Introduction
Breast cancer is one of the most common malignant
tumors in female patients (1). Based
on molecular characterization of the estrogen receptor (ER),
progesterone receptor (PR), Ki67 antigen and human epidermal growth
factor receptor-2 (HER2), breast cancer is typically divided into
different types, including luminal A, luminal B, HER-2-positive,
basal-like and ‘normal-like’ breast tumors. Triple-negative breast
cancer (TNBC) is defined as a subset of basal-like breast cancer,
which is negative for ER, PR and HER2. Due to the lack of a
specific hormonal biomarker, surgical resection and chemotherapy
are currently the mainstays of systemic treatment for patients with
TNBC (2,3). A previous study has demonstrated that
TNBC exhibits higher rates of metastasis and recurrence compared
with other breast cancer subtypes (4). Thus, the development of new therapeutic
targets for the treatment of TNBC is essential.
MicroRNA (miRNA/miR) is a class of small non-coding
RNA molecules of ~22 nucleotides typically acting as vital
regulators of target mRNA transcripts by binding to 3′-untranslated
regions (3′-UTRs) (5). miRNA
molecules have been reported to be deregulated in various cancer
types, including TNBC, suggesting that they may be involved in
cancer development and progression (6). A number of studies have demonstrated
that miR-497 is downregulated in various malignant tumors, such as
thyroid cancer (7), lung cancer
(8) and hepatocellular carcinoma
(9). In addition, miR-497 levels
have been shown to be significantly reduced in breast cancer tissue
and cell lines compared with matched non-cancerous breast tissue
samples and MCF-10A cells, respectively (10). Several downstream targets of miR-497
have only recently been identified, such as Bcl2, SRY-box
transcription factor 5, proline, glutamate and leucine rich protein
1, and insulin-like growth factor 1 receptor (IGF1R) (11–13).
Moreover, the function and mechanism of miR-497 in TNBC remain
largely undetermined. Yes-associated protein 1 (YAP1) is a major
downstream transducer of the Hippo pathway commonly identified as
an oncogene (14). Previous studies
have demonstrated that YAP1 is involved in various physiological
process, including cell proliferation, cell apoptosis, stem cell
differentiation and tumorigenesis (15,16).
Specifically, when the Hippo pathway is inactive, YAP1 translocates
to the nucleus, binds to other transcription factors, such as TEA
domain transcription factor 4 (TEAD4), and drives the expression of
target anti-apoptotic and proliferation genes (17). Furthermore, upon activation of the
pathway, YAP1 is phosphorylated by the phosphorylated and activated
form of large tumor suppressor (LATS) 1/2, leading to cytoplasmic
retention of YAP1 by the 14-3-3 protein or degradation (17). Previous studies, including own
research, have confirmed that dysregulation of YAP1 results in
tumorigenesis, including in TNBC (18–21).
Several miRNA molecules, such as miR-195-5p (22), miR-630 (23) and miR-1285-3p (24) were identified as YAP1 regulators.
Nevertheless, further understanding of the post-transcriptional
control of YAP1 in TNBC is necessary.
Therefore, the aim of the present study was to
examine the function and mechanism of miR-497 in TNBC. The
expression of miR-497 in was evaluated in tissue samples from
patients with TNBC. The findings of the current study may provide a
potential target for the treatment of TNBC.
Materials and methods
Clinical samples
In the present study, 36 pairs of TNBC and adjacent
non-cancerous tissue samples were collected from patients in the
Department of Breast and Thyroid Surgery of Shanghai No. 10
People's Hospital (Shanghai, China). All samples were snap-frozen
in liquid nitrogen. None of the patients received any cancer
treatment prior to surgery. TNBC diagnosis was based on a
pathological report of ER, PR and cerbb-2 expression status, as
well as a fluorescence in situ hybridization report of HER2
expression status. TNBC is defined as a tumor that is
ER−PR−HER2−. When the cerbb-2
status in a pathological report is negative or 1+, HER2−
is indicated. When the cerbb-2 status is 2+ or 3+, the HER2 status
is detected by FISH. The study protocols were approved by the
Institutional Ethics Committees of Shanghai No. 10 People's
Hospital (approval no. SHSY-IEC-KY-4.0/17-83/01).
Samples were collected from 36 female patients with
TNBC between December 2014 and December 2018 randomly selected from
the Department of Thyroid and Breast Surgery of Shanghai No. 10
People's Hospital. The patients had a median age of 57 years (age
range, 32–87 years). These patients did not receive any
chemotherapy, radiotherapy or other comprehensive treatment before
operation. The adjacent normal breast tissues were >5 cm away
from the edge of the tumor. The blood vessels and adipose tissue
around the tissues were removed. The tissues were chopped up with a
scalpel and quickly put into a cryopreservation tube and into a
portable liquid nitrogen tank. At the end of the day, the specimens
were transferred to the laboratory liquid nitrogen tank for
preservation. In order to avoid the degradation of tissue RNA by
exogenous RNase, the samples were stored at −80°C immediately after
tissue collection.
For survival analysis, the identification of the
cut-off point to distinguish between high or low miR-497 expression
was obtained by drawing a receiver operating characteristic curve.
The sensitivity, specificity and Youden index were calculated
(sensitivity, 0.909; specificity, 0.778; area under the curve,
0.854). The cut-off point (cut-off, 3.68) of miR-497 expression was
located in the maximum of the Youden index, and this value was
considered as the cut-off point to distinguish between high or low
miR-497 expression. Kaplan-Meier analysis and log-rank test were
used to evaluate the association between miR-497 expression and the
overall survival of patients with TNBC.
Cell culture and transfection
The human TNBC cell lines MDA-MB-231, MDA-MB-468,
MCF-7 and SKBR3, and 293T cells were purchased from The Cell Bank
of Type Culture Collection of The Chinese Academy of Sciences. The
cells were maintained in Dulbecco's Modified Eagle's Medium (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), penicillin (100
units/ml) and streptomycin (100 µg/ml). The human normal breast
epithelial cell line, MCF-10A, was purchased from Shanghai
Zhongqiao Xinzhou Biotechnology Co., Ltd. The MCF-10A cells were
cultured in Mammary Epithelial Cell Medium (ScienCell Research
Laboratories, Inc.). All cells were incubated at 37°C with 5%
CO2. miR-497 mimics (sense, 5′-UGUUUGGUGUCACACGACGAC-3′
and antisense, 5′-GUCGUCGUGUGACAACCAAACA-3′) and non-specific
negative control (NC; sense, 5′-UUCUCCGAACGUGUCACGUTT-3′, and
antisense, 5′-ACGUGACACGUUCGGAGAATT-3′) oligos were purchased from
Guangzhou RiboBio Co., Ltd. For transfection, actively growing
MDA-MB-231 cells (25×105 cells/well) were plated into
6-well plates (BD Biosciences) and cultured with serum- and
antibiotic-free DMEM. When cell density achieved 30–40% confluence,
cells were transfected with 100 nM miR-497 mimics or NC using
Lipofectamine® (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. All cells were
incubated at 37°C with 5% CO2 for 5 h, and complete
media was changed 5 h after transfection. All cells were incubated
at 37°C with 5% CO2 for 48 h before subsequent
experimentation.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from cells or tissues using
the TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). cDNA was synthesized using the PrimeScript RT
kit for mRNA and Prime-Script miRNA cDNA Synthesis kit (both Takara
Bio, Inc.) for miRNA, according to the manufacturer's instructions.
qPCR was carried out using the SYBR® FAST qRT-PCR Master
Mix kit (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol on a 7900HT fast RT-PCR instrument
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The primer
sequences were designed and synthesized by Guangzhou RiboBio Co.,
Ltd. The primer sequences were as follows: miR-497 forward,
5′-GTGCAGGGTCCGAGGT-3′ and reverse, 5′-TAGCCTGCAGCACACTGTGGT-3′; U6
forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′; YAP1 forward,
5′-AGAACAATGACGACCAATAGCTC-3′ and reverse,
5′-GTCGATGGCTAGTCGTAGCATCGAT-3′; and GAPDH forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-TGCTAGCTGGCATGCCCGATCGATC-3′. Relative mRNA and miRNA levels
were normalized to GAPDH and U6, respectively, and were obtained
from the threshold cycle (Cq) values using the 2−ΔΔCq
method (25). qPCR parameters for
mRNA and miRNA quantification were as follows: 2 min at 95°C,
followed by 40 cycles of 30 sec at 95°C and 45 sec at 60°C. Each
sample was tested in triplicate.
