Introduction
Thyroid cancer is a leading cause of mortality among
endocrine tumors (1). In total,
~562,000 individuals were diagnosed with thyroid cancer worldwide
in 2018 (2). Although significant
advances have been made in the therapeutic strategies used to
improve the long-term survival of patients with thyroid cancer,
~20% of patients with thyroid cancer experience recurrence and
distant metastasis within 10 years (3,4). Since
thyroid cancer usually progresses slowly, it has no obvious
different clinical manifestations from benign thyroid diseases,
which often leads to misdiagnosis and untimely treatment (5). Nowadays, increasing numbers of
molecular biomarkers have been identified as diagnostic and
therapeutic biomarkers in cancer, which can improve the early
detection of cancer and decreased the mortality rate (6,7). For
example, mircroRNA-203 (miR-203) promoted estrogen
receptor-positive breast cancer growth and stemness, and may be
used a therapeutic target for breast cancer (8). In addition, overexpression of c-Fos
enhanced tumor growth in vivo and the stemness in head and
neck squamous cell carcinoma (HNSCC) cells, and this may hold
potential as a cancer stem-like cell-directed therapeutic approach
to improve HNSCC treatment (9).
Therefore, it remains an urgent requirement to determine novel
sensitive and specific biomarkers for the diagnosis, clinical
treatment and prognosis of thyroid cancer.
Long non-coding RNAs (lncRNAs) are >200
nucleotides in length and lack protein coding ability. It has been
widely reported that the dysregulated expression of lncRNA is
associated with multiple biological functions and participates in
the development of multiple cancer types, including thyroid cancer.
For instance, a previous study reported that the upregulated
expression levels of lncRNA X inactive specific transcript (XIST)
accelerated cell proliferation and tumor growth in thyroid cancer
(10). The overexpression of lncRNA
metastasis-associated lung adenocarcinoma transcript 1 contributed
to the angiogenic process of thyroid cancer via the regulation of
fibroblast growth factor 2 secretion (11). Furthermore, lncRNA n340790 promoted
thyroid cancer tumorigenesis by modulating miR-1254 expression
(12). Thus, to determine potential
novel therapeutic treatments for thyroid cancer, it remains
important to identify novel tumor-associated lncRNAs and to
determine their biological role and mechanisms of action.
The lncRNA FTX transcript XIST regulator (FTX),
located in the XIST gene locus, has been reported to act as a tumor
promoter in various types of cancer, including glioma, lung
adenocarcinoma, colorectal cancer, osteosarcoma and gastric cancer,
where it was found to be closely associated with a poor prognosis
(13–17). In addition, the expression levels of
FTX were found to be significantly upregulated in sporadic
medullary thyroid cancer (18).
However, to the best of our knowledge, the clinical
characteristics, biological functions and underlying mechanism of
FTX in thyroid cancer have yet to be determined.
The current study aimed to determine the expression
levels of FTX in thyroid cancer and to elucidate the biological
functions of FTX in thyroid cancer cell proliferation, migration,
invasion and apoptosis. The results of the present study provide
novel evidence of the potential role of FTX in the development and
progression of thyroid cancer, which may provide a novel insight
into future therapeutic directions for patients with thyroid
cancer.
Materials and methods
Patient studies
A total of 58 thyroid cancer and adjacent normal
tissues (3 cm away from the tumor) were obtained from patients
(mean age, 51 years; age range, 41–62 years) during surgery at The
Anhui No. 2 Provincial People's Hospital (Hefei, China) between
March 2015 and January 2017. All patients enrolled in the present
study had not received radiotherapy or chemotherapy prior to
surgery. All samples were stored in liquid nitrogen before use.
Each patient provided written informed consent prior to
participation in the study. The patient experimental protocols were
approved by The Ethical Committee of Anhui No. 2 Provincial
People's Hospital.
Cell lines and culture
Thyroid cancer cell lines (FTC-236, SW-1736 and
8305C) and a normal human thyroid epithelial cell line
(Nthy-ori3-1) were purchased from The Cell Bank of Type Culture
Collection of The Chinese Academy of Sciences, and tested for
mycoplasma and authenticated by STR profiling. Cells were cultured
in DMEM supplemented with 10% FBS (both Gibco; Thermo Fisher
Scientific, Inc.), and maintained at 37°C in a humidified
atmosphere containing 5% CO2.
Cell transfection
Short hairpin (sh)RNAs targeting FTX (shRNA1,
5′-GCUGAUCUGUGAGCUAGCUCU-3′; shRNA2, 5′-GUGAGCUUGUACUGUUACAUC-3′;
and shRNA3, 5′-GGCUUGUUCUGCUAGAUCUGU-3′), and negative control (NC)
scrambled shRNA (shNC; 5′-UGUGAGAUGCAGCCUCUAC-3′) were inserted
into pGPU6/Neo plasmids (Shanghai GenePharma Co., Ltd.).
pcDNA3.1-FTX (oe-FTX) and control pcDNA (vector) were also
purchased from Shanghai GenePharma Co., Ltd.. Cells were
transfected with 10 nM plasmids using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.). The transfection
efficiency was analyzed using reverse transcription-quantitative
PCR (RT-qPCR) after 48 h of transfection.
RT-qPCR
Total RNA was extracted from tissues, thyroid cancer
cell lines (FTC-236, SW-1736 and 8305C) and a normal human thyroid
epithelial cell line (Nthy-ori3-1) using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was reverse
transcribed into cDNA using a PrimeScript RT reagent kit (Takara
Bio, Inc.) according to the manufacturer's instructions. qPCR was
subsequently performed using SYBR® Premix Ex Taq™
reagent (Takara Bio, Inc.). The following primer sequences were
used for the qPCR: FTX forward, 5′-GTGTCTCTCTCTCTCTCTCTCTT-3′ and
reverse, 5′-CCTCTTCAGCAGTAGCATAGTT-3′; TGF-β1 forward,
5′-GGACATCAACGGGTTCACTA-3′ and reverse, 5′-GCCATGAGAAGCAGGAAAG-3′;
and GAPDH forward, 5′-ATTCCATGGCACCGTCAAGGCTGA-3′ and reverse,
5′-TTCTCCATGGTGGTGAAGACGCCA-3′. The thermocycling conditions were
as follows: Pre-denaturation at 95°C for 1 min, followed by 40
cycles of 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec. The
expression levels were quantified using the 2−ΔΔCq
method (19) and normalized to GAPDH
expression levels.
