Introduction
Liver cancer is one of the most common types of
cancer and is a serious threat to human health, with 854,000 new
cases of liver cancer and 810,000 associated deaths globally in
2015, contributing to 20,578,000 disability-adjusted life-years
(1). After surgery, the incidence of
tumor recurrence and metastasis was high from 1990–2012 globally,
at 33–100% (2) and the prognosis is
poor (3). In recent years, surgical
techniques have developed with the improvement of diagnostic
methods. However, the clinical outcome of patients with
unresectable disease is suboptimal with a median survival of only
12 months and no patient survived beyond 5 years from 1986–2003 in
the USA (4,5). Therefore, it is important to identify
novel therapeutic targets and improve the diagnosis and treatment
of patients with liver cancer.
Long non-coding RNA (lncRNA) is an RNA molecule with
>200 nucleotides and lacking protein-coding potential. Research
has implicated lncRNAs may function as oncogene or tumor suppressor
genes by regulating the transcription of protein-coding genes or
altering chromatin structure (6,7).
Moreover, lncRNAs have been recognized as diagnostic molecular
biomarkers or therapeutic targets of cancer (8,9). For
example, lncRNA H19, SNHG11 and PTCSC3 are abnormally expressed in
tumor tissues and cell lines, and can serve as biomarkers for the
diagnosis and prognosis of numerous solid tumors, including breast
(10), colorectal (11) and gastric cancer (12). lncRNAs are involved in the
development and progression of malignant tumors by regulating
cancer cell proliferation, migration, invasion, apoptosis,
angiogenesis and immune escape (13). Previously, prostate cancer-associated
transcript 6 (PCAT6) has been identified as an lncRNA involved in
the regulation of lung cancer progression (14). Subsequently, several studies have
highlighted the abnormal expression of lncRNA PCAT6 in various
malignant tumors, including gastrointestinal stromal tumor, colon
cancer and osteosarcoma, suggesting that PCAT6 may play an
essential role in tumor progression (15–17).
Although the oncogenic roles of PCAT6 have been demonstrated in
several tumors, little is known about the functionality and
mechanism of PCAT6 in liver cancer. Moreover, evidence has shown
that the exerted actions of micro (mi)RNAs depend on the modulation
of upstream molecules, including lncRNAs, and downstream mRNAs
(18). Evidence has suggested that
microRNA (miRNA/miR) expression is abnormal in different types of
tumor tissues and it is well-known that miRNAs play notable roles
in tumor development and cancer therapy (19). To date, low expression levels of
miR-326 have been observed in HCC tissues and cell lines, and
miR-326 has been found to exert a regulatory effect on the
proliferation, migration and invasion of HCC cells (20). Nevertheless, to the best of the
authors knowledge, there is currently no evidence regarding the
interaction between PCAT6 and miR-326 in liver cancer.
In the present study, based on the Gene Expression
Profiling Interactive Analysis (GEPIA) database, the expression of
lncRNA PCAT6 between liver cancer tissues and adjacent noncancerous
liver tissues was analyzed. Furthermore, the functional role and
mechanism of PCAT6 was explored by performing a series of
experiments in vitro and in vivo. Taken together,
therapeutic interventions targeting PCAT6 could be developed as a
new strategy for the treatment of liver cancer.
Materials and methods
Patients and specimens
All tissue specimens (ANT, adjacent normal tissues;
HCCT, liver cancer tissues) were collected from 117 patients with
liver cancer, which were histologically confirmed by an experienced
pathologist (GS) at The Department of Herpetological Surgery,
Zhejiang Cancer Hospital (Hangzhou, China) between January 2015 and
January 2017. The age range of 117 patients was 45–80 years (mean
age, 58 years old), 68 were females and 49 were males. The
inclusion criteria were as follows: i) ≥18 Years old; ii)
histologically diagnosed with liver cancer and were amenable to
surgery; iii) had never undergone any type of anti-cancer therapy,
such as chemotherapy and radiotherapy, before surgery; iv) clinical
data were complete. The exclusion criteria were as follows:
Patients who also had other i) malignant tumors; ii) severe
cardiovascular and cerebrovascular diseases and iii) hematological
diseases. Tissue specimens were immediately kept in RNA Keeper
Tissue Stabilizer (Vazyme Biotech Co., Ltd.) after surgical
resection and then stored at −80°C. The study protocol was reviewed
and approved by The Ethics Board of Zhejiang Cancer Hospital
(Hangzhou, China). The study was conducted as per the principles
stated in the Declaration of Helsinki and local ethical guidance
documents (21). Written informed
consent was obtained from all patients from whom specimens were
collected.
GEPIA database
GEPIA (http://gepia.cancer-pku.cn/) is a database that
provides key interactive and customizable functions, including
differential expression analysis, and patient survival analysis
(22). Following submission of an
analysis request, GEPIA can provide the visual image results for
users. The gene symbol PCAT6 and hnRNPA2B1 were submitted on GEPIA
for differential expression analysis between data from liver cancer
tissues and adjacent normal tissues, and provided results
consisting of box plots and survival analysis. A total of 364 cases
of liver cancer patients were divided into groups according to the
median expression of PCAT6 or hnRNPA2B1: A high PCAT6 or hnRNPA2B1
expression group and low PCAT6 expression group. The median of
relative PCAT6 expression level was 1.5, so the number of patients
with high PCAT6 expression were 182 (>1.5) and the number of
patients with low PCAT6 expression were 182 (≤1.5). The median of
relative hnRNPA2B1 expression level was 8.2, so the number of
patients with high hnRNPA2B1 expression were 182 (>8.2) and the
number of patients with low hnRNPA2B1 expression were 182
(≤8.2).
Cell culture and transfection
Human liver cancer cell lines MHCC97H, HepG2 and
Huh7 and the normal liver epithelial cells THLE-3 and 293T cells
were obtained from The American Type Culture Collection and
cultured according to their recommendations. The liver cancer cell
lines were authenticated by short tandem repeat profiling.
PCAT6 short hairpin (sh)RNA and shRNA scrambled
control (sh-NC), pcDNA3.1-PCAT6 and empty vector (pcDNA3.1-NC),
miR-326 mimic and a scrambled negative control (NC mimic), miR-326
inhibitor (miR-Inh) and a scrambled negative control (miR-NC),
hnRNPA2B1 shRNA and scrambled control (sh-NC) were purchased from
GenePharm Co., Ltd. Both HepG2 and Huh7 cells were seeded into
seeded in six-well plates (2×105 cells/well) and then
transfected with an oligonucleotide (50 nM miRNA mimic/inhibitor)
or plasmid (2 µg) using Lipofectamine® 3000 reagent
(Thermo Fisher Scientific, Inc.) at 37°C for 24 h. In vivo
experiments, the lentiviral vectors carrying shRNA for PCAT6
(sh-PCAT6) or negative control (sh-NC) were synthesized by RiboBio
Co., Ltd.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from liver cancer tissues,
adjacent non-cancerous tissues, liver cancer cell lines and normal
liver epithelial cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) as described in a
previous study (23). cDNA was
synthesized from 1 µg of total RNA using the PrimeScript™ 1st
Strand cDNA synthesis kit (Takara Bio, Inc.) according to the
manufacturers instructions. qPCR was performed in an ABI 7500
instrument (Thermo Fisher Scientific, Inc.) using
SYBR®-Green Real-Time PCR Master Mix (Takara Bio, Inc.)
following the manufacturers protocols. The PCR reaction conditions
were initial denaturation at 95°C for 10 min, followed by 40 cycles
of 95°C for 15 sec and 60°C for 30 sec. The following primer
sequences were used for qPCR: PCAT6, Forward:
5′-CAGGAACCCCCTCCTTACTC-3′ and reverse: 5′-CTAGGGATGTGTCCGAAGGA-3′;
miR-326, forward: 5′-GGCGCCCAGAUAAUGCG-3′, reverse:
5′-CGTGCAGGGTCCGAGGTC-3′; hnRNPA2B1, forward:
5′-GCTGTAGCAAGAGAGGAATCTGGA-3′ and reverse:
5′-GCTTCTTCACAGTTACATGAGCCC-3′; GAPDH, forward:
5′-AGCCACATCGCTCAGACAC-3′ and reverse: 5′-GCCCAATACGACCAAATCC-3′;
U6, forward: 5′-CTCGCTTCGGCAGCACA-3′ and reverse:
5′-AACGCTTCACGAATTTGCGT-3′. The expression levels of PCAT6,
hnRNPA2B1 and miR-326 were calculated using the 2−ΔΔCq
method (24) and normalized to GAPDH
and U6 as appropriate.
