Introduction
Breast cancer (BC) is a malignant growth that
develops and spreads from the mammary system and is the leading
cause of mortality among women (6.6% mortality rate) worldwide in
2018 (1,2). Although numerous advanced technologies
such as breast-conserving surgery, mastectomy, chemotherapy and
radiation therapy are employed to treat various stages of BC, the
5-year survival rate of patients diagnosed during 2009 and 2015
remains low (98% for stage I, 92% for stage II, 75% for stage III,
and 27% for stage IV), especially among patients with stage IV BC,
partly due to the high recurrence rate (3,4). For
this reason, new diagnostic and therapeutic targets for BC need to
be urgently identified.
MicroRNAs (miRNAs) are a class of endogenous small
RNA molecules with 20–25 nucleotides that have been documented to
affect the expression of their target genes (5,6). In
addition, a growing number of studies have reported that miRNAs can
serve as oncogenes or tumor suppressor genes (7). As a diagnostic and therapeutic targets,
miRNAs can regulate the tumorigenesis of various types of cancer,
including BC (8–10). In BC, miR-1287-5p and miR-137 have
been identified to inhibit BC progression (11,12).
miR-1298-5p was first reported to regulate the proliferation and
migration of vascular smooth muscle cells by targeting connexin 43
(13). Further studies have revealed
that miR-1298-5p is expressed at low levels in tissues and cells of
a number of types of cancer, such as glioma (13), gastric cancer (14), bladder cancer (15) and lung cancer (16). In addition, this miR-1298-5p
contributes to the proliferation, migration and invasion of various
cancer cell types, including glioma, gastric cancer, bladder cancer
and lung cancer (14–17). A previous BC study has reported that
the overexpression of miR-1298 targets disintegrin and
metalloproteinase domain-containing protein 9 (ADAM9) and inhibits
malignant processes in vivo and in vitro (18). The tumor-suppressive function of
miR-1298-5p has been acknowledged in the tumorigenesis of several
types of cancer, such as glioma (13) and lung cancer (16). However, the roles of miR-1298-5p and
its downstream regulators in BC development need to be further
investigated.
The E2F family of transcription factors regulate the
expression of crucial genes involved in the cell cycle,
differentiation and apoptosis, such as pRB, p107 and p130 (19,20). E2F
transcription factor 1 (E2F1) was the first member of the E2F
family to be cloned, and has been demonstrated to modulate cell
proliferation and apoptosis by activating its downstream target
genes, such as p53 and DP-1 (21–23).
Considering the importance of cell proliferation and apoptosis in
the development of carcinoma, the effects of E2F1 on tumorigenesis
have been investigated in previous studies. For instance, E2F1 has
been demonstrated to serve a crucial role in the metastasis and
aggressiveness of gastric (24),
lung (25) and prostate (26) cancer, glioma (27) and hepatocellular carcinoma (28). In addition, E2F1 can serve as a tumor
promoter or suppressor; the dual function of E2F1 has been observed
in BC development (29–31). Notably, numerous studies have
reported the critical role of the miRNA/E2F1 axis in the
carcinogenesis of BC (32–34). In myeloid cells, bioinformatics
analysis has revealed that E2F1 may be targeted by miR-1298-5p to
regulate the cell cycle (35).
However, no studies have confirmed whether miR-1298-5p may target
E2F1 to contribute to BC progression to date.
The present study aimed to investigate the
regulatory role of miR-1298-5p and its potential target E2F1 in the
pathogenesis of BC. It was hypothesized that an association existed
between BC and miR-1298-5p. The results of the present study may
provide new targets for BC diagnosis and treatment.
Materials and methods
Clinical samples
Paired breast cancer and adjacent normal tissue
specimens were collected from 35 patients with BC who underwent
surgery prior to therapeutic intervention at The First Bethune
Hospital of Jilin University (Changchun, China) between February
2017 and December 2019. The age range of the patients was 28 to 54
years (mean ± SD, 40.75±12.37 years). All subjects provided written
informed consent before specimen collection, and all experiments
were approved by the Ethics Committee of The First Bethune Hospital
of Jilin University (approval no. 201021-005). The
clinicopathological characteristics of the patients are presented
in Table I.
 | Table I.Clinicopathological characteristics
of 35 patients with breast cancer. |
Table I.
Clinicopathological characteristics
of 35 patients with breast cancer.
|
| Cases (n=35) |
|---|
|
|
|
|---|
| Characteristic | Number | Frequency (%) |
|---|
| Onset age,
years |
|
|
|
<40 | 15 | 42.9 |
|
≥40 | 20 | 57.1 |
| Tumor site |
|
|
|
Unilateral | 32 | 91.4 |
|
Bilateral | 3 | 8.6 |
| Pathological
diagnosis |
|
|
| Ductal
carcinoma | 28 | 80.0 |
| Lobular
carcinoma | 5 | 14.3 |
| Other
neoplasm | 2 | 5.7 |
| Estrogen
receptor |
|
|
|
Positive | 6 | 17.1 |
|
Negative | 29 | 82.9 |
| HER-2/neu |
|
|
|
Positive | 9 | 25.7 |
|
Negative | 26 | 74.3 |
| Pathological
stage |
|
|
| I | 8 | 22.9 |
| II | 20 | 57.1 |
|
III | 6 | 17.1 |
| IV | 1 | 2.9 |
Cell lines and culture
Human BC cell lines T-47D, SK-BR-3, MDA-MB-231, MCF7
and BT-474, and the normal breast cell line MCF10A were purchased
from the American Type Culture Collection. MCF10A cells were
maintained in Mammary Epithelial Cell Growth Basal Medium (cat. no.
CC3150; Lonza Group, Ltd.) supplemented with 13 mg/ml bovine
pituitary extract (Thermo Fisher Scientific, Inc.), 0.5 mg/ml
hydrocortisone (Sigma-Aldrich; Merck KGaA), 10 µg/ml human
epidermal growth factor (Lonza Group, Ltd.), 5 mg/ml bovine insulin
(Sigma-Aldrich), 100 ng/ml cholera toxin (cat. no. C8052;
Sigma-Aldrich; Merck KGaA), and 100 U/ml penicillin-streptomycin
Mixtures (Lonza Group, Inc.). The RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), and 100 U/ml
penicillin-streptomycin Mixtures was used to culture the BC cells.
All cell lines were cultured or incubated at 37°C in an incubator
with 5% CO2.
Reverse transcription-quantitative PCR
(RT-qPCR)
The RNA from the tissues or cells was extracted
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). The expression of miR-1298-5p was detected using
the All-in-One™ miRNA RT-qPCR Detection kit (GeneCopoeia, Inc.).
The PrimeScript™ RT reagent kit (Takara Bio, Inc.) and
SYBR® Premix Ex Taq (Takara Bio, Inc.) were used to
perform the reverse transcription and qPCR, respectively, to
determine the expression levels of E2F1. The thermocycling
parameters were as follows: Initial denaturation at 95°C for 30
sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 30 sec.
All procedures were performed according to the manufacturer's
instructions. U6 and GAPDH were used as the normalization controls
for miR-1298-5p and E2F1, respectively. The relative expression
levels were calculated using the 2−ΔΔCq method (36). The primer sequences are presented in
Table II.
 | Table II.Primer sequences for reverse
transcription-quantitative PCR. |
Table II.
Primer sequences for reverse
transcription-quantitative PCR.
| Target | Primer sequences
(5′-3′) |
|---|
| miR-1298-5p | F:
GCCGTTCATTCGGCTGTCC |
|
| R:
GTGCAGGGTCCGAGGTATTC |
| U6 | F:
CTCGCTTCGGCAGCACA |
|
| R:
AACGCTTCACGAATTTGCGT |
| E2F1 | F:
ACGCTATGAGACCTCACTGAA |
|
| R:
TCCTGGGTCAACCCCTCAAG |
| GAPDH | F:
AGCCACATCGCTCAGACAC |
|
| R:
GCCCAATACGACCAAATCC |
Cell transfection
The miR-1298-5p mimics, miR-1298-5p inhibitor, E2F1
small interfering RNA (siRNA) and their corresponding negative
controls (NC) were designed and purchased from Guangzhou RiboBio
Co., Ltd.; the sequences are presented in Table SI. T47D and SKBR3 cells in the
logarithmic growth phase were digested with trypsin and collected
by centrifugation at 3,850 × g and 37°C for 5 min. The cells were
resuspended in the culture medium and seeded into 6- or 96-well
plates at a density of 1×106 or 5×103
cells/well. Following culture with 5% CO2 at 37°C for 12
h, 100 nM miR-1298-5p mimics, inhibitor, E2F1 siRNA or the
corresponding NC were transfected into the T47D and SKBR3 cells
using the Lipofectamine® 2000 transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 6 h, and
the culture medium was replaced. After 48 h, the transfection
efficiency was determined by RT-qPCR. The transfected cells were
subsequently harvested and prepared for further assays.
