Introduction
Lung cancer is the most common malignancy overall
and accounted for 11.6% of the total number of cancer cases
worldwide in 2018 (1). Furthermore,
~80% lung cancer cases are non-small cell lung cancer (NSCLC), and
lung adenocarcinoma is the most common subtype of NSCLC (1). Tumor metastasis is the main cause of
clinical treatment failure and mortality (2). Lung adenocarcinoma is a type of lung
cancer that easily metastasizes. Most patients with lung cancer who
do not smoke suffer from lung adenocarcinoma with epidermal growth
factor receptor (EGFR) mutations (3). In China, the proportion of women with
lung cancer is increasing, while ~80% of Chinese women with lung
cancer do not smoke (4).
Understanding the underlying molecular mechanism of lung
adenocarcinoma is useful to improve the therapeutic regimen and
effect of treatment, particularly for patients who do not
smoke.
Tumor metastasis is a complex pathophysiological
process in which vascular microenvironment remodelling plays a key
role in the formation of the tumor metastasis microenvironment
(5,6). Angiogenesis is an important
pathological and physiological basis for the growth and invasion of
tumor cells (7,8). Vascular endothelial cells (VECs) play
an important role in the process of angiogenesis (9). Studies have reported that the
components secreted by tumor cells can stimulate angiogenesis. For
example, the outer vesicles derived from osteosarcoma cells can
induce angiogenesis (10), and the
exosomes secreted by ovarian cancer cell ovacar-3 can promote the
expression and secretion of vascular endothelial growth factor
(VEGF) in endothelial cells (ECs), thereby enhancing the
proliferative and migratory abilities of ECs (11).
Platelets (PLTs) also play an important role in
tumor growth and metastasis (12–14). For
example, activated PLTs can encapsulate tumor cells, enhance the
ability of tumor cells to cope with blood flow shear force and
escape the killing of the immune system, mediate the adhesion and
extravasation of tumor cells and vascular ECs (15), and participate in the regulation of
tumor angiogenesis (16–18). PLTs are activated by tumor cells and
exhibit higher expression of pro-angiogenic factors, such as von
Willebrand factor, vascular endothelial growth factor (VEGF) and
sphingosine-1-phosphate (19).
However, antiplatelet therapy often leads to physiological
coagulation abnormalities, increasing the risk of bleeding
(20). Activated PLTs contain
various active biomolecules, which promote the proliferation of
tumor cells (20). Thus, it is
speculated that the interaction between tumor cells and PLTs can
promote the proliferation, migration and tube formation of vascular
ECs. However, further investigations are required to confirm this
hypothesis.
In the present study, the lung adenocarcinoma cell
line, H1975 with EGFR mutation, was isolated from a woman with lung
adenocarcinoma who does not smoke. A co-culture in vitro
system was used to simulate the interaction between H1975 cells and
PLTs and evaluate its impact on the proliferation, migration and
tube formation of vascular ECs to lay the foundation for future
studies on the mechanism and effect of drug intervention among
patients with lung adenocarcinoma who do not smoke.
Materials and methods
Cell culture
The human lung adenocarcinoma cell line, H1975, and
HUVECs were kindly provided by the Cell Bank of Type Culture
Collection of the Chinese Academy of Sciences. Cells were
maintained in RPMI-1640 medium (Thermo Fisher Scientific, Inc.)
supplemented with 1% penicillin/streptomycin and 10% fetal bovine
serum (FBS; Thermo Fisher Scientific, Inc.), at 37°C in a
humidified atmosphere with 95% air and 5% CO2.
Preparation of PLT
A total of 10 ml of venous whole blood was extracted
from healthy adult volunteer, using acid citrate dextrose (15% v/v;
lot, 0803A20; Beijing Leagene Biotechnology Co., Ltd.) as an
anticoagulant (21). Following
centrifugation at 190 × g for 20 min at room temperature in a
horizontal centrifuge, PLT-rich plasma was obtained by carefully
extracting the supernatant, which was centrifuged at 650 × g for 10
min at room temperature. PLTs were washed in citrate-glucose-sodium
buffer (CGS; 14.7 mM trisodium citrate, 33.3 mM glucose, 123 mM
NaCl, pH 7.0) and centrifuged at 600 × g for 5 min at room
temperature. After washing twice with CGS, PLTs were resuspended
using prewarmed RPMI-1640 medium without FBS. Subsequently, a
suspension with 3.0×108 PLTs/ml was made and immediately
used for cell experiment (17). All
participants provided oral consent after fully explanatory
statements of the study.
Generation of cell derived supernatant
(SN)
A total of two types of supernatants were generated,
namely, H1975 cell-derived supernatant (SN_H) and supernatants
derived from H1975 cells co-cultured with PLT (SN_HP). Briefly,
H1975 cells were cultured to confluence in a 75 cm2
petri dish, and the culture medium was replaced with RPMI-1640
medium without FBS. Following incubation for 24 h at 37°C, the cell
supernatant was collected in an aseptic tube and centrifuged at
1,800 × g for 10 min at room temperature to eliminate cell debris,
and SN_H was subsequently frozen at −80°C. Similarly, H1975 cells
were cultured to confluence in a 75 cm2 petri dish, and
cells were thoroughly washed with PBS following removal of the
culture medium. Following incubation with PLT suspension and
RPMI-1640 medium without FBS for 24 h at 37°C, SN_HP were harvested
in aseptic tubes and frozen at −80°C following centrifugation at
1,800 × g for 10 min at room temperature. Supernatants were used
for the wound healing and tube formation assays, which consisted of
three groups: Control stands for single-cultured HUVECs, Exp_H
stands for HUVECs co-cultured with SN_H and Exp_HP stands for
HUVECs co-cultured with SN_HP.
Construction of the co-culture system
(CCS)
In total, three groups (control, Exp_H and Exp_HP)
were set up, and each group consisted of an upper chamber and a
lower chamber (22). For the control
group, HUVECs (1×104 cells per well for 24-well plates
and 1×105 cells per well for 6-well plates) were seeded
into the upper chambers with RPMI-1640 medium, while RPMI-1640
medium supplemented with 10% FBS was added to the lower chambers.
For the Exp_H group, HUVECs (1×104 cells per well for
24-well plates and 1×105 cells per well for 6-well
plates) were seeded into the upper chambers with RPMI-1640 medium,
H1975 cells (1×104 cells per well for 24-well plates and
1×105 cells per well for 6-well plates) and RPMI-1640
medium supplemented with 10% FBS were added to the lower chambers.
For the Exp_HP group, HUVECs (1×104 cells per well for
24-well plates and 1×105 cells per well for 6-well
plates) were seeded into the upper chambers with RPMI-1640 medium,
and H1975 cells (1×104 cells per well for 24-well plates
and 1×105 cells per well for 6-well plates), PLTs
(2×106 cells per well for 24-well plates and
2×107 cells per well for 6-well plates) and RPMI-1640
medium supplemented with 10% FBS were added to the lower
chambers.
The upper chamber inoculated with HUVECs was
transferred to the corresponding lower chamber. Control stands for
single-cultured HUVECs, Exp_H stands for HUVECs co-cultured with
H1975 cells and Exp_HP stands for HUVECs co-cultured with H1975
cells and PLTs. Based on CCS, cell viability, cell resistance, and
Transwell migration measurements were performed using 24-well
Transwell culture plates, and morphological observation, cell cycle
analysis, VEGF and VEGF receptor 2 (VEGFR2) expression measurements
were performed using 6-well Transwell culture plates. The specific
process of each detection method is described subsequently.
Detection of PLT activation
PLTs were incubated with SN_H for 2 h at 37°C in a
humidified atmosphere with 5% CO2, centrifuged at 2,000
× g for 5 min at room temperature, and resuspended in 100 ml PBS.
Cells were subsequently incubated in the dark with phycoerythrin
(PE) isotype (lot, 12471482; Thermo Fisher Scientific, Inc.) and
PE-labelled anti-P-selectin (1:20 dilution; lot, 2265654; Thermo
Fisher Scientific, Inc.) at room temperature for 30 min, and
subsequently fixed with 1% paraformaldehyde (PFA) at room
temperature for 10 min. Cells were centrifuged at 400 × g for 5 min
at room temperature and resuspended in 500 ml of PBS. P-selectin
was measured using a FACSCalibur flow cytometer (Becton-Dickinson
and Company) and analyzed using CXP analysis software (version 2.2;
Beckman Coulter, Inc.).
Cell Counting Kit-8 (CCK-8) assay
A total of three groups were designed based on CCS.
For the control group, 1×104 HUVECs were seeded into the
upper chambers (Corning, Inc.) with 0.5 ml RPMI-1640 medium, while
1 ml of RPMI-1640 medium supplemented with 10% FBS was added to the
lower chambers. For the Exp_H group, 1×104 H1975 cells
were seeded into the lower chambers based on the control. For the
Exp_HP group, 2×106 PLTs were seeded into the lower
chambers based on the Exp_H.
