|
1
|
Koo SL, Wang WW and Toh HC: Cancer
Immunotherapy-The target is precisely on the cancer and also not.
Ann Acad Med Singap. 47:381–387. 2018.PubMed/NCBI
|
|
2
|
Meng J, Zhou Y, Lu X, Bian Z, Chen Y, Zhou
J, Zhang L, Hao Z, Zhang M and Liang C: Immune response drives
outcomes in prostate cancer: Implications for immunotherapy. Mol
Oncol. 15:1358–1375. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balachandran VP, Beatty GL and Dougan SK:
Broadening the impact of immunotherapy to pancreatic cancer:
Challenges and opportunities. Gastroenterology. 156:2056–2072.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parkin J and Cohen B: An overview of the
immune system. Lancet. 357:1777–1789. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perales-Puchalt A, Wojtak K, Duperret EK,
Yang X, Slager AM, Yan J, Muthumani K, Montaner LJ and Weiner DB:
Engineered DNA vaccination against follicle-stimulating hormone
receptor delays ovarian cancer progression in animal models. Mol
Ther. 27:314–325. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pedersen M, Westergaard MCW, Milne K,
Nielsen M, Borch TH, Poulsen LG, Hendel HW, Kennedy M, Briggs G,
Ledoux S, et al: Adoptive cell therapy with tumor-infiltrating
lymphocytes in patients with metastatic ovarian cancer: A pilot
study. OncoImmunology. 7:e15029052018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mitchell DM, Ravkov EV and Williams MA:
Distinct roles for IL-2 and IL-15 in the differentiation and
survival of CD8+ effector and memory T cells. J Immunol.
184:6719–6730. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jaeckel E, Kretschmer K, Apostolou I and
von Boehmer H: Instruction of Treg commitment in peripheral T cells
is suited to reverse autoimmunity. Semin Immunol. 18:89–92. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brisslert M, Bokarewa M, Larsson P, Wing
K, Collins LV and Tarkowski A: Phenotypic and functional
characterization of human CD25+ B cells. Immunology. 117:548–557.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim HP, Imbert J and Leonard WJ: Both
integrated and differential regulation of components of the
IL-2/IL-2 receptor system. Cytokine Growth Factor Rev. 17:349–366.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smith FO, Downey SG, Klapper JA, Yang JC,
Sherry RM, Royal RE, Kammula US, Hughes MS, Restifo NP, Levy CL, et
al: Treatment of metastatic melanoma using interleukin-2 alone or
in conjunction with vaccines. Clin Cancer Res. 14:5610–5618. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lopes JE, Fisher JL, Flick HL, Wang C, Sun
L, Ernstoff MS, Alvarez JC and Losey HC: ALKS 4230: A novel
engineered IL-2 fusion protein with an improved cellular
selectivity profile for cancer immunotherapy. J Immunother Cancer.
8:e0006732020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Attridge K, Wang CJ, Wardzinski L,
Kenefeck R, Chamberlain JL, Manzotti C, Kopf M and Walker LS: IL-21
inhibits T cell IL-2 production and impairs Treg homeostasis.
Blood. 119:4656–4664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zimmerman RJ, Aukerman SL, Katre NV,
Winkelhake JL and Young JD: Schedule dependency of the antitumor
activity and toxicity of polyethylene glycol-modified interleukin 2
in murine tumor models. Cancer Res. 49:6521–6528. 1989.PubMed/NCBI
|
|
15
|
Rosenberg SA: IL-2: The first effective
immunotherapy for human cancer. J Immunol. 192:5451–5458. 2014.