Cell proliferation assays
For the MTT assays, transfected cells
(2×103 cells/well) were first plated into 96-well
plates. Cell proliferation was detected at 24, 48, 72 and 96 h
detected using an MTT kit (Sigma-Aldrich; Merck KGaA) according to
the manufacturer's instructions. The 490-nm optical density was
measured using a microplate reader.
For the colony formation assays, transfected cells
(500 cells/well) were transferred to six-well plates. The cell
culture was terminated after 7–10 days or when the colonies were
visible. The plates were washed twice with PBS, and the colonies
were fixed in 95% ethanol for 10 min at room temperature, dried and
stained with 0.1% crystal violet solution for 10 min at room
temperature, and each plate was washed three times with water.
Transwell assays
After 48 h from transfection, cells of each group
were harvested and made into single cell suspension at a density of
5×103/ml using serum-free medium. The Transwell assays
were carried out using a 24-well insert with Matrigel-coated upper
chambers (BD Biosciences). The transfected cells were plated into
the upper chamber in serum-free medium. Medium containing 10% FBS
was added to the lower chamber. The 24-well Matrigel-coated
chambers were incubated at 37°C for 24 h. Subsequently, cells were
fixed with 4% paraformaldehyde for 10 min at room temperature and
stained with a 0.05% crystal violet solution for 10 min at room
temperature. Representative images were obtained using a light
microscope (magnification, ×200) and stained cells were counted in
five randomly selected fields.
Cell cycle analysis
The transfected cells were harvested and fixed in
70% ethanol overnight at 4°C. After washing twice with cold PBS,
250 µl of a 0.05 g/l propidium iodide (PI; Beyotime Institute of
Biotechnology) staining solution was added into each sample,
followed by incubation for 30 min at room temperature. The cell
cycle distribution data was acquired using a FACSCanto II flow
cytometer (BD Biosciences) and analyzed using ModFit LT 3.2 (Verity
Software House, Inc.).
Apoptosis assay
Cells transfected with miR-497 mimics or NC were
incubated in six-well plates for 24 h. The cells were subsequently
stained with fluorescein (FITC)-conjugated Annexin V and propidium
iodide (BD Biosciences) for 30 min at room temperature. The Annexin
V incubation reagent (100 µl) was prepared by combining 10 µl of
the 10X binding buffer (BD Biosciences), 10 µl of propidium iodide
(PI), 1 µl of Annexin V-FITC and 79 µl of deionized, distilled
H2O. The cells were gently resuspended in the Annexin V
incubation reagent at a concentration of
105−106 cells/100 µl. The rate of apoptosis
was detected using a FACSCanto II flow cytometer (BD Biosciences)
and analyzed using the CellQuest Pro software v1.0 (BD
Biosciences).
Dual-luciferase reporter assay
293T cells were seeded in 12-well plates (BD
Biosciences) and cultured until the cells reached 80–90%
confluence. The 3′-UTR segments of the YAP1 mRNA sequence
containing the predicted miR-497 binding sites were amplified by
PCR using the PrimerStar kit (Takara Bio, Inc.) according to the
manufacturer's protocol. The corresponding mutant constructs were
created by mutating the seed regions of the miR-497-binding sites
(5′-UGC UGC U-3′ to 5′-ACG AGC A-3′). Fragments were subcloned into
the XhoI site in the 3′-UTR of Renilla luciferase of
the psiCHECK-2 reporter vector (Shanghai Aibo Si Biological
Technology Co., Ltd.). Cells were transiently co-transfected with
0.2 µg psiCHECK-2/YAP1 3′-UTR or psiCHECK-2/YAP1 3′-UTR mutant
reporter plasmids together with 100 nmol/l miR-497 mimic or miR-NC
using Lipofectamine 2000, according to the manufacturer's
instructions. After 48 h, firefly and Renilla luciferase
activities were measured using a Dual Luciferase Assay (Promega
Corporation). Firefly luciferase activity was normalized to
Renilla luciferase activity, and the ratio of
firefly/Renilla luciferase activity was presented.
Western blot analysis
Cells were washed in ice-cold PBS and resuspended in
RIPA lysis buffer (100 µl/well; Beyotime Institute of
Biotechnology). Subsequently, the cells were collected and
centrifuged at 12,000 × g for 30 min at 4°C (Eppendorf 5804R;
Eppendorf). Supernatants were collected and the protein
concentrations were quantified using a BCA protein assay kit
(Beyotime Institute of Biotechnology). Protein samples were
denatured with 5X SDS loading buffer (Beyotime Institute of
Biotechnology) at 100°C for 10 min. Total protein (20 µg/lane)was
separated by 8% SDS-PAGE (Beyotime Institute of Biotechnology) and
transferred to a 0.45-µm nitrocellulose membrane (Beyotime
Institute of Biotechnology). Following 60 min of blocking at room
temperature with 5% skimmed milk, the membrane was incubated
overnight at 4°C with primary antibodies against YAP1 (1:1,000;
cat. no. BS1701; Bioworld Technology, Inc.) and β-actin (1:1,000;
cat. no. AP0060; Bioworld Technology, Inc.). After washing with
PBS-Tween (0.2%), blots were washed and incubated for 1 h at room
temperature with the anti-mouse secondary fluorescence antibody
(1:2,000; cat. no. 00002-1; Bioworld Technology, Inc.). Finally,
immunoreactive protein bands were detected with an Odyssey Scanning
system (LI-COR Biosciences).
Database analysis
Online databases were used to elucidate the target
genes of miR-497 in breast cancer. Database analysis was performed
using starBase3.0 (http://starbase.sysu.edu.cn) and TargetScan 7.2
(http://www.targetscan.org) according to
the guidelines of the websites.
Statistical analysis
Data were presented as the mean ± SD from at least
three independent experiments. All statistical analyses were
carried out using GraphPad Prism version 6.0 (GraphPad Software,
Inc.) and SPSS version 20.0 (IBM Corp.). All experiments were
independently repeated three times. The differences between two
paired groups (normal vs. tumor tissues) were assessed using paired
Student's t-test. The differences between two unpaired groups were
assessed using unpaired Student's t-test. Differences between
multiple cell lines were assessed using one-way ANOVA followed by
the Least Significant Difference test. Differences between tumor
stages were assessed using Kruskal-Wallis test followed by
Steel-Dwass' post-hoc test. Survival curves were analyzed using the
Kaplan-Meier method and the log-rank test. P<0.05 was considered
to indicate a statistically significant difference.
