Introduction
Colon cancer is the third most frequently diagnosed
cancer and the second leading cause of cancer death in the United
States (1). In the United States,
104,610 new cases of colon cancer were reported in 2020 (1). Among them, approximately 53,200
mortalities were reported annually in the United States (1). Overall, the incidence and death rates
for colon cancer are on the decline (2). However, this trend is not observed in
younger individuals from 15–35 years old (1–3). The two
major causes of colon cancer are chromosomal instability and
serrated neoplasia (3). Treatment
options for colon cancer include endoscopic and surgical local
excision, preoperative radiotherapy and chemotherapy (3). However, chemoresistance remains an
obstacle to the treatment of colon cancer (4,5).
MicroRNA (miRNA/miR) plays a significant role in
complex physiological and pathological processes through the
regulation of target gene expression via binding to their
3′-untranslated region (UTR) (6).
Usually, each miRNA has multiple target mRNA transcripts, resulting
in various effects, such as cell proliferation, apoptosis,
migration and invasion (7). Previous
studies have highlighted the link between miRNA and the
chemoresistant phenotype of different tumor types, which is
mediated by abnormal regulation of apoptosis (8), cell cycle distribution (9) and activity of drug efflux transporters
(10). Modulating the expression of
miRNA using specific mimics or inhibitors normalizes gene
regulatory networks and signaling pathways, thus sensitizing
cancerous cells to chemotherapy (11). Therefore, miRNA-based gene therapy
may represent an attractive approach for cancer therapy (11,12).
In various types of cancer, such as cervical cancer
(13), non-small cell lung cancer
(14), gastric cancer (15), glioma (16), colorectal cancer (CRC) (17), oral squamous cell carcinoma (18), hepatocellular carcinoma (19), prostate cancer (20), acute myeloid leukemia (21) and rectal cancer (22), miR-188-5p is downregulated. Moreover,
transfection of miR-188-5p mimics reduces proliferation and
migration while promoting apoptosis in A549 and H2126 cells
(14). In in vivo xenograft
models, miR-188-5p inhibits tumor growth of non-small cell lung
cancer (14). Additionally,
miR-188-3p regulates the expression of myeloid/lymphoid or
mixed-lineage leukemia 4 (MLLT4) and promotes the migration of
HCT116 and HRT18 cancer cells (17);
it has also been proposed as a novel independent prognostic factor
in patients with CRC (17). In
locally advanced rectal cancer, miR-188-5p is associated with a
complete pathological response to the neoadjuvant chemoradiotherapy
(22). However, the role of miR-188
in drug-resistant cancer cells has yet to be examined.
Ras GTPase-activating protein 1 (RASA1; also known
as p120/RasGAP) was the first identified RasGAP protein. RASA1 has
been implicated in a number of biological processes, including
actin filament polymerization, cell apoptosis and migration
(23). RASA1 acts as a cancer
suppressor gene in several types of tumor (24–32).
RASA1 suppresses the effect of Ras proteins by enhancing their weak
intrinsic GTPase activity, leading to an increase in the levels of
the inactive, GDP-bound form of Ras (33). This causes aberrant intracellular
signaling through the Ras-RAF-ERK pathway (33). A previous study has demonstrated that
RASA1 protein levels are significantly decreased in colon cancer
cells and that RASA1 is a target of miR-21, which promotes the
malignancy of colon cancer cells (34). The upregulation of RASA1 inhibits
cell proliferation and inhibits the RAS signaling pathway (34). Moreover, the expression levels of
miR-223 and RASA1 are inversely correlated in tumor tissue from
patients with CRC (35). RASA1 is
also a validated target of miR-335, which is downregulated in CRC
(36). Several studies have also
indicated that onco-miR molecules, such as miR-21 and miR-182,
promote tumor angiogenesis and lymph node metastasis by targeting
RASA1 (37,38).
The present study aimed to explore the molecular
role of miR-188-5p in oxaliplatin (OXA) resistance. miR-188-5p may
enhance colon cancer cell chemosensitivity by promoting the
expression of RASA1, providing a new insight into treatment options
for OXA-resistant colon cancer.
Materials and methods
Cell culture and treatment
The human SW480 colon cancer cell line was purchased
from American Type Culture Collection. To induce OXA resistance in
SW480 cells (SW480/OXA), SW480 cells were seeded into a 24-well
plate at a density of 1×105 cells/well and cultured in
the presence of 0.5 mM OXA (Sigma-Aldrich; Merck KGaA; cat. no.
O9512) for 24 h. Subsequently, the medium was replaced with
OXA-free medium, and the cells were cultured for three days. From
day 4, the drug concentration increase started. This procedure was
continued for 6 months with a drug concentration increase at 0.4
µM/month, (0.5–2.5 mM) and the medium was changed at every other
day. After appropriately increasing the concentration of OXA over a
period of 6 months, a stable drug-resistant cell line SW480/OXA was
obtained. Cells were cultured in Dulbecco's modified Eagle's medium
(DMEM; Sigma-Aldrich; Merck KGaA) with 10% Fetal Bovine Serum (FBS;
Gibco; Thermo Fisher Scientific Inc.) and 1%
penicillin/streptomycin solution at 37°C with 5% CO2
saturated humidity.
Hoechst 33342 staining
SW480 and SW480/OXA cells (2×105/well)
were seeded into a fibronectin-coated 12-well plate. After cell
transfection, 5 µg/ml Hoechst 33342 staining solution (Beijing
Solarbio Science & Technology Co., Ltd.; cat. no. C0031) was
added to the cells, which were incubated for 20 min at room
temperature. The cells were then washed three times with PBS.
Coverslips were sealed and visualized under a confocal microscope
(T1-SAM microscope; Nikon Corporation).
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was isolated from SW480 and SW480/OXA
cells (5×105 cells/well in a 6-well plate) using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.;
cat. no. 15596026) according to the manufacturer's instructions.
First-strand cDNA was synthesized using the High-Capacity cDNA
Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.; cat. no. 4368814) according to the manufacturer's
protocol. SYBR Green PCR Master mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.; cat. no. A25741) was used to perform
RT-qPCR. The qPCR thermocycling conditions were as follows: Initial
denaturation at 94°C for 5 min, followed by 40 cycles of
denaturation at 94°C for 15 sec, annealing at 60°C for 25 sec and
extension at 72°C for 30 sec. U6 and GAPDH were used as internal
references for normalization. The 2−ΔΔCq method was
applied to calculate relative mRNA levels (36). The primer sequences used in the study
were: i) GAPDH forward, 5′-TGTTCGTCATGGGTGTGAAC-3′ and reverse,
5′-ATGGCATGGACTGTGGTCAT-3′; ii) RASA1 forward,
5′-CAGTGGACGAAGGTGACTCT-3′ and reverse, 5′-AGGCGTTCTTCTGCTATCGT-3′;
iii) U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′; and iv) miR-188-5p forward,
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCCCTCCAC-3′ and reverse,
5′-ACACTCCAGCTGGGCATCCCTTGCATGGTGG-3′.
