Introduction
Benign prostatic hyperplasia (BPH) is prostate
hyperplasia, which is one of the most common diseases of
middle-aged and elderly males clinically at present (1). Currently, the global lifetime
prevalence rate of BPH is 26.2%, and the morbidity in urban areas
is markedly higher than that in rural areas (2). However, with the change of disease
incidence, a great number of studies have revealed that BPH is
diagnosed at younger ages (3,4). BPH is
a benign disease with a slow progression; however, it is possible
to have a worsening crisis if it is not properly controlled
(5). It can cause bladder neck
contracture, and repeated attacks cause urinary tract infection and
lower urinary tract obstruction. Symptoms of BPH patients include
obvious urination abnormalities and urinary incontinence (6). More serious cases may lead to prostate
cancer, endangering the life and health of patients (7). At present, conservative treatment is
mainly adopted for BPH clinically, and the most commonly used drugs
include 5α-reductase inhibitor, α1-receptor blocker and M receptor
antagonist, which can achieve certain efficacy for moderate and
severe cases (8). However, patients
who fail to receive conservative treatment and meet surgical
criteria select surgery for treatment. After prostatectomy,
patients are likely to experience dysuria, paruria, epididymitis,
and some may experience erectile dysfunction, interfering with
their sexual activity, which seriously affects their quality of
life (9). Therefore, timely
treatment in the early stage of BHP progression has a crucial
impact on the prognosis of patients. However, the diagnosis of BHP
requires a series of examinations including rectal digital
examination, B-ultrasound, residual urine and urinalysis (10) that are not conducive to early
diagnosis. Therefore, fully understanding the pathogenesis of BPH
is the key to prevent and treat BPH in the future.
Aldo-keto reductase family 1 member B10 (AKR1B10) is
located in the human chromosome 7q33 region. It can encode a
protein consisting of 316 amino acid residues, belonging to a
member of the aldo-keto reductase superfamily (11). To date, research on AKR1B10 has
mainly focused on liver and breast cancer. Research has revealed
that the mRNA level of AKR1B10 had a certain predictive value for
the recurrence of hepatitis B virus-related liver cancer patients,
and the positive rate of AKR1B10 in red blood cells of breast
cancer patients was markedly higher than that of healthy
individuals (12,13). However, its influence on BPH remains
unclear. AKR1B10 is a secretory protein belonging to a
lysosome-mediated non-classical protein-secretion pathway (14). Khan et al (15) considered that the aberrant expression
of nonclassical secretory proteins may be the key to BPH.
Therefore, it was theorized that AKR1B10 may be a key gene
affecting BPH, with marked significance for future clinical
diagnosis and treatment. In order to verify this theory, the
present study provided a reliable basis for future clinical study
by exploring the effect and mechanism of AKR1B10 in BPH.
Materials and methods
General data
In total, 142 BPH patients (51.2±11.6 years old) and
140 healthy individuals (50.8±8.9 years old) undergoing physical
examination from March 2017 to March 2019 were selected as the
research subjects. BPH patients were selected as the research group
and healthy people undergoing physical examination were regarded as
the control group. This experiment was approved by the Ethics
Committee of the First Hospital of Hunan University of Chinese
Medicine. All the aforementioned subjects signed informed consent
forms.
Inclusion and exclusion criteria
The inclusion criteria were as follows: i) patients
aged 20–70; ii) patients conforming to the clinical manifestation
of BPH and diagnosed with BPH after examination at the First
Hospital of Hunan University of Chinese Medicine; iii) patients
with complete medical case data; and iv) patients who agreed to
cooperate and participate in the investigation and study of the
First Hospital of Hunan University of Chinese Medicine. The
exclusion criteria were as follows: i) patients with tumor,
cardiovascular and cerebrovascular diseases, as well as other
autoimmune and infectious diseases; ii) patients with liver and
renal insufficiency due to organ failure; iii) patients with
gastrointestinal dysfunction; iv) patients without neurogenic
bladder dysfunction; v) diabetics; vi) patients who received
surgery, radiotherapy or chemotherapy, or drugs that affect the
function of bladder exfoliation or cause LUTS within half a year
before admission; vii) patients with nervous system diseases; viii)
patients with enuresis; ix) patients with a drug allergy; x)
patients with physical disabilities unable to take care of
themselves, bedridden; xi) patients with mental disorders and low
treatment compliance; xii) patients transferred from one hospital
to another. Inclusion and exclusion criteria in the control group
were as follows: i) all the results from the physical examination
at the First Hospital of Hunan University of Chinese Medicine were
normal; ii) no previous major medical history; iii) patients who
agreed to cooperate and participate in the investigation and study
of the First Hospital of Hunan University of Chinese Medicine.
Animal data
Twenty clean-grade 6-week-old male Srague-Dawley
rats were used as experimental subjects and purchased from Beijing
Charles River Laboratory Animal Technology Co., Ltd. with
certification number SCXK (Beijing) 2016-0011. weighing (210±20) g,
were kept in cages (5 in one cage) and maintained at a temperature
of 29±2°C, a humidity of 40–50% and a 12-h light/dark cycle. Food
and water were provided ad libitum. This experiment was
approved by the Animal Ethics Committee of the First Hospital of
Hunan University of Chinese Medicine.
Cell data
Human prostate hyperplasia cells line BPH-1 was
purchased from BeNa Culture Collection (BNCC339850). It was
adjusted to 1×105 cells/ml, transfected and cultured at
37°C under 95% oxygen and 5% CO2.
