Introduction
Esophageal cancer is one of the most aggressive
types of cancer, with high morbidity and mortality worldwide
(1). There are two most common
histological subtypes: Squamous cell carcinoma and adenocarcinoma.
Despite marked improvements of therapies, the overall survival rate
of patients with esophageal cancer remains unsatisfactory (2). The poor prognosis of esophageal cancer
is attributed to early metastasis (3). Although it has been determined that
certain tumor suppressors and oncogenes serve a key role in the
occurrence and development of esophageal cancer, it is urgent to
identify molecular markers to improve the early diagnosis and
prognosis of esophageal cancer (3).
Mitochondrial calcium uniporter (MCU) has been
identified as a channel responsible for mitochondrial
Ca2+ absorption, which may control cell energy
metabolism, ROS production and programmed cell death (4). All cancer tissues exhibit moderate to
strong MCU immunostaining (5).
Increasing evidence has suggested that MCU serves an important role
in cancer metastasis. For example, MCU is highly expressed in
metastatic breast cancer and induces cancer metastasis through the
Warburg effect (6). MCU-knockdown
significantly suppresses cell migration and invasion in
triple-negative breast cancer xenografts and decreases tumor
growth, lymph node infiltration and lung metastasis (7). MCU is closely associated with
metastasis and poor prognosis of patients with hepatocellular
carcinoma (8). MCU expression is
upregulated in pancreatic cancer tissues compared with in normal
tissues, which may be partly mediated by the HINT2 protein
(9). Furthermore, MCU is involved in
various cellular biological processes. For example, MCU loss
hinders cell cycle progression and proliferation (10). However, the expression and clinical
implications of MCU in esophageal cancer remain unclear. In the
present study, the expression levels of MCU were detected in 110
esophageal cancer tissues and its role in esophageal cancer was
analyzed.
Materials and methods
Bioinformatics analysis
The expression levels of MCU were assessed between
tumor and normal samples in various types of cancer via Gene
Expression Profiling Interactive Analysis (GEPIA; http://gepia2.cancer-pku.cn/#index). MCU
expression between esophageal cancer samples (n=182) and normal
tissues (n=286) was analyzed in The Cancer Genome Atlas (TCGA;
http://portal.gdc.cancer.gov/) normal
and GTEx datasets (http://www.gtexportal.org/home/). The differences in
MCU expression among different tumor stages were compared in
TCGA-Esophageal carcinoma (ESCA) dataset (https://portal.gdc.cancer.gov/projects?filters=%7B%22op%22%3A%22and%22%2C%22content%22%3A%5B%7B%22op%22%3A%22in%22%2C%22content%22%3A%7B%22field%22%3A%22projects.project_id%22%2C%22value%22%3A%5B%22TCGA-ESCA%22%5D%7D%7D%5D%7D).
Patients and specimens
Patients with esophageal cancer who underwent
esophagectomy were enrolled in the General Hospital of Ningxia
Medical University (Yinchuan, China) between January 2016 to
December 2017. The inclusion criteria were as follows: i) Patients
were diagnosed with esophageal cancer by at least two experienced
pathologists according to histopathology examination; and ii)
patients did not receive chemotherapy or radiation therapy before
surgery. Finally, a total of 110 patients (mean age, 58.6 years old
and age range, 42.6–75.3 years old) with esophageal cancer were
included in the study. All tumor and adjacent normal tissues were
collected from patients and the distance of the normal tissues was
at least 5 cm from the tumor tissues. All specimens were
immediately transferred to liquid nitrogen after surgery, and
stored at −80°C until use. Clinical information including sex, age,
depth of invasion, lymph node metastasis, TNM stage and distant
metastasis was also obtained. The present study strictly followed
the guidelines of the Declaration of Helsinki. All patients
provided written informed consent. The study was approved by the
ethics committee of General Hospital of Ningxia Medical University
(approval no. 2016049).
Hematoxylin and eosin (H&E)
staining
Esophageal cancer and normal tissue specimens were
fixed by 4% paraformaldehyde solution at 4°C for 24 h. After being
embedded in paraffin, the tissues were cut into 20-µm pieces. The
sections were stained with 0.5% hematoxylin for 5 min at 37°C and
0.5% eosin solution for 5 min at 37°C. Neutral gum was utilized for
sealing and images were observed under an light microscope (Olympus
Corporation) (magnification, ×200).
Immunohistochemistry
Fresh tissue specimens were fixed with 4%
paraformaldehyde overnight at 4°C and dehydrated using different
concentrations of alcohol. The specimen was incubated in a 1:1
mixture of 100% alcohol and xylene for 30 min at room temperature
and then transferred to xylene for 30 min at 37°C. Subsequently,
the tissues were embedded in paraffin, and the tissue sections were
cut to a thickness of 4 µm. The sections were placed in a 60°C
incubator for 90 min and then quickly placed in xylene I for 15 min
and xylene II for 15 min at 37°C. Subsequently, the tissue sections
were placed in absolute ethanol I, absolute ethanol II, 95, 90, 80,
70 and 50% ethanol for 5 min each at 37°C, followed by deionized
water for 5 min. Antigen retrieval was performed using the
high-pressure method (11). After
washing 3 times with 1X PBS, the sections were incubated with 3%
hydrogen peroxide at 37°C for 20 min to eliminate endogenous
peroxidase activity. After washing again, the sections were blocked
with 2% BSA (Beyotime Institute of Biotechnology) at 37°C for 2 h.
The tissue sections were incubated with primary antibodies
including anti-MCU (1:100; cat. no. sc-515930; Santa Cruz
Biotechnology, Inc.), anti-VEGF (1:100; cat. no. 26381-1-AP;
ProteinTech Group, Inc.), anti-Vimentin (1:150; cat. no. ab92547;
Abcam), anti-E-cadherin (1:200; cat. no. ab40772; Abcam), anti-MMP2
(1:100; cat. no. 10373-2-AP; ProteinTech Group, Inc.), and
anti-N-cadherin (1:100; cat. no. ab18203; Abcam) overnight at 4°C,
followed by secondary antibody (1:200; cat. no. ab150077; Abcam)
incubation at 37°C for 1 h. The sections were stained with DAB for
5 min and hematoxylin for 2 min at 37°C. Subsequently, the sections
were placed in hydrochloric acid monoethanol for color separation
for 30 sec and turned back to blue with 1X PBS for 2 min. After
dehydration and transparency, neutral gum was added to the tissue
sections, followed by air drying.
Immunohistochemistry scoring
assessment
Double-blind readings were performed by two
experienced pathologists. The positive expression of target
proteins was the appearance of brown particles on the cell membrane
and/or cytoplasm. In the present study, semi-quantitative results
were used to evaluate the percentage of positive cells and staining
intensity, respectively (12). The
number of positively stained cells was observed under 5 high-power
fields using a light microscope (Olympus Corporation)
(magnification, ×200) on each slide, and then the percentage of
positive cells was counted and scored as follows: 0, <5%
Positive cells; 1, 5–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100%.
Staining intensity was scored as follows: 0, Colorless; 1, light
yellow; 2, claybank; and 3, sepia. These two scores were multiplied
to obtain the positive grade: 0, Negative (−); 1–4, weak positive
(+); 5–8, positive (++); and 9–12, strong positive (+++). The
optical density of target proteins was measured using ImageJ
software v. 1.48 (National Institutes of Health).