Cell counting kit-8 (CCK-8) assay
After 24-h post-transfection, both HepG2 and Huh7
cells (2×103 cells/well) were seeded in 96-well plates
and cultured in DMEM medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.).
When the density of the cells reached 70%, cells were transfected
with PCAT6 shRNA and sh-NC, miR-326 mimic and NC mimic, miR-326
inhibitor and inhibitor NC, or hnRNPA2B1 shRNA and vector. After 48
h of transfection, 10 µl CCK-8 solution (Beyotime Institute of
Biotechnology) was added to each well. After a 2-h incubation at
37°C, the absorbance of each well was assessed at 450 nm using a
microplate reader (Thermo Fisher Scientific, Inc.).
Colony formation assay
Both HepG2 and Huh7 cells were transfected with
sh-hnRNPA2B1 and sh-NC, sh-PCAT6 and sh-NC, miR-326 inhibitor and
inhibitor NC, pcDNA3.1-PCAT6 and (pcDNA3.1-NC), and cultured under
standard conditions. After that, the cells were diluted and seeded
into six-well plates at the density of 3,000 cells per well for 14
days. The cells were then stained with 0.5% crystal violet
(Beyotime Institute of Biotechnology) at 37°C for 45 min. The
colony number was observed with an inverted microscope
(magnification, ×10; Thermo Fisher Scientific, Inc.) and counted
using ImageJ software (version 4.0; National Institutes of
Health).
Western blotting
To extract total protein, both HepG2 and Huh7 cells
were lysed with RIPA Lysis Buffer (Beyotime Institute of
Biotechnology) as described previously (25). The lysate was centrifuged at 1,200 ×
g at 4°C for 10 min. The protein amount in the supernatant was
quantified using a BCA assay. Subsequently, protein specimens (40
µg/lane) were added onto SDS-PAGE, and electrophoresis at 60 V.
Proteins were then transferred to a polyvinylidene difluoride
membrane (EMD Millipore). After blocking with 5% (w/v) non-fat dry
milk at 37°C for 1 h, the membranes were incubated with primary
antibodies against β-actin (1:2,000 dilution; cat. no. ab8226) and
hnRNPA2B1 (1:1,000 dilution; cat. no. ab31645) at 4°C overnight.
All antibodies were purchased from Abcam. Next, HRP-conjugated
secondary antibodies (1:5,000 dilution; cat. no. HRP-60008,
ProteinTech Group, Inc.) were added and incubated for 1 h at room
temperature. The protein bands were detected using
chemiluminescence (Pierce; Thermo Fisher Scientific, Inc.) and
visualized on a Tanon 5200 Imaging system (Tanon Science &
Technology Co., Ltd.).
Transwell chamber assay
A Transwell chamber (8-µm pore, 24-well plate;
Corning, Inc.) with insert membranes coated with diluted Matrigel
at 37°C for 1 h was used. HepG2 or Huh7 cells at 24-h
post-transfection in serum-free medium (1×105 cells)
were added to the upper chamber and cultured at 37°C for 24 h. The
bottom chamber contained a medium with 10% FBS. After incubation at
37°C for 24 h, the insert membranes were cut and stained with
crystal violet at 37°C for 30 min (Beijing Solarbio Science &
Technology Co., Ltd.) and images were captured using an inverted
microscope (magnification, ×100). The number of invading cells were
counted in three wells per group.
Bioinformatics
Starbase version 2.0 (http://starbase.sysu.edu.cn/index.php) (26) were browsed to identify the miRNAs
that bind lncRNA PCAT6 and hnRNPA2B1.
Dual-luciferase reporter gene
assay
The wild-type (WT) sequence of PCAT6 or the
hnRNPA2B1 3 untranslated region (UTR) containing binding sites of
miR-326 was cloned into pmirGLO (Promega Corporation) to form the
corresponding luciferase reporter vectors (WT-PCAT6 and hnRNPA2B1
3UTR-WT). The mutant (MUT)-PCAT6 and hnRNPA2B1 3UTR-MUT were
obtained by mutating the seed sites. 293T cells were seeded into
24-well plates at 1×105 cells/cm2. When the
cell density reached 70–80%, reporter gene plasmids and miR-326 or
control vector (GenePharm Co., Ltd.) were co-transfected into 293T
cells using Lipofectamine® 3000 (Thermo Fisher
Scientific, Inc.) at 37°C for 48 h. The relative luciferase
activities were detected using the Dual-Luciferase Reporter assay
kit (Promega Corporation) according to the manufacturers protocol.
Luciferase activity was measured and normalized to Renilla
luciferase activity. The sequences of miRNA mimic/inhibitor were as
follows: miR-NC, 5′-UUCUCCGAACGUUCACGUTT-3′; miR-326 mimics,
5′-AGGAUGUCUAAAUGUUUGUUA-3′ and miR-326 inhibitor,
5′-AAGAAGUGCACCAUGUUUGUUU-3′.
Xenograft model
A total of 20 BALB/c nude mice (male; 5-weeks-old,
20–22 g) were obtained from the Hunan SJA Laboratory Animal Co.,
Ltd. All mice had access to food and water ad libitum, and
temperature in the cages was maintained at 24±1°C with a 12/12 h
light/dark cycle and 40–50% humidity. Transfected HepG2 cells
(1×107) were resuspended in 100 µl PBS and
subcutaneously injected into the flanks of male BALB/c mice (n=10)
as previously described (27). Tumor
volume was detected every 4 days and calculated as: Length (mm) ×
width2 (mm2)/2. After tumor growth for 28
days, mice (weight, 21–24 g) were euthanized via cervical
dislocation. Tumor samples were weighed and harvested to examine
the expression of miR-326 and hnRNPA2B1. The animal experiments
were conducted under the approval of The Animal Care and Use
Committee of Zhejiang Cancer Hospital (approval no.
IRB-2019-5).
Statistical analysis
All experiments were repeated at least three times.
Data are presented as the mean ± standard deviation (unless
otherwise shown). The median expression of PCAT6 and hnRNPA2B1 in
all human liver cancer samples were considered as the cut-off to
classify groups into PCAT6- and hnRNPA2B1-high and PCAT6-and
hnRNPA2B1-low expression. Statistical analysis was performed using
GraphPad Prism 8 (GraphPad Software, Inc.), and differences between
groups were analyzed using one-way ANOVA and Tukeys post hoc tests.
A paired or unpaired two-tailed Students t-test was used to compare
differences between two groups of paired tissues or unpaired cells,
respectively. The correlation relationships among PCAT6, miR-326
and hnRNPA2B1 were analyzed using the Pearsons correlation
coefficient. Survival analysis was performed using log-rank tests
and presented on Kaplan-Meier plots. Survival curves with
cross-over were compared using the Renyi test. P<0.05 was
considered to indicate a statistically significant difference.