Cell transfection
The miR-188-5p inhibitor
(5′-CCCUCCACCAUGCAAGGGAUG-3′; 2′-OMe-modified), negative control
(NC) NC inhibitor (5′-CAGUACUUUUGUGUAGUACAA-3′), miR-188-5p mimics
(sense, 5′-CAUCCCUUGCAUGGUGGAGGG-3′; antisense,
5′-CUCCACCAUGCAAGGGAUGUU-3′), NC mimics (sense,
5′-UUCUCCGAACGUGUCACGUTT-3′; antisense,
5′-ACGUGACACGUUCGGAGAATT-3′). The mimic and inhibitor NCs were
scrambled sequences. RASA1 small interfering (si)RNA
(5′-CAGTTTATGATGGGAGGCCGGTATT-3′), siRNA negative control (siNC,
5′-TTCTCCGAACGTTTCACGTTT-3′), RASA1 overexpression plasmid
(pcDNA3.1-RASA1) and empty vector control (pcDNA3.1) were purchased
from Shanghai GenePharma Co. Ltd. Cells were seeded in a 12-well
plate (4×104 cells/well) and incubated overnight at 37°C
with 5% CO2. At 70% confluency, cells were transfected
with 100 pmol miR-188-5p mimics or NC mimics at room temperature
for 48 h using Lipofectamine® 2000 (cat. no. 11668-500;
Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. SW480 and SW480/OXA cells were
transfected with 50 pmol si-RASA1 or co-transfected with 50 pmol
miR-188-5p inhibitor and si-RASA1. After 48 h, cells were collected
for subsequent experiments.
Bioinformatics analysis
For the determination of potential target genes of
miR-188-5p, various bioinformatics tools, including TargetScan
(http://www.targetscan.org), miRDB
(http://www.mirdb.org/) and PITA (http://genie.weizmann.ac.il/pubs/mir07/mir07_data.html)
databases were used. TargetScan was used to predict the binding
mRNAs (218 mRNAs), similarly, 340 mRNAs and 229 mRNAs were
predicted in miRDB and PITA databases, respectively. When the
common mRNAs from the three databases were assessed, 6 mRNAs were
obtained. RASA1 was one of the predicted target genes of
miR-188-5p.
Luciferase activity assay
Luciferase reporter constructs were obtained by
ligating the wild-type (WT) or mutant (MUT) version of the RASA1
3′-untranslated region (UTR) to the psiECHECK-2 vector containing a
luciferase reporter gene (Promega Corporation; cat. no. C8021).
SW480 and SW480/OXA cells (5×105/well) were seeded into
6-well plates and co-transfected with RASA1 WT or MUT and with
miR-188-5p mimics or NC mimics using Lipofectamine 2000®
(Invitrogen; Thermo Fisher Scientific, Inc.; cat. no. 11668-500). A
Luciferase Reporter Gene Detection kit (Sigma-Aldrich; Merck KGaA;
LUC1-1KT) was used to detect luciferase activity 48 h after
transfection. Luciferase activity was normalized to the
Renilla luciferase activity. Experiments were set up in
triplicate and performed independently three times.
Western blot analysis
SW480 and SW480/OXA cells (1×106
cells/6-cm dish) were collected and lysed in lysis buffer
containing phosphorylase inhibitor cocktail (Roche; Diagnostics;
cat. no. 04693124001) and phenylmethanesulfonyl fluoride. Protein
concentration was measured using the bicinchoninic acid (BCA)
Protein Concentration Determination kit (Beyotime Institute of
Biotechnology) following the manufacturer's instructions. An equal
amount (30 µg) of each protein sample was resolved by SDS-PAGE on
8–12% gels, then transferred to PVDF membranes. Subsequently, the
membranes were blocked using 5% skimmed milk powder for 1 h at room
temperature, then incubated with primary antibodies at 4°C
overnight. The primary antibodies were specific for p21 (Abcam;
cat. no. ab227443; 1:1,000), RASA1 (Abcam; cat. no. ab2922;
1:1,000), Rs-GTP (Abcam; cat. no. ab69747; 1:2,000) and GAPDH
(Abcam; cat. no. ab9485; 1:5,000). Goat anti-rabbit (Sigma-Aldrich;
Merck KGaA; cat. no. 1:5,000) or goat anti-mouse IgG-HRP
(Sigma-Aldrich; Merck KGaA; cat. no. AP-308P; 1:5,000) secondary
antibodies were then added at room temperature for 1 h. The
expression of the target proteins was normalized to the GAPDH
internal control. The protein bands were visualized using an
enhanced chemiluminescence kit (Beyotime Institute of
Biotechnology) according to the manufacturer's instructions and
quantified using the Gel-Pro-Analyzer v.4.0 software (Media
Cybernetics, Inc.)
Immunofluorescence assay
SW480 and SW480/OXA cells (2×105/well)
were seeded on a fibronectin-coated 12-well plate. After
transfection with indicated mimics or inhibitors, the cells were
fixed in 4% paraformaldehyde at room temperature for 10 min, then
blocked with 1% BSA in PBS. The slides were incubated with a
RASA1-specific primary antibody (1:1,000; Abcam; cat. no. ab2922)
at room temperature for 1 h, then washed three times with PBS. An
Alexa Fluor 555-conjugated goat anti-mouse secondary antibody
(1:2,000; Abcam; cat. no. ab150114) was then added at room
temperature for 40 min. The slides were washed with PBS three times
and then stained with DAPI at room temperature for 10 min. For
fluorescence visualisation, the Nikon C1 confocal microscope was
used (Nikon Corporation; magnification, ×100).
Apoptosis assay
SW480 and SW480/OXA cells (2×105/well)
were seeded onto a 6-well plate and transfected with siRNA or
plasmid as indicated. After 48 h, the Annexin V-FITC apoptosis
detection kit (BD Biosciences; cat. no. 556547) was used to detect
apoptotic cells with Annexin V-FITC and propidium iodide (PI)
double staining according to the manufacturer's instructions. Gated
Annexin V+/PI+ cells as late stage apoptosis
and Annexin V+/PI− cells as early stage
apoptosis were assessed. The analysis was performed using a BD
FACScan® II flow cytometer (BD Biosciences), and the
data were analyzed using CellQuest Pro software version 5.1
(Becton, Dickinson and Company).
Cell cycle analysis
Cells (2×105 cells/well) were seeded onto
a 6-well plate and transfected 12 h later. The cells were harvested
48 h after transfection, fixed in ice-cold 70% ethanol for at 4°C
for 12 h, washed with PBS, then stained with 5 mg/ml propidium
iodide (Sigma-Aldrich; Merck KGaA, cat. no. P4170) or isotype
control antibody IgG2A (10 µl/106 cells; R&D
Systems; cat. no. IC003A) in PBS supplemented with RNase A (Roche
Diagnostics; cat. no. 10154105103) for 30 min at room temperature.
The flow cytometry data were acquired using a
FACSCalibur® (BD Biosciences) flow cytometer. Flow
cytometry data were analyzed using Summit v.5.3 (DAT/EM Systems
International). A histogram was plotted according to the
distribution of nuclear DNA content and the frequency of cells in
each phase of the cell cycle was determined based on their DNA
ploidy profile.