Western blot analysis
Tissues and cells were lysed by RIPA cell lysate
(Beyotime Institute of Biotechnology) plus protease inhibitor PMSF
and phosphatase inhibitor cocktail for 40 min on ice, followed by
centrifugation at 12,000 × g for 15 min at 4°C. Subsequently, the
supernatant was harvested and stored at −80°C. The BCA method was
used to measure the total protein concentration according to the
instructions of the BCA™ Protein Assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Protein (30 µg) was loaded per lane and protein
samples were separated via 12% SDS-PAGE and then transferred to a
PVDF membrane (IPVH00010; EMD Millipore). The membrane was blocked
with 5% skimmed milk at room temperature for 2 h and was then
incubated with primary antibodies including anti-MCU (1:500; cat.
no. sc-515930; Santa Cruz Biotechnology, Inc.), anti-VEGF (1:1,000;
cat. no. 26381-1-AP; ProteinTech Group, Inc.), anti-hypoxia
inducible factor (HIF)-1α (1:1,000; cat. no. 20960-1-AP;
ProteinTech Group, Inc.) anti-Vimentin (1:2,000; cat. no. ab92547;
Abcam), anti-E-cadherin (1:1,000; cat. no. ab40772; Abcam),
anti-MMP2 (1:1,000; cat. no. ab181286; Abcam), anti-N-cadherin
(1:1,000; cat. no. ab20760; Abcam) and anti-β-actin (1:2,000; cat.
no. 20536-1-AP; ProteinTech Group, Inc.) at 4°C overnight. After
washing the membrane, the membrane was incubated with a horseradish
peroxidase (HRP) secondary antibody (1:2,000; cat. no. ab7090;
Abcam) at room temperature for 2 h. The protein band was visualized
using BeyoECL Plus (cat. no. KGP1121; Nanjing KeyGen Biotech Co.,
Ltd.). ImageJ software v.1.48 (National Institutes of Health) was
used for quantifying the expression of target proteins.
Immunofluorescence
In brief, the tissue sections were incubated with
unconjugated-labeled primary antibodies including anti-MCU (1:100;
cat. no. sc-515930; Santa Cruz Biotechnology, Inc.),
anti-E-cadherin (1:100; cat. no. ab40772; Abcam) and anti-Vimentin
(1:100; cat. no. ab92547; Abcam) overnight at 4°C, followed by
Alexa Fluor® 488-conjugated (1:100; cat. no. ZF-0512;
OriGene Technologies, Inc.) and Alexa Fluor®
594-conjugated (1:100; cat. no. ZF-0513; OriGene Technologies,
Inc.) secondary antibodies at room temperature for 2 h. For nuclear
counterstaining, cells were incubated with DAPI (1:100; cat. no.
ab104139; Abcam) for 5 min at room temperature. The images were
acquired by confocal microscopy (General Electric Company)
(magnification, ×200). The results were determined using ImageJ
software v.1.48 (National Institutes of Health).
Cell culture
Two human esophageal cancer KYSE-150 and TE-1 cell
lines (American Type Culture Collection) were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific Inc.) with 10% FBS (Gibco;
Thermo Fisher Scientific Inc.), 100 U/ml penicillin and 100 U/ml
streptomycin at 37°C and 5% CO2.
Transfection
Cells were seeded onto a 6-well plate. KYSE-150 and
TE-1 cells were treated with different concentrations (10, 20, 30,
50, 70, 100 and 200 µM) of the MCU agonist Spermine (cat. no.
18041; Cayman Chemical Company) at 37°C for 48 h. A small
interfering RNA (siRNA) against MCU (si-MCU) was designed as
follows: si-MCU#1 sense, 5′-CAUAAAGGAGCCAAAAAGUCA-3′ and antisense,
5′-ACUUUUUGGCUCCUUUAUGGA-3′; si-MCU#2 sense,
5′-GGGAAUUGACAGAGUUGCU-3′ and antisense, 5′-AGCAACUCUGUCAAUUCCC-3′.
Meanwhile, the sequences of the siRNA negative control (si-NC;
scrambled RNA) were as follows: sense, 5′-GCCUAAGAACGACAAAUCA-3′
and antisense, 5′-UGAUUUGUCGUUCUUAGGC-3′. A total of 5 nM of si-MCU
or si-NC was transfected into cells via Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific Inc.) at room
temperature for 48 h. After 48 h, western blotting was used to
examine the transfection effects.
Colony formation assay
Cells of each group were collected 24 h after
transfection and were inoculated in 35-mm culture dishes (500–1,000
cells/dish). These cells were incubated at 37°C in a 5%
CO2 incubator for 14 days. The culture medium was
changed every 4–5 days. Following rinsing with lX PBS for 3 times,
the cells were fixed with methanol for 10 min at room temperature
and stained with 0.5% crystal violet for 20 min at room
temperature. Following rinsing and drying, the colonies were
photographed and counted. A total of 50 cells were considered as a
colony.
Cell Counting Kit-8 (CCK-8) assay
Cells were seeded into a 96-well plate. After 48 h
from transfection, cell viability was determined using the CCK-8
reagent (Dojindo Molecular Technologies, Inc.) for 1 h. The
absorbance at 450 nm was assessed utilizing a multiscan
spectrum.
Transwell assay
After 24 h from transfection, cells were starved
with serum-free RPMI-1640 medium for 6–8 h. The digested cells were
washed twice with serum-free RPMI-1640 medium and were then seeded
in the upper chamber of a 24-well Transwell insert
(1.5×109 cells/well). A total of 600 µl RPMI-1640 medium
containing 20% FBS was added to the lower chamber. The transfected
cells were cultured at 37°C with 5% CO2 for 24 h.
Afterwards, the cells were fixed using 4% paraformaldehyde for 15
min at 37°C and stained using Giemsa for 30 min at 37°C. Migratory
cells in lower chamber were observed under a light microscope
(Olympus Corporation) (magnification, ×200; scale bar 50 µm).
Statistical analysis
All statistical analysis was performed using SPSS
19.0 software (IBM Corp.) and GraphPad Prism 8.0 (GraphPad
Software, Inc.). The results were expressed as the mean ± SD from
at least three independent experiments. The comparisons between two
groups were analyzed using unpaired Student's t-test and one-way
analysis of variance followed by Tukey's post-hoc test was
performed for multiple comparisons. The association between MCU
expression and clinical traits was analyzed using the χ2
test. Furthermore, Pearson's correlation analysis was used to
analyze the correlation between MCU expression and other proteins.
P<0.05 and correlation coefficient |r|>0.3 were considered to
indicate a statistically significant difference.
Results
MCU expression is upregulated in
esophageal cancer
According to GEPIA, MCU was highly expressed in
esophageal cancer, kidney renal papillary cell carcinoma,
pancreatic adenocarcinoma and stomach adenocarcinoma among various
types of cancer (Fig. 1A). Box plots
visualized the differences in MCU expression between esophageal
cancer samples (n=182) and normal tissues (n=286) from TCGA in
Fig. 1B (P<0.05). Additionally,
there was a significant difference in MCU expression among
different stages of esophageal cancer (Fig. 1C). A total of 110 patients with
esophageal cancer were included in the present study. Fig. 1D shows the representative images of
H&E staining results of esophageal cancer tissues and the
corresponding adjacent normal tissues. Compared with normal
epithelium cells of esophageal tissues, tumor cells had large
nuclei and were in tight contact with neighbor cells. A total of 8
pairs of esophageal cancer and adjacent normal tissues were
randomly selected for western blot analysis. As expected, MCU
protein expression was significantly higher in esophageal cancer
tissues compared with in adjacent normal tissues (Fig. 2A and B).
