Introduction
Colorectal cancer (CRC) is the third most common
malignancy worldwide, accounting for 9% of cancer-associated deaths
in both men and women in the United States (1). In 2020, the total number of new cases
of CRC reported in the United States was 147,910, with 53,200
deaths, the latter roughly one-third of the former. The CRC
morbidity rate is highest in the >65-year age group (exceeding
50%), with a mortality rate of ~70%. Notably, the incidence of CRC
among individuals aged <50 years has now reached 12%. With
respect to tumor location, the incidence of colon cancer is 3.5
times greater than the incidence of rectal cancer (2). In 2020, data from The Global Cancer
Observatory revealed that between 2014 and 2018, the incidence of
CRC increased at an annual rate of ~8% (3). Although the prognosis of CRC has
improved greatly in recent years, with advances in medicine and
technology, cancer relapse, drug resistance, metastasis and other
clinical features still result in high mortality in patients with
CRC (4,5). Therefore, an improved understanding of
the molecular mechanisms underlying CRC carcinogenesis and
progression is essential for the development of specific markers
and effective therapeutic strategies for patients with CRC.
Yes-associated protein 1 (YAP) is a transcriptional
coactivator and regulator of the Hippo signaling pathway (6). The YAP gene is located on human
chromosome 11q22 and is a candidate oncogene (7). As a transcription coactivator, YAP can
negatively regulate the Hippo signaling pathway, which is involved
in the regulation of organ size by modulating cellular polarity,
proliferation and apoptosis (8).
When the Hippo signaling pathway is activated, YAP is
phosphorylated and accumulates in the cytoplasm by binding to the
14-3-3 protein, which inhibits downstream target gene transcription
(9). Conversely, when the Hippo
signaling pathway is blocked or inactivated, YAP cannot be
phosphorylated. Instead, it is transported to the nucleus, where it
interacts with the sequence-specific transcription factor TEAD, and
other transcription factors, to promote the transcription and
expression of target genes associated with cellular proliferation,
such as baculoviral inhibitor of apoptosis repeat-containing 5
(BIRC5)/survivin (10). This causes
cells to proliferate uncontrollably, resulting in tumorigenesis.
Hence, YAP expression is upregulated in various cancer types,
including lung (11), gastric
(12), ovarian (13), colorectal (7), and breast cancer (14).
The Hippo signaling pathway plays a crucial role in
cellular differentiation, proliferation, apoptosis and
tumorigenesis (15). Previous
studies have shown that the Hippo signaling pathway acts as a tumor
suppressor in multiple tumor types, including breast cancer
(16), oral squamous cell carcinoma
(17), hepatocellular carcinoma
(18), and ovarian cancer (19). Furthermore, the Hippo signaling
pathway negatively regulates YAP (20), whose expression is an important
prognostic factor in CRC (8). As a
transcriptional coactivator, YAP can induce the expression of
various negative regulators of apoptosis, including members of the
inhibitor of apoptosis protein family, such as BIRC5/survivin
(21). The Hippo/YAP and
Wnt/β-catenin signaling pathways interact with each other to
maintain cellular stability, and are associated with the apoptosis
and proliferation of CRC cells (22). Moreover, β-catenin is an important
transcriptional coactivator that regulates downstream targets in
the Wnt/β-catenin pathway that are involved in cellular
differentiation, proliferation and apoptosis (23). BIRC5/survivin is a target gene of the
Hippo/YAP and Wnt/β-catenin signaling pathways. The aim of the
present study was to investigate the role and molecular mechanism
of YAP in the occurrence and development of CRC.
Materials and methods
Patients and tissue samples
A total of 181 consecutive patients with newly
diagnosed, pathologically confirmed, CRC were recruited from the
Department of Pathology at the First Affiliated Hospital of Anhui
Medical University (Hefei, China), between November 2007 and
December 2013. The study was approved by the Ethics Committee of
Anhui Medical University, and written informed consent was obtained
from each participant. None of the patients had received
preoperative chemotherapy or radiotherapy. The cohort included 112
men and 69 women, of which the median age was 57.5 years (range,
31–89); 44, 105 and 32 patients had well, moderately and poorly
differentiated adenocarcinomas, respectively; 82 patients showed
evidence of lymph node metastasis on pathological examination.
Furthermore, 26 patients presented with Duke's stage A, 53 Duke's
stage B, and 102 Duke's stage C-D disease.
A total of 30 normal colorectal mucosa samples were
taken at a distance of >5.0 cm from the tumor margin. All
specimens were fixed with 10% neutral formalin at 25°C for 24 h,
embedded in paraffin, cut into 4.0-µm-thick serial sections, and
used for immunohistochemistry. Complete clinical data were
available for all patients. Follow-up information was obtained by
telephone or from outpatient records. Complete follow-up data were
available for 82 patients (patients who had died due to
unsuccessful treatment before the time point were considered as
having complete follow-up data).
Cell culture and transfection
RKO (ATCC® CRL-2577™) and LoVo
(ATCC® CCL-229™) cells were obtained from the Cell Bank
of the Chinese Academy of Sciences (Shanghai, China), and were
maintained in 6-well plates [2×105 cells per 2.0 ml
culture medium; 90% of DMEM supplemented with 10% fetal bovine
serum (both HyClone; Cytiva)] in a humidified incubator at 37°C (5%
CO2). Once the cells had reached 30–50% confluence, the
siRNA-Lipofectamine mixture was prepared per the following steps:
i) 5.0 µl Lipofectamine® 2000 (Invitrogen, Thermo Fisher
Scientific Inc.) was diluted in 250 µl Opti-MEM/well, gently
aspirated 3–5 times, and then incubated at 37°C for 5 min; ii) 5.0
µl siRNA (100 nM) was diluted in 250 µl Opti-MEM/well (Invitrogen;
Thermo Fisher Scientific, Inc.; final concentration, 50 nM); iii)
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was incubated for 10 min, mixed with the siRNA,
and allowed to stand at 25°C for 20 min; iv) the siRNA-Lip mixture
was added to the cells and transfection medium to a total volume of
2.0 ml; v) the plate was gently shaken until thoroughly mixed, and
incubated at 37°C (5% CO2) for 6 h; and vi) the medium
was replaced with 2.0 ml fresh culture medium, and incubated for a
further 24 h. The siRNA sequences were as follows: YAP1 sense,
5′-GGUGAUACUAUCAACCAAATT-3′; and antisense,
5′-UUUGGUUGAUAGUAUCACCTT-3′; scramble siRNA control sense,
5′-UUCUCCGAACGUGUCACGUTT-3′; and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′.