Statistical analysis
The data are presented as the mean ± SD and were
analyzed using SPSS 15.0 (SPSS, Inc.) All results are
representative of at least three independent experiments unless
stated otherwise. Unpaired two-tailed Student's t-test was used for
comparisons between two groups. For multi-group comparisons,
one-way ANOVA followed by Tukey's post hoc test was used. P<0.05
was considered to indicate a statistically significant
difference.
Results
Inhibition of miR-188-5p upregulates
RASA1 expression
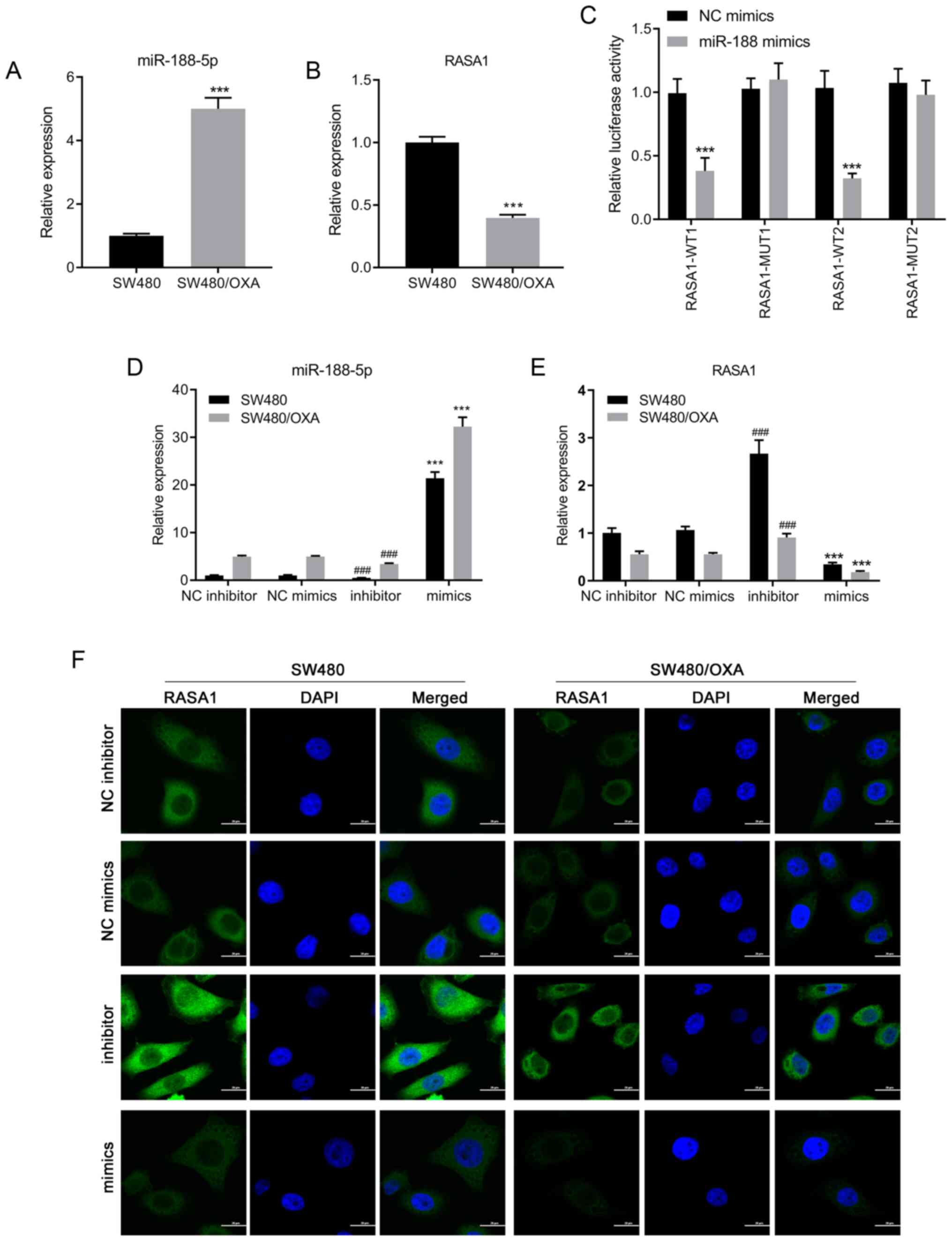
RT-qPCR was performed using SW480 and SW480/OXA
cells to determine the expression levels of miR-188-5p. As shown in
Fig. 1A, the expression of
miR-188-5p was significantly upregulated in SW480/OXA cells
compared with that in SW480 cells (P<0.001), suggesting that the
expression of miR-188-5p may be associated with drug resistance in
colon cancer cells. Bioinformatics analysis predicted that
miR-188-5p potentially bound to RASA1 mRNA. There was a targeted
binding association between miR-188-5p and RASA1. Moreover, mRNA
expression levels of RASA1 were decreased in SW480/OXA cells
compared with those in SW480 cells (Fig.
1B; P<0.001). To confirm whether RASA1 was a direct target
of miR-188-5p, a luciferase assay was performed. Co-transfection
with miR-188-5p mimics significantly reduced the luciferase
activity of WT RASA1, but not MUT RASA1, compared with NC mimics
(Fig. 1C; P<0.001). These results
indicated that RASA1 was a target of miR-188-5p.
SW480 or SW480/OXA cells were transfected with
miR-188-5p mimics or inhibitor to examine the regulatory effects of
miR-188-5p on RASA1 expression. Transfection with miR-188-5p mimics
significantly increased miR-188-5p expression and reduced RASA1
expression, compared with the respective negative controls. By
contrast, miR-188-5p inhibitor transfection reduced miR-188-5p
expression and increased RASA1 expression (Fig. 1D and E; all P<0.001). Moreover,
immunofluorescence was used to detect the expression of RASA1, and
the results suggested that transfection with miR-188-5p mimics
decreased the expression of RASA1 at the protein level (Fig. 1F). Thus, inhibition of miR-188-5p
increased the expression of its target gene, RASA1, both in SW480
and in SW480/OXA cells.
Targeting miR-188-5p promotes
apoptosis in SW480/OXA cells
Transfections with miR-188-5p mimics or inhibitor
were performed in SW480 or SW480/OXA cells to further examine the
role of miR-188-5p on the apoptosis of colon cancer cells. The
miR-188-5p mimics significantly decreased the protein expression
levels of RASA1, compared with NC mimics; whereas miR-188-5p
inhibitor significantly increased the expression of RASA1, compared
with NC inhibitor (P<0.001; Figs.
2A and S1A). In addition, the
expression of p21 decreased in miR-188-5p mimic-transfected cells
and increased in miR-188-5p inhibitor-transfected cells, compared
with that in NC mimics and NC inhibitor, respectively (Fig. 2A). Moreover, the expression of p21
was lower in miR-188-5p mimic-transfected SW480/OXA cells compared
with that in mimic-transfected SW480 cells (Figs. 2A and S2A). As RASA1 suppresses the function of
Ras and enhances the weak intrinsic GTPase activity of Ras proteins
(34), the protein expression levels
of Ras-GTP were then examined. The results demonstrated that
miR-188-5p mimics significantly promoted the protein expression
levels of Ras-GTP compared with NC mimics; whereas miR-188-5p
inhibitor significantly decreased the expression of Ras-GTP
compared with NC inhibitor (P<0.001 and P<0.01, respectively;
Figs. 2A and S1A).The expression of Ras-GTP changed
following miR-188-5p mimic or inhibitor transfection (Figs. 2A and S2A).