Cell counting kit-8 (CCK-8) assay
Cell proliferation was examined using a Cell
Counting kit-8 (CCK-8) assay (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. The transfected cells
were seeded into 96-well plates at a density of 1×104
cells/well and cultured at 37°C with 5% CO2 for 0, 24,
48 or 72 h. Following the incubation, 10 µl CCK-8 reagent (Dojindo
Molecular Laboratories, Inc.) was added/well. The absorbance of
each well was measured at a wavelength of 450 nm using a microplate
reader (Olympus Corporation).
Cell migration and invasion
assays
Transwell chambers (8.0-µm pore size; EMD Millipore)
precoated with Matrigel (invasion) or without Matrigel (migration)
were used. A total of 5×104 transfected cells were
plated into the upper chambers of Transwell plates in serum-free
DMEM (Gibco; Thermo Fisher Scientific, Inc.), while DMEM
supplemented with 20% FBS (Gibco; Thermo Fisher Scientific, Inc.)
was added into the lower chambers. Following 24 h of incubation,
cells were fixed with 4% paraformaldehyde for 20 min at room
temperature and stained with 0.1% crystal violet for 20 min at room
temperature. The migratory and invasive cells were counted in five
randomly selected fields of view using a light microscope
(magnification, ×200; Zeiss GmbH). All experiments were performed 3
times.
TUNEL assay
A TUNEL assay was used to analyze cell apoptosis.
Briefly, cells were fixed with 4% paraformaldehyde for 1 h at 4°C
and permeabilized with 0.1% Triton X-100 following treatment. TUNEL
assay solution (Invitrogen; Thermo Fisher Scientific, Inc.) was
subsequently incubated with the cells for 1 h at 37°C. Next, the
TUNEL-stained cells were counterstained with DAPI (2 µg/ml;
Beyotime Institute of Biotechnology) under antifade mounting medium
for 15 min at room temperature. Images were acquired from five
randomly selected fields of view using a fluorescence microscope
(magnification, ×200). TUNEL-positive cells were counted using a
cell counter (BD Biosciences).
Cell cycle distribution assay
A total of 1×104 transfected FTC-236 and
8305C cells were fixed with 75% ethanol at 4°C for 12 h and washed
with PBS twice. Cells were subsequently resuspended in staining
buffer containing 450 µl PI and 50 µl RNaseA in the dark for 30 min
at room temperature. Cell cycle distribution was analyzed using a
FACSCalibur flow cytometer (BD Biosciences). Cell cycle analysis
was conducted using ModFit 2.0 software (BD Biosciences).
Xenograft tumor model
A total of 6 male nude mice (age, 4–6 weeks; weight,
~20 g) were obtained for the xenograft assays. The mice were
maintained under specific pathogen-free conditions, with free
access to water and food, at a room temperature of 26–28°C,
humidity of 60±10% and under a 12-h light/dark cycle. A total of
100 µl PBS containing FTC-236 cells (5×106) transfected
with shNC or shRNA1 was injected into the back of each mouse. The
volume of the xenograft tumors was measured every 7 days. After 28
days, all mice were euthanized by cervical dislocation after deep
anesthesia with isoflurane (2% for induction and maintenance;
Baxter Healthcare Corporation), and the xenograft tumors were
excised and weighed. The tumor volume was calculated using the
following formula: V=1/2 × L × W2. The maximum tumor
diameter was 15 mm. Animal experimental protocols were approved by
the Animal Welfare Committee of Anhui No. 2 Provincial People's
Hospital, and all experimental procedures were performed according
to the guidelines from the National Institutes of Health.
Immunohistochemistry (IHC)
The tissue expression of TGF-β1 was evaluated via
IHC based on the intensity and the proportion of positively stained
cells, as previously described (19). Tumor tissues were fixed with 10%
paraformaldehyde at room temperature for 12 h, embedded in
paraffin, and sliced into 4-µm thick sections. Sections were
blocked with immunol staining blocking buffer (cat. no. P0102;
Beyotime Institute of Biotechnology) at room temperature for 1 h
and then incubated with primary antibody against TGF-β1 (1:1,000;
cat. no. ab215715; Abcam) overnight at 4°C, followed by incubation
with secondary antibody (1:2,000; cat. no. ab205718; Abcam) at room
temperature for 20 min. The sections were stained with
3,3′-diaminobenzidine and counterstained with hematoxylin for 5 min
at room temperature. Images were captured under a light microscope
(Olympus Corporation; magnification, ×200).
Western blotting
Total protein was extracted from thyroid cancer
cells (TC-236 and 8305C) using RIPA lysis buffer (Beyotime
Institute of Biotechnology) at 48 h post transfection. Protein
concentrations were determined using a BCA protein assay kit
(Beyotime Institute of Biotechnology). The protein (10 µg/lane) was
quantified and separated on a 10% gel via SDS-PAGE. The separated
proteins were subsequently transferred onto polyvinylidene fluoride
membranes (Bio-Rad Laboratories, Inc.) and blocked with 5% skimmed
milk for 2 h at room temperature. The membranes were then incubated
with the following primary antibodies overnight at 4°C: Anti-Bcl-2
(1:1,000; cat. no. ab32124), anti-Bax (1:1,000; cat. no. ab32503),
anti-TGF-β1 (1:1,000; cat. no. ab215715) and anti-GAPDH (1:1,000;
cat. no. ab8245) (all Abcam). Following the primary antibody
incubation, the membranes were incubated with horseradish
peroxidase-conjugated secondary antibody (1:1,000; cat. nos.
ab205719 and ab205718; Abcam) for 1 h at room temperature. Protein
bands were visualized using an ECL reagent (Cytiva).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 6.0 (GraphPad Software, Inc.). Data are presented as the mean
± SD of three independent experiments. A χ2 test was
used to determine the association between the expression levels of
FTX and the clinicopathological features of the patients.
Statistical differences between thyroid cancer and adjacent normal
tissues were analyzed using a paired Student's t-test, while the
differences between experiment and control groups were analyzed
using an unpaired Student's t-test. A one-way ANOVA followed by
Tukey's post hoc test was used to determine statistical differences
among multiple groups. The overall survival rate was analyzed using
the Kaplan-Meier method and a log-rank test. The patients were
divided into high and low expression groups based on the mean value
of FTX expression in patients with thyroid cancer. P<0.05 was
considered to indicate a statistically significant difference.
