Introduction
The movement of ions across a biological membrane is
a crucial physiological process necessary for maintaining cellular
homeostasis. Such processes involve different types of membrane
proteins which can move ions either down their concentration
gradient or against their concentration gradient (1). The latter is considered an active
transport mechanism, achieved by dephosphorylating ATP for each
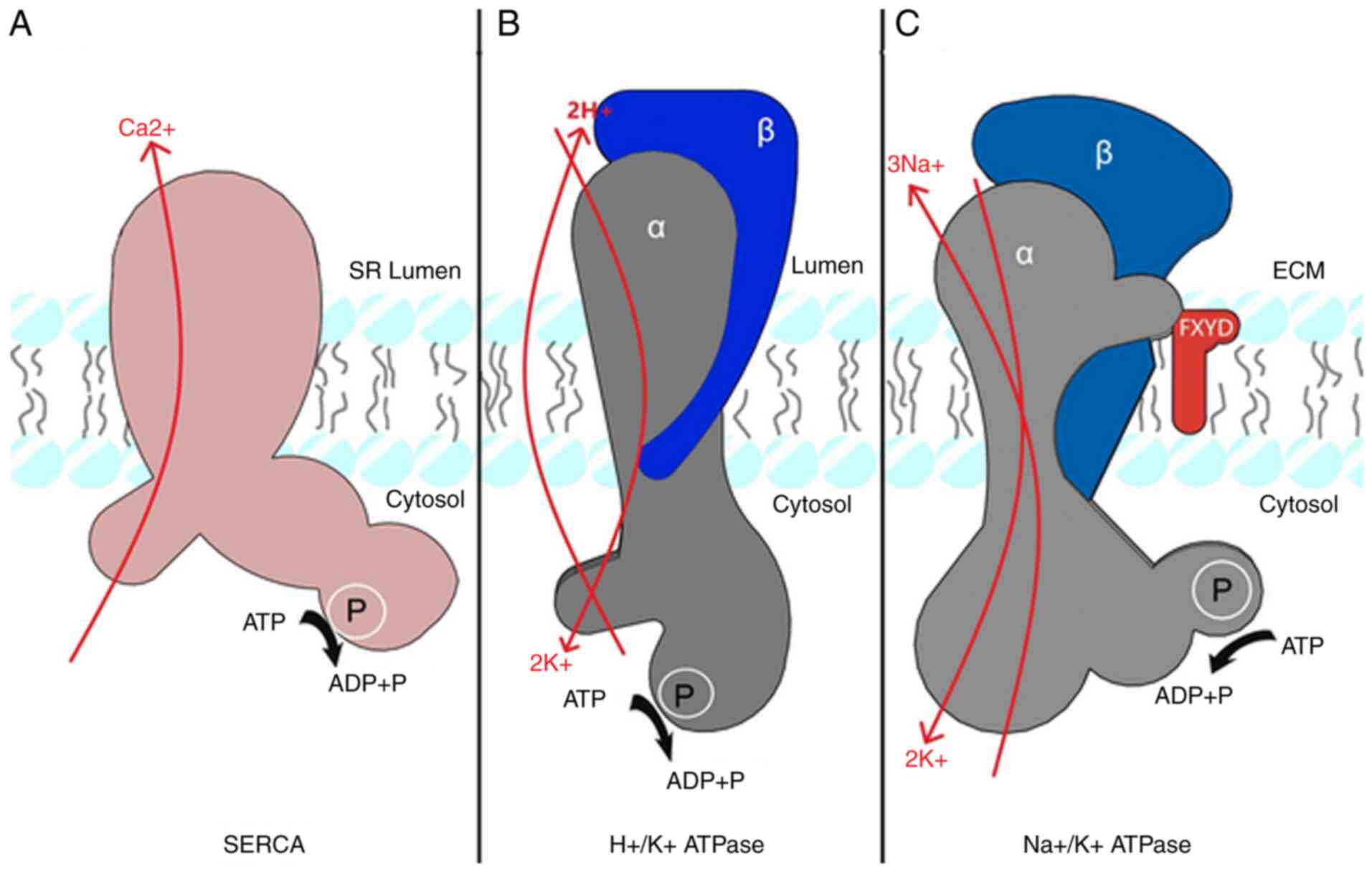
pump cycle, a mechanism by which a family of pumps known as P-type
ATPases are known to operate with (Fig.
1) (2,3). Cancer treatments currently include
different approaches such as surgery, chemotherapy, radiotherapy,
immunotherapy or other processes that are characterized to be
debilitating and traumatic for a patient with a direct effect on
his quality of life (4). Therefore,
specific characterized molecular targets could be used for the
designing or isolation of novel chemical agents that could
potentially be able to affect the tumor cell proliferation through
manipulation of those specific molecular targets. P-type ATPases
could be considered as such potential targets, as they are proteins
regulated by different functional means such as phosphorylation,
drug inhibition or activation and/or ion concentration sensitivity
(2,3,5).
The P-type ATPases are divided into five families
(P1 to P5) that can be further classified to one or more subgroups
A, B, C, D (5,6). P-class pumps are proteins, present in
all eukaryotic cells, as well as bacteria and are commonly
integrated within the lipid bilayer of the cellular membranes
(3,5). An essential segment of ATPases is the
α-subunit that is known to be involved in the enzymatic activity of
the pump but also the transport of ions across membranes. The pump
expresses variations in structural confirmation depending on the
factional stage of the protein found at any time during the
transportation or translocation process of the involved ions
(5,7). It is well characterized that
phosphorylation of the P site results in a conformational change
exposing the enzymatic sites (8).
Additional domains are also expressed in pumps such as the R
(regulatory) domain involved in auto inhibition of the pump.
Three members of this family have been extensively
studied in vitro, in vivo and in clinical studies, the
sodium (Na+)/potassium (K+)-ATPase (NKA),
sarcoendoplasmic reticulum calcium (Ca2+) ATPase
(SERCA), and the proton (H+)/K+ ATPase (HKA)
(3). NKA, HKA and SERCA are three
among many transmembrane proteins that play a significant role in
essential cellular functions, e.g., relaxation of muscle tissue or
restoration of stomach acidity (9,10). A
relationship on a molecular level, between pump behaviour and
cancer development/inhibition was also reported. Such an effect
could be justified by alterations (mutations) in genetic material,
abnormal regulation of pump protein translation or stimulation of
some cellular pathways and repression of others (11–16).
It is also known that cancer cells exhibit an acidic
environment, extracellular build-up of H+ and production of lactic
acid, creating numerous complications (10). These complications include changes in
both metastatic and proliferation behaviour but can additionally
result in resistance of cancer cells to chemotherapeutic drugs
(17). Given the ability of P-type
pumps to directly affect ion homeostasis, they are currently being
explored as antineoplastic or antitumor targets/biomarkers as able
to reverse the changes found in cancer cell environments (10).
This review aims to: (A) provide an understanding of
the significance of P-Type ATPases in essential functions of a
cell, (B) describe the outcomes when they are inhibited during
pathogenesis and specifically cancer (Table I), (C) determine the use of those
protein-pumps as potential biological cancer biomarkers and (D)
probably propose future advancements.
 | Table I.Listing of clinical effects of
P-class pumps in cancer, containing drug name and target, as well
as the upregulated/downregulated pump. |
Table I.
Listing of clinical effects of
P-class pumps in cancer, containing drug name and target, as well
as the upregulated/downregulated pump.
| First author,
year | Drug | Type of cancer | Pump outcome
PETH | Cellular
alterations PETH | (Refs.) |
|---|
| Yang et al,
2014 | Oleandrin | Human colorectal
cancer (in vitro) | ↓NKA (α3) | ↑K+
intracellularly ↓Ca2+ intracellularly | (52) |
| McConkey et
al, 2000 | Oleandrin | Prostate cancer
(in vitro, in vivo) | ↓NKA | ↑K+
intracellularly ↓Ca2+ intracellularly | (133) |
| Newman et
al, 2007 | Oleandrin | Pancreatic cancer
(in vitro) | ↓NKA | ↓phospho Akt
↑phospho ERK ↓G2/M cell | (11) |
| Li et al,
2011 | pNaktide
(peptide) | Prostate cancer
(in vivo) | ↑NKA (α1) | ↑K+
intracellularly ↓Ca2+ intracellularly ↓Src | (22) |
| Garcia et
al, 2015 | Perillyl
alcohol | Glioblastoma (in
vitro) | ↓NKA (α1) | ↑K+
intracellularly ↓Ca2+ intracellularly ↓JNK | (53) |
| Alevizopoulos et
al, 2016 | Istaroxime | Prostate cancer
(in vitro, in vivo) | ↓NKA | ↑K+
intracellularly ↓Ca2+ intracellularly Caspase-3 ↓c-Myc
expression | (128) |
| Li et al,
2011 | Bufalin (CG) | Hepatocallular
carcinoma (in vitro) | ↓NKA (α3) | ↑Akt ↓FOXO3a ↑ERK
↑K+ intracellularly ↓Ca2+
intracellularly | (134) |
| Numazawa et
al, 1994 | Bufalin (CG) | Leukaemia (in
vitro) | ↓NKA (α3) | ↑K+
intracellularly ↓Ca2+ intracellularly | (135) |
| Sun et al,
2019 | Bufalin (CG) | Colorectal cancer
(in vitro) | ↓NKA (α3) | ↑K+
intracellularly ↓Ca2+ intracellularly ↓NF-κB ↓HIF-1α
↓Cell cycle G2/M | (136) |
| Yu et al,
2008 | Cinobufagin
(CG) | Prostate cancer
(in vitro) | ↓NKA | ↑K+
intracellularly ↓Ca2+ intracellularly ↑Caspase-3
Cytochrome c release | (137) |
| Ihenetu et
al, 2007 | Digoxin (CG) | Leukemia (in
vitro) | ↓NKA | ↑K+
intracellularly ↓Ca2+ intracellularly ↑FasL | (12) |
| Prassas et
al, 2011 | Digitoxin (CG) | Pancreatic cancer
(in vitro) | ↓NKA | ↑Src ↑MAPK
signalling ↑K+ intracellularly ↓Ca2+
intracellularly | (55) |
| Hsu et al,
2016 | Digoxin (CG) | Ovarian clear cell
carcinoma (in vitro, in vivo) | ↓NKA | ↓FXYD2
↑K+ intracellularly ↓Ca2+
intracellularly | (62) |
| Kang et al,
2016 | Latonoside C
(CG) | Colorectal cancer
(in vitro) | ↓NKA | ↑K+
intracellularly ↓Ca2+ intracellularly Repair
mitochondrial membrane potential | (138) |
| Liu et al,
2013 | Ouabain (CG) | Lung cancer (in
vitro) | ↓NKA | ↑K+
intracellularly ↓Ca2+ intracellularly | (56) |
| Huang et al,
2004 | Ouabain (CG) | Prostate cancer
(in vitro) | ↓NKA (α) | ↑K+
intracellularly ↓Ca2+ intracellularly Restore
mitochondrial membrane potential | (139) |
| Ono et al,
2016 | Ouabain (CG) | Brain glioblastoma
(in vivo) | ↓NKA (α) | ↑K+
intracellularly ↓Ca2+ intracellularly | (140) |
| Li et al,
2019 | Digoxin (CG) | Glioblastoma (in
vitro, in vivo) | ↓NKA (β2) | ↑K+
intracellularly ↓Ca2+ intracellularly Cell cycle arrest
at G2/M phase | (59) |
| Rocha et al,
2016 | 21-benzylidene
digoxin (CG) | Cervical cancer and
colon carcinoma (in vitro) | ↓NKA (α1) | DNA damage ↑p21
↓Cyclin A, Bcl-2 and Bcl-XL | (141) |
| Denmeade and
Isaacs, 2015 | Thapsigargin | Prostate cancer
(in vitro, in vivo) | ↓SERCA | ↓Ca2+
intracellularly Cytochrome c release | (142) |
| Mahalingam et
al, 2016 | Mipsagargin | Hepatocellular
carcinoma (phase i) | ↓SERCA | ↓Ca2+
intracellularly | (81) |
| Fan et al,
2014 | Curcumin Analogue
(F36) | Colorectal
carcinoma (in vitro) | ↓SERCA | ↓Ca2+
intracellularly | (143) |
| Denmeade et
al, 2003 | PSA-Thapsigargin
prodrug | Metastatic prostate
cancer (in vitro, in vivo) | ↓SERCA | ↓Ca2+
intracellularly | (83) |