View Article : Google Scholar : PubMed/NCBI
|
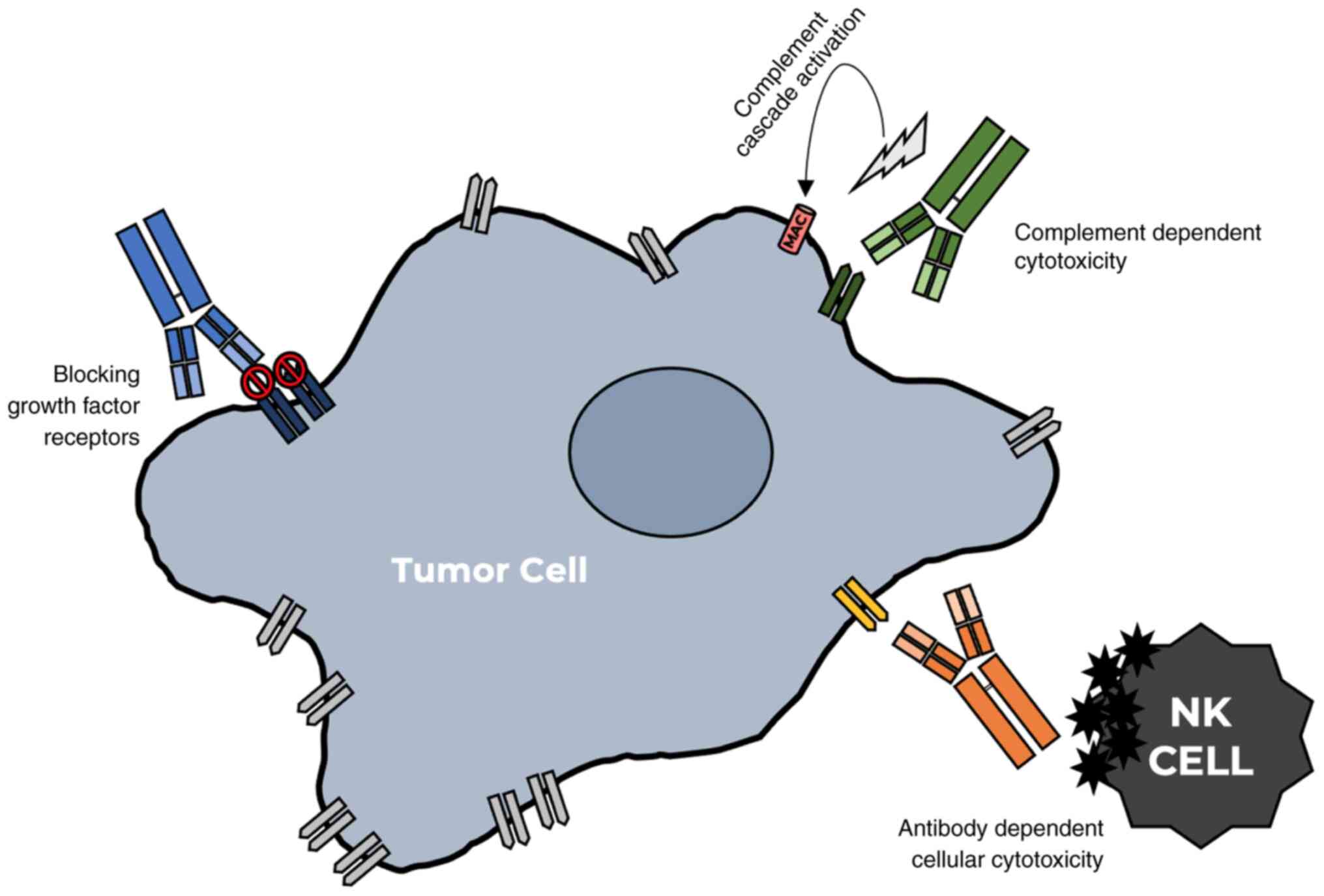
|
16
|
Grimm EA, Mazumder A, Zhang HZ and
Rosenberg SA: Lymphokine-activated killer cell phenomenon. Lysis of
natural killer-resistant fresh solid tumor cells by interleukin
2-activated autologous human peripheral blood lymphocytes. J Exp
Med. 155:1823–1841. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dudley ME, Wunderlich JR, Yang JC, Sherry
RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE,
Mavroukakis SA, et al: Adoptive cell transfer therapy following
non-myeloablative but lymphodepleting chemotherapy for the
treatment of patients with refractory metastatic melanoma. J Clin
Oncol. 23:2346–2357. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krieg C, Létourneau S, Pantaleo G and
Boyman O: Improved IL-2 immunotherapy by selective stimulation of
IL-2 receptors on lymphocytes and endothelial cells. Proc Natl Acad
Sci USA. 107:11906–11911. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nasreddine G, El-Sibai M and Abi-Habib RJ:
Cytotoxicity of [HuArgI (co)-PEG5000]-induced arginine deprivation
to ovarian cancer cells is autophagy dependent. Invest New Drugs.