The upper chamber inoculated with HUVECs was
transferred onto the corresponding lower chamber. Following
incubation for 1, 2, 3 and 4 days, CCK-8 reagent (lot, 69112500;
Biosharp Life Sciences) was used to detect HUVEC proliferation, and
a microplate reader (Molecular Devices, LLC) was used to measure
the optical density (OD) at a wavelength of 450 nm after another 2
h of incubation with the CCK-8 reagent. The OD values are
represented as the mean absorbance for three wells from each
group.
Cell cycle analysis
A total of three groups were designed based on CCS.
For the control group, 1×105 HUVECs were seeded into the
upper chambers with 2 ml of RPMI-1640 medium, while 3 ml of
RPMI-1640 medium supplemented with 10% FBS was added to the lower
chambers. For the Exp_H group, 1×105 H1975 cells were
seeded into the lower chambers at the same time as the control. For
the Exp_HP group, 2×107 PLTs were seeded into the lower
chambers based on the Exp_H.
The upper chamber inoculated with HUVECs was
transferred to the corresponding lower chamber. Following
incubation for 3 days at 37°C, cells were washed twice with cold
PBS and harvested. Subsequently, cells were treated with
ribonuclease A (Rnase A; lot, ST576; Beyotime Institute of
Biotechnology) for 30 min at 37°C, and then treated with propidium
iodide (PI; lot, 7007583; Biosharp Life Sciences) in the dark for
30 min at 4°C. The fluorescence intensity of cells was determined
using a FACSCalibur flow cytometer (Becton-Dickinson and Company).
Cell cycle phase distribution was calculated using ModFit LT
software (Verity Software House, Inc.).
Morphological observation
Grouping design and cell culture conditions were the
same as those used for cell cycle analysis. Briefly, HUVECs were
collected following incubation for 3 days at 37°C, fixed in 3%
glutaraldehyde (lot, 2191108; Ted Pella, Inc.) for 10 h at 4°C,
postfixed in 1% osmium tetroxide (lot, 4008-182802-100118; Ted
Pella, Inc.) for 1 h at 4°C, dehydrated in graded ethanol at room
temperature, and subsequently embedded in Epon. Thin sections were
mounted on copper grids, stained with lead citrate (lot: 180705;
Ted Pella, Inc.) for 30 min at room temperature. Transmission
electron microscopy (JEM 1400; JEOL, Ltd.) was used to observe the
morphological changes in the HUVECs.
Cell resistance measurement
Grouping design and cell culture conditions were the
same as those for the CCK-8 assay. Following incubation for 1, 2, 3
and 4 days, cell resistance was continuously measured using a meter
for the epithelium instrument (RE1600, Beijing Jinhongtai
Technology Co., Ltd.). The impedance value could be used to reflect
the degree of cell resistance, that is, high impedance indicates
tight junctions, while low impedance indicates loose junctions
(23).
Wound healing assay
Cell suspension containing 1×106 HUVECs
(2 ml) was added to a 6-well plate. The monolayer of cells was
scratched in the middle with a pipette tip when cells grew to
80–90% of confluence and washed twice with sterile PBS to remove
debris. Cells were subsequently cultured with 2 ml of SN_H, SN_HP
and RPMI-1640 medium without FBS to form the Exp_H, Exp_HP and
control groups, respectively. The cells were incubated at 37°C, and
the scratches were observed using the CKX41 inverted light
microscope (magnification, ×40; Olympus Corporation) at 0 and 12 h.
ImageJ software (version 1.52a; National Institutes of Health) was
used to measure the gap distance of the wound and compute the
relative migration rate.
Transwell migration assay
Grouping design and cell culture conditions were the
same as those for the CCK-8 assay. Briefly, the lower chambers were
added with 1 ml of RPMI-1640 medium (10% FBS) per well for the
control group, 1 ml of RPMI-1640 medium (10% FBS) plus
1×104 H1975 per well for the Exp_H group, 1 ml of
RPMI-1640 medium (10% FBS) plus 1×104 H1975 and
2×106 PLTs per well for the Exp_HP group, and the upper
chambers were added with 1×104 HUVECs plus 0.5 ml
RPMI-1640 medium per well for all three groups. The upper chambers
were taken out following incubation for 24 h at 37°C, the culture
medium was drained and cells were washed with PBS. Cells were
subsequently fixed with 4% PFA for 30 min at room temperature, and
then stained in the dark with 10% crystal violet for 30 min at room
temperature. Stained cells were counted in five randomly selected
fields using a fluorescence microscope (DMI6000B; Leica
Microsystems, Inc.) in the bright field (magnification, ×200), and
the number of migrated cells were counted using ImageJ
software.
Tube formation assay
Cell suspension containing 5×105 HUVECs
(2 ml) was added to a 6-well plate, and the cell supernatant was
replaced with RPMI-1640 medium, SN_H, and SN_HP following
incubation for 24 h at 37°C to form the control, Exp_H and Exp_HP
groups. Following incubation for an additional 24 h at 37°C, HUVECs
were harvested and diluted to 1×105 cells/ml using
RPMI-1640 medium supplemented with 10% FBS. A total of 60 µl
Matrigel (8–12 mg/ml; cat. no. 356234; Corning, Inc.) per well was
added into a 96-well plate at 4°C and solidified at 37°C for 30–45
min. Subsequently, HUVEC suspensions were plated at a concentration
of 1×104 cells/well in a 96-well plate coated with
Matrigel. Tube formation of HUVECs was observed under an inverted
light microscope (magnification, ×40; CKX41; Olympus Corporation)
following incubation for 3 and 9 h at 37°C.
ELISA detection
Grouping design and cell culture conditions were the
same as those used for cell cycle analysis. Briefly, the culture
medium was replaced with RPMI-1640 medium without FBS on the 3rd
day. Following incubation for 16 h at 37°C, VEGF expression in the
supernatant of the upper chamber was quantitatively measured using
the human VEGF ELISA kit (cat. no. H044-1; Nanjing Jiancheng
Bioengineering Institute).
Western blot analysis
Grouping design and cell culture conditions were the
same as those used for cell cycle analysis. Briefly, the culture
medium was replaced with RPMI-1640 medium without FBS on the third
day. Following incubation for 16 h at 37°C, VEGFR2 expression in
the lysate of HUVECs was detected via western blotting. HUVECs were
lysed in RIPA lysis buffer (cat. no. P00138, Beyotime Institute of
Biotechnology). After quantification by Bradford assay, the protein
samples were mixed with 5X loading buffer. Equal amounts of protein
(20–30 µg/lane) were separated using 10% SDS-PAGE and transferred
onto nitrocellulose membranes (Pall Corporation). The membranes
were blocked using 5% skimmed milk for 1 h at room temperature and
incubated with primary antibodies against VEGFR2 (cat. no.
ab134191; 1:3,000 dilution; Abcam) and β-actin (cat. no. TA-09;
1:1,000 dilution; OriGene Technologies, Inc.) overnight at 4°C.
After incubated with the appropriate horseradish peroxidase-labeled
IgG (cat. no. A0208, targeting anti-VEGFR2; 1:10,000 dilution;
Beyotime Institute of Biotechnology; cat. no. ZB-2205, targeting
anti-β-actin; 1:10,000 dilution; OriGene Technologies, Inc.) at
room temperature for 1 h, the proteins were detected with enhanced
chemiluminescence reagent (lot, 21064112; Biosharp Life Sciences).
TBS with Tween-20 (0.05%) was used for membrane washing. The
Electrophoresis Gel Imaging Analysis System and integrated analysis
software (version 1.00.0018, GV 6000plus; Guangzhou Biolight
Biotechnology, Ltd.) was used to detect the immunoreactive
protein.