Results
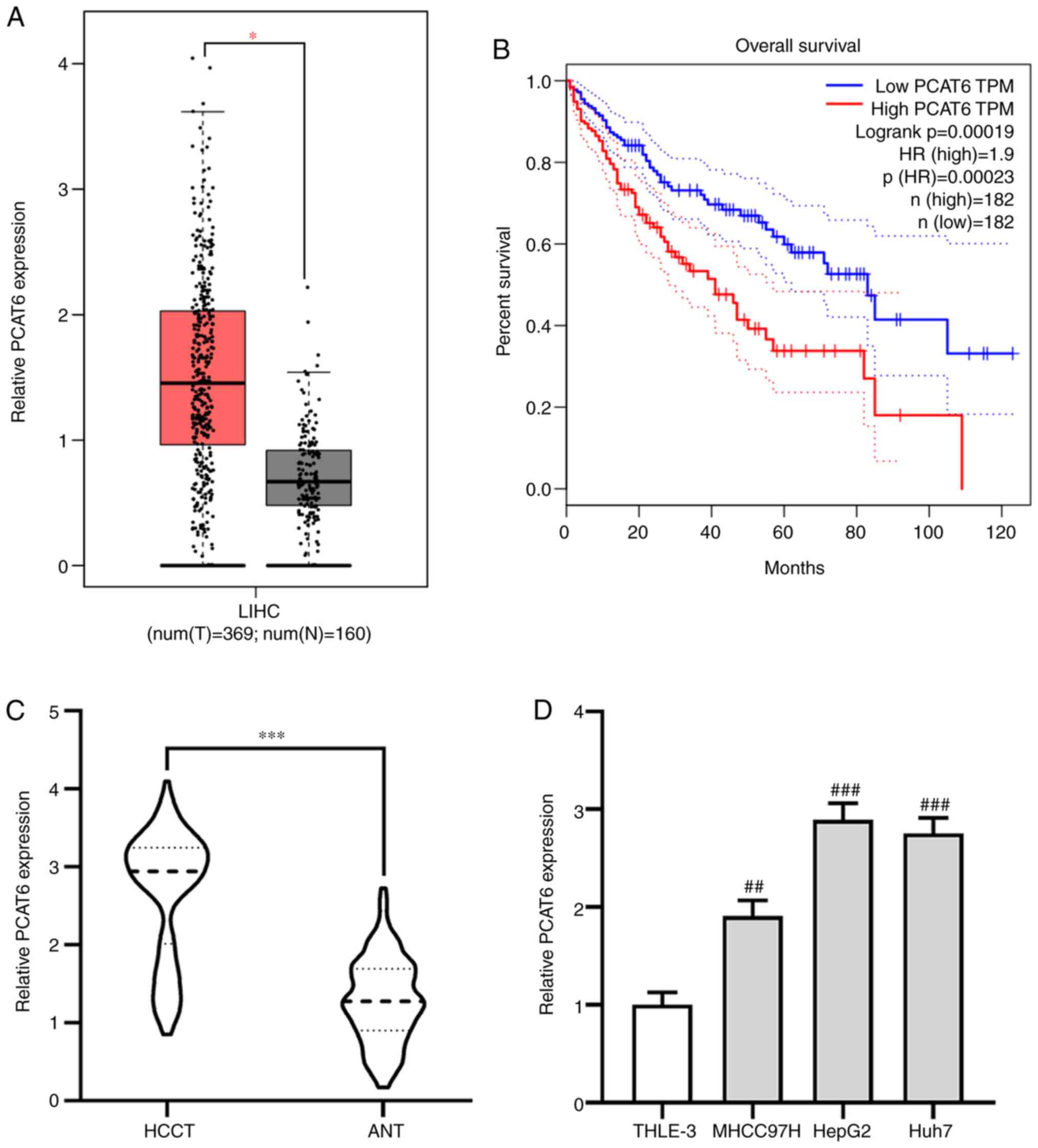
PCAT6 is upregulated and associated
with the progression of liver cancer
The effect of PCAT6 on the progression of liver
cancer was investigated using the GEPIA database. It was found that
the expression of PCAT6 was significantly elevated in liver cancer
tissues (HCCT) compared with adjacent normal tissues (ANT;
P<0.05; Fig. 1A). Moreover,
patients with liver cancer who had a high level of PCAT6 exhibited
a shorter overall survival (P<0.001; Fig. 1B). The expression pattern of PCAT6 in
117 pairs of HCCT and matching ANT was further investigated using
RT-qPCR. It was found that PCAT6 expression was upregulated in HCCT
compared with the ANT (P<0.001; Fig.
1C).Additionally, PCAT6 expressions in liver cancer cell lines
MHCC97H, HepG2 and Huh7 were significantly enhanced compared with
expression in THLE-3 normal liver epithelial cells, especially
HepG2 and Huh7 cells (MHCC97H P<0.01; HepG2 and Huh7 P<0.001;
Fig. 1D). Taken together, these data
suggested that PCAT6 may act as an oncogene in the progression of
liver cancer.
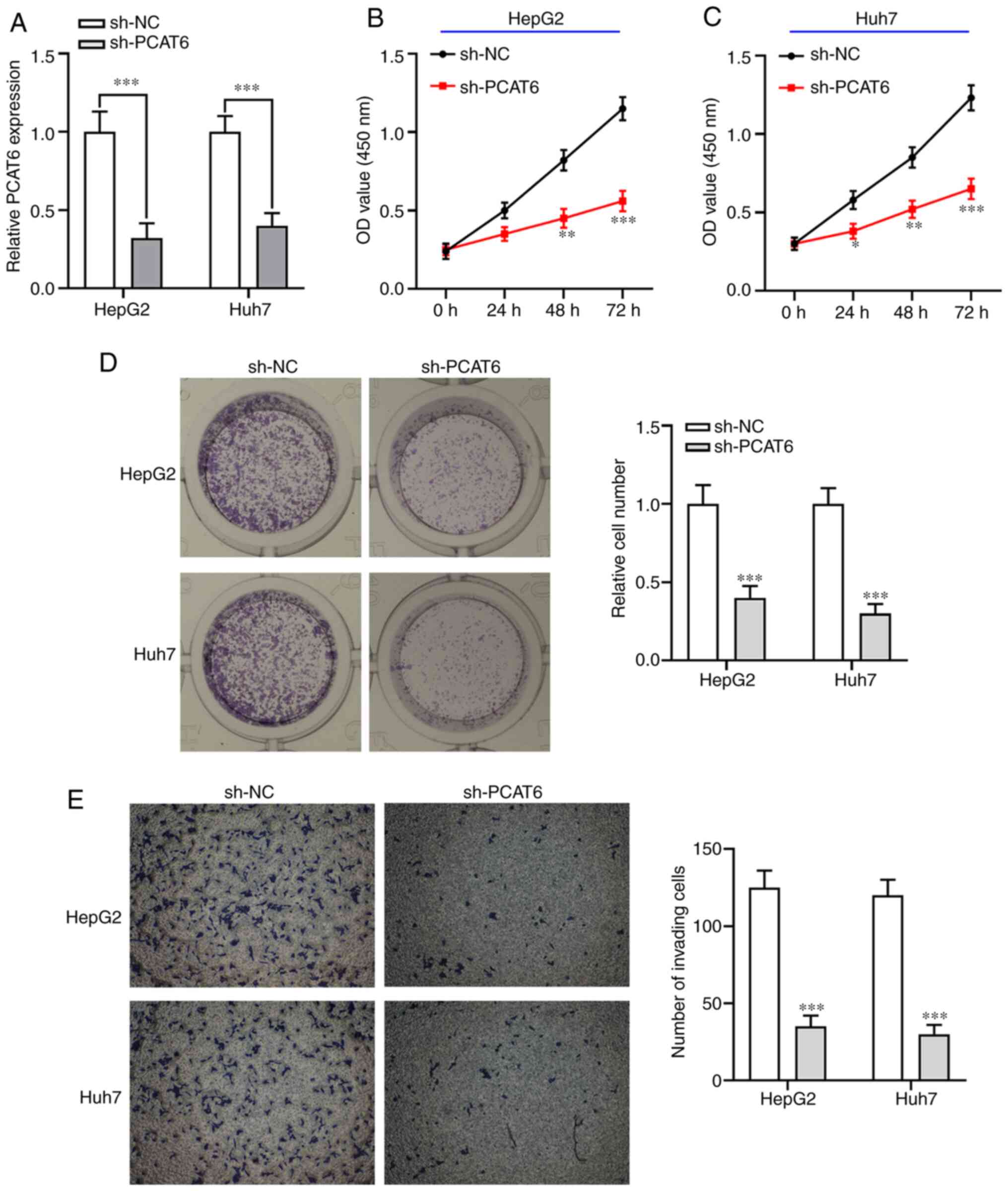
Knockdown of PCAT6 inhibits liver
cancer cell proliferation and invasion
To investigate the biological functions of PCAT6 in
liver cancer cells in vitro, sh-PCAT6 was transfected into
both HepG2 and Huh7 cells to decrease its expression (P<0.001;
Fig. 2A). The CCK-8 assay showed
that knockdown of PCAT6 significantly inhibited liver cancer cell
proliferation ability compared with the sh-NC group (P<0.05;
Fig. 2B and C). Colony formation
assays showed that PCAT6-knockdown markedly reduced the number of
liver cancer cell colonies compared with the sh-NC group
(P<0.001; Fig. 2D). Furthermore,
inhibition of PCAT6 weakened the invasion capacity of both HepG2
and Huh7 cells (P<0.001; Fig.
2E). These data indicated that knockdown of PCAT6 exhibited an
antitumor function in liver cancer by suppressing the proliferation
and invasion abilities of both HepG2 and Huh7 cells.
miR-326 is a target gene of PCAT6
To further probe the underlying mechanism of PCAT6
function in liver cancer, the bioinformatics database, Starbase,
was used to predict potential miRNAs that may be downstream target
genes of PCAT6. Bioinformatics analysis showed that miR-326 could
be a candidate target gene of PCAT6, as it has a potential binding
site for PCAT6 (Fig. 3A). Notably,
RT-qPCR revealed that the expression of miR-326 was lower in the
HCCT group compared with that in the ANT group (P<0.001;
Fig. 3B). A negative correlation was
also found between miR-326 and PCAT6 in ANT group (P<0.001;
Fig. 3C). Subsequently, the
luciferase reporter assay showed that overexpression of miR-326
significantly reduced the luciferase activity of 293T cells
transfected with the WT-PCAT6 vector (P<0.001; Fig. 3D), while the luciferase activity of
the MUT-PCAT6 was not changed. Moreover, RT-qPCR showed that
knockdown of PCAT6 markedly enhanced the expression of miR-326 in
both HepG2 and Huh7 cells (P<0.001; Fig. 3E). Collectively, these results
indicated that miR-326 was a target gene of PCAT6.