38:10–19. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ingersoll SB, Ahmad S, McGann HC, Banks
RK, Stavitzski NM, Srivastava M, Ali G, Finkler NJ, Edwards JR and
Holloway RW: Cellular therapy in combination with cytokines
improves survival in a xenograft mouse model of ovarian cancer. Mol
Cell Biochem. 407:281–287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ingersoll SB, Patel S, Caballero L, Ahmad
S, Edwards D, Holloway RW and Edwards JR: Synergistic cytotoxicity
of interferonalpha-2b and interleukin-2 in combination with PBMC
against ovarian cancer: Development of an experimental model for
cellular therapy. Gynecol Oncol. 112:192–198. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Di Scala M, Gil-Fariña I, Olagüe C, Vales
A, Sobrevals L, Fortes P, Corbacho D and González-Aseguinolaza G:
Identification of IFN-γ-producing T cells as the main mediators of
the side effects associated to mouse interleukin-15 sustained
exposure. Oncotarget. 7:49008–49026. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miller JS, Morishima C, McNeel DG, Patel
MR, Kohrt HEK, Thompson JA, Sondel PM, Wakelee HA, Disis ML, Kaiser
JC, et al: A First-in-Human Phase I Study of subcutaneous
outpatient recombinant human IL15 (rhIL15) in adults with advanced
solid tumors. Clin Cancer Res. 24:1525–1535. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Conlon KC, Lugli E, Welles HC, Rosenberg
SA, Fojo AT, Morris JC, Fleisher TA, Dubois SP, Perera LP, Stewart
DM, et al: Redistribution, hyperproliferation, activation of
natural killer cells and CD8 T cells, and cytokine production
during First-in-Human clinical trial of recombinant human
Interleukin-15 in patients with cancer. J Clin Oncol. 33:74–82.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rubinstein MP, Kovar M, Purton JF, Cho JH,
Boyman O, Surh CD and Sprent J: Converting IL-15 to a superagonist
by binding to soluble IL-15R{alpha}. Proc Natl Acad Sci USA.
103:9166–9171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ochoa MC, Fioravanti J, Rodriguez I,
Hervas-Stubbs S, Azpilikueta A, Mazzolini G, Gúrpide A, Prieto J,
Pardo J, Berraondo P and Melero I: Antitumor immunotherapeutic and
toxic properties of an HDL-Conjugated Chimeric IL-15 fusion
protein. Cancer Res. 73:139–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mortier E, Quéméner A, Vusio P, Lorenzen
I, Boublik Y, Grötzinger J, Plet A and Jacques Y: Soluble
interleukin-15 receptor alpha (IL-15R alpha)-sushi as a selective
and potent agonist of IL-15 action through IL-15R beta/gamma.
Hyperagonist IL-15 × IL-15R alpha fusion proteins. J Biol Chem.
281:1612–1619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ochoa MC, Minute L, López A, Pérez-Ruiz E,
Gomar C, Vasquez M, Inoges S, Etxeberria I, Rodriguez I, Garasa S,
et al: Enhancement of antibody-dependenT cellular cytotoxicity of
cetuximab by a chimeric protein encompassing interleukin-15.
Oncoimmunology. 7:e13935972017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rhode PR, Egan JO, Xu W, Hong H, Webb GM,
Chen X, Liu B, Zhu X, Wen J, You L, et al: Comparison of the
superagonist complex, ALT-803, to IL15 as cancer immunotherapeutics
in animal models. Cancer Immunol Res. 4:49–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Romee R, Cooley S, Berrien-Elliott MM,
Westervelt P, Verneris MR, Wagner JE, Weisdorf DJ, Blazar BR, Ustun
C, DeFor TE, et al: First-in-human phase 1 clinical study of the
IL-15 superagonist complex ALT-803 to treat relapse after
transplantation. Blood. 131:2515–2527. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rosser CJ, Nix L, Ferguson L, Hernandez L
and Wong HC: Phase Ib trial of ALT-803, an IL-15 superagonist, plus
BCG for the treatment of BCG-naïve patients with
non-muscle-invasive bladder cancer. J Clin Oncol. 36 (Suppl
6):5102021. View Article : Google Scholar
|
|
32
|
Timmerman JM, Byrd JC, Andorsky DJ, Yamada
RE, Kramer J, Muthusamy N, Hunder N and Pagel JM: A phase I
dose-finding trial of recombinant interleukin-21 and rituximab in
relapsed and refractory low grade B-cell lymphoproliferative
disorders. Clin Cancer Res. 18:5752–5760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fioravanti J, Di Lucia P, Magini D, Moalli
F, Boni C, Benechet AP, Fumagalli V, Inverso D, Vecchi A, Fiocchi
A, et al: Effector CD8+ T cell-derived interleukin-10 enhances
acute liver immunopathology. J Hepatol. 67:543–548. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Koski A, Kangasniemi L, Escutenaire S,
Pesonen S, Cerullo V, Diaconu I, Nokisalmi P, Raki M, Rajecki M,
Guse K, et al: Treatment of cancer patients with a serotype 5/3
chimeric oncolytic adenovirus expressing GMCSF. Mol Ther.
18:1874–1884. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Spaapen RM, Leung MY, Fuertes MB, Kline
JP, Zhang L, Zheng Y, Fu YX, Luo X, Cohen KS and Gajewski TF:
Therapeutic activity of High-Dose Intratumoral IFN-β requires
direct effect on the tumor vasculature. J Immunol. 193:4254–4260.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Herndon TM, Demko SG, Jiang X, He K,
Gootenberg JE, Cohen MH, Keegan P and Pazdur R: U.S. Food and Drug
Administration Approval: Peginterferon-alfa-2b for the adjuvant
treatment of patients with melanoma. Oncologist. 17:1323–1328.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bellobuono A, Mondazzi L, Tempini S,
Silini E, Vicari F and Idéo; G. Ribavirin and interferon-alpha
combination therapy vs interferon-alpha alone in the retreatment of
chronic hepatitis C, : A randomized clinical trial. J Viral Hepat.