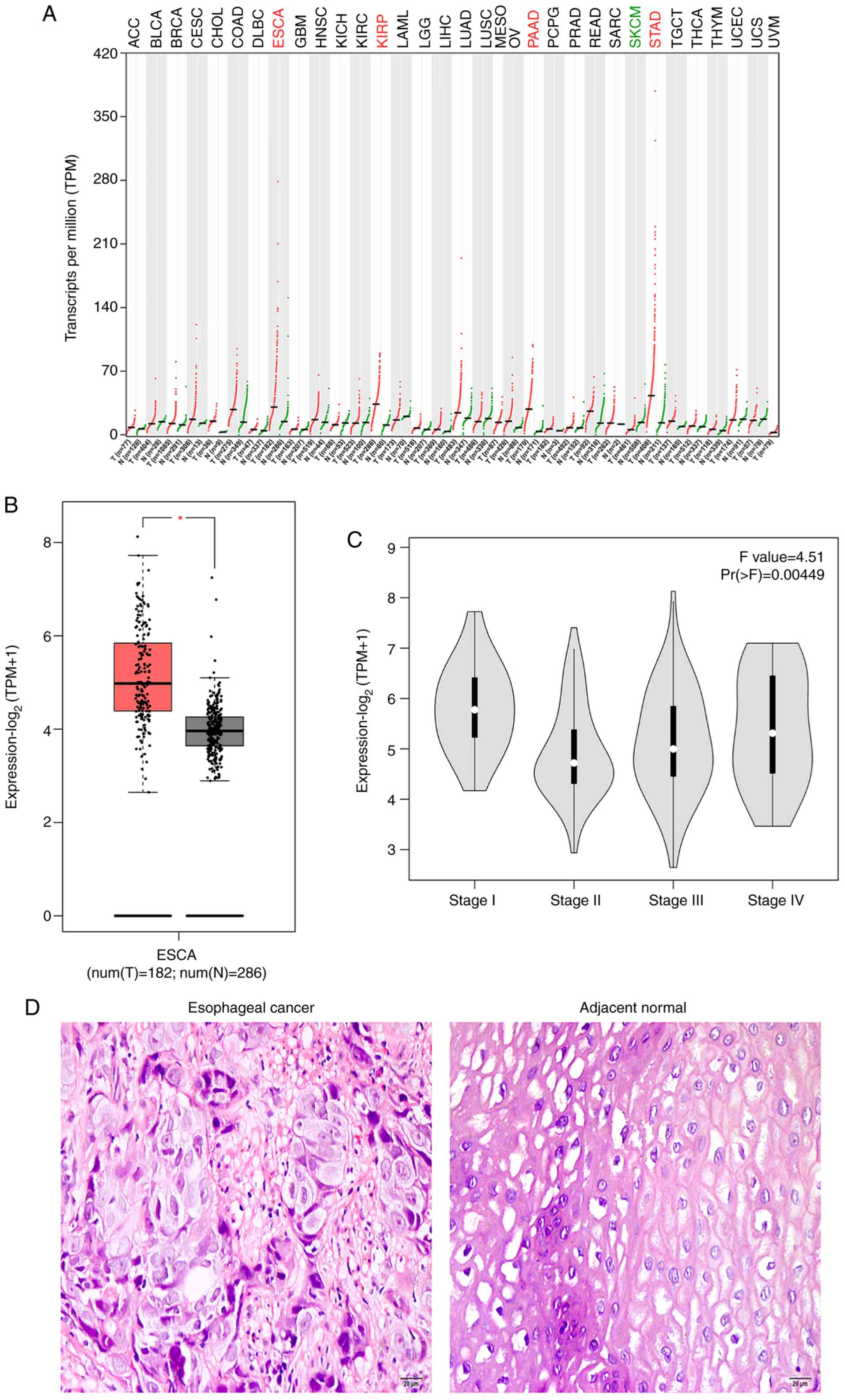 | Figure 1.MCU upregulation in esophageal cancer
using Gene Expression Profiling Interactive Analysis. (A) Patterns
of MCU expression in various types of cancer. (B) Box plots showing
the upregulation of MCU expression in esophageal cancer.
*P<0.05. (C) Violin diagram showing the difference in MCU
expression among different stages of esophageal cancer. (D)
Representative images of hematoxylin and eosin staining results of
esophageal cancer and corresponding adjacent normal tissues. Scale
bar, 20 µm; magnification, ×200. Red represented the upregulation
of MCU expression in this cancer type while green represented the
downregulation of MCU expression in this cancer type. MCU,
mitochondrial calcium uniporter; ACC, adrenocortical carcinoma;
BLCA, bladder urothelial carcinoma; BRCA, breast invasive
carcinoma; CESC, cervical squamous cell carcinoma; CHOL,
cholangiocarcinoma; COAD, colon adenocarcinoma; DLBC, lymphoid
neoplasm diffuse large B-cell lymphoma; ESCA, esophageal carcinoma;
GBM, glioblastoma multiforme; HNSC, head and neck squamous cell
carcinoma; KICH, kidney chromophobe; KIRC, kidney renal clear cell
carcinoma; KIRP, kidney renal papillary cell carcinoma; LAML, acute
myeloid leukemia; LGG, brain lower grade glioma; LIHC, liver
hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung
squamous cell carcinoma; MESO, mesothelioma; OV, ovarian serous
cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PCPG,
pheochromocytoma and paraganglioma; PRAD, prostate adenocarcinoma;
READ, rectum adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous
melanoma; STAD, stomach adenocarcinoma; TGCT, testicular germ cell
tumors; THCA, thyroid carcinoma; THYM, thymoma; UCEC, uterine
corpus endometrial carcinoma; UCS, uterine carcinosarcoma; UVM,
uveal melanoma. |
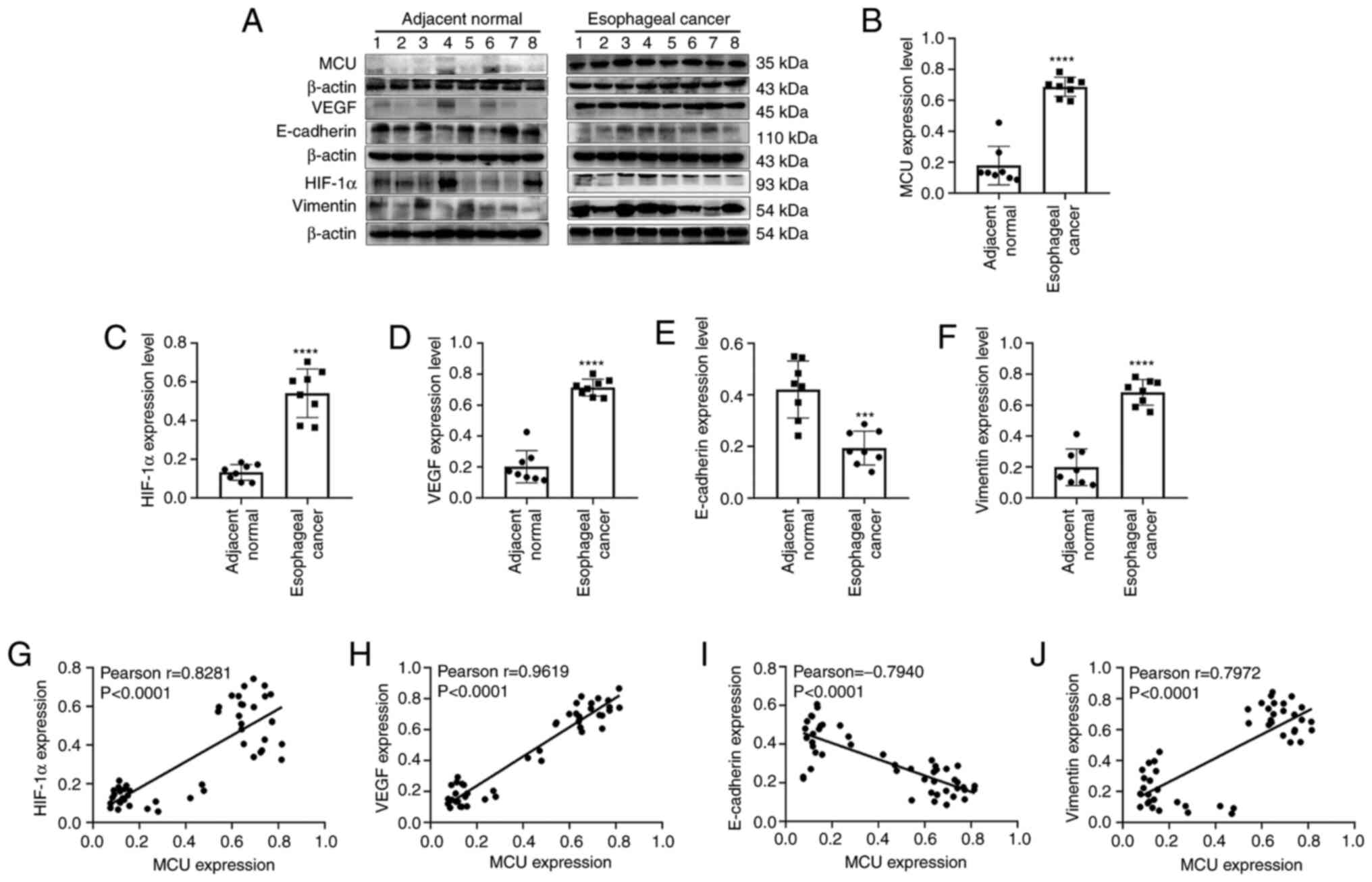 | Figure 2.Western blotting results showing the
protein expression levels of MCU, HIF-1α, VEGF, E-cadherin and
Vimentin in esophageal cancer and adjacent normal tissues. (A)
Representative images of western blots. Protein expression levels
of (B) MCU, (C) HIF-1α, (D) VEGF, (E) E-cadherin and (F) Vimentin
between esophageal cancer and adjacent normal tissues. Correlation
between MCU and (G) HIF-1α, (H) VEGF, (I) E-cadherin and (J)
Vimentin expression. Each experiment was repeated three times.