Statistical analysis
Statistical analysis was performed using SPSS 23.0
software (IBM Corp.). All experiments were performed in triplicate
and data are presented as the mean ± SD. Unpaired Student's t-test
was used to compare the expression rate of P-selectin between the
group of P-selectin-PE and the control group, while one-way ANOVA
followed by Newman-Keuls post hoc test were used to compare
differences between three groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
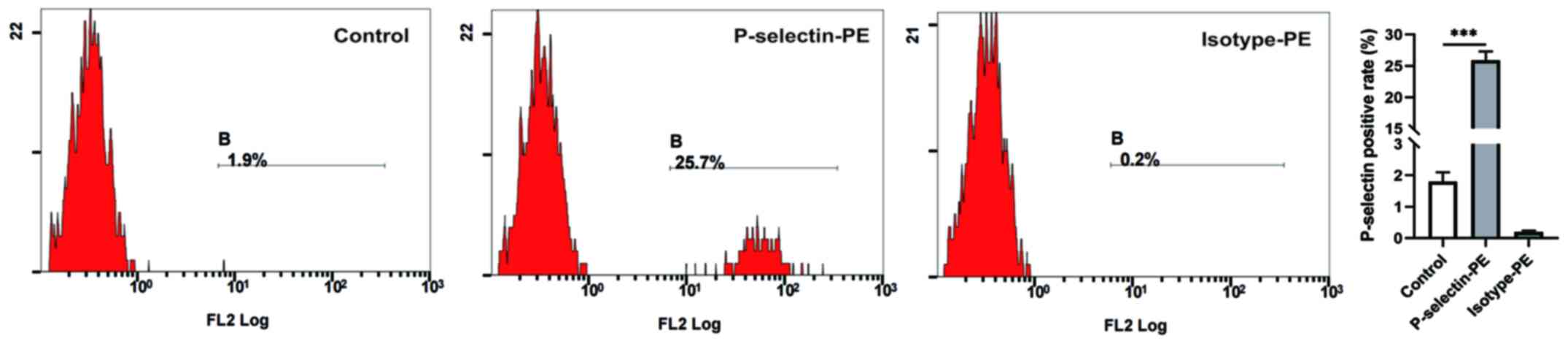
PLTs were activated by H1975
cells
PLT activation is a key step in the crosstalk
between tumor cells and PLTs (24).
In the present study, P-selectin expression was detected on the PLT
surface via flow cytometry. Fig. 1
depicts the different levels of the PLT activation marker,
P-selectin, among the three groups. P-selectin expression was
higher in PLTs incubated with SN_H (P-selectin-PE: 25.9±1.4%)
compared with the control group (1.8±0.3%, P<0.001), suggesting
that PLTs can be activated by H1975 cells.
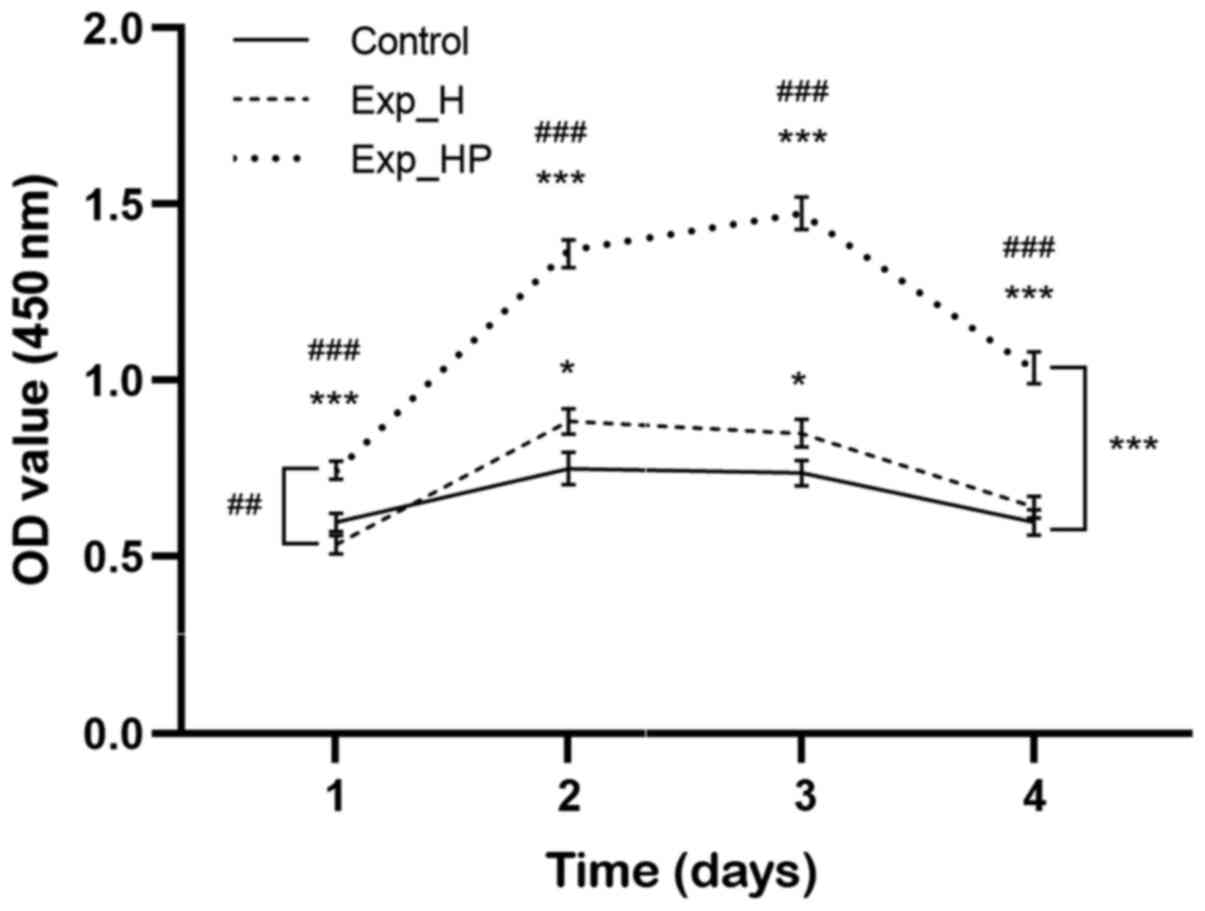
HUVEC activity improves following
co-culture with PLTs and H1975 cells
The CCK-8 assay was performed to determine whether
co-culture with PLTs enhances H1975 cell improvement in HUVEC
activity. The activity of HUVECs in the Exp_H group exhibited a
slight increase compared with the control group, while HUVECs in
the Exp_HP group exhibited a significant increase compared with the
Exp_H (P<0.01) and control group (P<0.001), respectively
(Fig. 2). Further analysis results
showed that the activity of HUVECs in the Exp_HP group was higher
than that of the Exp_H at all four timepoints (P<0.001).
Similarly, the activity of Exp_HP group was stronger than that of
the control at all timepoints (P<0.001); however, the activity
of the Exp_H group was higher than that of the control only at day
2 and day 3 (P<0.05). Notably, HUVEC activity in the Exp_HP
group reached a peak after 3 days of incubation and subsequently
decreased. These results suggested that the activity of HUVECs
could be improved by PLT-H1975 crosstalk.
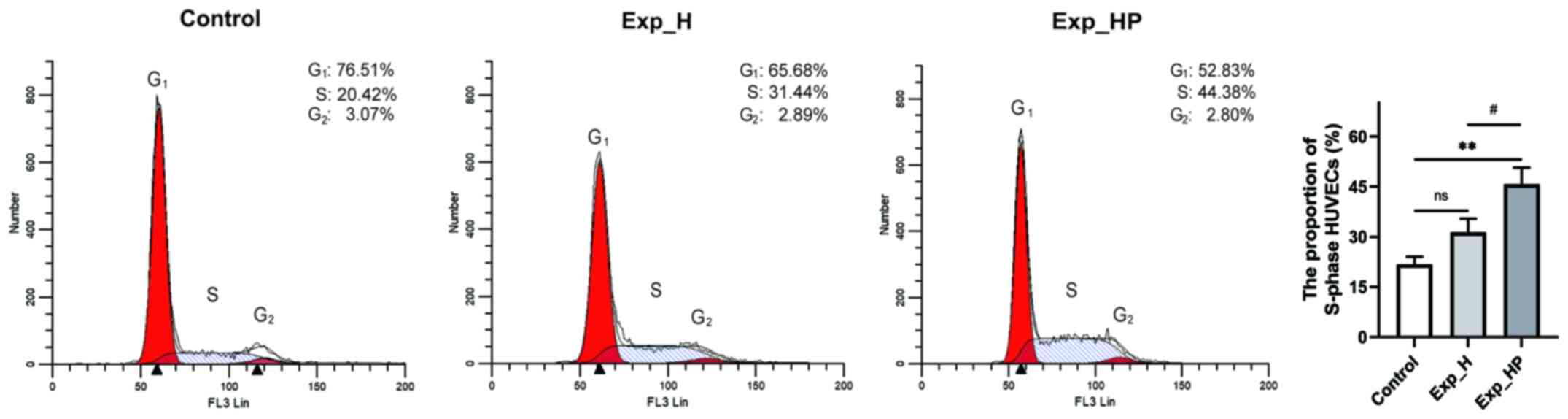
Co-culture with PLTs and H1975 cells
increases the proportion of S-phase HUVECs
S-phase is an important phase of cell proliferation
that reflects the active state of cell proliferation (25). Thus, it was hypothesized that the
proportion of S-phase HUVECs would increase following incubation
with SN_H or SN_HP. The results demonstrated that the proportion of
HUVECs in the S-phase in the Exp_H group (31.51±4.07%) was higher
compared with the control group (21.75±2.34%, P=0.048), and the
proportion of HUVECs in the S-phase was highest in the Exp_HP group
(45.73±4.95%; P<0.01 vs. control; P<0.05 vs. Exp_H; Fig. 3). These results suggested that the
proliferation of HUVECs could be increased by PLT-H1975
crosstalk.
