Introduction
Pancreatic cancer is one of the most common human
malignancies and a leading cause of cancer-related mortality
worldwide. Patients with pancreatic cancer often have a poor
prognosis. Despite advances in oncology, the overall 5-year
survival rates of this cancer have not significantly improved for
decades (1). The lack of adequate
and credible interventions is the key cause of the high mortality
rate in pancreatic cancer. Gemcitabine is the most effective and
extensively used chemotherapeutic in pancreatic cancer thus far.
However, the overall survival is dismal and is in part caused by
gemcitabine resistance in pancreatic cancer (2). New therapeutic agents for the treatment
of pancreatic cancer are thus urgently needed.
The metastatic progression of cancer cells requires
the remodeling of the actin cytoskeleton. The altered expression of
key regulatory proteins of the actin cytoskeleton, such as
cortactin (CTTN), contributes to carcinogenesis (3). CTTN is involved in various cell
functions, including actin polymerization, the formation of cell
motility structures such as podosomes and invadopodia, and
extracellular matrix (ECM)-protein deposition. These functions of
CTTN can lead to deregulated cell migration, invasion, and
metastasis (4).
AHCC® is a standardized extract of
Lentinula edodes mycelia. AHCC® comprises
polysaccharides, amino acids, minerals, and lipids enriched in α
1,4-glucans (5). The anti-oxidant,
anti-tumor, and immunomodulatory potentials of AHCC®
were described in several studies (6,7).
Previously we reported that AHCC® downregulated
tumor-associated proteins involved in pancreatic carcinogenesis
(8–11). We demonstrated that following
administration of AHCC®, the level of heat-shock protein
27 (HSP27), heat shock factor 1 (HSF1), sex-determining region
Y-box 2 (SOX-2), and CUB domain-containing protein 1 (CDCP1) were
significantly reduced in pancreatic cancer cells. Moreover, our
proteomic analysis revealed that HSP27 expression was strongly
related to gemcitabine resistance in pancreatic cancer (12). Similarly, SOX-2 and CDCP1 are highly
expressed in malignant tissues and involved in tumor invasion and
metastasis (8,11). Together, these findings suggested
that AHCC® might suppress the proteins involved in
chemoresistance and malignant progression of pancreatic cancer.
Therefore, we hypothesized that AHCC® might inhibit CTTN
expression and have an anti-metastatic potential in pancreatic
carcinogenesis.
In the present study, we investigated CTTN mRNA
expression levels in pancreatic cancer tissues from The Cancer
Genome Atlas (TCGA) databases using an online bioinformatics
platform. Next, we examined whether AHCC® suppressed
cell migration and CTTN expression in KLM1-R cells using an
in-vitro wound-healing assay and western blotting,
respectively.
Materials and methods
mRNA expression analysis of CTTN in
pancreatic cancer patients
The CTTN mRNA expression in pancreatic cancer
tissues was analyzed from the TCGA and GTEx databases, using the
Gene Expression Profiling Interactive Analysis (GEPIA2) platform
(13). The GEPIA2 platform was also
utilized to perform the association analyses of the CTTN expression
level in pancreatic cancer with clinical characteristics, including
pathological stages and Kaplan-Meier survival plots. The mRNA
expression cut-off criteria were selected as follows: LogFC
cut-off=1; P-value cut-off=0.05; datasets=pancreatic ductal
adenocarcinoma; and matched normal data=match TCGA normal and GTEx.
The quartile cut-off was selected for survival plot analysis.
P<0.05 is considered to indicate a statistically significant
difference.
Cancer cell line and conditions
The KLM1-R pancreatic cancer cell line is
gemcitabine-resistant. It has been established at the Department of
Surgery and Science, Kyushu University Graduate School of Medical
Science, and derived from the gemcitabine-sensitive pancreatic
cancer cell line KLM1, that was exposed to gemcitabine. In brief,
the KLM1 cells cultured at an initial density of 1×106
cells on 6-well flat-bottomed plates containing 2 ml medium for 1
day were treated with 10 µg/ml gemcitabine for 1 week. Cells were
then cultured in a gemcitabine-free medium for 2 weeks to recover
cell density. After repeating the above treatment 4×, a
gemcitabine-resistant cell line, KLM1-R was established. KLM1-R
exhibited stable characteristics with respect to growth rate,
morphology, and drug resistance (14). The cells were then kept in RPMI-1640
medium supplemented with 10% fetal bovine serum (inactivated at
56°C for 30 min), 2 mM L-glutamine, 1.5 g/l sodium bicarbonate, 10
mM N-2-hydroxyehylpiperazine-N′-2-ethanesulfonic acid (HEPES), and
1.0 mM sodium pyruvate, in a CO2 incubator.
Preparation of AHCC®
AHCC® was kindly provided by Amino Up
Co., Ltd. (Sapporo, Japan). AHCC® was dissolved in
RPMI-1640 medium to a final concentration of 10 mg/ml,
filter-sterilized, and stored at 4°C in aliquots. Fresh
AHCC® solution was used in each experiment.
In vitro wound-healing assay
The inhibition of cell migration by AHCC®
was investigated by in vitro wound-healing assay as
described previously (15). Briefly,
cells were allowed to grow to full confluence in 24-well plates,
and then a vertical wound was created with a 10 µl pipette tip.
