein
and ribosomal protein lateral stalk subunit P0. The expression
levels (Cq) of the target genes were normalized to the reference
genes ribosomal protein (RPL; cat. no. QT00075012), TATA
box-binding protein (TBP; cat. no. QT00000721), and
beta-2-microglobulin (B2M; cat. no. QT00088935) (all Qiagen GmbH)
(ΔCq), and the difference between the ΔCq value of the test and
control samples produced the ΔΔCq value. Finally, the expression
ratio was expressed as 2−ΔΔCq (21). The following QuantiTect primer assays
(Qiagen GmbH) were used for qPCR: Matrix metalloproteinases (MMP)2
(cat. no. QT00088396), MMP9 (cat. no. QT00040040), Bax (cat. no.
QT00997381), Bcl-2 antagonist/killer-1 (Bak) (cat. no. QT00228508),
Noxa (cat. no. QT00074438), and survivin (BIRC5) (cat. no.
QT00081186). The following primers were used: Bcl-2 forward,
5′-GGAGGATTGTGGCCTTCTTTG-3′ and reverse,
5′-GCCGGTTCAGGTACTCAGTCAT-3′; and TNF-related apoptosis-inducing
ligand receptor 2 (TRAIL-R2) forward, 5′-ACCAACGAGCTGAAGCAGATG-3′
and reverse, 5′-CAAGCAATGCCACTTTTGGA-3′. Untreated cells served as
the reference.
Western blot analysis
Whole cell protein extracts were prepared 48 h
post-bortezomib pretreatment and 5 Gy IR from spheroid cultures
using a lysis buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM
NaF, 1 mM ethylenediaminetetraacetic acid, 1% nonylphenol-40 and 1
mM Na3VO4], a protease and phosphatase
inhibitor cocktail (PPC1010; Sigma-Aldrich; Merck KGaA) and
subsequent sonication. Protein concentration was determined with
the Pierce BCA Protein Assay kit (Thermo Fisher Scientific, Inc.).
A total of 10–20 µg of proteins were separated by 12% sodium
dodecyl sulfate polyacrylamide gel electrophoresis and blotted on
Amersham™ Protran™ Premium 0.45 µM nitrocellulose membranes
(Cytiva). For microtubule-associated protein 1A/1B-light chain 3
(LC3)BI–II immunoblotting, the cells were treated with the
lysosomal protease inhibitors E64d and pepstatin A (10 µg/ml; both
Sigma-Aldrich; Merck KGaA), which were added to the cell culture
medium to inhibit the autophagic flux and the degradation of
LC3B-II, and improve the representation of the LC3B-II levels. All
other blots were incubated with primary antibodies against the
apoptotic markers Bax, Bcl-2, Noxa, TRAIL-R2, cleaved
poly(ADP-ribose) polymerase (PARP), survivin and its downstream
components heat shock protein 90 (HSP90), X-linked inhibitor of
apoptosis protein (XIAP), smad 2 and smad 3, the DNA damage marker
phospho-histone γH2AX and β-actin as the loading control. All
antibody information, dilutions and incubation times are listed in
Table I. Subsequently, the blots
were incubated with a horseradish peroxidase-conjugated secondary
antibody (Dako; Agilent Technologies, Inc.) for 1 h at room
temperature, followed by overlay with Amersham™ ECL™ prime western
blotting detection reagent (Cytiva). The resulting
chemiluminescence signals were detected with the ChemiDocTouch
Imaging System (Bio-Rad Laboratories, Inc.), and the images were
processed with ImageLab 5.2 software (Bio-Rad Laboratories,
Inc.).
 | Table I.Antibodies used for the western blot
analysis. |
Table I.
Antibodies used for the western blot
analysis.
|
| Primary
antibody | Secondary
antibody |
|---|
|
|
|
|
|---|
| Target | Supplier and cat.
no. | Dil. | Inc. | Target | Supplier and cat.
no. | Dil. | Inc. |
|---|
| Bax | CS #5023 | 1:1,000 | ON 4°C | Anti-rabbit | Dako P0448 | 1:3,000 | 1 h 4°C |
| Bcl-2 | CS #3498 | 1:1,000 | ON 4°C | Anti-rabbit | Dako P0448 | 1:3,000 | 1 h 4°C |
| Noxa | Thermo 1-41000 | 1:1,000 | ON 4°C | Anti-mouse | Dako P0447 | 1:3,000 | 1 h 4°C |
| TRAIL-R2 | CS #3696 | 1:1,000 | ON 4°C | Anti-rabbit | Dako P0448 | 1:3,000 | 1 h 4°C |
| cPARP | Thermo 436400 | 1:2000 | ON 4°C | Anti-mouse | Dako P0447 | 1:3,000 | 1 h 4°C |
| LC3BI–II | CS #2775 | 1:1,000 | ON 4°C | Anti-rabbit | Dako P0448 | 1:3,000 | 1 h 4°C |
| Survivin | CS #2802 | 1:1,000 | ON 4°C | Anti-mouse | Dako P0447 | 1:3,000 | 1 h 4°C |
| HSP90 | CS #4877 | 1:3,000 | ON 4°C | Anti-rabbit | Dako P0448 | 1:3,000 | 1 h 4°C |
| XIAP | CS #2045 | 1:3,000 | ON 4°C | Anti-rabbit | Dako P0448 | 1:3,000 | 1 h 4°C |
| Smad2 | sc-101153 | 1:1,000 | ON 4°C | Anti-mouse | Dako P0447 | 1:3,000 | 1 h 4°C |
| Smad3 | sc-101154 | 1:1,000 | ON 4°C | Anti-mouse | Dako P0447 | 1:3,000 | 1 h 4°C |
| γH2AX | CS #2577 | 1:1,000 | ON 4°C | Anti-rabbit | Dako P0448 | 1:3,000 | 1 h 4°C |
| β-actin | sc-47778 | 1:10,000 | ON 4°C | Anti-mouse | Dako P0447 | 1:15,000 | 1 h 4°C |
Gene expression profiling
Gene expression profiling was performed using the
Thermo Fisher Ion AmpliSeq RNA workflow. cDNA of 50 ng RNA from
chondrosarcoma spheroid cultures was synthesized using the
SuperScript IV™ Vilo™ Master Mix (cat. no. 11756500; Thermo Fisher
Scientific, Inc.; 25°C for 10 min, 50°C for 10 min, 85°C for 5 min
and hold at 10°C). AmpliSeq PCR and library preparation was
performed using the Ion AmpliSeq Library Kit Plus and an Ion
AmpliSeq RNA Panel (thermocycling conditions: 99°C for 2 min,
followed by 27 cycles of 99°C for 15 sec and 60°C for 4 min, and
hold at 10°C). Sequencing was performed on an Ion S5XL benchtop
sequencer using the 540 Chip kit and the 200 base pair workflow
(single-end; all Thermo Fisher Scientific, Inc.) to a total depth
of ~1 million reads per sample. The amplicon sequencing depth was
determined using the AmpliSeq RNA Ion Torrent Suite Plugin (version
4.4.0.4), and individual gene expression was calculated as amplicon
reads per million total reads (RPM). The heatmap of the RNA
sequencing data is presented as fold-change regarding the
expression without IR (control) and 1 or 24 h post-IR of key player
genes in terms of base excision repair (BER), mismatch mediated
repair (MMR), nucleotide excision repair (NER), homology directed
repair (HDR) and the nonhomologous end-joining (NHEJ). The
sequencing coverage and the quality statistics of the AmpliSeq RNA
sequencing are presented in Table
SI. The corresponding raw data are presented in Table SII.