Untransfected cells were used as the blank control group.
Cell viability assay
The Cell Counting Kit-8 (CCK-8; cat. no. B34304;
Bimake) was used to evaluate the viability of the T47D and SKBR3
cells according to the manufacturer's instructions. The cells were
harvested and plated in 96-well plates at a density of
3×103 cells/well following transfection and cultured for
1, 2, 3 and 4 days. At each time point, 10 µl CCK-8 solution was
added into each cell well and incubated for 2 h at 37°C with 5%
CO2. The absorbance at 450 nm was identified using a
microplate reader (Bio-Rad Laboratories, Inc.).
Cell proliferation assay
The DNA synthesis capacity was determined using the
5-bromo-2′-deoxyuridine (BrdU) Cell Proliferation ELISA kit (Abcam)
according to the manufacturer's instructions to measure the
proliferative capacity of T-47D and SK-BR-3 cells. The transfected
cells were collected with trypsin and plated in 96-well plates at a
density of 2×105 cells/well. Subsequently, 20 µl 1X BrdU
labeling solution was added to the each well to label newly
synthetized DNA and incubated at 37°C for 2 h. Following the
incubation, the medium was replaced with 200 µl Fixing Solution and
incubated at room temperature for 30 min. The cells were washed
thrice with 1X Wash Buffer, 100 µl anti-BrdU monoclonal antibody
solution was added, and the cells were incubated at room
temperature for 1 h. Subsequently, the cells were consecutively
incubated with 100 µl Peroxidase Goat Anti-Mouse IgG Conjugate and
100 µl of TMB substrate solution at room temperature for 30 min.
Finally, the absorbance at 450 nm was measured using a microplate
reader.
Caspase-3 activity assay
The caspase-3 activity of the T-47D and SK-BR-3
cells was examined using the Caspase-3 Colorimetric Assay kit
(Medical and Biological Laboratories Co., Ltd.) according to the
manufacturer's instructions. First, 2×105 transfected
cells were collected and lysed using lysis buffer. Subsequently, 50
µl of the cell lysates were added to 96-well plates, and 50 µl
reaction buffer and 5 µl caspase-3 substrate were added into each
cell well. The mixture was incubated at 37°C for 1 h. Finally, the
absorbance at 405 nm was measured using a microplate reader. The
enzymatic activity of caspase-3 normalized to that of the control
group was used for statistical analysis.
Apoptosis assay
The Annexin V/propidium iodide (PI) double staining
kit (BD Biosciences) was used to stain the cells according to the
manufacturer's protocol. T-47D and SK-BR-3 cells were seeded in
24-well plates at a density of 3×105 cells/well,
transfected and collected at 48 h post-transfection. Subsequently,
the cells were washed twice with cold PBS and resuscitated in 1X
binding buffer. The cells were then stained in the dark with 5 µl
Annexin V-FITC for 15 min and 5 µl PI for 10 min at room
temperature. Apoptosis was detected using a BD FACSVerse flow
cytometer (BD Biosciences) and analyzed using FlowJo v9.96 (FlowJo
LLC).
Cell adhesion assay
The cell adhesion assay was performed as previously
described. Briefly, the transfected T-47D and SK-BR-3 cells were
harvested by trypsin digestion and plated into 96-well plates
pre-coated with 50 µl of 10 µg/ml type I collagen (BD Biosciences)
at a density of 5×103 cells/well. Following culture for
1 h at 37°C, the culture medium was removed, and the wells were
carefully rinsed with PBS to remove the non-adherent cells.
Subsequently, the remaining cells were added 10 µl/well MTT
solution (Roche) for 4 h incubation at 37°C. Finally, the
absorbance at 570 nm was recorded using a microplate reader. The
relative cell adhesive ability normalized to that of the control
group was used for statistical analysis.
Wound healing assay
The transfected T-47D and SK-BR-3 cells were
harvested, seeded in 12-well plates (1×105 cells/well)
and cultured in a 5% CO2 atmosphere at 37°C for 12 h.
Subsequently, a wound was produced in the cell monolayer with the
sterile tip of a 200-µl pipette when the cell confluence was
>90%, and the floating cells were rinsed with PBS. The cells
were cultured in serum-free medium at 37°C with 5% CO2
for 24 h. Images were captured at 0 and 24 h using an inverted
light microscope with a camera (magnification, ×100). ImageJ
version 1.49 software (National Institutes of Health) was used to
analyze the wound width. The migratory rate of the cells was
calculated as follows: Migratory rate (%)=(W0 h-W24
h)/W0 h ×100%, where W is the width of the wound
at each time point.
Bioinformatics analysis
TargetScan 7.1 (http://www.targetscan.org/vert_71/) and miRWalk
(http://mirwalk.umm.uni-heidelberg.de/) were employed
to predict the target genes of miR-1298-5p. The common target genes
between the two databases were overlapped using Venny 2.1.0
(https://bioinfogp.cnb.csic.es/tools/venny/) and
uploaded to the STRING database (https://string-db.org/) for further analyses. UALCAN
(http://ualcan.path.uab.edu/index.html) was used to
assess the expression levels of the screened genes in breast
invasive carcinoma cells according to the data from The Cancer
Genome Atlas (TCGA) database. The 5-year prognosis of BC was
analyzed using the Kaplan-Meier plotter (http://kmplot.com/analysis/) to further identify the
key genes.
Luciferase reporter assay
The wild-type (Wt) 3′untranslated region (UTR) of
E2F1 was amplified and subcloned into the psiCHECK™-2 plasmid
(Promega Corporation) between the sites of the restriction enzymes
(XhoI and NotI) and the firefly luciferase coding
sequence. The QuikChange II XL Site-Directed Mutagenesis kit (cat.
no. 200521; Agilent Technologies, Inc.) was used to introduce
mutations into the seed sequence of E2F1 to establish a mutated
plasmid (Mut). The Lipofectamine 2000 Transfection Reagent was used
to co-transfect the T-47D and SK-BR-3 cells with the psiCHECK™
reporter vectors containing 400 ng Wt or Mut construct and 100 nM
miR-1298-5p mimics or the mimic-NC. After a transfection period of
48 h, the culture medium was collected, and the luciferase activity
was assayed using a Dual-Luciferase Reporter Assay system (Promega
Corporation). The relative luciferase activity was calculated by
normalizing the luminescence intensity of the firefly luciferase
activity to that of the Renilla luciferase activity.
Western blot assay
The proteins from T47D and SKBR3 cells were
extracted at 48 h post-transfection using the RIPA lysis buffer
(Beyotime Institute of Biotechnology), and the protein
concentration was determined using the Pierce BCA Protein Assay kit
(Thermo Fisher Scientific, Inc.). The proteins (40 µg/lane) was
separated by 10% SDS-PAGE and transferred to a PVDF membrane
(MilliporeSigma). The membrane was blocked with 5% BSA for 2 h at
room temperature and incubated overnight at 4°C for immunoblotting
with the primary rabbit polyclonal anti-E2F1 (cat. no. ab137415),
rabbit monoclonal anti-E-cadherin (cat. no. ab76319), rabbit
monoclonal anti-vimentin (cat. no. ab92547) and rabbit monoclonal
anti-GAPDH (cat. no. ab181602) (all 1:1,000; all Abcam).