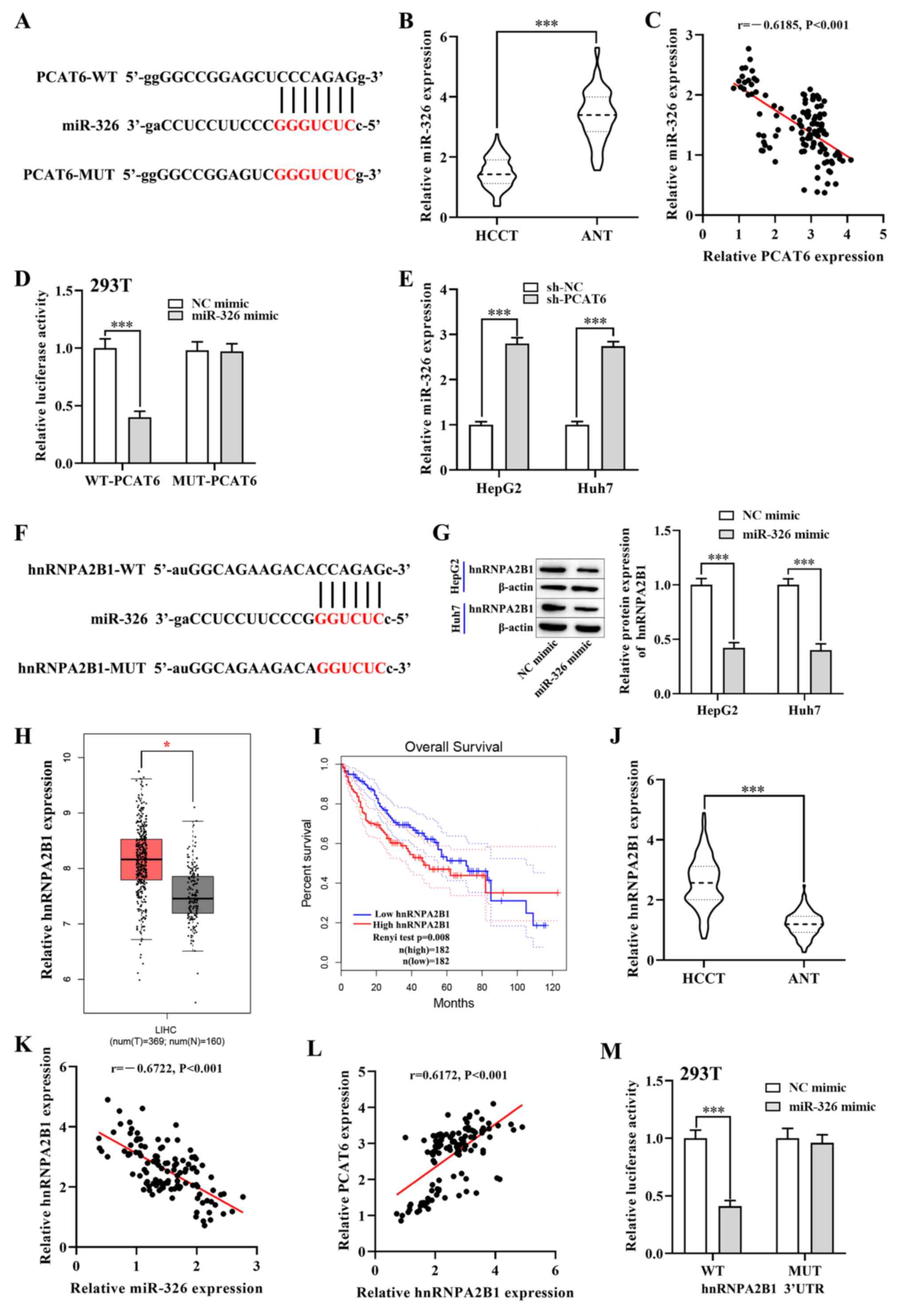 | Figure 3.PCAT6 promotes hnRNPA2B1 expression
in liver cancer cells by targeting miR-326. (A) Starbase predicted
binding sites between miR-326 and PCAT6. (B) RT-qPCR detected the
expression of miR-326 in HCCT (n=117) and matched ANT. (C) Pearsons
correlation analysis between PCAT6 and miR-326 in liver cancer
tissues. (D) Luciferase reporter assays were performed in 293T
cells to detect the binding affinity of miR-326 to the PCAT6
3UTR-WT or MUT. (E) Expression of miR-326 in both HepG2 and Huh7
cells after transfection with sh-PCAT6 or sh-NC examined using
RT-qPCR. (F) Starbase database binding site predictions between
miR-326 and hnRNPA2B1. (G) Protein levels of hnRNPA2B1 in both
HepG2 and Huh7 cells after transfection with miR-326 mimic and NC
mimic were examined using western blotting. (H) Expression of
hnRNPA2B1 in liver cancer tissues (n=369) and normal tissues
(n=160) from the GEPIA database. (I) Association between hnRNPA2B1
expression and overall survival rate of patients with liver cancer
was analyzed using GEPIA database. (J) RT-qPCR detected the mRNA
levels of hnRNPA2B1 in HCCT (n=117) and matched ANT. (K and L)
Correlation between hnRNPA2B1 and PCAT6 or miR-326 in liver cancer
tissues. (M) Dual-luciferase reporter assay verified the negative
regulatory association between miR-326 and hnRNPA2B1 in 293T cells.
*P<0.05 and ***P<0.001 compared with respective control.
PCAT6, prostate cancer-associated transcript 6; hnRNPA2B1,
heterogeneous nuclear ribonucleoprotein A2B1; GEPIA, Gene
Expression Profiling Interactive Analysis; RT-qPCR, reverse
transcription-quantitative PCR; HCCT, liver cancer tissue; ANT,
adjacent normal tissue; miR-, microRNA; WT, wild-type; MUT, mutant;
NC, negative control; HR, hazard ratio; LIHC; UTR, untranslated
region. |
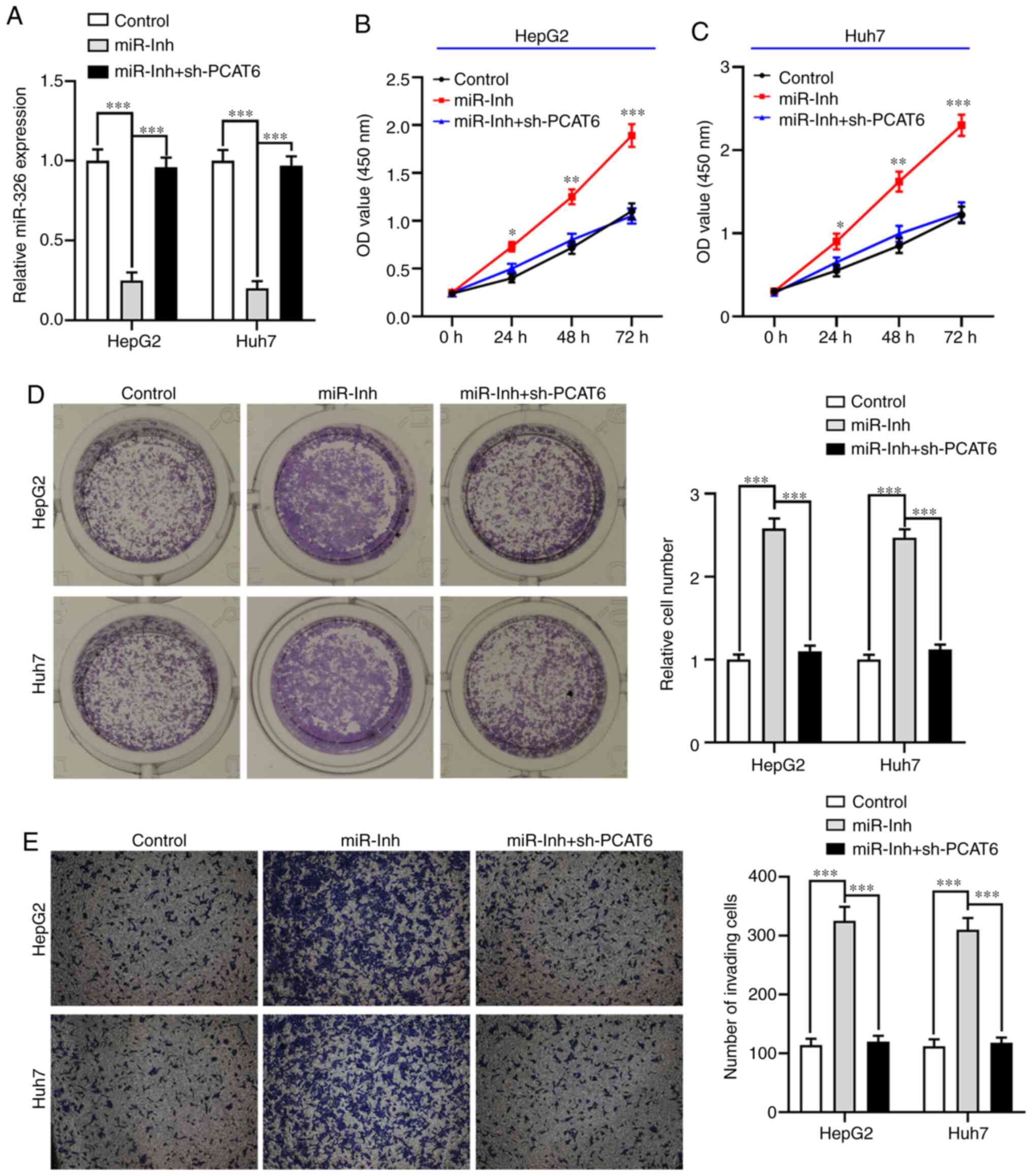
Inhibition of miR-326 abrogates the
inhibitory effect of PCAT6-silencing on liver cancer cell
proliferation and invasion
To further explore whether PCAT6 modulates the
malignant phenotype of liver cancer cells by targeting miR-326,
HepG2 and Huh7 cells were transfected with miR-Inh or miR-Inh +
sh-PCAT6 (P<0.001; Fig. 4A). The
CCK-8 assay showed that inhibition of miR-326 significantly
promoted the proliferation ability of both HepG2 and Huh7 cells
compared with the control group (P<0.05; Fig. 4B and C). On the other hand, knockdown
of PCAT6 reversed this effect. Similarly, the clone formation of