| Jia et al,
2016 | Artemisin | Gallbladder cancer
(in vitro, in vivo) | ↓SERCA | ↓ERK ↓CDK ↓Cyclin
D1 ↓Ca2+ intracellularly | (144) |
| Riganti et
al, 2009 | Artemisin | Colon cancer (in
vitro) | ↓SERCA | ↓Ca2+
intracellularly ↓doxorubicin intracellularly ↑P-glycoprotein | (86) |
| Riganti et
al, 2009 | Parthenolide | Colon cancer (in
vitro) | ↓SERCA | ↓Ca2+
intracellularly ↓doxorubicin intracellularly ↑P-glycoprotein | (86) |
| De Ford et
al, 2016 | Clerodanee ditepene
casearin J. | T-cell acute
lymphoblastic leukemia (in vitro) | ↓SERCA | ↓Ca2+
intracellularly ↓Oxidative stress ↑Notch1 | (87) |
|
Madreiter-Sokolowski et al,
2016 | Resveratrol,
Piceatannol and oligomycin A | Cervical
adenocarcinoma (in vitro) | ↓SERCA | ↓Ca2+
intracellularly Reverse Loss of mitochondrial membrane
potential | (131) |
| Wong et al,
2013 | Saikosaponin-d | Cervical cancer and
breast cancer | ↓SERCA | ↓Ca2+
intracellularly ↑CaMKKβ-AMP, (AMPK), (mTOR) signaling cascade
↑Unfolded protein responses | (13) |
| Jakab et al,
2014 | Sch28080 | Leukemia (in
vitro) | ↓HKA (α1) | ↑H+
intracellularly | (100) |
| Yeo, 2004 | Pantoprazole | Gastric cancer
(in vitro, in vivo) | ↓HKA (α) | ↑H+
intracellularly | (108) |
| Zhang et al,
2013 | Trametenolic acid
B | Gastric cancer
(in vitro, in vivo) | ↓HKA | ↑H+
intracellularly | (107) |
| Gu et al,
2014 | Rabeprazole | Gastric cancer | ↓HKA | ↑H+
intracellularly | (10) |
| Zhang et al,
2013 | Lansoprazole | Breast cancer | ↓HKA | ↑H+
intracellularly | (107) |
| Lindner et
al, 2014 | Esomeprazole | Esophageal
cancer | ↓HKA | ↑H+
intracellularly | (109) |
Methodology
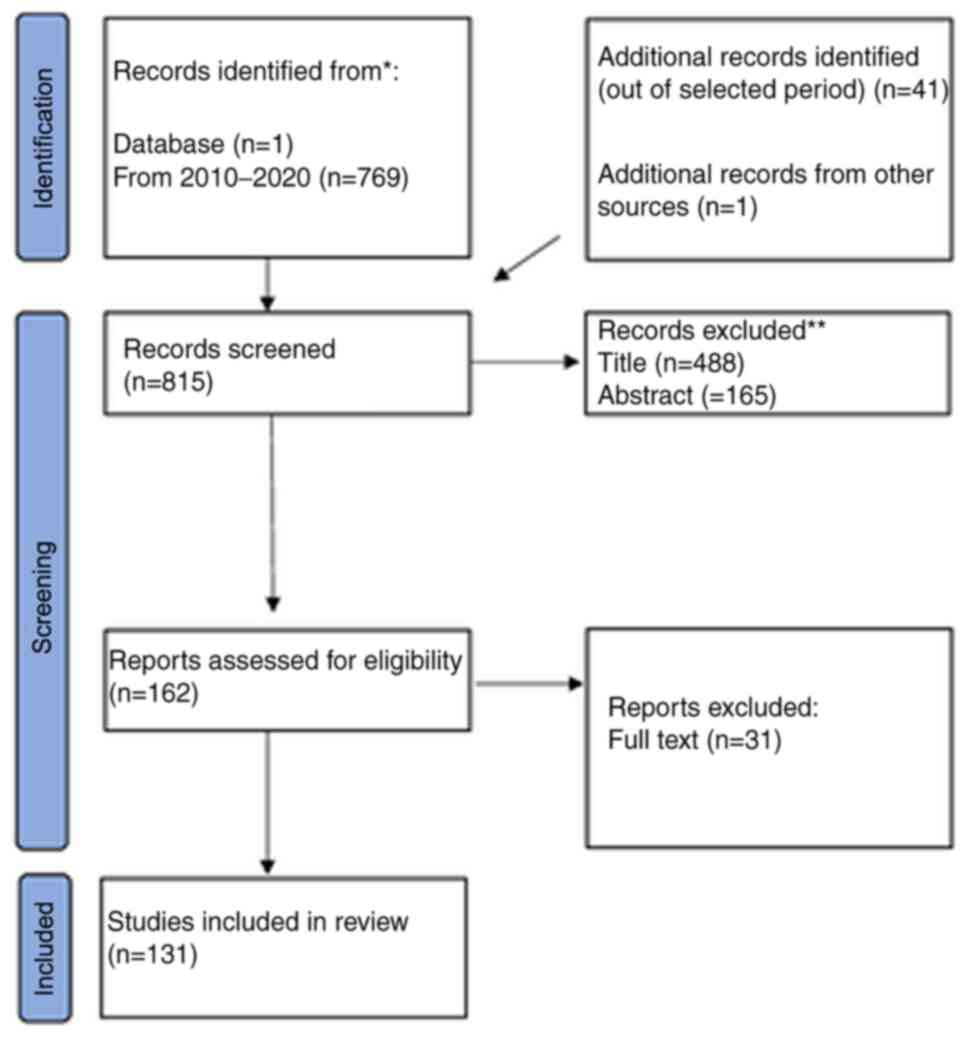
Evidence regarding P-class pumps was reviewed in
in vitro and in vivo studies, as well as in clinical
trials, in order to describe their quaternary structure, function,
and if they communicate any anticancer effects. A literature search
was performed in PubMed to identify relevant articles published
from January 2010 until December 2020 and in ClinicalTrials.gov database for any relevant trials.
The search was based on the following words and terms:
‘Na+/K+ ATPase’, ‘SERCA’,
‘H+/K+ ATPase’, ‘P-type pumps’ in association
with the term ‘cancer’ (Fig. 2). The
relevant papers found were then searched for discussing the
structure, function and the potential involvement of the searched
functional entities in cancer treatment. The references of the
relevant articles were also accessed and reviewed. Additionally,
expression data was collected from the UALCAN http://ualcan.path.uab.edu/ resource to evaluate the
degree of gene expression of the pumps in 24 separate cell lines,
comparing expression levels in cancer cells to healthy cells
(18).
NKA
NKA structure and function
NKA is crucial for cell function, serving as a
signal transducer involved in cell adhesion (14). Ions move against the concentration
gradient in order to form and protect the action potential which is
vital for many physiological processes in a number of organs. NKA
channel is present in the waste management in kidneys, sperm
mobility and neuronal action potential production. The regulation
of the neuron action potentials is also due to NKA maintaining the
concentrations of Na+ and K+ at constant
disequilibrium. When a neuron generates an impulse, a sudden shift
is created from resting to active state due to a rapid movement of
ions across the membrane.
For every NKA cycle an ATP is hydrolysed followed by
three sodium ions binding to the pump to be exported from the cell
in exchange of two potassium ions binding to be imported into the
cytosol, resulting in a net positive charge extracellularly
(19). The NKA transmembrane protein
is composed of the α, β and FXYD subunits, which are subdivided
into various isoforms (Fig. 1).
Action potentials are affected by NKA exertions, through
polarization/depolarization actions known to generate electrical
signals (8,19,20).
These subunits express in an heterologous manner and the isoform
combinations vary in different tissues (21). The α subunit, as previously
described, is the catalytic subunit (7). This structure has a mass of 110-kDa and
it is transcribed by 1,000 amino acids and it spans the membrane
ten times exhibiting functional domains intracellularly and
extracellularly (e.g., A,N,P,R) (8).
This subunit is comprised of four isoforms, α1-α4. The α1 subunit
gene ATP1A, of NKA is expressed in most tissues as it plays a key
role in signal transduction, cell/cell adhesion and tight junctions
(Fig. 3A and B) (22). Its expression is prominent in the
ependymal cells of the spinal cord and as well as in neurons of the
ventral horn (23). The α2 (ATP1A2)
subunit is highly expressed in cardiac T-tubules, skeletal and
smooth muscle, lung, astrocytes and adipose tissue (24). The α3 (ATP1A3) subunit is mostly
expressed in ventricular myocardial cells, axons and dendrites,
while the α4 (ATP1A4) in the testes and epididymis (25–29). The
β subunit has four separate isoforms (ATP1B1-ATP1B4), all highly
glycosylated, situated mostly extracellularly (33–36 kDa) (Fig. 3) (30). The β subunits attract K+
and incorporate the binding site for cardiac glycosides (31). Expressed in skeletal muscle, heart
muscle and human brain is the β1 subunit, which is also a
constituent of cell-cell adhesion. The β2 (32 kDa) subunit is more
commonly expressed in glial cells but expressed in myocardial cells
and erythrocytes, and is additionally an essential enzyme in cell
adhesion in the central nervous system (32,33). The
third enzyme is the β3 subunit expressed predominately in
oligodendrocytes of brain white matter and the optic nerve; it is
also detected in testes (30,34).
Finally, β4 varies from the other β isoforms, as it is situated
mostly intracellularly (31,35,36) and
is encountered in skeletal and cardiac muscle (37).
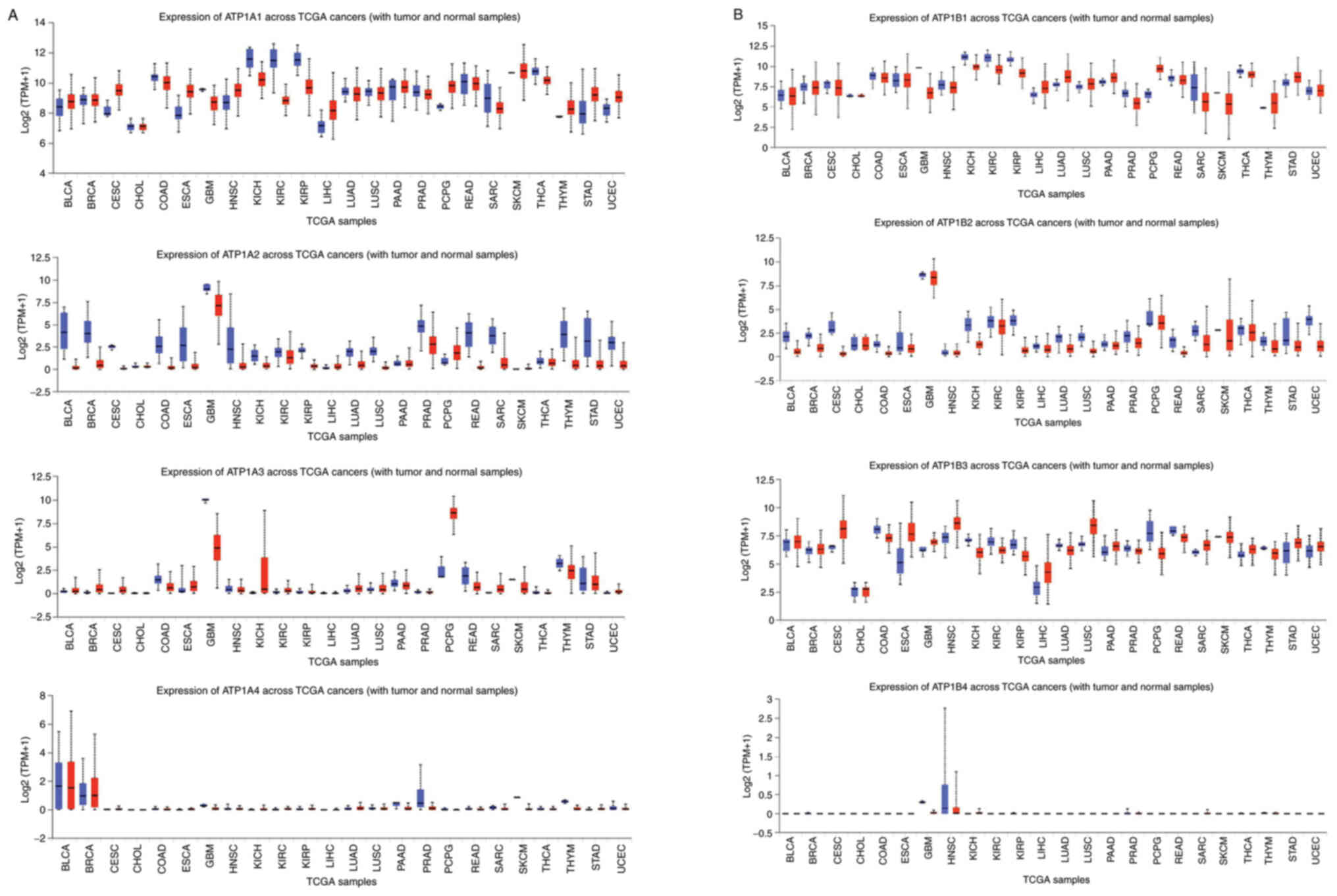 | Figure 3.Expression levels of all eight NKA
isoforms in 24 types of cancer (red) and their respective healthy
tissues (blue). (A) ATP1A1, ATP1A2, ATP1A3 and ATP1A4. (B) ATP1B1,
ATP1B2, ATP1B3 and ATP1B4. Data were collected and permitted for
publication from the UALCAN resource. TPM, transcripts per million;
TCGA, The Cancer Genome Atlas; NKA, Na+/K+
ATPase; BLCA, bladder urothelial carcinoma; BRCA, breast invasive
carcinoma; CESC, cervical squamous cell carcinoma; CHOL,
cholangiocarcinoma; COAD, colon adenocarcinoma; ESCA, esophageal
carcinoma; GBM, glioblastoma multiforme; HNSC, head and neck
squamous carcinoma; KICH, kidney chromophobe; KIRC, kidney renal
clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma;
LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma;
LUSC, lung squamous cell carcinoma; PAAD, pancreatic
adenocarcinoma; PRAD, prostate adenocarcinoma; PCPG,
pheochromocytoma and paragangliosarcoma; READ, rectal
adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; THCA,
thyroid carcinoma; THYM, thymoma; STAD, stomach adenocarcinoma;
UCEC, uterine corpus endometrial carcinoma. |
A third unit of NKA, FXYD, is a significant subunit,
sometimes referred to as an accessory unit due to its scarce
presence (16,20). This protein is transcribed by the
FXYD genes and seven isoforms have been described thus far,
FXYD1-FXYD7 (8). FXYD1
(phospholemman) is absent in cardiac tissue resulting in reduced
NKA activity, indicating that FXYD1 regulates NKA in this tissue
(38). FXYD2 (γ subunit) affects
Na+ reabsorption in the thick ascending limb in the
medulla of the kidney and has a low affinity for Na+
(39). FXYD3 (mammary tumor marker)
is expressed in breast, lung, small intestine but also stomach,
uterus and thymus (40).