Subsequently, the membranes were washed with TBST containing 0.05%
Tween-20, and a HRP-conjugated goat anti-rabbit IgG H&L
secondary antibody (1:5,000; cat. no. ab6721; Abcam) was added and
incubated for 2 h at room temperature. The blots were visualized
using an enhanced chemiluminescence kit (Thermo Fisher Scientific,
Inc.). GAPDH served as the reference control. Densitometry was
conducted using ImageJ 1.8.0 (National Institutes of Health)
Statistical analysis
Data are presented as the mean ± SD. Statistical
analysis was performed using GraphPad Prism 8.0 (GraphPad Software,
Inc.). Paired Student's t-test and one-way or two-way ANOVA with
Tukey's post hoc test were applied to compare the statistical
differences between variables. The correlation between miR-1298-5p
and E2F1 was analyzed by Pearson's correlation analysis. In each
experiment, three biological repeats were performed. P<0.05 was
considered to indicate a statistically significant difference.
Results
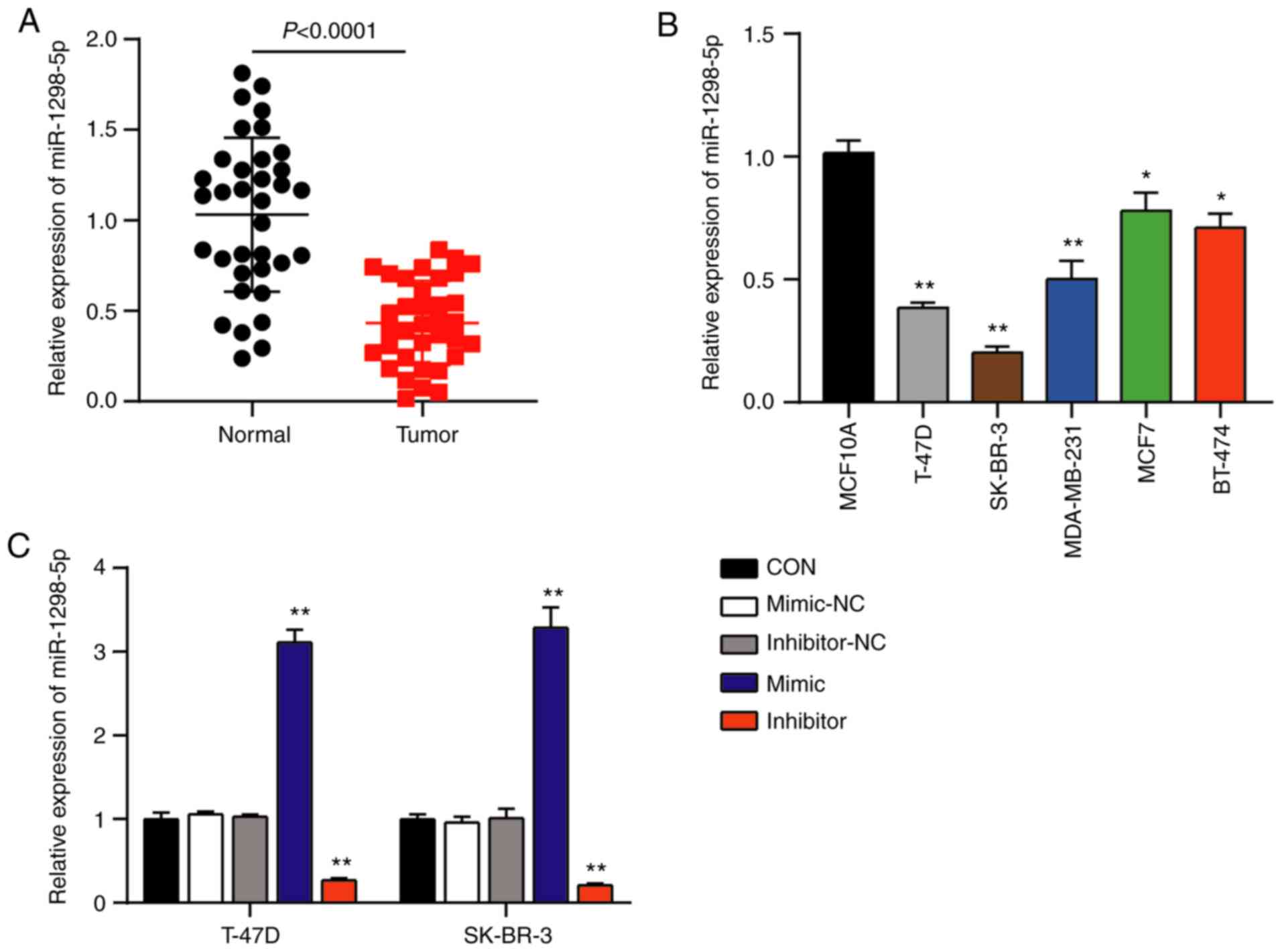
miR-1298-5p is expressed at low levels
in the BC tissues and cells
The potential role of miR-1298-5p in BC was assessed
using RT-qPCR in order to detect the expression levels of
miR-1298-5p in the BC and normal breast tissues. The results
demonstrated that the expression levels of miR-1298-5p were
downregulated by 60% in BC tissues compared with those in the
normal breast tissues (Fig. 1A). In
addition, the expression levels of miR-1298-5p were decreased in
the human BC cell lines T-47D, SK-BR-3, MDA-MB-231, MCF7 and BT-474
compared with those in the normal breast cell line MCF10A (Fig. 1B). To further analyze the association
between miR-1298-5p and BC, the miR-1298-5p mimic and inhibitor
were designed and transfected into T-47D and SK-BR-3 BC cells.
RT-qPCR analysis revealed a 3.2-fold increase in the expression
levels of miR-1298-5p in the mimic group and a 0.7-fold decrease in
the inhibitor group compared with those in the control groups,
indicating high transfection efficiency (Fig. 1C). Since the effects of
co-transfection with mimic-NC and inhibitor-NC were similar to
those of single NC transfections, the co-transfection of mimic-NC
and inhibitor-NC was used as the control for the subsequent
experiments. Overall, these results suggested that miR-1298-5p
expression was reduced in the BC.
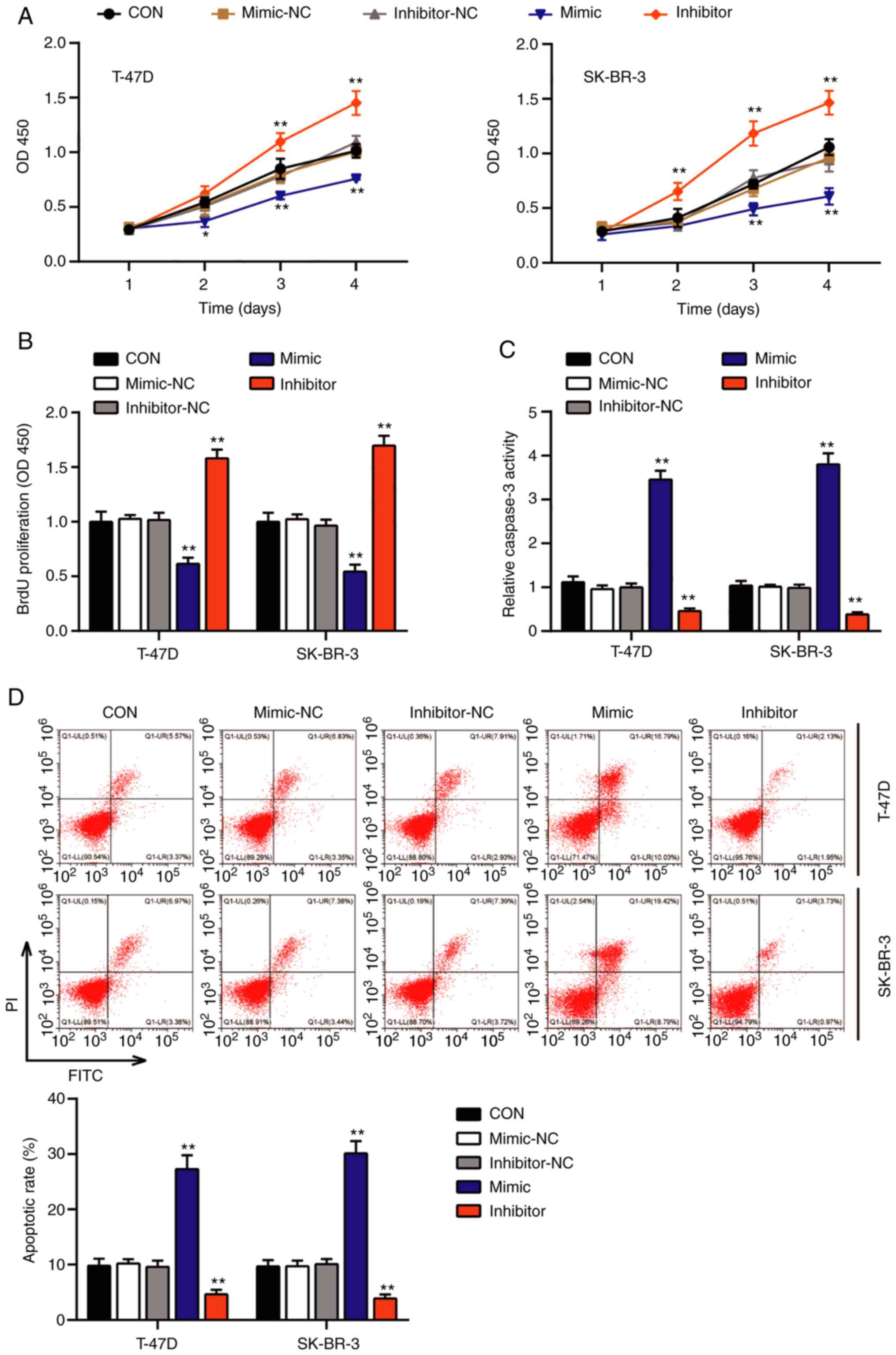
Effects of miR-1298-5p in BC
progression
The present study further investigated the function
of miR-1298-5p in the tumorigenesis of BC by detecting the
viability and proliferative ability of T-47D and SK-BR-3 cells
transfected with the miR-1298-5p mimic, inhibitor or NC. The
results of the CCK-8 assay demonstrated that BC cells transfected
with the miR-1298-5p mimic exhibited a decline in the cell
viability following culture for 3 and 4 days compared with that in
the control group; however, the viability of the two BC cell lines
was significantly enhanced following transfection with the
miR-1298-5p inhibitor (Fig. 2A).