both HepG2 and Huh7 cells when transfected with miR-Inh was
markedly increased compared with the control group (P<0.001;
Fig. 4D), while the number of liver
cancer cell colonies were reduced by knocking down both miR-326 and
PCAT6. Moreover, an elevated invasive ability of both HepG2 and
Huh7 cells was observed when miR-326 was suppressed, which was
abrogated by inhibition of PCAT6 (P<0.001; Fig. 4E). These results demonstrated that
PCAT6-silencing inhibited cell proliferation and invasion by
upregulating miR-326 in liver cancer.
hnRNPA2B1 is a target of miR-326 in
liver cancer cells
Next, the target of miR-326 was investigated using
Starbase, which predicted that hnRNPA2B1 was a target gene
(Fig. 3F). Considering the critical
roles of hnRNPA2B1 in a number of malignant tumors (28), hnRNPA2B1 was selected as the target
of miR-326. Notably, the GEPIA database showed that the mRNA level
of hnRNPA2B1 was upregulated in liver cancer tissues compared with
normal tissues (P<0.05; Fig. 3H).
Moreover, the elevated expression of hnRNPA2B1 in patients with
liver cancer resulted in a shorter overall survival rate
(P<0.01; Fig. 3I). Likewise, the
RT-qPCR analysis showed that the mRNA expression of hnRNPA2B1 in
the HCCT group was higher compared with that in the ANT group
(P<0.001; Fig. 3J). In addition,
Pearsons correlation analysis revealed that the mRNA expression of
hnRNPA2B1 was negatively correlated with miR-326 expression in HCC
tumor tissues (P<0.001; Fig. 3K),
while a positive correlation between hnRNPA2B1 and PCAT6 in liver
cancer tissues was observed (P<0.001; Fig. 3L). To further investigate this
correlation, the luciferase reporter assay showed that miR-326
mimics significantly reduced the luciferase activity of
WT-hnRNPA2B1 3UTR, while this inhibition was blocked by mutation of
the potential binding domains (P<0.001; Fig. 3M). Western blotting showed that the
protein level of hnRNPA2B1 was significantly downregulated in both
HepG2 and Huh7 cells after transfection with the miR-326 mimic
(P<0.001; Figs. 3G and S1A). Overall, these results suggested that
miR-326 targeted and inhibited the expression of hnRNPA2B1.
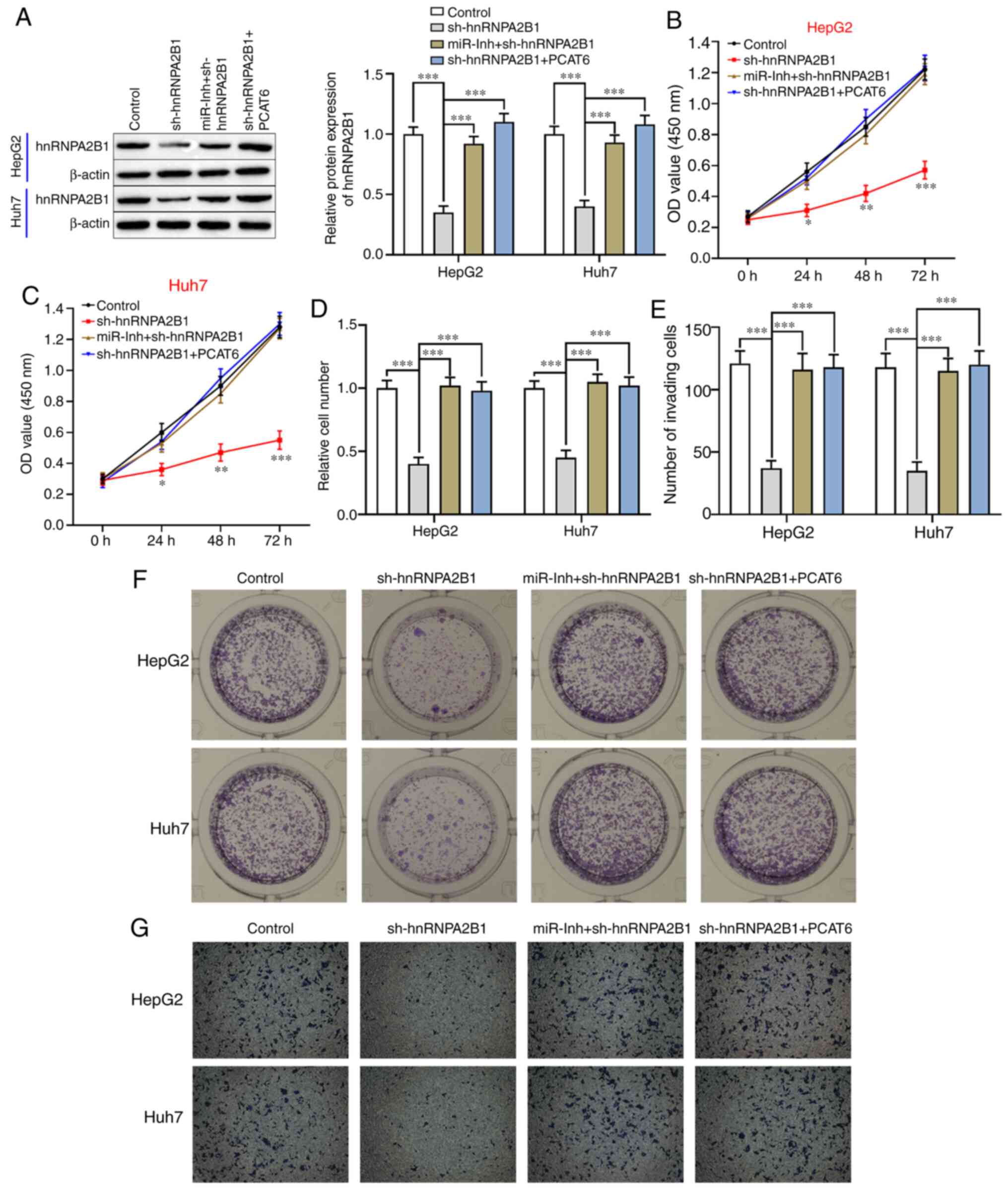
PCAT6 promotes the proliferation and
invasion of liver cancer cells via the miR-326/hnRNPA2B1 axis
Whether PCAT6 exhibited an antitumor effect on liver
cancer cells via regulation of the miR-326/hnRNPA2B1 axis was
further investigated. As shown in Figs.