Additionally, FXYD3 decreases K+ affinity, spans the
membrane twice instead of once, affects β subunit glycosylation and
its specificity is low as it is also associated with the β subunit
of HKA (41,42). The final locus of Na+
reabsorption in the kidneys is from the collecting duct where FXYD4
(channel-inducing factor) is expressed as part of NKA, instead of
FXYD2 (43). FXYD4 has a high
affinity to Na+ therefore ensuring that Na+
is reabsorbed into the bloodstream (42). Dysadherin (FXYD5) can double the
volume of NKA activity (44).
Phosphohippolin, also known as FXYD6, is transcribed in the
cerebellum and the posterior lobe of the brain (lobules VI–IX) and
is involved in brain neuronal excitability (45). It is additionally expressed in
kidney, liver, lung and testis (45). Finally, FXYD7 affects NKA
excitability in glial cells and neurons of the brain, by regulating
K+ movement (41,46). The combination in which the different
isoforms of FXYD co-exist with the various isoforms of α and β,
depends on the tissue in question (47).
NKA significance in cancer
The role of NKA in cancer has been supported by a
growing number of studies, including in vivo, in vitro and
genetic studies that have evaluated either the differential
expression of NKA subunits, or the effects of different NKA
modulators in malignancy.
Transcription and/or translation of the ion pump
protein may either be increased or decreased as a response to the
environmental settings created by cancerous cells. The gene
expression of each NKA subunit in twenty-four different cancer
types was compared to their expression in healthy tissue (Fig. 3A and B) (18). This data is constructive on two
fronts: i) It denotes novel biomarkers; ii) It recognises potential
treatment targets based on cancer geography. Expression of ATP1A1
in cancerous esophageal cells is significantly greater than in
healthy esophageal cells and the same was found true for the
expression of this subunit in cervical squamous carcinoma, thymoma,
paraganlgioma etc. When evaluating the expression of ATP1A1 in
glioblastoma multiforme was found decreased compared to healthy
cells. Ideally, these characteristic patterns in protein subunit
expression can be manipulated for diagnostic purposes. For example,
FXYD3 demonstrates overexpression in gastric adenocarcinomas,
prostate carcinoma, pancreatic cancer and in breast tumors as
opposed to their healthy counterparts (48). Inhibiting FXYD3 expression can result
in down-regulation of cancer cell proliferation, without affecting
the invasion rate or cellular apoptosis (49).
As previously reported mutations on specific genes
can affect the expression and hence transcription of the NKA
subunit, such as ATP1B2 deletions in breast cancer or the deletion
of ATPA1A and ATP1B1 in epithelial-mesenchymal transition which can
result in cancer and fibrosis (14,50).
This was also true for the α3β1 subunit (isozyme) that was found to
be abundant in colon cancer cells that metastasised to the liver,
but absent in healthy liver tissue.
Expression is not the only factor affected in cancer
cells. Intracellular localization of subunits has been recorded as
well, where ATP1A1 is expressed near the peri-nuclear site in
cancer environments, but in healthy tissue it it positioned on the
basolateral side of cells instead (51). In normal cells, such as in the lung
and colon, ATP1A3 is present close to the cytoplasmic membrane, in
contrast to its peri-nuclear location in cancer cells of both
tissue types. These modifications in intracellular localisation of
the α1 and α3 can possibly serve as biomarkers and/or as possible
treatment targets (52). As an
example, the Oleandrin has been shown to express anti-proliferative
activity promoting cell death in undifferentiated CaCo-2 cells
(52).
As we have previously discussed the α1 subunit is
involved in multiple cell functions including the regulation effect
on Src (a protein kinase that promotes cell proliferation and
invasion) (22). These findings
indicated that α1 expression decreases in prostate cancer and human
metastatic prostate cell lines (Fig. 3A
and B PRAD) while Src activity increased, favouring cancer
progression. An in vivo study illustrated that
administration of pNaktide peptide could restore the α1 expression
with a consequent inhibition of Src action, that further decreased
proliferation, migration and/or invasion (Table I) (22). Opposing evidence by in vivo
experiments on glioblastoma cells displayed elevated expression of
α1 which suppressed Src activity. However, when Src fails to
phosphorylate JNK (protein Kinase) the glioblastoma cells
proliferate freely, further supporting the influence of Src on
cancer cell death. Administering perillyl alcohol, an NKA
inhibitor, α1 is downregulated resulting in JNK phosphorylation and
initiating glioblastoma cell death (53). Src was further reported able to
affect MAPK and p38 proteins (53).
Cardiac glycosides (CGs) are natural compounds
commonly used in treating heart failure and cardiac arrhythmias;
display anticancer effects by decreasing K+, thus
increasing Na+ and Ca2+ intracellularly; and
modify the behaviour of multiple cellular pathways, such as IL-8
and TNF-α/NF-Κb that are associated with angiogenesis, metastasis
or apoptosis (54,55). Ouabain is a CG known to inhibit NKA.
Treatment of A549 cells, a lung cancer cell line, with ouabain
demonstrated low expression of both N-cadherin and vimentin, both
known as epithelial-mesenchymal transition biomarkers, hence
indicating decrease of A549 cell migration (56). It has also been shown that ouabain
inhibits migration via an epidermal growth factor pathway that
causes cytotoxicity in PC-3 (prostate cancer) cells by creating
reactive oxygen species (ROS) or upregulating the prostate
apoptosis response (56). CGs and
their relationship with cancer are listed in Fig. 3A and B. It is also worth noting that
the α1 subunit shows low affinity for ouabain which means that the
specific drug is more effective when certain alternative are
present (56,57).
In medulloblastoma, kidney carcinoma cells,
colorectal cancer and liver metastases, tumorigenesis is favoured
by inhibiting β1. The β1 subunit expression is additionally
reported to inhibit tumorigenicity in MSV-MDCK cells and able to
regulate the ERK pathway (regulating signalling molecules)
activation (8,57). NKA β1 also affects cellular growth
factors such as TGF-β2, as ion pump inhibition
upregulates TGF-β2 (a growth factor cytokine), activity.
Therefore the relationship between pump and disease are eminent,
presenting it as a potential drug treatment target (58).
The expression of β2 subunits is greatly upregulated
in cancer (59,60). in vitro down-regulation of β2
expression results in cell cycle arrest in glioblastoma multiforme
(GBM) at the G2/M phase with increased Ca2+
intracellularly, through the sodium/calcium exchanger, resulting in
cell apoptosis (59).
The adhesion molecule on glia (β2/AMOG) is a
glycoprotein that accompanies the β2 subunit. As β2/AMOG expression
decreases in cancer cells the grade of malignancy increases,
adhesion is lost and migration is increased (61). Alternatively, the degree of protein
pump expression may be useful for prognosis, as β2 appears to be
greatly expressed in primary GBM compared to secondary GBM
(60). The opposite is revealed for
β3, as it is more upregulated in secondary GBM than primary. This
pattern may benefit as a prognostic marker (61).
The NKA protein and specifically the FXYD2, is
significantly upregulated in ovarian clear cell carcinoma (OCCC)
but is scarce to non-existent in surrounding healthy ovarian cells
(62). This characteristic increase
may be manipulated as a biomarker for OCCC prognostic purposes.
Tumor growth is suppressed due to FXYD2 inhibition by digoxin and
digitoxin as autophagy-mediated cell death is stimulated (62). Previous studies report a
characteristic increase in FXYD3 expression in endometrial cancer,
breast tumor, pancreatic and prostate cancer while in lung cancer
the expression of the protein was significantly downregulated;
possibly suggesting FXYD3 as a biomarker for the aforementioned
cancer types (63,64). The same can be supported for FXYD6 in
cholangiocarcinoma, while in hepatocellular carcinoma
downregulating FXYD6 inhibited NKA activity resulting in a decrease
in tumor volume, revealing the protein as a potential treatment
target (65,66). In a case of ovarian cancer, FXYD5
involvement in cell adhesion is believed to drive cancer cell
migration but may also be a useful mortality predictor (67).
SERCA pump
SERCA pump structure and function
The SERCA is a 110 kDa power-driven P-type pump that
transports two Ca2+ ions from the cytoplasm to the
sarco/endoplasmic reticulum lumen per ATP dephosphorylated,
balancing free Ca2+ in both eukaryotes and prokaryotes
(68). SERCA is also composed of a
ten transmembrane α subunit segments (Fig. 1) (69). Three SERCA homologous genes
transcribe to three subunits; SERCA1 (gene: ATP2A1), SERCA2
(ATP2A2), and SERCA3(ATP2A3) (70,71). The
isoform which will be expressed is determined by alternative
splicing of genes, is cell-type dependent and they respond
differently to drugs even though they are approximately 75%
homologous (70). SERCA1 consists of
two transcripts; 1a is mainly expressed in adult skeletal muscle
whereas 1b is present in fetal skeletal muscle (68,72,73).
SERCA2 also consists of two transcripts. The 2a transcript is found
in adult skeletal and cardiac muscle. SERCA2b is abundant in all
adult and fetal cardiac and skeletal muscles. SERCA3 is divided
into three isoforms, SERCA3a-3c, all of which are expressed in
non-muscle cells (68,70,73).
Signal transduction via intracellular free
Ca2+-concentration [(Ca2+)i]
elevation is one of the fundamental events observed in cell
regulation. Systems including muscle cells, neurons, immune system
cells as well as plant cells and protozoa utilize
Ca2+-signalling.
Free calcium is an important second messenger for
the cell. The ER acts as a reservoir and releases the ion into the
cytosol upon signaling where it is involved in proliferation, cell
death and gene regulation. In neurons, it is the most common
intracellular messenger, it is activated by neurotransmitters
dopamine and etc. whereas in T cells it is stimulated by the TCR
pathway and promotes T cell immunity.
While Ca2+ concentration is known to be
greater extracellularly (cytosol) than intracellularly (lumen), a
disordered homeostasis is indicative of pathological conditions,
including cancer (68). This action
is crucial for a number of cellular processes such as gene
transcription, cell proliferation and cell death (68,74,75).
Given that SERCA is found in fundamental structures of the cell and
is involved in the regulation of cellular metabolism, any pump
inhibitor recognised to have anti-tumor effects must be prescribed
as a pro-drug to ensure only cancerous cells are affected and
leaving healthy cells unharmed (72).
SERCA significance in cancer
The consequence of inhibiting SERCA results in
depletion of the Ca2+ concentration in mitochondria that
can lead to the collapse of the electrochemical proton gradient,
thus triggering numerous signalling pathways resulting in cell
death (76). Use of SERCA pump
inhibitors in cancer has been verified through in vitro and
in vivo studies, with the most commonly used inhibitors
being thapsigargin, artemisinin parthenolide, resveratrol and
saikosaponin (13,77). In early stages of cancer, a
noticeable decrease in SERCA3 is presented by breast cancer, colon
cancer and lung adenocarcinoma rendering inhibitors impractical
(71,78).
Thapsigargin is a sesquiterpene lactone isolated
from Thapsia garganica, which naturally represses ATPase
activity, induces endoplasmic reticulum (ER) stress by preventing
Ca2+ from leaving the cytosol to enter the ER but also
exhibit anti-autophagic effects hence long-lived cytosolic proteins
are not broken down (68). While
SERCA transmembrane helices are in the E2 conformational state,
Thapsigargin can bind to the pump and prevents its return to the
initial E1 state capable of binding Ca2+(more
prominently binds to SERCA1b) (68).