Similarly, the BrdU assay revealed a 0.4-fold reduction in the DNA
synthesis levels in the T-47D and SK-BR-3 cells transfected with
the miR-1298-5p mimic and a 1.5–1.8-fold increase in the DNA
synthesis in the cells transfected with the miR-1298-5p inhibitor
compared with those in the control groups (Fig. 2B). These results suggested that
miR-1298-5p suppressed the ability of the BC cells to proliferate.
Furthermore, the results of the caspase-3 activity assay
demonstrated that the BC cell lines transfected with the
miR-1298-5p mimic displayed a 3-fold increase in the caspase-3
activity compared with that in the control groups, whereas the
inhibition of miR-1298-5p reduced the caspase-3 activity by
0.5-fold (Fig. 2C). In addition, the
changes in the apoptotic rate were detected by flow cytometry, and
the results were similar to those observed in the caspase-3
activity assay; the miR-1298-5p mimics increased, whereas
inhibition of miR-1298-5p reduced the apoptotic rate compared with
that observed in the control groups (Fig. 2D). These results demonstrated the
promotive effect of miR-1298-5p on BC cell apoptosis. Subsequently,
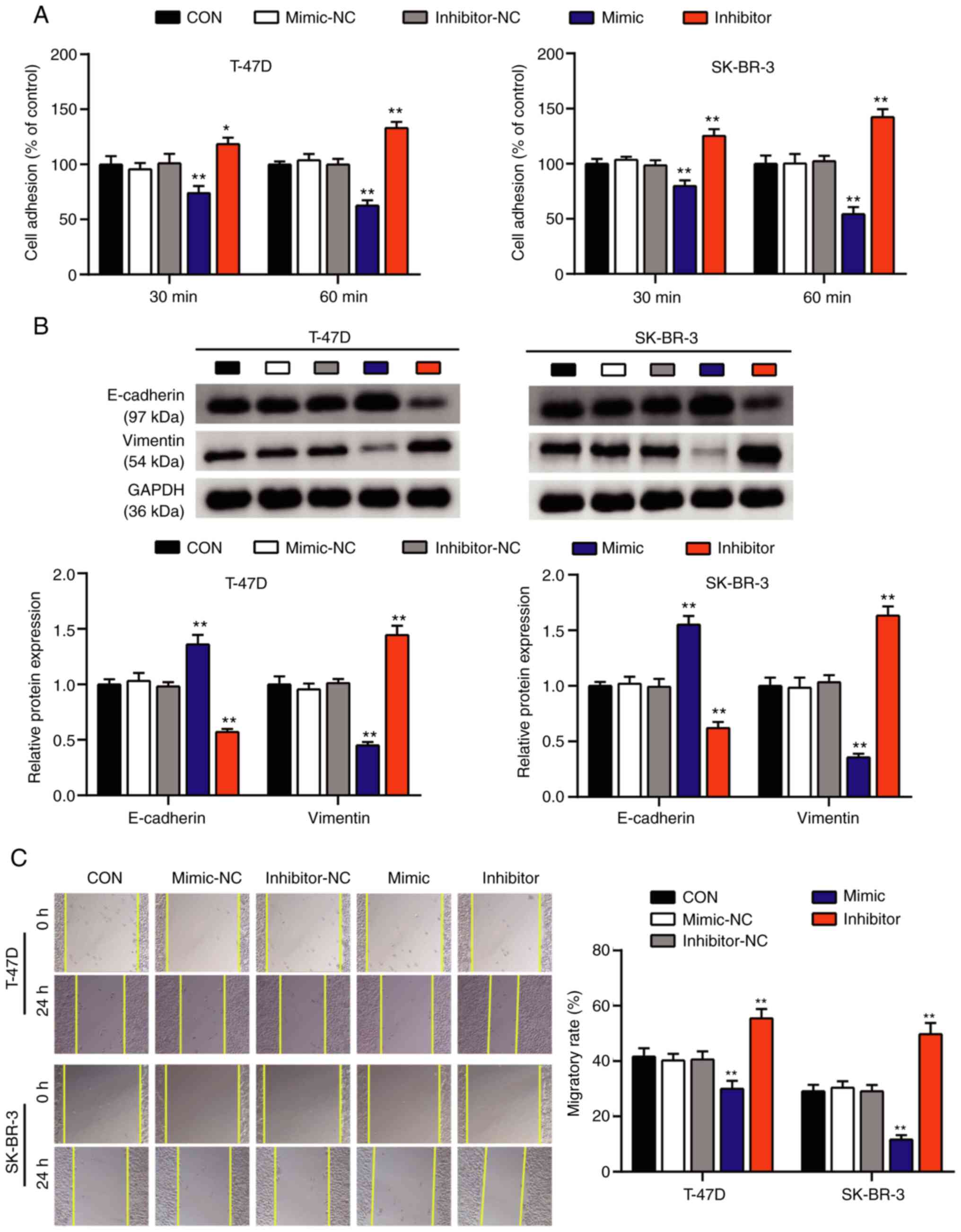
the cell adhesion and wound healing assays were performed to
evaluate the effects of miR-1298-5p on the adhesive capacity of BC
cells. The results of the cell adhesion assay revealed that the
percentage of adherent T-47D and SK-BR-3 cells exhibited a 0.4-fold
reduction in the miR-1298-5p mimic group and a 1.4-fold increase in
the miR-1298-5p inhibitor group compared with that in the control
group (Fig. 3A). Western blot assay
evaluated the protein expression levels of vimentin and E-cadherin,
which are associated with cell adhesion (37). The results of this assay demonstrated
that the protein expression levels of vimentin in the miR-1298-5p
mimic group decreased, whereas those of E-cadherin increased
compared with those in the control group. The effects of the
miR-1298-5p inhibitor on the protein expression levels of vimentin
and E-cadherin were opposite to those in the mimic group (Fig. 3B). In addition, according to the
results of the wound healing assay, the migratory rate of T-47D and
SK-BR-3 cells was reduced in the miR-1298-5p mimic group and
increased in the miR-1298-5p inhibitor group compared with that in
the control groups (Fig. 3C). Taken
together, these results suggested that miR-1298-5p inhibited the
proliferation, adhesion and migration of BC cells and induced
apoptosis.
