Introduction
Over the years, several therapeutic advances have
been made to cure malignant lymphomas, especially, non-Hodgkin's
lymphoma (NHL) which is much less predictable compared to Hodgkin's
lymphomas. Of importance, diffuse large B-cell lymphoma (DLBCL)
represents the most common NHL subtype, which poses a major
clinical challenge due to its heterogeneity, variable efficacy,
multiple side effects, and frequent relapses.
Recent studies using programmed cell death 1 (PD-1)
blockade have shown some promising outcomes in the phase I trial of
B-cell non-Hodgkin's lymphoma (B-NHL) (1,2). PD-1
and its ligand (PD-L1/L2) have also been pronounced as diagnostic
and prognostic determinant in lymphomas (3). This can be evident from
immunohistochemistry-based studies where the variable expression of
PD-L1 has been observed in classical Hodgkin's lymphoma (87–100%)
(4), diffuse large B-cell lymphoma
(11–31%) (5,6) and Burkitt's lymphoma (0%) (7).
Increasing the clinical utility of PD blockades
alone or with combination therapies is currently gaining momentum,
and in this context, cytokine-induced killer (CIK) cells are
emerging as a new potential partner. CIK cells, as heterogeneous
subset of ex vivo expanded T lymphocytes, exhibit
cytotoxicity toward tumor cells thus contribute to prolong the
survival in cancer patients (8–10). The
use of autologous and allogeneic CIK cells in the clinical trials
of acute myeloid leukemia, chronic myeloid leukemia, and chronic
lymphocytic leukemia had already demonstrated an innovative
clinical perspective (11–13). However, the cytotoxicity of CIK cells
against B-NHL has not been fully elucidated. Also, the efficacy of
combining PD-1 blockade with CIK cells in B-NHL (in vitro or
in vivo) remains unclear.
Therefore, we aimed to investigate the cytotoxic
potential of CIK cells in B-NHL cell lines and further elucidate
the relative contribution of PD-1/PD-L1 inhibitors towards
CIK-mediated antitumor immune response. Given that PD-1 can
suppress immune inactivation, whereas CD40L can promote it, we also
examined the effects of CD40L blockade under the same experimental
conditions.
Materials and methods
Cell culture and generation of CIK
cells
CIK cells were cultured in RPMI-1640 medium (PAN
Biotech) supplemented with 10% heat-inactivated FBS, 1%
penicillin/streptomycin, and 2.5% HEPES (Gibco; Thermo Fisher
Scientific, Inc.). Human B-lymphoblast cell lines: DAUDI (Burkitt's
lymphoma, DSMZ) and SU-DHL-4 (diffuse large B-cell lymphoma, ATCC)
were used in the study. RPMI-1640 medium supplemented with 10%
heat-inactivated FBS (Sigma-Aldrich Chemie GmbH) and 1%
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.) was
used to culture tumor cells (37°C, 5% CO2). All cell
lines were mycoplasma negative as confirmed by MycoAlert™
mycoplasma detection kit (Lonza).
CIK cells generation and
expansion
For CIK cell generation, peripheral blood
mononuclear cells (PMBCs) were derived from buffy coats of healthy
volunteers received from the Blutspendedienst at the University
Hospital Bonn. Approval of the ethics committee of the University
Hospital Bonn was obtained, including signed informed consent from
the volunteers. CIK cells were generated as previously described
protocol (14). Briefly, a standard
gradient density centrifugation using Pancoll (Pan-Biotech) was
performed. Subsequently, the PBMC layer containing the lymphocytes
was removed and washed one time with PBS/0.4% EDTA, and treated
with an erythrocyte lysis buffer (Biolegend). With sequential
addition of IFN-γ 1,000 IU/ml on day 0, and 50 ng/ml monoclonal
antibody against CD3 (anti-CD3 mAb) and 100 IU/ml interleukin-1β
(IL-1β) and 600 IU/ml interleukin-2 (IL-2) on the next days, the
cells were expanded.
PD-L1 and PD-L2 cell surface
expression detection by flow cytometry
CIK cells at day 14 were cultured with
5×105 CFSE-labeled DAUDI and/or SU-DHL-4 cells,
primarily, at E/T (effector cell-CIK cell/target cell-tumor cell)
ratio of 5:1 for 24 h. PE-conjugated anti-PD-L1, BV421-conjugated
anti-PD-L2, and PerCP-conjugated 7-AAD (BD Bioscience) were used
for the flow cytometric detections. CIK cells were generated from
different donors (n=3).