4:185–191. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gogas H, Ioannovich J, Dafni U,
Stavropoulou-Giokas C, Frangia K, Tsoutsos D, Panagiotou P, Polyzos
A, Papadopoulos O, Stratigos A, et al: Prognostic significance of
autoimmunity during treatment of melanoma with interferon. N Engl J
Med. 354:709–718. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fioravanti J, González I, Medina-Echeverz
J, Larrea E, Ardaiz N, González-Aseguinolaza G, Prieto J and
Berraondo P: Anchoring interferon alpha to apolipoprotein A-I
reduces hematological toxicity while enhancing immunostimulatory
properties. Hepatology. 53:1864–1873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cauwels A, Van Lint S, Paul F, Garcin G,
De Koker S, Van Parys A, Wueest T, Gerlo S, Van der Heyden J,
Bordat Y, et al: Delivering Type I interferon to dendritic cells
empowers tumor eradication and immune combination treatments.
Cancer Res. 78:463–474. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Palladino MA, Bahjat FR, Theodorakis EA
and Moldawer LL: Anti-TNF-alpha therapies: The next generation. Nat
Rev Drug Discov. 2:736–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Creaven PJ, Plager JE, Dupere S, Huben RP,
Takita H, Mittelman A and Proefrock A: Phase I clinical trial of
recombinant human tumor necrosis factor. Cancer Chemother
Pharmacol. 20:137–144. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zheng L, Fisher G, Miller RE, Peschon J,
Lynch DH and Lenardo MJ: Induction of apoptosis in mature T cells
by tumour necrosis factor. Nature. 377:348–351. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kahn JO, Kaplan LD, Volberding PA, Ziegler
JL, Crowe S, Saks SR and Abrams DI: Intralesional recombinant tumor
necrosis factor-alpha for AIDS-associated Kaposi's sarcoma: A
randomized, double-blind trial. J Acquir Immune Defic Syndr.
2:217–223. 1989.PubMed/NCBI
|
|
45
|
Manusama ER, Nooijen PT, Stavast J,
Durante NM, Marquet RL and Eggermont AM: Synergistic antitumour
effect of recombinant human tumour necrosis factor alpha with
melphalan in isolated limb perfusion in the rat. Br J Surg.
83:551–555. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lejeune FJ, Liénard D, Matter M and Rüegg
C: Efficiency of recombinant human TNF in human cancer therapy.
Cancer Immun. 6:62006.PubMed/NCBI
|
|
47
|
van Horssen R, Ten Hagen TL and Eggermont
AM: TNF-alpha in cancer treatment: Molecular insights, antitumor
effects, and clinical utility. Oncologist. 11:397–408. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Herzberg B, Campo MJ and Gainor JF: Immune
checkpoint inhibitors in non-small cell lung cancer. Oncologist.
22:81–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Delgobo M and Frantz S: Heart failure in
cancer: Role of checkpoint inhibitors. J Thorac Dis. 10 (Suppl
35):S4323–S4334. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wolchok JD, Chiarion-Sileni V, Gonzalez R,
Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D,
Ferrucci PF, et al: Overall survival with combined nivolumab and
ipilimumab in advanced melanoma. N Engl J Med. 377:1345–1356. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kamath SD and Kumthekar PU: Immune
checkpoint inhibitors for the treatment of central nervous system
(CNS) metastatic disease. Front Oncol. 8:4142018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Heinzerling L, Ott PA, Hodi FS, Husain AN,
Tajmir-Riahi A, Tawbi H, Pauschinger M, Gajewski TF, Lipson EJ and
Luke JJ: Cardiotoxicity associated with CTLA4 and PD1 blocking
immunotherapy. J Immunother Cancer. 4:502016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sznol M, Postow MA, Davies MJ, Pavlick AC,
Plimack ER, Shaheen M, Veloski C and Robert C: Endocrine-related
adverse events associated with immune checkpoint blockade and
expert insights on their management. Cancer Treat Rev. 58:70–76.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hassel JC, Heinzerling L, Aberle J, Bähr
O, Eigentler TK, Grimm MO, Grünwald V, Leipe J, Reinmuth N, Tietze
JK, et al: Combined immune checkpoint blockade
(anti-PD-1/anti-CTLA-4): Evaluation and management of adverse drug
reactions. Cancer Treat Rev. 57:36–49. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Simonaggio A, Michot JM, Voisin AL, Le
Pavec J, Collins M, Lallart A, Cengizalp G, Vozy A, Laparra A,
Varga A, et al: Evaluation of readministration of immune checkpoint
inhibitors after immune-related adverse events in patients with
cancer. JAMA Oncol. 5:1310–1317. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Santini FC, Rizvi H, Plodkowski AJ, Ni A,
Lacouture ME, Gambarin-Gelwan M, Wilkins O, Panora E, Halpenny DF,
Long NM, et al: Safety and efficacy of re-treating with
immunotherapy after immune-related adverse events in patients with
NSCLC. Cancer Immunol Res. 6:1093–1099. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kluger HM, Zito CR, Turcu G, Baine MK,
Zhang H, Adeniran A, Sznol M, Rimm DL, Kluger Y, Chen L, et al:
PD-L1 studies across tumor types, its differential expression and
predictive value in patients treated with immune checkpoint
inhibitors. Clin Cancer Res. 23:4270–4279. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Fan J, Shang D, Han B, Song J, Chen H and
Yang JM: Adoptive cell transfer: Is it a promising immunotherapy
for colorectal cancer? Theranostics. 8:5784–5800. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wrangle J, Paulos CM, Smith TW, Nishimura
MI and Rubinstein MP: Inducible enhancement of T cell function and
anti-tumor activity after adoptive transfer. Mol Ther.