Results
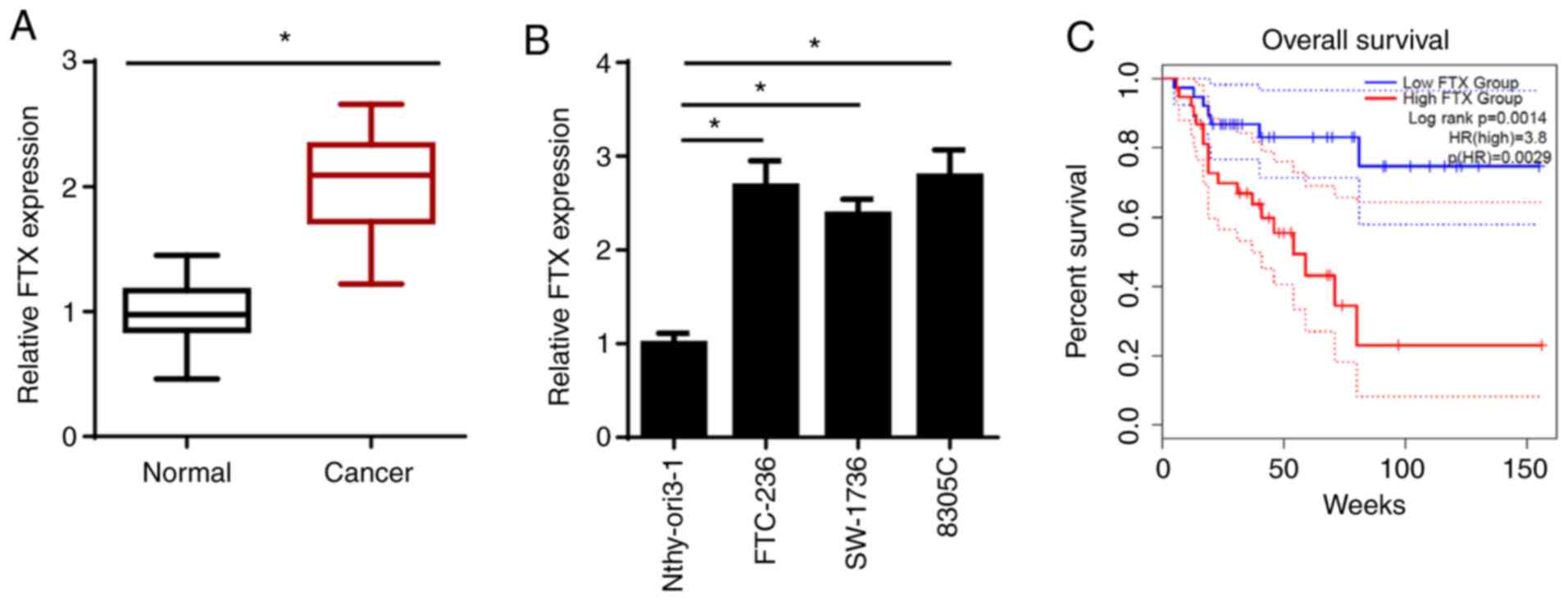
FTX expression levels are upregulated
in thyroid cancer tissues and cell lines, and upregulated FTX
expression is associated with an unfavorable prognosis
To determine the biological functions of FTX, the
expression levels of FTX were analyzed in thyroid cancer and
adjacent normal tissues using RT-qPCR. As shown in Fig. 1A, FTX expression levels were
significantly upregulated in thyroid cancer tissues compared with
those in adjacent normal tissues. Similarly, the expression levels
of FTX were significantly upregulated in thyroid cancer cell lines
(FTC-236; differentiated thyroid carcinoma, SW-1736; anaplastic
thyroid carcinoma, and 8305C; anaplastic thyroid carcinoma)
compared with those in the normal human thyroid epithelial cell
line, Nthy-ori3-1. FTC-236 and 8305C cell lines were chosen for use
in subsequent experiments, as FTX expression levels were the
highest in these two cell lines (Fig.
1B). To determine the association between FTX expression levels
and the prognosis of patients with thyroid cancer, Kaplan-Meier
analysis was performed. As shown in Fig.
1C, patients with thyroid cancer and high FTX expression levels
exhibited a lower overall survival rate compared with those
patients with low expression levels. In addition, FTX expression
levels were associated with the clinical stage and lymph node
metastasis, but not with sex, age, pathological type or tumor size
(Table I). These results suggested
that FTX expression levels may be upregulated in thyroid cancer and
associated with a poor prognosis in patients with this disease.
 | Table I.Clinical characteristics and FTX
expression of patients with thyroid cancer. |
Table I.
Clinical characteristics and FTX
expression of patients with thyroid cancer.
|
| FTX expression |
|
|---|
|
|
|
|
|---|
| Variable | Low, n | High, n | P-value |
|---|
| All cases | 23 | 35 |
|
| Age, years |
|
|
|
|
<50 | 12 | 17 | >0.05 |
| ≥50 | 11 | 18 |
|
| Sex |
|
|
|
|
Male | 10 | 15 | >0.05 |
|
Female | 13 | 20 |
|
| Pathological
type |
|
|
|
|
Papillary adenocarcinoma | 9 | 11 | >0.05 |
|
Follicular adenocarcinoma | 14 | 24 |
|
| Lymph node
metastasis |
|
|
|
|
Yes | 18 | 23 | <0.05 |
| No | 5 | 12 |
|
| Tumor size, cm |
|
|
|
|
<3 | 11 | 16 | >0.05 |
| ≥3 | 12 | 19 |
|
| Tumor stage |
|
|
|
|
I/II | 13 | 10 | <0.05 |
|
III/IV | 10 | 25 |
|
Knockdown of FTX inhibits
proliferation and migration, and induces apoptosis in thyroid
cancer cells
To determine the biological functions of FTX in
thyroid cancer cells, FTX expression levels were knocked down in
FTC-236 and 8305C cells using three shRNAs. Due to the greatest
knockdown efficiency, shRNA1 and shRNA3 were selected for use in
subsequent experiments (Fig. 2A).