After the cell debris was removed, a fresh RPMI-1640 complete
medium supplemented 10% FBS with various concentrations of
AHCC® [0 (control), 1, 5 and 10 mg/ml] was added. Cells
were incubated at 37°C and 5% CO2 for 24 h and images
were captured using a microscope at three time points (3, 6, and 24
h). Wound healing was observed at different time points within the
scraped line, and representative images of scraped lines were
captured using a phase-contrast microscope at ×40 magnification.
The migration area was measured and analyzed with ImageJ software
(version 1.48) (16), and the
experiments were conducted in triplicate.
Western blot analysis
KLM1-R cells were treated with or without
AHCC® (10 mg/ml) for 48 h in vitro. After
treatment and washing three times, proteins were extracted from
cells using lysis buffer (50 mM Tris HCl, pH 7.5; 10 mM EDTA, pH
7.5; 165 mM NaCl; 10 mM NaF; 1% Nonidet P-40; 1 mM PMSF; 1 mM
NaVO3; 10 µg/ml leupeptin; and 10 µg/ml aprotinin). The
lysis reaction was carried out for 1 h at 4°C. The samples were
centrifuged at 15,000 rpm for 30 min at 4°C, and the supernatant
was used as a sample. Protein concentration was quantified by
Lowry's protein assay. Fifteen micrograms of the protein samples
were used for western blot analysis. Sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried
out in pre-cast gels (4–20% gradient of polyacrylamide;
Mini-PROTEAN TGX Gels; Bio-Rad). After electrophoresis, gels were
transferred electrophoretically onto polyvinylidene difluoride
membranes (Immobilon-P; Millipore) and blocked for 1 h with
Tris-buffered saline with 0.1% Tween-20 solution (TBS-T) containing
5% skimmed milk. Blocked membranes were washed twice with
TBS-T.
The following primary antibodies were used: Rabbit
monoclonal antibody against cortactin (dilution 1:1,000, #CST3502;
Cell Signaling Technology, Beverly, MA, USA) and rabbit polyclonal
antibody against actin (dilution 1:5,000, #sc1616R; Santa Cruz
Biotechnology, Inc.). Membranes were incubated with the primary