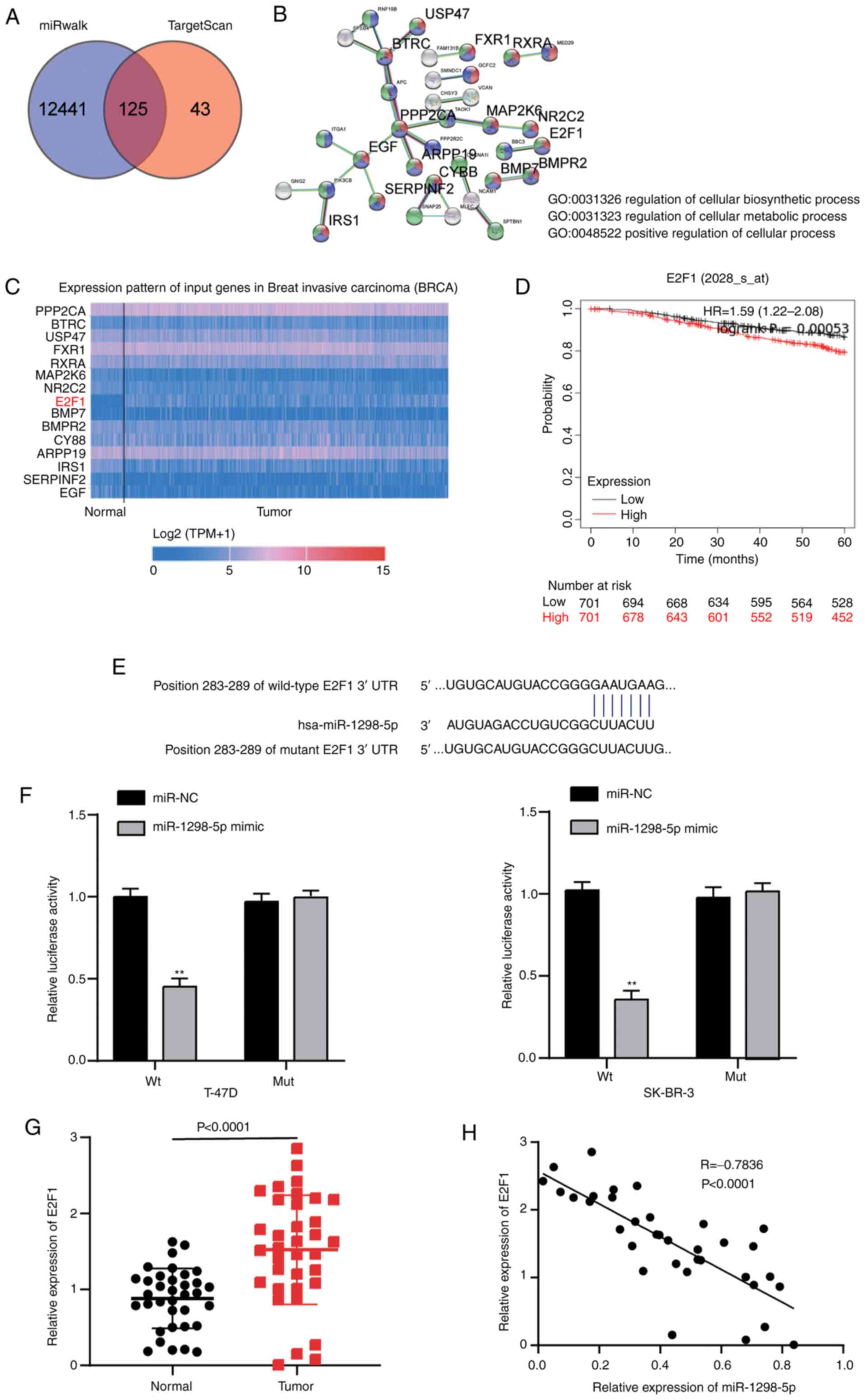
E2F1 is a target gene of miR-1298-5p
in BC cells
TargetScan and miRWalk databases were used to
predict the potential target genes of miR-1298-5p. Following Venny
2.1.0 analysis, a total of 125 target genes overlapped between the
miRWalk and TargetScan results (Fig.
4A). These genes were further analyzed using the STRING
database, and 15 key genes involved in ‘regulation of cellular
biosynthetic process’, ‘cellular metabolic process’ and ‘positive
regulation of cellular process’ were discovered (Fig. 4B). Among these genes, higher
expression levels of E2F1 were observed in breast carcinoma samples
in TCGA database (Fig. 4C). The
Kaplan-Meier plotter analysis demonstrated that high expression
levels of E2F1 were associated with a poor prognosis of BC
(Fig. 4D). Therefore, E2F1 was
selected as the gene of interest for further analysis. In addition,
the TargetScan results revealed that the E2F1 3′UTR contained a
putative recognition sequence (GAAUGAA) targeted by miR-1298-5p
(Fig. 4E). To assess the ability of
miR-1298-5p to directly target the 3′UTR of E2F1, the luciferase
activity assay was performed. Following transfection with the
miR-1298-5p mimic, the luciferase activity of T-47D and SK-BR-3
cells containing the Wt construct decreased by 0.6-fold compared
with that in the cells-transfected with the mimic-NC. However, the
luciferase activity of the T-47D and SK-BR-3 cells transfected with
the Mut construct was not affected by the miR-1298-5p mimic
(Fig. 4F). Furthermore, the
expression levels of E2F1 were significantly upregulated in the
breast tumor tissues compared with those in normal breast tissues
(Fig. 4G). In addition, the
expression levels of E2F1 were negatively correlated with those of
miR-1298-5p in the breast tumor tissues (Fig. 4H). These results demonstrated that
E2F1 was a direct target of miR-1298-5p in BC cells.
miR-1298-5p targets E2F1 to affect BC
cell malignant behaviors
To test whether miR-1298-5p regulated the
proliferation, adhesion, migration and apoptosis by targeting E2F1
in BC cells, T-47D and SK-BR-3 cell lines were transfected with the
E2F1 siRNA and the miR-1298-5p inhibitor separately or together.
The results of RT-qPCR and western blot analyses demonstrated that
the mRNA and the protein levels of E2F1 were downregulated by
~0.5-fold in the T47D and SKBR3 cells transfected with the E2F1
siRNA compared with those in the control groups (Fig. 5A and B). However, the expression
levels of the E2F1 mRNA and protein increased in the two BC cell
lines following transfection with the miR-1298-5p inhibitor
compared with those in the control group. The cells co-transfected
with the E2F1 siRNA and the miR-1298-5p inhibitor exhibited no
significant changes in the E2F1 expression levels compared with
those in the control group, indicating that these two molecules may
offset each other (Fig. 5A and B).
The results of further experiments also demonstrated that the
silencing of E2F1 reduced the viability and proliferative capacity
of T-47D and SK-BR-3 cells compared with those in the control
groups. However, the inhibition of miR-1298-5p reversed this effect
(Fig. 5C and D). In addition, the
silencing of E2F1 increased the caspase-3 activity levels of T-47D
and SK-BR-3 cells by ~3-fold compared with those in the control
groups. However, no significant changes were observed in the cells
co-transfected with the E2F1 siRNA and miR-1298-5p inhibitor
(Fig. 5E). Similarly, the apoptotic
rates of T-47D and SK-BR-3 cells increased after silencing E2F1
compared with those in the control groups, whereas no significant
changes were present in the cells co-transfected with the E2F1
siRNA and miR-1298-5p inhibitor (Fig.