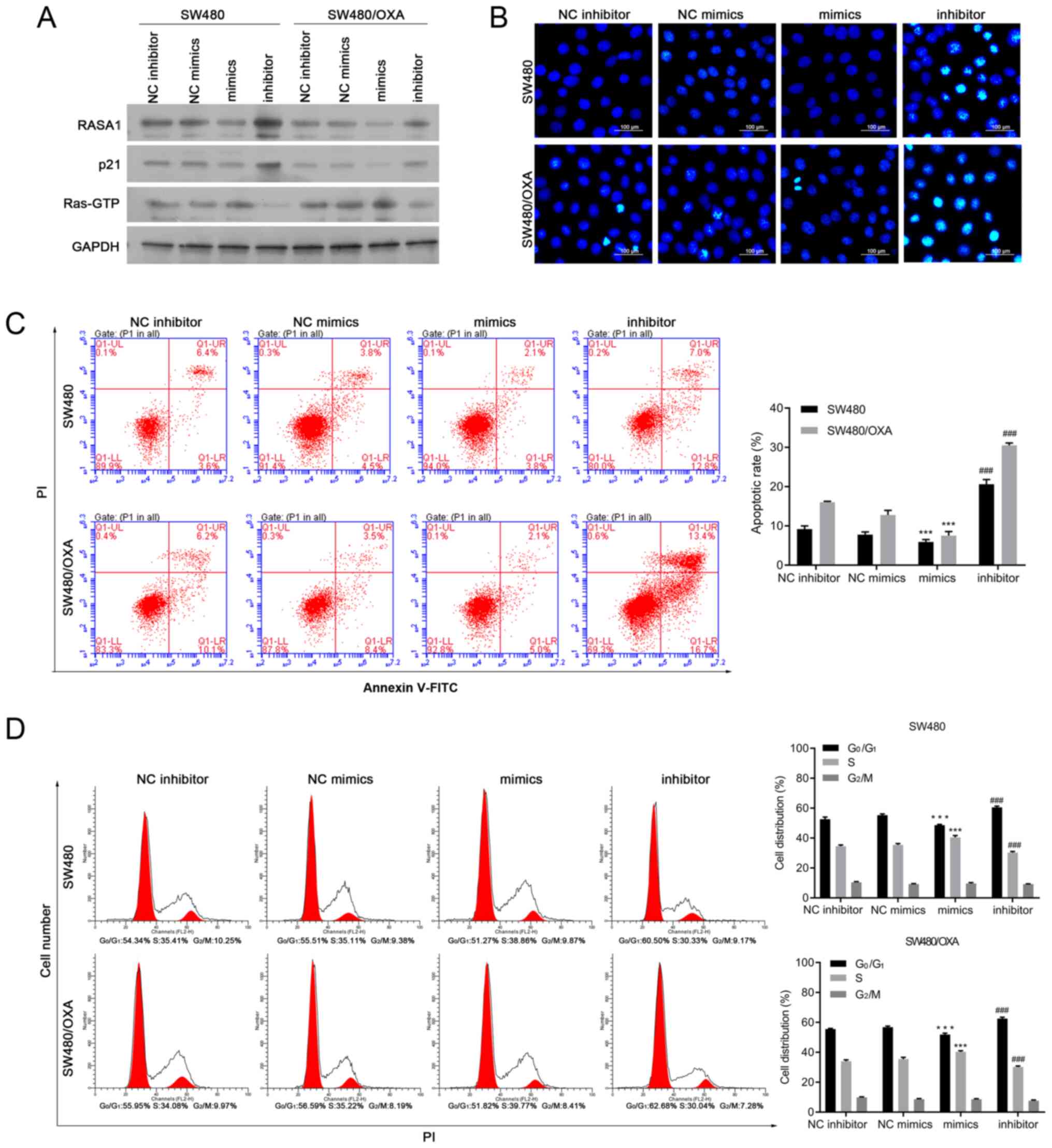 | Figure 2.Downregulation of miR-188-5p induces
cell apoptosis in SW480/OXA cells. (A) Protein expression levels of
RASA1, p21, and Ras-GTP in SW480 or SW480/OXA cells transfected
with miR-188-5p mimics or inhibitors. (B) Hoechst 33342 staining of
SW480 or SW480/OXA cells transfected with miR-188-5p mimics or
inhibitor. Scale bar, 100 µm. (C) Apoptosis of SW480 or SW480/OXA
cells transfected with miR-188-5p mimics or inhibitor.
***P<0.001 vs. NC mimics; ###P<0.001 vs. NC
inhibitor. (D) Cell cycle distribution was analyzed by flow
cytometry. ***P<0.001 vs. NC mimics, ###P<0.001
vs. NC inhibitor. miR, microRNA; oxa, oxaliplatin; RASA1, Ras
GTPase-activating protein 1; PI, propidium iodide; FITC,
fluorescein isothiocyanate; NC, negative control. |
Moreover, in the Hoechst 33342 staining and Annexin
V/PI assays, transfection with miR-188-5p mimics inhibited cell
apoptosis compared with transfection with NC mimics, whereas
miR-188-5p inhibitor promoted cell apoptosis compared with NC
inhibitor in SW480 and SW480/OXA cells (P<0.01 and P<0.001,
respectively; Figs. 2B and C and
S2A). These data suggested that
targeting miR-188-5p promoted apoptosis in both SW480 and SW480/OXA
cells.
The effect of miR-188-5p on cell cycle progression
was also determined. Transfection with miR-188-5p mimics reduced
the G0G1-phase cell population and increased
the proportion of cells in the S phase, while miR-188-5p inhibitor
arrested cells at the G1 phase (Fig. 2D; all P<0.001). These data
suggested that targeting miR-188-5p delays the cell cycle.S1B),
compared with the respective negative controls. In addition, the
overexpression of RASA1 decreased the expression of Ras-GTP,
whereas siRASA1 transfection increased it, both in SW480 and
SW480/OXA cells (Figs. 3B and
S2B). and significantly Conversely,
the expression of p21 decreased (Figs.
3B and S1B) following RASA1
silencing increased in RASA1-overexpressing SW480 and SW480/OXA
cells (P<0.01and P<0.001, respectively; Figs. 3B and S1B).