Results
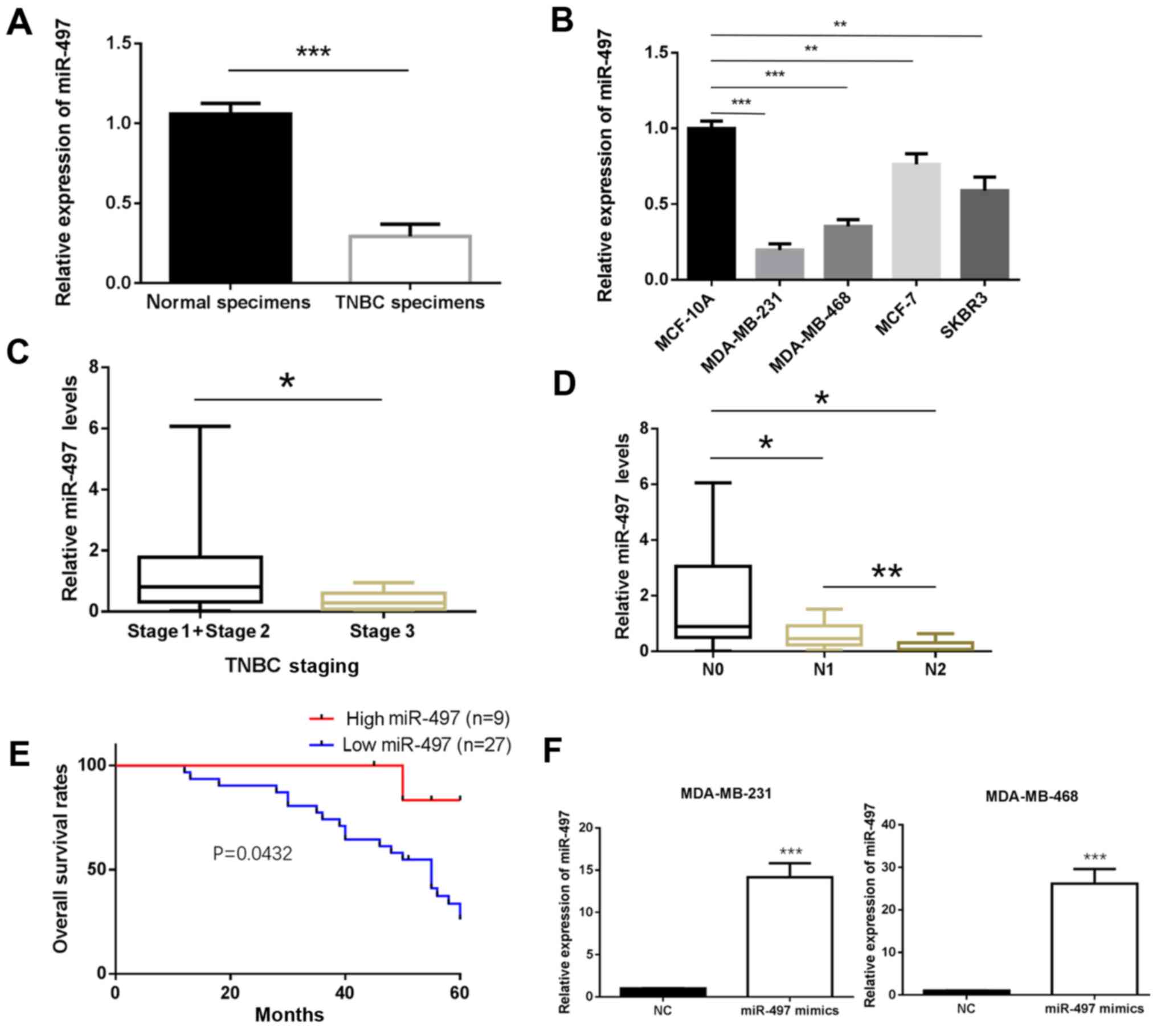
miR-497 expression is reduced in TNBC
tissue samples, which is associated with poor prognosis in patients
with TNBC
The expression of miR-497 in the TNBC and matched
non-cancerous breast tissue samples was determined by RT-qPCR. The
results showed that miR-497 was significantly downregulated in TNBC
tissue compared with normal tissue samples (P<0.001; Fig. 1A). Furthermore, the expression of
miR-497 was significantly decreased in the MDA-MB-231 and
MDA-MB-468 cell lines compared with MCF-10A (P<0.01; Fig. 1B). The experiments carried out in the
MCF-7 (Luminal A type) and SKBR3 (HER-2-overexpressing type) cells
also exhibited a similar trend (P<0.001; Fig. 1B). To further investigate the
clinicopathological and prognostic significance of miR-497, the
clinical data of the 36 patients with TNBC was collected and
analyzed together with the miR-497 levels. As demonstrated in
Fig. 1C and D, the expression of
miR-497 was significantly lower in the TNBC patients with an
advanced TNM stage of the disease (P<0.05; Fig. 1C) and in those with lymph node
metastasis compared with those without (P<0.05; Fig. 1D). The Kaplan-Meier method was used
to evaluate the association between the expression of miR-497 and
the survival rate of patients with TNBC. Patients with low
expression of miR-497 had a shorter survival compared to those with
high expression (P<0.05; Fig.
1E). The MDA-MB-231 and MDA-MB-468 cells were transfected with
miR-497 mimics for subsequent experiments. RT-qPCR analysis
confirmed that miR-497 expression was upregulated in miR-497
mimics-transfected MDA-MB-231 and MDA-MB-468 cells compared with in
cells transfected with miR-NC (P<0.001; Fig. 1F).
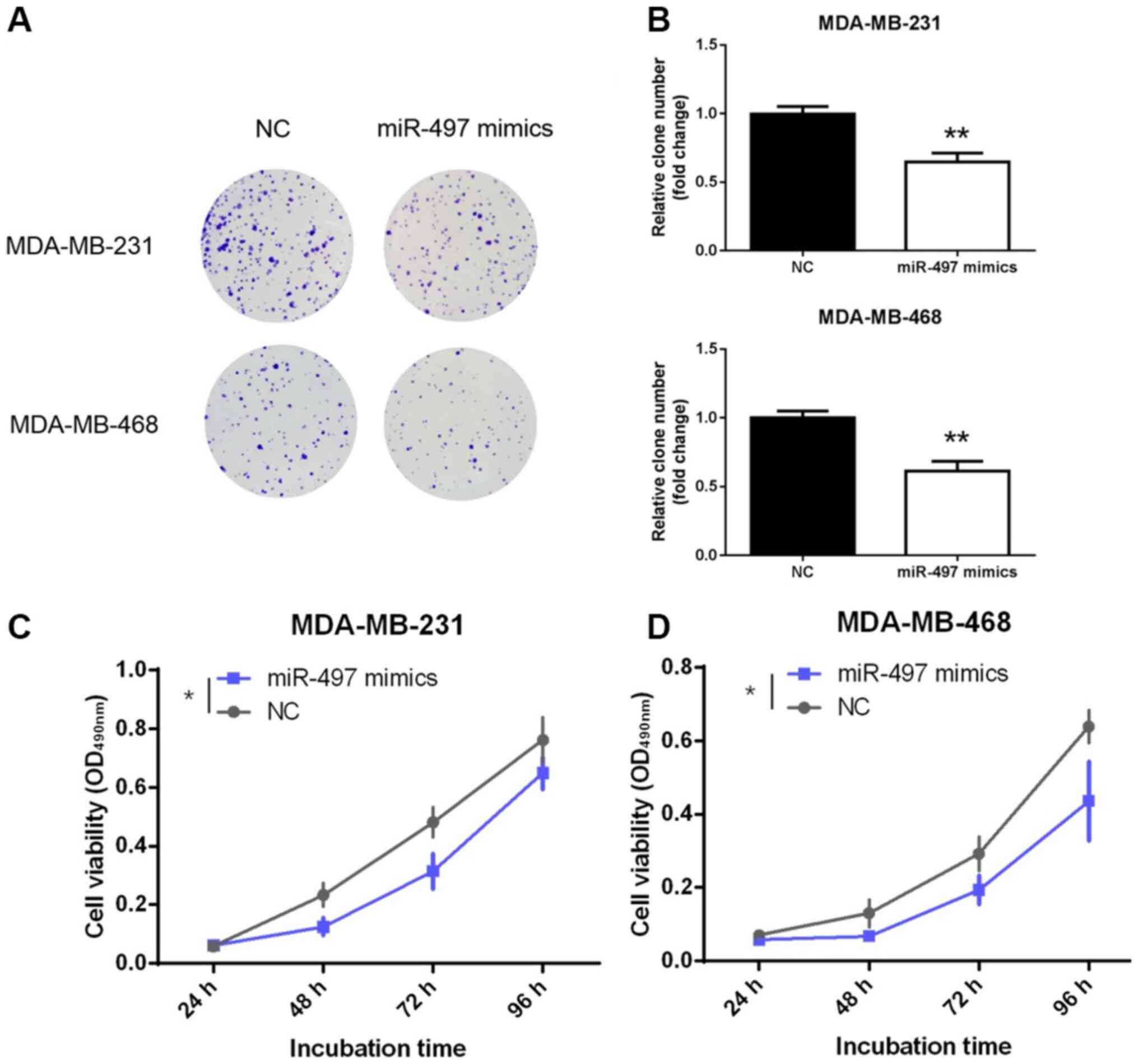
miR-497 inhibits proliferation of TNBC
cells
Decreased expression of miR-497 in TNBC tissues may
reflect tumor-suppressing role in TNBC. To further explore the
function of miR-497, its effect on the proliferation of TNBC cells
was evaluated. MDA-MB-231 and MDA-MB-468 cells were transfected
with miR-497 mimics or NC. Notably, the representative results
obtained from the colony formation assay showed fewer colonies in
the miR-497 mimics group compared with the NC group (P<0.01;
Fig. 2A and B). Moreover, the MTT
assay also demonstrated that overexpression of miR-497
significantly inhibited the viability of the MDA-MB-231 and
MDA-MB-468 cell lines compared with NC at the 96-h time point
(P<0.05; Fig. 2C and D). These
findings confirmed that miR-497 inhibited the proliferation and
viability of the TNBC cells in vitro.