Immunocytochemistry
For immunocytochemical staining, chondrosarcoma
cells were pretreated with the IC50 concentration of 5
nM bortezomib for 24 h and irradiated with 0 (control) and 5 Gy. At
1 and 24 h post-IR, the cells were fixed with 4% paraformaldehyde
for 30 min. The slides were incubated with a γH2AX antibody (Merck
KGaA; cat. no. 05-636; 1:200) for 1 h, the bridge antibody (Dako;
cat. no. P0260; 1:200) for 30 min, the polymer
(rabbit-ON-rodent-horseradish peroxidase; Biocare Medical, LLC;
RMR622H; ready to use) for 30 min and aminoethyl carbazole
substrate chromogen (Dako; Agilent Technologies, Inc.) for 3 min
(all room temperature). The reaction was stopped with
phosphate-buffered saline, and hematoxylin core staining was
performed. Images with ×100 magnification were captured under an
Olympus BX51 transmitted-light microscope (Olympus Corporation) and
five areas were analyzed per slide.
Statistical analysis
Data are presented as the mean ± SD or SEM as
indicated. SigmaPlot 14.0 (Systat Software, Inc.) was used to
generate the figures and graphs. Comparisons between untreated
control, bortezomib- and IR-treated cells were performed by one way
ANOVA followed by post hoc analysis using the Holm-Sidak test.
Two-sided P<0.05 was considered to indicate a statistically
significant difference.
Results
Increasing IR intensities affect
spheroid cultures
The preparation of 3D spheroid cultures using the
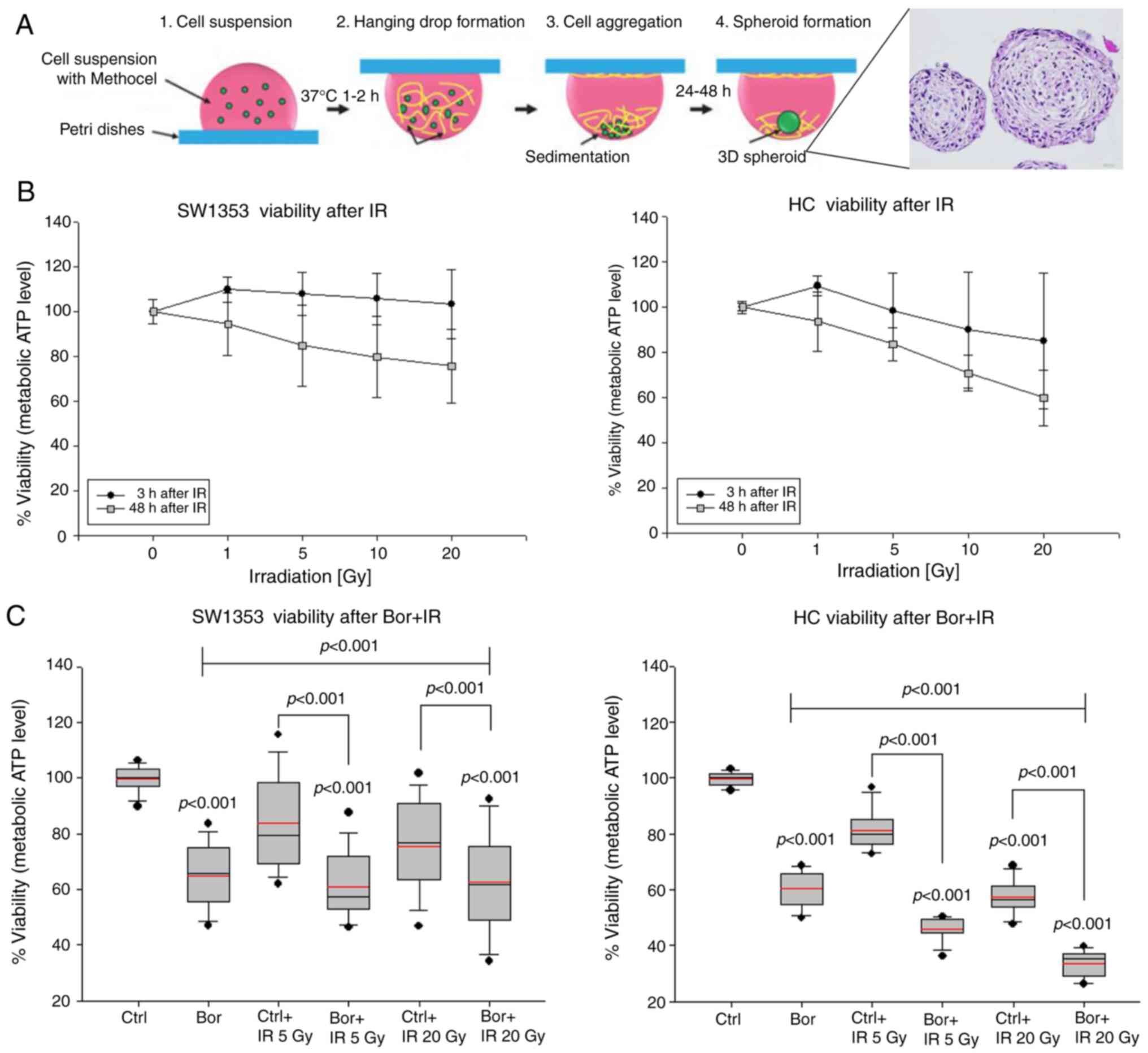
hanging drop method is schematically presented in Fig. 1A. Chondrosarcoma cells (SW1353) and
HCs formed clearly defined spheroids after 24–48 h.
Increasing IR doses only marginally reduced the
viability of SW1353 3D spheroids at 3 h; the reduction in HC
viability appeared to be more pronounced compared with that
observed in chondrosarcoma cells (Fig.
1B). Additionally, this downward trend of viability appeared
also to be more pronounced in HC at 48 h compared with that in the
chondrosarcoma cells. Treatment with the IC50
concentration of bortezomib significantly reduced viability in the
two cell types (Fig. 1C). Treatment
with the respective IC50 concentrations (16) reduced the cell viability to
65.04±11.1% for SW1353 and to 60.37±6.10% for HC. Additional 5 or
20 Gy IR caused a further significant reduction to 58.61±12.24% for
SW1353 and 33.77±4.56% for HC, respectively. Therefore, HC
exhibited higher sensitivity for both treatments compared with that
of the chondrosarcoma cells.