Cell viability assays
Despite the different detection principles for
cytotoxicity evaluation, we used two parallel approaches (MTT assay
and CCK-8 cell viability assay) to avoid any misleading results.
Both assays were performed, as described by the manufacturer (MTT
assay: Sigma-Aldrich; CCK-8 cell viability assay: Dojindo). In MTT
assay, the detection was measured at 560 nm using a plate reader
(BMG Labtech) and the data were normalized to the amount of CIK
cells used. While, the OD measurements of CCK-8 assay were taken at
450 nm. Untreated tumor cells (as negative control) and CIK cells
co-cultured with tumor cells (as positive control) at different E/T
ratios of 0.1:1, 1:1, 3:1 were used in the experimental setup.
Blockade of receptor-ligand
interaction
To block the PD-1 receptor present on CIK cells, a
polyclonal human PD-1 antibody (R&D Systems) was used and CIK
cells were incubated with 3–12 µg/ml anti-rhPD-1 antibody (for 2 h)
before incubation with the tumor cells. Similarly, a polyclonal
human B7-H1/PD-L1 antibody (R&D Systems) was used to block
PD-L1 expressed by the tumor cell lines. Here again, tumor cells
were incubated with 1–5 µg/ml anti-B7-H1/PD-L1 for 2 h before
incubation with CIK cells. In case of CD40L expressed on the tumor
cells, a human CD40L/TNFSF5 monoclonal antibody (R&D Systems)
was used and the tumor cells were incubated with 0.03–0.1 µg/ml
anti-rhCD40L for 2 h before incubation with CIK cells.
Human IFN-γ ELISA
To determine the amount of IFN-γ produced by CIK
cells, a Duoset® Human IFN-γ ELISA kit (R&D Systems)
was used, as described by the manufacturer. The final measurements
were performed at 450 and 540 nm wavelengths and the data were