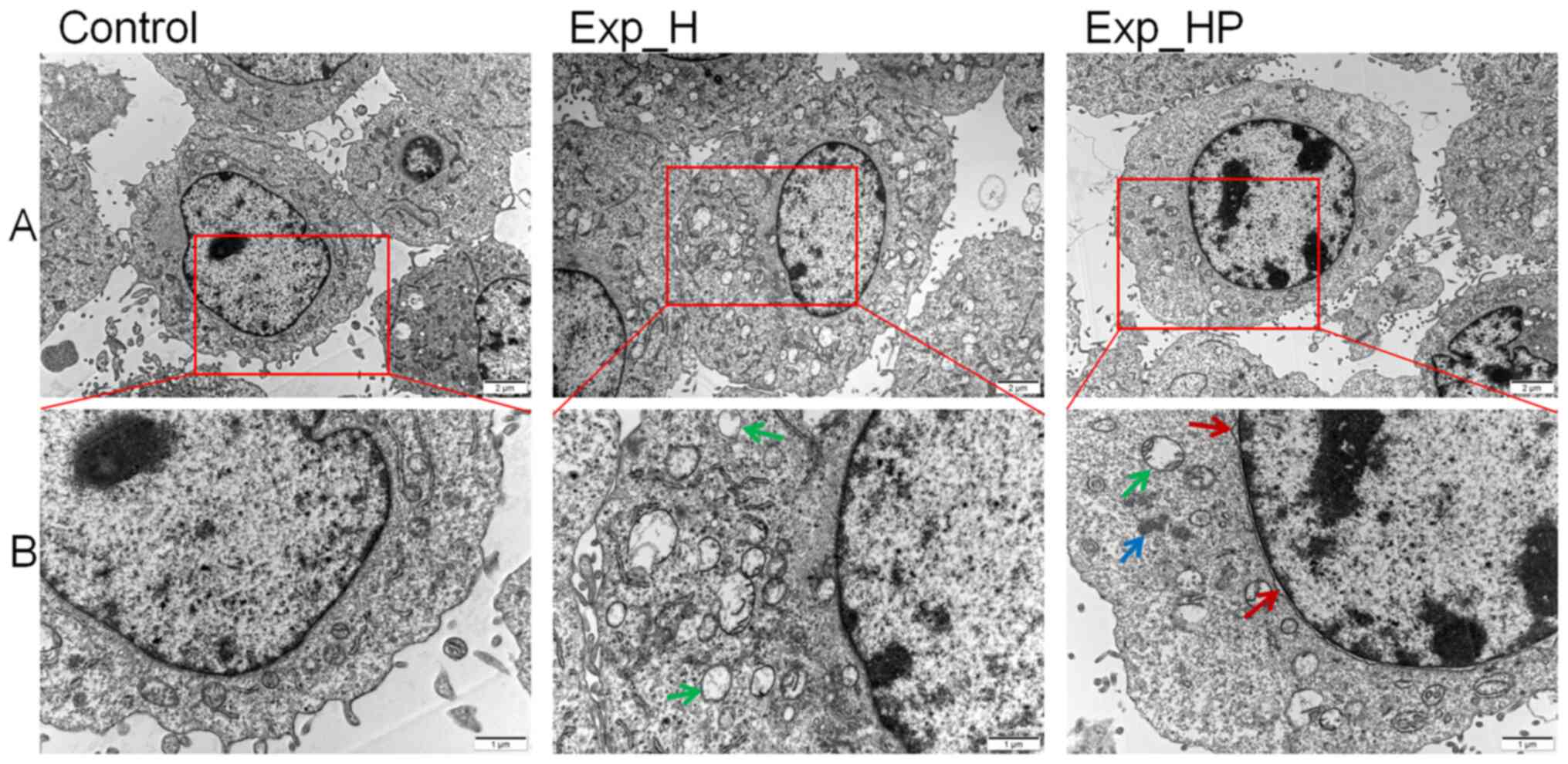
HUVEC morphology changes following
co-culture with PLTs and H1975 cells
It is well-known that tumors have dense blood
vessels but incomplete structures (26). Thus, it was hypothesized that the
internal structure of HUVECs that proliferate rapidly under SN_HP
stimulation may be abnormal. The morphological changes were
observed following incubation for 3 days as the results of CCK-8
assay indicated that HUVECs were at the vigorous growth stage after
3 days of incubation. Fig. 4 depicts
the morphological changes of HUVECs observed via transmission
electron microscopy. The results demonstrated that more vacuolation
of mitochondria was observed in the Exp_H group compared with the
control group. In addition, more types of abnormal changes were
observed in the Exp_HP group, such as mitochondrial vacuolation,
fluffy structure, enlarged nucleus and nuclear membrane deformity.
These results suggested that the ultrastructure of HUVECs could be
damaged by PLT-H1975 crosstalk.
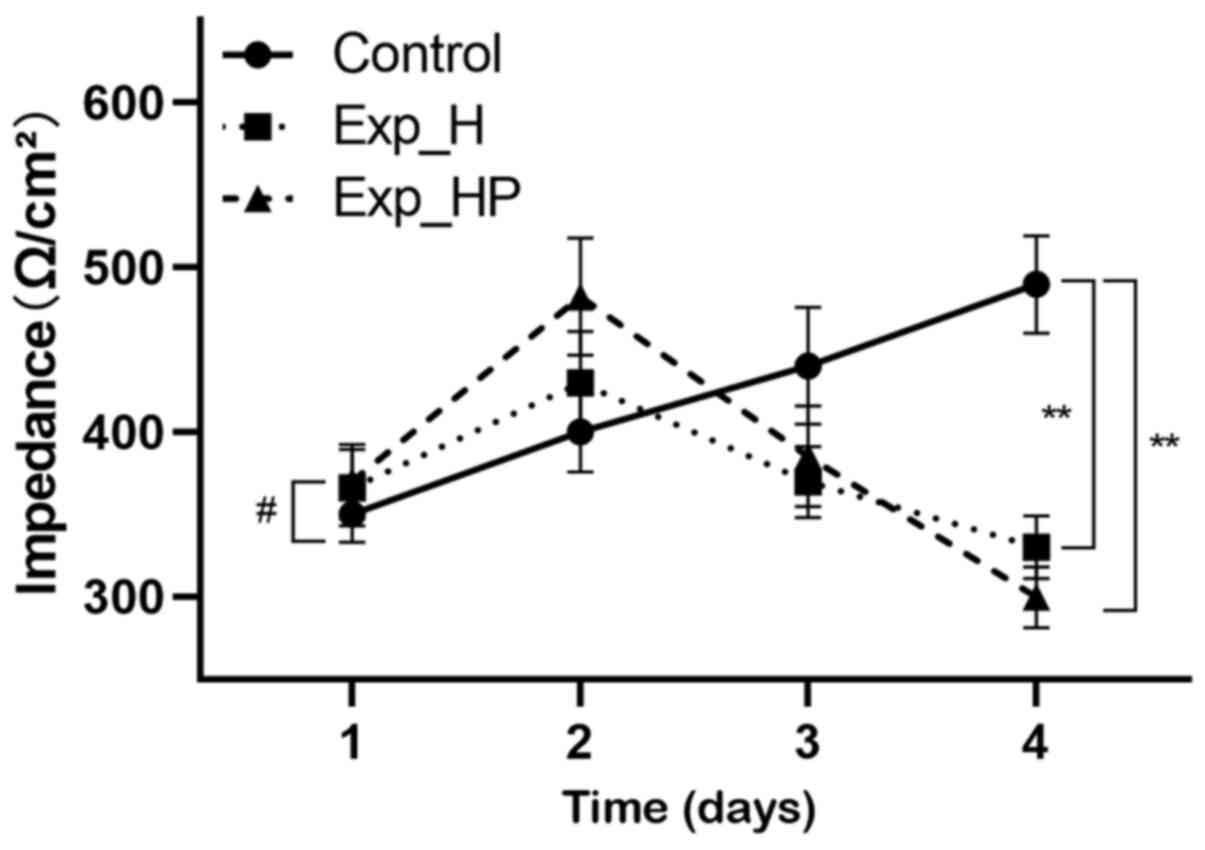
HUVEC resistance decreases following
co-culture with PLTs and H1975 cells
Abnormal structure may decrease the tightness of
intercellular connections, and transepithelial electrical
resistance can reflect the connections between ECs (27). It was hypothesized that the
transepithelial electrical resistance of HUVECs would decrease in
the Exp_HP group. Fig. 5 depicts
HUVEC resistance on 4 different incubation days. The results
demonstrated that resistance continuously increased in the control
group, with significant differences between the time points. While
the trends of resistance in the Exp_H and Exp_HP groups were
different compared with the control group (P<0.01). Notably,
resistance in the Exp_HP and Exp_H groups reached a peak after 2
days of incubation and subsequently decreased at a rapid rate. The
difference between the Exp_H and Exp_HP groups was also significant
(P<0.05). These results suggested that the tightness of HUVECs
junction could be decreased by PLT-H1975 crosstalk.