Therefore, Thapsigargin elevates intracellular free calcium
[(Ca2+)i], through inhibition of the endoplasmic
reticulum Ca-ATPase which repossesses calcium from the cytosol
(79,80). It has been reported that increase of
[Ca2+]i in keratinocytes is associated with
differentiation and can be inhibited by 3 nM of thapsigargin.
Thapsigargin has been reported to promote apoptosis
in human hepatoma cells via initiating apoptosis of cells, DNA
fragmentation and chromatin condensation (80,81).
Similarly, recent clinical studies with mipsagargin, a prodrug of
thapsigargin, have supported its use against multiple solid tumors
with strong evidence for treatment of hepatocellular carcinoma
(Table I) (82). A PSA-Thapsigargin prodrug was
administered to nude mice xenograft models diagnosed with prostate
cancer and significantly reduced tumor size (81,82).
However, a prodrug was used to ensure the effects of thapsigargin
on SERCA would not be activated unless bound to prostate tissue,
despite its location (83).
Thapsigargin also inflicts tumor cell death more sufficiently when
administered with a metabolic inhibitor of glucose, in in
vivo parental cancer cells (84,85).
SERCA pump disregulation renders chemotherapeutic
drugs useless due to the environmental conditions created inside a
cell. Artemisinin is a plant-derived drug proven effective in
triggering a pro-apoptotic pathway in colorectal adenocarcinoma
cells and increases Ca2+ levels intracellularly
(86). Doxorubicin is a chemotherapy
drug proven effective against HT29, Hep G2, MCF-7 and LoVo cells
while expressing cytotoxic effects. Artemisin and parthenolide
activate CaMKII which sets off a cascade of events resulting in
increased resistance of cancer cells against Doxorubicin, therefore
suggesting they should not be administered synergistically.
Alternatively, a combination of parthenolide with a product known
as clerodane diterpene casearin J, was effective in initiating
T-cell acute lymphoblastic leukemia cell apoptosis by inhibiting
simultaneously NF-κB and SERCA respectively (87). Parthenolides ability to inhibit
SERCA, hence restoring Ca2+ intracellularly, also
assists the successful functioning of 5-fluorouracil (chemotherapy
drug) (86,88).
ATP2A2 and ATP2A3 expression is suppressed in human
breast cancer and human dermal fibroblast cell lines. Resveratrol,
a polyphenol molecule, tested in both these cancer cell lines, is
capable of reestablishing ATP2A3 expression therefore decreasing
cytosol Ca2+ and consequently decreasing proliferation
and triggering apoptosis. Drugs that bind to pumps may be subunit
specific, like resveratrol which restores ATP2A3 and not ATP2A2
(89).
As previously mentioned inhibiting pumps can lead to
a cascade of cellular alterations. This was also observed when
saikosaponin-d, a SERCA inhibitor that promoted autophagy via
Ca2+ build-up intracellularly induces ER stress and
results in CaMKKβ-AMPK-mTORs signalling activation in cervical and
breast cancer cells (13).
Alterations occur in gene expression of all three
SERCA subunits when comparing cancer and healthy samples (Fig. 4). Analysing these gene expressions
may indicate specific subunits to be used as biomarkers for tissue
specific diagnosis of cancer as the previously discussing pumps.
For instance, in liver cancer, ATP2A2 is highly expressed compared
to healthy cells.
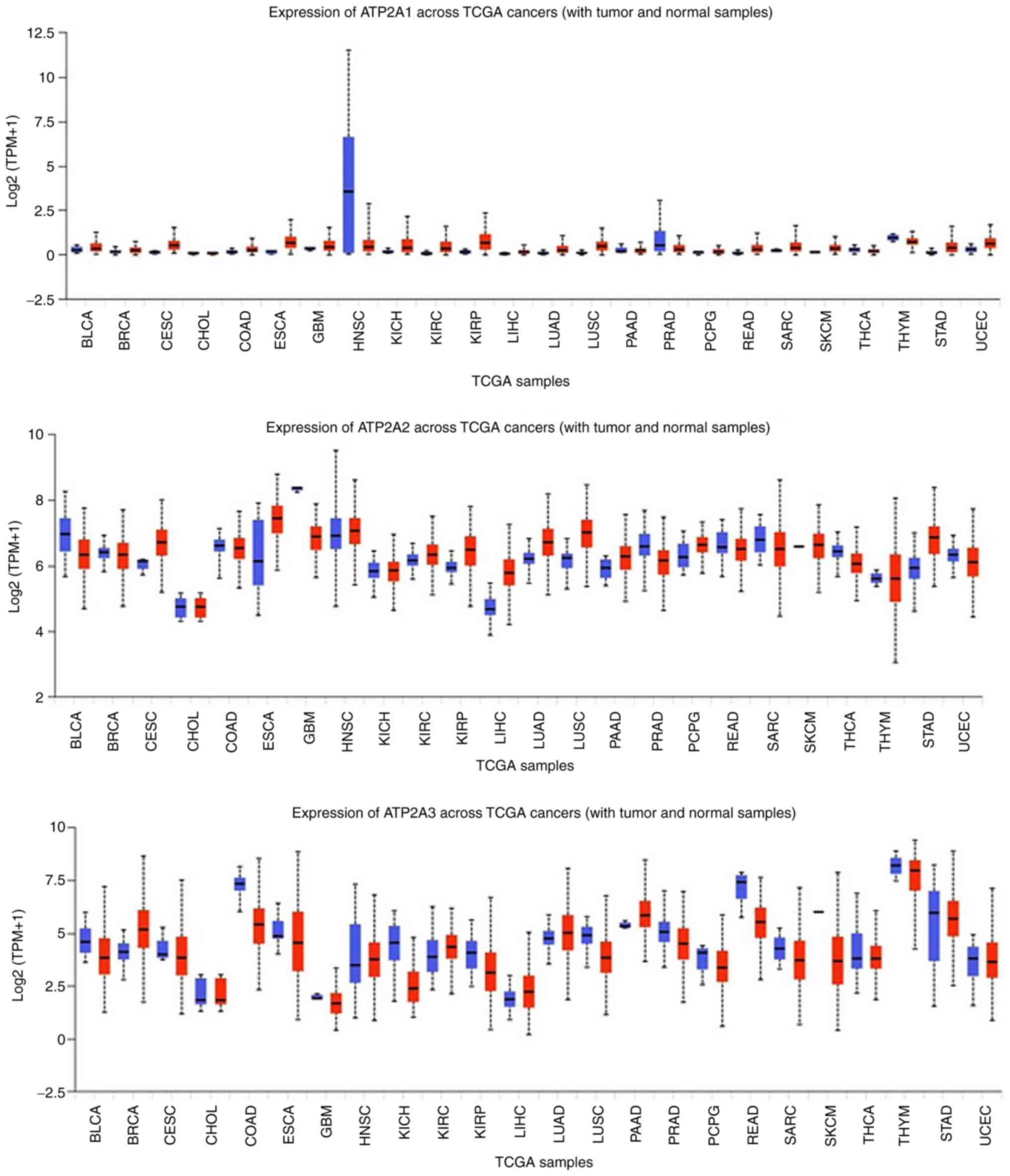 | Figure 4.Expression levels of SERCA in 24
cancer tissues compared with in healthy tissues. Data were
collected and permitted for publication from the UALCAN resource.
TPM, transcripts per million; TCGA, The Cancer Genome Atlas; SERCA,
sarcoendoplasmic reticulum calcium ATPase; BLCA, bladder urothelial
carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous
cell carcinoma; CHOL, cholangiocarcinoma; COAD, colon
adenocarcinoma; ESCA, esophageal carcinoma; GBM, glioblastoma
multiforme; HNSC, head and neck squamous carcinoma; KICH, kidney
chromophobe; KIRC, kidney renal clear cell carcinoma; KIRP, kidney
renal papillary cell carcinoma; LIHC, liver hepatocellular
carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell
carcinoma; PAAD, pancreatic adenocarcinoma; PRAD, prostate
adenocarcinoma; PCPG, pheochromocytoma and paragangliosarcoma;
READ, rectal adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous
melanoma; THCA, thyroid carcinoma; THYM, thymoma; STAD, stomach
adenocarcinoma; UCEC, uterine corpus endometrial carcinoma. |
HKA
HKA structure and function
The HKA is a heterodimer composed of two subunits, α
and β, which are expressed by two separate genes (ATP4A, ATP4B)
(90). The ATP4A gene encodes for
the gastric α subunit, composed of 10 transmembrane helices and
housing the pump inhibitor-binding site intracellularly, known as
cysteine 813. When referring to a separate α subunit located
outside the gastric tissue, such as the prostate and skin, it is
known as the ATP12A (110 kDA) (91).
The ATP4B gene encodes for the β subunit (35 kDA) which is highly
glycosylated transmembrane protein and protects the enzyme from the
stomachs' acidic environment (92).
Apart from the parietal cells of the stomach, the
HKA is also found in the distal nephrons of kidneys, prostate, skin
and the placenta (93). ATP4A and
ATP4B are highly expressed in stomach and esophageal tissue
(Fig. 5), which is expected given
the action of HKA in maintaining pH in both organs. However, both
HKA subunits are scarce in other tissues, which may explain, at
least in part, why studies on this ion pump are limited.
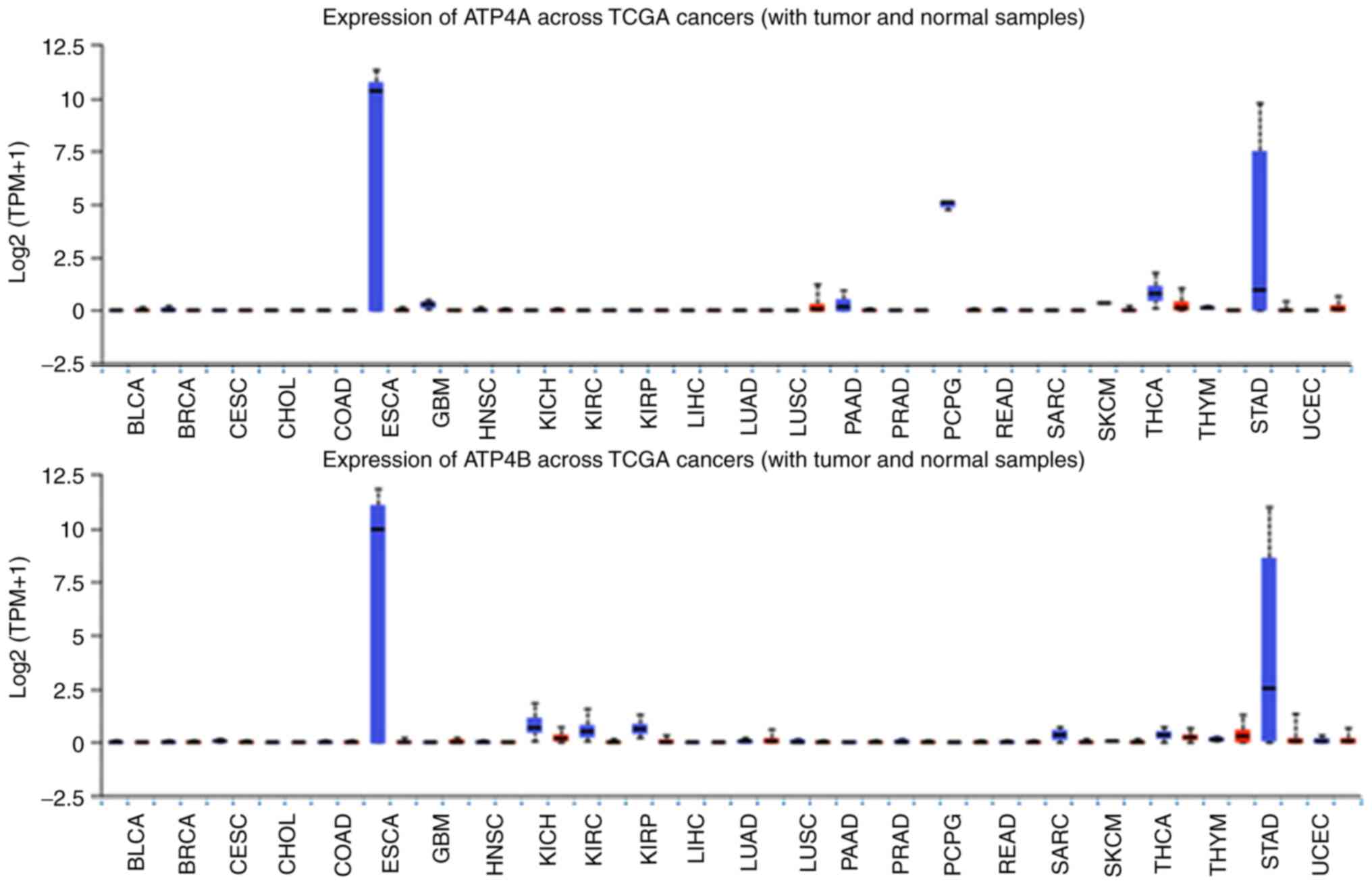 | Figure 5.Expression levels of HKA in cancer
compared with in healthy tissues across 24 types of cancer.