Effects of IR on cell cycle
distribution
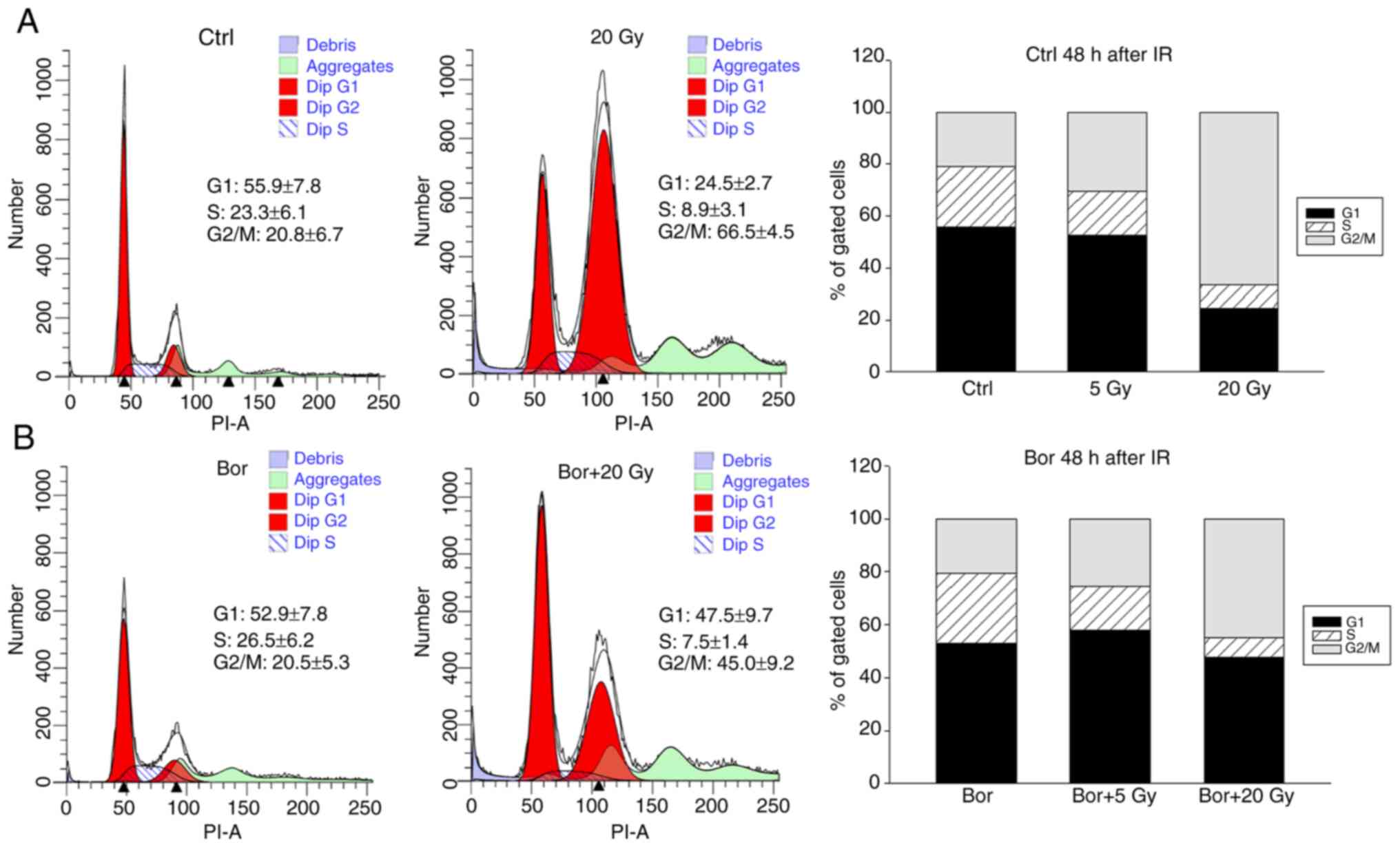
Flow cytometry analysis was performed to determine
the cell cycle profiles of chondrosarcoma spheroid cultures exposed
to 5 and 20 Gy IR with or without their respective IC50
concentrations of bortezomib for 48 h (16). Untreated cells were used as controls.
The percentages and the corresponding significance levels of cells
in various cell cycle phases are listed in Table II. Representative flow cytometry
plots of untreated chondrosarcoma cells and those irradiated with
20 Gy are presented in Fig. 2A, and
plots of the corresponding bortezomib-treated groups are presented
in Fig. 2B. The graphical
representations of the G0/G1, S and
G2/M values are shown in stacked bars. High-dose IR
induced a significant increase in the number of cells in the
G2/M phase compared with that in the control group,
which was accompanied by a decrease in the number of G1
and S phase cells, indicating a persisting G2/M phase
arrest at 48 h post-IR. Notably, a co-treatment with bortezomib
reduced the G2/M phase arrest.
 | Table II.Cell cycle distribution of
chondrosarcoma cells 48 h after 5 and 20 Gy IR. |
Table II.
Cell cycle distribution of
chondrosarcoma cells 48 h after 5 and 20 Gy IR.
|
| Cell cycle
distribution at 48 h, % |
|---|
|
|
|
|---|
| Group | G1 | S |
G2/M |
|---|
| Ctrl | 55.9±7.8 | 23.6±6.1 | 20.8±6.7 |
| Ctrl + 5 Gy IR | 52.5±13.5 | 17.1±5.8 | 30.4±9.1 |
| Ctrl + 20 Gy
IR |
24.5±2.7a |
8.9±3.1a |
66.5±4.5a |
| Bor | 52.9±7.8 | 26.5±6.2 | 20.5±5.3 |
| Bor + 5 Gy IR | 57.9±6.6 |
16.6±4.6b | 25.5±10.1 |
| Bor + 20 Gy IR |
47.5±9.7c |
7.5±1.4a |
45.0±9.2a,d |
Induction of apoptosis by bortezomib
and IR treatment
In order to investigate a possible sensitization to
IR of chondrosarcoma cells by pretreatment with bortezomib, RT-qPCR
was performed for the detection of the relative expression levels
of the proapoptotic markers Bax and Bak, the antiapoptotic marker
Bcl-2, the proapoptotic antagonist Noxa and the death receptor
TRAIL-R2 (Fig. 3A). The mRNA levels
of the proapoptotic genes Bax and Bak were significantly increased
by bortezomib treatment compared with those in the control group.
IR achieved higher or similar levels of Bax and Bak, respectively,
compared with those in bortezomib-treated cells. Notably,
co-treatment with bortezomib and IR decreased the Bax and Bak mRNA
levels compared with those in cells treated with IR alone. By
contrast, bortezomib reduced the Bcl-2 and Noxa mRNA levels in
non-irradiated cells, whereas in IR-treated cells, bortezomib
significantly enhanced the 5 Gy-induced effect. In addition, IR
increased the protein expression of TRAIL-R2 by 2-fold (Fig. 3B). Annexin V/PI flow cytometric
measurements revealed only a limited increase in the
Annexin+/PI− cells between the untreated and
the co-treatment groups Fig. 3C). In
addition, there was no significant increase in early or late
apoptotic cells between the control and Bor + 20 Gy groups. The
lack of apoptotic induction was further verified with a
PARP/cleaved PARP immunoblot (Fig.
3D).