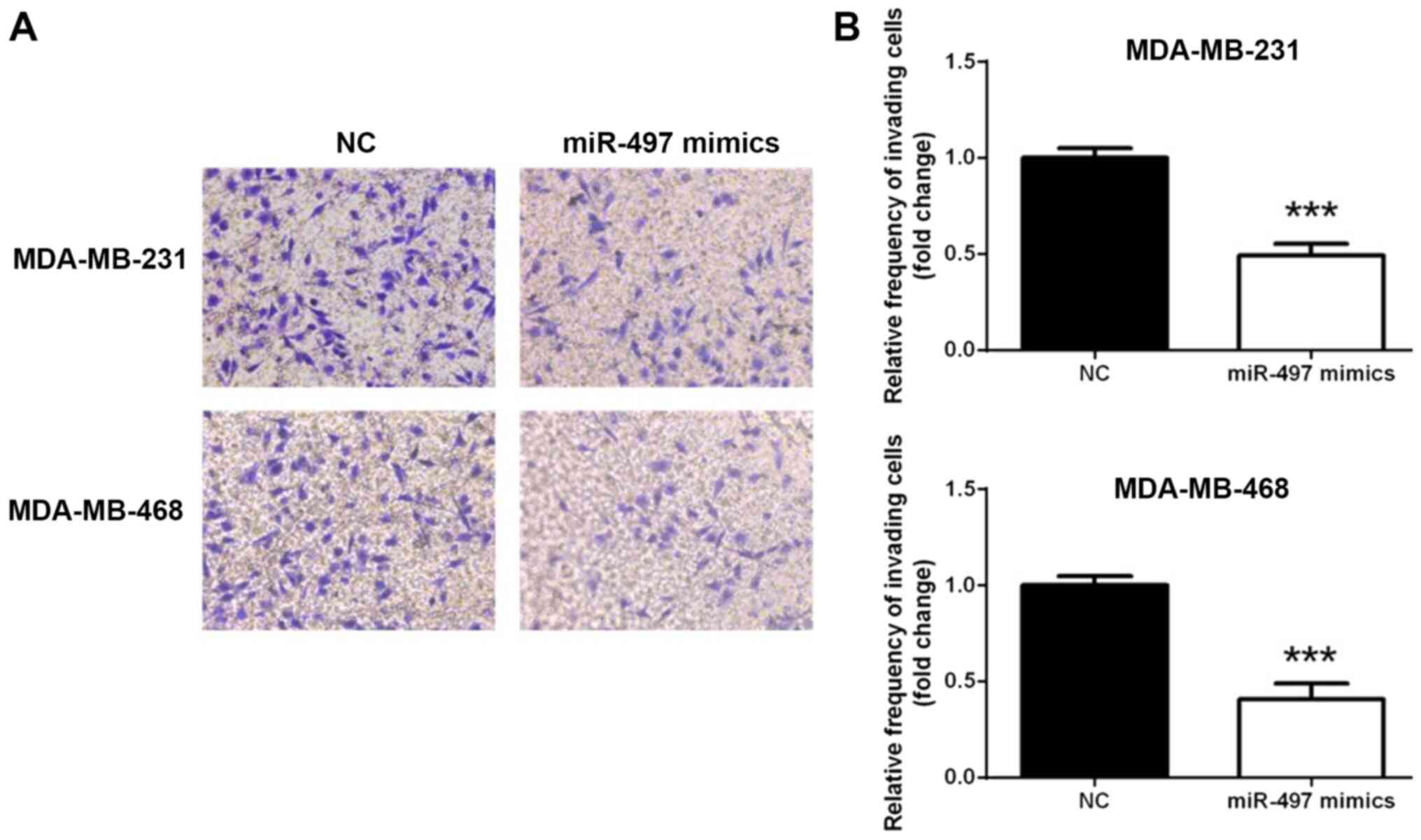
miR-497 inhibits the invasion of TNBC
cells
To investigate whether miR-497 overexpression
affected the TNBC cell invasion, a Transwell assay was conducted.
As shown in Fig. 3, the MDA-MB-231
and MDA-MB-468 cell lines exhibited similar trends in terms of
invasion (Fig. 3A). The number of
cells penetrating the membrane significantly decreased 24 h
following transfection with miR-497 mimics compared with the NC
(P<0.001; Fig. 3B). This
observation indicated that overexpression of miR-497 suppressed
TNBC cell invasion in vitro.