antibody overnight at 4°C, washed three times with TBS-T, and
incubated with horseradish peroxidase-conjugated secondary antibody
(dilution 1:10,000; Jackson Immuno-Research Laboratories Inc.) for
1 h at room temperature. Bands of cortactin and actin were
visualized by the enhanced chemiluminescence system (Clarity™
Western ECL Substrate; Bio-Rad) and LuminoGraph I (ATTO
Corporation, Tokyo, Japan) and recorded using ImageSaver6 software
(ATTO Corporation) (17). Cortactin
and actin levels were quantified by analyzing each band intensity
using CS Analyzer4 software (ATTO Corporation). Each experiment was
performed in triplicate. Data are expressed as mean ± standard
error (SE) of the target protein ratio to actin protein.
Literature review of the effects of
AHCC® in different cancers
Two databases, namely PubMed and Scopus, were
screened for relevant articles and were limited to articles
published in English. Data were extracted from the databases on May
22, 2021, without applying any time restrictions. The formulated
search strategy was used in the databases: (Active hexose
correlated compound [MeSH Terms]) OR (AHCC [MeSH Terms]) AND
(cancer [MeSH Terms]) OR (neoplasm [MeSH Terms]). After a
comprehensive analysis, 17 studies were selected (8–11,18–30)
Statistical analysis
Statistical analysis was performed on a database
using IBM SPSS Statistics v22 (IBM Corp.). All data are expressed
as the mean ± SE. When ANOVA indicated differences among the
groups, multiple comparisons among each experimental group were
performed using Bonferroni's method. Mann-Whitney U test was
performed between groups of AHCC®-treated and untreated
samples. All statistical experiments were performed three times,
and P<0.05 was considered to indicate a statistically
significant difference.
Results
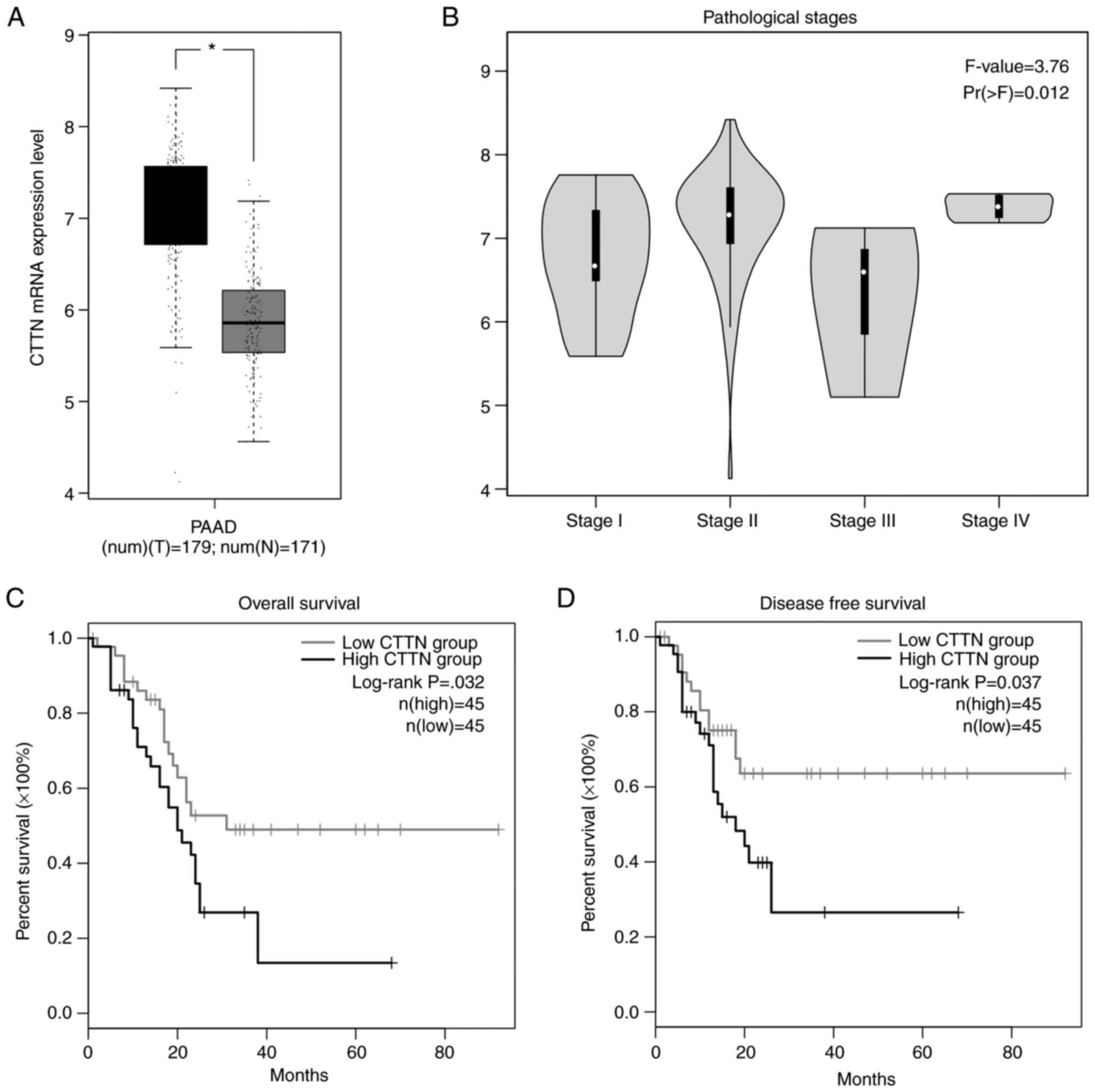
CTTN mRNA expression is inversely
correlated with prolonged patient survival
The CTTN mRNA expression level in pancreatic cancer