***P<0.001 and ****P<0.0001. MCU, mitochondrial calcium
uniporter; HIF, hypoxia inducible factor. |
MCU expression is significantly
correlated with HIF-1α/VEGF/E-cadherin/Vimentin expression in
esophageal cancer
Tumor metastasis is the main cause of esophageal
cancer mortality (13).
Epithelial-mesenchymal transition (EMT) is associated with
downregulation of E-cadherin and upregulation of vimentin
expression, which contributes to tumor invasion and metastasis
(14). Meanwhile, the HIF-1α/VEGF
signaling pathway, which can mediate the EMT process, has been
considered a key target for treating cancer metastasis (14). Thus, the expression levels of HIF-1α,
VEGF, E-cadherin and Vimentin were detected between 8 pairs of
esophageal cancer tissues and adjacent normal tissues using western
blotting. HIF-1α (Fig. 2C) and VEGF
(Fig. 2D) protein expression was
significantly higher in esophageal cancer compared with in adjacent
normal tissues. Additionally, low E-cadherin expression was
observed in esophageal cancer tissues (Fig. 2E), while vimentin had higher
expression levels in esophageal cancer than in adjacent normal
tissues (Fig. 2F). Correlation
analysis was then performed, revealing that there were positive
correlations between MCU expression and HIF-1α (Pearson r=0.8281;
P<0.0001; Fig. 2G) and VEGF
(Pearson r=0.9619; P<0.0001; Fig.
2H) expression. Additionally, a negative correlation was
observed between MCU and E-cadherin expression in esophageal cancer
tissues (Pearson r=−0.7940; P<0.0001; Fig. 2I). Furthermore, MCU was positively
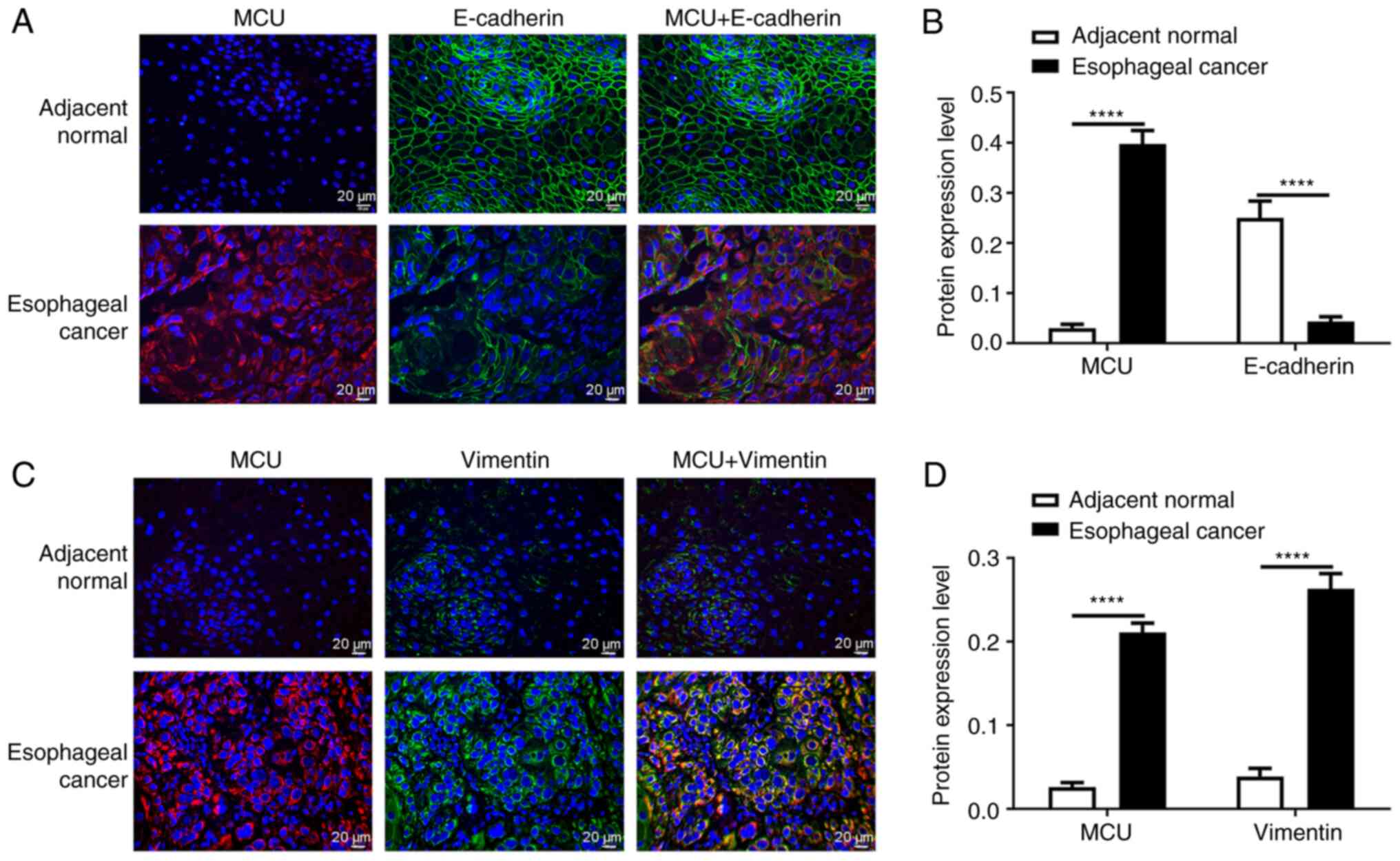
correlated with Vimentin (Pearson r=0.7972; P<0.0001; Fig. 2J). Subsequently, immunofluorescence
analysis of MCU, E-cadherin and Vimentin was also performed in
esophageal cancer and adjacent normal tissues. Consistent with the
western blot results, the immunofluorescence results revealed that
MCU protein expression was significantly higher and E-cadherin
protein expression was significantly lower in esophageal cancer
compared with in adjacent normal tissues (Fig. 3A and B). As shown in Fig. 3C and D, both MCU and Vimentin
exhibited higher expression levels in esophageal cancer than in
adjacent normal tissues.
Association between MCU expression and
clinicopathological features of patients with esophageal
cancer
The expression and localization of MCU were detected
by immunohistochemistry in 110 patients with esophageal cancer.