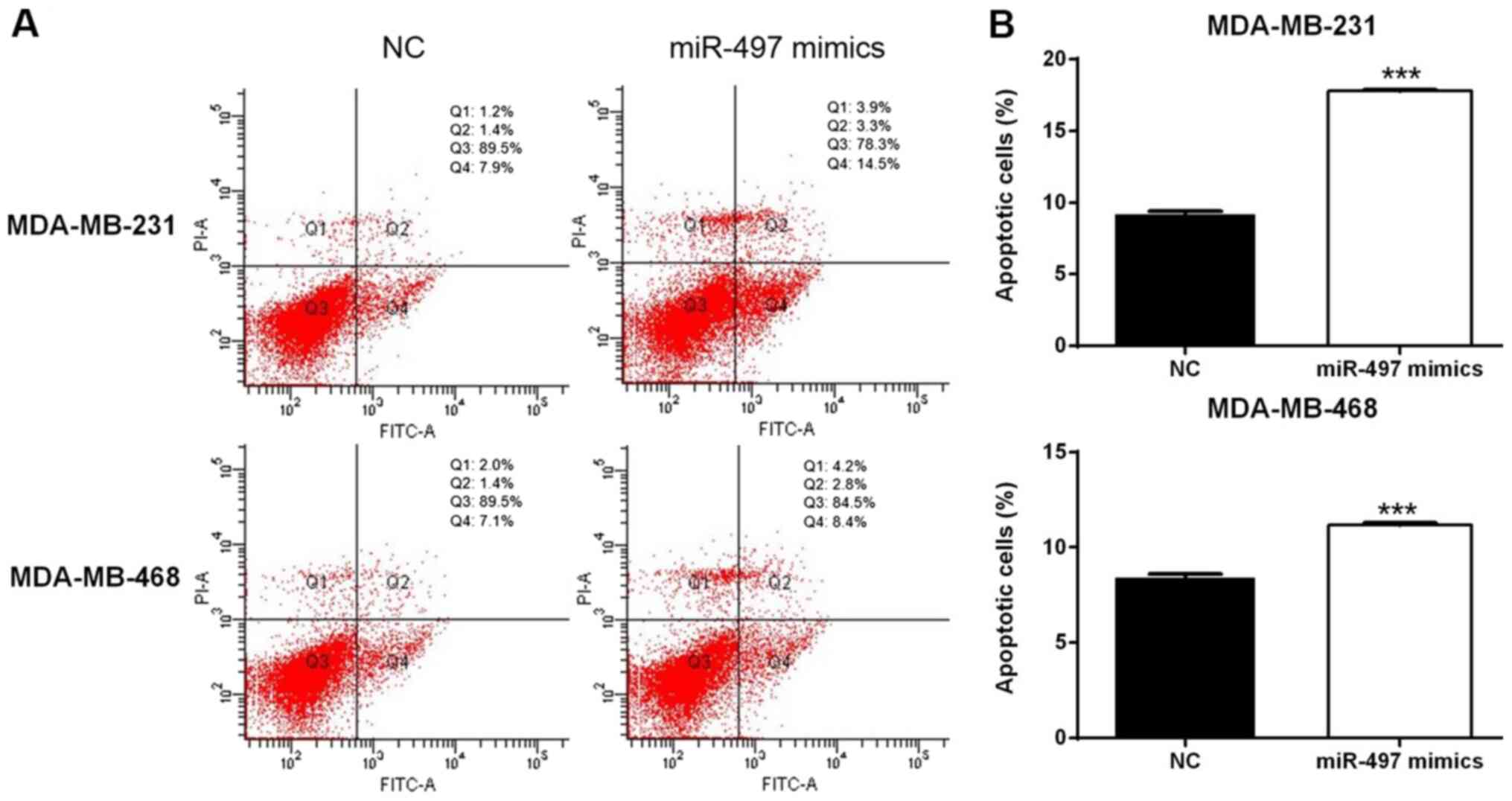
miR-497 disrupts the cell cycle and
induces apoptosis in TNBC cells
Flow cytometry suggested that overexpression of
miR-497 significantly increased the apoptosis rate in the
MDA-MB-231 and MDA-MB-468 cells compared with the respective NC
cells (Fig. 4). In addition, the
cell cycle evaluation demonstrated that the percentage of cells in
the G0/G1 phase increased in the miR-497
mimics group compared with the NC group in both MDA-MB-231 and
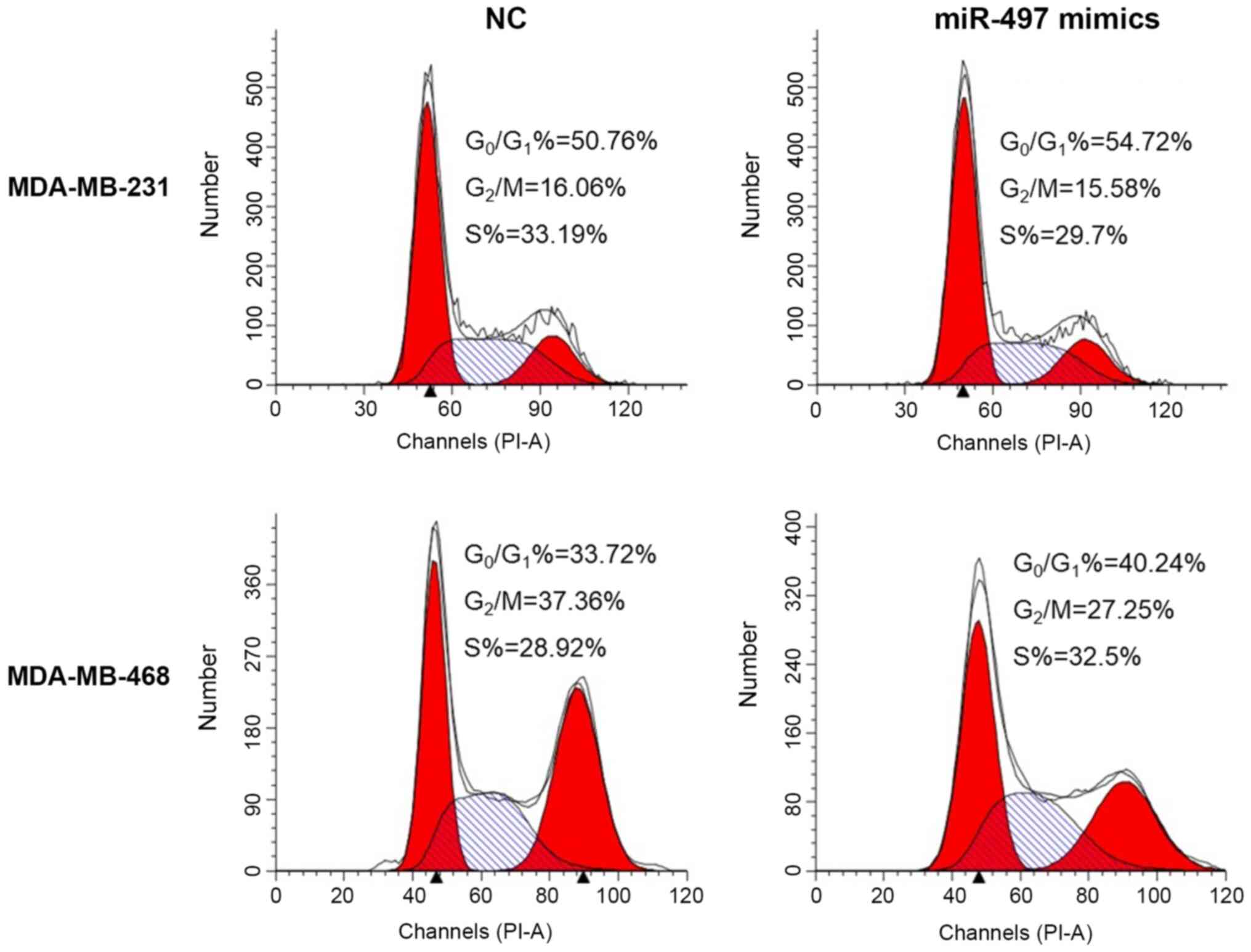
MDA-MB-468 cell lines (Fig. 5).
Thus, the upregulation of miR-497 could impact the cell cycle
distribution and induce apoptosis in TNBC cells.