The proliferation rates of FTC-236 and 8305C cells transfected with
shRNA1 and shRNA3 were analyzed using a CCK-8 assay; the results
revealed that FTX knockdown significantly decreased cell
proliferation in the thyroid cancer cells (Fig. 2B). In addition, the results of the
Transwell assays demonstrated that the knockdown of FTX attenuated
the migratory and invasive abilities of the thyroid cancer cells
(Fig. 2C and D). Moreover, according
to the results of the TUNEL assay, FTX knockdown also alleviated
the apoptosis of the thyroid cancer cells (Fig. 2E). Western blot analysis revealed
that the knockdown of FTX upregulated the protein expression levels
of Bax and downregulated Bcl-2 expression levels in the thyroid
cancer cells (Fig. 2F). In addition,
flow cytometric analysis found that FTX knockdown initiated cell
cycle arrest in the G0/G1 phase (Fig. 2G). These data indicated that FTX
knockdown may repress the progression of thyroid cancer.
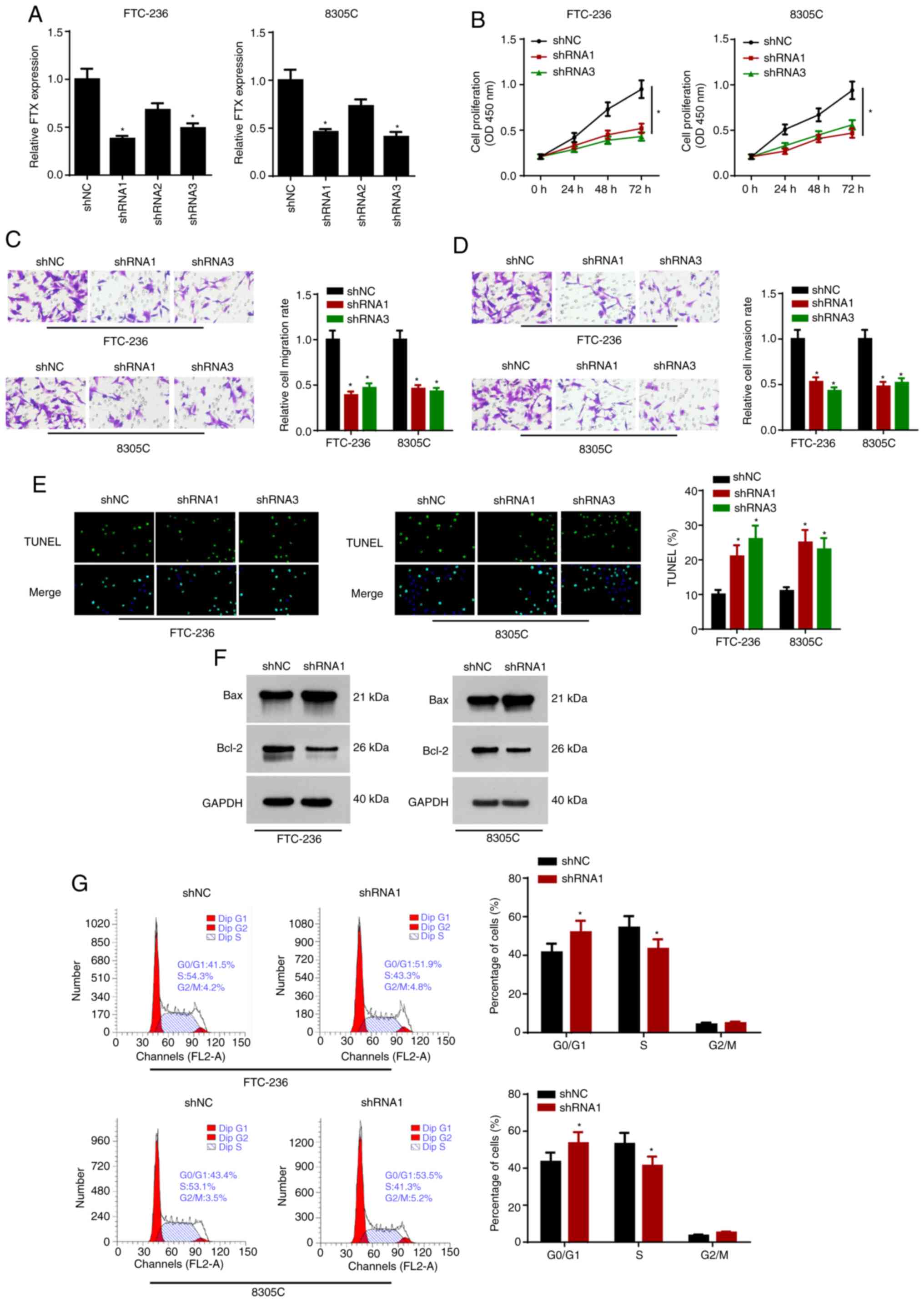 | Figure 2.FTX depletion inhibits proliferative
and metastatic capacities, and induces apoptosis of thyroid cancer
cells. FTC-236 and 8305C cells were transfected with shNC, shRNA1,
shRNA2 and shRNA3, respectively. (A) The transfection efficacy of
shNC, shRNA1, shRNA2 and shRNA3 was detected by reverse
transcription-quantitative PCR. (B) Proliferation of FTC-236 and
8305C cells was measured by CCK-8 assay at 0, 24, 48 and 72 h. (C
and D) Transwell assay exhibited the migration and invasion of
FTC-236 and 8305C cells (magnification, ×200). (E) Apoptosis of
FTC-236 and 8305C cells was analyzed by TUNEL assay (magnification,
×200). (F) Protein expression levels of Bax and Bcl-2 in thyroid
cancer cells transfected with sh-NC or shRNA1 were detected by
western blot analysis. (G) The cell cycle of transfected FTC-236
and 8305C cells was assessed by flow cytometry analysis. *P<0.05
vs. shNC. FTX, FTX transcript X inactive specific transcript
regulator; sh, short hairpin; NC, negative control. |
Overexpression of FTX accelerates
proliferation, migration and invasion, and alleviates apoptosis in
thyroid cancer cells
The effect of the overexpression of FTX on thyroid
cancer cells was investigated by transfecting FTC-236 and 8305C
cells with vector or oe-FTX, and the transfection efficiency was
verified using RT-qPCR (Fig. 3A).