Methods
Reverse transcription-quantitative
(RT-q)PCR detection method
In total, 5 ml fasting venous blood was drawn from
patients in the research group and the control group before
treatment. The blood was left 30 min at room temperature and
centrifuged 10 min (1,505 × g, 4°C), and the upper serum was
obtained and stored in a refrigerator at −80°C for later use. The
PCR method was used to detect the expression of AKR1B10 and NF-κB
in the serum of patients. The collected serum was extracted with an
EasyPure miRNA kit (cat. no. ER601-01; Beijing TransGen Biotech
Co., Ltd.) according to the manufacturer's instructions, and
purity, concentration and integrity of the extracted total RNA was
tested by ultraviolet spectrophotometer and agarose gel
electrophoresis. The total RNA was reverse transcribed using
TransScript® miRNA RT Enzyme Mix and 2×TS miRNA Reaction
Mix (cat. no. AQ321-01; Beijing TransGen Biotech Co., Ltd.), and
the operation steps were strictly in accordance with the
manufacturer's kit. Then, PCR amplification was carried out. The
PCR reaction system was as follows: 1 µl cDNA, 0.4 µl each of
upstream and downstream primers, 10 µl 2×TransTaq® Tip
Green qPCR SuperMix, 0.4 µl Passive Reference Dye (50X),
ddH2O supplemented to 20 µl. The PCR reaction conditions
were as follows: Pre-denaturation at 94°C for 30 sec, denaturation
at 94°C for 5 sec, annealing at 60°C for 30 sec, 40 cycles in
total. Each sample was assessed in triplicate, and the experiment
was carried out 3 times. GAPDH was used as an internal reference
and the 2−ΔΔcq method was used for data analysis
(16). Primer sequences are
presented in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
|
| Reverse | Forward |
|---|
| AKR1B10 |
CAACACGTTACAGGCCCTCC |
ACCAGCACGCATTGTTGAGA |
| NF-κB |
TGAGAAGAGGGAGAGCAAGGAAGTC |
ACAGAAGCAGGCTGGAGGTAAGG |
| GAPDH |
GCGTCAAAGGTGGAGGAGTG |
TCAAGAAGGTGGTGAAGCAGG |
Detection of serum markers
Prostate specific antigen (PSA), epidermal growth
factor (EGF), interleukin (IL)-6 and tumor necrosis factor (TNF)-α
in peripheral blood of patients in the research group were
detected. PSA was tested by the laboratory of the First Hospital of
Hunan University of Traditional Chinese medicine and EGF, IL-6 and
TNF-α were measured through enzyme-linked immunosorbent assay
(ELISA). The EGF kit (cat. no. EH0009) was provided by Wuhan Fine
Biotech Co., Ltd., the IL-6 kit (cat. no. SEKH-0013) was purchased
from Solarbio Life Sciences, and the TNF-α kit (cat. no. JLC7047)
was purchased from Shanghai Jingkang Bioengineering Co., Ltd., and
were used according to the manufacturer's instructions.
Diagnostic value prediction
The levels of AKR1B10 and NF-kB of both groups were
analyzed by ROC curve, and the area under curve (AUC) and the
sensitivity and specificity of the two methods for predicting BPH
were analyzed.
Modeling method
Twenty rats were randomized into two groups (n=10),
and one group was employed as the normal group and the other group
was used as the model group to carry out BPH modeling; the method
was carried out according to a study from Ishola et al
(17): 10% chloral hydrate was
injected intraperitoneally at 350 mg/kg for anesthesia. After
complete anesthesia, the rat hair was removed; after routine
disinfection, the abdominal cavity was opened, bilateral testicles
were removed through the scrotum, and the skin was sutured after
ligation and hemostasis at the stump. Rats recovered on their own 7
days after castration, and testosterone propionate (5 mg/kg/time)
was injected subcutaneously on day 8, once a day, for 28 days. On
day 29, the rats were anesthetized by intraperitoneal injection of
chloral hydrate (as aforementioned) and then euthanized by cervical
dislocation.
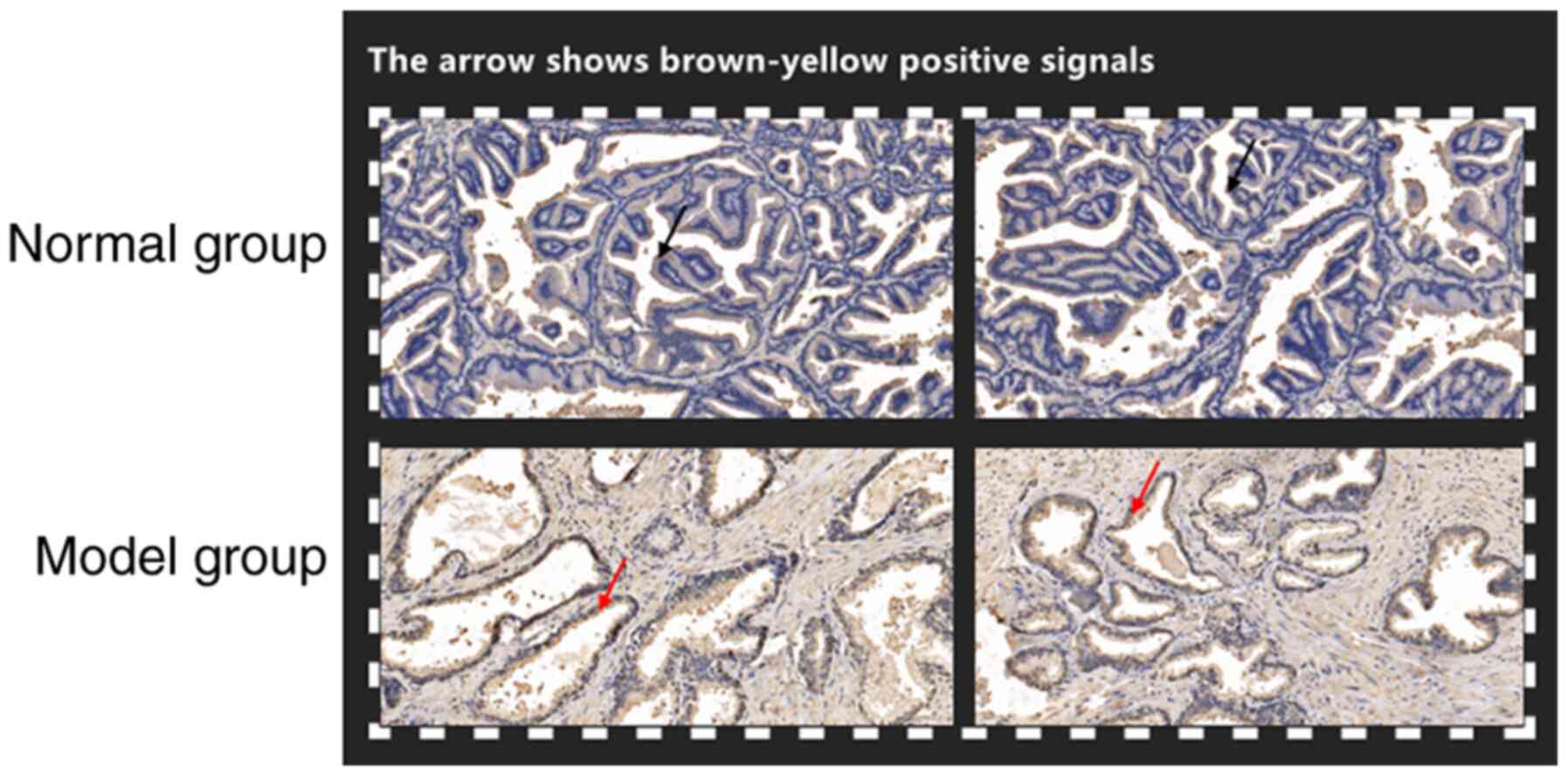
Immunohistochemical detection
method
The prostate tissues of the rats were obtained after
they were sacrificed, and one part was fixed in 4% paraformaldehyde
(4°C, 24 h) and the other part was frozen in liquid nitrogen. Then,
routine sampling, dehydration, paraffin embedding with 4-µm thick
sections, and IHC staining (37°C, 1–2 h) were carried out
(cytoplasmic staining; AKR1B10 monoclonal antibody; 1:500; cat. no.