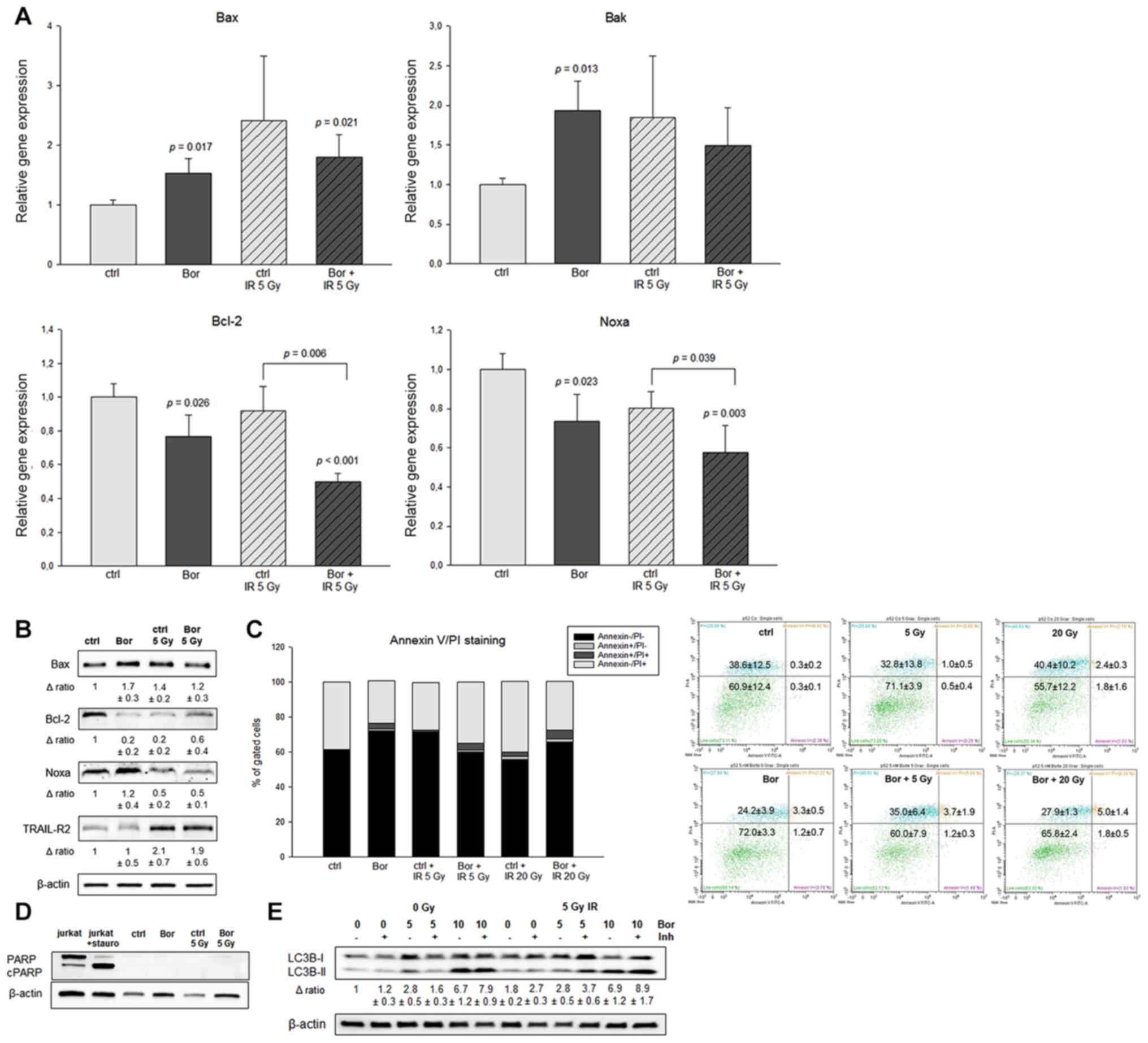 | Figure 3.Effects of bortezomib and IR on
apoptotic induction. (A) mRNA expression analysis of pro- and
antiapoptotic markers was performed at 48 h post-IR. The expression
levels of the proapoptotic markers Bax and Bak were significantly
increased by bortezomib treatment compared with those in the
control group, and further enhanced by co-treatment with IR. In
addition, bortezomib reduced the mRNA expression levels of Bcl-2
and Noxa compared with those in the control group. IR increased the
relative expression levels of TRAIL-R2 (n=5, mean ± SEM). (B)
Western blot analyses were used to verify the results at the
protein level. β-actin was used as loading control (n=3). (C)
Annexin V/PI flow cytometric analysis at 48 h identified no
significant changes following treatment with bortezomib or IR
(n=3). Representative measurements of the control and bortezomib +
20 Gy groups are presented. (D) The PARP/cleaved PARP
immunoblotting revealed no apoptotic induction. (E) Western blot
analysis of the autophagy marker LC3BI–II in SW1353 cells treated
with 5 and 10 nM bortezomib for 24 h. Lysosomal protease inhibitors
blocked the autophagic flux and inhibited the degradation of
LC3B-II. Additional IR induced no changes. β-actin was used as the
loading control (n=2). IR, ionizing radiation; Ctrl, control; Bor,
bortezomib; PARP, poly (ADP-ribose) polymerase; TRAIL-R2,
TNF-related apoptosis-inducing ligand receptor 2; LC3,
microtubule-associated protein 1A/1B-light chain 3; Inh, E64d and
pepstatin A. |
To determine the potential interplay between
apoptosis and autophagy, immunoblotting of LC3B was performed. As
demonstrated in Fig. 3E, increasing
concentrations of bortezomib significantly increased the expression
levels of LC3B-II compared with those in the untreated cells. The
lysosomal protease inhibitors E64d and pepstatin A inhibited the
degradation of LC3B-II. IR, however, did not increase the
expression of LC3B-II.