Immunohistochemistry
Briefly, sections were deparaffinized and rehydrated
in a descending alcohol series (100, 90, 75 and 50%). Endogenous
peroxidase was blocked with 0.3% hydrogen peroxide. The sections
were boiled in sodium citrate buffer (0.01 M, pH 6.0) for 15 min
(120°C, 0.097 MPa) for antigen retrieval. After cooling to room
temperature for 30 min, the sections were blocked with 5% normal
goat serum at 25°C for 1 h, followed by incubation with a YAP1
antibody (1:100; cat. no. ab205270; Abcam) in blocking buffer at
4°C overnight. The next day, the sections were incubated with Goat
Anti-Rabbit IgG H&L (HRP) secondary antibody (1:5,000; cat. no.
ab7090; Abcam) at 25°C for 30 min. The slides were developed with
3,3-diaminobenzidine solution at 25°C for 5 min, and counterstained
with hematoxylin. The total YAP immunostaining score was obtained
through multiplying staining power by positive cell percentage. The
score standard for staining intensity was based on different
degrees of tissue staining without specific background staining: No
visible staining, 0; light yellow, 1; tan, 2; and dun, 3. The score
standard for positive cell evaluation criteria was as follows:
Positive cells ≤5%, 0; >5% but ≤25%, 1; >25% but ≤50%, 2;
>50% but ≤75%, 3; and >75%, 4.
RNA extraction and reverse
transcription-quantitative (RT-q) PCR
Total RNA was extracted from cell samples using
RNAiso Plus reagent (Takara Bio, Inc.). Reverse transcription (RT)
was performed using PrimeScript RT-polymerase (Takara Bio, Inc.),
The RT procedure comprised 42°C for 2 min for gNDA eraser and 37°C
for 15 min for 6 cycles, followed by 85°C for 5 sec. qPCR was
performed to amplify the cDNA templates using the SYBR Premix Ex
Taq II kit (Takara Bio, Inc.). The qPCR thermocycling conditions
were as follows: 95°C for 10 sec during the hold stage, followed by
95°C for 5 sec and 60°C for 30 sec for 40 cycles. β-actin was used
as the reference gene, and each sample was run in triplicate. Data
were normalized to the expression of β-actin, and relative
expression was calculated using the 2−ΔΔCq method
(24). The primer sequences were as
follows: YAP forward, 5′-TGGGACTCAAAATCCAGTGTC-3′ and reverse,
5′-CCATCTCCTTCCAGTGTTCC-3′; and β-actin, forward
5′-GGCATCCACGAAACTACCTT-3′ and reverse,
5′-CGGACTCGTCATACTCCTGCT-3′.
Western blot analysis
Total protein was extracted from whole-cell lysates
using RIPA buffer (Invitrogen; Thermo Fisher Scientific Inc.)
containing protease inhibitors, and protein concentration was
determined by BCA analysis (Thermo Fisher Scientific Inc.). A 30-µg
sample of protein per lane was separated by denaturing 8–10%
SDS-PAGE and transferred PVDF membrane (EMD Millipore). The
membranes were blocked with 5% skim milk in PBST at room
temperature for 1 h, and then incubated with an anti-YAP1 antibody
(1:1,000; cat. no. ab205270; Abcam) at 4°C overnight. The next day,
the membranes were washed three times with PBST (PBS containing
0.1% Tween-20) for 5 min each, and then incubated with rabbit
anti-sheep IgG H&L (HRP) (1:10,000; cat. no. ab6747; Abcam) at
25°C for 1 h. Enhanced chemiluminescence reagents (Thermo Fisher
Scientific, Inc.) were used to detect the immunoreactive bands.
Each experiment was performed in triplicate and repeated three
times. The gray values of the blots were determined using ImageJ
software version 1.47 (National Institutes of Health) for
statistical analysis.
Cell Counting Kit-8 assay
Cells transfected with scramble control and
YAP-siRNA were incubated in 96-well plates (10,000 cells per plate,
5 wells per group) in 100 µl medium. The medium was replaced with
fresh culture medium daily. After 0, 24, 48, 72 and 96 h, 10 µl
CCK-8 (Invitrogen; Thermo Fisher Scientific, Inc.) was added to the
appropriate wells, and the plate was incubated at 37°C for 1 h. The
MTT formazan crystals were solubilized with 100 µl dimethyl
sulfoxide (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for
4 h. The optical density (OD) at a wavelength of 570 nm was
measured using a microplate reader (Thermo Fisher Scientific,
Inc.). Cell proliferation inhibition rate was calculated as
follows: [(control group OD value-experimental group A
value)/control group OD value] × 100%. The experiment was repeated
three times, and cell proliferation curves were constructed using
Prism version 8.0 (GraphPad Software, Inc.).
Flow cytometry
The apoptotic cells were detected using a
FACSCalibur flow cytometer (BD Biosciences) using FlowJo software
version 10 (FlowJo LLC). The cells were digested with 0.25%
trypsin-EDTA. The Annexin V-FITC Apoptosis Detection Kit
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to detect
apoptotic cells induced by 10 mmol/l 5-fluorouracil (5-FU;
Sigma-Aldrich) for 24 h at 37°C (5% CO2). Cells were
washed twice with precooled phosphate-buffered saline and the cell
concentration was adjusted to 5×105/ml. The cells were
then resuspended in 400 µl 1X Annexin V. Annexin V-FITC staining
solution (5.0 µl) was added at 4°C for 15 min, followed by 10 µl
propidium iodide staining solution, and incubated at 2–8°C for 5
min. The experiment was repeated three times.
Bioinformatics analysis
Gene Expression Profiling Interactive Analysis
(GEPIA) was used to mine the expression and gene regulation network
of YAP in CRC. LinkedOmics was used to identify differential gene
expression associated with YAP, and to analyze Kyoto Encyclopedia
of Genes and Genomes (KEGG) pathways. Moreover, colon
adenocarcinoma and rectum adenocarcinoma data were downloaded from
The Cancer Genome Atlas for further evaluation of YAP expression in
a wider cohort of patients with colorectal cancer, according to
different clinical and pathological features; however, no other
significant association was found (data not shown), thus only data
from GEPIA were include in the current study.