H00057016; Abnova). The pathological changes of tissues were
observed under an optical microscope and images were captured for
analysis. Positive cell markers were revealed in tissue sections
with pale yellow to tan cells. The staining intensity was scored
based on the staining characteristics of most cells (the staining
depth was compared with the background staining): 0 for
non-staining, 1 point for pale yellow, 2 points for yellow-brown
and 3 for tan. The percentage of positive cells referred to the
average number of positive cells in 3 fields (×200) of certain
cells: 0–5% was 0, 6–25% was 1 point, 26–50% was 2 points, 51–75%
was 3 points, and >75% was 4 points.
Western blot detection methods
The total protein was extracted from frozen prostate
tissue by lysis method (cat. no. R0010; Solarbio Life Sciences),
and its concentration was assessed by BCA method and adjusted to 4
µg/µl. The protein was separated by 12% SDS-PAGE electrophoresis
and then transferred to a PVDF membrane (Molecular weight standard:
Lanes 1, 3, 5 and 7; calf liver lysate: Lanes 2, 4, 6 and 8;
loading amount per lane 20 µg; at 4°C for 10 min). The membrane was
stained with 0.2% Ponceau S working solution for 10 min at 4°C),
immersed 5 min in PBST and then washed, blocked for 2 h at 25°C
with 5% skimmed milk powder, and finally sealed overnight at 4°C
after adding caspase-3 (1:1,000; cat. no. R-1344-100; Biosensis,
Ltd.), caspase-9 (1:1,000; cat. no. 3016-30T; BioVision, Inc.), and
GPD1 (1:1,000; cat. no. H00002819-A01; Abnova) primary antibodies.
Subsequently, after it was washed to remove the primary antibody,
horseradish peroxidase-labeled goat anti-rabbit secondary antibody
(1:5,000) was added (cat. no. A-11034; Thermo Fisher Scientific,
Inc.), and the protein was incubated for 1 h at 37°C. Then, the
membrane was rinsed 3 times with PBS, 5 min each time. The protein
bands on the membrane were developed in a dark room using an
enhanced chemiluminescence reagent (product no. BL523B; Biosharp
Life Sciences), and the excess liquid on the membrane was absorbed
with a filter paper. The luminescent protein bands were scanned and
the gray value was analyzed using Quantity One (v4.6.6; Bio-Rad
Laboratories, Inc.). The relative expression of each protein was
calculated as follows: Relative protein expression=the gray value
of the target protein band/the β-actin protein band.
Cell culture
The BPH-1 cell line was placed in a culture medium
containing 90% RPMI-1640 medium+10% FBS and cultured at 37°C and 5%
CO2. When the adherent growth and fusion reached 85%,
25% pancreatin was added for digestion. Then, the BPH-1 cell line
was placed in a culture medium for continuous culture to complete
passage. The concentration of the primer sequences was 10 µmol/l,
and AKR1B10-mimics (overexpression sequence: Forward,
5′-CGGGGTACCAGATTCAACCAAAGCCAACTCATC-3′ and reverse,
5′-CCGCTCGAGGTAGAAGTCTCACGTCCTGCTCTC-3′); AKR1B10-mimics-NC
(forward,
5′-CCAACTTTTGGCTGTGTTGAATTTGAAGAGTGAGCATGAACAAGCAGAAACTCCAATGATAC-3′
and reverse,
5′-GTATCATTGGAGTTTCTGCTTGTTCATGCTCACTCTTCAAATTCAACACAGCCAAAAGTTGG-3′);
and AKR1B10-inhibitor (inhibitory expression sequence: Forward,
5′-CGGGGTACCATGATGGACTTGGAGCTGC-3′ and reverse,
5′-CCGCTCGAGCTAGTTTTTCTTAACATCTGGCTTC-3′); AKR1B10-inhibitor-NC
(forward, 5′-CAACAGAGAGCAGGACGTGAGACT-3′ and reverse,
5′-GCATCTTGGCTTTGGTACTGAGCTC-3′) were used to transfect cells with
a Lipofectamine 2000 kit (cat. no. 11668019; Thermo Fisher
Scientific, Inc.), and the operation steps were strictly carried
out in accordance with the kit instructions. The primer sequences
were designed by Thermo Fisher Scientific, Inc. and their size was
~1,100 bp. The transfection temperature was 37°C, and 36 h after
transfection, the endogenous peroxidase was cleared by hydrogen
peroxide disinfector (PCR laboratory hydrogen peroxide disinfector;
Shenzhen Runlian Huanbao Technology Co., Ltd.).
Cell Counting Kit-8 (CCK-8)
detection
Cells were collected 24 h after transfection,
adjusted to 4×106 cells/well and inoculated on 96-well
plates. Then, after being cultured for 0, 24, 48 and 72 h, 10 µl
CCK-8 (product no. BS350B; Biosharp Life Sciences) solution and 90
µl basic medium (DMEM) were added to each well, and cultured for 2
h at 37°C. Finally, the OD values in each group were measured at an
absorbance of 450 nm using an enzyme reader.
Flow cytometry
The transfected cells were digested with 0.25%
trypsin, washed twice with PBS, added using 100 µl binding buffer
and prepared into 1×106 cells/ml suspension. Annexin
V-FITC and PI (product no. 40302ES20; Shanghai Yeasen Biotechnology
Co., Ltd.) used according to the manufacturer's instructions, were
sequentially added, and incubated 5 min at room temperature under
dark conditions. Detection was performed using a FC500MCL flow
cytometer and CytExpert software (version 2.0; Beckman Coulter,
Inc.). FlowJo version 10.0 (Tree Star, Inc.) was also used for
analysis. The experiment was repeated 3 times and data were
averaged.