analyzed using MARS data analysis (BMG Labtech).
Statistical analysis
Unpaired, two-tailed Student's t-test was performed
to evaluate the effect of CIK incubation with tumor cells on
PD-L1/PD-L2 expression compared with the untreated tumor group at
different ratios. One-way ANOVA with Turkey's post-hoc test was
performed to compare multiple groups. A 4-PL non-linear regression
model was used to calibrate the data for the ELISA assay.
Statistical analysis was performed using Prism software (GraphPad
Prism version 5.0 f) and a value of P<0.05 was considered
significant (*P<0.05; **P<0.005; ***P<0.001).
Results
CIK cells displayed cytotoxicity
towards B-cell non-Hodgkin leukemia cells
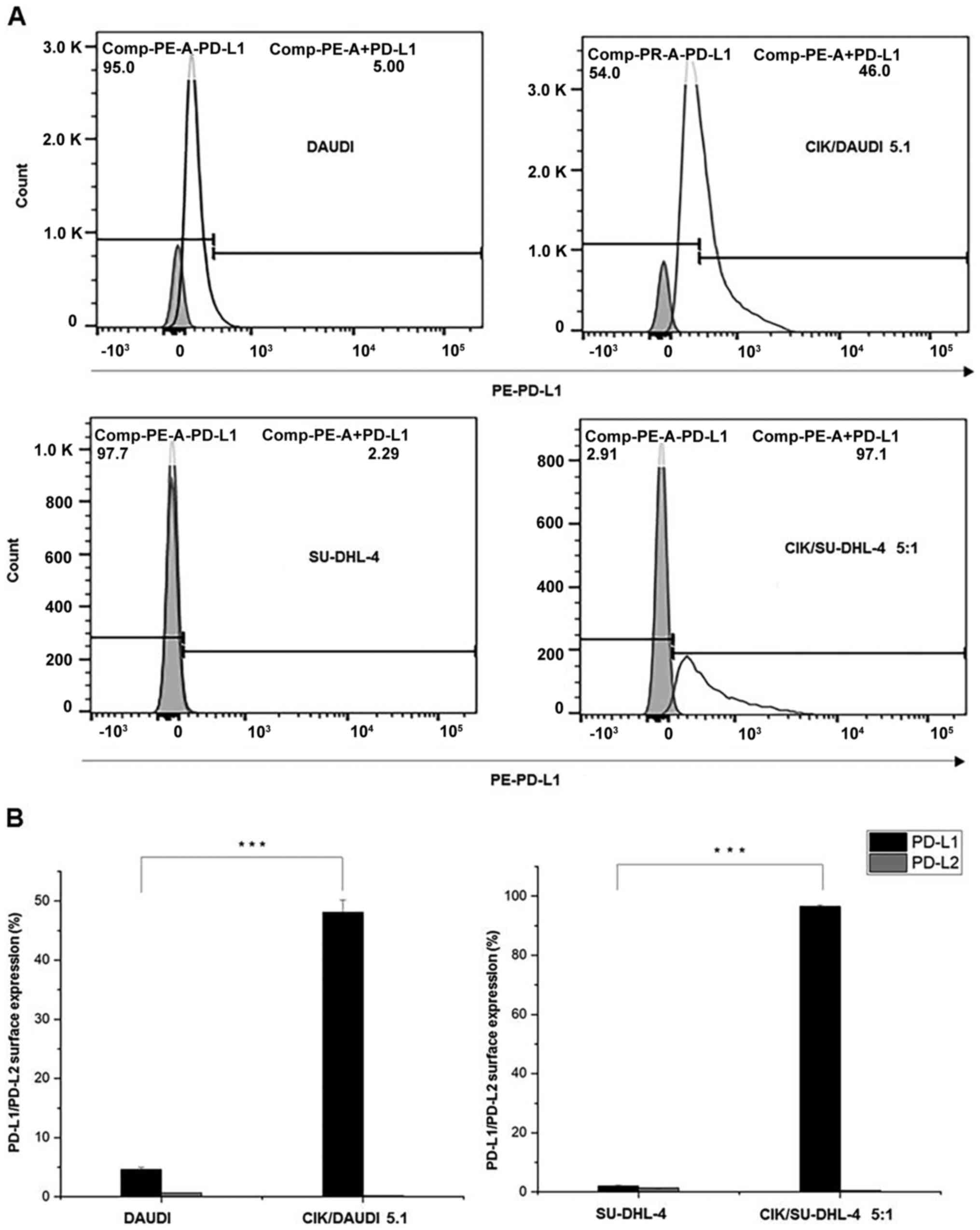
In both cell lines, very low levels of PD-L1 (DAUDI:
4.62±0.39%; SU-DHL-4: 1.97±0.37%) and PD-L2 (<2%) were observed.
However, a significant increase in PD-L1 (DAUDI: 48.13±2.01%;
SU-DHL-4: 96.57±0.47%) was observed when CIK cells were co-cultured
with them (24 h, E/T ratio of 5:1) (Fig.
1A and 1B). Noticeably, PD-L2
levels remained unchanged.
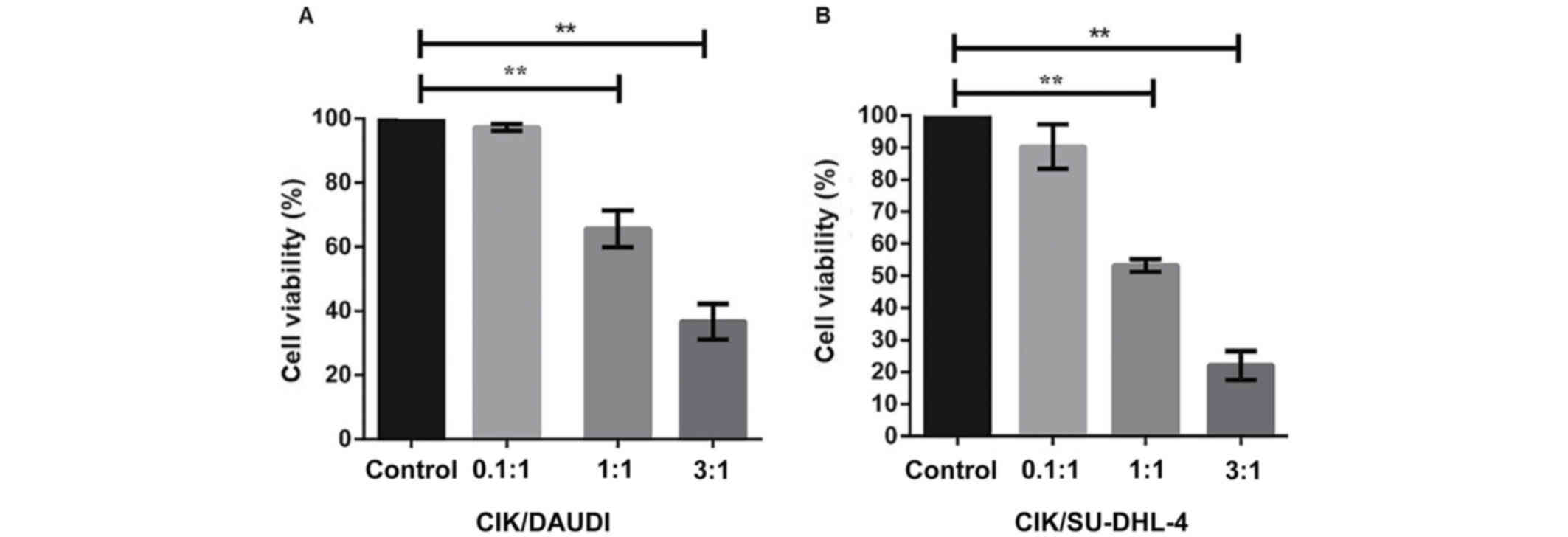
Additionally, we determined the percentage of viable
cells at varying target cell-to-effector cell ratios (E/T ratios:
0.1:1, 1:1, 3:1) by using the MTT assay. We found that DAUDI cells
were more viable compared to SU-DHL-4 (Fig. 2A and B). Notably, at E/T ratio of
1:1, the viability of DAUDI cells was found to be reduced (by 35%)
compared to the positive control, while it was decreased severely
(by 50%) in case of SU-DHL-4. A further increase in the E/T ratio
to 3:1 resulted in a continued decrease in cell viability of
SU-DHL-4 by >70%.