25:1995–1996. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rohaan MW, Wilgenhof S and Haanen JBAG:
Adoptive cellular therapies: The current landscape. Virchows Arch.
474:449–461. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mitchison NA: Studies on the immunological
response to foreign tumor transplants in the mouse. I. The role of
lymph node cells in conferring immunity by adoptive transfer. J Exp
Med. 102:157–177. 1955. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fefer A: Immunotherapy and chemotherapy of
Moloney sarcoma virus-induced tumors in mice. Cancer Res.
29:2177–2183. 1969.PubMed/NCBI
|
|
63
|
Rosenberg SA and Terry WD: Passive
immunotherapy of cancer in animals and man. Adv Cancer Res.
25:323–388. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kono K, Ichihara F, Iizuka H, Sekikawa T
and Matsumoto Y: Expression of signal transducing T-cell receptor
zeta molecules after adoptive immunotherapy in patients with
gastric and colon cancer. Int J Cancer. 78:301–305. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lu TL, Pugach O, Somerville R, Rosenberg
SA, Kochenderfer JN, Better M and Feldman SA: A Rapid cell
expansion process for production of engineered autologous CAR-T
cell therapies. Hum Gene Ther Methods. 27:209–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Xiao L, Cen D, Gan H, Sun Y, Huang N,
Xiong H, Jin Q, Su L, Liu X, Wang K, et al: Adoptive transfer of
NKG2D CAR mRNA-Engineered natural killer cells in colorectal cancer
patients. Mol Ther. 27:1114–1125. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Vivier E, Raulet DH, Moretta A, Caligiuri
MA, Zitvogel L, Lanier LL, Yokoyama WM and Ugolini S: Innate or
adaptive immunity? The example of natural killer cells. Science.
331:44–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Morvan MG and Lanier LL: NK cells and
cancer: You can teach innate cells new tricks. Nat Rev Cancer.
16:7–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Basar R, Daher M and Rezvani K:
Next-generation cell therapies: The emerging role of CAR-NK cells.
Blood Adv. 4:5868–5876. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhou J, Bethune MT, Malkova N, Sutherland
AM, Comin-Anduix B, Su Y, Baltimore D, Ribas A and Heath JR: A
kinetic investigation of interacting, stimulated T cells identifies
conditions for rapid functional enhancement, minimal phenotype
differentiation, and improved adoptive cell transfer tumor
eradication. PLoS One. 13:e01916342018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hinrichs CS, Borman ZA, Cassard L,
Gattinoni L, Spolski R, Yu Z, Sanchez-Perez L, Muranski P, Kern SJ,
Logun C, et al: Adoptively transferred effector cells derived from
naive rather than central memory CD8+ T cells mediate superior
antitumor immunity. Proc Natl Acad Sci USA. 106:17469–17474. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
De Sanctis F, Trovato R and Ugel S:
Anti-telomerase T cells adoptive transfer. Aging (Albany NY).
9:2239–2240. 2017. View Article : Google Scholar
|
|
73
|
Kondo T, Imura Y, Chikuma S, Hibino S,
Omata-Mise S, Ando M, Akanuma T, Iizuka M, Sakai R, Morita R and
Yoshimura A: Generation and application of human induced-stem cell
memory T cells for adoptive immunotherapy. Cancer Sci.
109:2130–2140. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Foley KC, Nishimura MI and Moore TV:
Combination immunotherapies implementing adoptive T-cell transfer
for advanced-stage melanoma. Melanoma Res. 28:171–184. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Abi-Habib RJ, Singh R, Leppla SH, Greene
JJ, Ding Y, Berghuis B, Duesbery NS and Frankel AE: Systemic
anthrax lethal toxin therapy produces regressions of subcutaneous
human melanoma tumors in athymic nude mice. Clin Cancer Res.