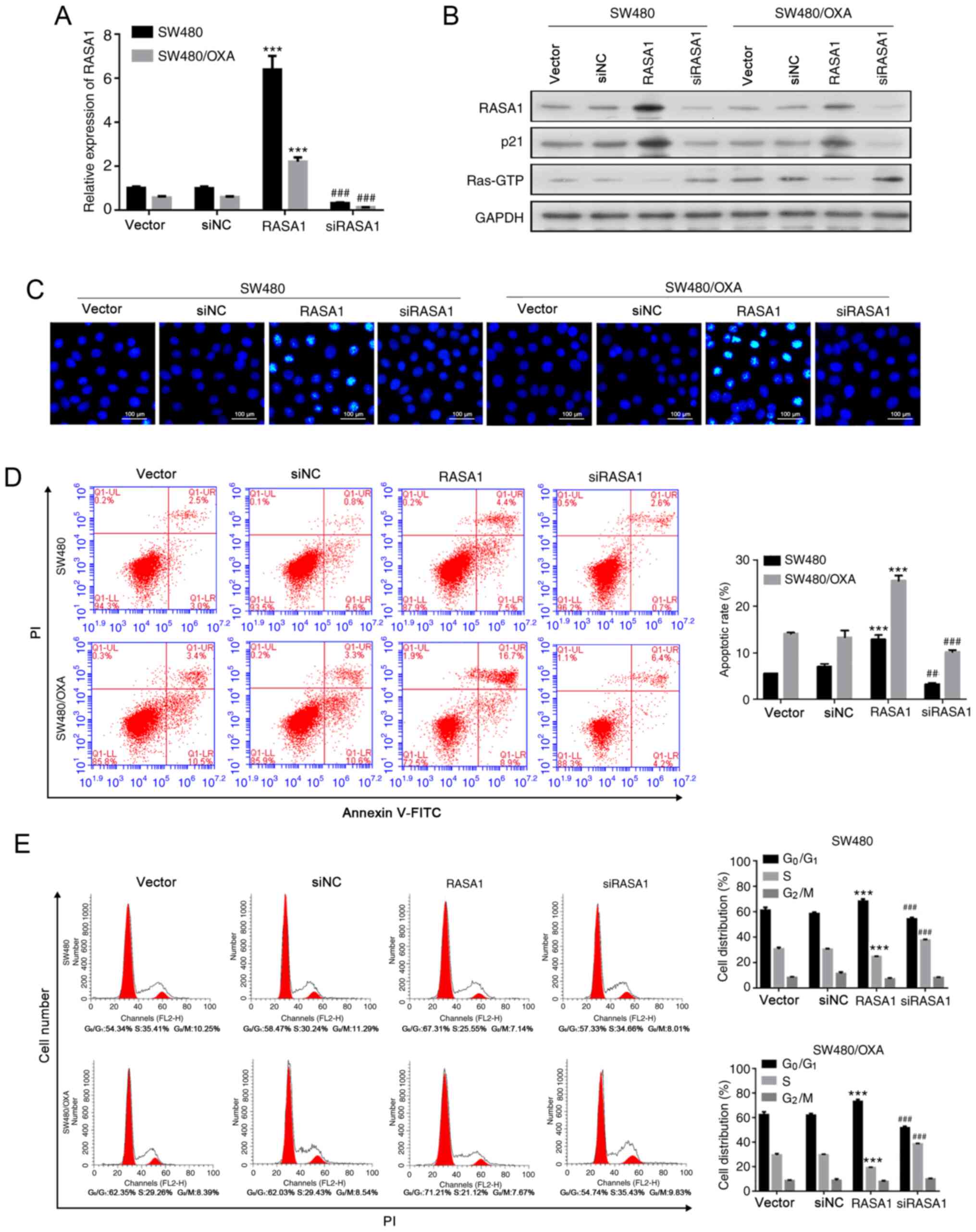 | Figure 3.Overexpression of RASA1 accelerates
apoptosis and inhibits the cell cycle. (A) Relative mRNA expression
of RASA1 in SW480 or SW480/OXA cells transfected with RASA1
overexpression plasmid or RASA1 siRNA. ***P<0.001 vs. vector,
###P<0.001 vs. siNC group. (B) RASA1, p21, and
Ras-GTP protein expression in SW480 or SW480/OXA cells transfected
with RASA1 plasmid or RASA1 siRNA. (C) Hoechst 33342 staining in
SW480 or SW480/OXA cells transfected with RASA1 plasmid or RASA1
siRNA. Scale bar, 100 µm. (D) Apoptosis rate in SW480 or SW480/OXA
cells following transfection with RASA1 plasmid or RASA1 siRNA.
***P<0.001 vs. vector, ###P<0.001 vs. siNC group.
(E) Cell cycle distribution was analyzed by flow cytometry.
***P<0.001 vs. vector; ##P<0.01,
###P<0.001 vs. siNC. Oxa, oxaliplatin; RASA1, Ras
GTPase-activating protein 1; siNC, siRNA negative control; PI,
propidium iodide; FITC, fluorescein isothiocyanate. |
Furthermore, apoptosis was decreased following RASA1
silencing (Figs. 3C and D and
S2B; P<0.005). However, RASA1
overexpression promoted apoptosis, both in SW480 and SW480/OXA
cells (Figs. 3C and D and S2B; P<0.001). In addition, SW480 and
SW480/OXA cells accumulated in the G0G1 phase
of the cell cycle following RASA1 overexpression, and the frequency
of S-phase cells was reduced (Fig.
3E; P<0.001). By contrast, the frequency of
G0G1 cells decreased, whereas the frequency
of S-phase cells increased following RASA1 silencing (Fig. 3E; P<0.001). These findings further
confirmed that RASA1 induced the cell apoptosis of colon cancer
cells.
Suppression of miR-188-5p promotes
SW480/OXA cell apoptosis by increasing RASA1 expression
Co-transfection experiments were performed to
determine whether the increase in apoptosis induced by the
miR-188-5p inhibitor was dependent on RASA1. As shown in Figs. 4A and S1C RASA1 and p21 were upregulated
following miR-188-5p mimic transfection, while the transfection
with miR-188-5p inhibitor inhibited the expression of RASA1 and
p21, compared with cells transfected with the mimics and empty
vector control (P<0.001).