Downregulation of HKA expression was markedly observed in
esophageal carcinoma and stomach adenocarcinoma, and was less
distinct in thyroid carcinoma. Overall expression in all other
tissues appeared to be limited. Data were collected and permitted
for publication from the UALCAN resource. TPM, transcripts per
million; TCGA, The Cancer Genome Atlas; HKA,
H+/K+ ATPase; BLCA, bladder urothelial
carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous
cell carcinoma; CHOL, cholangiocarcinoma; COAD, colon
adenocarcinoma; ESCA, esophageal carcinoma; GBM, glioblastoma
multiforme; HNSC, head and neck squamous carcinoma; KICH, kidney
chromophobe; KIRC, kidney renal clear cell carcinoma; KIRP, kidney
renal papillary cell carcinoma; LIHC, liver hepatocellular
carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell
carcinoma; PAAD, pancreatic adenocarcinoma; PRAD, prostate
adenocarcinoma; PCPG, pheochromocytoma and paragangliosarcoma;
READ, rectal adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous
melanoma; THCA, thyroid carcinoma; THYM, thymoma; STAD, stomach
adenocarcinoma; UCEC, uterine corpus endometrial carcinoma. |
The HKA expresses characteristic structural changes
once activated, known as E1 and E2 conformations, leading to the
transportation of H+ into the lumen while K+ is removed
and released into the cytoplasm side of the enzyme (94–96).
Therefore, when the protein is open to the cytoplasmic side then it
is in the E1 phase, when it is open to the extracellular
environment then it is in the E2 phase (92). At an almost neutral pH (~6),
2H+ and 2K+ are pumped whereas in acidic
conditions (pH ~3), 1H+ and 1K+ are
transported. For all this to happen, energy from dephosphorylation
is required, giving HKA ion pumps the ability to succour the acidic
environment in organs such as the stomach (97).
H/K ATPase significance in cancer
The behavior of HKA subunits suggests that ATP4A is
more stable when its counterpart, ATP4B is present (98). The absence of ATP4A alone however,
resulted in hyperplasia, metaplasia and an imbalance in growth
factor in mice in chronic cases suffering from hypergastrinemia and
achlorhydria (99). Ion pumps may
not always have a direct effect on the pathology of the tissue.
Pepsin expresses both inflammatory and carcinogenic effects in the
hypopharynx and larynx when active, consequently an acidic
environment created by HKA has been proven to activate pepsin
freely present in the vicinity or stored in vesicles (98). In the case of rectal cancer,
omeprazole improves efficacy of chemotherapy and relieves patients
from chemotherapy side effects. As mentioned, these inhibitors bind
to the α subunit causing conformational changes which cease ATPase
action, hence the pump remains inactive (100). This favourable co-administration of
proton pump inhibitor (PPI)-chemotherapeutic drug was noticeable
within a human leukemia cell line (HL60 cells), where butyrate was
administered inhibiting histone deacetylase (located on ATP12A)
favouring apoptosis. However, SCH28080 was required first for
inhibiting pump activity and restoring pH intracellularly (100).
As mentioned above, chemotherapy drugs are often
rendered incompetent in eliminating cancer cells due to the greatly
acidic environment expressed in tumors (101). Hence, PPIs such as omeprazole,
pantoprazole and lansoprazole, serve to restore proton movement
across cellular membranes, thus restoring the pH to levels where
chemotherapy drugs can actively express their anticancer effects
(91,102). In breast cancer cell lines
doxorubicin was significantly more cytotoxic to when administered
in combination with PPIs (103).
The same was perceived when PPIs were synergistically administered
with raloxifene to T47D cells (103), another breast cancer cell line,
suppressing cell growth compared to the raloxifene alone. PPI
administration was also evaluated in Prostate cancer patients, yet
no significant results indicated their effectiveness as a cancer
drug (104). HKA (coded by ATP4A,
ATP4B and ATP12A) is expressed in pancreatic ducts and is
responsible for regulating the secretion of pancreatic fluid but is
also expressed in pancreatic cancer cells. Administrating PPIs to
pancreatic ductal adenocarcinoma (PDA) and pancreatic stellate
cells inhibited proliferation in vitro experiments while
in vivo testing on PDA had brought the GO/G1 cell cycle at a
halt, hence decreasing both tumor growth and fibrosis (105).
Fig. 5 illustrates
that ATP4B is exceedingly reduced in gastric cancer (GC) compared
to its high expression in healthy gastric cells and this is also
corroborated by the literature (106). This often causes problems in terms
of distorting chemotherapeutic drug behaviour. ATP4B can be
restored using 5′-aza-2′-deoxycytidine (a DNA methyltransferase
inhibitor) or trichostatin A (an histone deacetylase inhibitor),
consequently reinstating the β subunit further restoring pH to
homeostatic levels, hence creating a suitable environment for
docetaxel (chemotherapeutic drug) to function (106).
Trametenolic acid B (TAB) targets HKA in healthy
individuals and rectifies pH in gastric environments reversing
gastric ulcers diseases. The inhibitory effects of TAB also promote
apoptosis in human gastric carcinoma (HGC-27 cells) and reduce cell
viability both in vitro and in vivo (107). Model dose and pH need to be
detailed for the drug to be effective without affecting surrounding
healthy cells.
Another PPI is pantoprazole that is activated in
acidic environments expressed in tumors, and has been reported
capable of inducing apoptosis in MKN-45 cells and RGM-1 cells
(108). Evaluation in a GC
xenograft indicated suppressed tumor growth and promotion of
apoptosis through covalent binding of pantoprazole to the cysteine
residues on the α subunit of HKA, manipulating the extracellular
signal-regulated kinases pathway (108). Pantoprazole also proved to reduce
chemoresistance to fluorouracil (a chemotherapeutic drug) in both
in vitro and in vivo studies in colorectal cancer
cells (102). Rabeprazole on the
other hand was found to be effective in treating gastric cancer, by
preventing ERK 1/2 phosphorylation and simultaneously inhibiting
HKA, creating a less acidic environment (10).
Esophageal cancer cells express a sensitivity
towards chemotherapeutics when exposed to esomeprazole (ESO) as
tumor survival is reduced and metastasis is obstructed (in
vitro) (109). ESO proved to
alter the expression of miRNAs involved in chemo-resistance as it
is a proton pump inhibitor preventing protons from entering the
lumen of a cell, resulting in an intracellular basic state
(109,110). This action also sensitizes human
osteosarcoma cells to cisplatin, another chemotherapeutic drug
(110). ESO has also proved
effective when administered along with doxorubicin.
Doxorubicin-esomeprazole combination was significantly more
effective than either drug separately (111). Esomeprazole prevents H+
from moving intracellularly, therefore causing a build-up of
protons in the tumorigenic cells creating a toxic environment,
hence favouring the action of doxorubicin (111).
Efavirenz, a reverse transcriptase inhibitor, is an
antiviral and anti-tumor drug whose action is impaired in acidic
conditions. Lansoprazole is a drug that is activated by the acidic
environment in cancer cells and its function is to inhibit HKA.
Once the HKA is inhibited then the acidic pH can be overturned to a
state where efavirenz can perform. Efavirenz prevents migration of
tumor cells and decreases proliferation in human melanoma cells
(112). Lansoprazole is also
effective in promoting apoptosis and inhibiting proliferation in
breast cancer cell lines (MDA-MB-231), while inhibiting tumor
growth in vivo (107).
Dexlansoprazole, tenatoprazole, revaprazan and
vonoprazan are all PPIs used for treating esophageal reflux
disease, with evidence suggesting faster HKA inhibition compared to
other PPIs (113–115). However, to the best of our
knowledge, they have yet to be evaluated as potential anticancer
drugs. Rohitukine, on the other hand, expresses both antiulcer and
anticancer properties, but it is uncertain if the anticancer
effects are due to HKA pump inhibition (116). Maintaining HKA activity is crucial
as additional studies point out that failure to treat disorders
such as Helicobacter pylori infection, which renders HKA
inactive, may lead to gastric cancer (64).
Compared to NKA and SERCA, HKA subunits expressed
in normal and cancer cells have no significant alterations across
the 24 cell lines studied using bioinformatic tools, with the
exception of gastric and esophageal cells (Fig. 5). This pinpoints the specific
subunits to be explored as potential targets for treatment or as
biomarkers.
Clinical studies of P-class pump
modulators
Na/K ATPase
Previous studies have shown that NKA expression is
altered in various tumors, including drug-resistant tumors, marking
it as a potential target by developing NKA modulators (97,117,118).
For this reason, different NKA inhibitors, such as perillyl alcohol
and cardiac glycosides, are currently being used in a number of
clinical trials, to assess their effect on different cancers
(63,119,120).
Despite their narrow therapeutic window, cardiac glycosides have
exhibited acceptable tolerance, so far. In addition to their direct
cytotoxic effects and anti-proliferative properties, different NKA
modulators can overcome multiple mechanisms of cancer cells that
often lead to failure of existing chemotherapeutic agents (117).
In particular, in one study with
biochemically-relapsed prostate cancer, digoxin was well-tolerated,
but was not of additional benefit compared to that observed in
controls reported in previous data (historical controls) (121). A study of patients with BRAF-wild
type metastatic melanoma, showed a better response to trametinib
when administered with digoxin, compared to studies of trametinib
alone (119). In addition, effects
of lapatinib on digoxin were assessed in a series of patients with
HER2-positive breast cancer, showing that lapatinib significantly
increased the absorption of digoxin, thus underscoring the
necessity for dose adjustment. No effects on the tumor were
reported (120).
SERCA pump
As Ca2+ regulates different cellular
functions, including proliferation and differentiation, disruption
of Ca2+ pathways may lead to the development of
anti-cancer therapies. To this end, altered function of SERCA pumps
have shown to contribute to cancer development (70). Among SERCA inhibitors, thapsigargin
and its prodrug, mipsagargin, are used in different stages of
clinical trials. A study of mipsagargin in patients with advanced
solid tumors exhibited acceptable tolerability and safety, whereas
cancer stabilization (defined as progression-free survival) was
recorded, particularly in patients with hepatocellular carcinoma
(81).
H/K ATPase
The clinical usefulness of the HKA is evident in
the number of clinical trials that involve PPIs. PPIs can reduce
tumor acidity, thus allowing other drugs to reach cancer cells, but
also exhibit direct tumor cell toxicity themselves. Additionally,
by affecting the acidic environment of the tumor, potentiate the
pharmacokinetics of antitumor drugs (102). For this reason, most clinical
studies include PPI in combinations for treating malignancies.
Combination of high-dose PPIs and aspirin significantly improved
composite end-point outcomes in patients with Barrett's esophagus
(122). High dose PPI treatment
enhanced antitumor effects (translated as time to progression) of
chemotherapy in patients with metastatic breast cancer (102). Patients who received PPI during
chemotherapy for colorectal cancer, had significantly lower rates
of nausea and vomiting compared to patients who did not receive PPI
(102). Although pantoprazole did
not affect the pharmacokinetics of intravenous doxorubicin in
patients with solid tumors, esomeprazole reduced the
pharmacokinetic properties of pazopanib in patients with various
solid tumors, suggesting decreased absorption of oral pazopanib
when the gastric pH increases (106,108).
Concluding remarks and future
perspectives
The aim of this review was to assess literature
sources and determine if there is efficient evidence to support the
implication that P-class pumps should be targeted in cancer
treatments (Table SI). Overall, ion
homeostasis can affect an organ's natural function, diseased or
healthy. Research shows that cancer cell environments express ion
imbalances (10) and further
evidence, presented above, suggest one cause to be the involvement
of p-class pumps. Subsequently, the functional roles of NKA, SERCA
and HKA (ion pumps) as potential biomarkers but also as probable
targets against tumorigenesis and progression were evaluated.
Two of the major setbacks of chemotherapeutic drugs
is the hostile environments generated by cancer cells resulting in
chemoresistance and toxicity created by the drugs themselves
(110,111,117).
Numerous reports present evidence of pump inhibitors administered
synergistically with chemotherapy drugs overcome the hostile
environment and result in inducing cell cycle arrest, decreasing
proliferation and angiogenesis in various cancer types and
primarily induce apoptosis. Nonetheless, other inhibitors
administered in synergy with specific chemotherapeutic drugs have
proven to have the unfavorable opposite effect. Some pump
inhibitors administered do not express anti-cancerous effects per
se but simply layout a more friendly or more hostile environment
for the chemotherapeutic drug or cancer cells (100) respectively. Henceforth, further
research is necessary to cross-examine which combinations of
anticancer drugs to pump inhibitors express a favorable effect on
which specific type of cancer cells (103).