Statistical analysis
The Pearson's χ2 test was used to
determine the association between the expression levels of YAP and
the clinicopathological characteristics of patients with CRC. A
two-tailed unpaired t-test was used to compare the differences
between two groups. One-way ANOVA followed by Tukey's multiple
comparison test was used to compare cellular proliferation ability
between different cell types. Survival curves were plotted using
the Kaplan-Meier method and compared using the log-rank test. All
statistical analyses were performed using SPSS v23.0 (IBM Corp.),
and P<0.05 was considered to indicate a statistically
significant difference.
Results
Upregulation of YAP in CRC
tissues
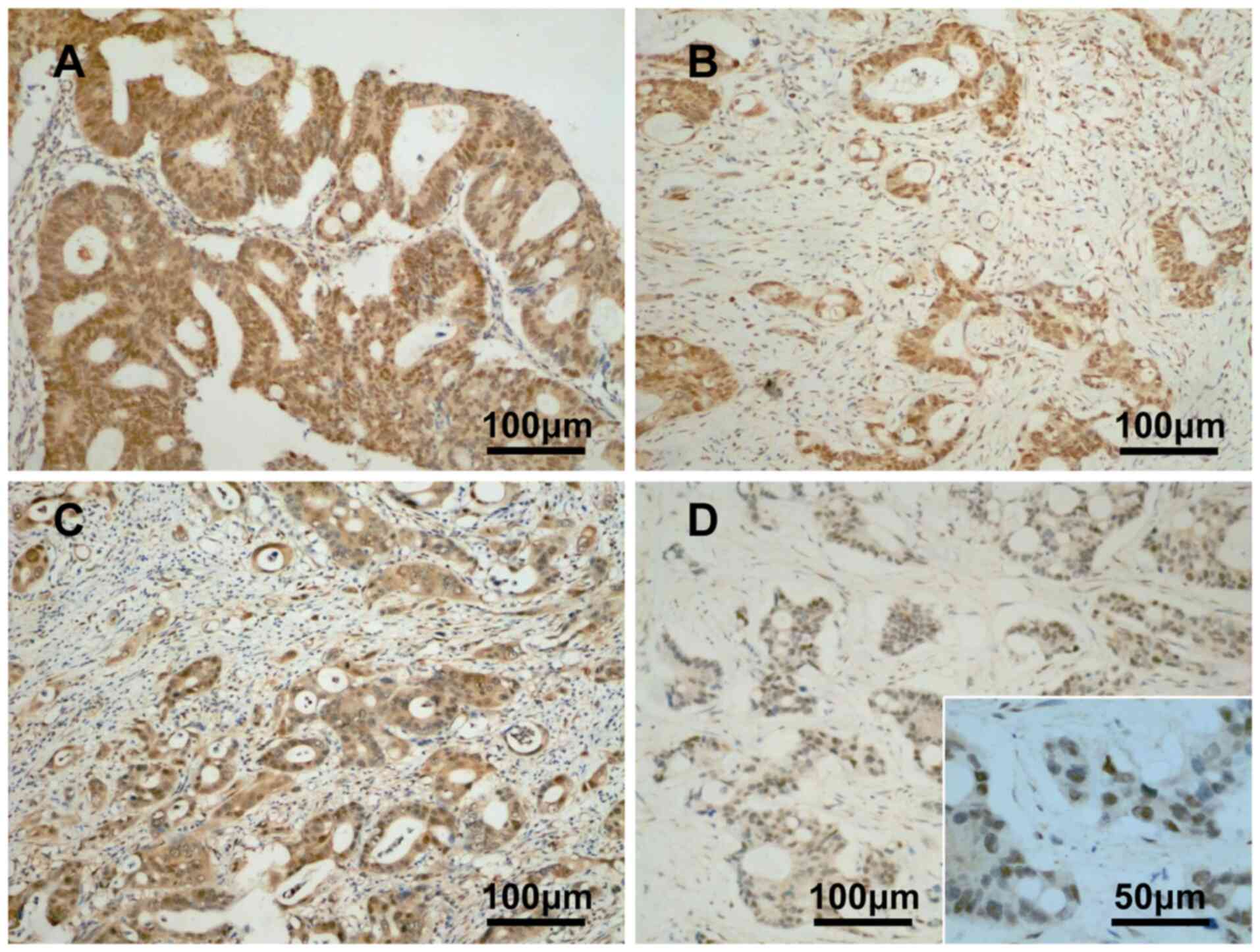
The expression profile of YAP protein is as follows:
Cytoplasmic and nuclear staining of YAP was positive in
well-differentiated CRC tissues (Fig.
1A); diffuse nuclear expression of YAP was positive in
moderately differentiated CRC tissues (Fig. 1B); cytoplasmic and nuclear staining
of YAP was positive in moderate to poorly differentiated CRC
tissues (Fig. 1C); and nuclear
expression of YAP was positive in poorly differentiated CRC tissues
(Fig. 1D). The positive expression
rate of YAP was 73.5% (n=133/181), with a low expression rate of
25.9% (n=47/181) and a high expression rate of 47.5% (n=86/181).
Among the 30 normal colorectal mucosa samples, only five stained
positive for YAP with low plasma expression (16.7%). No nuclear or
nuclear plasma staining was detected. The positive expression rate
of YAP was significantly higher in CRC tissues (n=133/181) than in
normal colorectal mucosa samples (16.7%) (P<0.0001). These
findings were consistent with the results of bioinformatics
analysis (Fig. 2A).