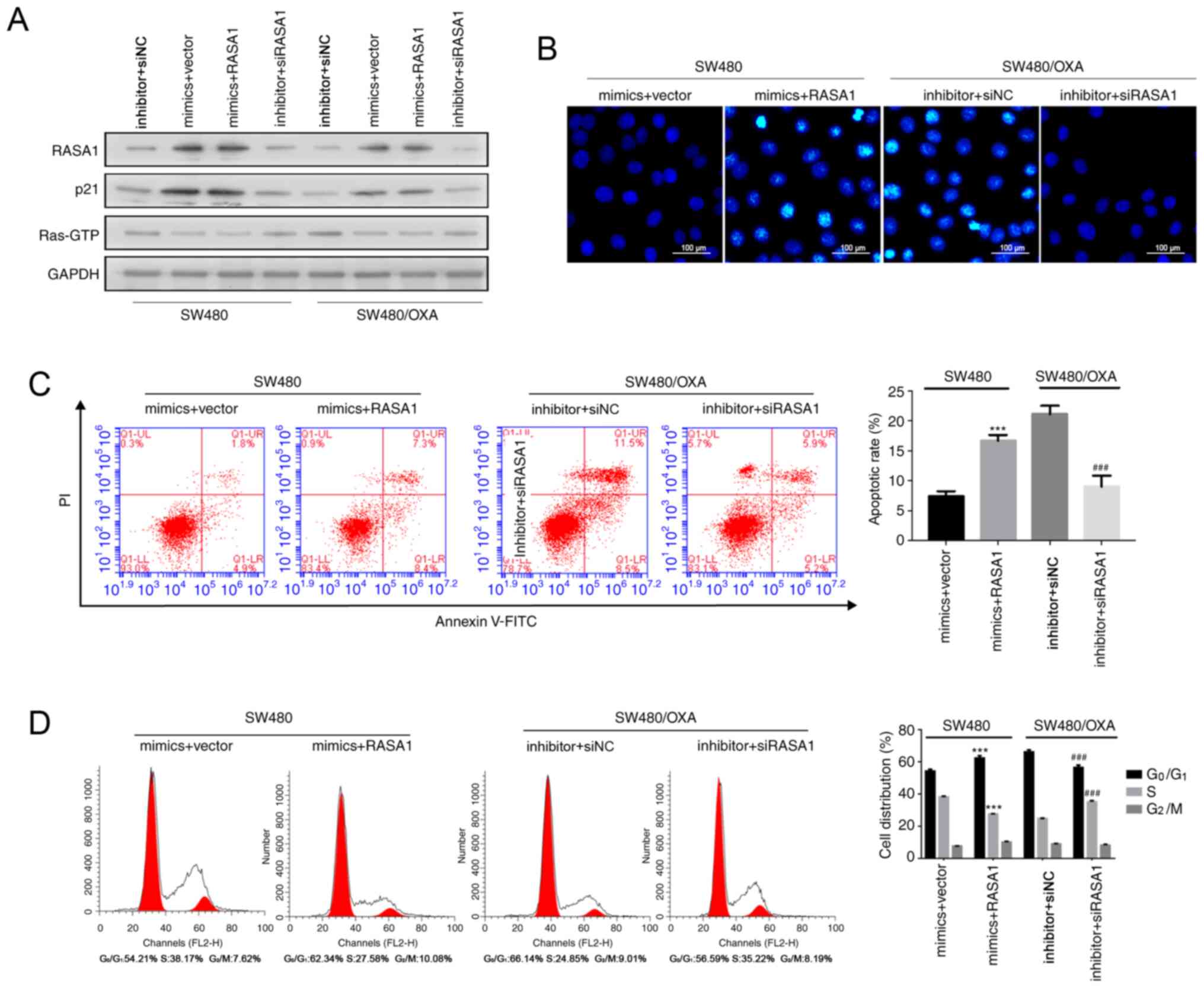 | Figure 4.Downregulation of miR-188-5p promotes
SW480/OXA cell apoptosis by increasing RASA1 expression. (A) RASA1,
p21, and Ras-GTP protein expression in SW480 or SW480/OXA cells
following co-transfection with RASA1 plasmid or RASA1 siRNA and
with miR-188-5p or inhibitor. (B) Hoechst 33342 staining in the
different transfection groups. Scale bar, 100 µm. (C) Apoptosis
rate in the different transfection groups. (D) Cell cycle
distribution was analyzed by flow cytometry. ***P<0.001 vs.
mimics + vector group; ###P<0.001 vs. inhibitor +
siNC group. miR, microRNA; oxa, oxaliplatin; RASA1, Ras
GTPase-activating protein 1; NC, negative control siNC, siRNA
negative control; PI, propidium iodide; FITC, fluorescein
isothiocyanate. |
Hoechst 33342 staining and Annexin V assays showed
that overexpression of RASA1 enhanced apoptosis in miR-188-5p
mimic-treated SW480 cells, compared with cells transfected with the
mimics and empty vector control, whereas RASA1 silencing combined
with miR-188-5p inhibitor transfection reduced the apoptosis rate
(Figs. S2C, 4B and C; P<0.001). Moreover, RASA1
overexpression increased the frequency of cells in the
G0G1 phase while decreasing the frequency of
cells in the S phase of the cell cycle in miR-188-5p
mimic-transfected SW480 cells (Fig.
4D). By contrast, siRASA1 and miR-188-5p inhibitor transfection
resulted in the opposite effect (Fig.
4D; P<0.001). These results indicated that the inhibition of
miR-188-5p induced SW480/OXA cell apoptosis by enhancing RASA1
expression.
Discussion
miR-188-5p was significantly upregulated in both
SW480 and SW480/OXA cells. Transfection with miR-188-5p resulted in
significant inhibition of SW480 and OXA-induced cell apoptosis,
whereas both SW480 and SW480/OXA apoptosis significantly increased
following miR-188-5p inhibition. Moreover, miR-188-5p transfection
downregulated RASA1 expression. Moreover, the overexpression of
miR-188-5p conferred OXA resistance, while downregulation of
miR-188-5p sensitized resistant cells to OXA, which was blocked by
inhibition of RASA1. In summary, the present study suggested that
miR-188-5p could enhance colon cancer cell chemosensitivity by
promoting the expression of RASA1.
Accumulating evidence has demonstrated the role of
miR-188-5p in cancer development. However, the expression of
miR-188-5p in OXA-resistant colon cancer cells has not been
characterized. In the present study, the expression of miR-188-5p
significantly increased in OXA-resistant colon cancer cells. A
previous miRNA sequencing study by Zhao et al (14) involving 228 patients, six miRNA
candidates, including miR-188-3p, were identified as strong
predictors of patient survival. In addition, miR-188-3p promotes
CRC cell migration both in vitro and in vivo partly
by regulating MLLT4 expression, and high miR-188-3p expression is
considered an independent prognostic factor of colon cancer
(17). Moreover, a previous study
reported the role of miR-188-5p in rectal cancer and response to
neoadjuvant radio- and chemotherapy (22). The results of the present study were
partly consistent with previous studies showing that miR-188-5p
promoted colon cancer progression (22,39,40). In
the present study, miR-188-5p inhibition promoted apoptosis,
whereas the upregulation of miR-188-5p inhibited apoptosis in
SW480/OXA cells, suggesting that targeting miR-188-5p promoted
apoptosis in both SW480 and SW480/OXA cells. Moreover, cell cycle
arrest in the G0G1 phase was induced, while
apoptosis was increased following miR-188-5p inhibition. These
results were consistent with previous studies showing that
miR-188-5p controlled cell cycle progression via downregulation of
multiple cyclins and cyclin-dependent kinase complexes involved in
the G1/S transition (20,41).
Although previous studies have indicated that miR-188-5p serves as
a tumor suppressor and is downregulated in multiple types of cancer
(15,42), miR-188-5p was upregulated in
OXA-resistant colon cancer and RASA1 silencing abrogated the
increase in cell apoptosis induced by the miR-188-5p inhibitor in
the present study. Hence, miR-188-5p may enhance colon cancer cell
chemosensitivity by promoting the expression of RASA1.
Of the several potential target genes for miR-188-5p
predicted by TargetScan, PITA and miRDB Human database, RASA1 was
selected for the present study. RASA1 acts as a suppressor of Ras
function and enhances the weak intrinsic GTPase activity of Ras
proteins, resulting in the inactive GDP-bound form of Ras (23). Previous studies have suggested that
RASA1 acts as a tumor suppressor in hepatocellular carcinoma
(42), human bronchial epithelial
cells (43), breast cancer (44) and melanoma (45). In colon cancer cells, RASA1 is
significantly downregulated, and has been identified as a target
gene of miR-21, miR-223 and miR-335 (34,36).