Enhanced cytotoxicity of CIK cells in
B-NHL induced by anti-PD-1 antibodies
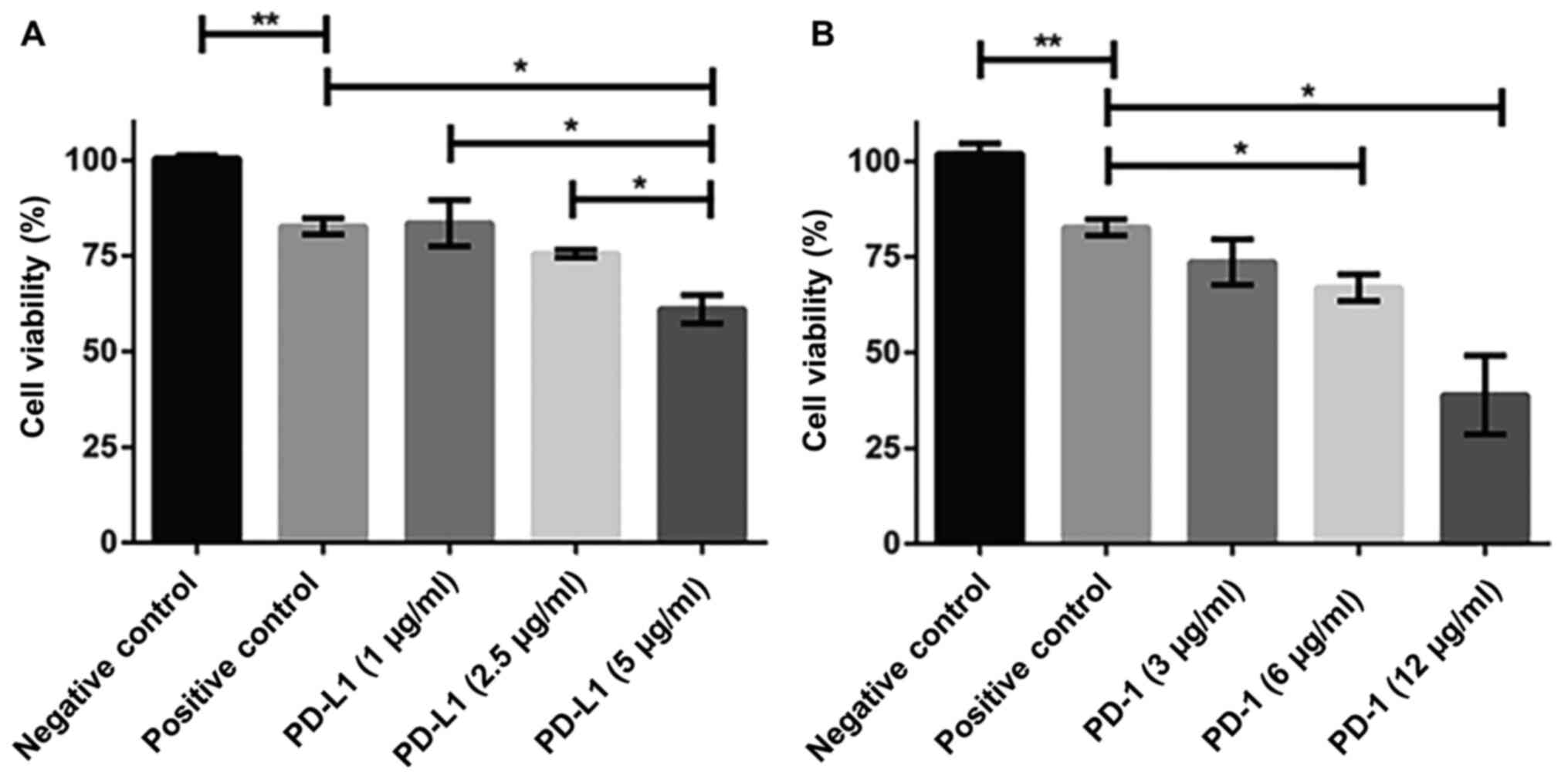
To investigate the amount of antibody that could
effectively block PD-1 and PD-L1 we performed a series of
titrations with them and examined the cell viability using CCK-8
assay. The use of 5 µg/ml PD-L1 antibody significantly decreased
the cell viability (~60%) compared to positive control in DAUDI
cells (after being cultured with CIK cells for 24 h) (Fig. 3A). 12 µg/ml PD-1 was required to
achieve the comparable results (Fig.