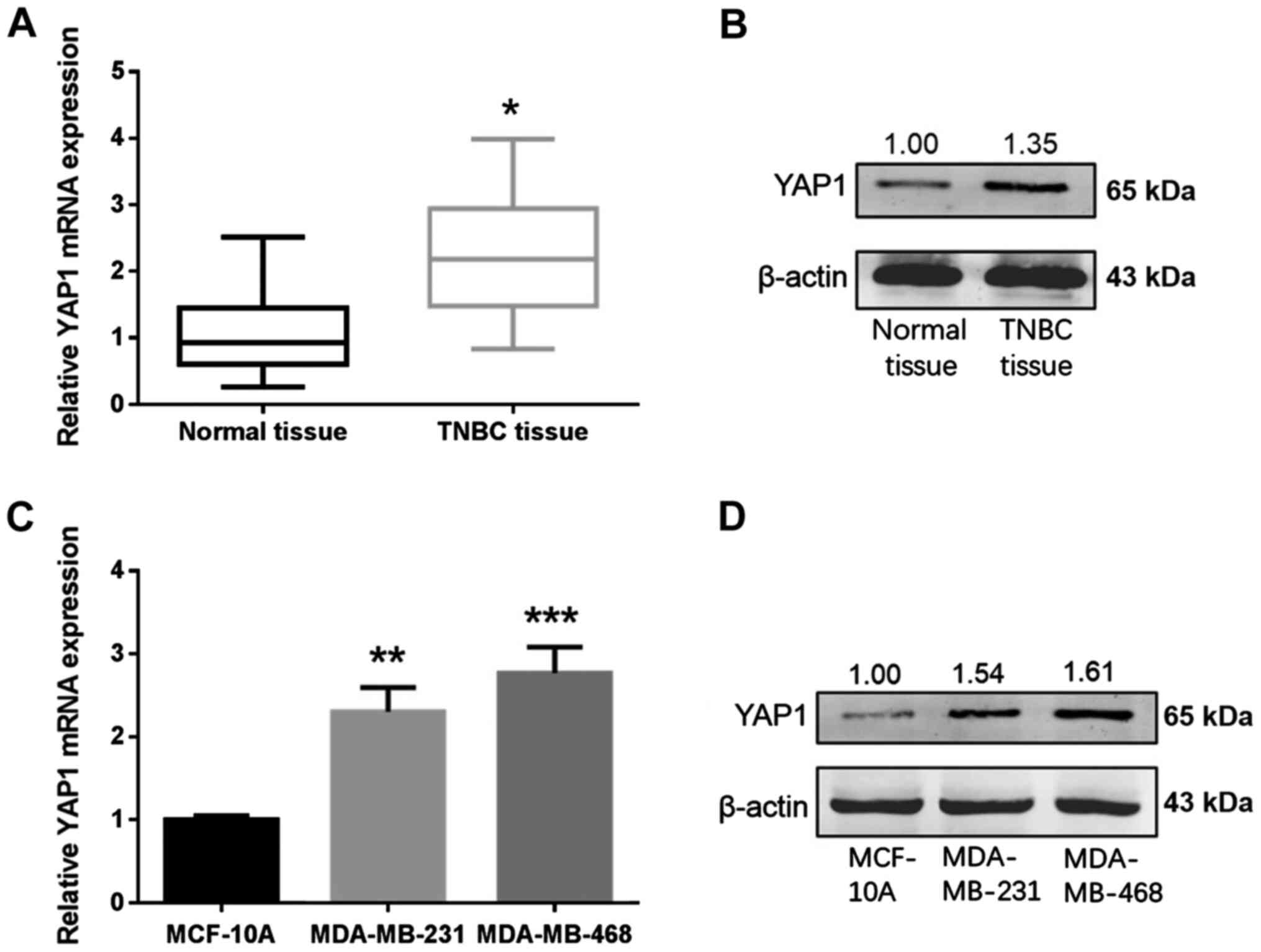
YAP1 is upregulated in TNBC and is a
direct target for miR-497 in TNBC cells
Online databases were used to elucidate the
mechanism of miR-497 in breast cancer. Based on the analysis
carried out in databases, miR-497 exhibited a high prediction score
for interaction with YAP1. The expression levels of YAP1 in 36
paired samples from patients with TNBC and cell lines were measured
using RT-qPCR and western blotting. YAP1 mRNA and protein levels
were increased in the TNBC tissues in comparison with normal
tissues (Fig. 6A and B). YAP1 mRNA
and protein levels were higher in the TNBC cells (MDA-MB-231 and
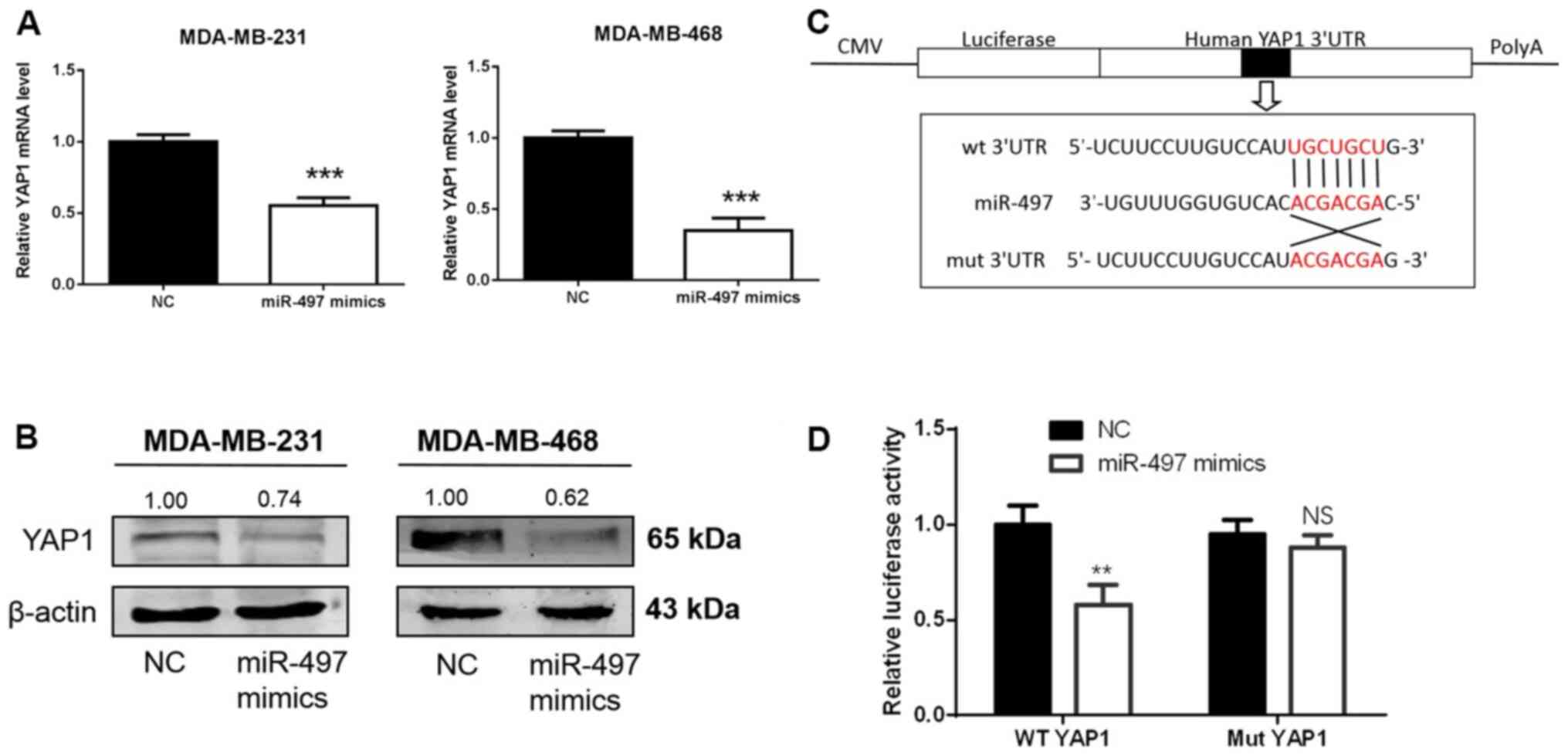
MDA-MB-468) compared with MCF-10A cells (Fig. 6C and D). To determine whether miR-497
regulates endogenous YAP1 at the mRNA or protein levels, miR-497
mimics or NC were transfected into the MDA-MB-231 and MDA-MB-468
cells, and the levels of YAP1 mRNA and protein were detected 48 h
following transfection. YAP1 mRNA and protein levels in the
MDA-MB-231 and MDA-MB-468 cells were markedly downregulated after
transfection with miR-497 mimics (Fig.
7A and B). To establish whether miR-497 directly targeted YAP1,
a luciferase reporter assay was carried out in 293T cells (Fig. 7C). In the wt YAP1 group, luciferase
activity decreased following transfection with the miR-497 mimics
compared with NC, while no evident differences were found in the
mut YAP1 groups (Fig. 7D). Thus,
miR-497 binds to the 3′-UTR of YAP1 mRNA, suggesting that YAP1 is a
direct target of miR-497 in TNBC cells.