Complication, besides some inconclusive outcomes,
have surfaced through the literature where reports imply
development of gastric cancer after long-term PPI treatment
(123–125). One additional aspect to be
considered is that PPIs are commonly metabolised by CYP-mediated
isoforms that express drug-drug interactions, meaning other drugs
may bind to the same CYP-active sites occupying them or even
causing some alterations in the proteins structure. Such actions
may render CYP unresponsive when PPIs try to attach, therefore
concomitant medication should be considered when administering
these drugs (126). Reference to
toxicity was scarce throughout the literature, as the majority of
reported studies were performed in vitro, however it is a
significant feature to consider when selecting drugs for medical
administration both short-term and long-term.
This review has also collected data indicating
p-type pump expression is altered in tumor cells, affecting various
cellular pathways which are involved in cell cycle, apoptosis,
angiogenesis and metastasis, activation of MAPK, Caspase 3, ERK
phosphorylation, downregulation/inhibition of IL-8, TNF-α/NF-κB, f
and Bcl-2 (14,53,54,85,127–129).
The main finding from the bioinformatics data collected (TCGA
database) indicates that the differential expression of NKA, HKA
and SERCA pump subunits is not following a specific expression
pattern. These subunits expressed in a tissue specific manner with
variations of high and low expression in cancer vs normal and vice
versa.
Further evaluation of p-class pumps currently
administered for other disorders (e.g., HKA in gastric reflux) may
assist in determining the exact stage of cellular pathways affected
by these pumps hence determining whether they can potentially
contribute as novel cancer treatments (100,130).
An additional gap identified in the literature is the absence of
the exact pump subunits targeted by the drugs as this would help in
better understanding and evaluating the behavior of the inhibitors.
An example was presented with resveratrol established to
specifically target the ATP2A3 subunit leading to its extensive
evaluation and progression into clinical trials (89).
More detailed conclusions could have been drawn
with more in-depth analysis of how the biochemical pathways
involved are related to both cancer and the p-class pumps.
Nevertheless, with this collection it is feasible to identify gaps
which remain and directions in which to progress, such as the need
for further studies to elucidate the safety and clinical impact of
the above findings. The clinical studies section does refer to some
additional drugs which are not analysed in detail in the review.
There have been other collective reviews on p-class pumps (77,89,130–132).
To the best of the authors knowledge no other review has brought
together evidence from the literature on the behavior of P-class
pumps in-vivo, in-vitro and the clinical environment. The
progress made so far towards including p-class pumps as targets
against cancer or as architects which utilize chemotherapeutic
drugs has been depicted above.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
ST and AY conceptualized the study. ST, AY, CT, AZ,
EJ and IP collected and interpreted the data. ST, AY and CT drafted
the manuscript. All authors revised the manuscript for important
intellectual content, and read and approved the final manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AMOG
|
adhesion molecule on glia
|
|
CaMK
|
Ca2+/calmodulin-dependent
kinase
|
|
CG
|
cardiac glycoside
|
|
ER
|
endoplasmic reticulum
|
|
HKA
|
hydrogen potassium ATPase
|
|
NKA
|
sodium potassium ATPase
|
|
OCCC
|
ovarian clear cell carcinoma
|
|
PDA
|
pancreatic ductal adenocarcinoma
|
|
PPI
|
proton pump inhibitor
|
|
SERCA
|
sarcoendoplasmic reticulum calcium
ATPase
|
|
TAB
|
trametenolic acid B
|
References
|
1
|
Lauger P: Dynamics of ion transport
systems in membranes. Physiol Rev. 67:1296–1331. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pedersen PL: Transport ATPases into the
year 2008: A brief overview related to types, structures, functions
and roles in health and disease. J Bioenerg Biomembr. 39:349–355.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Apell HJ: Structure-function relationship
in P-type ATPases-a biophysical approach. Rev Physiol Biochem
Pharmacol. 150:1–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Litan A and Langhans SA: Cancer as a
channelopathy: Ion channels and pumps in tumor development and
progression. Front Cell Neurosci. 9:862015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Palmgren MG and Nissen P: P-type ATPases.
Annu Rev Biophys. 40:243–266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Palmgren MG and Axelsen KB: Evolution of
P-type ATPases. Biochim Biophys Acta. 1365:37–45. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kühlbrandt W: Biology, structure and
mechanism of P-type ATPases. Nat Rev Mol Cell Biol. 5:282–295.
2004. View Article : Google Scholar
|
|
8
|
Clausen MV, Hilbers F and Poulsen H: The
structure and function of the Na,K-ATPase isoforms in health and
disease. Front Physiol. 8:3712017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Toyoshima C, Nakasako M, Nomura H and
Ogawa H: Crystal structure of the calcium pump of sarcoplasmic
reticulum at 2.6 A resolution. Nature. 405:647–655. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gu M, Zhang Y, Zhou X, Ma H, Yao H and Ji
F: Rabeprazole exhibits antiproliferative effects on human gastric
cancer cell lines. Oncol Lett. 8:1739–1744. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Newman RA, Kondo Y, Yokoyama T, Dixon S,
Cartwright C, Chan D, Johansen M and Yang P: Autophagic cell death
of human pancreatic tumor cells mediated by Oleandrin, a
lipid-soluble cardiac glycoside. Integr Cancer Ther. 6:354–364.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ihenetu K, Qazzaz HM, Crespo F,
Fernandez-Botran R and Valdes R Jr: Digoxin-Like immunoreactive
factors induce apoptosis in human acute T-cell lymphoblastic
leukemia. Clin Chem. 53:1315–1322. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wong VK, Li T, Law BY, Ma ED, Yip NC,
Michelangeli F, Law CK, Zhang MM, Lam KY, Chan PL and Liu L:
Saikosaponin-d, a novel SERCA inhibitor, induces autophagic cell
death in apoptosis-defective cells. Cell Death Dis. 4:e7202013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rajasekaran SA, Huynh TP, Wolle DG,
Espineda CE, Inge LJ, Skay A, Lassman C, Nicholas SB, Harper JF,
Reeves AE, et al: Na,K-ATPase subunits as markers for
epithelial-mesenchymal transition in cancer and fibrosis. Mol
Cancer Ther. 9:1515–1524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dang D and Rao R: Calcium-ATPases: Gene
disorders and dysregulation in cancer. Biochim Biophys Acta.
1863:1344–1350. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arimochi J, Ohashi-Kobayashi A and Maeda
M: Interaction of Mat-8 (FXYD-3) with Na+/K+-ATPase in colorectal
cancer cells. Biol Pharm Bull. 30:648–654. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De Milito A and Fais S: Tumor acidity,
chemoresistance and proton pump inhibitors. Future Oncol.
1:779–786. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yiallouris A, Stephanou A and Patrikios I:
Anticancer properties of Na+/K+-ATPase: A mini review. Asian J Sci
Technol. 7:2864–2868. 2015.
|
|
20
|
Chakraborti S and Dhalla NS: Regulation of
membrane Na+-K+ ATPase. Springer International Publishing; Cham:
2016, View Article : Google Scholar
|
|
21
|
Dyla M, Kjærgaard M, Poulsen H and Nissen
P: Structure and mechanism of P-Type ATPase ion pumps. Annu Rev
Biochem. 89:583–603. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Z, Zhang Z, Xie JX, Li X, Tian J, Cai
T, Cui H, Ding H, Shapiro JI and Xie Z: Na/K-ATPase mimetic
pNaKtide peptide inhibits the growth of human cancer cells. J Biol
Chem. 286:32394–32403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Edwards IJ, Bruce G, Lawrenson C, Howe L,
Clapcote SJ, Deuchars SA and Deuchars J: Na+/K+ ATPase α1 and α3
isoforms are differentially expressed in α- and ү-motoneurons. J
Neurosci. 33:9913–9919. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pirahanchi Y, Jessu R and Aeddula NR:
Physiology, sodium potassium pump. StatPearls. StatPearls
Publishing; Treasure Island, FL: 2021
|
|
25
|
Lingrel JB and Kuntzweiler T:
Na+,K(+)-ATPase. J Biol Chem. 269:19659–19662. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kaplan JH: Biochemistry of Na,K-ATPase.
Annu Rev Biochem. 71:511–535. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pietrini G, Matteoli M, Banker G and
Caplan MJ: Isoforms of the Na,K-ATPase are present in both axons
and dendrites of hippocampal neurons in culture. Proc Natl Acad Sci
USA. 89:8414–8418. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nawata J, Ohno I, Isoyama S, Suzuki J,
Miura S, Ikeda J and Shirato K: Differential expression of alpha 1,
alpha 3 and alpha 5 integrin subunits in acute and chronic stages
of myocardial infarction in rats. Cardiovasc Res. 43:371–381. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Underhill DA, Canfield VA, Dahl JP, Gros P
and Levenson R: The Na,K-ATPase alpha4 gene (Atp1a4) encodes a
ouabain-resistant alpha subunit and is tightly linked to the alpha2
gene (Atp1a2) on mouse chromosome 1. Biochemistry. 38:14746–14751.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Makita N, Bennett PB Jr and George AL Jr:
Voltage-gated Na+ channel beta 1 subunit mRNA expressed in adult
human skeletal muscle, heart, and brain is encoded by a single
gene. J Biol Chem. 269:7571–7578. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hilbers F, Kopec W, Isaksen TJ, Holm TH,
Lykke-Hartmann K, Nissen P, Khandelia H and Poulsen H: Tuning of
the Na,K-ATPase by the beta subunit. Sci Rep. 6:204422016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mobasheri A, Trujillo E, Arteaga MF and
Martín-Vasallo P: Na(+), K(+)-ATPase subunit composition in a human
chondrocyte cell line; evidence for the presence of α1, α3, β1, β2
and β3 isoforms. Int J Mol Sci. 13:5019–5034. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sundaram SM, Safina D, Ehrkamp A, Faissner
A, Heumann R and Dietzel ID: Differential expression patterns of
sodium potassium ATPase alpha and beta subunit isoforms in mouse
brain during postnatal development. Neurochem Int. 128:163–174.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Malik N, Canfield VA, Beckers MC, Gros P
and Levenson R: Identification of the mammalian Na,K-ATPase 3
subunit. J Biol Chem. 271:22754–22758. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pestov NB, Zhao H, Basrur V and Modyanov
NN: Isolation and characterization of BetaM protein encoded by
ATP1B4-a unique member of the Na,K-ATPase β-subunit gene family.
Biochem Biophys Res Commun. 412:543–548. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mijatovic T, Dufrasne F and Kiss R:
Na+/K+-ATPase and cancer. Pharm Pat Anal. 1:91–106. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hundal HS, Marette A, Ramlal T, Liu Z and
Klip A: Expression of beta subunit isoforms of the Na+,K(+)-ATPase
is muscle type-specific. FEBS Lett. 328:253–258. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jia LG, Donnet C, Bogaev RC, Blatt RJ,
McKinney CE, Day KH, Berr SS, Jones LR, Moorman JR, Sweadner KJ and
Tucker AL: Hypertrophy, increased ejection fraction, and reduced
Na-K-ATPase activity in phospholemman-deficient mice. Am J Physiol
Heart Circ Physiol. 288:H1982–H1988. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jones DH, Li TY, Arystarkhova E, Barr KJ,
Wetzel RK, Peng J, Markham K, Sweadner KJ, Fong GH and Kidder GM:
Na,K-ATPase from mice lacking the gamma subunit (FXYD2) exhibits
altered Na+ affinity and decreased thermal stability. J Biol Chem.
280:19003–19011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Morrison BW, Moorman JR, Kowdley GC,
Kobayashi YM, Jones LR and Leder P: Mat-8, a novel
phospholemman-like protein expressed in human breast tumors,
induces a chloride conductance in xenopus oocytes. J Biol Chem.
270:2176–2182. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Crambert G, Li C, Swee LK and Geering K:
FXYD7, mapping of functional sites involved in endoplasmic
reticulum export, association with and regulation of Na,K-ATPase. J
Biol Chem. 279:30888–30895. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Geering K: Function of FXYD proteins,
regulators of Na, K-ATPase. J Bioenerg Biomembr. 37:387–392. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mayan H, Farfel Z and Karlish SJD: Renal
Mg handling, FXYD2 and the central role of the Na,K-ATPase. Physiol
Rep. 6:e138432018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lubarski I, Pihakaski-Maunsbach K, Karlish
SJ, Maunsbach AB and Garty H: Interaction with the Na,K-ATPase and
tissue distribution of FXYD5 (related to ion channel). J
Biol Chem. 280:37717–37724. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kadowaki K, Sugimoto K, Yamaguchi F, Song
T, Watanabe Y, Singh K and Tokuda M: Phosphohippolin expression in
the rat central nervous system. Brain Res Mol Brain Res.