tissues was analyzed using the GEPIA2 platform. The results
demonstrated that the CTTN mRNA expression level was significantly
increased in pancreatic cancer tissues when compared to normal
pancreatic tissues (Fig. 1A).
Further analysis of TCGA pancreatic cancer data in GEPIA2 showed
that CTTN expression was positively correlated with the
pathological disease stages, underlying their prognostic value for
pancreatic cancer (Fig. 1B;
P=0.012). The Kaplan-Meier survival plots demonstrated a
significant relation with elevated CTTN expression in pancreatic
cancer. The high level of CTTN is inversely correlated with
prolonged patient survival (Fig. 1C;
P=0.032) and disease-free survival states in patients with
pancreatic cancer (Fig. 1D;
P=0.037).
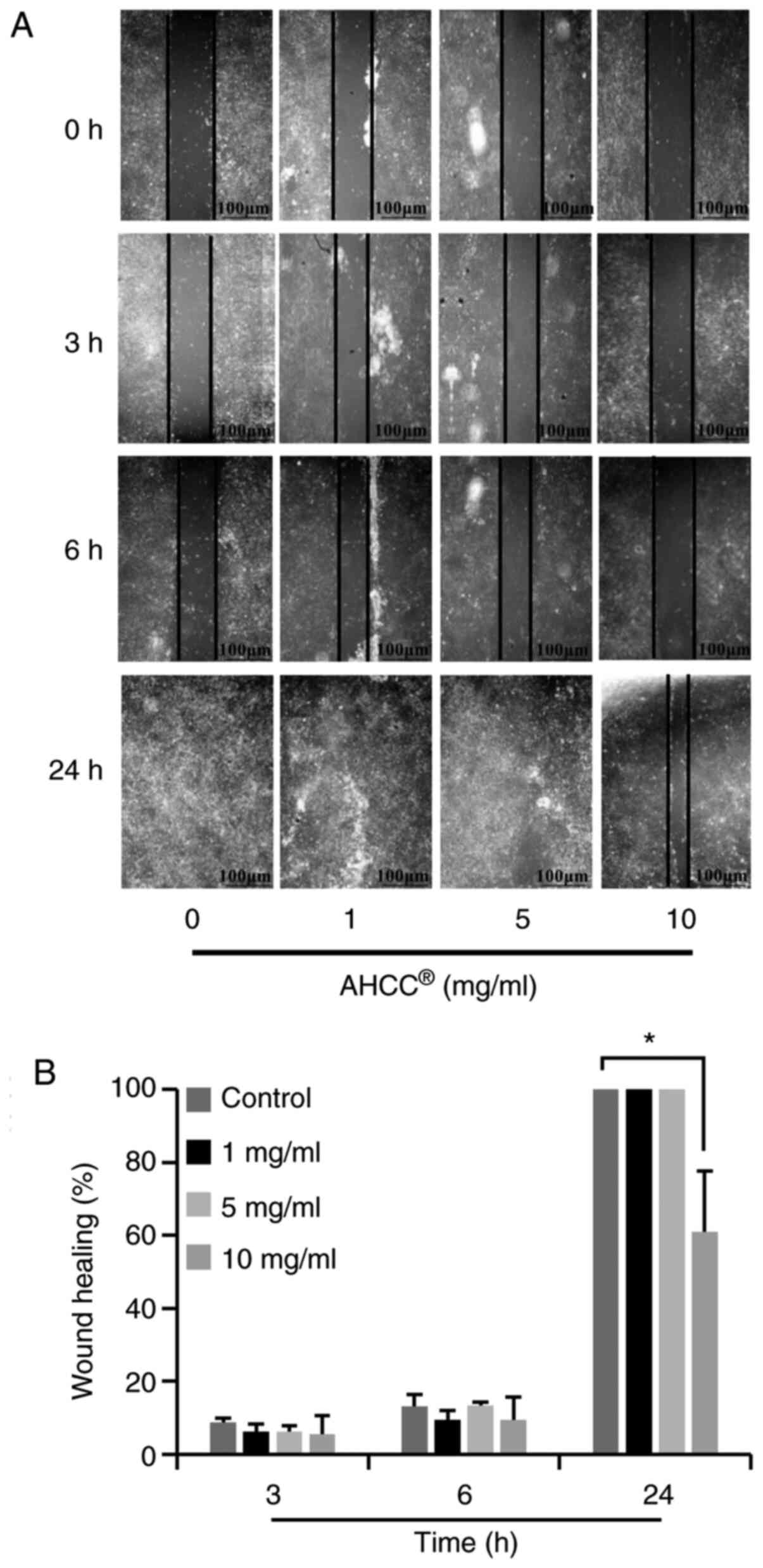
AHCC® inhibited migration
of KLM1-R cells
The effects of AHCC® on KLM1-R cell
migration were assessed by wound healing assay. As shown in
Fig. 2A, photomicrographs taken 24 h
after wounding showed delayed wound closure by KLM1-R cells treated
with AHCC® at concentrations ranging from 0 to 10 mg/ml.
Quantification of the wound closure over time revealed a
significant inhibitory effect of AHCC® on KLM1-R cell
motility at the concentrations of 10 mg/ml (Fig. 2B; P=0.000). Beyond these dosages [0
(control), 1, and 5 mg/ml], adjacent cells migrated toward the
scratched space on plates, and the gap was closed as time passed.
These data indicate that AHCC® effectively inhibits the
migration of KLM1-R cells.
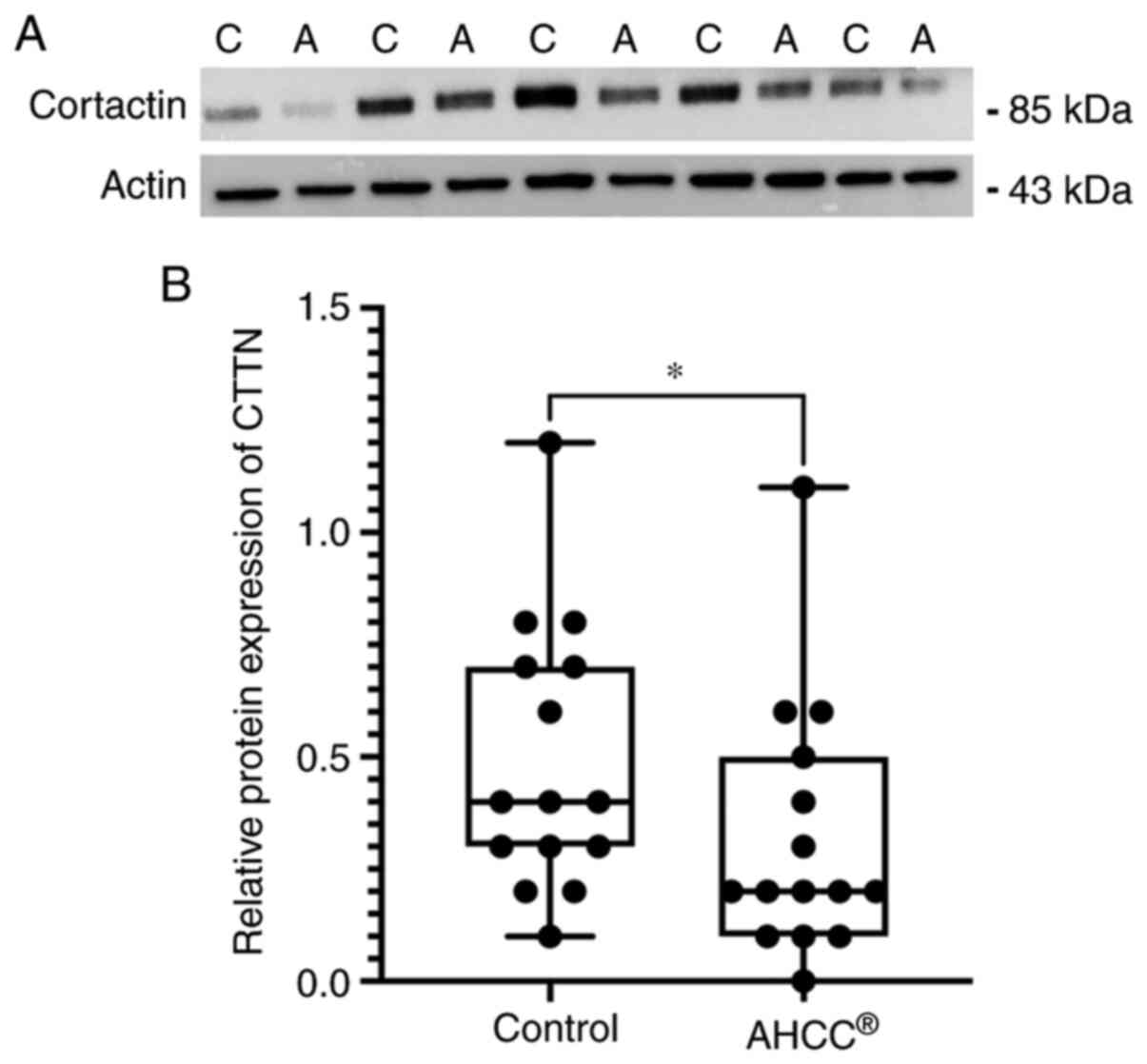
AHCC® treatment decreased
CTTN protein levels in KLM1-R cells
To evaluate the effect of AHCC® on the
CTTN expression, we analyzed the intracellular proteins from KLM1-R
cells treated with or without AHCC® by western blot
analysis with primary antibodies against CTTN and actin. The
protein expression of CTTN was reduced by AHCC®
treatment in KLM1-R cells, whereas actin was almost the same in all
cells (Fig. 3A). In addition, the
ratio of intensities of CTTN/actin in KLM1-R cells was measured.