Outcome measures
The outcome measures were as follows: i) the AKR1B10
and NF-κB levels in peripheral blood and the levels of PSA, EGF,
IL-6 and TNF-α in the two groups; ii) the correlation between
AKR1B10 and PSA, EGF, IL-6, TNF-α in the research group; iii) the
AKR1B10 and NF-κB levels in peripheral blood and prostate tissue of
rats; iv) the proliferation and apoptosis of transfected cells and
the NF-κB protein expression.
Statistical analysis
The results were analyzed by SPSS 24.0 (Shanghai
Yuchuang Network Technology Co., Ltd.) and all graphical results
were drawn using GraphPad 8 (Shenzhen Qiruitian Software Technology
Co., Ltd.). The counting data were expressed in the form of a rate,
and the chi-square test was used for comparison between groups. The
measurement data were expressed in the form of the mean ± standard
deviation (SD). Inter-group comparisons were analyzed by
Mann-Whitney U test, multi-group comparisons were assessed by
one-way ANOVA and LSD post hoc test, and multiple time-points were
compared using repeated measures ANOVA and Bonferroni post hoc
test. The diagnostic predictive value was analyzed by receiver
operating characteristic (ROC) curve, and Pearson correlation
coefficient was used for correlation analysis. P<0.050 was
considered to indicate a statistically marked difference.
Results
Comparison of general data
There was no obvious difference in age, BMI,
smoking, drinking, exercise habits, place of residence, nationality
and family medical history between the research group and the
control group (P>0.050; Table
II)
 | Table II.Comparison of general data between
research group and control group [n (%)]. |
Table II.
Comparison of general data between
research group and control group [n (%)].
|
| Research group
(n=142) | Control group
(n=140) | t or
χ2 | P-value |
|---|
| Age (years) |
|
| 0.744 | 0.458 |
|
|
56.6±7.6 |
57.3±8.2 |
|
|
| BMI
(kg/cm2) |
|
| 0.918 | 0.360 |
|
|
24.62±2.16 |
24.87±2.41 |
|
|
| Smoking |
|
| 0.919 | 0.338 |
| Yes | 95
(66.90) | 86
(61.43) |
|
|
| No | 47
(33.10) | 54
(38.57) |
|
|
| Drinking |
|
| 0.689 | 0.407 |
|
Yes | 92
(64.79) | 84
(60.00) |
|
|
| No | 50
(35.21) | 56
(40.00) |
|
|
| Exercise
habits |
|
| 0.982 | 0.322 |
|
Yes | 22
(15.49) | 28
(20.00) |
|
|
| No | 120 (84.51) | 112 (80.00) |
|
|
| Place of
residence |
|
| 1.735 | 0.188 |
| Cities
and towns | 118 (83.10) | 124 (88.57) |
|
|
|
Countryside | 24
(16.90) | 16
(11.43) |
|
|
| Nationality |
|
| 0.13 | 0.719 |
|
Han | 138 (97.18) | 135 (96.43) |
|
|
|
Minority | 4
(2.82) | 5
(3.57) |
|
|
| Family medical
history |
|
| 1.151 | 0.283 |
|
Yes | 36
(25.35) | 28
(20.00) |
|
|
| No | 106 (74.65) | 112 (80.00) |
|
|
Comparison of AKR1B10 and NF-κB
expression in peripheral blood
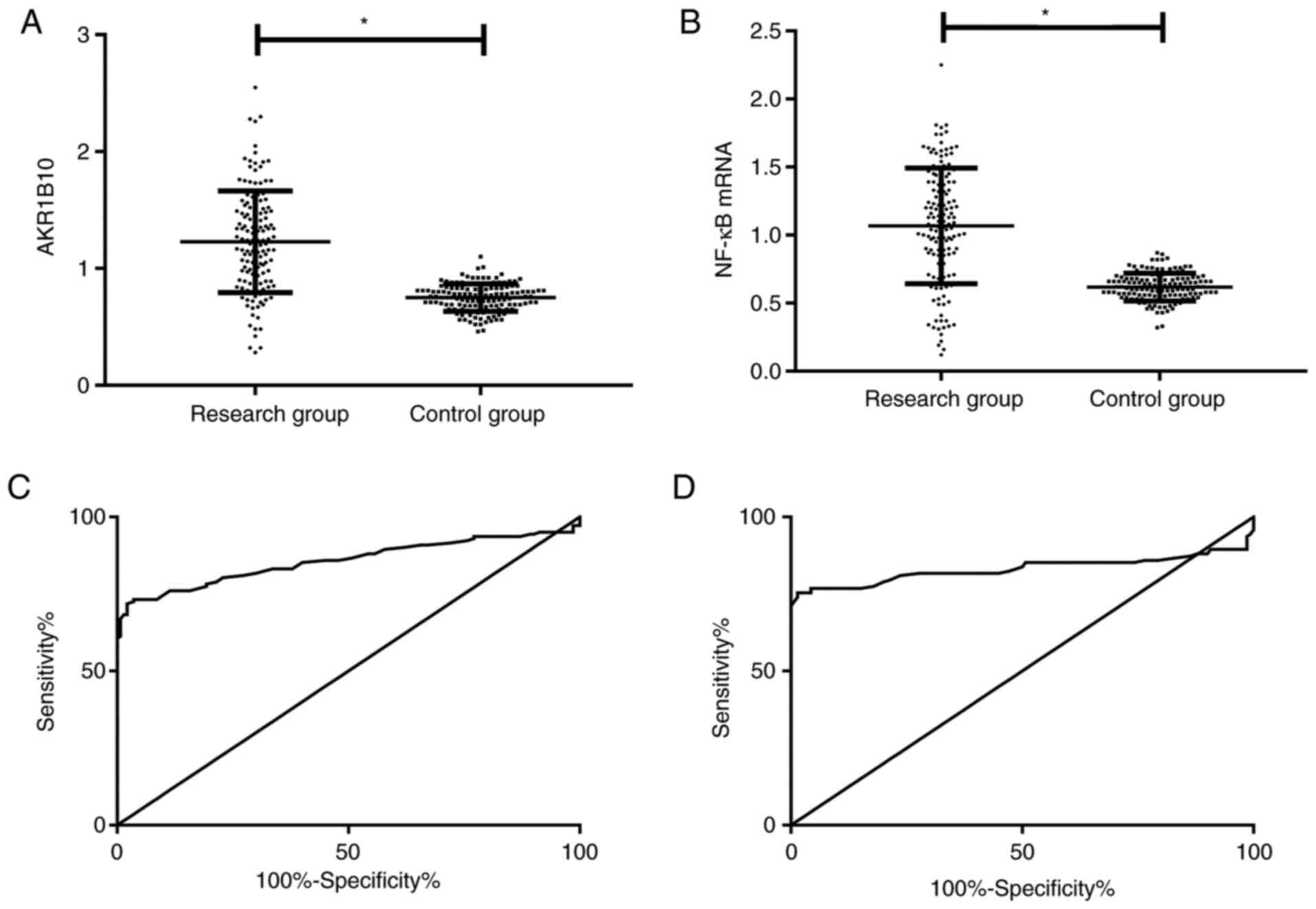
AKR1B10 and NF-κB in the peripheral blood of the
research group were markedly higher than those of the control group
(P<0.001). ROC curve analysis revealed that when the cut-off
value was 0.955, AKR1B10 had a sensitivity of 71.83% and a
specificity of 97.86% for predicting BPH; when the cut-off value
was 0.840, the sensitivity and specificity of NF-κB mRNA were 75.35
and 98.57% (Table III and Fig. 1).
 | Table III.Diagnostic values of AKR1B10 and
NF-κB for BPH. |
Table III.