125:105–112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Béguin P, Cambert G, Monnet-Tschudi F,
Uldry M, Horesberger JD, Garty H and Geering K: FXYD7 is a
brain-specific regulator of Na,K-ATPase alpha1-beta isozymes. EMBO
J. 21:3264–3273. 2002. View Article : Google Scholar
|
|
47
|
Yamaguchi F, Yamaguchi K, Tai Y, Sugimoto
K and Tokuda M: Molecular cloning and characterization of a novel
phospholemman-like protein from rat hippocampus. Brain Res Mol
Brain Res. 86:189–192. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhu ZL, Zhao ZR, Zhang Y, Yang YH, Wang
ZM, Cui DS, Wang MW, Kleeff J, Kayed H, Yan BY and Sun XF:
Expression and significance of FXYD-3 protein in gastric
adenocarcinoma. Dis Markers. 28:63–69. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Grzmil M, Voigt S, Thelen P, Hemmerlein B,
Helmke K and Burfeind P: Up-regulated expression of the MAT-8 gene
in prostate cancer and its siRNA-mediated inhibition of expression
induces a decrease in proliferation of human prostate carcinoma
cells. Int J Oncol. 24:97–105. 2004.PubMed/NCBI
|
|
50
|
Arcangeli A, Crociani O, Lastraioli E,
Masi A, Pillozzi S and Becchetti A: Targeting ion channels in
cancer: A novel frontier in antineoplastic therapy. Curr Med Chem.
16:66–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Baker Bechmann M, Rotoli D, Morales M,
Maeso Mdel C, García Mdel P, Ávila J, Mobasheri A and
Martín-Vasallo P: Na,K-ATPase isozymes in colorectal cancer and
liver metastases. Front Physiol. 7:92016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang P, Cartwright C, Efuet E, Hamilton
SR, Wistuba II, Menter D, Addington C, Shureiqi I and Newman RA:
Cellular location and expression of Na+,K+-ATPase α subunits affect
the anti-proliferative activity of oleandrin. Mol Carcinog.
53:253–263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Garcia DG, de Castro-Faria-Neto HC, da
Silva CI, de Souza e Souza KF, Gonçalves-de-Albuquerque CF, Silva
AR, de Amorim LM, Freire AS, Santelli RE, Diniz LP, et al:
Na/K-ATPase as a target for anticancer drugs: Studies with perillyl
alcohol. Mol Cancer. 14:1052015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Slingerland M, Cerella C, Guchelaar HJ,
Diederich M and Gelderblom H: Cardiac glycosides in cancer therapy:
From preclinical investigations towards clinical trials. Invest New
Drugs. 31:1087–1094. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Prassas I, Karagiannis GS, Batruch I,
Dimitromanolakis A, Datti A and Diamandis EP: Digitoxin-induced
cytotoxicity in cancer cells is mediated through distinct kinase
and interferon signaling networks. Mol Cancer Ther. 10:2083–2093.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu N, Li Y, Su S, Wang N, Wang H and Li
J: Inhibition of cell migration by ouabain in the A549 human lung
cancer cell line. Oncol Lett. 6:475–479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bogdanov A, Moiseenko F and Dubina M:
Abnormal expression of ATP1A1 and ATP1A2 in breast cancer.
F1000Res. 6:102017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Mony S, Lee SJ, Harper JF, Barwe SP and
Langhans SA: Regulation of Na,K-ATPase β1-subunit in
TGF-β2-mediated epithelial-to-mesenchymal transition in human
retinal pigmented epithelial cells. Exp Eye Res. 115:113–122. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li S, Dai Z, Yang D, Li W, Dai H, Sun B,
Liu X, Xie X, Xu R and Zhao X: Targeting β2 subunit of
Na+/K+-ATPase induces glioblastoma cell
apoptosis through elevation of intracellular Ca2. Am J
Cancer Res. 9:1293–1308. 2019.PubMed/NCBI
|
|
60
|
Rotoli D, Cejas MM, Maeso MC,
Pérez-Rodríguez ND, Morales M, Ávila J, Mobasheri A and
Martín-Vasallo P: The Na, K-ATPase β-Subunit isoforms expression in
glioblastoma multiforme: Moonlighting roles. Int J Mol Sci.
18:23692017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sun MZ, Kim JM, Oh MC, Safaee M, Kaur G,
Clark AJ, Bloch O, Ivan ME, Kaur R, Oh T, et al: Na+/K+-ATPase
β2-subunit (AMOG) expression abrogates invasion of
glioblastoma-derived brain tumor-initiating cells. Neuro Oncol.
15:1518–1531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hsu IL, Chou CY, Wu YY, Wu JE, Liang CH,
Tsai YT, Ke JY, Chen YL, Hsu KF and Hong TM: Targeting FXYD2 by
cardiac glycosides potently blocks tumor growth in ovarian clear
cell carcinoma. Oncotarget. 7:62925–62938. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li Y, Zhang X, Xu S, Ge J, Liu J, Li L,
Fang G, Meng Y, Zhang H and Sun X: Expression and clinical
significance of FXYD3 in endometrial cancer. Oncol Lett. 8:517–522.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Xue Y, Lai L, Lian W, Tu X, Zhou J, Dong
P, Su D, Wang X, Cao X, Chen Y and Wang Q: SOX9/FXYD3/Src Axis is
critical for ER + breast cancer stem cell function. Mol
Cancer Res. 17:238–249. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chen X, Sun M, Hu Y, Zhang H, Wang Z, Zhou
N and Yan X: FXYD6 is a new biomarker of cholangiocarcinoma. Oncol
Lett. 7:393–398. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gao Q, Chen X, Duan H, Wang Z, Feng J,
Yang D, Song L, Zhou N and Yan X: FXYD6: A novel therapeutic target
toward hepatocellular carcinoma. Protein Cell. 5:532–543. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Raman P, Purwin T, Pestell R and Tozeren
A: FXYD5 is a marker for poor prognosis and a potential driver for
metastasis in ovarian carcinomas. Cancer Inform. 14:113–119. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Casemore D and Xing C: SERCA as a target
for cancer therapies. Integr Cancer Sci Therap. 2:100–103.
2015.
|
|
69
|
Aubier M and Viires N: Calcium ATPase and
respiratory muscle function. Eur Respir J. 11:758–766.
1998.PubMed/NCBI
|
|
70
|
Chemaly ER, Troncone L and Lebeche D:
SERCA control of cell death and survival. Cell Calcium. 69:46–61.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Arbabian A, Brouland JP, Apáti Á, Pászty
K, Hegedűs L, Enyedi Á, Chomienne C and Papp B: Modulation of
endoplasmic reticulum calcium pump expression during lung cancer
cell differentiation. FEBS J. 280:5408–5418. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Colomer-Saucedo JB, Loulousis MM, Copello
VA, Krager SL, Tischkau SL and Copello JA: Pharmacological
targeting of SERCA in breast cancer. FASEB J. 34 (Suppl):S12020.
View Article : Google Scholar
|
|
73
|
Primeau JO, Armanious GP, Fisher ME and
Young HS: The SarcoEndoplasmic reticulum calcium ATPase. Subcell
Biochem. 87:229–258. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sacchetto R, Bertipaglia I, Giannetti S,
Cendron L, Mascarello F, Damiani E, Carafoli E and Zanotti G:
Crystal structure of sarcoplasmic reticulum Ca2+-ATPase (SERCA)
from bovine muscle. J Struct Biol. 178:38–44. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Stewart TA, Yapa KT and Monteith GR:
Altered calcium signaling in cancer cells. Biochim Biophys Acta.
1848:2502–2511. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Celsi F, Pizzo P, Brini M, Leo S, Fotino
C, Pinton P and Rizzuto R: Mitochondria, calcium and cell death: A
deadly triad in neurodegeneration. Biochim Biophys Acta.
1787:335–344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yousef M, Vlachogiannis IA and Tsiani E:
Effects of resveratrol against lung cancer: In vitro and in vivo
studies. Nutrients. 9:12312017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Papp B, Brouland JP, Arbabian A, Gélébart
P, Kovács T, Bobe R, Enouf J, Varin-Blank N and Apáti A:
Endoplasmic reticulum calcium pumps and cancer cell
differentiation. Biomolecules. 2:165–186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Sagara Y, Wade JB and Inesi G: A
conformational mechanism for formation of a dead-end complex by the
sarcoplasmic reticulum ATPase with thapsigargin. J Biol Chem.
267:1286–1292. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Jaskulska A, Janecka AE and Gach-Janczak
K: Thapsigargin-from traditional medicine to anticancer drug. Int J
Mol Sci. 22:42020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Mahalingam D, Wilding G, Denmeade S,
Sarantopoulas J, Cosgrove D, Cetnar J, Azad N, Bruce J, Kurman M,
Allgood VE and Carducci M: Mipsagargin, a novel thapsigargin-based
PSMA-activated prodrug: Results of a first-in-man phase I clinical
trial in patients with refractory, advanced or metastatic solid
tumours. Br J Cancer. 114:986–994. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Gu J, Liu H, Fu T and Xu Y: Thapsigargin
increases apoptotic cell death in human hepatoma BEL-7404 cells.
Cell Res. 5:59–65. 1995. View Article : Google Scholar
|
|
83
|
Denmeade SR, Jakobsen CM, Janssen S, Khan
SR, Garrett ES, Lilja H, Christensen SB and Isaacs JT:
Prostate-specific antigen-activated thapsigargin prodrug as
targeted therapy for prostate cancer. J Natl Cancer Inst.
95:990–1000. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Søhoel H, Jensen AM, Møller JV, Nissen P,
Denmeade SR, Isaacs JT, Olsen CE and Christensen SB: Natural
products as starting materials for development of second-generation
SERCA inhibitors targeted towards prostate cancer cells. Bioorg Med
Chem. 14:2810–2815. 2006. View Article : Google Scholar
|
|
85
|
Park KC, Kim SW, Jeon JY, Jo AR, Choi HJ,
Kim J, Lee HG, Kim Y, Mills GB, Noh SH, et al: Survival of cancer
stem-like cells under metabolic stress via CaMK2α-mediated
upregulation of sarco/endoplasmic reticulum calcium ATPase
expression. Clin Cancer Res. 24:1677–1690. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Riganti C, Doublier S, Viarisio D,
Miraglia E, Pescarmona G, Ghigo D and Bosia A: Artemisinin induces
doxorubicin resistance in human colon cancer cells via
calcium-dependent activation of HIF-1alpha and P-glycoprotein
overexpression. Br J Pharmacol. 156:1054–1066. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
De Ford C, Heidersdorf B, Haun F, Murillo
R, Friedrich T, Borner C and Merfort I: The clerodane diterpene
casearin J induces apoptosis of T-ALL cells through SERCA
inhibition, oxidative stress, and interference with Notch1
signaling. Cell Death Dis. 7:e20702016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kim SL, Kim SH, Trang KT, Kim IH, Lee SO,
Lee ST, Kim DG, Kang SB and Kim SW: Synergistic antitumor effect of
5-fluorouracil in combination with parthenolide in human colorectal
cancer. Cancer Lett. 335:479–486. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Izquierdo-Torres E, Rodríguez G,
Meneses-Morales I and Zarain-Herzberg A: ATP2A3 gene as an
important player for resveratrol anticancer activity in breast
cancer cells. Mol Carcinog. 56:1703–1711. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Vander Stricht DV, Raussens V, Oberg KA,
Ruysschaert JM and Goormaghtigh E: Difference between the E1 and E2
conformations of gastric H+/K+-ATPase in a multilamellar lipid film
system: Characterization by fluorescence and ATR-FTIR spectroscopy
under a continuous buffer flow. Eur J Biochem. 268:2873–2880. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Streif D, Iglseder E, Hauser-Kronberger C,
Fink KG, Jakab M and Ritter M: Expression of the non-gastric H+/K+
ATPase ATP12A in normal and pathological human prostate tissue.
Cell Physiol Biochem. 28:1287–1294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Abe K, Tani K, Nishizawa T and Fujiyoshi
Y: Inter-subunit interaction of gastric H+,K+-ATPase prevents
reverse reaction of the transport cycle. EMBO J. 28:1637–1643.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Sachs G, Shin JM, Vagin O, Lambrecht N,
Yakubov I and Munson K: The gastric H,K ATPase as a drug target:
Past, present, and future. J Clin Gastroenterol. 41 (Suppl
2):S226–S242. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ward RM and Kearns GL: Proton pump
inhibitors in pediatrics: Mechanism of action, pharmacokinetics,
pharmacogenetics, and pharmacodynamics. Paediatr Drugs. 15:119–131.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Sakai H, Fujii T and Takeguchi N:
Proton-potassium (H(+)/K(+)) ATPases: Properties and roles in
health and diseases. Met Ions Life Sci. 16:459–483. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Dubey V, Han M, Kopec W, Solov'yov IA, Abe
K and Khandelia H: K+ binding and proton redistribution
in the E2P state of the H+, K+-ATPase. Sci
Rep. 8:127322018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Shin JM, Munson K, Vagin O and Sachs G:
The gastric HK-ATPase: Structure, function, and inhibition.
Pflugers Arch. 457:609–622. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
McCormick CA, Samuels TL, Battle MA,
Frolkis T, Blumin JH, Bock JM, Wells C, Yan K, Altman KW and
Johnston N: H+/K+ATPase expression in the larynx of
laryngopharyngeal reflux and laryngeal cancer patients.