5F). In addition, the results of the cell adhesion assay
demonstrated that the adhesive ability of T-47D and SK-BR-3 cells
was significantly inhibited when E2F1 was silenced; however, the
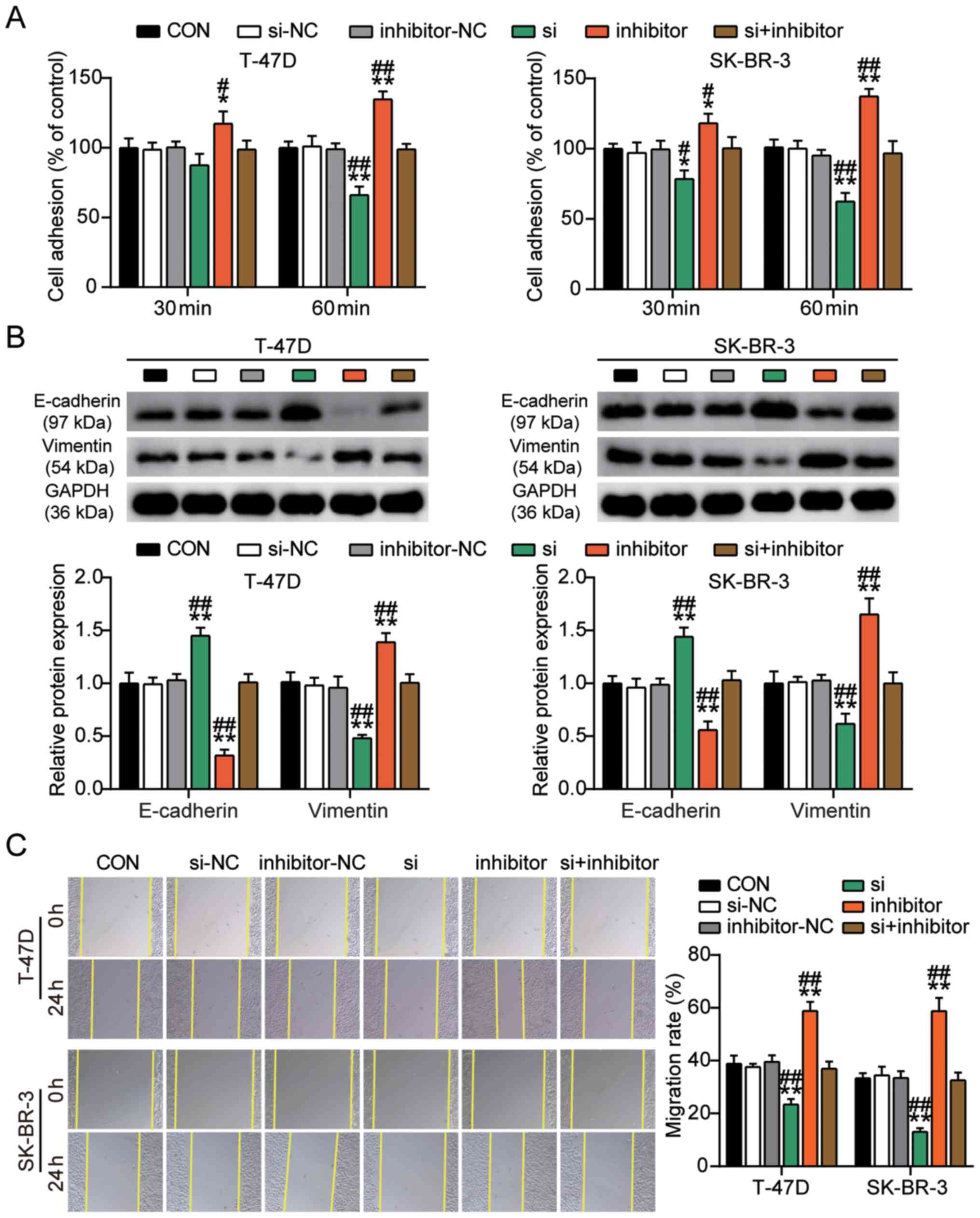
miR-1298-5p inhibitor reversed this effect (Fig. 6A). Western blotting results revealed
that following E2F1 interference, the protein expression levels of
vimentin decreased, whereas the levels of E-cadherin increased
compared with those in the control groups. However, co-transfection
with si-E2F1 and the miR-1298-5p inhibitor reversed the effects of
si-E2F1 on the expression of vimentin and E-cadherin in T-47D and
SK-BR-3 cells (Fig. 6B). In the cell
migration assay, the silencing of E2F1 inhibited cell migration
compared with that in the control group, and this inhibitory effect
was reversed by co-transfection with the miR-1298-5p inhibitor
(Fig. 6C). Taken together, these
results suggested that by targeting E2F1, miR-1298-5p exerted not
only suppressive effects on the proliferation, adhesion and
migration of BC cells, but also promotive effects on BC cell
apoptosis.
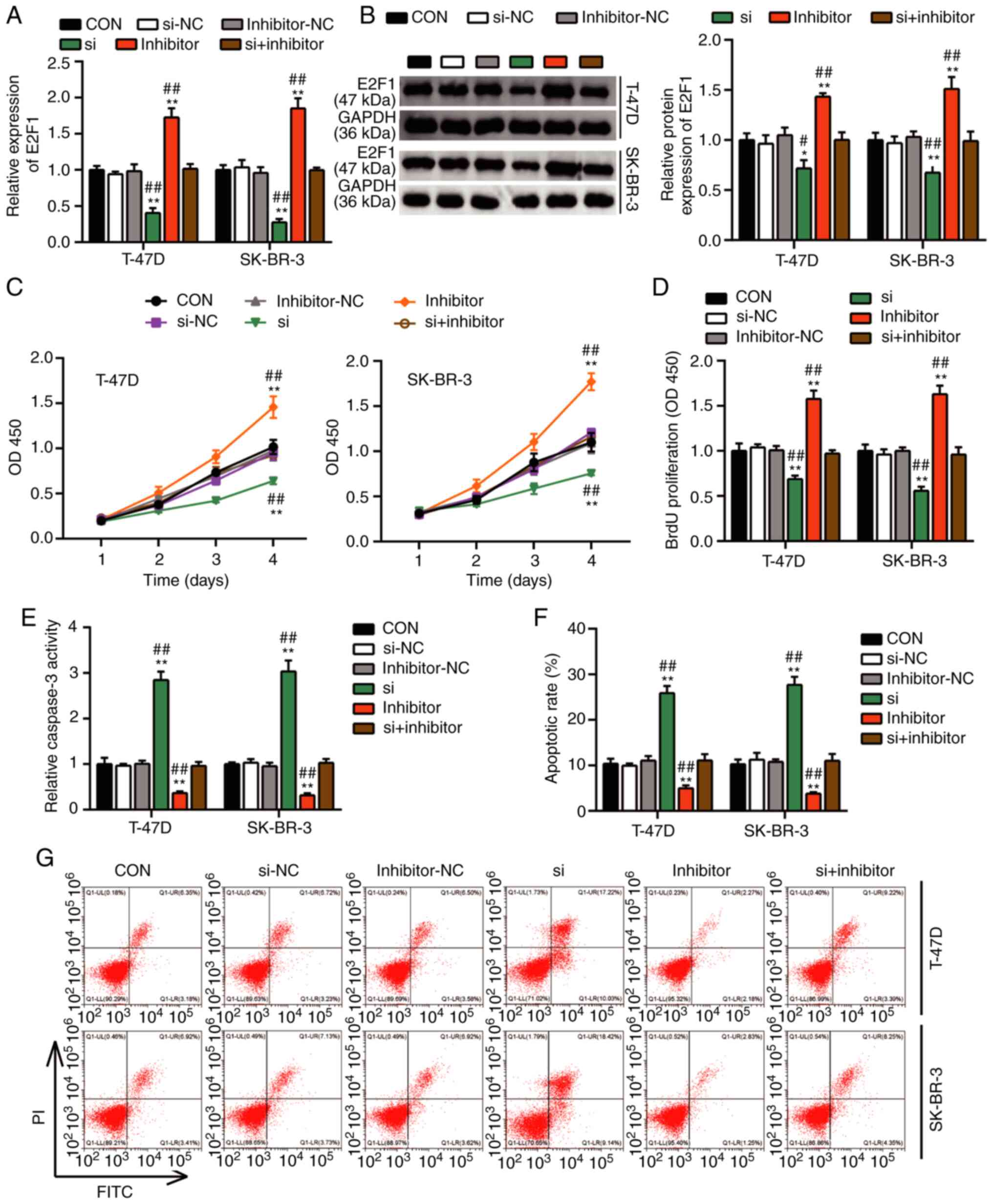 | Figure 5.miR-1298-5p promotes breast cancer
cell proliferation and represses apoptosis by downregulating E2F1.