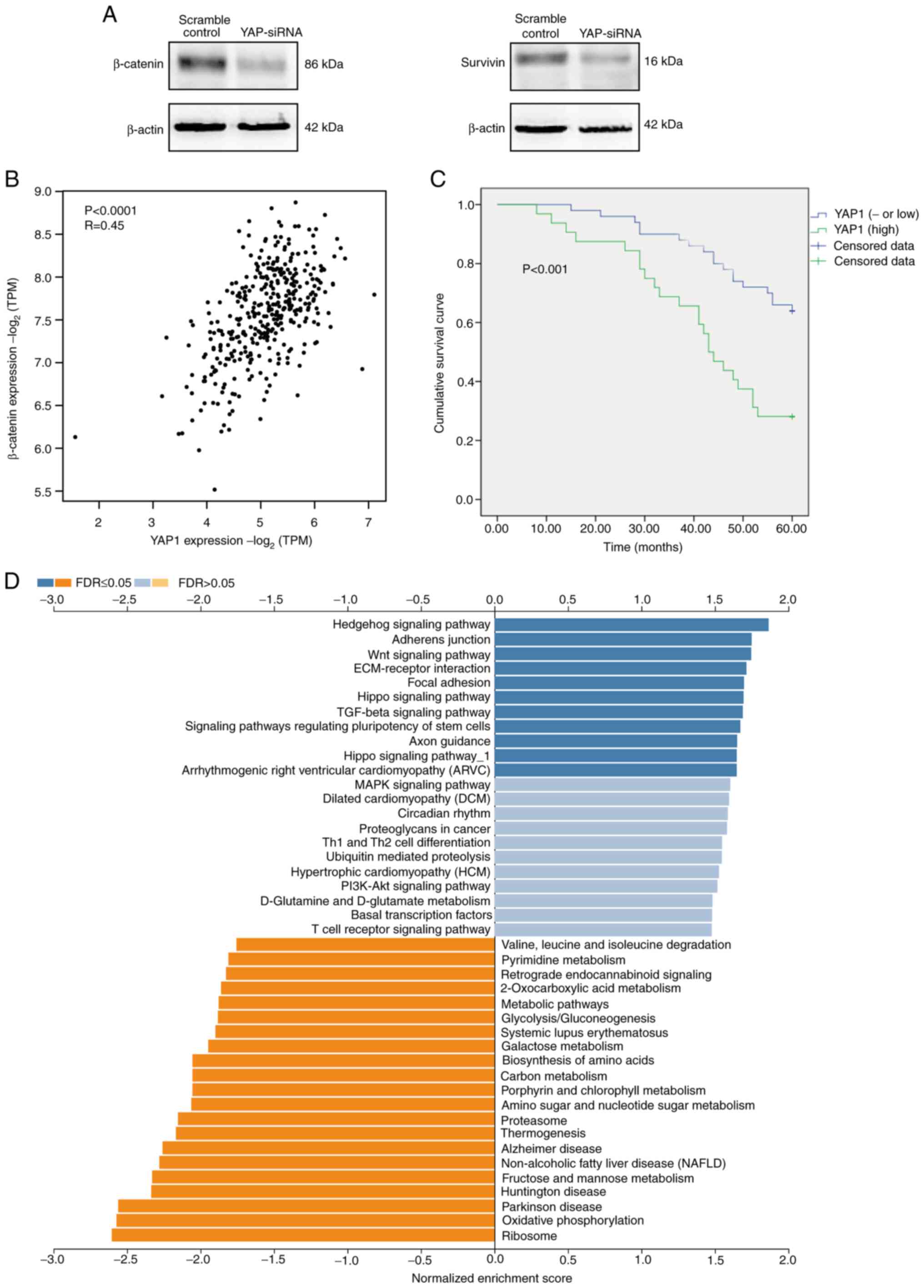
 | Figure 2.Expression of YAP protein is
upregulated in CRC tissues, which is correlated with the aggressive
characteristics of CRC. (A) Relative expression of YAP in CRC
tissues and normal colorectal mucosa samples. (B) Correlation of
YAP expression with M stage, pathological stage, microsatellite
instability phenotype and N stage. (C) Validation of YAP expression
in normal control group, YAP-siRNA group, scramble control group,
and 5-FU group using western blot analysis and reverse
transcription-quantitative PCR in RKO and LoVo cells. (D) RKO and
LoVo cell proliferation detected by MTT assay after transfection
with YAP-siRNA. (E) Rate of RKO cell apoptosis detected by flow
cytometry 48 h after transfection with YAP-siRNA: (a) Normal
control group, (b) Scramble control group, (c) YAP-siRNA
transfected group, and (d) 5-FU group. (F) Rate of LoVo cell
apoptosis detected by flow cytometry 48 h after transfection with
YAP-siRNA: (a) Normal control group, (b) Scramble control group,
(c) YAP-siRNA transfected group, and (d) 5-FU group. YAP,
yes-associated protein; CRC, colorectal cancer; siRNA, small
interfering RNA; 5-fluorouracil; MSS, microsatellite stable; MSI-H,
microsatellite instability-high. YAP, yes-associated protein; CRC,
colorectal cancer; siRNA, small interfering RNA; 5-fluorouracil;
MSS, microsatellite stable; MSI-H, microsatellite
instability-high. |
Upregulation of YAP is correlated with
the aggressive characteristics of CRC
The expression of YAP in CRC was found to correlate
with histological differentiation, lymph node metastasis and Duke's
stage (P<0.05). However, it was not correlated with other
clinicopathological characteristics of CRC, such as age, sex and T
stage (Table I). Furthermore, YAP
was highly expressed in both the cytoplasm and nucleus, as well as
nuclear plasma of CRC tissues. Nuclear and nuclear
plasma-positivity were associated with lymph node metastasis and
Duke's stage (P<0.05). No significant correlations with other
clinicopathological characteristics were reported. Cytoplasmic
positivity was not significantly correlated with any of the
clinicopathological characteristics analyzed in the present study
(Table II). In addition,
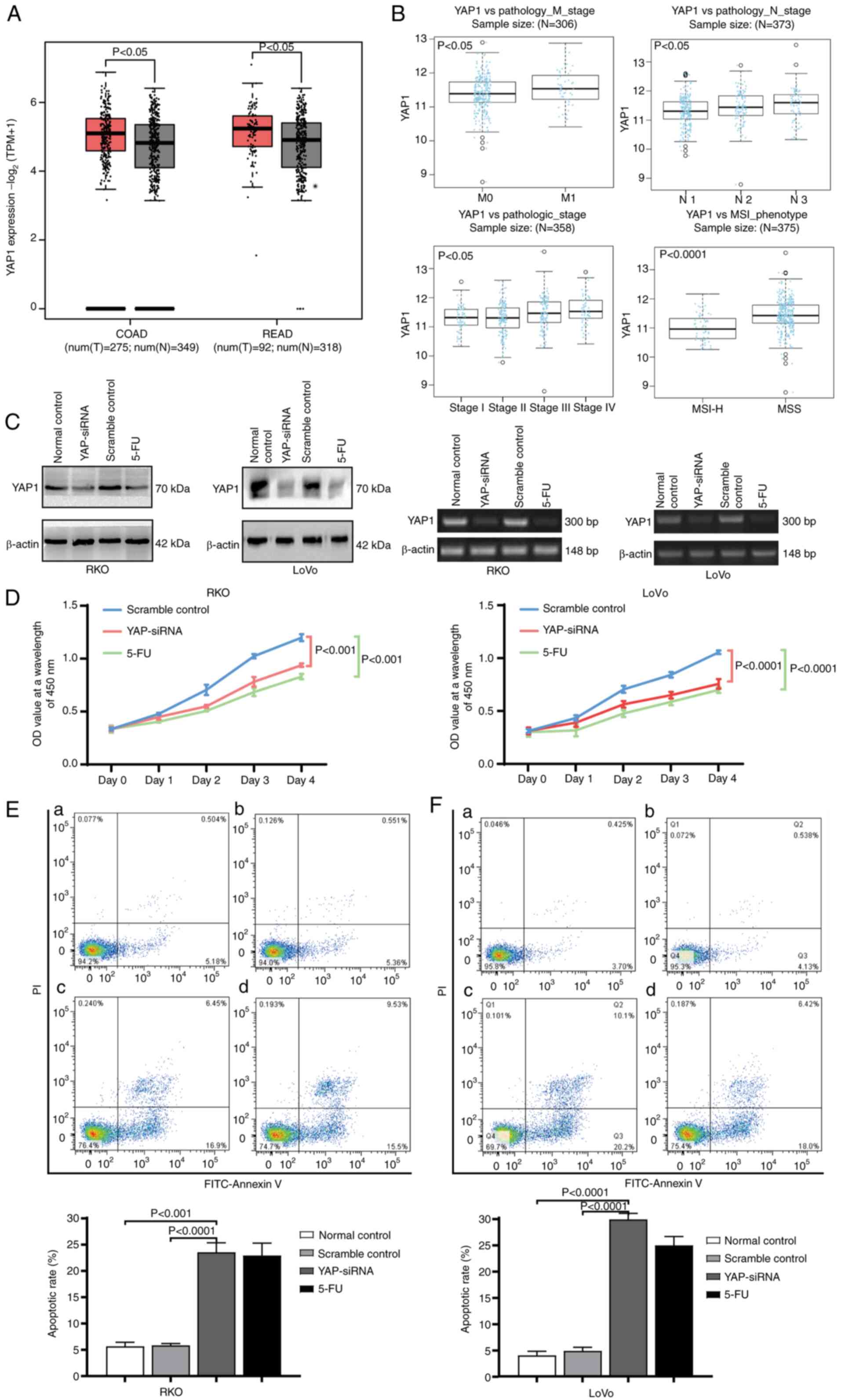
bioinformatics analysis revealed that the relative expression of
the YAP gene was positively correlated with M stage
(P<0.05; Wilcoxon test), N stage (P<0.05; Kruskal-Wallis
Test) and pathological stage (P<0.05; Kruskal-Wallis Test).
Furthermore, the expression level of the YAP gene was higher
in patients with microsatellite stable (MSS)-CRC than in those with
microsatellite instability-high (MSI-H)-CRC (P<0.0001; Wilcoxon
test) (Fig. 2B).
 | Table I.Association between YAP expression and
clinicopathological characteristics of patients with CRC
(n=181). |
Table I.