Among 110 esophageal cancer specimens, 88 had positive expression
of MCU (including weak positive, positive or strong positive
expression) and others had negative expression of MCU (Table I). The association between MCU
expression and clinical characteristics, including sex, age, depth
of invasion, lymph node metastasis, TNM stage and distant
metastasis, was analyzed. As shown in Table I, MCU expression was significantly
associated with depth of invasion (P=0.031), lymph node metastasis
(P=0.027), TNM stage (P=0.036) and distant metastasis (P=0.008).
However, there was no association between MCU expression and sex
(P=0.332) and age (P=0.381) (Table
I). Furthermore, MCU was mainly expressed in the cytoplasm.
Representative results of MCU staining are shown in Fig. 4A. Significantly higher MCU expression
was detected in esophageal cancer tissues compared with in adjacent
normal tissues (Fig. 4B).
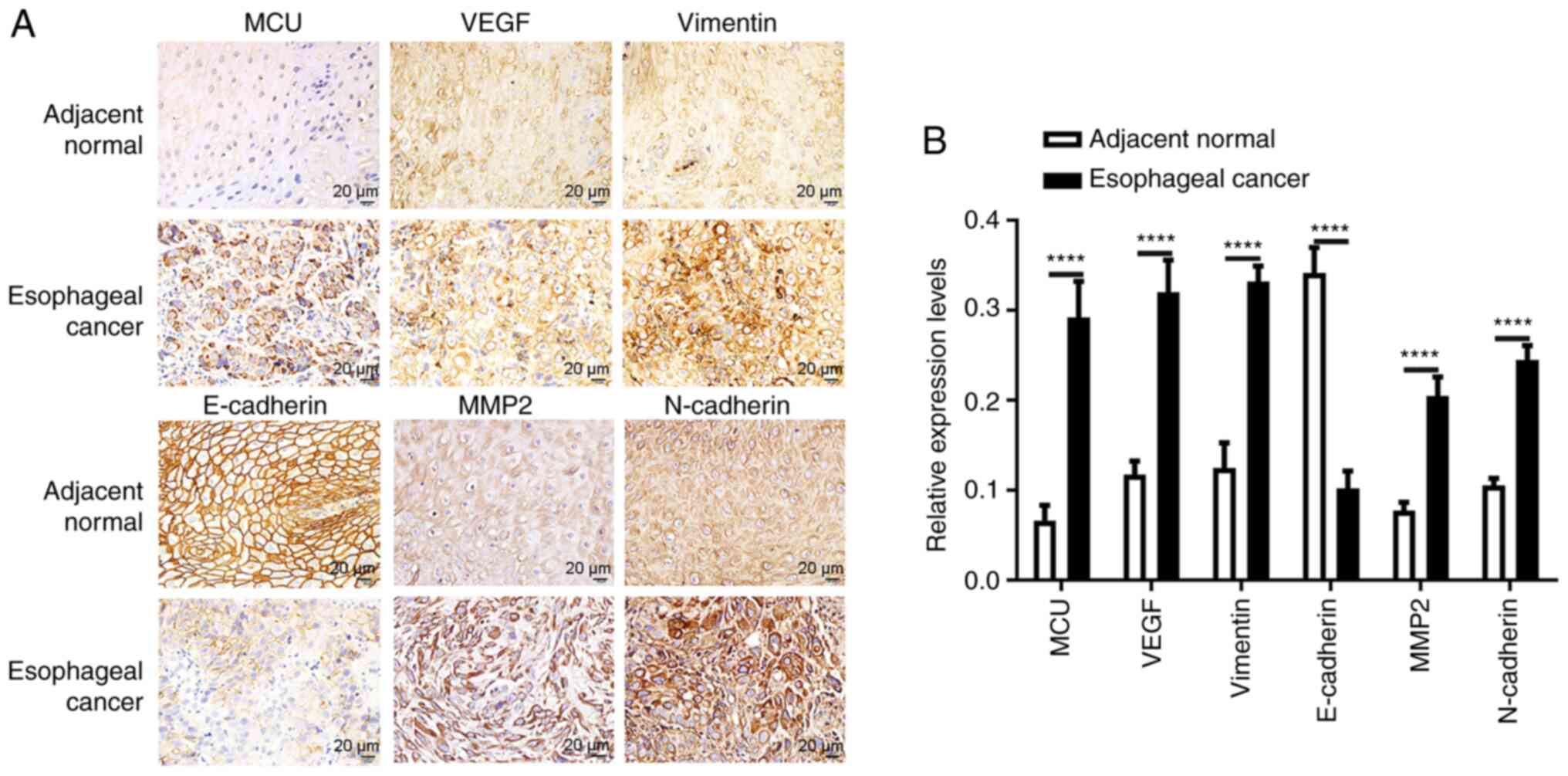 | Figure 4.Immunohistochemistry results of MCU,
VEGF, Vimentin, E-cadherin, MMP2 and N-cadherin in esophageal
cancer and adjacent normal tissues. (A) Representative images of
immunohistochemistry results. (B) Expression levels of MCU, VEGF,
Vimentin, E-cadherin, MMP2 and N-cadherin between esophageal cancer
and adjacent normal tissues. Scale bar, 20 µm; magnification, ×200.
****P<0.0001. MCU, mitochondrial calcium uniporter. |
 | Table I.Association between
clinicopathological parameters and MCU expression in 110 patients
with esophageal cancer. |
Table I.
Association between
clinicopathological parameters and MCU expression in 110 patients
with esophageal cancer.
|
|
| MCU
expressionc, n |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinical
parameters | Total, n | Positive
(n=88) | Negative
(n=22) | χ2 | P-value |
|---|
| Sex |
|
|
|
|
|
|
Male | 65 | 54 | 11 | 0.940 | 0.332 |
|
Female | 45 | 34 | 11 |
|
|
| Age, years |
|
|
|
|
|
|
<60 | 66 | 51 | 15 | 0.767 | 0.381 |
|
≥60 | 44 | 37 | 7 |
|
|
| Depth of
invasion |
|
|
|
|
|
|
T1/T2 | 42 | 38 | 4 | 4.660 | 0.031a |
|
T3/T4 | 68 | 50 | 18 |
|
|
| Lymph node
metastasis |
|
|
|
|
|
| N0 | 38 | 26 | 12 | 4.865 | 0.027a |
|
N1/N2/N3 | 72 | 62 | 10 |
|
|
| TNM stage |
|
|
|
|
|
|
I–II | 39 | 27 | 12 | 4.380 | 0.036a |
|
III–IV | 71 | 61 | 10 |
|
|
| Distant
metastasis |
|
|
|
|
|
|
Absent | 43 | 29 | 14 | 6.959 | 0.008b |
|
Present | 67 | 59 | 8 |
|
|
Immunohistochemistry results of VEGF,
Vimentin, E-cadherin, MMP2 and N-cadherin in esophageal cancer
As aforementioned, MCU expression was significantly
correlated with VEGF and EMT-associated proteins. Hence, the
protein expression levels of VEGF, Vimentin, E-cadherin, MMP2 and
N-cadherin between esophageal cancer and adjacent normal tissues
were further examined by immunohistochemistry. Consistent with the
aforementioned results, higher expression levels of VEGF and
Vimentin were detected in esophageal cancer compared with in
adjacent normal tissues (Fig. 4A and
B). Furthermore, E-cadherin expression was significantly lower
in esophageal cancer than in adjacent normal tissues, while MMP2
and N-cadherin protein expression was significantly upregulated in
esophageal cancer tissues compared with in adjacent normal tissues
(Fig. 4A and B), indicating that
these proteins may be involved in the development of esophageal
cancer.
MCU promotes VEGF, MMP2, Vimentin and
N-cadherin, and inhibits E-cadherin in esophageal cancer cells
Western blotting was performed to validate the
transfection effect of si-MCU in KYSE-150 and TE-1 cells. As shown
in Fig. 5A and B, MCU protein
expression was significantly suppressed in KYSE-150 and TE-1 cells.