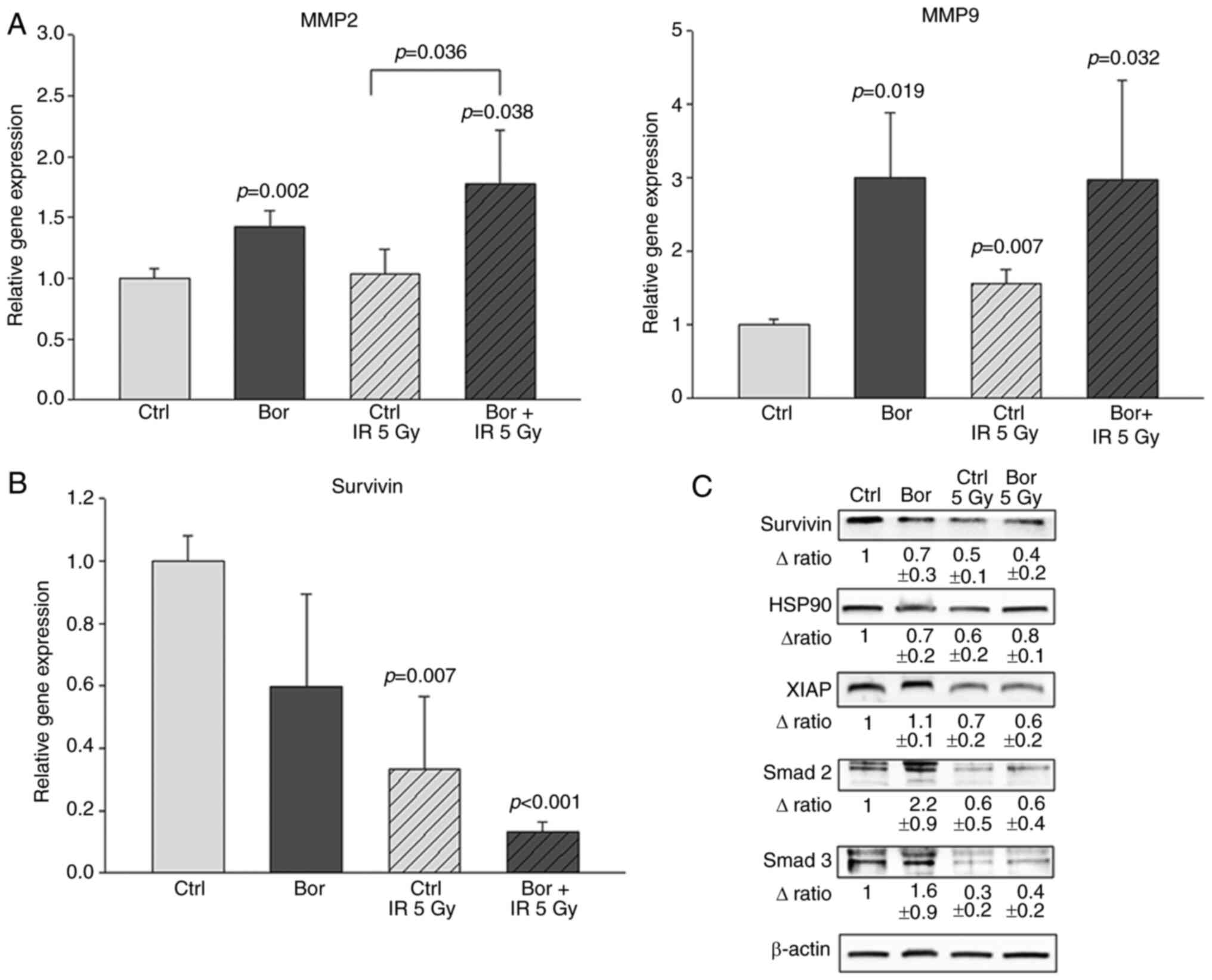
Expression of MMPs following
bortezomib and IR treatment
Chondrosarcoma spheroid cultures were irradiated
with 5 Gy following pretreatment with bortezomib, and total RNA was
isolated and analyzed by qPCR. The results revealed that treatment
with bortezomib or IR alone and the combined treatment
significantly increased the expression levels of MMP2 and MMP9
compared with those in the control cells (Fig. 4A).
Synergistic effects of bortezomib and
IR on the survivin signaling pathway
Following bortezomib treatment and IR, RNA was
isolated from the cells, and the relative expression of the BIRC5
gene was determined using RT-qPCR. The expression levels of BIRC5
mRNA were significantly decreased by both bortezomib and IR
treatment compared with those in the control group. In addition,
co-treatment with bortezomib and IR resulted in an additive
reduction (Fig. 4B). Western blot
analyses of survivin and its downstream proteins were used to
verify these results at the protein level (Fig. 4C). Compared with those in the control
cells, treatment with bortezomib and IR reduced the protein
expression levels of HSP90 in chondrosarcoma spheroid cultures, and
the expression levels of XIAP, Smad2 and Smad3 were reduced by IR
(Fig. 4C).
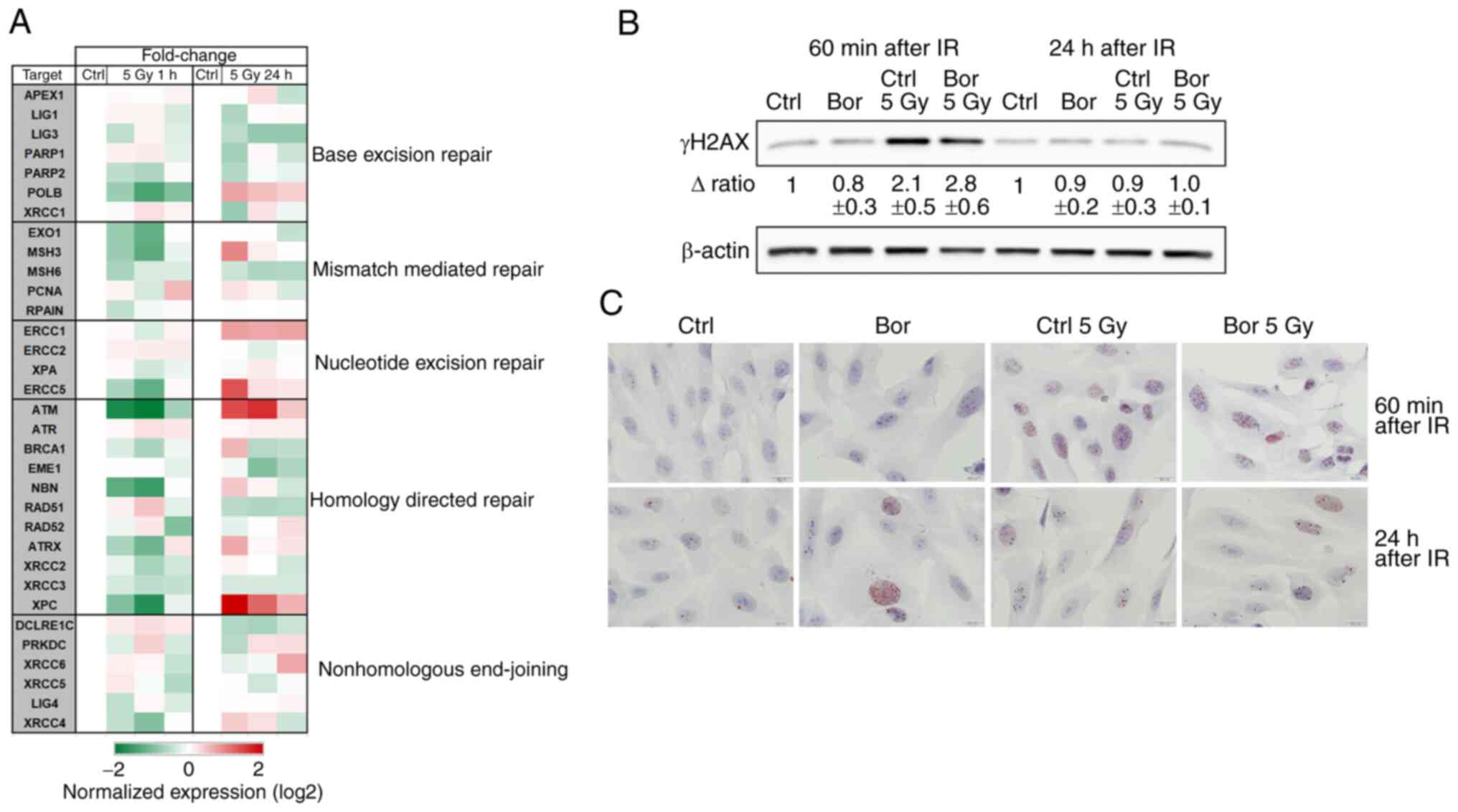
DNA repair mechanisms following
treatment with bortezomib and IR
To determine the potential involvement of bortezomib
in radiation therapy resistance, DNA damage and the corresponding
DNA repair genes were studied. The results demonstrated that at 24
h post-IR, not only the HDR, but also the NER pathway is positively
regulated compared with those in the untreated controls (Fig. 5A). The number of double-strand breaks
was determined by quantifying the levels of γH2AX at 1 and 24 h
post-bortezomib pretreatment and IR (Fig. 5B). Chondrosarcoma cells exhibited a
significant upregulation in γH2AX levels compared with those in the
untreated control cells at 1 h post-IR independent of bortezomib,
whereas at 24 h post-IR, the levels of this indirect marker of DNA
damage returned to the baseline. These observations were confirmed
by immunohistochemical staining (Fig.