Association between YAP expression and
clinicopathological characteristics of patients with CRC
(n=181).
|
|
| Yes-associated
protein |
|
|
|---|
|
|
|
|
|
|
|---|
| Features | n | − | + | χ2
value | P-value |
|---|
| Age |
|
|
|
|
|
|
<60 | 79 | 20 | 59 | 0.104 | 0.747 |
|
≥60 | 102 | 28 | 74 |
|
|
| Sex |
|
|
|
|
|
|
Male | 112 | 31 | 81 | 0.203 | 0.653 |
|
Female | 69 | 17 | 52 |
|
|
|
Differentiation |
|
|
|
|
|
|
High | 44 | 20 | 24 | 10.740 | 0.005 |
|
Moderate | 105 | 21 | 84 |
|
|
|
Low | 32 | 7 | 25 |
|
|
| T stage |
|
|
|
|
|
|
T1-T2 | 63 | 18 | 45 | 0.209 | 0.648 |
|
T3-T4 | 118 | 30 | 88 |
|
|
| Lymph node
metastasis |
|
|
|
|
|
| No | 99 | 34 | 65 | 6.865 | 0.009 |
|
Yes | 82 | 14 | 68 |
|
|
| Duke's stage |
|
|
|
|
|
| A | 26 | 15 | 11 | 23.901 |
<0.0001 |
| B | 53 | 19 | 34 |
|
|
|
C+D | 102 | 14 | 88 |
|
|
 | Table II.Relation between different location
of YAP and clinicopathological parameters and β-catenin expression
pattern in CRC. |
Table II.
Relation between different location
of YAP and clinicopathological parameters and β-catenin expression
pattern in CRC.
|
|
| YAP |
|
| YAP |
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Features | n=181 | - (48) | Lowa (47/181) | Highb (86/181) | χ2
value | P-value | - (48) | Cytoplasm
(58/181) | Nucleus and
nucleus/plasma (75/181) | χ2
value | P-value |
|---|
|
Differentiation |
|
|
|
|
|
|
|
|
|
|
|
|
High | 44 | 20 | 14 | 10 | 0.056 | 0.451 | 20 | 11 | 13 | 2.090 | 0.352 |
|
Moderate | 105 | 21 | 25 | 59 |
|
| 21 | 34 | 50 |
|
|
|
Low | 32 | 7 | 8 | 17 | 16.816 |
<0.0001 | 7 | 13 | 12 | 4.422 | 0.110 |
| Lymph node
metastasis |
|
|
|
|
|
|
|
|
|
|
|
| No | 99 | 34 | 27 | 38 | 0.060 | 0.807 | 34 | 31 | 34 | 0.054 | 0.817 |
|
Yes | 82 | 14 | 20 | 48 | 7.304 | 0.007 | 14 | 27 | 41 | 4.531 | 0.003 |
| Duke's stage |
|
|
|
|
|
|
|
|
|
|
|
| A | 26 | 15 | 5 | 6 | 1.117 | 0.572 | 15 | 5 | 6 | 2.291 | 0.318 |
| B | 53 | 19 | 16 | 18 |
|
| 19 | 18 | 16 |
|
|
|
C+D | 102 | 14 | 26 | 62 | 17.332 |
<0.0001 | 14 | 35 | 53 | 11.030 | 0.004 |
| β-catenin nucleus
expression |
|
|
|
|
|
|
|
|
|
|
|
| + | 103 | 10 | 26 | 67 | 0.019 | 0.800 | 16 | 28 | 59 | 0.120 | 0.109 |
| − | 78 | 38 | 21 | 19 | 0.403 |
<0.0001 | 32 | 30 | 16 | 0.370 |
<0.0001 |
| β-catenin cytoplasm
expression |
|
|
|
|
|
|
|
|
|
|
|
| + | 45 | 8 | 7 | 30 | 0.137 | 0.067 | 11 | 13 | 21 | 0.039 | 0.603 |
| − | 136 | 40 | 40 | 56 | 0.221 | 0.003 | 37 | 45 | 54 | 0.061 | 0.414 |
| β-catenin
nucleus/plasma expression |
|
|
|
|
|
|
|
|
|
|
|
| + | 22 | 10 | 5 | 7 | 0.027 | 0.713 | 7 | 9 | 6 | 0.066 | 0.378 |
| − | 159 | 38 | 42 | 79 | 0.117 | 0.117 | 40 | 50 | 69 | 0.107 | 0.152 |
YAP-knockdown cell line
construction
RKO and LoVo cells were transfected with YAP-siRNA
and scramble control, and the expression levels of YAP were
determined by western blotting and RT-qPCR 48 h after transfection.