After overexpression of MCU using Spermine, VEGF, Vimentin and
N-cadherin expression was significantly increased in KYSE-150 cells
(Fig. 5C-H). MCU overexpression did
not significantly alter MMP2 expression. However, their expression
levels were significantly suppressed and E-cadherin expression was
significantly promoted in KYSE-150 cells after transfection with
si-MCU (Fig. 5C-I). Similar results
of MCU, VEGF, MMP2, Vimentin, N-cadherin and E-cadherin expression
were obtained in TE-1 cells treated with Spermine or transfected
with si-MCU (Fig. 5C and J-O).
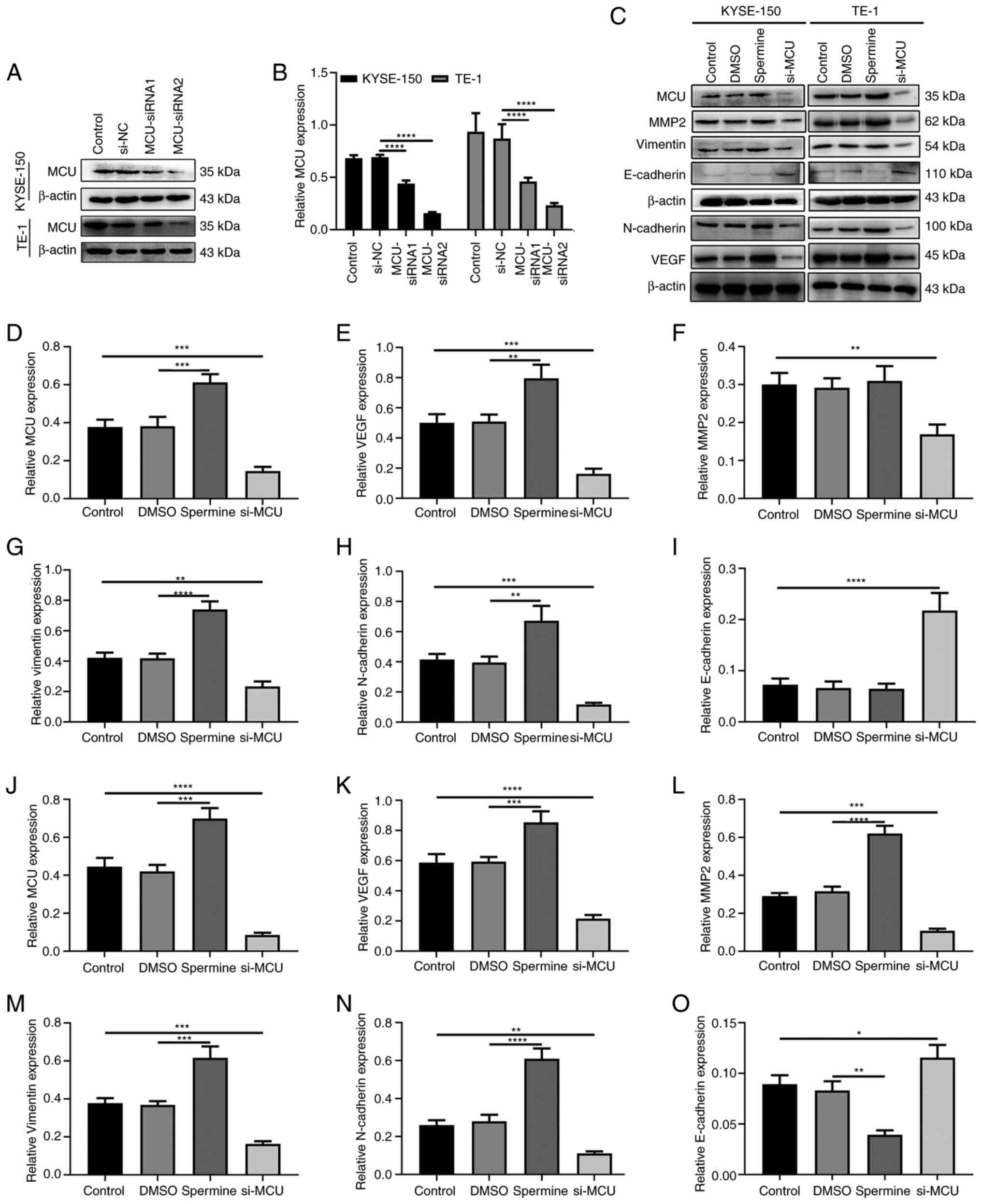 | Figure 5.MCU promotes VEGF, MMP2, Vimentin and
N-cadherin expression, and inhibits E-cadherin expression in
esophageal cancer cells. (A and B) Western blotting was used to
validate the effect of si-MCU transfection in KYSE-150 and TE-1
cells. (C) Representative images of western blotting for the
protein expression levels of VEGF, MMP2, Vimentin, N-cadherin and
E-cadherin in KYSE-150 and TE-1 cells treated with Spermine or
transfected with si-MCU. Quantification of protein expression
levels of (D) MCU, (E) VEGF, (F) MMP2, (G) Vimentin, (H) N-cadherin
and (I) E-cadherin in KYSE-150 cells treated with Spermine or
si-MCU. Quantification of protein expression levels of (J) MCU, (K)
VEGF, (L) MMP2, (M) Vimentin, (N) N-cadherin and (O) E-cadherin in
TE-1 cells treated with Spermine or si-MCU. β-actin was used as a
reference control. *P<0.05; **P<0.01; ***P<0.001;
****P<0.0001. MCU, mitochondrial calcium uniporter; si/siRNA,
small interfering RNA; NC, negative control. |
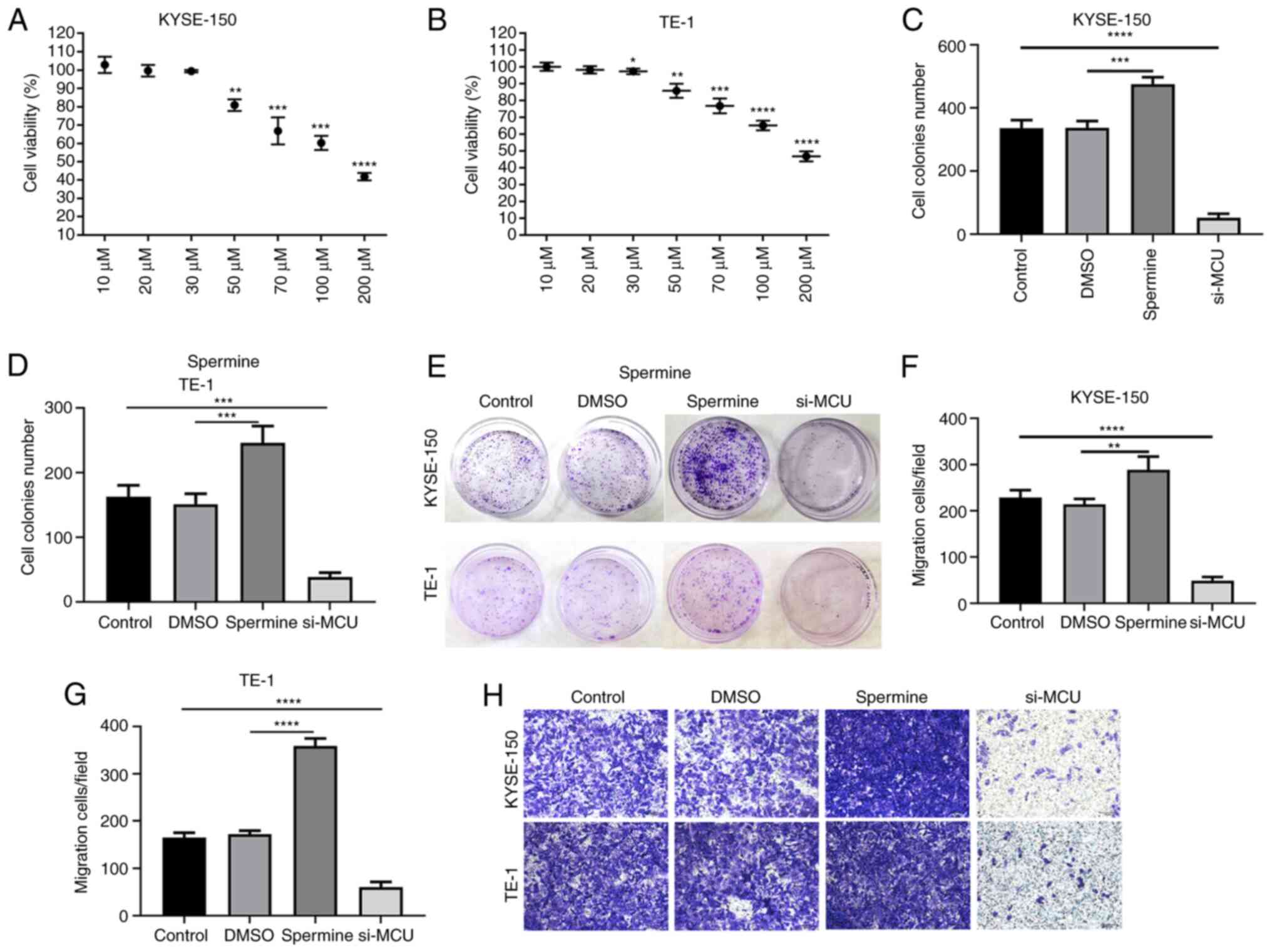
MCU accelerates the proliferation and
migration of esophageal cancer cells
The cellular function of MCU in esophageal cancer
was further explored. CCK-8 results indicated that the viability of
KYSE-150 and TE-1 cells was significantly inhibited with Spermine
in a concentration-dependent manner (Fig. 6A and B). The concentration of 30
µm/ml Spermine was chosen as the optimal concentration for further
analysis. As shown in Fig. 6C-E, MCU
overexpression significantly promoted the proliferation of KYSE-150
and TE-1 cells, while opposite results were observed after
transfection with si-MCU. Transwell assay results demonstrated that
the migration of KYSE-150 and TE-1 cells was significantly promoted
by MCU overexpression, while it was significantly suppressed after
si-MCU transfection (Fig. 6F-H).
Discussion
The development of esophageal cancer is a complex
process involving multiple steps and multiple factors. Despite
surgical resection and other novel treatments, patients with
esophageal cancer are usually diagnosed in the middle and late
stages, leading to a high mortality and poor prognosis (15). Moreover, lymph node metastasis is an
important event for esophageal cancer, since it may contribute to a
high recurrence rate (16,17). The present study identified that MCU
was significantly highly expressed in esophageal cancer. Its high
expression promoted the proliferation, migration and EMT of
esophageal cancer cells. Thus, high MCU expression may contribute
to esophageal cancer metastasis.
The present study analyzed MCU expression in
patients with esophageal cancer by western blotting,
immunohistochemistry and immunofluorescence. Moreover, the
association between MCU expression and clinical characteristics was
analyzed. The current data suggested that MCU expression was higher
in esophageal cancer tissues compared with normal tissues. In 110
cases, there were significant associations between MCU expression
and TNM stage and lymph node metastasis. It has been identified
that the prognosis of esophageal cancer is greatly affected by
lymph node metastasis, which is associated with poor survival
(18). The current results indicated
that high MCU expression was associated with the invasiveness of
esophageal cancer, thereby serving an important role in the
metastasis of esophageal cancer.
According to the present western blotting results,
MCU expression was significantly correlated with HIF-1α and VEGF
expression, and with the EMT process in esophageal cancer. In the
present study, a positive correlation was identified between MCU
and HIF-1α expression. Similar results were observed in breast