Discussion
Breast cancer is the most common malignant tumor in
female patients. The incidence and mortality of breast cancer in
China has markedly increased in recent years (26). It is noteworthy that the five-year
disease-free survival rate of TNBC is the lowest among all
molecular types of breast cancer (26). The health and quality of life of
patients with TNBC is significantly challenged upon the occurrence
of recurrence or metastasis. Thus, the determination of new targets
to classify TNBC as well as the development of appropriate
treatment are important. Recently, various miRNA molecules have
been reported to be aberrantly expressed during the occurrence and
development of TNBC. Some of these miRNA molecules are upregulated
in TNBC tissue, such as miR-155, miR-21, whereas others are
downregulated, including miR-10b, miR-125b and miR-145 (27). Sánchez-González et al
(28) found that low miR-149
expression was associated with reduced macrophage infiltration and
patient survival in lymph node-positive TNBC. Moreover, Lee et
al (29) reported that miR-137
was markedly downregulated in TNBC tissue, and proposed this miRNA
as a target for the treatment of TNBC.
The purpose of the present study was to explore the
potential function and mechanism of miR-497 in TNBC. The results
indicated that expression of miR-497 was downregulated in TNBC
specimens and in the MDA-MB-231 and MDA-MB-468 cell lines. The
clinical outcome analysis demonstrated that low expression of
miR-497 was associated with advanced TNM stage, lymph node
metastasis and reduced patient survival. Therefore, it was
hypothesized that miR-497 may function as a tumor suppressor in
TNBC. To confirm this, miR-497 mimics were transfected into the
MDA-MB-231 and MDA-MB-468 cells to overexpress miR-497. miR-497
markedly suppressed the growth and invasion of the transfected TNBC
cells in vitro. Furthermore, overexpression of miR-497 also
induced apoptosis and arrested the cell cycle in the
G0/G1 phase. Overall, the results of the
current study demonstrated that miR-497 exhibited anti-cancer
properties, which might attenuate the progression of TNBC. These
results were partly analogous to those of previous studies
concerning other types of cancer and molecular types of breast
cancer (30–33).
Online databases were used to establish the
molecular mechanisms of miR-497 in the progression of TNBC. YAP1
was identified as the potential targets of miR-497. A luciferase
reporter assay to validate the regulation of the putative target
YAP1 by miR-497. The results suggested that miR-497 specifically
bound to the 3′-UTR of the YAP1 mRNA. Several targets of miR-497
have been previously identified in various cancer types. For
instance, Shen et al (30)
found that miR-497 significantly inhibited Bcl-w levels in breast
cancer. Subsequently, Bcl-2 (10),
cyclin E1 (31), VEGFR2 (32) and IGF-1R (33) were identified as targets of miR-497.
Moreover, YAP1 was identified as a target of miR-497 in thyroid
papillary carcinoma (7). It has also
been reported that some long non-coding RNA molecules could bind to
miR-497. For example, Li et al (34) suggested that DLX6-AS1 regulated the
progression of neuroblastoma by targeting YAP1 via miR-497.
Moreover, Duan et al (35)
reported that LINC02476 promoted the malignant phenotype of
hepatocellular carcinoma by sponging miR-497. However, our study is
the first to show the relevance of miR-497 modulation in TNBC.
YAP1 is a major downstream transducer of the Hippo
signaling pathway, which is known as a critical player in multiple
human cancer types, including TNBC (36). The Hippo signaling pathway comprises
numerous components and its upstream genes include mammalian
Ste20-like kinases 1/2 (MST1/2) and LATS1/2. Moreover, the
downstream genes include YAP1 and transcriptional coactivator with
the PDZ-binding motif (37). Guo
et al (38) determined that
the overexpression of YAP1 was associated with poor prognosis of
breast cancer patients and induces breast cancer cell growth by
inhibiting PTEN. A recent study also reported that YAP-independent
mechanotransduction drives breast cancer progression, suggesting a
new mechanism underlying the role of YAP1 in breast cancer
(39). In the present study, YAP1
mRNA and protein levels were upregulated in TNBC tissue and cell
lines. Several miRNA molecules have been previously found to be
critical upstream regulators of YAP1. For instance, Li et al
(40) established that miR-141-3p
regulated the proliferation and senescence of stem cells from
apical papilla by targeting YAP1. Furthermore, Chen et al
(41) demonstrated that miR-590-5p
suppressed chemoresistance of hepatocellular carcinoma by targeting
the expression of YAP1. Our previous study also suggested that
miR-506 inhibited cell growth and disrupted the cell cycle by
targeting YAP in breast cancer cells (19). In the current study, YAP1 mRNA and
protein levels in the MDA-MB-231 and MDA-MB-468 cells were
downregulated following overexpression of miR-497, indicating that
miR-497 was an upstream regulator of YAP1.
This study had certain limitations. Firstly, only 36
cases of TNBC tissues could be obtained due to the limited number
of TNBC patients in our hospital. The reliability based on these
samples is relatively low and the results require validation in a
larger number of samples. Secondly, in vivo experiments were
not conducted due to the limitations of the laboratory conditions.
Lastly, the downstream proteins of YAP1 should be explored to gain
more comprehensive understanding of the role of miR-497 in
TNBC.
In conclusion, the present study demonstrated that
miR-497 was downregulated in TNBC tissue samples and cells. It was
also confirmed that miR-497 inhibited cell proliferation and
migration via direct regulation of YAP1 expression. This suggests
that miR-497 may represent a potential therapeutic target for
TNBC.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from The
National Natural Science Foundation of China (grant. no. 82172240),
Shanghai Municipal Health Bureau of Shanghai (grant. no. 201640097)
and Shanghai Science and Technology Commission Guidance Project
(grant. no. 17411967200).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL, KH and LF made substantial contributions to
conception and design. JJ performed the experiments. YL drafted the
manuscript. YL and KH confirmed the authenticity of the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocols were approved by the
Institutional Ethics Committees of Shanghai No. 10 People's
Hospital (approval no. SHSY-IEC-KY-4.0/17-83/01). Written informed
consent was obtained from the participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cardoso F, Harbeck N, Barrios CH, Bergh J,
Cortés J, El Saghir N, Francis PA, Hudis CA, Ohno S, Partridge AH,
et al: Research needs in breast cancer. Ann Oncol. 28:208–217.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhai Q, Li H, Sun L, Yuan Y and Wang X:
Identification of differentially expressed genes between triple and
non-triple-negative breast cancer using bioinformatics analysis.
Breast Cancer. 26:784–791. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mouh FZ, Mzibri ME, Slaoui M and Amrani M:
Recent progress in triple negative breast cancer research. Asian
Pac J Cancer Prev. 17:1595–1608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuan Q, Zheng L, Liao Y and Wu G:
Overexpression of CCNE1 confers a poorer prognosis in
triple-negative breast cancer identified by bioinformatic analysis.
World J Surg Oncol. 19:862021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pratama MY, Pascut D, Massi MN and
Tiribelli C: The role of microRNA in the resistance to treatment of
hepatocellular carcinoma. Ann Transl Med. 7:5772019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ding L, Gu H, Xiong X, Ao H, Cao J, Lin W,
Yu M, Lin J and Cui Q: MicroRNAs involved in carcinogenesis,
prognosis, therapeutic resistance and applications in human
triple-negative breast cancer. Cells. 8:14922019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng H, Dong H, Feng J, Tian H, Zhang H
and Xu L: miR-497 inhibited proliferation, migration and invasion
of thyroid papillary carcinoma cells by negatively regulating YAP1
expression. Onco Targets Ther. 11:4711–4721. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang Q, Li H, Dai X, Zhao D, Guan B and
Xia W: miR-497 inhibits the proliferation and migration of A549
non-small-cell lung cancer cells by targeting FGFR1. Mol Med Rep.
20:3959–3967. 2019.PubMed/NCBI
|
|
9
|
Xu GS, Li ZW, Huang ZP, Brunicardi FC, Jia
F, Song C, Zou HJ and Sun RF: MiR-497-5p inhibits cell
proliferation and metastasis in hepatocellular carcinoma by
targeting insulin-like growth factor 1. Mol Genet Genomic Med.