The results of the cell proliferation assay showed that FTX
overexpression promoted the proliferation of the thyroid cancer
cells (Fig. 3B). In addition, the
results of the Transwell assays demonstrated that the
overexpression of FTX expression markedly enhanced the migration
and invasion of the thyroid cancer cells (Fig. 3C and D). Furthermore, the
overexpression of FTX attenuated the apoptosis of thyroid cancer
cells (Fig. 3E). In addition, the
overexpression of FTX also significantly increased cell
proliferation, migration and invasion, but inhibited the apoptosis
of Nthy-ori3-1 cells (Fig. 3F-I).
These results indicated that the overexpression of FTX may promote
the proliferation, migration and invasion, but attenuate the
apoptosis of thyroid cancer cells.
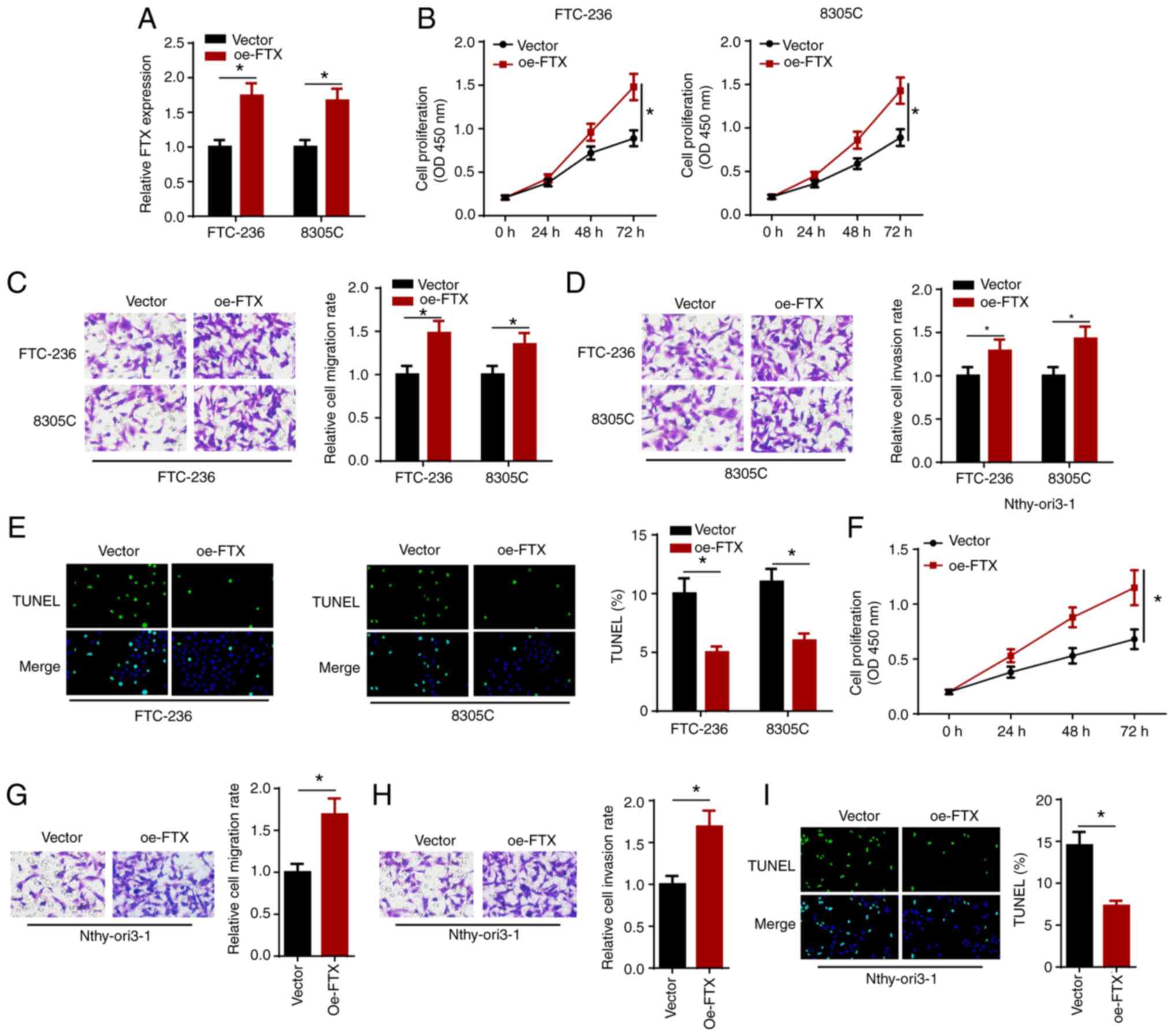 | Figure 3.FTX overexpression accelerates
proliferation, migration and invasion, and alleviates apoptosis of
thyroid cancer cells. FTC-236 and 8305C cells were transfected with
vector and oe-FTX, respectively. (A) The transfection efficacy of
vector and oe-FTX was detected by reverse
transcription-quantitative PCR. (B) Viability of FTC-236 and 8305C
cells was measured by CCK-8 assay at 0, 24, 48 and 72 h. (C and D)
Transwell assay exhibited the migration and invasion of FTC-236 and
8305C cells (magnification, ×200). (E) Apoptosis of FTC-236 and
8305C cells was analyzed by TUNEL assay (magnification, ×200).
(F-I) The cell viability, migration, invasion and apoptosis
(magnification, ×200) of Nthy-ori3-1 cells transfected with FTX
overexpression plasmid were analyzed by functional assays.