12:7437–7443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kong LY, Abou-Ghazal MK, Wei J,
Chakraborty A, Sun W, Qiao W, Fuller GN, Fokt I, Grimm EA,
Schmittling RJ, et al: A novel inhibitor of signal transducers and
activators of transcription 3 activation is efficacious against
established central nervous system melanoma and inhibits regulatory
T cells. Clin Cancer Res. 14:5759–5768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Weiss T, Weller M, Guckenberger M, Sentman
CL and Roth P: NKG2D-Based CAR T cells and radiotherapy exert
synergistic efficacy in glioblastoma. Cancer Res. 78:1031–1043.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Al Hassan M, Fakhoury I, El Masri Z,
Ghazale N, Dennaoui R, El Atat O, Kanaan A and El-Sibai M:
Metformin treatment inhibits motility and invasion of glioblastoma
cancer cells. Anal Cell Pathol (Amst). 2018:59174702018.
|
|
79
|
Khoury O, Ghazale N, Stone E, El-Sibai M,
Frankel AE and Abi-Habib RJ: Human recombinant arginase I
(Co)-PEG5000 [HuArgI (Co)-PEG5000]-induced arginine depletion is
selectively cytotoxic to human glioblastoma cells. J Neurooncol.
122:75–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Dudley ME and Rosenberg SA:
Adoptive-cell-transfer therapy for the treatment of patients with
cancer. Nat Rev Cancer. 3:666–675. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Miao Y, Yang H, Levorse J, Yuan S, Polak
L, Sribour M, Singh B, Rosenblum MD and Fuchs E: Adaptive immune
resistance emerges from tumor-initiating stem cells. Cell.
177:1172–1186.e14. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Agudo J, Park ES, Rose SA, Alibo E,
Sweeney R, Dhainaut M, Kobayashi KS, Sachidanandam R, Baccarini A,
Merad M and Brown BD: Quiescent tissue stem cells evade immune
surveillance. Immunity. 48:271–285.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Brown JA, Yonekubo Y, Hanson N,
Sastre-Perona A, Basin A, Rytlewski JA, Dolgalev I, Meehan S,
Tsirigos A, Beronja S and Schober M: TGF-β-induced quiescence
mediates chemoresistance of tumor-propagating cells in squamous
cell carcinoma. Cell Stem Cell. 21:650–664.e8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Tey SK: Adoptive T-cell therapy: Adverse
events and safety switches. Clin Transl Immunology. 3:e172014.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yang JC: Toxicities associated with
adoptive T-cell transfer for cancer. Cancer. 21:506–509. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Miliotou AN and Papadopoulou LC: CAR
T-cell therapy: A new era in cancer immunotherapy. Curr Pharm
Biotechnol. 19:5–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Maeng HM and Berzofsky JA: Strategies for
developing and optimizing cancer vaccines. F1000Res 8: F1000
Faculty Rev-654. 2019. View Article : Google Scholar
|
|
88
|
Gatti-Mays ME, Redman JM, Collins JM and
Bilusic M: Cancer vaccines: Enhanced immunogenic modulation through
therapeutic combinations. Hum Vaccines Immunother. 13:2561–2574.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Manthorpe M, Cornefert-Jensen F, Hartikka
J, Felgner J, Rundell A, Margalith M and Dwarki V: Gene therapy by
intramuscular injection of plasmid DNA: Studies on firefly
luciferase gene expression in mice. Hum Gene Ther. 4:419–431. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Walters JN, Ferraro B, Duperret EK,
Kraynyak KA, Chu J, Saint-Fleur A, Yan J, Levitsky H, Khan AS,
Sardesai NY and Weiner DB: A Novel DNA vaccine platform enhances
neo-antigen-like T cell responses against WT1 to break tolerance
and induce anti-tumor immunity. Mol Ther. 25:976–988. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lopes A, Vanvarenberg K, Kos Š, Lucas S,
Colau D, Van den Eynde B, Préat V and Vandermeulen G: Combination
of immune checkpoint blockade with DNA cancer vaccine induces
potent antitumor immunity against P815 mastocytoma. Sci Rep.
8:157322018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Paston SJ, Brentville VA, Symonds P and
Durrant LG: Cancer vaccines, adjuvants, and delivery systems. Front
Immunol. 12:6279322021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Gamat-Huber M, Jeon D, Johnson LE, Moseman
JE, Muralidhar A, Potluri HK, Rastogi I, Wargowski E, Zahm CD and
McNeel DG: Treatment combinations with DNA vaccines for the
treatment of Metastatic Castration-Resistant Prostate Cancer
(mCRPC). Cancers (Basel). 12:28312020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Li L and Petrovsky N: Molecular mechanisms
for enhanced DNA vaccine immunogenicity. Expert Rev Vaccines.