7:e008602019. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei C, Luo Q, Sun X, Li D, Song H, Li X,
Song J, Hua K and Fang L: MicroRNA-497 induces cell apoptosis by
negatively regulating Bcl-2 protein expression at the
posttranscriptional level in human breast cancer. Int J Clin Exp
Pathol. 8:7729–7739. 2015.PubMed/NCBI
|
|
11
|
Li G, Wang K, Wang J, Qin S, Sun X and Ren
H: miR-497-5p inhibits tumor cell growth and invasion by targeting
SOX5 in non-small-cell lung cancer. J Cell Biochem.
120:10587–10595. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Li K, Lin X, Yao Z, Wang S, Xiong
X, Ning Z, Wang J, Xu X, Jiang Y, et al: Metformin induces human
esophageal carcinoma cell pyroptosis by targeting the miR-497/PELP1
axis. Cancer Lett. 450:22–31. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma W, Feng W, Tan J, Xu A, Hu Y, Ning L,
Kang Y, Wang L and Zhao Z: miR-497 may enhance the sensitivity of
non-small cell lung cancer cells to gefitinib through targeting the
insulin-like growth factor-1 receptor. J Thorac Dis. 10:5889–5897.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Overholtzer M, Zhang J, Smolen GA, Muir B,
Li W, Sgroi DC, Deng CX, Brugge JS and Haber DA: Transforming
properties of YAP, a candidate oncogene on the chromosome 11q22
amplicon. Proc Natl Acad Sci U S A. 103:12405–12410. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan D: The hippo signaling pathway in
development and cancer. Dev Cell. 19:491–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johnson R and Halder G: The two faces of
hippo: Targeting the hippo pathway for regenerative medicine and
cancer treatment. Nat Rev Drug Discov. 13:63–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng Y and Pan D: The hippo signaling
pathway in development and disease. Dev Cell. 50:264–282. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deng X and Fang L: VGLL4 is a
transcriptional cofactor acting as a novel tumor suppressor via
interacting with TEADs. Am J Cancer Res. 8:932–943. 2018.PubMed/NCBI
|
|
19
|
Hua K, Yang W, Song H, Song J, Wei C, Li D
and Fang L: Up-regulation of miR-506 inhibits cell growth and
disrupt the cell cycle by targeting YAP in breast cancer cells. Int
J Clin Exp Med. 8:12018–12027. 2015.PubMed/NCBI
|
|
20
|
Cao L, Sun PL, Yao M, Jia M and Gao H:
Expression of YES-associated protein (YAP) and its clinical
significance in breast cancer tissues. Hum Pathol. 68:166–174.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu C, Li L, Zhang Z, Bi M, Wang H, Su W,
Hernandez K, Liu P, Chen J, Chen M, et al: A non-canonical Role of
YAP/TEAD is required for activation of estrogen-regulated enhancers
in breast cancer. Mol Cell. 75:791–806.e8. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu X, Zhou Y, Ning YE, Gu H, Tong Y and
Wang N: MiR-195-5p inhibits malignant progression of cervical
cancer by targeting YAP1. Onco Targets Ther. 13:931–944. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Q, Ding J, Nan G, Lyu Y and Ni G:
LncRNA NOC2L-4.1 functions as a tumor oncogene in cervical cancer
progression by regulating the miR-630/YAP1 pathway. J Cell Biochem.
120:16913–16920. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu XH, Dai J, Shang HL, Zhao ZX and Hao
YD: miR-1285-3p is a potential prognostic marker in human
osteosarcoma and functions as a tumor suppressor by targeting YAP1.
Cancer Biomark. 25:1–10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thakur V and Kutty RV: Recent advances in
nanotheranostics for triple negative breast cancer treatment. J Exp
Clin Cancer Res. 38:4302019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Piasecka D, Braun M, Kordek R, Sadej R and
Romanska H: MicroRNAs in regulation of triple-negative breast
cancer progression. J Cancer Res Clin Oncol. 144:1401–1411. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sánchez-González I, Bobien A, Molnar C,
Schmid S, Strotbek M, Boerries M, Busch H and Olayioye MA: miR-149
suppresses breast cancer metastasis by blocking paracrine
interactions with macrophages. Cancer Res. 80:1330–1341. 2020.
View Article : Google Scholar
|
|
29
|
Lee SJ, Jeong JH, Kang SH, Kang J, Kim EA,
Lee J, Jung JH, Park HY and Chae YS: MicroRNA-137 inhibits cancer
progression by targeting Del-1 in triple-negative breast cancer
cells. Int J Mol Sci. 20:61622019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen L, Li J, Xu L, Ma J, Li H, Xiao X,
Zhao J and Fang L: miR-497 induces apoptosis of breast cancer cells
by targeting Bcl-w. Exp Ther Med. 3:475–480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luo QF, Li XY, Gao Y, Long Y, Chen L,
Huang YX and Fang L: MiRNA-497 regulates cell growth and invasion
by targeting cyclin E1 in breast cancer. Cancer Cell Int.
13:952013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tu Y, Liu L, Zhao D, Liu Y, Ma X, Fan Y,
Wan L, Huang T, Cheng Z and Shen B: Overexpression of miRNA-497
inhibits tumor angiogenesis by targeting VEGFR2. Sci Rep.
5:138272015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo ST, Jiang CC, Wang GP, Li YP, Wang CY,
Guo XY, Yang RH, Feng Y, Wang FH, Tseng HY, et al: MicroRNA-497
targets insulin-like growth factor 1 receptor and has a tumour
suppressive role in human colorectal cancer. Oncogene.
32:1910–1920. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li C, Wang S and Yang C: Long non-coding
RNA DLX6-AS1 regulates neuroblastoma progression by targeting YAP1
via miR-497-5p. Life Sci. 11:1176572020. View Article : Google Scholar
|
|
35
|
Duan Y, Zhao M, Jiang M, Li Z and Ni C:
LINC02476 promotes the malignant phenotype of hepatocellular
carcinoma by sponging miR-497 and increasing HMGA2 expression. Onco
Targets Ther. 13:2701–2710. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Andrade D, Mehta M, Griffith J,
Panneerselvam J, Srivastava A, Kim TD, Janknecht R, Herman T,
Ramesh R and Munshi A: YAP1 inhibition radiosensitizes triple
negative breast cancer cells by targeting the DNA damage response
and cell survival pathways. Oncotarget. 8:98495–98508. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheng H, Zhang Z, Rodriguez-Barrueco R,
Borczuk A, Liu H, Yu J, Silva JM, Cheng SK, Perez-Soler R and
Halmos B: Functional genomics screen identifies YAP1 as a key
determinant to enhance treatment sensitivity in lung cancer cells.
Oncotarget. 7:28976–28988. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo L, Chen Y, Luo J, Zheng J and Shao G:
YAP1 overexpression is associated with poor prognosis of breast
cancer patients and induces breast cancer cell growth by inhibiting
PTEN. FEBS Open Bio. 9:437–445. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee JY, Chang JK, Dominguez AA, Lee HP,
Nam S, Chang J, Varma S, Qi LS, West RB and Chaudhuri O:
YAP-independent mechanotransduction drives breast cancer
progression. Nat Commun. 10:18482019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Z, Ge X, Lu J, Bian M, Li N, Wu X, Li
Y, Yan M and Yu J: MiR-141-3p regulates proliferation and
senescence of stem cells from apical papilla by targeting YAP. Exp
Cell Res. 383:1115622019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen M, Wu L, Tu J, Zhao Z, Fan X, Mao J,
Weng Q, Wu X, Huang L, Xu M and Ji J: miR-590-5p suppresses
hepatocellular carcinoma chemoresistance by targeting YAP1
expression. EBioMedicine. 35:142–154. 2018. View Article : Google Scholar : PubMed/NCBI
|