The CTTN/actin intensity ratio was significantly different between
AHCC®-treated and untreated KLM1-R cells (Fig. 3B; P=0.049). These results suggested
that CTTN was down-regulated by AHCC®−treatment in
vitro.
AHCC® improve treatment
outcome and reduces chemotherapeutic adverse effects
The literature review was performed to investigate
the effects of AHCC® in different cancers. The search
strategy resulted in 48 potentially eligible studies, with 16 in
PubMed and 32 in Scopus. After removing the duplicates, the first
screening resulted in 37 studies singled out for evaluation. After
a comprehensive analysis, 17 studies were chosen based on the
criteria which were set. The effects of AHCC® and its
potential clinical relevance in different cancers were explored, as
shown in Table I. In brief,
AHCC® induces apoptosis, inhibits cellular proliferation
and malignant progression in various cancer types. The apoptotic
effects of AHCC® have been described to be mediated via
both intrinsic and extrinsic apoptotic mechanisms. In addition,
AHCC® was shown to decrease the levels of cellular
reactive oxygen species (ROS). The inhibition of ROS via
administration of AHCC® has been suggested to be
involved in maintaining cellular integrity and preventing
ROS-induced carcinogenesis. Meanwhile, it was also found that in
combination with conventional anti-tumor agents, AHCC®
improves the immune system and prevents immune invasion of cancer
cells. These immunomodulatory effects of AHCC® are
described to be involved in prolonging patient survival and
reducing chemotherapeutic-related adverse effects.
 | Table I.Effects of AHCC® on
different cancers. |
Table I.
Effects of AHCC® on
different cancers.
| Author (year) | Effects | Cancer types | (Refs.) |
|---|
| Kuhara et
al, 2018 | Downregulates CDCP1
levels; inhibits cell migration and malignant progression | Pancreatic
cancer | (8) |
| Suenaga et
al, 2014 | Downregulates HSP27
levels; inhibits cancer cell proliferation | Pancreatic
cancer | (9) |
| Tokunaga et
al, 2015 | Downregulates HSF1
levels | Pancreatic
cancer | (10) |
| Nawata et
al, 2014 | Downregulates SOX-2
levels | Pancreatic
cancer | (11) |
| Matsushita et
al, 1998 | Inhibits tumor
metastasis and improves treatment outcome when combined with
anti-tumor agents | Adenocarcinoma | (18) |
| Cowawintaweewat
et al, 2006 | Prolongs patient
survival; decreases serum AST and ALT levels; increases IL-2 and
neopterin levels | Hepatocellular
carcinoma | (19) |
| Hunter et
al, 2011 | Inhibits tumor
metastasis and improves treatment outcome when combined with
anti-tumor agents | Ovarian cancer | (20) |
| Hangai et
al, 2013 | Inhibits
chemotherapy-related adverse effects; inhibits cellular
inflammation | Breast cancer | (21) |
| Ito et al,
2014 | Decreases the
salivary level of HHV-6 following chemo therapy; reduces
chemotherapeutical-related adverse effects | Colon, pancreatic,
lung and ovarian cancer | (23) |
| Ignacio et
al, 2015 | Maintains cellular
ROS level and increases antioxidant production; inhibits
tumor-related cytokine production | B6 melanoma murine
model | (24) |
| Cao et al,
2015 | Induces apoptosis
and inhibition of cellular proliferation mice | Hepatoma
tumor-bearing | (25) |
| Yanagimoto et
al, 2016 | Reduces
chemotherapy-related adverse effects; Reduces CRP and albumin
levels; Improves patient outcomes | Pancreatic
cancer | (26) |
| Graham et
al, 2017 | Inhibits cellular
proliferation and migration; upregulates tumor suppressor protein
miR-335 and contributes to prevent immune invasion | Breast cancer | (27) |
| Fatehchand et
al, 2017 | Induces apoptosis
through involvement with both extrinsic and intrinsic
mechanisms | Acute myeloblastic
leukemia | (28) |
| Choi et al,
2018 | Induces apoptosis
and proliferation; inhibits STAT3 phosphorylation; induces SHP-1
and inhibits cyclin D1, Bcl-2, Mcl-1, survivin and VEGF levels | Ovarian cancer | (29) |
| Suknikhom et
al, 2017 | Increases CD+T cell
population and improves patient outcome | Ovarian cancer | (30) |
Discussion
In the present study, we demonstrated that CTTN mRNA
expression was significantly higher in pancreatic cancer than in
normal tissues. The higher CTTN expression was significantly
correlated with the pathological stages of pancreatic cancer. The
Kaplan-Meier survival plots showed that elevated CTTN expression
levels are inversely associated with prolonged patient survival.