(A) Reverse transcription-quantitative PCR analysis of E2F1
expression levels in transfected T-47D and SK-BR-3 cell lines. (B)
Western blot analysis of E2F1 protein expression levels in
transfected T-47D and SK-BR-3 cells. (C) The viability of
transfected T-47D and SK-BR-3 cells was analyzed following culture
for 1, 2, 3 and 4 days by Cell Counting Kit-8 assay. (D) BrdU
assay-based proliferation analysis of transfected T-47D and SK-BR-3
cells. (E) Caspase-3 activity assay was performed to evaluate the
apoptotic rate of transfected T-47D and SK-BR-3 cells. (F and G)
The apoptotic rate of transfected T-47D and SK-BR-3 cells was
analyzed by flow cytometry assay. Data are presented as the mean ±
SD and were analyzed by one- or two-way ANOVA. *P<0.05 and
**P<0.001 vs. CON; #P<0.05 and
##P<0.001 vs. si + inhibitor. miR, microRNA; E2F1,
E2F transcription factor 1; CON, blank control; NC, negative
control; si, small interfering RNA targeting E2F1; OD, optical
density; BrdU, 5-bromo-2′-deoxyuridine; PI, propidium iodide. |
Discussion
Numerous studies have reported that miRNAs regulate
the tumorigenesis and metastasis of BC by targeting genes
associated with cancer progression (8,38) such
as miR-106b-5p targeting CNN1 in breast cancer (39), miR-944 targeting MACC1 in colorectal
cancer (40) and miR-548c targeting
Twist in ovarian cancer (41). In
BC, miR-1298 has been demonstrated to inhibit malignant cell
behaviors by targeting ADAM9 (18).
In contrast to the aforementioned study, the results of the present
study demonstrated that miR-1298-5p targeted E2F1 and negatively
affected the proliferation, adhesion, migration and apoptosis of BC
cells. These results may be instrumental in developing a potential
strategy to repress the tumorigenesis of BC. In addition, the
results of the present study revealed that the expression levels of
miR-1298-5p were aberrantly downregulated in BC tissues and cells
compared with those in normal breast tissues and cells,
respectively. This result was in agreement with previous studies
that described the participation of miR-1298-5p in regulating the
progression of gastric, bladder and lung cancer (15,17,42).
Cell proliferation, adhesion, migration and apoptosis are widely
used to determine the tumorigenic and metastatic potential of
cancer cells (43,44). The effects of miR-1298-5p on cell
proliferation, adhesion, migration and apoptosis were evaluated in
the current study, and the results demonstrated that miR-1298-5p
suppressed the tumorigenic capacity of BC. These results were
similar and consistent with previous reports on the effect of
miR-1298-5p in other cancer types (15,17,42).
Thus, the present study validated the tumor-suppressive role of
miR-1298-5p in cancer development.
The present study also aimed to determine the
molecular mechanism underlying the regulation of BC cell behaviors
by miR-1298-5p. Solomon et al (35) predicted the targeting relationship
between miR-1298-5p and E2F1 by bioinformatics analysis in human
myeloma cells, but did not validate this association by functional
cell experiments. Following target scanning and bioinformatics
analysis, the present study identified E2F1 as a potential target
of miR-1298-5p; this was consistent with the aforementioned study.
Notably, the results of the present study demonstrated that E2F1
levels were upregulated in BC tissues compared with those in normal
breast tissues, and they were negatively correlated with the
expression levels of miR-1298-5p in BC. The luciferase activity
assay demonstrated that E2F1 was a direct target of miR-1298-5p in
BC cells. In addition, the results of the functional assays
suggested that by inhibiting E2F1 expression, miR-1298-5p exerted
its repressive role in the proliferation, adhesion and migration of
BC cells and its promotive role in apoptosis. Previous studies have
reported E2F1 as a key regulator in the tumorigenesis of various
types of cancer, including BC (24,29,45).
Both oncogenic and tumor-suppressive functions of E2F1 have been
demonstrated in BC cells (30,31). The
results of the present study revealed that E2F1 may act as an
oncogene and contribute to the pathogenesis of BC.
Despite the aforementioned results, the signaling
pathway downstream of the miR-1298-5p/E2F1 axis that may regulate
the aggressiveness of breast tumors was not explored in the present
study. As a regulator of the cell cycle, E2F1 directly promotes the
transcription of P73, which consecutively activates the
proapoptotic target genes (p53 and CDKN2A) and induces apoptosis in
BC and other types of cancer including cervical cancer,
retinoblastoma and prostate cancer (46–50). The
effects of the miR-1298-5p/E2F1 axis on BC cells were assessed
under in vitro conditions in the current study. Therefore,
in future research, it is pertinent to construct a BC animal model
and verify these results under in vivo conditions. The serum
levels of miR-1298-5p in patients with BC and healthy volunteers
should also be measured to evaluate the potential value of
miR-1298-5p in the diagnosis of BC. Furthermore, long non-coding
RNAs (lncRNAs) have been recently demonstrated to affect BC
progression (51). However, the
lncRNA regulatory mechanism for miR-1298-5p has not been reported.
In the future, the upstream regulation mechanism of miR-1298-5p in
BC needs to be explored.
In conclusion, the results of the present study
suggested that miR-1298-5p may affect BC progression by targeting
E2F1. miR-1298-5p exerted not only suppressive effects on the
proliferation, adhesion and migration of BC cells, but also
promotive effects on BC cell apoptosis by targeting E2F1. These
results may assist in providing new targets for the treatment and
diagnosis of BC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DWH and ZMF designed the experiments, and confirm
the authenticity of all the raw data. JZ and CYH conducted the
experiments and wrote the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Ethic Committee of The First Bethune Hospital of
Jilin University (Jilin, China) approved the study (approval no.
17K038-009). Written informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeSantis CE, Ma J, Gaudet MM, Newman LA,
Miller KD, Goding Sauer A, Jemal A and Siegel RL: Breast cancer
statistics, 2019. CA Cancer J Clin. 69:438–451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harbeck N and Gnant M: Breast cancer.
Lancet. 389:1134–1150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ebert MS and Sharp PA: Roles for microRNAs
in conferring robustness to biological processes. Cell.
149:515–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wozniak M, Mielczarek A and Czyz M: miRNAs
in Melanoma: Tumor suppressors and oncogenes with prognostic
potential. Curr Med Chem. 23:3136–3153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ding L, Gu H, Xiong X, Ao H, Cao J, Lin W,
Yu M, Lin J and Cui Q: MicroRNAs involved in carcinogenesis,
prognosis, therapeutic resistance and applications in human
triple-negative breast cancer. Cells. 8:14922019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu H: MicroRNAs in breast cancer
initiation and progression. Cell Mol Life Sci. 69:3587–3599. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schwarzenbacher D, Klec C, Pasculli B,
Cerk S, Rinner B, Karbiener M, Ivan C, Barbano R, Ling H,
Wulf-Goldenberg A, et al: MiR-1287-5p inhibits triple negative
breast cancer growth by interaction with phosphoinositide 3-kinase
CB, thereby sensitizing cells for PI3Kinase inhibitors. Breast
Cancer Res. 21:202019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ying X, Sun Y and He P: MicroRNA-137
inhibits BMP7 to enhance the epithelial-mesenchymal transition of
breast cancer cells. Oncotarget. 8:18348–18358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu W, Wang M, Yin H, Yao C, He Q, Yin L,
Zhang C, Li W, Chang G and Wang S: MicroRNA-1298 is regulated by
DNA methylation and affects vascular smooth muscle cell function by
targeting connexin 43. Cardiovasc Res. 107:534–545. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu X, Ban Y, Zhao Z, Pan Q and Zou J:
MicroRNA-1298-3p inhibits proliferation and invasion of glioma
cells by downregulating Nidogen-1. Aging (Albany NY). 12:7761–7773.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Du Z, Wu J, Wang J, Liang Y, Zhang S,
Shang Z and Zuo W: MicroRNA-1298 is downregulated in non-small cell
lung cancer and suppresses tumor progression in tumor cells. Diagn
Pathol. 14:1322019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xue G, Lin X, Wu JF, Pei D, Wang DM, Zhang
J and Zhang WJ: Identification of key genes of papillary thyroid
carcinoma by integrated bioinformatics analysis. Biosci Rep.
40:BSR202015552020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qiu ZK, Liu N, Zhao SF, Ding AP, Cheng G,
Qiu WS and Qi WW: MiR-1298 expression correlates with prognosis and
inhibits cell proliferation and invasion of gastric cancer. Eur Rev
Med Pharmacol Sci. 22:1672–1679. 2018.PubMed/NCBI
|
|
18
|
Chen W, Lu Q, Li S, Zhang X and Xue X:
microRNA-1298 inhibits the malignant behaviors of breast cancer
cells via targeting ADAM9. Biosci Rep. 40:BSR202012152020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Attwooll C, Lazzerini Denchi E and Helin
K: The E2F family: Specific functions and overlapping interests.