5C) and indicated rapid and efficient DNA damage repair in
chondrosarcoma cells.
Discussion
In patients with chondrosarcoma with unfavorable
initial characteristics, a high grade and incomplete resection,
postoperative radiotherapy is an expedient option; it reduces the
risk of local recurrence and improves 5-year disease-free survival
from 64.2 to 71.8% (22).
Chondrosarcomas exhibit variable response to irradiation, possibly
due to their strong genetic heterogeneity, which is also evident in
different chondrosarcoma cell lines (23,24). In
this context, it is particularly important to gain new insights
into the underlying tumor biology of chondrosarcoma. The use of 3D
spheroid cultures represents the in vivo environment more
accurately compared with conventional 2D monolayer cultures; 3D
cultivation mimics the architecture of the donor tissue and enables
both cell-cell and cell-matrix interactions (25). Therefore, the present study utilized
the hanging drop 3D spheroid model. The viability of the two cell
types used in the present study was only moderately affected by
increasing IR intensity. Co-treatment with bortezomib appeared to
be the main trigger for the observed decrease in the viability of
chondrosarcoma cells. During radiotherapy, the co-treatment may
positively affect the therapeutic efficacy of 5 Gy IR.
DNA structure checkpoints are necessary in the
regulation of the cell cycle; they cause cell cycle arrest in
response to incomplete replication or DNA damage. Known as the
‘restriction point’ in human cells, this point is defined as the
moment after which cells are committed to entering and progressing
through the cell cycle independent of signals from the environment
(26). The G2/M phase
delay in tumor cells following IR enables sufficient time for
repair processes and ensures cell survival of otherwise lethal DNA
damage (27). A previous study has
reported that X-ray irradiation induces a G2/M phase
arrest in HeLa cancer cells and is associated with the increase of
the expression levels of checkpoint kinase 1 and the reduction of
those of cyclin-dependent kinase 1 (28,29). The
results of the present study revealed that both untreated and
bortezomib pretreated spheroid cultures exhibited G2/M
accumulation following IR. This replication arrest may enable the
chondrosarcoma cells to regenerate more efficiently. The changes in
cell cycle distribution observed in other cell systems following
bortezomib treatment alone, however, were not be detected in
chondrosarcoma cells in the present study (30,31).
An important indicator of possible damage in tumor
cells is the induction of apoptosis. Bcl-2 family proteins exert
undergo pro- or anti-apoptotic activities and regulate the
mitochondrial apoptosis pathway by controlling outer mitochondrial
membrane permeability (32).
Reacting to a variety of types of damage or stress, certain Bcl-2
family members induce Bax or Bak proapoptotic protein activation at
the mitochondrion (32). Bax and Bak
homo-oligomerize and function in the outer mitochondrial membrane
in pore formation, via which proapoptotic molecules escape; this
results in the development of the morphological characteristics of
apoptosis, including DNA laddering, condensed nuclei and
phosphatidylserine exposure to the outer plasma membrane leaflet
(33). In the present study, in
chondrosarcoma cells, both bortezomib treatment and IR increased
the expression levels of Bax and Bak compared with those in the
control group. Notably, the combination of the two treatments did
not lead to a synergistic enhancement of these effects, whereas the
reduction in the expression levels of the antiapoptotic protein
Bcl-2 was significantly enhanced by the co-treatment with IR and
bortezomib. This was particularly notable at the protein level.
Noxa is an indirect activator of apoptosis, as it only neutralizes
the prosurvival members of the Bcl-2 family Mcl1 and Bcl-2,
preventing them from regulating the levels of Bax and Bak (34). The levels of Noxa were also
significantly decreased in the spheroid cultures by co-treatment
with IR and bortezomib compared with those in the control cells in
the present study. As a tumor necrosis factor receptor superfamily
protein, which is mainly localized in the cell membrane, TRAIL-R2
triggers caspase-8 activation in response to anticancer drug
treatment (35). The irradiation
intensity of 5 Gy increased the protein expression levels of
TRAIL-R2 2-fold compared what that in the controls. Despite the
regulation of these apoptotic genes, only a small increase in the
percentage of late apoptotic cells
(Annexin+/PI−) was observed 48 h after IR
and/or bortezomib treatment.
Autophagy has been discussed in the literature in
recent years as an alternative cell death mechanism to apoptosis
(36,37). During autophagy, the
microtubule-associated protein LC3B is localized on the membranes,
resulting in autophagosome membrane formation, and the level of
LC3B-II is a marker for autophagosome formation (38). Bortezomib increases the
autophagy-associated protein expression levels, including that of
as autophagy-related 5/12, LC3I–II and Beclin in chondrosarcoma
cells (16). However, an additional
increase in the levels of autophagy-associated proteins by IR was
not observed in the present study.
MMP2 and MMP9 are cancer-associated, secreted,
zinc-dependent endopeptidases, the secretion of which is elevated
in several types of human cancer such as liposarcoma, cervical,
pancreatic or prostate cancer; their elevated expression has been
associated with a poor prognosis (39). In the present study, bortezomib
treatment significantly increased the expression levels of MMP2 and
MMP9 compared with those in the untreated control cells, whereas
irradiation exerted limited effects.
The results of the present study demonstrated the
synergistic effects of bortezomib and IR on the mRNA and protein
levels of survivin. Survivin is the smallest member of the
inhibitor of apoptosis protein family (IAPs), and serves vital
roles in the regulation of cell division and the inhibition of
apoptosis via caspase activation blockade (40,41).
Survivin has been highlighted vital for chondrosarcoma survival
using small interfering RNA screening for 51 apoptosis-related
genes in chondrosarcoma cells (42).
The molecular chaperone Hsp90 participates in importing various
client proteins to the mitochondria and has been demonstrated to be
associated with survivin and other IAPs (43). Compared with that in the control
cells, treatment with bortezomib and IR reduced the protein
expression of HSP90 in chondrosarcoma spheroid cultures and thus
may have impeded the survivin pathway. As a potent inhibitor of
apoptosis, XIAP interacts directly with caspases to inhibit them
(44). The reduced expression levels
of survivin and XIAP caused by IR in the present study may lead to
the induction of apoptosis. TGF-β and its signaling effectors smad
2 and smad 3 are key factors in controlling tumor cell
proliferation and differentiation (45), and repress survivin expression at the
transcriptional level (46). Both
smad 2 and smad 3 exhibited a significant reduction in their
protein expression levels following IR compared with those in the
control group. Thus, the inhibition of the entire survivin pathway
was demonstrated, especially after IR, in the present study.