From our in vitro analysis, we showed that AHCC®
significantly suppressed KLM1-R cell migration. A significant
reduction of CTTN protein level was observed in cells treated with
AHCC® compared to control. The downregulation of CTTN
possibly causes the anti-tumor potential of AHCC® in
pancreatic cancer cells.
CTTN has been documented to play essential roles in
regulating actin cytoskeletal dynamics (31). The basic structure of CTTN consists
of four major domains: An N-terminal acidic (NTA), a central 6.5
tandems repeat, a proline-rich domain, and the C-terminal Src
homology 3 domains (SH3 domains) (31,32).
Those domains are modified and alter the interaction with binding
partners to promote actin polymerization during cell motility. This
then plays a central role in the formation of invadopodia, which
are actin-driven protrusive structures in invasive cancer cells
that degrade the ECM. The degraded ECM allows cancer cells to
migrate towards the distant organs resulting in metastasis
(33). The higher CTTN expression
was previously described in several cancers and correlated with
poor clinical outcomes in breast cancer, oral cancer, liver cancer,
colon cancer, and melanoma (4,31,33).
However, little is known about CTTN involvement in
the tumor progression of pancreatic cancer. A previous study has
shown that elevated CTTN expression in pancreatic cancer is
significantly involved with metastatic compared to primary tumors
(34). In addition, it was
demonstrated that inhibition of CTTN expression impaired the
migration and invasion potential of pancreatic cancer (34). Based on these findings, it is
imperative to regulate CTTN to treat pancreatic cancer. Our study
observed a significant reduction in cell migration and CTTN levels
in AHCC® treated cells compared to untreated cells. The
downregulation of CTTN possibly caused the reduced cell migration
observed in this study. It is, however, still unknown how
AHCC® downregulated CTTN levels in pancreatic cancer
cells.
In vitro, CTTN is overexpressed and activated
by Src-mediated tyrosine phosphorylation, which leads to increased
migration of fibroblasts and endothelial cells (35,36).
Phosphorylation of CTTN occurring primarily at tyrosine 421
(Tyr421) enhances the actin assembly during cytoskeletal remodeling
(37,38). The tyrosine phosphorylation of CTTN
correlates with the invadopodia activity necessary for matrix
degradation, cell migration, and invasion (39). The protein-tyrosine phosphatase SHP-1
is a negative regulator of multiple signal transduction pathways
and proposed to be a candidate tumor suppressor gene in various
cancers (40). The substrates that
are efficiently phosphorylated by Src kinase are, in turn,
efficient substrates for SHP-1. Consequently, SHP-1 can negatively
regulate Src-mediated autophosphorylation (41). Notably, a previous study has
demonstrated that treatment with AHCC® significantly
elevated SHP-1 in ovarian cancer, whereas elevated SHP-1
contributed to preventing the malignant progression of this cancer
(29). Since Src-kinase-mediated
phosphorylation is essential for the biological relevance of CTTN
in cancer progression, the elevated SHP-1 level via
AHCC® may control the expression and activation of CTTN
in pancreatic cancer. The other possible mechanisms cannot be ruled
out. Further studies are needed to clarify our speculation and
elucidate the anti-tumor potential of AHCC® in
pancreatic cancer.
Meanwhile, to explore potential anti-tumor effects
of AHCC® in other cancers, we performed a literature
review by using effective search engines. We found that
AHCC® can inhibit malignant progression by inducing
cellular apoptosis and inhibition of cellular proliferation.
Moreover, AHCC® decreases the cellular ROS levels and
maintains endothelial cellular plasticity, and prevents malignant
transformation. Importantly, we observed that administration of
AHCC® involved immune-modulatory functions, which
contribute to improving overall patient survival and reducing the
adverse effects of chemotherapeutics. Collectively, these findings
indicated that AHCC® plays pleiotropic roles in
preventing the onset of different cancers and may constitute an
attractive therapeutic agent.
In conclusion, our results suggested that
AHCC® has anti-metastatic effects in pancreatic cancer
cell lines via the downregulated CTTN level, and thus, this
compound exhibits for the treatment of pancreatic cancer. However,
a lack of adequate validation of the bioinformatics results in
tissue samples is a limitation of this study. Therefore, further
studies are needed to develop AHCC® as a complementary
and alternative therapeutic approach to treating pancreatic
cancer.
Acknowledgements
The authors would like to thank Dr Shin-ichiro
Maehara and Professor Yoshihiko Maehara at Kyushu University
(Fukuoka, Japan) for providing the KLM1-R cells.