*P<0.05. FTX, FTX transcript X inactive specific transcript
regulator; oe, overexpression. |
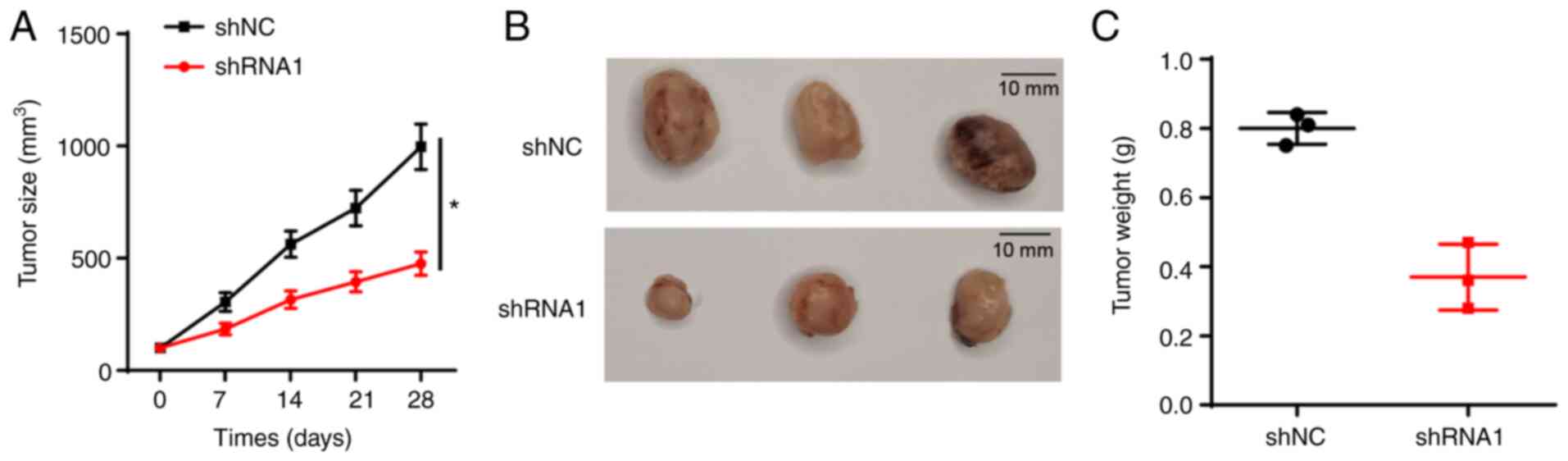
Knockdown of FTX represses thyroid
cancer growth in vivo
Due to the observed inhibitory effect of FTX
knockdown on thyroid cancer cell proliferation, the effect of FTX
knockdown on tumor growth in vivo was further analyzed using
xenograft models. The data indicated that xenograft tumors derived
from FTC-236 cells stably transfected with shRNA1 grew
significantly more slowly compared with those of the shNC group
(Fig. 4A), and the tumor volume and
weight were markedly decreased in the shRNA1 group compared with
the shNC group (Fig. 4B and C).
These findings suggested that the knockdown of FTX may suppress the
growth of thyroid cancer in vivo.
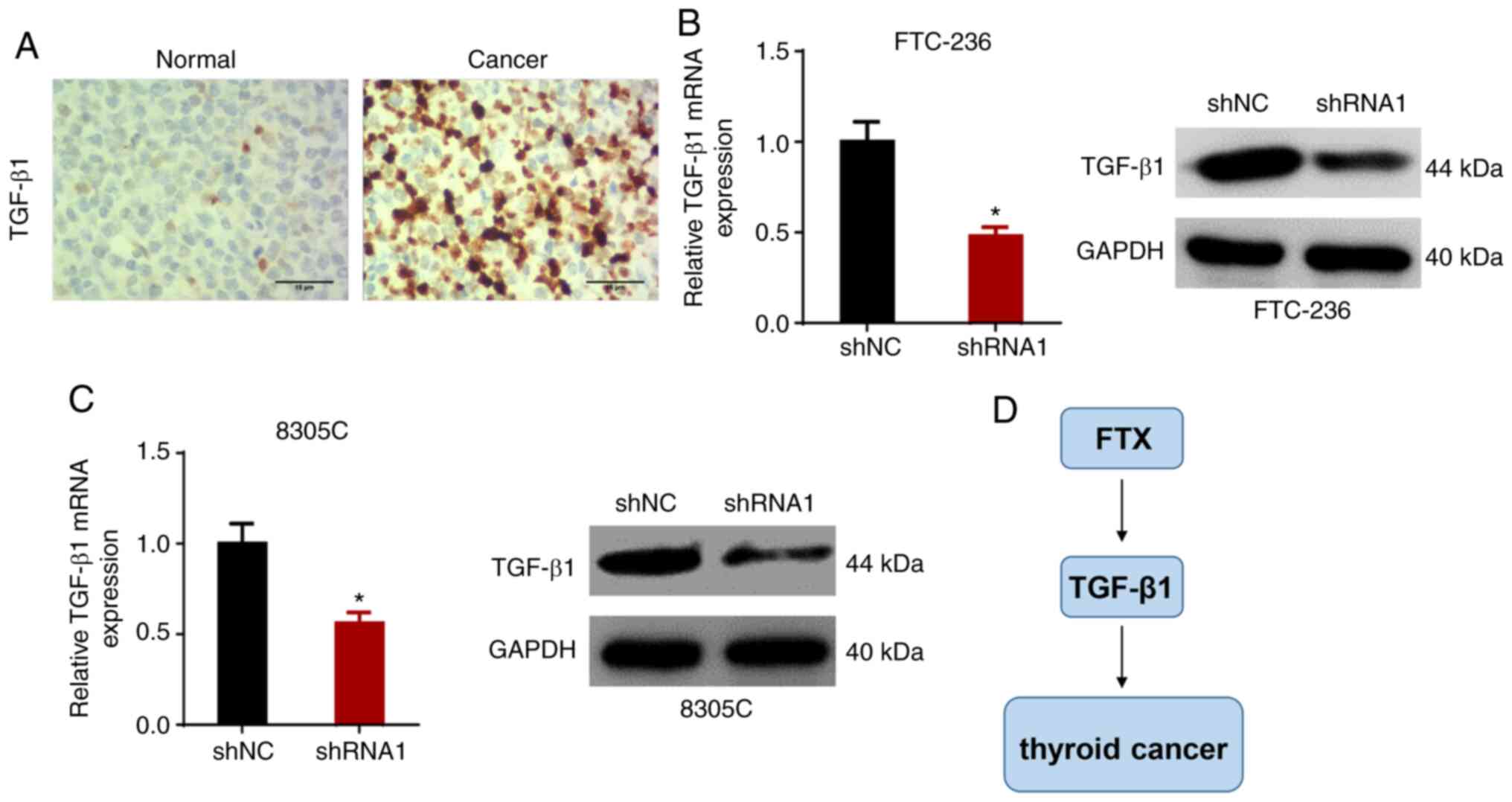
FTX positively regulates TGF-β1
expression levels in thyroid cancer cells
It was previously reported that the TGF-β signaling
pathway played an important role in promoting tumor metastasis
(20,21). Moreover, IHC showed that the
expression of TGF-β1 in thyroid cancer tissues was higher compared
with that in adjacent normal tissues (Fig. 5A). Therefore, in the present study,
it was hypothesized that FTX may promote the development of thyroid
cancer by modulating TGF-β1 expression. To validate this
hypothesis, the expression levels of TGF-β1 in FTC-236 and 8305C
cells transfected with shNC or shRNA1 were analyzed. As shown in
Fig. 5B and C, RT-qPCR and western
blot analysis revealed that the knockdown of FTX downregulated the
expression levels of TGF-β1. These results suggested that FTX may
positively regulate TGF-β1 expression levels in thyroid cancer
cells (Fig. 5D).