Western blot analysis showed that compared with the normal and
scramble control groups, YAP protein expression was markedly
reduced in the YAP-siRNA group 48 h after transfection.
Furthermore, the PCR results showed that compared with the normal
and scramble control groups, RKO and LoVo cell YAP DNA levels were
significantly reduced in the YAP-siRNA group, which was equivalent
to the 5-FU control group (Fig.
2C).
Association between YAP and the
proliferation of CRC cells
RKO and LoVo cell proliferation were detected 24 h
after transfection using the Cell Counting Kit-8 assay at 0-, 24-,
48-, 72- and 96-h time points. Cell viability is shown as a
proliferation curve based on the OD value at 450 nm. Compared with
the scramble control groups, the proliferation rates of the
YAP-siRNA RKO (P<0.001) and YAP-siRNA LoVo (P<0.0001) groups
were significantly decreased, and were similar to those of the 5-FU
groups (P<0.05; Fig. 2D).
Association between YAP and CRC cell
apoptosis
In the present study, apoptosis was represented as
the late plus the early apoptotic rate. The apoptotic rates of RKO
and LoVo cells were stained by Annexin V/PI and detected by flow
cytometry 48 h after transfection. The results showed that compared
with the normal control and scramble control groups, the apoptotic
rates of YAP-siRNA RKO and LoVo group were increased by 11.35%
(11.90±2.07%) and 16.9% (15.23±2.31%), respectively. The apoptotic
rates in YAP-siRNA RKO and LoVo groups were significantly higher
than those in the normal control and scramble control groups, and
were similar to that of the 5-FU group (Fig. 2E).
YAP is involved in the Hippo and
Wnt/β-catenin signaling pathways in CRC
Western blot analysis showed that compared with the
scramble control group, β-catenin and BIRC5/survivin protein
expression in RKO cells was reduced following transfection with
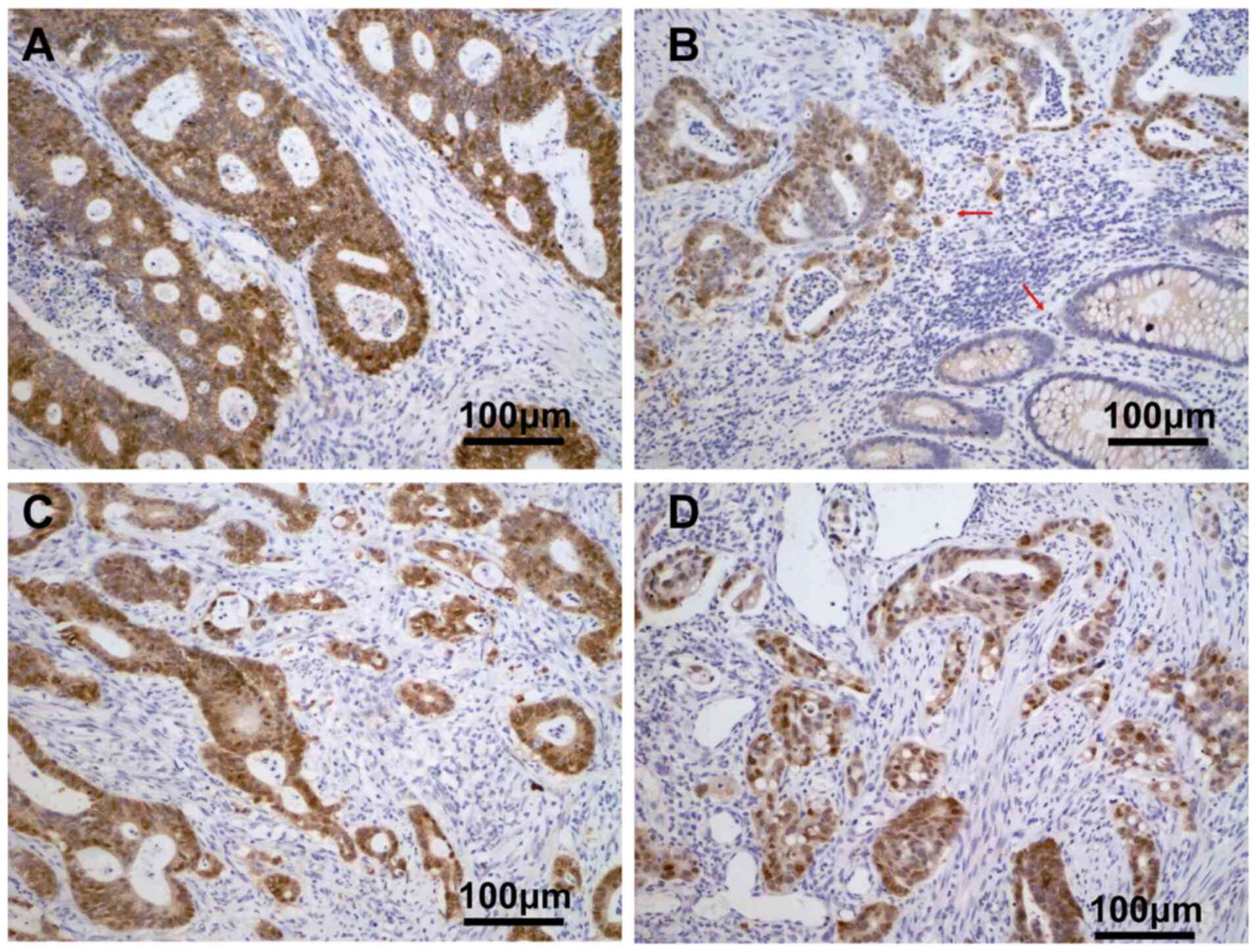
YAP-siRNA (Fig. 3A). Bioinformatics
analysis revealed that the expression levels of YAP and β-catenin
were significantly correlated in CRC (Fig. 3B), in agreement with the western blot
analysis. Immunohistochemical analysis showed that high YAP protein
expression was significantly correlated with the expression of
β-catenin in the nucleus and cytoplasm of CRC tissues, YAP
expression in the nucleus and nuclear plasma was associated with
the expression of β-catenin in the nucleus (P<0.05), (Fig. 4 and Table
II). Conversely, there was no association between YAP
expression in the nucleus and the expression of β-catenin in the
cytoplasm or nuclear plasma (Fig. 4
and Table II). Also, Kaplan-Meier
survival curves were used to characterize survival differences
categorized by YAP expression, and patients with high YAP
expression had a significantly lower mortality rate (n=32) compared
with those with low YAP expression (n= 50) (P<0.001) (Fig. 3C). KEGG cluster analysis showed that
differentially expressed genes with pathway annotation were mapped
to six significant pathways (P<0.05; Fig. 3D), including the Hippo and
Wnt/β-catenin signaling pathways. Consistent with the
protein-protein interaction results, KEGG pathway analysis
confirmed that these differentially expressed genes were primarily
enriched in the Hippo and Wnt/β-catenin signaling pathways.