Another important aspect of cancer therapy is the
regeneration behavior of tumor cells after radiotherapy.
Radiation-induced cell death mostly occurs due to DNA damage,
especially due to double-strand breaks (47). The gene expression profiling data in
the present study revealed that, supplementary to the HDR pathway,
as described in the literature for other types of cancer such as
breast or ovarian cancer (48), the
NER pathway, with the key players excision repair
cross-complementation group 1 and 5, is also activated in
chondrosarcoma cells after IR. Although cell viability was
decreased both after treatment with bortezomib and after
irradiation compared with that in untreated cells, increased DNA
repair following treatment was observed in the present study. Since
the levels of γH2AX are associated with DNA double-strand breaks,
it is the most common surrogate marker to study DNA damage
induction and the subsequent repair of the DNA lesion (49). In the present study, comparative
analysis at 1 and 24 h post-IR indicated efficient DNA repair in
human chondrosarcoma cells, returning to baseline levels within 24
h.
In conclusion, the results of the present study
demonstrated that co-treatment with bortezomib improved the
radiation sensitivity of chondrosarcoma cells to a limited extent.
The inhibition of the survivin signaling pathway revealed a novel
interesting aspect in the tumor biology of chondrosarcoma 3D
spheroid cultures and may provide a future option for clinical
exploitation.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The current study was supported by the Austrian
Science Foundation (grant no. P32103-B) and the Ludwig Boltzmann
Institute for Arthritis and Rehabilitation.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BL conceived and supervised the study. DGl, SKG and
NE performed the experiments. BL, DGl, BSF, BR, NE, AL and DGe
analyzed and interpreted the data. DGl, NE and BR confirm the
authenticity of all the raw data. BL, SKG, and DGe drafted and
revised the manuscript. BR, AL and DGe provided technical support.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fletcher CD, Bridge JA, Hogendoorn PC and
Mertens F; World Health Organization, : World Health Organization
Classification of Tumours of Soft Tissue and Bone. 4th edition.
IARC Press; Lyon: pp. 264–274. 2013
|
|
2
|
Chow WA: Chondrosarcoma: biology,
genetics, and epigenetics. F1000Res. 7:F1000 Faculty Rev-1826.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hug EB, Loredo LN, Slater JD, DeVries A,
Grove RI, Schaefer RA, Rosenberg AE and Slater JM: Proton radiation
therapy for chordomas and chondrosarcomas of the skull base. J
Neurosurg. 91:432–439. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hauptman JS, Barkhoudarian G, Safaee M,
Gorgulho A, Tenn S, Agazaryan N, Selch M and De Salles AA:
Challenges in linear accelerator radiotherapy for chordomas and
chondrosarcomas of the skull base: Focus on complications. Int J
Radiat Oncol Biol Phys. 83:542–551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bloch O and Parsa AT: Skull base
chondrosarcoma: Evidence-based treatment paradigms. Neurosurg Clin
N Am. 24:89–96. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Debus J, Schulz-Ertner D, Schad L, Essig
M, Rhein B, Thillmann CO and Wannenmacher M: Stereotactic
fractionated radiotherapy for chordomas and chondrosarcomas of the
skull base. Int J Radiat Oncol Biol Phys. 47:591–596. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Amorim Bernstein K, Liebsch N, Chen YL,
Niemierko A, Schwab JH, Raskin K, Lozano-Calderon SA, Cote G,
Harmon DC, Choy E, et al: Clinical outcomes for patients after
surgery and radiation therapy for mesenchymal chondrosarcomas. J
Surg Oncol. 114:982–986. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Amorim Bernstein K and DeLaney T:
Chordomas and chondrosarcomas-The role of radiation therapy. J Surg
Oncol. 114:564–569. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
DeLaney TF, Liebsch NJ, Pedlow FX, Adams
J, Weyman EA, Yeap BY, Depauw N, Nielsen GP, Harmon DC, Yoon SS, et
al: Long-term results of Phase II study of high dose photon/proton
radiotherapy in the management of spine chordomas, chondrosarcomas,
and other sarcomas. J Surg Oncol. 110:115–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Indelicato DJ, Rotondo RL, Begosh-Mayne D,
Scarborough MT, Gibbs CP, Morris CG and Mendenhall WM: A
prospective outcomes study of proton therapy for chordomas and
chondrosarcomas of the spine. Int J Radiat Oncol Biol Phys.
95:297–303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Holtzman AL, Rotondo RL, Rutenberg MS,
Indelicato DJ, Mercado CE, Rao D, Tavanaiepour D, Morris CG, Louis
D, Flampouri S, et al: Proton therapy for skull-base
chondrosarcoma, a single-institution outcomes study. J Neurooncol.
142:557–563. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu YH, Wu WS, Lin LC, Liu CS, Ho SY, Wang
BJ, Huang BM, Yeh YL, Chiu HW, Yang WL, et al: Bortezomib enhances
radiosensitivity in oral cancer through inducing autophagy-mediated
TRAF6 oncoprotein degradation. J Exp Clin Cancer Res. 37:912018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cui H, Qin Q, Yang M, Zhang H, Liu Z, Yang
Y, Chen X, Zhu H, Wang D, Meng C, et al: Bortezomib enhances the
radiosensitivity of hypoxic cervical cancer cells by inhibiting
HIF-1α expression. Int J Clin Exp Pathol. 8:9032–9041.
2015.PubMed/NCBI
|
|
14
|
Cacan E, Spring AM, Kumari A, Greer SF and
Garnett-Benson C: Combination treatment with sublethal ionizing
radiation and the proteasome inhibitor, bortezomib, enhances
death-receptor mediated apoptosis and anti-tumor immune Attack. Int
J Mol Sci. 16:30405–30421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang D, Qin Q, Jiang QJ and Wang DF:
Bortezomib sensitizes esophageal squamous cancer cells to
radiotherapy by suppressing the expression of HIF-1α and apoptosis
proteins. J XRay Sci Technol. 24:639–646. 2016.PubMed/NCBI
|
|
16
|
Lohberger B, Steinecker-Frohnwieser B,
Stuendl N, Kaltenegger H, Leithner A and Rinner B: The proteasome
inhibitor bortezomib affects chondrosarcoma cells via the
mitochondria-caspase dependent pathway and enhances death receptor
expression and autophagy. PLoS One. 11:e01681932016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ryu NE, Lee SH and Park H: Spheroid
culture system methods and applications for mesenchymal stem cells.