EMBO J. 23:4709–4716. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
DeGregori J and Johnson DG: Distinct and
overlapping roles for E2F family members in transcription,
proliferation and apoptosis. Curr Mol Med. 6:739–748. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ginsberg D: E2F1 pathways to apoptosis.
FEBS Lett. 529:122–125. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Helin K, Wu CL, Fattaey AR, Lees JA,
Dynlacht BD, Ngwu C and Harlow E: Heterodimerization of the
transcription factors E2F-1 and DP-1 leads to cooperative
trans-activation. Genes Dev. 7:1850–1861. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Strauss M, Lukas J and Bartek J:
Unrestricted cell cycling and cancer. Nat Med. 1:1245–1246. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu TP, Wang YF, Xiong WL, Ma P, Wang WY,
Chen WM, Huang MD, Xia R, Wang R, Zhang EB, et al: E2F1 induces
TINCR transcriptional activity and accelerates gastric cancer
progression via activation of TINCR/STAU1/CDKN2B signaling axis.
Cell Death Dis. 8:e28372017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang T, Chen X, Qiao W, Kong L, Sun D and
Li Z: Transcription factor E2F1 promotes EMT by regulating ZEB2 in
small cell lung cancer. BMC Cancer. 17:7192017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ren Z, Kang W, Wang L, Sun B, Ma J, Zheng
C, Sun J, Tian Z, Yang X and Xiao W: E2F1 renders prostate cancer
cell resistant to ICAM-1 mediated antitumor immunity by NF-κB
modulation. Mol Cancer. 13:842014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fueyo J, Gomez-Manzano C, Yung WK, Liu TJ,
Alemany R, McDonnell TJ, Shi X, Rao JS, Levin VA and Kyritsis AP:
Overexpression of E2F-1 in glioma triggers apoptosis and suppresses
tumor growth in vitro and in vivo. Nat Med. 4:685–690. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kent LN, Bae S, Tsai SY, Tang X,
Srivastava A, Koivisto C, Martin CK, Ridolfi E, Miller GC, Zorko
SM, et al: Dosage-dependent copy number gains in E2f1 and E2f3
drive hepatocellular carcinoma. J Clin Invest. 127:830–842. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Frietze S, Lupien M, Silver PA and Brown
M: CARM1 regulates estrogen-stimulated breast cancer growth through
up-regulation of E2F1. Cancer Res. 68:301–306. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun B, Wingate H, Swisher SG, Keyomarsi K
and Hunt KK: Absence of pRb facilitates E2F1-induced apoptosis in
breast cancer cells. Cell Cycle. 9:1122–1130. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Worku D, Jouhra F, Jiang GW, Patani N,
Newbold RF and Mokbel K: Evidence of a tumour suppressive function
of E2F1 gene in human breast cancer. Anticancer Res. 28:2135–2139.
2008.PubMed/NCBI
|
|
32
|
Lu G, Li Y, Ma Y, Lu J, Chen Y, Jiang Q,
Qin Q, Zhao L, Huang Q, Luo Z, et al: Long noncoding RNA LINC00511
contributes to breast cancer tumourigenesis and stemness by
inducing the miR-185-3p/E2F1/Nanog axis. J Exp Clin Cancer Res.
37:2892018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cataldo A, Cheung DG, Balsari A, Tagliabue
E, Coppola V, Iorio MV, Palmieri D and Croce CM: miR-302b enhances
breast cancer cell sensitivity to cisplatin by regulating E2F1 and
the cellular DNA damage response. Oncotarget. 7:786–797. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao YX, Liu HC, Ying WY, Wang CY, Yu YJ,
Sun WJ and Liu JF: microRNA372 inhibits proliferation and induces
apoptosis in human breast cancer cells by directly targeting E2F1.
Mol Med Rep. 16:8069–8075. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Solomon LA, Podder S, He J,
Jackson-Chornenki NL, Gibson K, Ziliotto RG, Rhee J and DeKoter RP:
Coordination of myeloid differentiation with reduced cell cycle
progression by PU.1 induction of MicroRNAs targeting cell cycle
regulators and lipid anabolism. Mol Cell Biol. 37:e00013–17. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Niknami Z, Muhammadnejad A, Ebrahimi A,
Harsani Z and Shirkoohi R: Significance of E-cadherin and Vimentin
as epithelial-mesenchymal transition markers in colorectal
carcinoma prognosis. EXCLI J. 19:917–926. 2020.PubMed/NCBI
|
|
38
|
Das PK, Siddika MA, Asha SY, Aktar S,
Rakib MA, Khanam JA, Pillai S and Islam F: MicroRNAs, a promising
target for breast cancer stem cells. Mol Diagn Ther. 24:69–83.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Z, Li TE, Chen M, Pan JJ and Shen KW:
miR-106b-5p contributes to the lung metastasis of breast cancer via
targeting CNN1 and regulating Rho/ROCK1 pathway. Aging.
12:1867–1887. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wen L, Li Y, Jiang Z, Zhang Y, Yang B and
Han F: miR-944 inhibits cell migration and invasion by targeting
MACC1 in colorectal cancer. Oncol Rep. 37:3415–3422. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun X, Cui M, Zhang A, Tong L, Wang K, Li
K, Wang X, Sun Z and Zhang H: MiR-548c impairs migration and
invasion of endometrial and ovarian cancer cells via downregulation
of Twist. J Exp Clin Cancer Res. 35:102016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li G, Sun L, Mu Z, Liu S, Qu H, Xie Q and
Hu B: MicroRNA-1298-5p inhibits cell proliferation and the
invasiveness of bladder cancer cells via down-regulation of
connexin 43. Biochem Cell Biol. 98:227–237. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Feliciano A, Garcia-Mayea Y, Jubierre L,
Mir C, Hummel M, Castellvi J, Hernández-Losa J, Paciucci R, Sansano
I, Sun Y, et al: miR-99a reveals two novel oncogenic proteins E2F2
and EMR2 and represses stemness in lung cancer. Cell Death Dis.
8:e31412017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhao W, Cui Y, Liu L, Qi X, Liu J, Ma S,
Hu X, Zhang Z, Wang Y, Li H, et al: Splicing factor derived
circular RNA circUHRF1 accelerates oral squamous cell carcinoma
tumorigenesis via feedback loop. Cell Death Differ. 27:919–933.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen X, Wu Q, Depeille P, Chen P, Thornton
S, Kalirai H, Coupland SE, Roose JP and Bastian BC: RasGRP3
mediates MAPK pathway activation in GNAQ mutant uveal melanoma.
Cancer Cell. 31:685–696.e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hua B, Li Y, Yang X, Niu X, Zhao Y and Zhu
X: MicroRNA-361-3p promotes human breast cancer cell viability by
inhibiting the E2F1/P73 signalling pathway. Biomed Pharmacother.
125:1099942020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Stiewe T and Putzer BM: Role of the
p53-homologue p73 in E2F1-induced apoptosis. Nat Genet. 26:464–469.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Peng X, Zhang Y, Gao J and Cai C: MiR-1258
promotes the apoptosis of cervical cancer cells by regulating the
E2F1/P53 signaling pathway. Exp Mol Pathol. 114:1043682020.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang Z, Liu W, Zhao L, Huang Z, Chen X,
Ma N, Xu J, Zhang W and Zhang Y: Retinoblastoma 1 protects T cell
maturation from premature apoptosis by inhibiting E2F1.
Development. 145:dev1581392018.PubMed/NCBI
|
|
50
|
Udayakumar T, Shareef MM, Diaz DA, Ahmed
MM and Pollack A: The E2F1/Rb and p53/MDM2 pathways in DNA repair
and apoptosis: Understanding the crosstalk to develop novel
strategies for prostate cancer radiotherapy. Semin Radiat Oncol.
20:258–266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xia W, Liu Y, Cheng T, Xu T, Dong M and Hu
X: Correction to: Down-regulated lncRNA SBF2-AS1 inhibits
tumorigenesis and progression of breast cancer by sponging
microRNA-143 and repressing RRS1. J Exp Clin Cancer Res. 39:602020.
View Article : Google Scholar : PubMed/NCBI
|