3B).
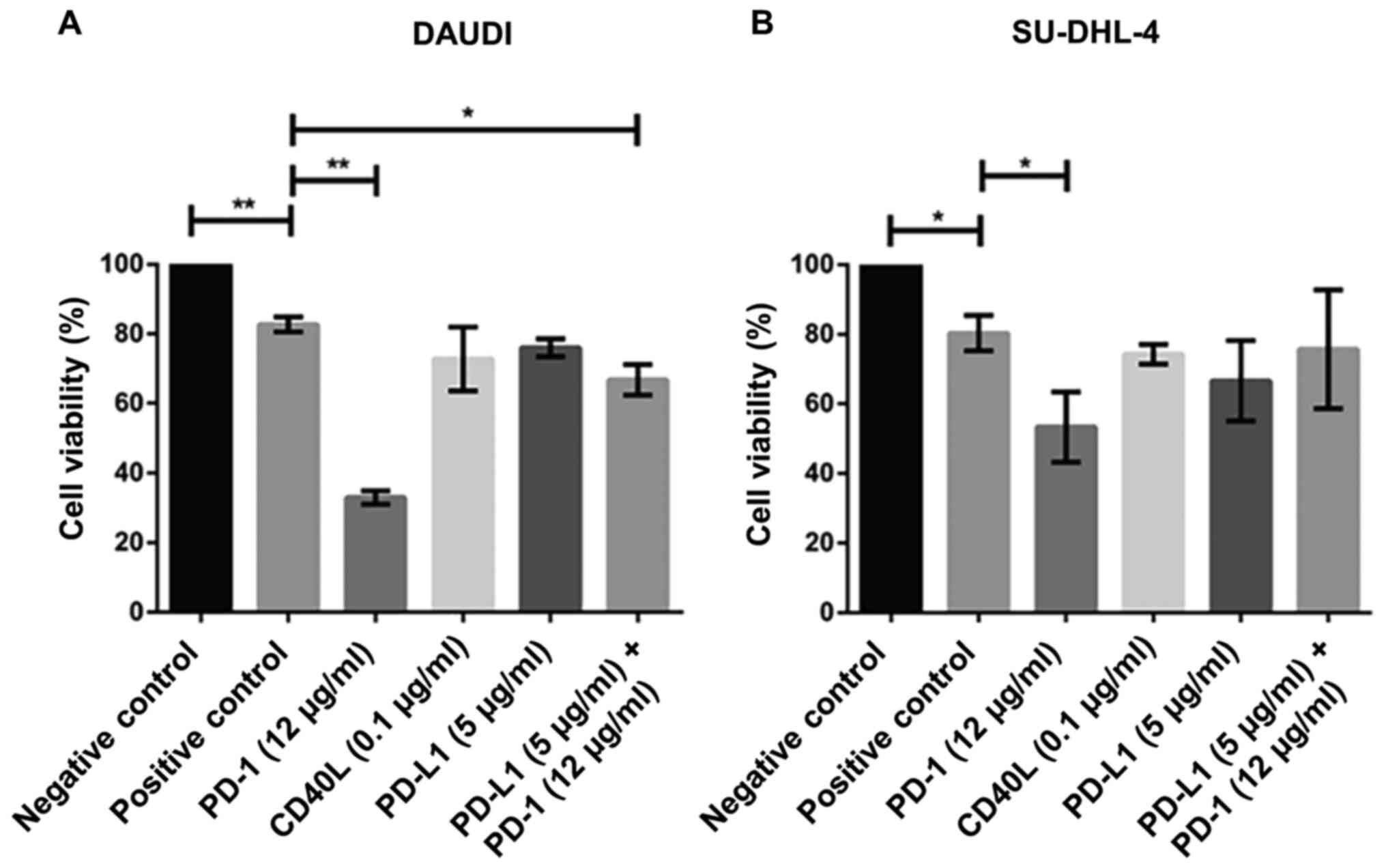
Notably, the cell viability in both cell lines
(DAUDI and SU-DHL-4) was severely impacted when co-cultured with
PD-1 blockade-activated CIK cells (Fig.