Diagnostic values of AKR1B10 and
NF-κB for BPH.
| Parameters | AKR1B10 | NF-κB |
|---|
| Cut-off | 0.955 | 0.840 |
| Sensitivity
(%) | 71.83 | 75.35 |
| Specificity
(%) | 97.86 | 98.57 |
| AUC | 0.857 | 0.831 |
| Std. Error | 0.025 | 0.029 |
| 95% CI | 0.809–0.906 | 0.774–0.888 |
| P-value | <0.001 | <0.001 |
Correlation between AKR1B10 in
peripheral blood and clinical indicators
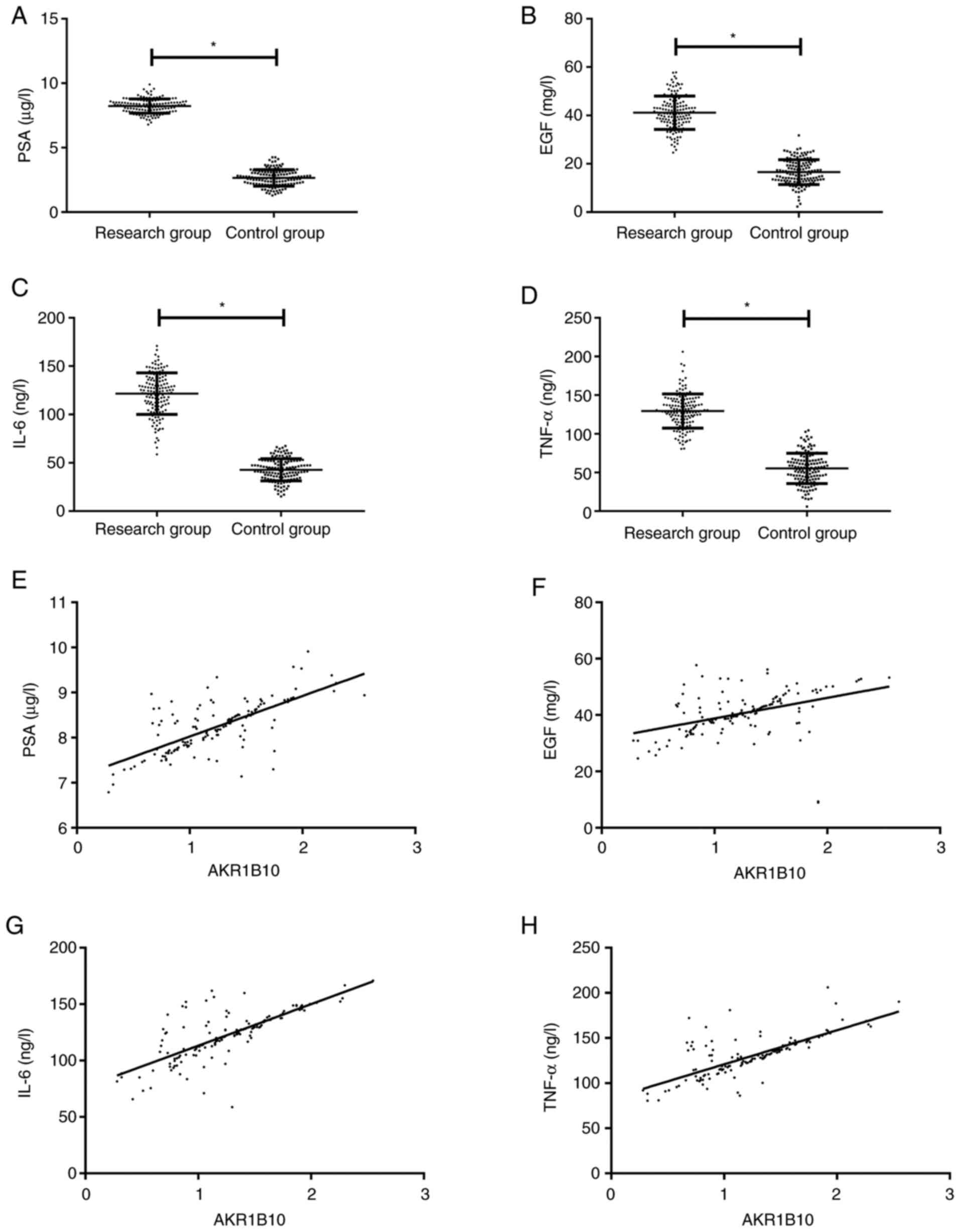
PSA, EGF, IL-6 and TNF-α in the peripheral blood of
the research group were dramatically higher than those of the
control group (P<0.001). Pearson correlation coefficient
analysis demonstrated that AKR1B10 in the research group was
positively correlated with PSA, EGF, IL-6 and TNF-α (r=0.704,
0.415, 0.745, 0.742, respectively; P<0.001; Fig. 2).