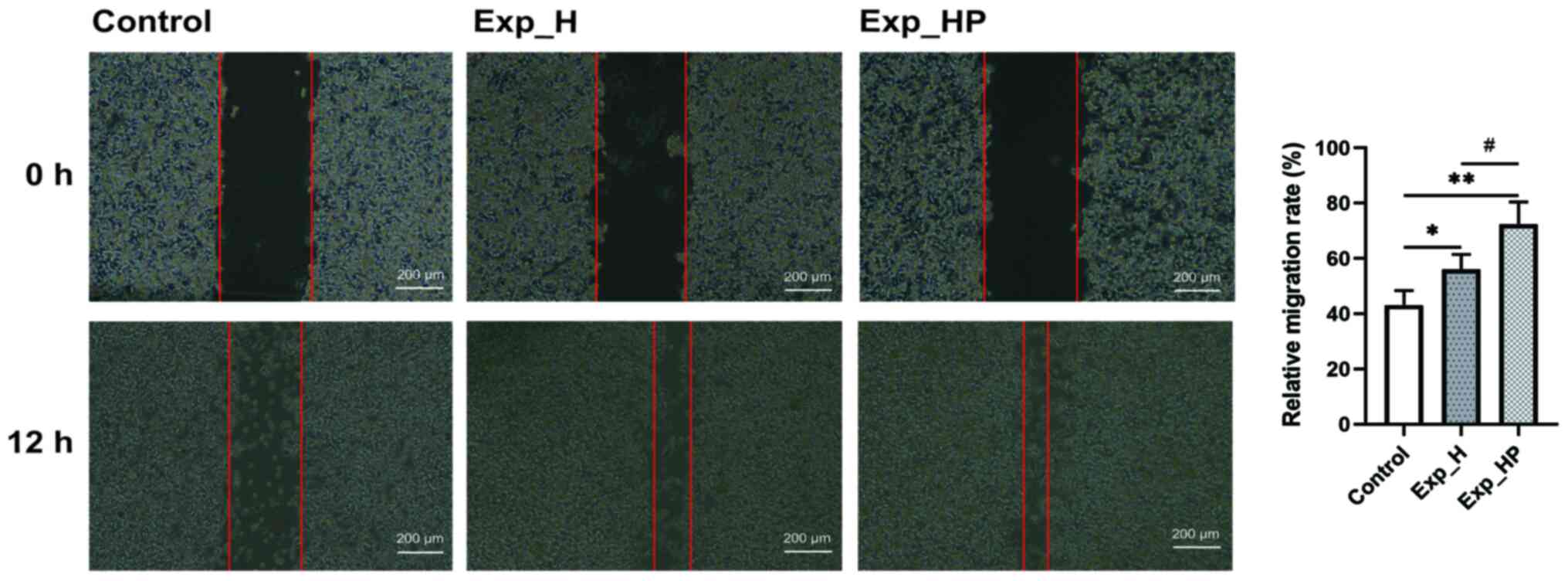
SN_HP increases the wound healing
capacity of HUVECs
The wound healing and Transwell assays were
performed to assess cell migration. The results demonstrated that
the relative migration rate of HUVECs was significantly higher in
the Exp_HP group, followed by the Exp_H group and the control group
(Fig. 6) with significant
differences among three groups: P<0.05 for Exp_HP vs. Exp_H and
Exp_H vs. control, P<0.01 for Exp_HP vs. control. These results
suggested that the wound healing capacity of HUVECs could be
promoted by PLT-H1975 crosstalk.
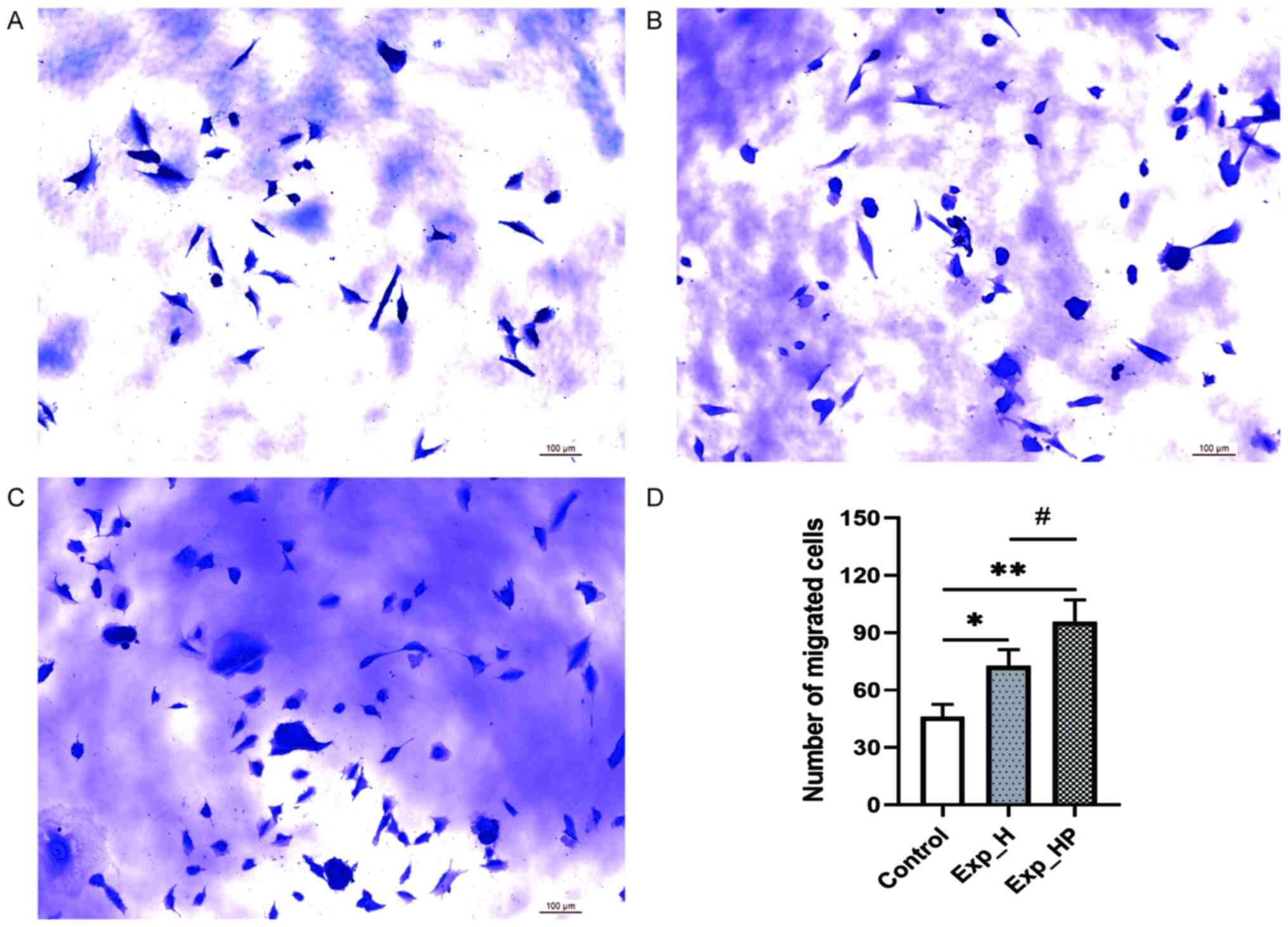
Co-culture with PLTs and H1975 cells
improves the migration of HUVECs
The microscopic results of the Transwell migration
assay are presented in Fig. 7.
Following co-culture with tumor cells and PLTs for 24 h, the
migratory ability of HUVECs was significantly higher in the Exp_HP
group compared with the control (P<0.01) and Exp_H groups
(P<0.05), and the migratory ability of HUVECs was significantly
higher in the Exp_H group compared with the control group
(P<0.05). These results were consistent with those of the wound
healing assay. These results suggested that the migration capacity
of HUVECs could be improved by PLT-H1975 crosstalk.