5A and S1B-E, the protein
levels of hnRNPA2B1 were significantly reduced in both HepG2 and
Huh7 cells after transfection with sh-hnRNPA2B1 (P<0.001),
whereas its expression was upregulated by inhibition of miR-326 or
upregulation of PCAT6 in hnRNPA2B1-silenced liver cancer cells.
Silencing of hnRNPA2B1 significantly decreased the proliferation
(P<0.05; Fig. 5B and C) and clone
formation abilities compared with the control in both HepG2 and
Huh7 cells (P<0.001; Fig. 5D and
E). However, the inhibitory effect of hnRNPA2B1-silencing on
liver cancer cell biological behavior was reversed by the combined
downregulation of miR-326 and hnRNPA2B1 or PCAT6-overexpression and
hnRNPA2B1-silencing (P<0.001, Fig.
5B-E). Moreover, the invasion capacity of HepG2 and Huh7 cells
was inhibited by knockdown of hnRNPA2B1 (P<0.001; Fig. 5F and G), an effect that could be
attenuated through the inhibition of miR-326 or upregulation of
PCAT6. Taken together, PCAT6-inhibition contributed to decreased
cell proliferation and invasion of liver cancer cells via
downregulation of hnRNPA2B1 and upregulation of miR-326.
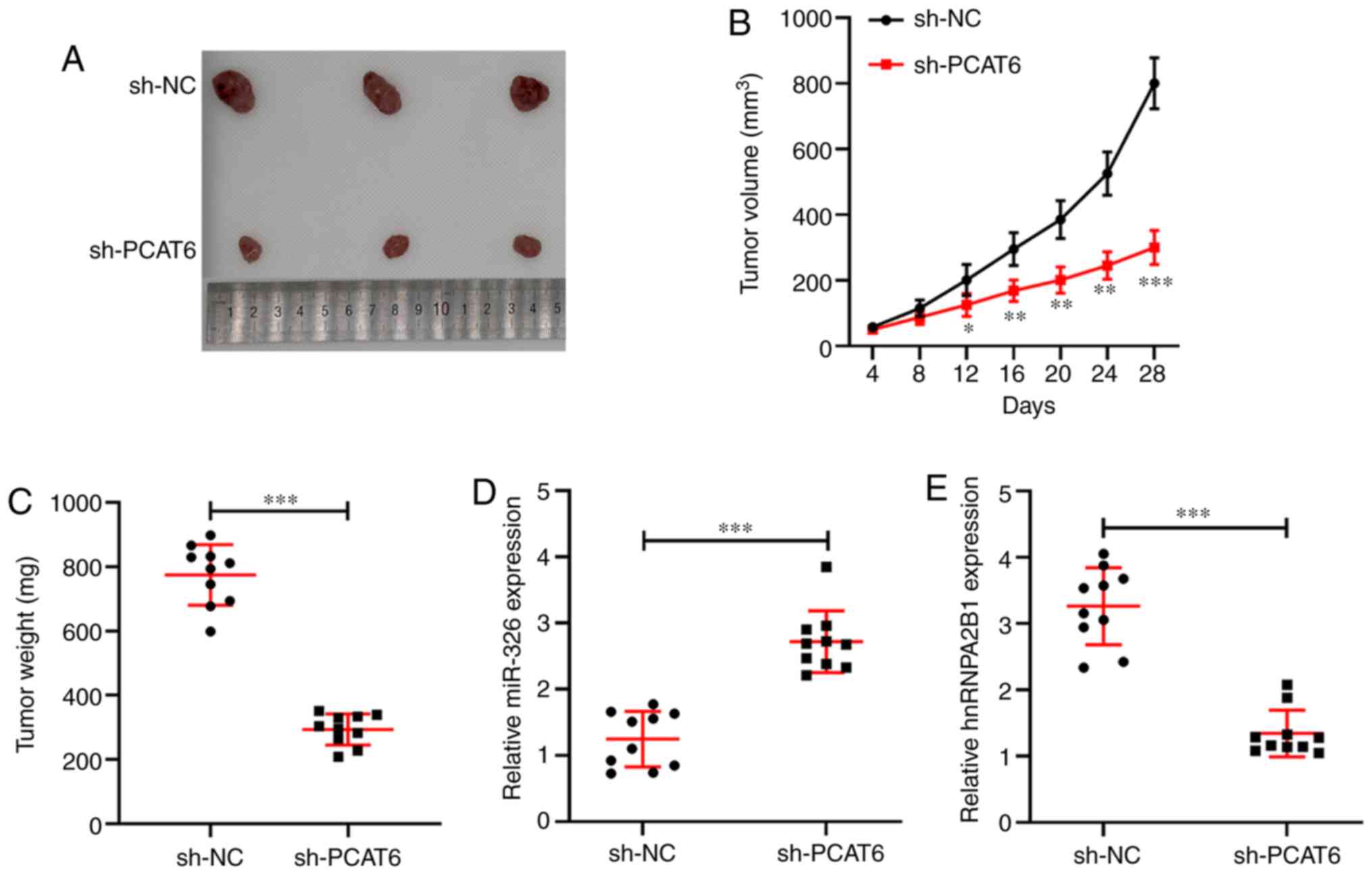
Knockdown of PCAT6 suppresses tumor
growth of liver cancer in vivo
Based on the antitumor effect of PCAT6-inhibition by
regulating the miR-326/hnRNPA2B1 axis in vitro, the role of
PCAT6 in vivo was further examined using a subcutaneous
xenograft model. As shown in Fig. 6A and
C, knockdown of PCAT6 significantly decreased tumor growth and
tumor weight (P<0.001) compared with the sh-NC group at 28 days.
Tumor volume in the sh-PCAT6 group was significantly lower compared
with in the sh-NC group (P<0.05, Fig.
6B). Moreover, RT-qPCR was performed to detect the expression
of miR-326 and hnRNPA2B1 in tumor tissues derived from the
xenograft mice model. PCAT6-silencing was found to enhance the
expression of miR-326 while it reduced hnRNPA2B1 expression
compared with the sh-NC group (P<0.001, Fig. 6D and E). Therefore, knockdown of
PCAT6 inhibited tumor growth by upregulating the inhibitory effect
of miR-326 on hnRNPA2B1 expression in liver cancer.
Discussion
Despite advances and progress in the field of liver
cancer research, liver cancer remains a worrisome burden worldwide
and the number of deaths due to liver cancer in China increased by
33.80% between in 1990–2015 (29–31). In
the present study, PCAT6 was upregulated in liver cancer tissues
and associated with poor outcomes in patients. Moreover,
loss-of-function experiments revealed that the knockdown of PCAT6
suppressed the proliferation and invasion capacities of liver
cancer cells in vitro and in vivo. Mechanistically,
inhibition of PCAT6 exerted an antitumor function in liver cancer
by targeting miR-326 and downregulating hnRNPA2B1 expression.
Studies have confirmed that lncRNA is a useful
biomarker for the early diagnosis and prognosis of numerous
diseases including cancer (32,33). For
example, Sun et al (34)
found five differentially expressed lncRNAs that are associated
with shorter survival time in The Cancer Genome Atlas. Cai et
al (35) reported that a nine
lncRNA signature acts as a biomarker to predict overall survival in
gastric cancer. Wan et al (36) demonstrated that serum PCAT6 could be
used as a diagnostic and prognostic biomarker in patients with
non-small-cell lung cancer. Moreover, lncRNAs may function as an
oncogene or tumor suppressor gene involved in the progression of
the malignant tumor by regulating the proliferation, invasion and
migration of cancer cells (6,37). For
instance, Wang et al (38)
demonstrated that a novel lncRNA MCM3AP antisense RNA 1 facilitates
tumor growth of HCC by promoting HCC cell proliferation and
reducing apoptosis. In the present study, PCAT6 was shown to be
overexpressed in liver cancer tissues and cell lines, and
inhibition of PCAT6 significantly decreased liver cancer cell
proliferation and invasion. Of note, a recent study reported that
PCAT6 serves as an oncogene to promote the development of
non-small-cell lung cancer by binding to the enhancer of zeste
homolog 2 (EZH2) and suppressing the expression of large tumor
suppressor homolog 2 (39). Lv et
al (40) showed that
PCAT6-overexpression promotes cell proliferation, migration,
invasion and reduces apoptosis through the activation of the
Wnt/β-catenin pathway in cervical cancer. Other studies have
demonstrated that knockdown of PCAT6 reverses chemoresistance of
cancer cells to 5-fluorouracil and enhances radiosensitivity in
breast cancer cells (41,42). The aforementioned results of the
present study indicated that the oncogene PCAT6 might be an
effective therapeutic target for patients with liver cancer.