Comparison of AKR1B10 and NF-κB
expression in rats
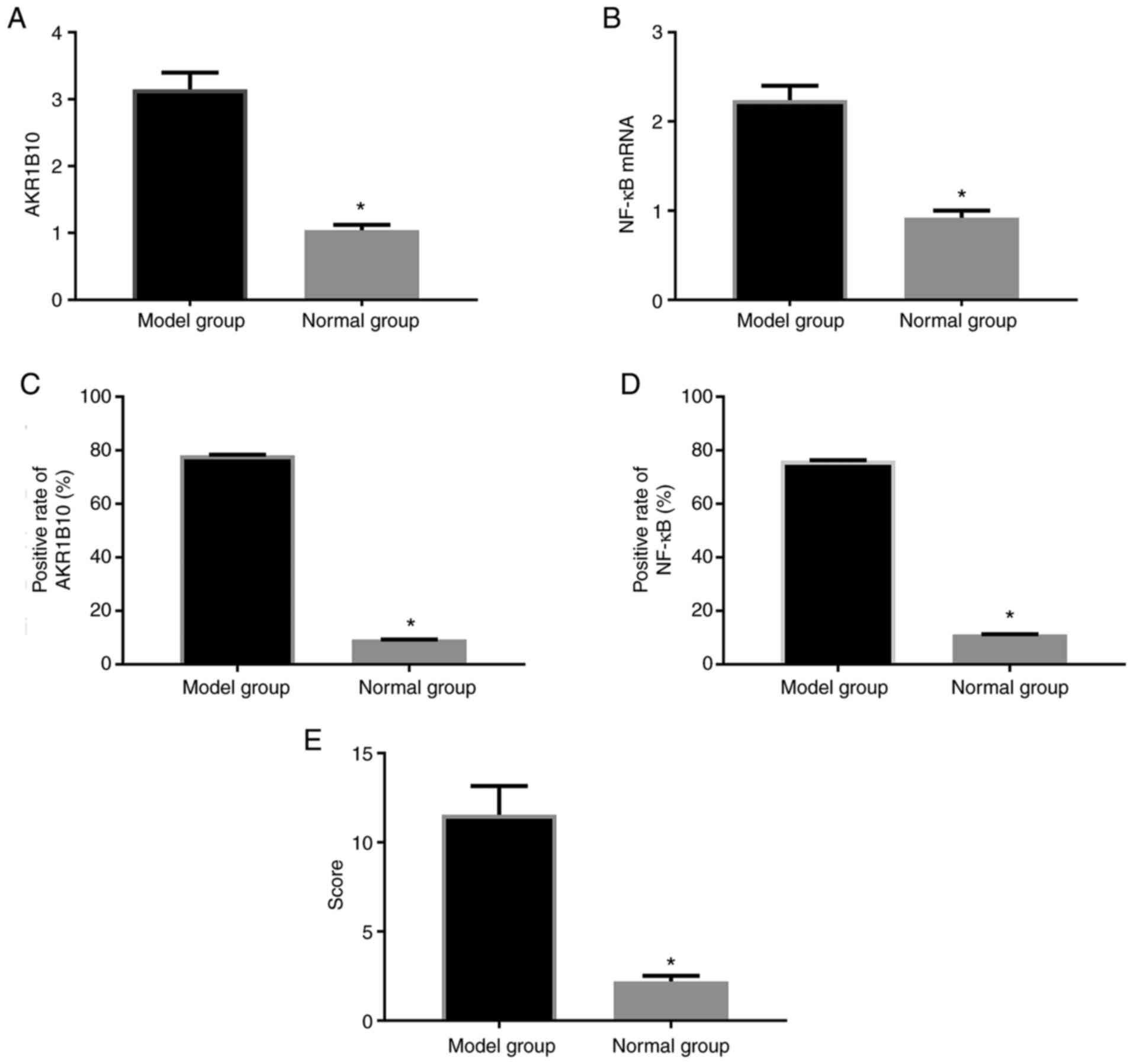
For the 10 modeling rats, all were successfully
modeled, with a modeling rate of 100.0%. There was no peritonitis
observed in the rats. AKR1B10 and NF-κB mRNA levels in the prostate
tissue of rats in the model group were obviously higher than those
in the normal group (P<0.001). The positive rates of AKR1B10 and
NF-κB in tissues were also markedly higher than those in the normal
group (P<0.001). Semi-quantitative analysis of tissue sections
revealed that the model group was higher than the control group
(P<0.001; Figs. 3 and 4).
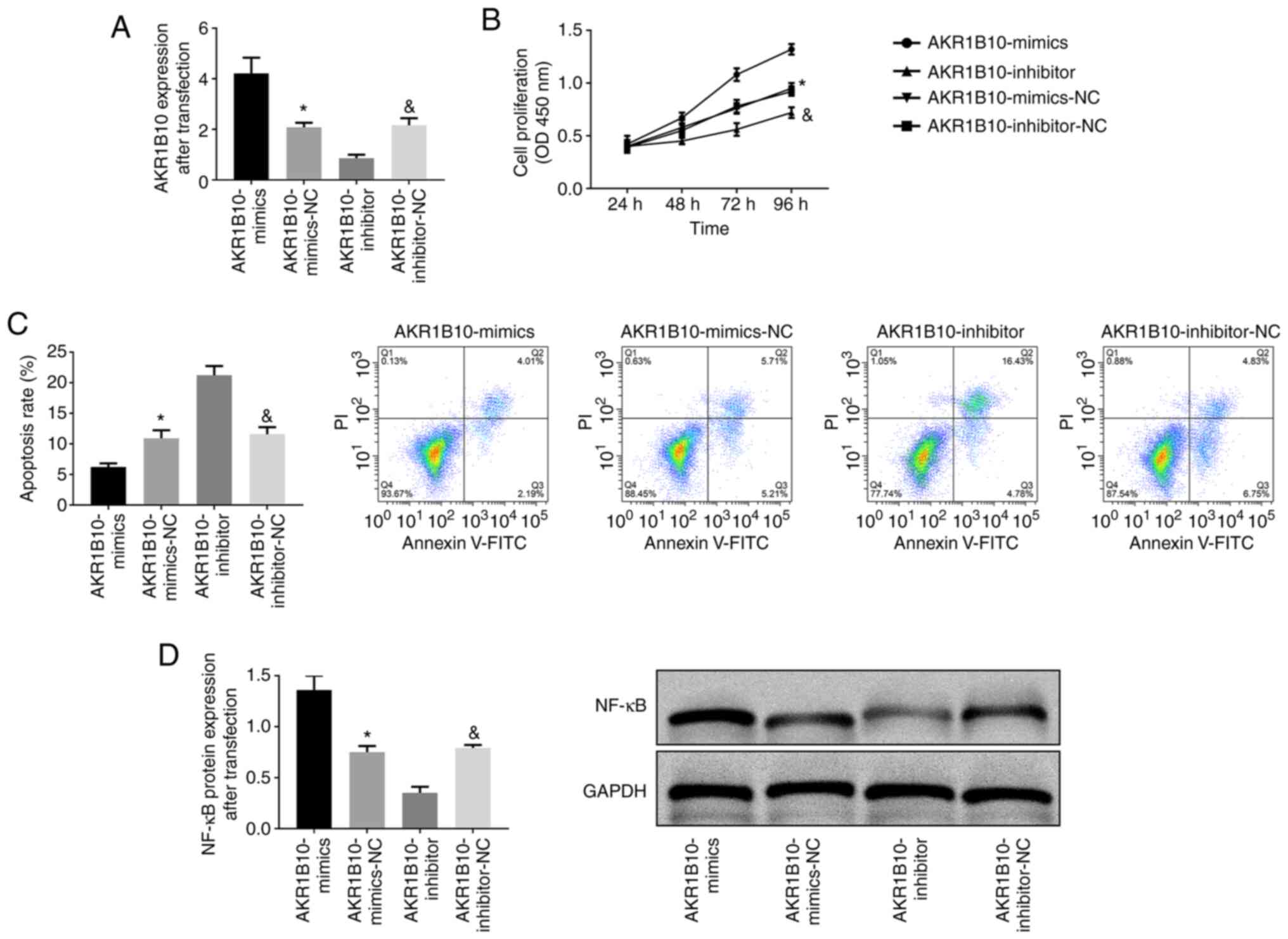
Effect of AKR1B10 on the biological
behavior of prostate hyperplasia cells
AKR1B10 was transfected into the BPH-1 cell line and
the expression in AKR1B10-mimics, AKR1B10-inhibitor and AKR1B10-NC
was detected. It was revealed that the AKR1B10-mimics group was
dramatically higher than the other two groups (AKR1B10-NC and
AKR1B10-inhibitor), while the AKR1B10-inhibitor group exhibited the
lowest expression (P<0.001). According to the CCK-8 experiment,
the cell proliferation of the AKR1B10-mimics group was the highest
among the three groups (P<0.001). Flow cytometric analysis
revealed that the apoptosis rate in the AKR1B10-mimics group was
the lowest among the three groups, and the apoptosis rate in the
AKR1B10-inhibitor group was the highest (P<0.001). Western
blotting revealed that the NF-κB protein expression in the
AKR1B10-mimics group increased, while that in the AKR1B10-inhibitor
group decreased (P<0.001; Fig.
5).
Discussion
BPH is currently one of the most common male
diseases among middle-aged and elderly men in the world. With the
increasingly serious aging of the global population, the morbidity
of BPH is also rising (16).
Understanding BPH pathogenesis is quite marked for future
prevention and treatment. AKR1B10, belonging to a class of carbonyl
compounds that reduces aldehydes and ketones, not only has a
protective effect on cells when they suffer from carbonyl toxicity
damage, but can also stabilize acetyl-CoA carboxylase α, block its
degradation process through the ubiquitination pathway, and cause
lipid synthesis in tumor cells (17). Previous studies revealed that AKR1B10
acted as a cancer-promoting factor in gastric cancer and breast
cancer (18,19). However, its role in BPH is ambiguous.
The present study is aimed to explore the role of AKR1B10 in BPH
and reveal its mechanism influencing the biological behavior
through NF-κB.
It demonstrated that the levels of AKR1B10 and NF-κB
mRNA in peripheral blood of BPH patients in the research group were
dramatically higher than those in the control group, suggesting
that the two may be involved in BPH development and progression. In
fact, when Ko et al (20) and
Hung et al (21) studied the
effects of AKR1B10 on oral cancer and lung adenocarcinoma, they
revealed that AKR1B10 was also markedly overexpressed, which