Discussion
In the present study, immunohistochemistry was used
to detect the expression of YAP in 181 CRC tissues. The results
indicated that the total positive expression rate of YAP was
significantly higher in CRC tissues than in normal colorectal
mucosa samples. Furthermore, the expression of YAP in CRC tissues
was associated with histological differentiation, Duke's stage and
lymph node metastasis, suggesting that aberrant YAP upregulation
plays an important role in the occurrence and development of CRC.
Previous studies have shown that patients with CRC with strong
nuclear localization of YAP have a poor prognosis (25,26). The
results of the present study showed that the expression of YAP in
the nucleus and nuclear plasma was associated with Duke's stage.
Although high levels of YAP expression were associated with
histological differentiation, Duke's stage and lymph node
metastasis, the cytoplasmic expression was not related to these
clinical characteristics. This suggests that strong nuclear
localization of YAP has greater clinical significance for the
occurrence and progression of CRC.
The upregulation of YAP is reported to be an
independent prognostic factor for some tumors, such as
hepatocellular carcinoma (27). In
the present study, the 5-year survival rate of patients in the high
YAP expression group was significantly lower than that of patients
in the low expression groups, suggesting that YAP plays an
important role in the prognosis of CRC. Microsatellites are short
tandem repeats that are distributed throughout the human genome.
MSI refers to DNA methylation or gene mutations that cause mismatch
repair gene deletions, resulting in the insertion or deletion of
microsatellite repeats. The subsequent changes in length are
closely related to the occurrence of tumors. Microsatellite
stability is divided into three types: MSI-H, MSS and MSI-L. In
terms of surgical efficacy, MSI-H is optimal, followed by MSS, with
MSI-L being the worst. The current analysis showed that the
expression level of YAP was higher in patients with MSS CRC than in
those with MSI-H CRC. This lends further support for the use of YAP
as an independent prognostic factor for the occurrence and
progression of CRC.
In vitro studies were also conducted to
determine the effect of YAP on the proliferation and apoptosis of
RKO and LoVo cells. siRNA experiments showed that YAP increased
proliferation and inhibited apoptosis in RKO and LoVo cells,
suggesting a tumor promoting role of YAP in CRC. Furthermore, to
the best of our knowledge, the present study is the first to show
that the carcinogenesis of YAP may be due to the interaction
between the Hippo and Wnt/β-catenin signaling pathways. The most
important factor in the role of YAP in promoting the occurrence and
progression of CRC may be the direct or indirect interactions of
YAP with β-catenin. In the present study, YAP expression was found
to be associated with the Wnt/β-catenin pathway, apoptosis and
proliferation in colorectal cancer cell lines (RKO and LoVo). RKO
is a poorly differentiated colon cancer cell line. RKO cells
possess wild-type, but not mutant p53-type, and lack the human
thyroid receptor nuclear receptor (28). The level of p53 protein in RKO cells
was higher than that in RKO-E6 cells. The cell line formed tumors
in nude mice and formed colonies in soft agar (29). Thus, experiments performed using the
RKO cell line are relatively stable, and the experimental results
are objective and reliable.
In conclusion, detecting the expression level of YAP
and its nuclear expression pattern in CRC tissues is indicative of
the prognosis of patients with CRC. The present study provides a
theoretical basis for molecular targeted therapy in CRC, by
targeting YAP.
Acknowledgements
The authors would like to thank Dr Yansong Ren
(Department of Pathology, The First Affiliated Hospital of USTC,
Division of Life Sciences and Medicine, University of Science and
Technology of China) for their technical support.
Funding
The present study was supported by the Anhui
Provincial Natural Science Foundation of China (grant no.
1808085MH283), the Science and Technology Fund Project of Anhui
Medical University, 2018 (grant no. 2018×kj085), and the Doctoral
Fund of Anhui Medical University, Anhui Province, P.R. China (grant
no. XJ201614).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LC and CZ designed the present study and wrote the
paper. ZB, QW and JC conducted the experiments and carried out the
statistical analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Research was conducted in accordance with the
Declaration of Helsinki. The study design was approved by the
Ethics Committee of Anhui Medical University. Written informed
consent was obtained from all participants for the use of their
clinical samples.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BIRC5
|
baculoviral inhibitor of apoptosis
repeat-containing 5
|
|
CRC
|
colorectal cancer
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
MSI
|
microsatellite instability
|
|
MSS
|
microsatellite stable
|
|
YAP
|
yes-associated protein.
|
References
|
1
|
DeSantis CE, Miller KD, Dale W, Mohile SG,
Cohen HJ, Leach CR, Goding Sauer A, Jemal A and Siegel RL: Cancer
statistics for adults aged 85 years and older, 2019. CA Cancer J
Clin. 69:452–467. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Goding Sauer A,
Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA and Jemal
A: Colorectal cancer statistics, 2020. CA Cancer J Clin.