Moreover, onco-miRNA molecules, such as microRNA-21 and
microRNA-182 promote tumor angiogenesis or lymph node metastasis by
targeting RASA1 (37,38). p21 is a tumor suppresser gene that
acts as negative regulator of the G1/S transition
(46). Downregulation of p21 is
observed in various human cancer types, including melanoma,
gastric, ovarian and colon cancers (47). In the present study, the expression
of miR-188-5p was negatively associated with that of RASA1 in
OXA-resistant colon cancer cells. The overexpression of RASA1
promoted apoptosis, induced the expression of p21 and delayed cell
cycle progression. Moreover, apoptosis in SW480 cells was reduced
following miR-188-5p mimic transfection, while overexpression of
RASA1 enhanced cell apoptosis in miR-188-5p mimic-transfected SW480
cells. By contrast, suppression of RASA1 abrogated cell apoptosis
in miR-188-5p inhibitor-transfected SW480/OXA cells. These results
suggested that miR-188-5p inhibition induced SW480/OXA cell
apoptosis by enhancing RASA1 expression.
In conclusion, the present findings suggest that
targeting miR-188-5p promoted apoptosis in SW480/OXA cells and
suppression of miR-188-5p promoted SW480/OXA cell apoptosis by
increasing RASA1 expression, thus providing new insight into
treatment options for OXA-resistant colon cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by The Guilin Scientific
Research and Technology Development Plan Project (grant no.
20170109-50).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW conceived and designed the study, and drafted and
revised the manuscript. XZ, XSL, ZS, SJ and XG performed the
experiments. XKL, XX, and LZ analyzed data and prepared the
figures. XZ and XSL edited and revised the manuscript. HW and XZ
confirmed the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
miR/miRNA
|
microRNA
|
|
OXA
|
oxaliplatin
|
|
RASA1
|
Ras GTPase-activating protein 1
|
|
UTR
|
untranslated region
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
The Lancet Gastroenterology Hepatology, .
Colorectal cancer screening: Is earlier better? Lancet
Gastroenterol Hepatol. 3:5192018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lai M, Liu G, Li R, Bai H, Zhao J, Xiao P
and Mei J: Hsa_circ_0079662 induces the resistance mechanism of the
chemotherapy drug oxaliplatin through the TNF-α pathway in human
colon cancer. J Cell Mol Med. 24:5021–5027. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou Y, Zhang J, Wang K, Han W, Wang X,
Gao M, Wang Z, Sun Y, Yan H, Zhang H, et al: Quercetin overcomes
colon cancer cells resistance to chemotherapy by inhibiting solute
carrier family 1, member 5 transporter. Eur J Pharmacol.
881:1731852020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meister G and Tuschl T: Mechanisms of gene
silencing by double-stranded RNA. Nature. 431:343–349. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garofalo M and Croce CM: MicroRNAs: Master
regulators as potential therapeutics in cancer. Annu Rev Pharmacol
Toxicol. 51:25–43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie Y, Tobin LA, Camps J, Wangsa D, Yang
J, Rao M, Witasp E, Awad KS, Yoo N, Ried T and Kwong KF:
MicroRNA-24 regulates XIAP to reduce the apoptosis threshold in
cancer cells. Oncogene. 32:2442–2451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamanaka S, Campbell NR, An F, Kuo SC,
Potter JJ, Mezey E, Maitra A and Selaru FM: Coordinated effects of
microRNA-494 induce G2/M arrest in human
cholangiocarcinoma. Cell Cycle. 11:2729–2738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu H, Wu H, Liu X, Evans BR, Medina DJ,
Liu CG and Yang JM: Role of MicroRNA miR-27a and miR-451 in the
regulation of MDR1/P-glycoprotein expression in human cancer cells.
Biochem Pharmacol. 76:582–588. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
D'Angelo E, Vicentini C, Agostini M, Kiss
A, Baffa R, Scarpa A and Fassan M: MicroRNAs as tools and effectors
for patient treatment in gastrointestinal carcinogenesis. Curr Drug
Targets. 16:383–392. 2015. View Article : Google Scholar
|
|
12
|
D'Angelo E, Zanon C, Sensi F, Digito M,
Rugge M, Fassan M, Scarpa M, Pucciarelli S, Nitti D and Agostini M:
MiR-194 as predictive biomarker of responsiveness to neoadjuvant
chemoradiotherapy in patients with locally advanced rectal
adenocarcinoma. J Clin Pathol. 71:344–350. 2018. View Article : Google Scholar
|
|
13
|
Gao C, Zhou C, Zhuang J, Liu L, Liu C, Li
H, Liu G, Wei J and Sun C: MicroRNA expression in cervical cancer:
Novel diagnostic and prognostic biomarkers. J Cell Biochem.
119:7080–7090. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao L, Ni X, Zhao L, Zhang Y, Jin D, Yin
W, Wang D and Zhang W: MiroRNA-188 Acts as tumor suppressor in
Non-Small-Cell Lung cancer by Targeting MAP3K3. Mol Pharm.
15:1682–1689. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peng Y, Shen X, Jiang H, Chen Z, Wu J, Zhu
Y, Zhou Y and Li J: MiR-188-5p suppresses gastric cancer cell
proliferation and invasion via Targeting ZFP91. Oncol Res.
27:65–71. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li N, Shi H, Zhang L, Li X, Gao L, Zhang
G, Shi Y and Guo S: MiR-188 inhibits glioma cell proliferation and
cell cycle progression through targeting β-Catenin. Oncol Res.
26:785–794. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pichler M, Stiegelbauer V,
Vychytilova-Faltejskova P, Ivan C, Ling H, Winter E, Zhang X,
Goblirsch M, Wulf-Goldenberg A, Ohtsuka M, et al: Genome-Wide miRNA
Analysis Identifies miR-188-3p as a novel prognostic marker and
molecular factor involved in colorectal carcinogenesis. Clin Cancer
Res. 23:1323–1333. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L and Liu H: MicroRNA-188 is
downregulated in oral squamous cell carcinoma and inhibits
proliferation and invasion by targeting SIX1. Tumour Biol.
37:4105–4113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fang F, Chang RM, Yu L, Lei X, Xiao S,
Yang H and Yang LY: MicroRNA-188-5p suppresses tumor cell
proliferation and metastasis by directly targeting FGF5 in
hepatocellular carcinoma. J Hepatol. 63:874–885. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang H, Qi S, Zhang T, Wang A, Liu R, Guo
J, Wang Y and Xu Y: MiR-188-5p inhibits tumour growth and
metastasis in prostate cancer by repressing LAPTM4B expression.