supports our present results. However, Sinreih et al
(22) demonstrated that AKR1B10 was
markedly reduced in endometrial cancer and speculated that it had
different effects in different diseases. AKR1B10 is a soluble
monomer oxidoreductase in the cytoplasm, which can catalyze various
endogenous and exogenous aldosterone-dependent NADH reduction
reactions, mainly existing in embryonic liver and hepatocellular
carcinoma tissues (23). However,
ROC curve analysis revealed that AKR1B10 had a good predictive
value for BPH occurrence and was positively associated with PSA,
EGF, IL-6, TNF-α in the research group, which further confirmed the
important influence of AKR1B10 on BPH occurrence. PSA, as the most
sensitive indicator to reflect prostate state, is currently the
preferred marker for diagnosing prostate cancer (24), and its physiological function is
mainly to prevent semen coagulation. Usually the level in healthy
individuals is extremely low, but PSA markedly increases once
prostate disease or urogenital system disease occurs (25). However, AKR1B10 was also revealed to
increase with PSA increase, which demonstrated that it had better
monitoring value for BPH development. Moreover, AKR1B10 is better
than PSA in that it has higher specificity and its increase is not
as marked as PSA, which also suggests that it can be used as a
reference indicator for future occurrence and development of BPH
clinically.
To further verify the relationship between AKR1B10,
NF-κB and BPH, a BPH rat model was established and it was observed
by section staining that the prostate tissue of rats exhibited a
positive staining rate of AKR1B10 and NF-κB compared to the tissue
obtained from the normal group of rats. In addition, it was also
determined that the prostate tissue of the BPH rat model had
markedly higher mRNA expression of AKR1B10 and NF-κB than that of
normal rats. Furthermore, by transfecting AKR1B10 into prostate
hyperplasia cells, we found that inhibiting AKR1B10 expression
reduced the proliferation and apoptosis rates of prostate
hyperplasia cells, and the protein expression of NF-κB also
decreased. Therefore, it was inferred that the mechanism involved
in the effect of AKR1B10 on BPH may be through NF-κB regulation.
Zhang et al (26) also
demonstrated that NF-κB had a promoting effect on prostate cancer,
which supported our experimental results.
The present study was designed to explore the
influence and mechanism of AKR1B10 on BPH. However, due to the
limited experimental conditions, deficiencies remain. The present
study focused on the influence of AKR1B10 on BPH through NF-κB, but
it does not exclude the possibility of other influences through
other pathways, which will be a key direction of our future
research. Moreover, due to the short experimental period, it is
impossible to ascertain how AKR1B10 affects the long-term prognosis
of BPH. We will conduct a more in-depth and comprehensive analysis
of the aforementioned limitations to obtain more concrete
experimental results.