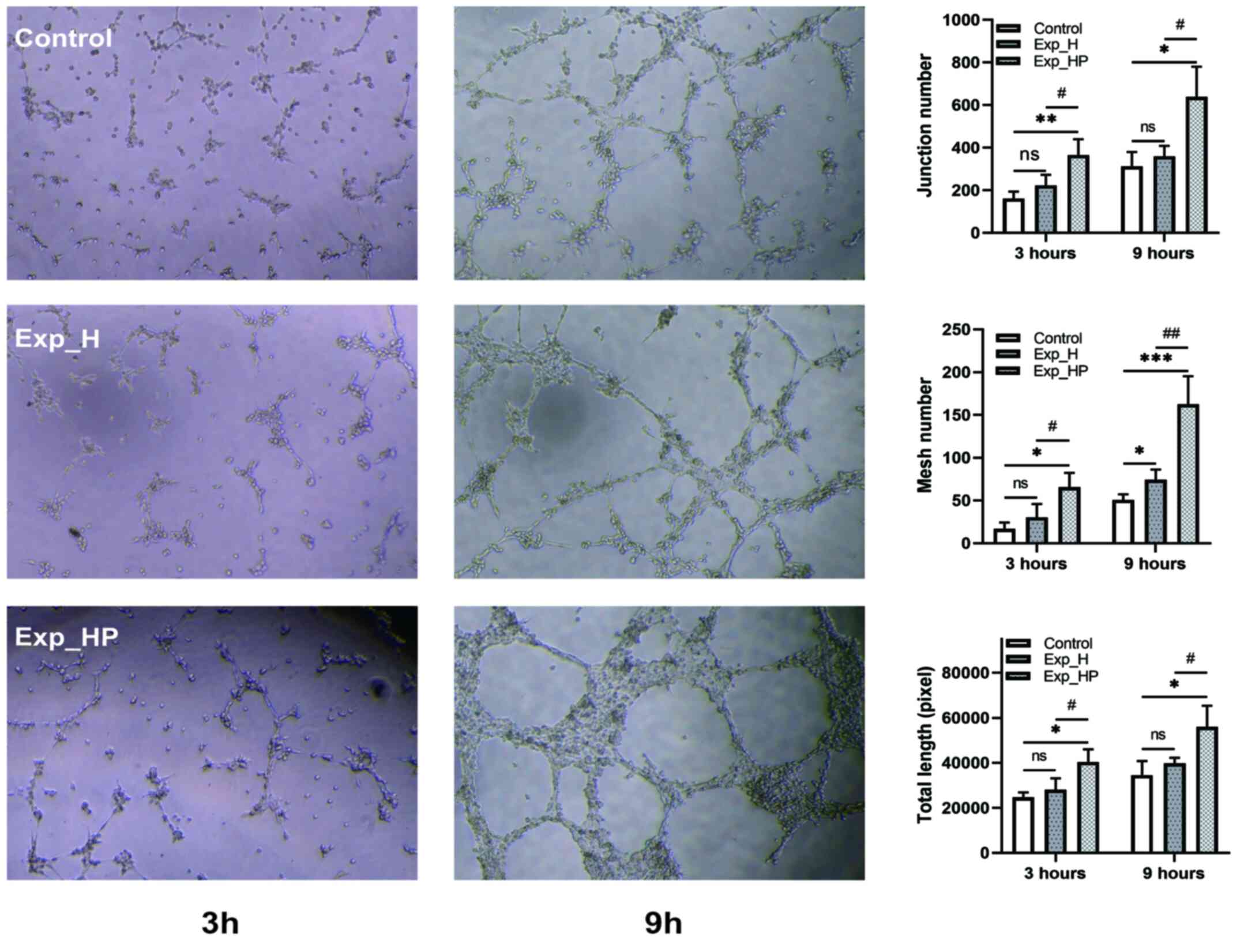
SN_HP promotes the tube-forming
ability of HUVECs
Based on the aforementioned results, it was revealed
that H1975-PLT crosstalk can promote the proliferation and
migration of HUVECs. Thus, it was hypothesized that crosstalk may
improve the tube formation ability of HUVECs. Fig. 8 demonstrates the tube formation
ability indicated by the junction number, mesh number and total
length of HUVECs among the three groups. The tube-forming ability
increased in each group with time. Furthermore, the function of
HUVECs in the Exp_HP group was significantly better compared with
the control group following 3 and 9 h of incubation (P<0.05),
especially for junction number 3 h after incubation (P<0.01) and
mesh number 9 h after incubation (P<0.001). Similarly, the
tube-forming ability in the Exp_HP group was significantly higher
compared with the Exp_H group following 3 and 9 h of incubation
(P<0.05), especially for mesh number 9 h after incubation
(P<0.01). However, no significant differences were observed
between the Exp_H and control groups except for mesh number 9 h
after incubation (P<0.05). These results suggested that the
tube-forming ability of HUVECs could be increased by PLT-H1975
crosstalk.
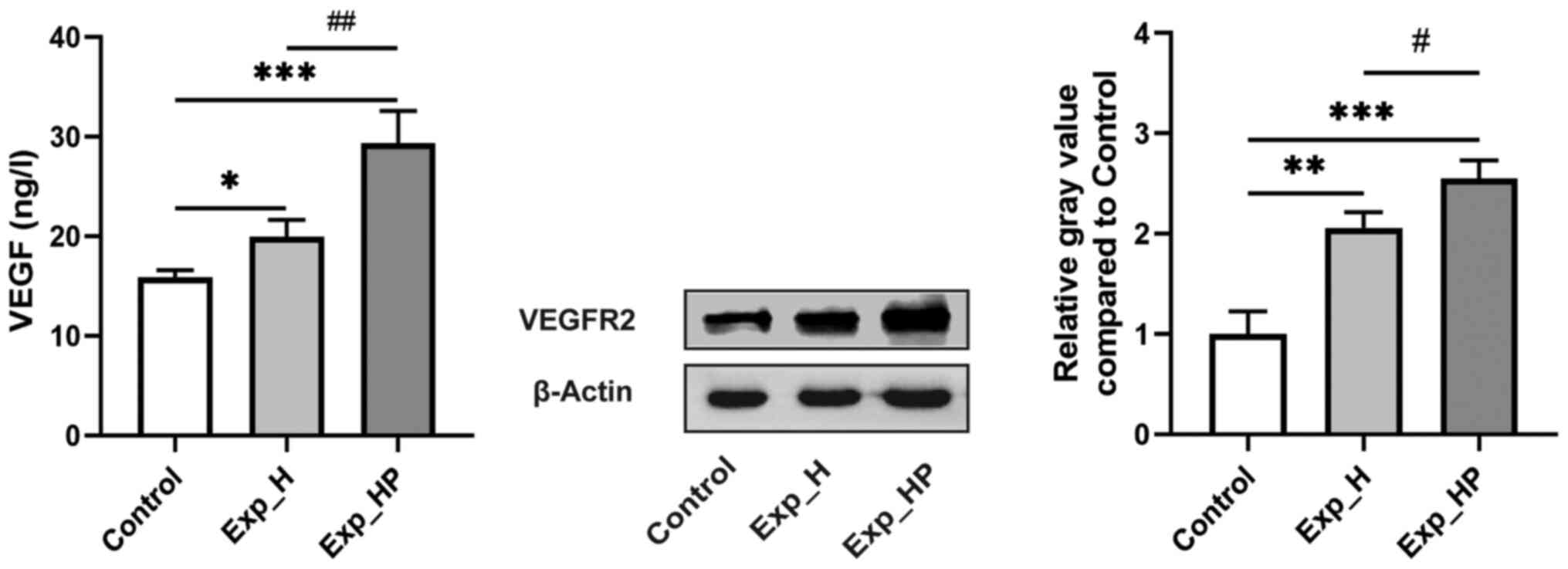
Co-culture with PLTs and H1975 cells
increases VEGF and VEGFR2 expression levels in HUVECs
VEGF is highly specific and the combination of VEGF
and VEGFR2 is the key mechanism that induces tumor angiogenesis
(28). It was hypothesized that
H1975-PLT crosstalk may enhance VEGF and VEGFR2 expression levels
in HUVECs. Fig. 9 depicts VEGF
content measured by ELISA and VEGFR2 expression detected via
western blotting. High VEGF expression was observed in the Exp_HP
group (29.32±3.25 ng/l), which was significantly higher compared
with the Exp_H (19.93±1.71 ng/l; P<0.01) and control (15.86±0.73
ng/l; P<0.001) groups. In addition, VEGF expression was
significantly higher in the Exp_H group compared with the control
group (P<0.05). Similarly, the highest expression of VEGFR2 was
observed in the Exp_HP group, followed by the Exp_H group and the
control group, with significant differences among three groups:
P<0.05 for Exp_HP vs. Exp_H, P<0.01 for Exp_H vs. control,
and P<0.001 for Exp_HP vs. control. These results suggested that
the expression levels of VEGF and VEGFR2 in HUVECs could be
improved by PLT-H1975 crosstalk.