15:313–329. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Jahanafrooz Z, Baradaran B, Mosafer J,
Hashemzaei M, Rezaei T, Mokhtarzadeh A and Hamblin MR: Comparison
of DNA and mRNA vaccines against cancer. Drug Discov Today.
25:552–560. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Bhuyan PK, Dallas M, Kraynyak K, Herring
T, Morrow M, Boyer J, Duff S, Kim J and Weiner DB: Durability of
response to VGX-3100 treatment of HPV16/18 positive cervical HSIL.
Hum Vaccin Immunother. 17:1288–1293. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lopes A, Vandermeulen G and Préat V:
Cancer DNA vaccines: Current preclinical and clinical developments
and future perspectives. J Exp Clin Cancer Res. 38:1462019.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Malonis RJ, Lai JR and Vergnolle O:
Peptide-based vaccines: Current progress and future challenges.
Chem Rev. 120:3210–3229. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Li W, Joshi MD, Singhania S, Ramsey KH and
Murthy AK: Peptide vaccine: Progress and challenges. Vaccines
(Basel). 2:515–536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Curry JM, Besmer DM, Erick TK, Steuerwald
N, Das Roy L, Grover P, Rao S, Nath S, Ferrier JW, Reid RW and
Mukherjee P: Indomethacin enhances anti-tumor efficacy of a MUC1
peptide vaccine against breast cancer in MUC1 transgenic mice. PLoS
One. 14:e02243092019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Pan J, Zhang Q, Palen K, Wang L, Qiao L,
Johnson B, Sei S, Shoemaker RH, Lubet RA, Wang Y and You M:
Potentiation of Kras peptide cancer vaccine by avasimibe, a
cholesterol modulator. EBioMedicine. 49:72–81. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhang R, Yuan F, Shu Y, Tian Y, Zhou B, Yi
L, Zhang X, Ding Z, Xu H and Yang L: Personalized neoantigen-pulsed
dendritic cell vaccines show superior immunogenicity to
neoantigen-adjuvant vaccines in mouse tumor models. Cancer Immunol
Immunother. 69:135–145. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Neek M, Kim TI and Wang SW: Protein-based
nanoparticles in cancer vaccine development. Nanomedicine.
15:164–174. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Rousseau RF, Hirschmann-Jax C, Takahashi S
and Brenner MK: Cancer vaccines. Hematol Oncol Clin North Am.
15:741–773. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Steinman RM and Cohn ZA: Identification of
a novel cell type in peripheral lymphoid organs of mice. I.
Morphology, quantitation, tissue distribution. J Exp Med.
137:1142–1162. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Santos PM and Butterfield LH: Dendritic
cell-based cancer vaccines. J Immunol. 200:443–449. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Alvarez-Dominguez C, Calderón-Gonzalez R,
Terán-Navarro H, Salcines-Cuevas D, Garcia-Castaño A, Freire J,
Gomez-Roman J and Rivera F: Dendritic cell therapy in melanoma. Ann
Transl Med. 5:3862017. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
de Gruijl TD, van den Eertwegh AJM, Pinedo
HM and Scheper RJ: Whole-cell cancer vaccination: From autologous
to allogeneic tumor- and dendritic cell-based vaccines. Cancer
Immunol Immunother. 57:1569–1577. 2008. View Article : Google Scholar
|
|
109
|
Fu C, Zhou L, Mi QS and Jiang A: DC-Based
vaccines for cancer immunotherapy. Vaccines (Basel). 8:7062020.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wculek SK, Amores-Iniesta J, Conde-Garrosa
R, Khouili SC, Melero I and Sancho D: Effective cancer
immunotherapy by natural mouse conventional type-1 dendritic cells
bearing dead tumor antigen. J Immunother Cancer. 7:1002019.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Lai X and Friedman A: Combination therapy
of cancer with cancer vaccine and immune checkpoint inhibitors: A
mathematical model. PLoS One. 12:e01784792017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Anassi E and Ndefo UA: Sipuleucel-T
(provenge) injection: The first immunotherapy agent (vaccine) for
hormone-refractory prostate cancer. P T. 36:197–202.
2011.PubMed/NCBI
|
|
113
|
Ayoub NM, Al-Shami KM and Yaghan RJ:
Immunotherapy for HER2-positive breast cancer: Recent advances and
combination therapeutic approaches. Breast Cancer (Dove Med Press).
11:53–69. 2019.PubMed/NCBI
|
|
114
|
Han Q, Wang Y, Pang M and Zhang J:
STAT3-blocked whole-cell hepatoma vaccine induces cellular and
humoral immune response against HCC. J Exp Clin Cancer Res.