Numerous studies have confirmed that lncRNA plays a
major role in regulating cancer progression by targeting miRNA to
regulate mRNA expression (43). For
example, Dong et al (44)
reported that silencing of PCAT6 restrains cell proliferation and
epithelial-mesenchymal transition of gastric cancer cells by
targeting miR-15a. Huang et al (16) confirmed that PCAT6 suppresses
apoptosis and contributes to cell proliferation by upregulating
anti-apoptotic protein apoptosis repressor with caspase recruitment
domain via EZH2 in colon cancer. Zhu et al (17) reported that PCAT6 promotes tumor
progression in osteosarcoma by regulating the miR-185-5p/TGFBR1/2
axis. The results of the present study showed that PCAT6 targeted
miR-326 to promote tumorigenesis of liver cancer by upregulating
the expression of hnRNPA2B1. Notably, a number of studies have
demonstrated that miR-326 acts as a tumor suppressor in cancer
progression (45,46). Lu et al (47) reported that miR-326 is a novel miRNA
signature for HCC diagnosis and prognosis. Meanwhile, evidence
suggests that miR-326 is involved in malignant phenotypes by
targeting downstream genes or signaling pathways. For instance,
overexpression of miR-326 targets LIM and SH3 protein 1 to suppress
HCC cell proliferation and invasion and promote apoptosis (48). Mo et al (49) demonstrated that miR-326 inhibits HCC
tumor growth by blocking the Akt/c-Myc pathway through targeting of
3-phosphoinositide-dependent protein kinase 1. Upregulation of
miR-326 reduces chemoresistance in numerous solid tumors, such as
HCC (50), gastric cancer (51) and lung adenocarcinoma (52). In the present study, miR-326 directly
targeted hnRNPA2B1, which is reported as an oncogene in HCC
(53). In the present study,
knockdown of hnRNPA2B1 significantly suppressed liver cancer cell
proliferation and invasion, which is also in agreement with a
previous study by Wang et al (54). Furthermore, previous studies
demonstrated that silencing of hnRNPA2B1 inhibits malignant
capability and induces apoptosis through downregulation of lin28B
and inactivation of the PI3K/Akt pathway in tumors (55,56).
Notably, the data from the present study showed that inhibition of
miR-326 or upregulation of PCAT6 significantly alleviated the
inhibitory effect of hnRNPA2B1 deletion on the proliferation and
invasion of liver cancer cells.
It was concluded that PCAT6 is upregulated in liver
cancer tissues and significantly associated with poor prognosis in
patients with liver cancer. Loss-of-function experiments
demonstrated that knockdown of PCAT6 exhibited an anti-tumorigenic
effect in liver cancer by downregulating hnRNPA2B1 expression
through the targeting of miR-326. The study provided a theoretical
basis for PCAT6 as a new diagnostic, prognostic and therapeutic
target for patients with liver cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by The Medicine and Health
Discipline Platform Project of Zhejiang Province (grant no.
2018RC019) and The Medicine and Health Science Project of Zhejiang
Province (grant no. 2020KY483).
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author on request.
Authors contributions
JL and GS designed the experiments. JL, JZ and WH
performed experiments. WH, HZ and ZZ analyzed the data. JZ and WH
confirmed the authenticity of all raw data. JL and JZ drafted the
manuscript. GS revised manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was approved by The Ethics Committee of
Zhejiang Cancer Hospital (Hangzhou, China; approval no.
IRB-2020-6). All patients have signed written informed consent. The
animal study was approved by the Animal Care and Use Committee of
the Zhejiang Cancer Hospital (approval no. IRB-2019-5).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ANT
|
adjacent normal tissue
|
|
CCK-8
|
Cell Counting Kit-8
|
|
GEPIA
|
Gene Expression Profiling Interactive
Analysis
|
|
HCCT
|
liver cancer tissue
|
|
HCC
|
hepatocellular carcinoma
|
|
hnRNPA2B1
|
heterogeneous nuclear
ribonucleoprotein A2B1
|
|
PCAT6
|
prostate cancer-associated transcript
6
|
|
MUT
|
mutant type
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
TCGA
|
The Cancer Genome Atlas
|
|
WT
|
wild-type
|
References
|
1
|
Global Burden of Disease Liver Cancer
Collaboration, ; Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu
MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, et al: The
burden of primary liver cancer and underlying etiologies from 1990
to 2015 at the global, regional, and national level: Results from
the global burden of disease study 2015. JAMA Oncol. 3:1683–1691.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mavros MN, Mayo SC, Hyder O and Pawlik TM:
A systematic review: Treatment and prognosis of patients with
fibrolamellar hepatocellular carcinoma. J Am Coll Surg.
215:820–830. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tan N, Zhu B, Shu H, Tao YF, Wu JR, Fang
M, Li CR, Chen ZQ and Ou C: Effect of lncRNA-BC200 on proliferation
and migration of liver cancer cells in vitro and in
vivo. Oncol Rep. 43:461–470. 2020.PubMed/NCBI
|
|
4
|
Stipa F, Yoon SS, Liau KH, Fong Y,
Jarnagin WR, DAngelica M, Abou-Alfa G, Blumgart LH and DeMatteo RP:
Outcome of patients with fibrolamellar hepatocellular carcinoma.
Cancer. 106:1331–1338. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kakar S, Burgart LJ, Batts KP, Garcia J,
Jain D and Ferrell LD: Clinicopathologic features and survival in
fibrolamellar carcinoma: Comparison with conventional
hepatocellular carcinoma with and without cirrhosis. Mod Pathol.
18:1417–1423. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arun G, Diermeier SD and Spector DL:
Therapeutic targeting of long non-coding RNAs in cancer. Trends Mol
Med. 24:257–277. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Feng W, Liu W, Kong X, Li L, He J,
Wang D, Zhang M, Zhou G, Xu W, et al: Circulating lncRNA ABHD11-AS1
serves as a biomarker for early pancreatic cancer diagnosis. J
Cancer. 10:3746–3756. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chi Y, Wang D, Wang J, Yu W and Yang J:
Long non-coding RNA in the pathogenesis of cancers. Cells.
8:10152019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhong G, Wang K, Li J, Xiao S, Wei W and
Liu J: Determination of serum exosomal H19 as a noninvasive
biomarker for breast cancer diagnosis. Onco Targets Ther.
13:2563–2571. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu W, Zhou G, Wang H, Liu Y, Chen B, Chen
W, Lin C, Wu S, Gong A and Xu M: Circulating lncRNA SNHG11 as a
novel biomarker for early diagnosis and prognosis of colorectal
cancer. Int J Cancer. 146:2901–2912. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang G, Chi N, Lu Q, Zhu D and Zhuang Y:
LncRNA PTCSC3 is a biomarker for the treatment and prognosis of
gastric cancer. Cancer Biother Radiopharm. 35:77–81. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yao Z, Zhang Y, Xu D, Zhou X, Peng P, Pan
Z, Xiao N, Yao J and Li Z: Research progress on long non-coding RNA
and radiotherapy. Med Sci Monit. 25:5757–5770. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wan L, Zhang L, Fan K, Cheng ZX, Sun QC
and Wang JJ: Knockdown of long noncoding RNA PCAT6 inhibits
proliferation and invasion in lung cancer cells. Oncol Res.
24:161–170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bai F, Zhang N, Fang W, He X, Zheng Y and
Gu D: PCAT6 mediates cellular biological functions in
gastrointestinal stromal tumor via upregulation of PRDX5 and
activation of Wnt pathway. Mol Carcinog. 59:661–669. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang W, Su G, Huang X, Zou A, Wu J, Yang
Y, Zhu Y, Liang S, Li D, Ma F and Guo L: Long noncoding RNA PCAT6
inhibits colon cancer cell apoptosis by regulating anti-apoptotic
protein ARC expression via EZH2. Cell Cycle. 18:69–83. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu C, Huang L, Xu F, Li P, Li P and Hu F:
LncRNA PCAT6 promotes tumor progression in osteosarcoma via
activation of TGF-β pathway by sponging miR-185-5p. Biochem Biophys
Res Commun. 521:463–470. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang X, Feng D, Li M, Zhou J, Li X, Zhao
D, Hao B, Li D and Ding K: Transcriptomic analysis of
mRNA-lncRNA-miRNA interactions in hepatocellular carcinoma. Sci
Rep. 9:160962019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gnoni A, Santini D, Scartozzi M, Russo A,
Licchetta A, Palmieri V, Lupo L, Faloppi L, Palasciano G, Memeo V,
et al: Hepatocellular carcinoma treatment over sorafenib:
Epigenetics, microRNAs and microenvironment. Is there a light at
the end of the tunnel? Expert Opin Ther Targets. 19:1623–1635.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He S, Yang J, Jiang S, Li Y and Han X:
Circular RNA circ_0000517 regulates hepatocellular carcinoma
development via miR-326/IGF1R axis. Cancer Cell Int. 20:4042020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Council for International Organizations of
Medical Sciences (CIOMS), . International Ethical Guidelines for
Biomedical Research Involving Human Subjects. CIOMS; Geneva: pp.
p602002
|
|
22
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng J, Luo J, Zeng H, Guo L and Shao G:
125I suppressed the Warburg effect via regulating
miR-338/PFKL axis in hepatocellular carcinoma. Biomed Pharmacother.