Discussion
Angiogenesis is the central marker of tumors and is
key for the progression and metastasis of solid tumors (6). HUVECs are a classic model system to
study angiogenesis (29). Previous
studies have demonstrated that tumor cells can improve the
proliferation and migration of VECs, and promote angiogenesis to a
certain extent (30–32); activated PLTs are also involved in
this process (16,17,33).
However, the effect of co-culture of tumor cells and PLTs on the
proliferation, migration and angiogenesis of HUVECs remains
unknown. To the best of our knowledge, the present study was the
first to investigate H1975-PLT crosstalk on the properties of
HUVECs based on CCS. The results demonstrated that the activity of
HUVECs significantly enhanced following co-culture with tumor cells
and PLTs, which can help illustrate the mechanism of tubular
formation of VECs around cancer cells.
Angiogenesis is induced when the tumor grows to 2 mm
in diameter; otherwise tumor cells die due to a lack of nutrition
(34). VECs serve a key role in
tumor angiogenesis (32). To
determine the effect of tumor-PLT crosstalk on the ability of VECs,
the present study assessed the proliferation, migration and tube
formation abilities of HUVECs and their molecular basis.
Proliferation reflects the basic functional state of
HUVECs. The present study investigated the effect of tumor-PLT
crosstalk on the proliferation of HUVECs by analyzing cell
activity, the proportion of S-phase cells, ultrastructure and cell
resistance. The results demonstrated that H1975-PLT crosstalk
significantly promoted HUVEC proliferation, with a higher OD value
detected via CCK-8 and a higher proportion of S-phase cells in the
cell cycle. However, more HUVEC ultrastructure anomalies and a
lower degree of tight connections were observed between HUVECs
following co-culture with H1975 cells and PLTs. These results are
consistent with the features of blood vessels in the tumor
microenvironment, which are dense, disordered and incomplete
(35–38). It is assumed that if the
proliferation of VECs is blocked or weakened, this reduces tumor
angiogenesis, which helps control tumor growth and metastasis
(9). Activated PLTs can directly
stimulate the proliferation of tumor cells and enhance the
proliferation of VECs (13,39). Thus, inhibition of PLT activation,
particularly tumor cell-induced PLT activation, may be a potential
target to prevent tumor-PLT crosstalk and inhibit the proliferation
of HUVECs.
Migration of VECs serves an important role in
angiogenesis (40). The present
study performed wound healing and Transwell assays to determine the
effect of H1975-PLT crosstalk on the migratory ability of HUVECs.
The results demonstrated that the migratory ability of HUVECs was
significantly stronger in the Exp_HP group compared with the
control group, suggesting that more HUVEC stimulation may be
produced in the Exp_HP group compared with the Exp_H and control
groups. The results of the present study were consistent with
previous findings (12,30). The results of the Transwell assay
demonstrated that inhibition of PLT activation may be a potential
target to inhibit the migration of HUVECs induced by H1975-PLT
crosstalk.
According to the aforementioned research results,
tumor-PLT crosstalk could promote HUVECs proliferation and
migration. It was speculated that tumor-PLT crosstalk can enhance
the tube formation ability of HUVECs. Tube-forming experiments are
a rapid and quantifiable method to measure angiogenesis in
vitro (41). The present study
investigated the effect of H1975-PLT crosstalk on HUVEC
angiogenesis using junction number, mesh number and total length as
indicators of tube-forming ability, and the results of the present
study confirmed that the tube-forming ability of HUVECs could be
promoted by H1975-PLT crosstalk. These results are consistent with
previous findings, suggesting that PLTs can promote tube formation
by adhering to ECs or releasing PLT microparticles (16,42).
Thus, PLTs activated by tumors may upregulate the expression levels
of P-selectin and GIIbIIIa and release more PLT microparticles to
promote tube formation (20,43). Taken together, these results suggest
that controlling H1975-PLT crosstalk can inhibit angiogenesis and
be used as a therapeutic target for tumor metastasis.
Several factors affect the proliferation, migration
and tube-forming abilities of HUVECs with complex mechanisms, and
the combination of VEGF and VEGFR2 is a key mechanism that induces
tumor angiogenesis (28). The
present study measured VEGF content in the HUVEC supernatant and
detected VEGFR2 expression in the lysate of HUVECs. The results
demonstrated that both VEGF and VEGFR2 expression levels were
highest in the Exp_HP group, which suggests that H1975-PLT
crosstalk may not only stimulate the expression levels of VEGF and
VEGFR2 in HUVECs (33) but also
promote their combination. Thus, H1975-PLT crosstalk may enhance
the proliferation, migration and tube formation of HUVECs. Notably,
the increased levels of VEGF may mediate the tumor promotion of
PLTs. This result is similar to a previous study that reported that
PLTs from patients with glioblastoma can promote angiogenesis of
tumor ECs and exhibit increased VEGF content and release (17).
The interaction between H1975 cells and PLTs plays
an important role in tumor angiogenesis by affecting the
characteristics of ECs, which suggests that inhibition of H1975-PLT
crosstalk may be a potential target for the treatment of
non-smoking patients with lung adenocarcinoma. However, further
studies are required to effectively interfere with tumor-PLT
crosstalk without affecting normal coagulation function. Thus,
prospective studies will investigate the effect of crosstalk
between A549 cells and PLTs on the properties of HUVECs, such as
proliferation, migration and tube formation, and evaluate antitumor
Chinese medicine, which can interfere with the interaction between
tumor cells and PLTs (44,45).
In conclusion, the results of the present study
demonstrated that the proliferation, migration and tube-forming
abilities of HUVECs co-cultured with H1975 and PLTs were
significantly higher compared with HUVECs cultured alone and better
than HUVECs co-cultured with only H1975. Taken together, these
results suggest that tumor cells interacting with PLTs may play an
important role in tumor angiogenesis by affecting or mediating
changes in the properties of ECs.
Acknowledgements
The authors would like to thank Mr. Ruishan Liu
(Anhui Medical University, Hefei, China), Dr Ying Cao (Anhui
University of Chinese Medicine, Hefei, China), Dr Hui Cheng (Anhui
University of Chinese Medicine, Hefei, China) for their technical
assistance.
Funding
The present study was funded by the Opening
Foundation of Key Laboratory of Xin'an Medicine, Ministry of
Education of Anhui University of Chinese Medicine (grant no.
2020×ayx05) and the Scientific Research Foundation of Education
Department of Anhui Province (grant nos. KJ2019A046 and
SK2020A0236).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
BL conceived the study and XD, JZ, TZ, DP and QL
designed the present study. BL, XD, XT, TZ and JZ performed the
experiments and statistical analysis. BL and XD drafted the initial
manuscript. BL, XD, XT, JZ, TZ and QL were involved in reviewing
and editing the manuscript for important intellectual content. BL,
DP and QL supervised the study and provided funding. BL and QL
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Scientific
Research Ethics Committee of the First Affiliated Hospital of Anhui
University of Traditional Chinese Medicine (approval no.
2020AHZY-01; Hefei, China). All participants provided oral consent,
since the expected risk was low for qualified participants
contributing 10 ml venous blood in the hospital, this method of
obtaining consent was approved by the Scientific Research Ethics
Committee and volunteers chose oral rather than written
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu C, Chen F, Wang X, Cai Z, Yang M, Zhong
Q, Feng J, Li J, Shen C and Wen Z: Pin2 telomeric repeat factor
1-interacting telomerase inhibitor 1 (PinX1) inhibits
nasopharyngeal cancer cell stemness: Implication for cancer
progression and therapeutic targeting. J Exp Clin Cancer Res.
39:312020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okudela K, Matsumura M, Arai H and Woo T:
The nonsmokers' and smokers' pathways in lung adenocarcinoma:
Histological progression and molecular bases. Cancer Sci. Jun
18–2021.(Epub ahead of print). doi: 10.1111/cas.15031. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seow WJ, Shu XO, Nicholson JK, Holmes E,
Walker DI, Hu W, Cai Q, Gao YT, Xiang YB, Moore SC, et al:
Association of untargeted urinary metabolomics and lung cancer risk
among never-smoking women in China. JAMA Netw Open. 2:e19119702019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aggarwal C, Somaiah N and Simon G:
Antiangiogenic agents in the management of non-small cell lung
cancer: Where do we stand now and where are we headed? Cancer Biol
Ther. 13:247–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rivera LB and Bergers G: CANCER. Tumor
angiogenesis, from foe to friend. Science. 349:694–695. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Abboodi M, An R, Weber M, Schmid R,
Klausing A, Horch RE, Boos AM and Kengelbach-Weigand A: Tumor type
dependent effects on the angiogenic abilities of endothelial cells
in an in vitro rat cell model. Oncol Rep. 42:350–360.