4A, B). For instance, the concentration of 12 µg/ml PD-1
antibody led to a significant decrease in cell viability in DAUDI
(~38%) and SU-DHL-4 (~50%) cells. To mention, no significant
difference was observed after treatment with PD-L1 (5 µg/ml) and/or
CD40L (0.1 µg/ml) antibodies. However, it cannot be excluded that
the high concentrations of CD40L (~1 µg/ml) may exert severe
cytotoxic effects. Interestingly, PD-1 combined with PD-L1 blockade
showed significant differences only when CIK cells were co-cultured
with DAUDI cells.
Blocking of the PD-1 receptor present
on CIK cells enhances the secretion of IFN-γ
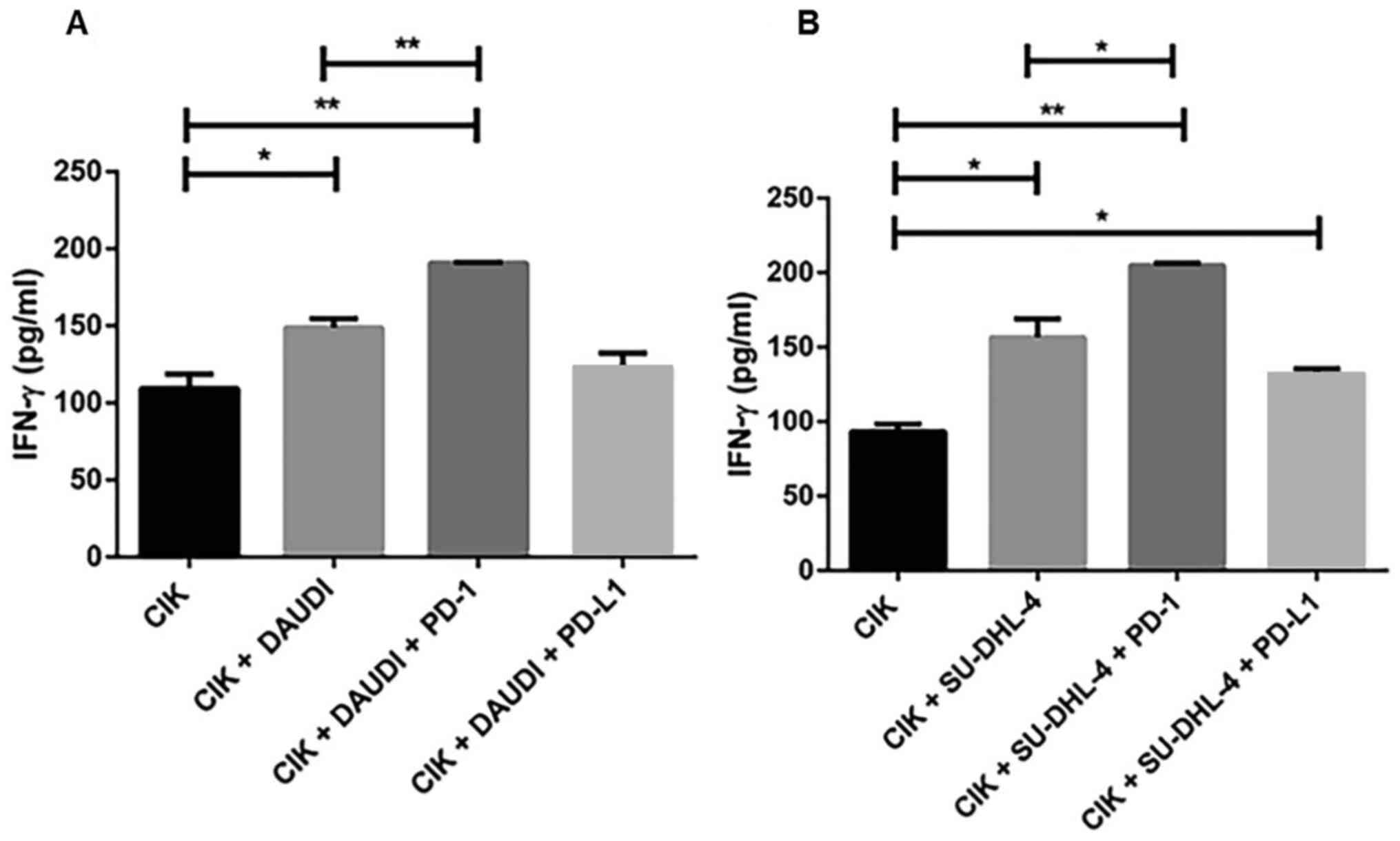
To evaluate the efficiency of PD-1 and PD-L1
blockade, we performed an IFN-γ ELISA assay and observed a
significant increase in IFN-γ secretion in both cell lines treated
with CIK alone and/or with PD-1 antibody (Fig. 5). In contrast, this tendency was not
observed for PD-L1. Similarly, we also examined the effects of
CD40L blockade but did not observe any significant changes.
Discussion
Cytokine-induced killer (CIK) cell therapy has
emerged as a promising option in cancer immunotherapy. There have
been growing numbers of clinical trials suggesting that CIK therapy
achieves a very convincing clinical response in a variety of
cancers (9). In the current study,
we investigated the cytotoxic capacity of CIK cells in two
frequently used human B-cell non-Hodgkin lymphoma cell lines
(SU-DHL-4, DAUDI). As a combinatorial approach, we also considered
PD-L1/PD-1 blockade in our analyses, as it has been previously
shown that combined therapy of CIK cells and PD-L1/PD-1 blockade
can delay tumor growth in murine gastric cancer model (15).
Our previous study has shown that PD-1 surface
expression on CD3+ CIK cells was 3.9±0.5% (16). In our current analysis, both cell
lines showed very low levels of PD-L1 and PD-L2, however, a
significant increase in PD-L1 (but not PD-L2) was observed when CIK
cells were co-cultured with them. To mention, the PD-L1/L2 levels
may vary in other B-NHL cell lines (>100 reported in ATCC
repositories), as suggested by Sharma et al the