cancer tissues (7). Furthermore,
silencing MCU can inhibit HIF-1α expression in colon cancer and
triple-negative breast cancer (7,19). These
findings indicated that HIF-1α may be regulated by MCU. In the
current study, it was revealed that MCU promoted VEGF, MMP2,
Vimentin and N-cadherin expression, and inhibited E-cadherin
expression in esophageal cancer cells. However, further studies
need to be performed. Tumor metastasis is the main cause of death
for most patients with esophageal cancer (20). HIF-1α activation is one of the most
important mechanisms that promotes metastasis and increases tumor
aggressiveness (21–23). Additionally, high VEGF expression was
found in esophageal cancer tissues, which may serve a major
angiogenic role in esophageal cancer. Several meta-analysis studies
have revealed that high VEGF expression is associated with poor
overall survival in patients with esophageal cancer (24,25).
Furthermore, it has been confirmed that VEGF is a downstream target
of HIF-1α in esophageal cancer (26). EMT is one of the main mechanisms for
inducing tumor invasion and metastasis (27,28). EMT
is a process in which epithelial cells lose their polarity and
acquire a mesenchymal phenotype (24,25).
Hypoxia induced by HIF-1α is an important microenvironment factor
that can induce the expression of certain EMT regulators and
coordinate the interaction between these EMT regulators (such as
E-cadherin and Vimentin) (29–31). The
extracellular matrix serves an important role in the development of
esophageal cancer (32). It has been
shown that certain molecules are involved in these events,
especially different collagen isoforms and enzymes associated with
their metabolism, such as MMPs (32). Different MMP isoforms, especially
MMP-2, −3, −7 and −9, have been reported to be involved in the
development of esophageal cancer (32). The invasion and metastasis of
esophageal cancer cells involve complex, continuous multi-step
processes. Previous studies have revealed that there is a close
association between MMP2 and the invasion and metastasis of
esophageal cancer cells (33–35).
High MMP2 expression was observed in esophageal cancer tissue
samples in the present study. Additionally, it has been found that
MCU promotes the activity of MMP2 and cell movement, thereby
promoting invasion, migration and metastasis of liver cancer cells
(8).
Metastasis is one of the most important causes of
death among patients with esophageal cancer and one of the
important malignant phenotypes of cancer (13). Patients with esophageal cancer are
prone to metastases to the lungs, brain and kidneys (36). The ability of cancer cells to invade
and migrate is the basis of cancer metastasis. Strong invasive and
migratory abilities help to break through the basement membrane and
enter the lymphatic system or blood vessels, which eventually lead
to the metastasis to lymph nodes or distant organs (13). The present results suggested that MCU
accelerated proliferation and migration of esophageal cancer
cells.
However, there were several limitations in the
current study. Firstly, this was a retrospective study conducted at
a single institution, with a relatively small sample size. The
present results need to be further confirmed based on larger sample
multi-center analysis. Secondly, the exact mechanism of esophageal
cancer metastasis caused by MCU dysregulation is unclear and
requires further study. In conclusion, the current data indicated
that MCU was frequently expressed in esophageal cancer. Further
research is required to confirm the application of MCU in the
diagnosis and treatment of esophageal cancer.
Overall, the present study described for the first
time the abnormal expression levels of MCU and its potential
clinical value in esophageal cancer, providing a new potential
biomarker and therapeutic target for esophageal cancer. However,
the role of MCU in esophageal cancer requires further study.
Acknowledgements
Not applicable.
Funding
The present study was funded by Project of Hebei
Medical Science Research Project (grant no. 20191127); Ningxia
Medical University School-level Project (grant no. XM2019072);
Natural Science Foundation of Ningxia (grant no. 2021AAC03343) and
Ningxia Hui Autonomous Region Key R&D Projects (grant no.