Funding
This work was supported in part by Grants-in-Aid
from the Ministry of Education, Science, Sports and Culture of
Japan (grant no. 17K07218).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SI, TK and YK conceived and designed the study and
performed the experiments. SI, BB, KK, HN, MK, IC and YK analyzed
and interpreted the data. SI wrote the initial draft of the
manuscript. BB, TK and YK contributed to critical revision of the
manuscript. All authors read and approved the final manuscript. SI
and YK confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Islam S, Kitagawa T, Baron B, Abiko Y,
Chiba I and Kuramitsu Y: ITGA2, LAMB3, and LAMC2 may be the
potential therapeutic targets in pancreatic ductal adenocarcinoma:
An integrated bioinformatics analysis. Sci Rep. 11:105632021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amrutkar M and Gladhaug IP: Pancreatic
cancer chemoresistance to gemcitabine. Cancers (Basel). 9:1572017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamaguchi H and Condeelis J: Regulation of
the cytoskeleton in cancer cell migration and invation. Biochim
Biophys Acta. 1773:642–652. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yin M, Ma W and An L: Cortactin in cancer
cell migration and invasion. Oncotarget. 8:88232–88243. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamiyama Y, Matsui Y, Kawaguchi Y, Kosuna
K and Wakame K: Active hexose correlated compound (AHCC).
Biotherapy. 14:959–964. 2000.
|
|
6
|
Kidd P: The use of mushroom glucans and
proteoglycans in cancer treatment. Altern Med Rev. 5:4–27.
2000.PubMed/NCBI
|
|
7
|
Ye SF, Ichimura K, Wakame K and Ohe M:
Suppressive effects of active hexose correlated compound on the
increased activity of hepatic and renal ornithine decarboxylase
induced by oxidative stress. Life Sci. 74:593–602. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuhara K, Tokuda K, Kitagawa T, Baron B,
Tokunaga M, Harada K, Terasaki M, Uehara O, Ohta T, Takai R, et al:
CUB domain-containing protein 1 (CDCP1) is down-regulated by active
hexose-correlated compound in human pancreatic cancer cells.
Anticancer Res. 38:6107–6111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suenaga S, Kuramitsu Y, Kaino S, Maehara
S-I, Maehara Y, Sakaida I and Nakamura K: Active hexose-correlated
compound down-regulates HSP27 of pancreatic cancer cells, and helps
the cytotoxic effect of gemcitabine. Anticancer Res. 34:141–146.
2014.PubMed/NCBI
|
|
10
|
Tokunaga M, Baron B, Kitagawa T, Tokuda K
and Kuramitsu Y: Active hexose-correlated compound down-regulates
heat shock factor 1, a transcription factor for HSP27, in
gemcitabine-resistant human pancreatic cancer cells. Anticancer
Res. 35:6063–6067. 2015.PubMed/NCBI
|
|
11
|
Nawata J, Kuramitsu Y, Wang Y, Kitagawa T,
Tokuda K, Baron B, Akada J, Suenaga S, Kaino S, Maehara S, et al:
Active hexose-correlated compound down-regulates sex-determining
region Y-box 2 of pancreatic cancer cells. Anticancer Res.
34:4807–4811. 2014.PubMed/NCBI
|
|
12
|
Mori-Iwamoto S, Kuramitsu Y, Ryozawa S,
Mikuria K, Fujimoto M, Maehara S, Maehara Y, Okita K, Nakamura K
and Sakaida I: Proteomics finding heat shock protein 27 as a
biomarker for resistance of pancreatic cancer cells to gemcitabine.
Int J Oncol. 31:1345–1350. 2007.PubMed/NCBI
|
|
13
|
Tang Z, Kang B, Li C, Chen T and Zhang Z:
GEPIA2: An enhanced web server for large-scale expression profiling
and interactive analysis. Nucleic Acids Res. 47:W556–W560. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maehara S, Tanaka S, Shimada M, Shirabe K,
Saito Y, Takahashi K and Maehara Y: Selenoprotein P, as a predictor
for evaluating gemcitabine resistance in human pancreatic cancer
cells. Int J Cancer. 112:184–189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Belkourchia F and Desrosiers R: The enzyme
L-isoaspartyl (D-aspartyl) methyltransferase promotes migration and
invasion in human U-87 MG and U-251 MG glioblastoma cell lines.
Biomed Pharmacother. 140:1117662021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Islam S, Uehara O, Matsuoka H, Kuramitsu
Y, Adhikari BR, Hiraki D, Toraya S, Jayawardena A, Saito I,
Muthumala M, et al: DNA hypermethylation of sirtuin 1 (SIRT1)
caused by betel quid chewing-a possible predictive biomarker for
malignant transformation. Clin Epigenetics. 12:122020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsushita K, Kuramitsu Y, Ohiro Y, Obara
M, Kobayashi M, Li YQ and Hosokawa M: Combination therapy of active
hexose correlated compound plus UFT significantly reduces the
metastasis of rat mammary adenocarcinoma. Anticancer Drugs.
9:343–350. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cowawintaweewat S, Manoromana S, Sriplung
H, Khuhaprema T, Tongtawe P, Tapchaisri P and Chaicumpa W:
Prognostic improvement of patients with advanced liver cancer after
active hexose correlated compound (AHCC) treatment. Asian Pac J
Allergy Immunol. 24:33–45. 2006.PubMed/NCBI
|
|
20
|
Hunter RJ, Fujii H, Wakame K, Gaikwad A,
Wolf JK and Smith JA: Evaluation of active hexose correlated
compound (AHCC) in combination with pegylated liposomal doxorubicin
for treatment of ovarian cancer. Int J Appl Res Nat Prod. 4:6–11.
2011.
|
|
21
|
Hangai S, Iwase S, Kawaguchi T, Kogure Y,
Miyaji T, Matsunaga T, Nagumo Y and Yamaguchi T: Effect of active
hexose-correlated compound in women receiving adjuvant chemotherapy
for breast cancer: A retrospective study. J Altern Complement Med.