70:145–164. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Garza Treviño EN, González PD, Valencia
Salgado CI and Martinez Garza A: Effects of pericytes and colon
cancer stem cells in the tumor microenvironment. Cancer Cell Int.
19:1732019. View Article : Google Scholar
|
|
5
|
Tang M, Wang H, Cao Y, Zeng Z, Shan X and
Wang L: Nomogram for predicting occurrence and prognosis of liver
metastasis in colorectal cancer: A population-based study. Int J
Colorectal Dis. 36:271–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zanconato F, Cordenonsi M and Piccolo S:
YAP and TAZ: A signalling hub of the tumour microenvironment. Nat
Rev Cancer. 19:454–464. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang R, Cai TT, Wu XJ, Liu YN, He J, Zhang
XS, Ma G and Li J: Tumour YAP1 and PTEN expression correlates with
tumour-associated myeloid suppressor cell expansion and reduced
survival in colorectal cancer. Immunology. 155:263–272. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pan Y, Tong JHM, Lung RWM, Kang W, Kwan
JSH, Chak WP, Tin KY, Chung LY, Wu F, Ng SSM, et al: RASAL2
promotes tumor progression through LATS2/YAP1 axis of hippo
signaling pathway in colorectal cancer. Mol Cancer. 17:1022018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moon S, Kim W, Kim S, Kim Y, Song Y,
Bilousov O, Kim J, Lee T, Cha B, Kim M, et al: Phosphorylation by
NLK inhibits YAP-14-3-3-interactions and induces its nuclear
localization. EMBO Rep. 18:61–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Felley-Bosco E and Stahel R: Hippo/YAP
pathway for targeted therapy. Transl Lung Cancer Res. 3:75–83.
2014.PubMed/NCBI
|
|
11
|
Tsuji T, Ozasa H, Aoki W, Aburaya S,
Yamamoto Funazo T, Furugaki K, Yoshimura Y, Yamazoe M, Ajimizu H,
Yasuda Y, et al: YAP1 mediates survival of ALK-rearranged lung
cancer cells treated with alectinib via pro-apoptotic protein
regulation. Nat Commun. 11:742020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu S, Zhang Y, Li Q, Zhang Z, Zhao G and
Xu J: CLDN6 promotes tumor progression through the YAP1-snail1 axis
in gastric cancer. Cell Death Dis. 10:9492019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan H, Li H, Li P, Li X, Lin J, Zhu L,
Silva MA, Wang X, Wang P and Zhang Z: Long noncoding RNA MLK7-AS1
promotes ovarian cancer cells progression by modulating
miR-375/YAP1 axis. J Exp Clin Cancer Res. 37:2372018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo L, Chen Y, Luo J, Zheng J and Shao G:
YAP1 overexpression is associated with poor prognosis of breast
cancer patients and induces breast cancer cell growth by inhibiting
PTEN. FEBS Open Bio. 9:437–445. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yin MX and Zhang L: Hippo signaling in
epithelial stem cells. Acta Biochim Biophys Sin (Shanghai).
47:39–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park J, Kim GH, Lee J, Phuong BTC, Kong B,
Won JE, Won GW, Lee YH, Han HD and Lee Y: MST2 silencing induces
apoptosis and inhibits tumor growth for estrogen receptor
alpha-positive MCF-7 breast cancer. Toxicol Appl Pharmacol.
408:1152572020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hasegawa K, Fujii S, Matsumoto S, Tajiri
Y, Kikuchi A and Kiyoshima T: YAP signaling induces PIEZO1 to
promote oral squamous cell carcinoma cell proliferation. J Pathol.
253:80–93. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, Wang X and Yang Y: Hepatic Hippo
signaling inhibits development of hepatocellular carcinoma. Clin
Mol Hepatol. 26:742–750. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Azar WJ, Christie EL, Mitchell C, Liu DS,
Au-Yeung G and Bowtell DDL: Noncanonical IL6 signaling-mediated
activation of YAP regulates cell migration and invasion in ovarian
clear cell cancer. Cancer Res. 80:4960–4971. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dey A, Varelas X and Guan KL: Targeting
the Hippo pathway in cancer, fibrosis, wound healing and
regenerative medicine. Nat Rev Drug Discov. 19:480–494. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma K, Xu Q, Wang S, Zhang W, Liu M, Liang
S, Zhu H and Xu N: Nuclear accumulation of Yes-associated protein
(YAP) maintains the survival of doxorubicin-induced senescent cells
by promoting survivin expression. Cancer Lett. 375:84–91. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kriz V and Korinek V: Wnt, RSPO and Hippo
signalling in the intestine and intestinal stem cells. Genes
(Basel). 9:202018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kahn M: Can we safely target the WNT
pathway? Nat Rev Drug Discov. 13:513–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Camargo FD, Gokhale S, Johnnidis JB, Fu D,
Bell GW, Jaenisch R and Brummelkamp TR: YAP1 increases organ size
and expands undifferentiated progenitor cells. Curr Biol.
17:2054–2060. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou D, Zhang Y, Wu H, Barry E, Yin Y,
Lawrence E, Dawson D, Willis JE, Markowitz SD, Camargo FD and
Avruch J: Mst1 and Mst2 protein kinases restrain intestinal stem
cell proliferation and colonic tumorigenesis by inhibition of
Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci USA.
108:E1312–E1320. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li H, Wang S, Wang G, Zhang Z, Wu X, Zhang
T, Fu B and Chen G: Yes-associated protein expression is a
predictive marker for recurrence of hepatocellular carcinoma after
liver transplantation. Dig Surg. 31:468–478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van Bree C, Franken NA, Snel FA, Haveman J
and Bakker PJ: Wild-type p53-function is not required for
hyperthermia-enhanced cytotoxicity of cisplatin. Int J
Hyperthermia. 17:337–346. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nagashima M, Shiseki M, Pedeux RM, Okamura
S, Kitahama-Shiseki M, Miura K, Yokota J and Harris CC: A novel
PHD-finger motif protein, p47ING3, modulates p53-mediated
transcription, cell cycle control, and apoptosis. Oncogene.
22:343–350. 2003. View Article : Google Scholar : PubMed/NCBI
|