36:1562017. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Sheikhi A, Jafarzadeh A, Kokhaei P and
Hojjat-Farsangi M: Whole tumor cell vaccine adjuvants: Comparing
IL-12 to IL-2 and IL-15. Iran J Immunol. 13:148–166.
2016.PubMed/NCBI
|
|
116
|
Xia L, Schrump DS and Gildersleeve JC:
Whole-Cell cancer vaccines induce large antibody responses to
carbohydrates and glycoproteins. Cell Chem Biol. 23:1515–1525.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Chen G, Gupta R, Petrik S, Laiko M,
Leatherman JM, Asquith JM, Daphtary MM, Garrett-Mayer E, Davidson
NE, Hirt K, et al: A feasibility study of cyclophosphamide,
trastuzumab, and an allogeneic GM-CSF-secreting breast tumor
vaccine for HER2+ Metastatic breast cancer. Cancer Immunol Res.
2:949–961. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Constantino J, Gomes C, Falcão A, Cruz MT
and Neves BM: Antitumor dendritic cell-based vaccines: Lessons from
20 years of clinical trials and future perspectives. Transl Res.
168:74–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Köhler G and Milstein C: Continuous
cultures of fused cells secreting antibody of predefined
specificity. Nature. 256:495–497. 1975. View Article : Google Scholar
|
|
120
|
Chung S, Lin YL, Reed C, Ng C, Cheng ZJ,
Malavasi F, Yang J, Quarmby V and Song A: Characterization of in
vitro antibody-dependenT cell-mediated cytotoxicity activity of
therapeutic antibodies-impact of effector cells. J Immunol Methods.
407:63–75. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wang W, Erbe AK, Hank JA, Morris ZS and
Sondel PM: NK Cell-Mediated Antibody-DependenT cellular
cytotoxicity in cancer immunotherapy. Front Immunol. 6:3682015.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Harris TJ and Drake CG: Primer on tumor
immunology and cancer immunotherapy. J Immunother Cancer. 1:122013.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Mayor M, Yang N, Sterman D, Jones DR and
Adusumilli PS: Immunotherapy for non-small cell lung cancer:
Current concepts and clinical trials. Eur J Cardiothorac Surg.
49:1324–1333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kimiz-Gebologlu I, Gulce-Iz S and
Biray-Avci C: Monoclonal antibodies in cancer immunotherapy. Mol
Biol Rep. 45:2935–2940. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Karlitepe A, Ozalp O and Avci CB: New
approaches for cancer immunotherapy. Tumour Biol. 36:4075–4078.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Sathyanarayanan V and Neelapu SS: Cancer
immunotherapy: Strategies for personalization and combinatorial
approaches. Mol Oncol. 9:2043–2053. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Posner J, Barrington P, Brier T and
Datta-Mannan A: Monoclonal antibodies: Past, present and future.
Handb Exp Pharmacol. 260:81–141. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zahavi D and Weiner L: Monoclonal
Antibodies in Cancer Therapy. Antibodies (Basel). 9:342020.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Loi S, Giobbie-Hurder A, Gombos A,
Bachelot T, Hui R, Curigliano G, Campone M, Biganzoli L, Bonnefoi
H, Jerusalem G, et al: Pembrolizumab plus trastuzumab in
trastuzumab-resistant, advanced, HER2-positive breast cancer
(PANACEA): A single-arm, multicentre, phase 1b-2 trial. Lancet
Oncol. 20:371–382. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
ClinicalTrials.gov, . A Dose Escalation
and Cohort Expansion Study of NKTR-214 in Combination With
Nivolumab and Other Anti-Cancer Therapies in Patients With Select
Advanced Solid Tumors (PIVOT-02). linicalTrials.gov Identifier:
NCT02983045. U.S.National Library of Medicine; Bethesda, MD: 2016,
https://clinicaltrials.gov/ct2/show/NCT02983045December
6–2016
|
|
131
|
ClinicalTrials.gov, . Bempegaldesleukin
and Pembrolizumab With or Without Chemotherapy in Locally Advanced
or Metastatic Solid Tumors (PROPEL). ClinicalTrials.gov Identifier:
NCT03138889. U.S.National Library of Medicine; Bethesda, MD: 2017,
https://clinicaltrials.gov/ct2/show/NCT03138889May
3–2017
|
|
132
|
Zimmer L, Goldinger SM, Hofmann L, Loquai
C, Ugurel S, Thomas I, Schmidgen MI, Gutzmer R, Utikal JS, Göppner
D, et al: Neurological, respiratory, musculoskeletal, cardiac and
ocular side-effects of anti-PD-1 therapy. Eur J Cancer. 60:210–225.
2016. View Article : Google Scholar : PubMed/NCBI
|