119:1094022019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeng H, Zheng J, Wen S, Luo J, Shao G and
Zhang Y: MicroRNA-339 inhibits human hepatocellular carcinoma
proliferation and invasion via targeting ZNF689. Drug Des Devel
Ther. 13:435–445. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42:D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li HW and Liu J: Circ_0009910 promotes
proliferation and metastasis of hepatocellular carcinoma cells
through miR-335-5p/ROCK1 axis. Eur Rev Med Pharmacol Sci.
24:1725–1735. 2020.PubMed/NCBI
|
|
28
|
Liu Y and Shi SL: The roles of hnRNP A2/B1
in RNA biology and disease. Wiley Interdiscip Rev RNA.
12:e16122021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen J, Zhang Y, Chen Y and Ding L:
Incidence trend of liver cancer: An analysis of 40 years data from
Qidong population-based cancer registry. Zhongguo Zhong Liu.
23:621–628. 2014.PubMed/NCBI
|
|
31
|
Sun Z, Chen T, Thorgeirsson SS, Zhan Q,
Chen J, Park JH, Lu P, Hsia CC, Wang N, Xu L, et al: Dramatic
reduction of liver cancer incidence in young adults: 28 year
follow-up of etiological interventions in an endemic area of China.
Carcinogenesis. 34:1800–1805. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kondo Y, Shinjo K and Katsushima K: Long
non-coding RNAs as an epigenetic regulator in human cancers. Cancer
Sci. 108:1927–1933. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Camacho CV, Choudhari R and Gadad SS: Long
noncoding RNAs and cancer, an overview. Steroids. 133:93–95. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun Y, Zhang F, Wang L, Song X, Jing J,
Zhang F, Yu S and Liu H: A five lncRNA signature for prognosis
prediction in hepatocellular carcinoma. Mol Med Rep. 19:5237–5250.
2019.PubMed/NCBI
|
|
35
|
Cai C, Yang L, Tang Y, Wang H, He Y, Jiang
H and Zhou K: Prediction of overall survival in gastric cancer
using a nine-lncRNA. DNA Cell Biol. 38:1005–1012. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wan L, Zhang L, Fan K and Wang JJ:
Diagnostic significance of circulating long noncoding RNA PCAT6 in
patients with non-small cell lung cancer. Onco Targets Ther.
10:5695–5702. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Peng WX, Koirala P and Mo YY:
LncRNA-mediated regulation of cell signaling in cancer. Oncogene.
36:5661–5667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Y, Yang L, Chen T, Liu X, Guo Y, Zhu
Q, Tong X, Yang W, Xu Q, Huang D and Tu K: A novel lncRNA
MCM3AP-AS1 promotes the growth of hepatocellular carcinoma by
targeting miR-194-5p/FOXA1 axis. Mol Cancer. 18:282019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shi X, Liu Z, Liu Z, Feng X, Hua F, Hu X,
Wang B, Lu K and Nie F: Long noncoding RNA PCAT6 functions as an
oncogene by binding to EZH2 and suppressing LATS2 in non-small-cell
lung cancer. EBioMedicine. 37:177–187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lv XJ, Tang Q, Tu YQ, Yan DD and Wei QC:
Long noncoding RNA PCAT6 regulates cell growth and metastasis via
Wnt/β-catenin pathway and is a prognosis marker in cervical cancer.
Eur Rev Med Pharmacol Sci. 23:1947–1956. 2019.PubMed/NCBI
|
|
41
|
Wu H, Zou Q, He H, Liang Y, Lei M, Zhou Q,
Fan D and Shen L: Long non-coding RNA PCAT6 targets miR-204 to
modulate the chemoresistance of colorectal cancer cells to
5-fluorouracil-based treatment through HMGA2 signaling. Cancer Med.
8:2484–2495. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shi R, Wu P, Liu M, Chen B and Cong L:
Knockdown of lncRNA PCAT6 enhances radiosensitivity in
triple-negative breast cancer cells by regulating miR-185-5p/TPD52
axis. Onco Targets Ther. 13:3025–3037. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lin C, Yuan G, Hu Z, Zeng Y, Qiu X, Yu H
and He S: Bioinformatics analysis of the interactions among lncRNA,
miRNA and mRNA expression, genetic mutations and epigenetic
modifications in hepatocellular carcinoma. Mol Med Rep.
19:1356–1364. 2019.PubMed/NCBI
|
|
44
|
Dong D, Lun Y, Sun B, Sun H, Wang Q, Yuan
G and Quan J: Silencing of long non-coding RNA PCAT6 restrains
gastric cancer cell proliferation and epithelial-mesenchymal
transition by targeting microRNA-15a. Gen Physiol Biophys. 39:1–12.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zou X, Zhong J, Li J, Su Z, Chen Y, Deng
W, Li Y, Lu S, Lin Y, Luo L, et al: miR-362-3p targets nemo-like
kinase and functions as a tumor suppressor in renal cancer cells.
Mol Med Rep. 13:994–1002. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang D, Wang H, Li Y and Li Q: MiR-362-3p
functions as a tumor suppressor through targeting MCM5 in cervical
adenocarcinoma. Biosci Rep. 38:BSR201806682018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lu M, Kong X, Wang H, Huang G, Ye C and He
Z: A novel microRNAs expression signature for hepatocellular
carcinoma diagnosis and prognosis. Oncotarget. 8:8775–8784. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hu S, Ran Y, Chen W, Zhang Y and Xu Y:
MicroRNA-326 inhibits cell proliferation and invasion, activating
apoptosis in hepatocellular carcinoma by directly targeting LIM and
SH3 protein 1. Oncol Rep. 38:1569–1578. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mo Y, He L, Lai Z, Wan Z, Chen Q, Pan S,
Li L, Li D, Huang J, Xue F and Che S: Gold nano-particles (AuNPs)
carrying miR-326 targets PDK1/AKT/c-myc axis in hepatocellular
carcinoma. Artif Cells Nanomed Biotechnol. 47:2830–2837. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ma J, Wang T, Guo R, Yang X, Yin J, Yu J,
Xiang Q, Pan X, Tang H and Lei X: Involvement of miR-133a and
miR-326 in ADM resistance of HepG2 through modulating expression of
ABCC1. J Drug Target. 23:519–524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Song W, Qian Y, Zhang MH, Wang H, Wen X,
Yang XZ and Dai WJ: The long non-coding RNA DDX11-AS1 facilitates
cell progression and oxaliplatin resistance via regulating
miR-326/IRS1 axis in gastric cancer. Eur Rev Med Pharmacol Sci.
24:3049–3061. 2020.PubMed/NCBI
|
|
52
|
Yu W, Peng W, Sha H and Li J:
Hsa_circ_0003998 promotes chemoresistance via modulation of miR-326
in lung adenocarcinoma cells. Oncol Res. 27:623–628. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mizuno H, Honda M, Shirasaki T, Yamashita
T, Yamashita T, Mizukoshi E and Kaneko S: Heterogeneous nuclear
ribonucleoprotein A2/B1 in association with hTERT is a potential
biomarker for hepatocellular carcinoma. Liver Int. 32:1146–1155.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang H, Liang L, Dong Q, Huan L, He J, Li
B, Yang C, Jin H, Wei L, Yu C, et al: Long noncoding RNA miR503HG,
a prognostic indicator, inhibits tumor metastasis by regulating the
HNRNPA2B1/NF-κB pathway in hepatocellular carcinoma. Theranostics.
8:2814–2829. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang Y, Wei Q, Tang Y, Wang Y, Luo Q, Zhao
H, He M, Wang H, Zeng Q, Lu W, et al: Loss of hnRNPA2B1 inhibits
malignant capability and promotes apoptosis via down-regulating
Lin28B expression in ovarian cancer. Cancer Lett. 475:43–52. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shi X, Ran L, Liu Y, Zhong SH, Zhou PP,
Liao MX and Fang W: Knockdown of hnRNP A2/B1 inhibits cell
proliferation, invasion and cell cycle triggering apoptosis in
cervical cancer via PI3K/AKT signaling pathway. Oncol Rep.
39:939–950. 2018.PubMed/NCBI
|