heterogeneity between cancer cell lines (in addition to
genetic-epigenetic variations) may lead to discrepancies in the
experimental data (17). In our
study we could show that the variation in cell viability was
entirely due to co-cultured CIK cells, thus the above-mentioned
factor can be excluded. This can also be evident from the CCK-8
assay data, where we used different titrations of PD-L1 and PD-1
antibodies and obtained the exact concentrations (PD-L1: 5 µg/ml,
PD-1: 12 µg/ml) required to obtain the comparable cytotoxicity
levels. In contrast to PD-L1, CD40L blockade did not show any
significant alteration, but it cannot be excluded that its high
concentrations might exert potent cytotoxic effects.
Arguably, the question remains whether PD-1 and PD-
L1 are comparable, as they are not fully interchangeable in the
clinical practice. In our analysis the cell viability was severely
impaired with PD-1-blocked CIK cells, in contrast to PD-L1 which
showed no significant differences. However, the cumulative effect
(PD-1 combined with PD-L1 blockade) was clearly seen when CIK cells
were co-cultured with DAUDI cells. Importantly, we observed a
significant increase in IFN-γ secretion in both cell lines when
treated with CIK alone and/or with PD-1 antibody. We therefore
suggest that in vivo experiments are warranted to undermine
the extent at which PD-1 inhibitor could be used to enhance the
antitumor activity of CIK cells in B-NHL.
Taken together, our in vitro data suggest
that CIK cells can exert a significant cytotoxic function against
B-NHL, and a combination of PD-1 inhibitors with CIK cells may
provide a potential therapeutic option for this particular
lymphoma.
Acknowledgements
Not applicable.
Funding
The CIO Aachen Bonn Köln Düsseldorf is supported by
the Deutsche Krebshilfe (grant. no. 70113470). This work was partly
funded by the Deutsche Forschungsgemeinschaft (DFG, German Research
Foundation) to MK (project no. 410853455).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
IGHSW conceived the study. YL performed flow
cytometry and MWJRB contributed to the MTT, CCK-8 and ELISA assays.