Cells. 8:E16202019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Edmondson R, Broglie JJ, Adcock AF and
Yang L: Three-dimensional cell culture systems and their
applications in drug discovery and cell-based biosensors. Assay
Drug Dev Technol. 12:207–218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khaitan D and Dwarakanath BS:
Multicellular spheroids as an in vitro model in experimental
oncology: Applications in translational medicine. Expert Opin Drug
Discov. 1:663–675. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dolznig H, Rupp C, Puri C, Haslinger C,
Schweifer N, Wieser E, Kerjaschki D and Garin-Chesa P: Modeling
colon adenocarcinomas in vitro a 3D co-culture system induces
cancer-relevant pathways upon tumor cell and stromal fibroblast
interaction. Am J Pathol. 179:487–501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Terlizzi M, Le Pechoux C, Salas S, Rapeaud
E, Lerouge D, Sunyach MP, Vogin G, Sole CV, Zilli T, Lutsyk M, et
al: Postoperative radiotherapy in patients with extracranial
chondrosarcoma, a joint study of the French Sarcoma Group and Rare
Cancer Network. Int J Radiat Oncol Biol Phys. 107:726–735. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Girard N, Lhuissier E, Aury-Landas J,
Cauvard O, Lente M, Boittin M, Baugé C and Boumédiene K:
Heterogeneity of chondrosarcomas response to irradiations with
X-rays and carbon ions: A comparative study on five cell lines. J
Bone Oncol. 22:1002832020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Jong Y, Ingola M, Briaire-de Bruijn IH,
Kruisselbrink AB, Venneker S, Palubeckaite I, Heijs BP,
Cleton-Jansen AM, Haas RL and Bovée JV: Radiotherapy resistance in
chondrosarcoma cells; a possible correlation with alterations in
cell cycle related genes. Clin Sarcoma Res. 9:92019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ravi M, Paramesh V, Kaviya SR, Anuradha E
and Solomon FD: 3D cell culture systems: Advantages and
applications. J Cell Physiol. 230:16–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barnum KJ and O'Connell MJ: Cell cycle
regulation by checkpoints. Methods Mol Biol. 1170:29–40. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krempler A, Deckbar D, Jeggo PA and
Löbrich M: An imperfect G2M checkpoint contributes to chromosome
instability following irradiation of S and G2 phase cells. Cell
Cycle. 6:1682–1686. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao H, Zhuang Y, Li R, Liu Y, Mei Z, He
Z, Zhou F and Zhou Y: Effects of different doses of X-ray
irradiation on cell apoptosis, cell cycle, DNA damage repair and
glycolysis in HeLa cells. Oncol Lett. 17:42–54. 2019.PubMed/NCBI
|
|
29
|
Marples B, Wouters BG and Joiner MC: An
association between the radiation-induced arrest of G2-phase cells
and low-dose hyper-radiosensitivity: A plausible underlying
mechanism? Radiat Res. 160:38–45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xing SS, Shen CC, Godard MP, Wang JJ, Yue
YY, Yang ST, Zhao Q, Zhang SB, Wang TX, Yang XL, et al: Bortezomib
inhibits C2C12 growth by inducing cell cycle arrest and apoptosis.
Biochem Biophys Res Commun. 445:375–380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gu JJ, Kaufman GP, Mavis C, Czuczman MS
and Hernandez-Ilizaliturri FJ: Mitotic catastrophe and cell cycle
arrest are alternative cell death pathways executed by bortezomib
in rituximab resistant B-cell lymphoma cells. Oncotarget.
8:12741–12753. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Edlich F: BCL-2 proteins and apoptosis:
Recent insights and unknowns. Biochem Biophys Res Commun.
500:26–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brunelle JK and Letai A: Control of
mitochondrial apoptosis by the Bcl-2 family. J Cell Sci.
122:437–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guikema JE, Amiot M and Eldering E:
Exploiting the pro-apoptotic function of NOXA as a therapeutic
modality in cancer. Expert Opin Ther Targets. 21:767–779. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamamoto Y, Tomiyama A, Sasaki N,
Yamaguchi H, Shirakihara T, Nakashima K, Kumagai K, Takeuchi S,
Toyooka T, Otani N, et al: Intracellular cholesterol level
regulates sensitivity of glioblastoma cells against
temozolomide-induced cell death by modulation of caspase-8
activation via death receptor 5-accumulation and activation in the
plasma membrane lipid raft. Biochem Biophys Res Commun.
495:1292e12992018. View Article : Google Scholar
|
|
36
|
Tompkins KD and Thorburn A: Regulation of
apoptosis by autophagy to enhance cancer therapy. Yale J Biol Med.
92:707–718. 2019.PubMed/NCBI
|
|
37
|
Tilija Pun N, Jang WJ and Jeong CH: Role
of autophagy in regulation of cancer cell death/apoptosis during
anti-cancer therapy: Focus on autophagy flux blockade. Arch Pharm
Res. 43:475–488. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mizushima N and Yoshimori T: How to
interpret LC3 immunoblotting. Autophagy. 3:542–545. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Roomi MW, Monterrey JC, Kalinovsky T, Rath
M and Niedzwiecki A: Patterns of MMP-2 and MMP-9 expression in
human cancer cell lines. Oncol Rep. 21:1323–1333. 2009.PubMed/NCBI
|
|
40
|
Brady SW, Zhang J, Tsai MH and Yu D:
PI3K-independent mTOR activation promotes lapatinib resistance and
IAP expression that can be effectively reversed by mTOR and Hsp90
inhibition. Cancer Biol Ther. 16:402–411. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen X, Duan N, Zhang C and Zhang W:
Survivin and tumorigenesis: Molecular mechanisms and therapeutic
strategies. J Cancer. 7:314–323. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
de Jong Y, van Oosterwijk JG,
Kruisselbrink AB, Briaire-de Bruijn IH, Agrogiannis G, Baranski Z,
Cleven AH, Cleton-Jansen AM, van de Water B, Danen EH, et al:
Targeting survivin as a potential new treatment for chondrosarcoma
of bone. Oncogenesis. 5:e2222016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kang BH, Plescia J, Song HY, Meli M,
Colombo G, Beebe K, Scroggins B, Neckers L and Altieri DC:
Combinatorial drug design targeting multiple cancer signaling
networks controlled by mitochondrial Hsp90. J Clin Invest.
119:454–464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Du J, Kelly AE, Funabiki H and Patel DJ:
Structural basis for recognition of H3T3ph and Smac/DIABLO
N-terminal peptides by human Survivin. Structure. 20:185–195. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Derynck R, Akhurst RJ and Balmain A:
TGF-beta signaling in tumor suppression and cancer progression. Nat
Genet. 29:117–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Song K, Shankar E, Yang J, Bane KL,
Wahdan-Alaswad R and Danielpour D: Critical role of a
survivin/TGF-β/mTORC1 axis in IGF-I-mediated growth of prostate
epithelial cells. PLoS One. 8:e618962013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Khanna KK and Jackson SP: DNA
double-strand breaks: Signaling, repair and the cancer connection.
Nat Genet. 27:247–254. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fontana AO, Augsburger MA, Grosse N,
Guckenberger M, Lomax AJ, Sartori AA and Pruschy MN: Differential
DNA repair pathway choice in cancer cells after proton- and
photon-irradiation. Radiother Oncol. 116:374–380. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sharma A, Singh K and Almasan A: Histone
H2AX phosphorylation: A marker for DNA damage. Methods Mol Biol.
920:613–626. 2012. View Article : Google Scholar : PubMed/NCBI
|