Oncotarget. 6:6092–6104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jinlong S, Lin F, Yonghui L, Li Y and
Weidong W: Identification of let-7a-2-3p or/and miR-188-5p as
prognostic biomarkers in cytogenetically normal acute myeloid
leukemia. PLoS One. 10:e01180992015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Della Vittoria Scarpati G, Falcetta F,
Carlomagno C, Ubezio P, Marchini S, De Stefano A, Singh VK,
D'Incalci M, De Placido S and Pepe S: A specific miRNA signature
correlates with complete pathological response to neoadjuvant
chemoradiotherapy in locally advanced rectal cancer. Int J Radiat
Oncol Biol Phys. 83:1113–1119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pamonsinlapatham P, Hadj-Slimane R,
Lepelletier Y, Allain B, Toccafondi M, Garbay C and Raynaud F:
p120-Ras GTPase activating protein (RasGAP): A multi-interacting
protein in downstream signaling. Biochimie. 91:320–328. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang XY, Guan M, Vigil D, Der CJ, Lowy DR
and Popescu NC: p120Ras-GAP binds the DLC1 Rho-GAP tumor suppressor
protein and inhibits its RhoA GTPase and growth-suppressing
activities. Oncogene. 28:1401–1409. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Calvisi DF, Ladu S, Conner EA, Seo D,
Hsieh JT, Factor VM and Thorgeirsson SS: Inactivation of Ras
GTPase-activating proteins promotes unrestrained activity of
wild-type Ras in human liver cancer. J Hepatol. 54:311–319. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mai A, Veltel S, Pellinen T, Padzik A,
Coffey E, Marjomäki V and Ivaska J: Competitive binding of Rab21
and p120RasGAP to integrins regulates receptor traffic and
migration. J Cell Biol. 194:291–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun D, Yu F, Ma Y, Zhao R, Chen X, Zhu J,
Zhang CY, Chen J and Zhang J: MicroRNA-31 activates the RAS pathway
and functions as an oncogenic MicroRNA in human colorectal cancer
by repressing RAS p21 GTPase activating protein 1 (RASA1). J Biol
Chem. 288:9508–9518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anand S, Majeti BK, Acevedo LM, Murphy EA,
Mukthavaram R, Scheppke L, Huang M, Shields DJ, Lindquist JN,
Lapinski PE, et al: MicroRNA-132-mediated loss of p120RasGAP
activates the endothelium to facilitate pathological angiogenesis.
Nat Med. 16:909–914. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Organ SL, Hai J, Radulovich N, Marshall
CB, Leung L, Sasazuki T, Shirasawa S, Zhu CQ, Navab R, Ikura M and
Tsao MS: p120RasGAP is a mediator of rho pathway activation and
tumorigenicity in the DLD1 colorectal cancer cell line. PLoS One.
9:e861032014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sharma SB, Lin CC, Farrugia MK, McLaughlin
SL, Ellis EJ, Brundage KM, Salkeni MA and Ruppert JM: MicroRNAs 206
and 21 cooperate to promote RAS-extracellular signal-regulated
kinase signaling by suppressing the translation of RASA1 and
SPRED1. Mol Cell Biol. 34:4143–4164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, Liu T, Sun Q, Niu M, Jiang Y and
Pang D: Downregulation of Ras GTPaseactivating protein 1 is
associated with poor survival of breast invasive ductal carcinoma
patients. Oncol Rep. 33:119–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Y, Qiu C, Tu J, Geng B, Yang J, Jiang T
and Cui Q: HMDD v2.0: A database for experimentally supported human
microRNA and disease associations. Nucleic Acids Res.
42:D1070–D1074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Siderovski DP and Willard FS: The GAPs,
GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int J
Biol Sci. 1:51–66. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gong B, Liu WW, Nie WJ, Li DF, Xie ZJ, Liu
C, Liu YH, Mei P and Li ZJ: MiR-21/RASA1 axis affects malignancy of
colon cancer cells via RAS pathways. World J Gastroenterol.
21:1488–1497. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun D, Wang C, Long S, Ma Y, Guo Y, Huang
Z, Chen X, Zhang C, Chen J and Zhang J: C/EBP-β-activated
microRNA-223 promotes tumour growth through targeting RASA1 in
human colorectal cancer. Br J Cancer. 112:1491–1500. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu Y, Yang H, Yuan L, Liu G, Zhang C, Hong
M, Liu Y, Zhou M, Chen F and Li X: Overexpression of miR-335
confers cell proliferation and tumour growth to colorectal
carcinoma cells. Mol Cell Biochem. 412:235–245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang L, Zhan X, Yan D and Wang Z:
Circulating MicroRNA-21 is involved in lymph node metastasis in
cervical cancer by targeting RASA1. Int J Gynecol Cancer.
26:810–816. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu YJ, Xu B and Xia W: Hsa-mir-182
downregulates RASA1 and suppresses lung squamous cell carcinoma
cell proliferation. Clin Lab. 60:155–159. 2014.PubMed/NCBI
|
|
39
|
Yan S, Yue Y, Wang J, Li W, Sun M, Gu C
and Zeng L: LINC00668 promotes tumorigenesis and progression
through sponging miR-188-5p and regulating USP47 in colorectal
cancer. Eur J Pharmacol. 858:1724642019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu J, Chen Z, Liu W, Zhang Y, Feng W, Yuan
Y, Ye J, Wang L, Cai S, He Y, et al: MicroRNA-188-5p targeting
Forkhead Box L1 promotes colorectal cancer progression via
activating Wnt/β-catenin signaling. Oncol Res. Nov 23–2020.(Epub
ahead of print). View Article : Google Scholar
|
|
41
|
Wu J, Lv Q, He J, Zhang H, Mei X, Cui K,
Huang N, Xie W, Xu N and Zhang Y: MicroRNA-188 suppresses G1/S
transition by targeting multiple cyclin/CDK complexes. Cell Commun
Signal. 12:662014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen YL, Huang WC, Yao HL, Chen PM, Lin
PY, Feng FY and Chu PY: Down-regulation of RASA1 is associated with
poor prognosis in human hepatocellular carcinoma. Anticancer Res.
37:781–785. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hayashi T, Desmeules P, Smith RS, Drilon
A, Somwar R and Ladanyi M: RASA1 and NF1 are preferentially
Co-mutated and define a distinct genetic subset of
smoking-associated non-small cell lung carcinomas sensitive to MEK
inhibition. Clin Cancer Res. 24:1436–1447. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Suarez-Cabrera C, Quintana RM, Bravo A,
Casanova ML, Page A, Alameda JP, Paramio JM, Maroto A, Salamanca J,
Dupuy AJ, et al: A Transposon-based analysis reveals RASA1 Is
involved in triple-negative breast cancer. Cancer Res.
77:1357–1368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sung H, Kanchi KL, Wang X, Hill KS,
Messina JL, Lee JH, Kim Y, Dees ND, Ding L, Teer JK, et al:
Inactivation of RASA1 promotes melanoma tumorigenesis via R-Ras
activation. Oncotarget. 7:23885–23896. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pines J and Rieder CL: Re-staging mitosis:
A contemporary view of mitotic progression. Nat Cell Biol. 3:E3–E6.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cai K and Dynlacht BD: Activity and nature
of p21(WAF1) complexes during the cell cycle. Proc Natl Acad Sci
USA. 95:12254–12259. 1998. View Article : Google Scholar : PubMed/NCBI
|