AS analyzed the data and revised the manuscript critically for
important intellectual content. RSW and MK contributed to study
design and revised the manuscript. AS, RSW, MK and IGHSW were
responsible for confirming the authenticity of the data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Approval of the ethics committee of the University
Hospital Bonn (Bonn, Germany) was obtained, including signed
informed consent from the healthy volunteers.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xu-Monette ZY, Zhou J and Young KH: PD-1
expression and clinical PD-1 blockade in B-cell lymphomas. Blood.
131:68–83. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goodman A, Patel SP and Kurzrock R:
PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev
Clin Oncol. 14:203–220. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Panjwani PK, Charu V, DeLisser M,
Molina-Kirsch H, Natkunam Y and Zhao S: Programmed death-1 ligands
PD-L1 and PD-L2 show distinctive and restricted patterns of
expression in lymphoma subtypes. Hum Pathol. 71:91–99. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roemer MG, Advani RH, Ligon AH, Natkunam
Y, Redd RA, Homer H, Connelly CF, Sun HH, Daadi SE, Freeman GJ, et
al: PD-L1 and PD-L2 genetic alterations define classical hodgkin
lymphoma and predict outcome. J Clin Oncol. 34:2690–2697. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen BJ, Chapuy B, Ouyang J, Sun HH,
Roemer MG, Xu ML, Yu H, Fletcher CD, Freeman GJ, Shipp MA and Rodig
SJ: PD-L1 expression is characteristic of a subset of aggressive
B-cell lymphomas and virus-associated malignancies. Clin Cancer
Res. 19:3462–3473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Menter T, Bodmer-Haecki A, Dirnhofer S and
Tzankov A: Evaluation of the diagnostic and prognostic value of
PDL1 expression in hodgkin and B-cell lymphomas. Hum Pathol.
54:17–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Andorsky DJ, Yamada RE, Said J, Pinkus GS,
Betting DJ and Timmerman JM: Programmed death ligand 1 is expressed
by non-hodgkin lymphomas and inhibits the activity of
tumor-associated T cells. Clin Cancer Res. 17:4232–4244. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmeel FC, Schmeel LC, Gast SM and
Schmidt-Wolf IG: Adoptive immunotherapy strategies with
cytokine-induced killer (CIK) cells in the treatment of
hematological malignancies. Int J Mol Sci. 15:14632–14648. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y and Schmidt-Wolf IGH: Ten-Year
update of the international registry on cytokine-induced killer
cells in cancer immunotherapy. J Cell Physiol. 235:9291–9303. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Ellinger J, Ritter M and
Schmidt-Wolf IGH: Clinical studies applying cytokine-induced killer
cells for the treatment of renal cell carcinoma. Cancers (Basel).
12:24712020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmidt-Wolf GD and Schmidt-Wolf IG:
Immunomodulatory gene therapy for haematological malignancies. Br J
Haematol. 117:23–32. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Linn YC, Lau LC and Hui KM: Generation of
cytokine-induced killer cells from leukaemic samples with in vitro
cytotoxicity against autologous and allogeneic leukaemic blasts. Br
J Haematol. 116:78–86. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kornacker M, Moldenhauer G, Herbst M,
Weilguni E, Tita-Nwa F, Harter C, Hensel M and Ho AD:
Cytokine-Induced killer cells against autologous CLL: Direct
cytotoxic effects and induction of immune accessory molecules by
interferon-gamma. Int J Cancer. 119:1377–1382. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu X, Zhang Y, Li Y and Schmidt-Wolf IGH:
Increase of antitumoral effects of cytokine-induced killer cells by
antibody-mediated inhibition of MICA shedding. Cancers (Basel).
12:18182020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dai C, Lin F, Geng R, Ge X, Tang W, Chang
J, Wu Z, Liu X, Lin Y, Zhang Z and Li J: Implication of combined
PD-L1/PD-1 blockade with cytokine-induced killer cells as a
synergistic immunotherapy for gastrointestinal cancer. Oncotarget.
7:10332–10344. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dehno MN, Li Y, Weiher H and Schmidt-Wolf
IGH: Increase in efficacy of checkpoint inhibition by
cytokine-induced-killer cells as a combination immunotherapy for
renal cancer. Int J Mol Sci. 21:30782020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sharma A, Reutter H and Ellinger J: DNA
methylation and bladder cancer: Where genotype does not predict
phenotype. Curr Genomics. 21:34–36. 2020. View Article : Google Scholar : PubMed/NCBI
|