Laryngoscope. 131:130–135. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Judd LM, Andringa A, Rubio CA, Spicer Z,
Shull GE and Miller ML: Gastric achlorhydria in
H/K-ATPase-deficient (Atp4a(−/-)) mice causes severe hyperplasia,
mucocystic metaplasia and upregulation of growth factors. J
Gastroenterol Hepatol. 20:1266–1278. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Jakab M, Hofer S, Ravasio A, Huber F,
Schmidt S, Hitzl W, Geibel JP, Fürst J and Ritter M: The putative
role of the non-gastric H+/K+-ATPase ATP12A (ATP1AL1) as
anti-apoptotic ion transporter: Effect of the H+/K+ ATPase
inhibitor SCH28080 on butyrate-stimulated myelomonocytic HL-60
Cells. Cell Physiol Biochem. 34:1507–1526. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Yan D, Hu Y, Li S and Cheng M: A model of
3D-structure of H+, K+-ATPase catalytic subunit derived by homology
modeling. Acta Pharmacol Sin. 25:474–479. 2004.PubMed/NCBI
|
|
102
|
Wang X, Liu C, Wang J, Fan Y, Wang Z and
Wang Y: Proton pump inhibitors increase the chemosensitivity of
patients with advanced colorectal cancer. Oncotarget.
8:58801–58808. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Ihraiz WG, Ahram M and Bardaweel SK:
Proton pump inhibitors enhance chemosensitivity, promote apoptosis,
and suppress migration of breast cancer cells. Acta Pharm.
70:179–190. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hálfdánarson ÓÖ, Pottegård A, Lund SH,
Ogmundsdottir MH, Ogmundsdottir HM and Zoega H: Use of proton pump
inhibitors and mortality among Icelandic patients with prostate
cancer. Basic Clin Pharmacol Toxicol. 126:484–491. 2020. View Article : Google Scholar
|
|
105
|
Tozzi M, Sørensen CE, Magni L, Christensen
NM, Bouazzi R, Buch CM, Stefanini M, Duranti C, Arcangeli A and
Novak I: Proton pump inhibitors reduce pancreatic adenocarcinoma
progression by selectively targeting H+,
K+-ATPases in pancreatic cancer and stellate cells.
Cancers (Basel). 12:6402020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Lin S, Lin B, Wang X, Pan Y, Xu Q, He JS,
Gong W, Xing R, He Y, Guo L, et al: Silencing of ATP4B of ATPase
H+/K+ transporting beta subunit by intragenic
epigenetic alteration in human gastric cancer cells. Oncol Res.
25:317–329. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhang Q, Huang N, Wang J, Luo H, He H,
Ding M, Deng WQ and Zou K: The H+/K+-ATPase inhibitory activities
of trametenolic acid B from trametes lactinea (Berk.) Pat, and its
effects on gastric cancer cells. Fitoterapia. 89:210–217. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Yeo M, Kim DK, Kim YB, Oh TY, Lee JE, Cho
SW, Kim HC and Hahm KB: Selective induction of apoptosis with
proton pump inhibitor in gastric cancer cells. Clin Cancer Res.
10:8687–8696. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Lindner K, Borchardt C, Schöpp M, Bürgers
A, Stock C, Hussey DJ, Haier J and Hummel R: Proton pump inhibitors
(PPIs) impact on tumour cell survival, metastatic potential and
chemotherapy resistance, and affect expression of
resistance-relevant miRNAs in esophageal cancer. J Exp Clin Cancer
Res. 33:732014. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ferrari S, Perut F, Fagioli F, Brach Del
Prever A, Meazza C, Parafioriti A, Picci P, Gambarotti M, Avnet S,
Baldini N and Fais S: Proton pump inhibitor chemosensitization in
human osteosarcoma: From the bench to the patients' bed. J Transl
Med. 11:2682013. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Goh W, Sleptsova-Freidrich I and Petrovic
N: Use of proton pump inhibitors as adjunct treatment for
triple-negative breast cancers. An introductory study. J Pharm
Pharm Sci. 17:439–446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Lugini L, Sciamanna I, Federici C, Iessi
E, Spugnini EP and Fais S: Antitumor effect of combination of the
inhibitors of two new oncotargets: Proton pumps and reverse
transcriptase. Oncotarget. 8:4147–4155. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wu DC, Kuo CH, Tsay FW, Hsu WH, Chen A and
Hsu PI: A pilot randomized controlled study of dexlansoprazole
mr-based triple therapy for Helicobacter pylori infection.
Medicine (Baltimore). 95:e26982016. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Otake K, Sakurai Y, Nishida H, Fukui H,
Tagawa Y, Yamasaki H, Karashima M, Otsuka K and Inatomi N:
Characteristics of the novel potassium-competitive acid blocker
vonoprazan fumarate (TAK-438). Adv Ther. 33:1140–1157. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Inatomi N, Matsukawa J, Sakurai Y and
Otake K: Potassium-competitive acid blockers: Advanced therapeutic
option for acid-related diseases. Pharmacol Ther. 168:12–22. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Singh N, Singh P, Shrivastva S, Mishra SK,
Lakshmi V, Sharma R and Palit G: Gastroprotective effect of
anti-cancer compound rohitukine: Possible role of gastrin
antagonism and H(+) K (+)-ATPase inhibition. Naunyn-Schmiedebergs
Arch Pharmacol. 385:277–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Mijatovic T, Dufrasne F and Kiss R:
Cardiotonic steroids-mediated targeting of the Na(+)/K(+)-ATPase to
combat chemoresistant cancers. Curr Med Chem. 19:627–646. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Bindu PH, Sastry GM, Murty US and Sastry
GN: Structural and conformational changes concomitant with the
E1-E2 transition in H(+)K(+)-ATPase: A comparative protein modeling
study. Biochem Biophys Res Commun. 319:312–320. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Frankel AE, Eskiocak U, Gill JG, Yuan S,
Ramesh V, Froehlich TW, Ahn C and Morrison SJ: Digoxin plus
trametinib therapy achieves disease control in BRAF wild-type
metastatic melanoma patients. Neoplasia. 19:255–260. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Koch KM, Smith DA, Botbyl J, Arya N,
Briley LP, Cartee L, White JH, Beyer J, Dar MM, Chung HC, et al:
Effect of lapatinib on oral digoxin absorption in patients. Clin
Pharmacol Drug Dev. 4:449–453. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Lin J, Zhan T, Duffy D, Hoffman-Censits J,
Kilpatrick D, Trabulsi EJ, Lallas CD, Chervoneva I, Limentani K,
Kennedy B, et al: A pilot phase II Study of digoxin in patients
with recurrent prostate cancer as evident by a rising PSA. Am J
Cancer Ther Pharmacol. 2:21–32. 2014.PubMed/NCBI
|
|
122
|
Jankowski JAZ, de Caestecker J, Love SB,
Reilly G, Watson P, Sanders S, Ang Y, Morris D, Bhandari P, Brooks
C, et al: Esomeprazole and aspirin in Barrett's oesophagus
(AspECT): A randomised factorial trial. Lancet. 392:400–408. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Cheung KS and Leung WK: Long-term use of
proton-pump inhibitors and risk of gastric cancer: A review of the
current evidence. Therap Adv Gastroenterol. 12:1756284819834512019.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Hammadi M, Adi M, John R, Khoder GA and
Karam SM: Dysregulation of gastric H,K-ATPase by cigarette smoke
extract. World J Gastroenterol. 15:4016–4022. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Robertson DJ, Larsson H, Friis S, Pedersen
L, Baron JA and Sørensen HT: Proton pump inhibitor use and risk of
colorectal cancer: A population-based, case-control study.
Gastroenterology. 133:755–760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Roche VF: The chemically elegant proton
pump inhibitors. Am J Pharm Educ. 70:1012006. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Inge LJ, Rajasekaran SA, Yoshimoto K,
Mischel PS, McBride W, Landaw E and Rajasekaran AK: Evidence for a
potential tumor suppressor role for the Na,K-ATPase beta1-subunit.
Histol Histopathol. 23:459–467. 2008.PubMed/NCBI
|
|
128
|
Alevizopoulos K, Dimas K, Papadopoulou N,
Schmidt EM, Tsapara A, Alkahtani S, Honisch S, Prousis KC, Alarifi
S, Calogeropoulou T, et al: Functional characterization and
anti-cancer action of the clinical phase II cardiac Na+/K+ ATPase
inhibitor istaroxime: In vitro and in vivo properties and cross
talk with the membrane androgen receptor. Oncotarget.
7:24415–22428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Chow DC and Forte JG: Functional
significance of the beta-subunit for heterodimeric P-type ATPases.
J Exp Biol. 198:1–17. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Peterson JA, Oblad RV, Mecham JC and
Kenealey JD: Resveratrol inhibits plasma membrane
Ca2+-ATPase inducing an increase in cytoplasmic calcium.
Biochem Biophys Rep. 7:253–258. 2016.PubMed/NCBI
|
|
131
|
Madreiter-Sokolowski CT, Gottschalk B,
Parichatikanond W, Eroglu E, Klec C, Waldeck-Weiermair M, Malli R
and Graier WF: Resveratrol specifically kills cancer cells by a
devastating increase in the Ca2+ coupling between the greatly
tethered endoplasmic reticulum and mitochondria. Cell Physiol
Biochem. 39:1404–1420. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Almasi S and El Hiani Y: Exploring the
therapeutic potential of membrane transport proteins: Focus on
cancer and chemoresistance. Cancers (Basel). 12:16242020.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
McConkey DJ, Lin Y, Nutt LK, Ozel HZ and
Newman RA: Cardiac glycosides stimulate Ca2+ increases and
apoptosis in androgen-independent, metastatic human prostate
adenocarcinoma cells. Cancer Res. 60:3807–3812. 2000.PubMed/NCBI
|
|
134
|
Li H, Wang P, Gao Y, Zhu X, Liu L, Cohen
L, Meng Z and Yang P: Na+/K+-ATPase α3 mediates sensitivity of
hepatocellular carcinoma cells to bufalin. Oncol Rep. 25:825–830.
2011.PubMed/NCBI
|
|
135
|
Numazawa S, Shinoki MA, Ito H, Yoshida T
and Kuroiwa Y: Involvement of Na+,K(+)-ATPase inhibition in K562
cell differentiation induced by bufalin. J Cell Physiol.
160:113–120. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Sun X, Ng TTH, Sham KWY, Zhang L, Chan
MTV, Wu WKK and Cheng CHK: Bufalin, a traditional Chinese medicine
compound, prevents tumor formation in two murine models of
colorectal cancer. Cancer Prev Res (Phila). 12:653–666. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Yu CH, Kan SF, Pu HF, Jea Chien E and Wang
PS: Apoptotic signaling in bufalin- and cinobufagin-treated
androgen-dependent and -independent human prostate cancer cells.
Cancer Sci. 99:2467–2476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Kang MA, Kim MS, Kim W, Um JH, Shin YJ,
Song JY and Jeong JH: Lanatoside C suppressed colorectal cancer
cell growth by inducing mitochondrial dysfunction and increased
radiation sensitivity by impairing DNA damage repair. Oncotarget.
7:6074–6087. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Huang YT, Chueh SC, Teng CM and Guh JH:
Investigation of ouabain-induced anticancer effect in human
androgen-independent prostate cancer PC-3 cells. Biochem Pharmacol.
67:727–733. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Ono Y, Chiba S, Yano H, Nakayama N, Saio
M, Tsuruma K, Shimazawa M, Iwama T and Hara H: Glycoprotein
nonmetastatic melanoma protein B (GPNMB) promotes the progression
of brain glioblastoma via Na+/K+-ATPase.
Biochem Biophys Res Commun. 481:7–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Rocha SC, Pessoa MT, Neves LD, Alves SL,
Silva LM, Santos HL, Oliveira SM, Taranto AG, Comar M, Gomes IV, et
al: 21-Benzylidene digoxin: A proapoptotic cardenolide of cancer
cells that up-regulates Na,K-ATPase and epithelial tight junctions.
PLoS One. 9:e1087762014. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Denmeade SR and Isaacs JT: The SERCA pump
as a therapeutic target: Making a ‘smart bomb’ for prostate cancer.
Cancer Biol Ther. 4:21–29. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Fan L, Li A, Li W, Cai P, Yang B, Zhang M,
Gu Y, Shu Y, Sun Y, Shen Y, et al: Novel role of Sarco/endoplasmic
reticulum calcium ATPase 2 in development of colorectal cancer and
its regulation by F36, a curcumin analog. Biomed Pharmacother.
68:1141–1148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Jia J, Qin Y, Zhang L, Guo C, Wang Y, Yue
X and Qian J: Artemisinin inhibits gallbladder cancer cell lines
through triggering cell cycle arrest and apoptosis. Mol Med Rep.
13:4461–4468. 2016. View Article : Google Scholar : PubMed/NCBI
|