Discussion
Although significant advances have been made in the
screening, diagnosis and treatment of thyroid cancer, the majority
of patients with thyroid cancer have an undesirable prognosis.
Numerous previous studies have attempted to further understand the
underlying molecular mechanisms of thyroid cancer; however, to the
best of our knowledge, the pathogenesis of thyroid cancer remains
unclear.
The dysregulated expression of lncRNAs has been
reported to be involved in multiple cellular activities, including
oncogenesis (22,23). In addition, a large number of lncRNAs
have been recognized as potential sensitive biomarkers for thyroid
cancer in the clinic. For example, lncRNA ST binding factor
2-antisense RNA 1 (AS1) accelerated the growth of papillary thyroid
cancer by modulating the miR-431-5p/CDK14 axis (24). The overexpression of opa interacting
protein 5-AS1 promoted thyroid cancer progression and predicted an
unfavorable prognosis (25). In
addition, lncRNA aspartyl-tRNA synthetase 1-AS1 contributed to
thyroid cancer development by interacting with miR-129 (26). Therefore, it is has been widely
suggested that lncRNAs may function as biomarkers for thyroid
cancer diagnosis and prognosis, and may represent novel targets for
thyroid cancer treatment.
FTX has been reported to play a role in various
types of cancer. In lung adenocarcinoma, the expression levels of
FTX were upregulated and promoted the progression of the cell lines
(27). In addition, FTX targeted
miR-342-3p in glioma to accelerate tumor growth and metastasis
(28). Another previous study
reported that the knockdown of FTX suppressed the progression of
renal cell carcinoma (29).
Moreover, FTX overexpression promoted tumorigenesis in osteosarcoma
by targeting the miR-214-5p/SOX4 signaling axis (30). Recently, Luzón-Toro et al
(18) reported that FTX was
significantly upregulated in sporadic medullary thyroid cancer. The
findings of the present study revealed that the expression levels
of FTX were upregulated in thyroid cancer tissues and cells, and
high FTX expression was associated with lymph node metastasis and
clinical stage. Moreover, the upregulated expression levels of FTX
favored a poor prognosis in patients with thyroid cancer and serum
FTX had a relatively high diagnostic value. Results of the
functional analysis demonstrated that the knockdown of FTX
significantly inhibited proliferation, invasion, migration and cell
cycle progression, and induced apoptosis in thyroid cancer cells.
In addition, the suppressive effects of FTX knockdown on thyroid
cancer progression were also confirmed using an in vivo
xenograft tumor assay. These results indicated that FTX may promote
the progression of thyroid cancer and may represent a novel
biomarker for the treatment and prognosis of thyroid cancer.
An increasing number of studies have reported that
TGF-β1 contributes to the progression of various cancer types,
including thyroid cancer. For example, forkhead box D3-AS1 promoted
the aggressive biological behaviors of thyroid cancer by activating
the TGF-β1/Smads signaling pathway (31). In addition, miR-483 targeted par-3
family cell polarity factor to enhance TGF-β1-induced migration and
invasion in thyroid cancer cells (32). To determine whether FTX exerted its
biological functions by regulating TGF-β1 in the present study,
RT-qPCR and western blotting were performed; the results revealed
that the knockdown of FTX downregulated the expression levels of
TGF-β1. These data indicated that FTX may regulate thyroid cancer
progression via TGF-β1.
However, there are several limitations to the
present study. First, the study only investigated the effect of FTX
on thyroid cancer. Therefore, the downstream targets or signaling
pathways of FTX should be further investigated in future studies.
Second, only a small number of mice were used for the in
vivo studies. Therefore, an increased number of mice should be
used in the in vivo xenograft model studies in the future to
improve the reliability of the experiments.
In conclusion, the findings of the present study
suggested that FTX may exert an important role in thyroid cancer
progression; therefore, FTX may represent a novel biomarker for the
diagnosis, treatment and prognosis of thyroid cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PW and HJ designed the present study. YZ and WW
performed the experiments. PW, YZ and WW analyzed the data and
prepared the figures. PW and HJ drafted the initial manuscript,
reviewed and revised the manuscript. All authors have read and
approved the manuscript. PW and HJ confirm the authenticity of all
the raw data.
Ethics approval and consent to
participate
Each patient provided written informed consent prior
to participation in the study. The present study was approved by
the Ethical Committee of Anhui No. 2 Provincial People's
Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Segev DL, Umbricht C and Zeiger MA:
Molecular pathogenesis of thyroid cancer. Surg Oncol. 12:69–90.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Siegel RL,
Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sipos JA and Mazzaferri EL: Thyroid cancer
epidemiology and prognostic variables. Clin Oncol (R Coll Radiol).
22:395–404. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Janovitz T and Barletta JA: Clinically
relevant prognostic parameters in differentiated thyroid carcinoma.