19:905–910. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haidari M, Zhang W and Wakame K:
Disruption of endothelial adherens junction by invasive breast
cancer cells is mediated by reactive oxygen species and is
attenuated by AHCC. Life Sci. 93:994–1003. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ito T, Urushima H, Sakaue M, Yukawa S,
Honda H, Hirai K, Igura T, Hayashi N, Maeda K, Kitagawa T and Kondo
K: Reduction of adverse effects by a mushroom product, active
hexose correlated compound (AHCC) in patients with advanced cancer
during chemotherapy-the significance of the levels of HHV-6 DNA in
saliva as a surrogate biomarker during chemotherapy. Nutr Cancer.
66:377–382. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ignacio RM, Kim CS, Kim YD, Lee HM, Qi XF
and Kim SK: Therapeutic effect of active hexose-correlated compound
(AHCC) combined with CpG-ODN (oligodeoxynucleotide) in B16 melanoma
murine model. Cytokine. 76:131–137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao Z, Chen X, Lan L, Zhang Z, Du J and
Liao L: Active hexose correlated compound potentiates the antitumor
effects of low-dose 5-fluorouracil through modulation of immune
function in hepatoma 22 tumor-bearing mice. Nutr Res Pract.
9:129–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yanagimoto H, Satoi S, Yamamoto T, Hirooka
S, Yamaki S, Kotsuka M, Ryota H, Michiura T, Inoue K, Matsui Y, et
al: Alleviating effect of active hexose correlated compound (AHCC)
on chemotherapy-related adverse events in patients with
unresectable pancreatic ductal adenocarcinoma. Nutr Cancer.
68:234–240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Graham ÉA, Mallet JF, Jambi M, Nishioka H,
Homma K and Matar C: MicroRNA signature in the chemoprevention of
functionally-enriched stem and progenitor pools (FESPP) by active
hexose correlated compound (AHCC). Cancer Biol Ther. 18:765–774.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fatehchand K, Santhanam R, Shen B,
Erickson EL, Gautam S, Elavazhagan S, Mo X, Belay T, Tridandapani S
and Butchar JP: Active hexose-correlated compound enhances
extrinsic-pathway-mediated apoptosis of acute myeloid leukemic
cells. PLoS One. 12:e01817292017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choi JY, Lee S, Yun SM, Suh DH, Kim K, No
JH, Jeong EH and Kim YB: Active hexose correlated compound (AHCC)
inhibits the proliferation of ovarian cancer cells by suppressing
signal transducer and activator of transcription 3 (STAT3)
activation. Nutr Cancer. 70:109–115. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Suknikhom W, Lertkhachonsuk R and Manchana
T: The effects of active hexose correlated compound (AHCC) on
levels of CD4+ and CD8+ in patients with epithelial ovarian cancer
or peritoneal cancer receiving platinum based chemotherapy. Asian
Pac J Cancer Prev. 18:633–638. 2017.PubMed/NCBI
|
|
31
|
Buday L and Downward J: Roles of cortactin
in tumor pathogenesis. Biochim Biophys Acta. 1775:263–273.
2007.PubMed/NCBI
|
|
32
|
Ammer AG and Weed S: Cortactin branches
out: Roles in regulating protrusive actin dynamics. Cell Motil
Cytoskeleton. 65:687–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He J, Xia TS and Wang S: Cortactin and
tumor invasiveness. Chin J Cancer Prev Treat. 22:72–75, 80.
2015.
|
|
34
|
Stock K, Borrink R, Mikesch JH, Hansmeier
A, Rehkämper J, Trautmann M, Wardelmann E, Hartmann W, Sperveslage
J and Steinestel K: Overexpression and Tyr421-phosphorylation of
cortactin is induced by three-dimensional spheroid culturing and
contributes to migration and invasion of pancreatic ductal
adenocarcinoma (PDAC) cells. Cancer Cell Int. 19:772019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Patel AS, Schlechter GL, Wasilenko WJ and
Somers KD: Overexpression of EMS1/cortactin in NIH3T3 fibroblasts
causes increased cell motility and invasion in vitro. Oncogene.
16:3227–3232. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang C, Liu J, Haudenschild CC and Zhan
X: The role of tyrosine phosphorylation of cortactin in the
locomotion of endothelial cells. J Biol Chem. 273:25770–25776.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tehrani S, Tomasevic N, Weed S, Sakowicz R
and Cooper J: Src phosphorylation of cortactin enhances actin
assembly. Proc Natl Acad Sci USA. 104:11933–11938. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Head JA, Jiang D, Li M, Zorn LJ, Schaefer
EM, Parsons JT and Weed SA: Cortactin tyrosine phosphorylation
requires Rac1 activity and association with the cortical actin
cytoskeleton. Mol Biol Cell. 14:3216–3229. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bowden ET, Onikoyi E, Tidwell R, Myoui A,
Yoneda T, Yamada KM and Mueller SC: Co-localization of cortactin
and phosphotyrosine identifies active invadopodia in human breast
cancer cells. Exp Cell Res. 312:1240–1253. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu C, Sun M, Liu L and Zhou W: The
function of the protein tyrosine phosphatase SHP-1 in cancer. Gene.
306:1–12. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Frank C, Burkhardt C, Imhof D, Ringel J,
Zschörnig O, Wieligmann K, Zacharias M and Böhmer FD: Effective
dephosphorylation of Src substrates by SHP-1. J Biol Chem.
279:11375–11383. 2004. View Article : Google Scholar : PubMed/NCBI
|