2019.PubMed/NCBI
|
|
10
|
Perut F, Roncuzzi L, Zini N, Massa A and
Baldini N: Extracellular nanovesicles secreted by human
osteosarcoma cells promote angiogenesis. Cancers (Basel).
11:7792019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ghorbanian M, Babashah S and Ataei F: The
effects of ovarian cancer cell-derived exosomes on vascular
endothelial growth factor expression in endothelial cells. EXCLI J.
18:899–907. 2019.PubMed/NCBI
|
|
12
|
Li N: Platelets in cancer metastasis: To
help the ‘villain’ to do evil. Int J Cancer. 138:2078–2087. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chiodoni C, Iezzi M, Guiducci C,
Sangaletti S, Alessandrini I, Ratti C, Tiboni F, Musiani P, Granger
DN and Colombo MP: Triggering CD40 on endothelial cells contributes
to tumor growth. J Exp Med. 203:2441–2450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gay LJ and Felding-Habermann B:
Contribution of platelets to tumour metastasis. Nat Rev Cancer.
11:123–134. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Borsig L: The role of platelet activation
in tumor metastasis. Expert Rev Anticancer Ther. 8:1247–1255. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim HK, Song KS, Chung JH, Lee KR and Lee
SN: Platelet microparticles induce angiogenesis in vitro. Br J
Haematol. 124:376–384. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Vito C, Navone SE, Marfia G, Abdel Hadi
L, Mancuso ME, Pecci A, Crisà FM, Berno V, Rampini P, Campanella R,
et al: Platelets from glioblastoma patients promote angiogenesis of
tumor endothelial cells and exhibit increased VEGF content and
release. Platelets. 28:585–594. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li R, Ren M, Chen N, Luo M, Deng X, Xia J,
Yu G, Liu J, He B, Zhang X, et al: Presence of intratumoral
platelets is associated with tumor vessel structure and metastasis.
BMC Cancer. 14:1672014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Campanella R, Guarnaccia L, Cordiglieri C,
Trombetta E, Caroli M, Carrabba G, La Verde N, Rampini P, Gaudino
C, Costa A, et al: Tumor-educated platelets and angiogenesis in
glioblastoma: Another brick in the wall for novel prognostic and
targetable biomarkers, changing the vision from a localized tumor
to a systemic pathology. Cells. 9:2942020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haemmerle M, Stone RL, Menter DG,
Afshar-Kharghan V and Sood AK: The platelet lifeline to cancer:
Challenges and opportunities. Cancer Cell. 33:965–983, 2018.21.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carrim N, Arthur JF, Hamilton JR, Gardiner
EE, Andrews RK, Moran N, Berndt MC and Metharom P: Thrombin-induced
reactive oxygen species generation in platelets: A novel role for
protease-activated receptor 4 and GPIbα. Redox Biol. 6:640–647.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mercatali L, La Manna F, Miserocchi G,
Liverani C, De Vita A, Spadazzi C, Bongiovanni A, Recine F, Amadori
D, Ghetti M, et al: Tumor-stroma crosstalk in bone tissue: The
osteoclastogenic potential of a breast cancer cell line in a
co-culture system and the role of EGFR inhibition. Int J Mol Sci.
18:E16552017. View Article : Google Scholar
|
|
23
|
Kraya R, Komin A and Searson P: On chip
bioelectric impedance spectroscopy reveals the effect of
p-glycoprotein efflux pumps on the paracellular impedance of tight
junctions at the blood-brain barrier. IEEE Trans Nanobioscience.
15:697–703. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
LI S, CHEN ZB, LÜ M and ZHU Y: Current
status and future of drugs targeting platelets-tumor cells
interactions. Yao Xue Xue Bao. 2:360–367. 2021.(In Chinese).
|
|
25
|
Mazurek A, Luo W, Krasnitz A, Hicks J,
Powers RS and Stillman B: DDX5 regulates DNA replication and is
required for cell proliferation in a subset of breast cancer cells.
Cancer Discov. 2:812–825. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jain RK: Antiangiogenesis strategies
revisited: From starving tumors to alleviating hypoxia. Cancer
Cell. 26:605–622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang YF, Meng YY, Ye J, Xia XJ, Li L, Dong
WJ, Wang HL and Liu YL: Co-culture of human breast adenocarcinoma
cells and human umbilical vein endothelial cells to mimic in vivo
tumor microenvironment. Yao Xue Xue Bao. 3:403–409. 2018.(In
Chinese).
|
|
28
|
Khan KA and Kerbel RS: Improving
immunotherapy outcomes with anti-angiogenic treatments and vice
versa. Nat Rev Clin Oncol. 15:310–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin X, Qiu W, Xiao Y, Ma J, Xu F, Zhang K,
Gao Y, Chen Q, Li Y, Li H, et al: MiR-199b-5p suppresses tumor
angiogenesis mediated by vascular endothelial cells in breast
cancer by targeting ALK1. Front Genet. 10:13972020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ishihara S, Onoda N, Noda S, Asano Y,
Tauchi Y, Morisaki T, Kashiwagi S, Takashima T and Ohira M:
Sorafenib inhibits vascular endothelial cell proliferation
stimulated by anaplastic thyroid cancer cells regardless of BRAF
mutation status. Int J Oncol. 55:1069–1076. 2019.PubMed/NCBI
|
|
31
|
Kaneda H, Arao T, Matsumoto K, De Velasco
MA, Tamura D, Aomatsu K, Kudo K, Sakai K, Nagai T, Fujita Y, et al:
Activin A inhibits vascular endothelial cell growth and suppresses
tumour angiogenesis in gastric cancer. Br J Cancer. 105:1210–1217.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo D, Xu S, Wang N, Jiang H, Huang Y, Jin
X, Xue B, Zhang C and Zhu X: Prodrug-embedded angiogenic
vessel-targeting nanoparticle: A positive feedback amplifier in
hypoxia-induced chemo-photo therapy. Biomaterials. 144:188–198.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Frezzetti D, Gallo M, Roma C, D'Alessio A,
Maiello MR, Bevilacqua S, Normanno N and De Luca A: Vascular
endothelial growth factor a regulates the secretion of different
angiogenic factors in lung cancer cells. J Cell Physiol.
231:1514–1521. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carmeliet P and Jain RK: Principles and
mechanisms of vessel normalization for cancer and other angiogenic
diseases. Nat Rev Drug Discov. 10:417–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jain RK: Normalization of tumor
vasculature: An emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nagy JA, Chang SH, Shih SC, Dvorak AM and
Dvorak HF: Heterogeneity of the tumor vasculature. Semin Thromb
Hemost. 36:321–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mierke CT: Cancer cells regulate
biomechanical properties of human microvascular endothelial cells.
J Biol Chem. 286:40025–40037. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schumacher D, Strilic B, Sivaraj KK,
Wettschureck N and Offermanns S: Platelet-derived nucleotides
promote tumor-cell transendothelial migration and metastasis via
P2Y2 receptor. Cancer Cell. 24:130–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang X, Hu J, Wu Z, Cafarello ST, Di
Matteo M, Shen Y, Dong X, Adler H, Mazzone M, Ruiz de Almodovar C,
et al: Protein phosphatase 2A mediates YAP activation in
endothelial cells upon VEGF stimulation and matrix stiffness. Front
Cell Dev Biol. 9:6755622021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Behnammanesh G, Durante ZE, Peyton KJ,
Martinez-Lemus LA, Brown SM, Bender SB and Durante W: Canagliflozin
inhibits human endothelial cell proliferation and tube formation.
Front Pharmacol. 10:3622019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pipili-Synetos E, Papadimitriou E and
Maragoudakis ME: Evidence that platelets promote tube formation by
endothelial cells on matrigel. Br J Pharmacol. 125:1252–1257. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wassmer SC, Taylor T, Maclennan CA,
Kanjala M, Mukaka M, Molyneux ME and Grau GE: Platelet-induced
clumping of Plasmodium falciparum-infected erythrocytes from
Malawian patients with cerebral malaria-possible modulation in vivo
by thrombocytopenia. J Infect Dis. 197:72–78. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin WF, Lu JY, Cheng BB and Ling CQ:
Progress in research on the effects of traditional Chinese medicine
on the tumor microenvironment. J Integr Med. 15:282–287. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li Q, Chen Y, Zhao D, Yang S, Zhang S, Wei
Z, Wang Y, Qian K, Zhao B, Zhu Y, et al: LongShengZhi Capsule
reduces carrageenan-induced thrombosis by reducing activation of
platelets and endothelial cells. Pharmacol Res. 144:167–180. 2019.
View Article : Google Scholar : PubMed/NCBI
|