In summary, high expression of AKR1B10 in BPH
promoted the proliferation and reduced the apoptosis of prostate
cells, and its mechanism might be through the regulation of
NF-κB.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81603634), the Hunan
Male Diseases Clinical Medical Research Center of Traditional
Chinese Medicine (grant no. 2018SK4012), the Hunan Clinical Medical
Technology Innovation Guidance Plan (grant no. 2017SK50304) and the
Hunan Scientific Research Project of Traditional Chinese Medicine
(grant no. 201732).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WX designed and conceived the study and wrote the
manuscript. YG performed the PCR, ELISA and western blotting
experiments. JZ was responsible for CCK-8 detection and flow
cytometry. RZ analyzed and interpreted the data of patients. QC
helped with the statistical analysis. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Hospital of Hunan University of Chinese
Medicine (Changsha, China). Patients who participated in this
research, signed the informed consent and had complete clinical
data. Signed written informed consents were obtained from the
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chughtai B, Forde JC, Thomas DD, Laor L,
Hossack T, Woo HH, Te AE and Kaplan SA: Benign prostatic
hyperplasia. Nat Rev Dis Primers. 2:160312016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Egan KB: The epidemiology of benign
prostatic hyperplasia associated with lower urinary tract symptoms:
Prevalence and incident rates. Urol Clin North Am. 43:289–297.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee YJ, Lee JW, Park J, Seo SI, Chung JI,
Yoo TK and Son H: Nationwide incidence and treatment pattern of
benign prostatic hyperplasia in Korea. Investig Clin Urol.
57:424–430. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim EH, Larson JA and Andriole GL:
Management of benign prostatic hyperplasia. Annu Rev Med.
67:137–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lim KB: Epidemiology of clinical benign
prostatic hyperplasia. Asian J Urol. 4:148–151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Al-Khalil S, Boothe D, Durdin T, Sunkara
S, Watkins P, Yang S, Haynes A and de Riese W: Interactions between
benign prostatic hyperplasia (BPH) and prostate cancer in large
prostates: A retrospective data review. Int Urol Nephrol. 48:91–97.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khazaei S, Rezaeian S, Ayubi E,
Gholamaliee B, Pishkuhi MA, Khazaei S, Mansori K, Nematollahi S,
Sani M and Hanis SM: Global prostate cancer incidence and mortality
rates according to the human development index. Asian Pac J Cancer
Prev. 17:3793–3796. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Corona G, Tirabassi G, Santi D, Maseroli
E, Gacci M, Dicuio M, Sforza A, Mannucci E and Maggi M: Sexual
dysfunction in subjects treated with inhibitors of 5α-reductase for
benign prostatic hyperplasia: A comprehensive review and
meta-analysis. Andrology. 5:671–678. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang L, Wu B, Zha Z, Zhao H, Yuan J,
Jiang Y and Yang W: Surgical margin status and its impact on
prostate cancer prognosis after radical prostatectomy: A
meta-analysis. World J Urol. 36:1803–1815. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Macey MR and Raynor MC: Diagnosis of
benign prostatic hyperplasia. Chapter 2. Prostatic Artery
Embolization. Isaacson AJ, Bagla S, Raynor MC and Yu H: Springer;
Cham: pp. 11–19. 2020, View Article : Google Scholar
|
|
11
|
He YC, Shen Y, Cao Y, Tang FQ, Tian DF,
Huang CF, Tao H, Zhou FL, Zhang B, Song L, et al: Overexpression of
AKR1B10 in nasopharyngeal carcinoma as a potential biomarker.
Cancer Biomark. 16:127–135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang YY, Qi LN, Zhong JH, Qin HG, Ye JZ,
Lu SD, Ma L, Xiang BD, Li LQ and You XM: High expression of AKR1B10
predicts low risk of early tumor recurrence in patients with
hepatitis B virus-related hepatocellular carcinoma. Sci Rep.
7:421992017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reddy KA, Kumar PU, Srinivasulu M, Triveni
B, Sharada K, Ismail A and Reddy GB: Overexpression and enhanced
specific activity of aldoketo reductases (AKR1B1 & AKR1B10) in
human breast cancers. Breast. 31:137–143. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nishinaka T, Miura T, Shimizu K and Terada
T: Identification and characterization of functional antioxidant
response elements in the promoter of the aldo-keto reductase
AKR1B10 gene. Chem Biol Interact. 276:160–166. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khan S, Bennit HF and Wall NR: The
emerging role of exosomes in survivin secretion. Histol
Histopathol. 30:43–50. 2015.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishola IO, Yemitan KO, Afolayan OO,
Anunobi CC and Durojaiye TE: Potential of Moringa oleifera
in the treatment of benign prostate hyperplasia: Role of
antioxidant defence systems. Med Princ Pract. 27:15–22. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao Z, Zhou B, Chen X, Huang D, Zhang X,
Wang Z, Huang H, Wang Y and Cao D: Statil suppresses cancer cell
growth and proliferation by the inhibition of tumor marker AKR1B10.
Anticancer Drugs. 25:930–937. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao HB, Xu Y, Chen LG, Guan TP, Ma YY, He
XJ, Xia YJ, Tao HQ and Shao QS: AKR1B10, a good prognostic
indicator in gastric cancer. Eur J Surg Oncol. 40:318–324. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ko HH, Cheng SL, Lee JJ, Chen HM, Kuo MY
and Cheng SJ: Expression of AKR1B10 as an independent marker for
poor prognosis in human oral squamous cell carcinoma. Head Neck.
39:1327–1332. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hung JJ, Yeh YC and Hsu WH: Prognostic
significance of AKR1B10 in patients with resected lung
adenocarcinoma. Thorac Cancer. 9:1492–1499. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sinreih M, Štupar S, Čemažar L, Verdenik
I, Frković Grazio S, Smrkolj Š and Rižner TL: STAR and AKR1B10 are
down-regulated in high-grade endometrial cancer. J Steroid Biochem
Mol Biol. 171:43–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Taskoparan B, Seza EG, Demirkol S, Tuncer
S, Stefek M, Gure AO and Banerjee S: Opposing roles of the
aldo-keto reductases AKR1B1 and AKR1B10 in colorectal cancer. Cell
Oncol (Dordr). 40:563–578. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Preston MA, Batista JL, Wilson KM,
Carlsson SV, Gerke T, Sjoberg DD, Dahl DM, Sesso HD, Feldman AS,
Gann PH, et al: Baseline prostate-specific antigen levels in
midlife predict lethal prostate cancer. J Clin Oncol. 34:2705–2711.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Filella X and Foj L: Prostate cancer
detection and prognosis: From prostate specific antigen (PSA) to
exosomal biomarkers. Int J Mol Sci. 17:17842016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang L, Zhang Q, Li L, Wang Z, Ying J,
Fan Y, He Q, Lv T, Han W, Li J, et al: DLEC1, a 3p tumor
suppressor, represses NF-κB signaling and is methylated in prostate
cancer. J Mol Med (Berl). 93:691–701. 2015. View Article : Google Scholar : PubMed/NCBI
|