Endocr Pathol. 29:357–364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thompson LD, Wieneke JA, Paal E, Frommelt
RA, Adair CF and Heffess CS: A clinicopathologic study of minimally
invasive follicular carcinoma of the thyroid gland with a review of
the English literature. Cancer. 91:505–24. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Federico C, Sun J, Muz B, Alhallak K,
Cosper PF, Muhammad N, Jeske A, Hinger A, Markovina S, Grigsby P,
et al: Localized Delivery of Cisplatin to Cervical cancer improves
its therapeutic efficacy and minimizes its side effect profile. Int
J Radiat Oncol Biol Phys. 109:1483–1494. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mohammad N, Singh SV, Malvi P, Chaube B,
Athavale D, Vanuopadath M, Nair SS, Nair B and Bhat MK: Strategy to
enhance efficacy of doxorubicin in solid tumor cells by
methyl-β-cyclodextrin: Involvement of p53 and Fas receptor ligand
complex. Sci Rep. 5:118532015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Muhammad N, Bhattacharya S, Steele R and
Ray RB: Anti-miR-203 suppresses ER-positive breast cancer growth
and stemness by targeting SOCS3. Oncotarget. 7:58595–58605. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Muhammad N, Bhattacharya S, Steele R,
Phillips N and Ray RB: Involvement of c-Fos in the promotion of
cancer stem-like cell properties in head and neck squamous cell
carcinoma. Clin Cancer Res. 23:3120–3128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu H, Deng H, Zhao Y, Li C and Liang Y:
LncRNA XIST/miR-34a axis modulates the cell proliferation and tumor
growth of thyroid cancer through MET-PI3K-AKT signaling. J Exp Clin
Cancer Res. 37:2792018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang JK, Ma L, Song WH, Lu BY, Huang YB,
Dong HM, Ma XK, Zhu ZZ and Zhou R: LncRNA-MALAT1 promotes
angiogenesis of thyroid cancer by modulating tumor-associated
macrophage FGF2 protein secretion. J Cell Biochem. 118:4821–4830.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qin Y, Xue B, Liu C, Wang X, Tian R, Xie
Q, Guo M, Li G, Yang D and Zhu H: NLRX1 mediates MAVS degradation
to attenuate the hepatitis C virus-induced innate immune response
through PCBP2. J Virol. 91:e01264–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li B, Ren P and Wang Z: Long non-coding
RNA Ftx promotes osteosarcoma progression via the epithelial to
mesenchymal transition mechanism and is associated with poor
prognosis in patients with osteosarcoma. Int J Clin Exp Pathol.
11:4503–4511. 2018.PubMed/NCBI
|
|
14
|
Zhang F, Wang XS, Tang B, Li PA, Wen Y and
Yu PW: Long non-coding RNA FTX promotes gastric cancer progression
by targeting miR-215. Eur Rev Med Pharmacol Sci. 24:3037–3048.
2020.PubMed/NCBI
|
|
15
|
Yang Y, Zhang J, Chen X, Xu X, Cao G, Li H
and Wu T: LncRNA FTX sponges miR-215 and inhibits phosphorylation
of vimentin for promoting colorectal cancer progression. Gene Ther.
25:321–330. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liang Y and Lu H: Long noncoding RNA FTX
is associated with prognosis of glioma patients. J Gene Med.
22:e32372020. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang W, Zhang B, Sun J, Liu Y, Bi Y and
Wei H: LncRNA FTX promotes the tumorigenesis of lung adenocarcinoma
by targeting miR-300. Panminerva Med. Jan 24–2020.(Epub ahead of
print). doi: 10.23736/S0031-0808.19.03823-0.
|
|
18
|
Luzón-Toro B, Villalba-Benito L, Fernández
RM, Torroglosa A, Antiñolo G and Borrego S: RMRP, RMST, FTX and
IPW: Novel potential long non-coding RNAs in medullary thyroid
cancer. Orphanet J Rare Dis. 16:42021. View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun DY, Wu JQ, He ZH, He MF and Sun HB:
Cancer-associated fibroblast regulate proliferation and migration
of prostate cancer cells through TGF-β signaling pathway. Life Sci.
235:1167912019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dai G, Sun B, Gong T, Pan Z, Meng Q and Ju
W: Ginsenoside Rb2 inhibits epithelial-mesenchymal transition of
colorectal cancer cells by suppressing TGF-β/Smad signaling.
Phytomedicine. 56:126–135. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choudhari R, Sedano MJ, Harrison AL,
Subramani R, Lin KY, Ramos EI, Lakshmanaswamy R and Gadad SS: Long
noncoding RNAs in cancer: From discovery to therapeutic targets.
Adv Clin Chem. 95:105–147. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chan JJ and Tay Y: Noncoding RNA:RNA
regulatory networks in cancer. Int J Mol Sci. 19:13102018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wen HL, Xu ZM, Wen D, Lin SY, Liang Y and
Xie JP: Long noncoding RNAs SET-binding factor 2-antisense RNA1
promotes cell growth through targeting miR-431-5p/CDK14 axis in
human papillary thyroid cancer. Kaohsiung J Med Sci. 36:808–816.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Q, Chen W, Luo R, Zhang Z, Song M, Chen
W, Yang Z, Yang Y, Guo Z and Yang A: Upregulation of OIP5-AS1
predicts poor prognosis and contributes to thyroid cancer cell
proliferation and migration. Mol Ther Nucleic Acids. 20:279–291.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng W, Tian X, Cai L, Shen YM, Cao QS,
Yang JY and Tian GY: LncRNA DARS-AS1 regulates microRNA-129 to
promote malignant progression of thyroid cancer. Eur Rev Med
Pharmacol Sci. 23:10443–10452. 2019.PubMed/NCBI
|
|
27
|
Huo X and Wang H, Huo B, Wang L, Yang K,
Wang J, Wang L and Wang H: FTX contributes to cell proliferation
and migration in lung adenocarcinoma via targeting miR-335-5p/NUCB2
axis. Cancer Cell Int. 20:892020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang W, Bi Y, Li J, Peng F, Li H, Li C,
Wang L, Ren F, Xie C, Wang P, et al: Long noncoding RNA FTX is
upregulated in gliomas and promotes proliferation and invasion of
glioma cells by negatively regulating miR-342-3p. Lab Invest.
97:447–457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He X, Sun F, Guo F, Wang K, Gao Y, Feng Y,
Song B, Li W and Li Y: Knockdown of long noncoding RNA FTX inhibits
proliferation, migration, and invasion in renal cell carcinoma
cells. Oncol Res. 25:157–166. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen H, Liu T, Ouyang H, Lin S, Zhong H,
Zhang H and Yang Y: Upregulation of FTX promotes osteosarcoma
tumorigenesis by increasing SOX4 expression via miR-214-5p. Onco
Targets Ther. 13:7125–7136. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen Y, Gao H and Li Y: Inhibition of
LncRNA FOXD3-AS1 suppresses the aggressive biological behaviors of
thyroid cancer via elevating miR-296-5p and inactivating
TGF-β1/Smads signaling pathway. Mol Cell Endocrinol.
500:1106342020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang X, Liu L, Deng X, Li D, Cai H, Ma Y,
Jia C, Wu B, Fan Y and Lv Z: MicroRNA 483-3p targets Pard3 to
potentiate TGF-β1-induced cell migration, invasion, and
epithelial-mesenchymal transition in anaplastic thyroid cancer
cells. Oncogene. 38:699–715. 2019. View Article : Google Scholar : PubMed/NCBI
|