2020BEG03001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FZ conceived and designed the study. YM, XW, YL and
WL conducted most of the experiments and data analysis, and wrote
the manuscript. YH, HY and RH conducted immunohistochemistry and
immunofluorescence experiments and data analysis and wrote and
revised the manuscript. FZ and YM confirmed the authenticity of all
the raw data. All authors reviewed and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
General Hospital of Ningxia Medical University (Yinchuan, China;
approval no. 2016049). All patients provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cai R, Wang P, Zhao X, Lu X, Deng R, Wang
X, Su Z, Hong C and Lin J: LTBP1 promotes esophageal squamous cell
carcinoma progression through epithelial-mesenchymal transition and
cancer-associated fibroblasts transformation. J Transl Med.
18:1392020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xi M, Yang Y, Zhang L, Yang H, Merrell KW,
Hallemeier CL, Shen RK, Haddock MG, Hofstetter WL, Maru DM, et al:
Multi-institutional analysis of recurrence and survival after
neoadjuvant chemoradiotherapy of esophageal cancer: Impact of
histology on recurrence patterns and outcomes. Ann Surg.
269:663–670. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baughman JM, Perocchi F, Girgis HS,
Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L,
Goldberger O, Bogorad RL, et al: Integrative genomics identifies
MCU as an essential component of the mitochondrial calcium
uniporter. Nature. 476:341–345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vultur A, Gibhardt CS, Stanisz H and
Bogeski I: The role of the mitochondrial calcium uniporter (MCU)
complex in cancer. Pflugers Arch. 470:1149–1163. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hall DD, Wu Y, Domann FE, Spitz DR and
Anderson ME: Mitochondrial calcium uniporter activity is
dispensable for MDA-MB-231 breast carcinoma cell survival. PLoS
One. 9:e968662014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tosatto A, Sommaggio R, Kummerow C,
Bentham RB, Blacker TS, Berecz T, Duchen MR, Rosato A, Bogeski I,
Szabadkai G, et al: The mitochondrial calcium uniporter regulates
breast cancer progression via HIF-1α. EMBO Mol Med. 8:569–585.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ren T, Zhang H, Wang J, Zhu J, Jin M, Wu
Y, Guo X, Ji L, Huang Q, Zhang H, et al: MCU-dependent
mitochondrial Ca2+ inhibits NAD(+)/SIRT3/SOD2 pathway to
promote ROS production and metastasis of HCC cells. Oncogene.
36:5897–5909. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen L, Sun Q, Zhou D, Song W, Yang Q, Ju
B, Zhang L, Xie H, Zhou L, Hu Z, et al: HINT2 triggers
mitochondrial Ca2+ influx by regulating the
mitochondrial Ca2+ uniporter (MCU) complex and enhances
gemcitabine apoptotic effect in pancreatic cancer. Cancer Lett.
411:106–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koval OM, Nguyen EK, Santhana V, Fidler
TP, Sebag SC, Rasmussen TP, Mittauer DJ, Strack S, Goswami PC, Abel
ED and Grumbach IM: Loss of MCU prevents mitochondrial fusion in
G1-S phase and blocks cell cycle progression and proliferation. Sci
Signal. 12:eaav14392019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sawaguchi A, McDonald KL and Forte JG:
High-pressure freezing of isolated gastric glands provides new
insight into the fine structure and subcellular localization of
H+/K+-ATPase in gastric parietal cells. J Histochem Cytochem.
52:77–86. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsieh CC, Hsu HS, Li AF and Chen YJ:
Clinical relevance of PD-L1 and PD-L2 overexpression in patients
with esophageal squamous cell carcinoma. J Thorac Dis.
10:4433–4444. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang W, Han Y, Zhao X, Duan L, Zhou W,
Wang X, Shi G, Che Y, Zhang Y, Liu J, et al: Advances in prognostic
biomarkers for esophageal cancer. Expert Rev Mol Diagn. 19:109–119.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li C, Wang Q, Shen S, Wei X and Li G:
HIF-1α/VEGF signaling-mediated epithelial-mesenchymal transition
and angiogenesis is critically involved in anti-metastasis effect
of luteolin in melanoma cells. Phytother Res. 33:798–807. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okadome K, Baba Y, Yagi T, Kiyozumi Y,
Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Watanabe M and Baba
H: Prognostic nutritional index, tumor-infiltrating lymphocytes,
and prognosis in patients with esophageal cancer. Ann Surg.
271:693–700. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dubecz A, Kern M, Solymosi N, Schweigert M
and Stein HJ: Predictors of lymph node metastasis in surgically
resected T1 esophageal cancer. Ann Thorac Surg. 99:1879–1886. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qu J, Shen C, Qin J, Wang Z, Liu Z, Guo J,
Zhang H, Gao P, Bei T, Wang Y, et al: The MR radiomic signature can
predict preoperative lymph node metastasis in patients with
esophageal cancer. Eur Radiol. 29:906–914. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Al-Kaabi A, van der Post RS, Huising J,
Rosman C, Nagtegaal ID and Siersema PD: Predicting lymph node
metastases with endoscopic resection in cT2N0M0 oesophageal cancer:
A systematic review and meta-analysis. United European
Gastroenterol J. 8:35–43. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun Y, Li M, Liu G, Zhang X, Zhi L, Zhao J
and Wang G: The function of Piezo1 in colon cancer metastasis and
its potential regulatory mechanism. J Cancer Res Clin Oncol.
146:1139–1152. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J, Luo A, Huang F, Gong T and Liu Z:
SERPINE2 promotes esophageal squamous cell carcinoma metastasis by
activating BMP4. Cancer Lett. 469:390–398. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu X, Lin J, Jiang M, He X, Wang K, Wang
W, Hu C, Shen Z, He Z, Lin H, et al: HIF-1α promotes the metastasis
of esophageal squamous cell carcinoma by targeting SP1. J Cancer.
11:229–240. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang NN, Zhu H, Zhang HJ, Zhang WF, Jin
HL, Wang L, Wang P, He GJ, Hao B and Shi RH: HIF-1α induces
VE-cadherin expression and modulates vasculogenic mimicry in
esophageal carcinoma cells. World J Gastroenterol. 20:17894–17904.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu Y, Zang Y, Zhao F, Li Z, Zhang J, Fang
L, Li M, Xing L, Xu Z and Yu J: Inhibition of HIF-1alpha by PX-478
suppresses tumor growth of esophageal squamous cell cancer in vitro
and in vivo. Am J Cancer Res. 7:1198–1212. 2017.PubMed/NCBI
|
|
24
|
Chen M, Cai E, Huang J, Yu P and Li K:
Prognostic value of vascular endothelial growth factor expression
in patients with esophageal cancer: A systematic review and
meta-analysis. Cancer Epidemiol Biomarkers Prev. 21:1126–1134.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peng J, Shao N, Peng H and Chen LQ:
Prognostic significance of vascular endothelial growth factor
expression in esophageal carcinoma: A meta-analysis. J Buon.
18:398–406. 2013.PubMed/NCBI
|
|
26
|
Li B, Xu WW, Han L, Chan KT, Tsao SW, Lee
NPY, Law S, Xu LY, Li EM, Chan KW, et al: MicroRNA-377 suppresses
initiation and progression of esophageal cancer by inhibiting CD133
and VEGF. Oncogene. 36:3986–4000. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qin T, Liu W, Huo J, Li L, Zhang X, Shi X,
Zhou J and Wang C: SIRT1 expression regulates the transformation of
resistant esophageal cancer cells via the epithelial-mesenchymal
transition. Biomed Pharmacother. 103:308–316. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taniguchi D, Saeki H, Nakashima Y, Kudou
K, Nakanishi R, Kubo N, Ando K, Oki E, Oda Y and Maehara Y: CD44v9
is associated with epithelial-mesenchymal transition and poor
outcomes in esophageal squamous cell carcinoma. Cancer Med.
7:6258–6268. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nahomi RB and Nagaraj RH: The role of
HIF-1α in the TGF-β2-mediated epithelial-to-mesenchymal transition
of human lens epithelial cells. J Cell Biochem. 119:6814–6827.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yeh YH, Hsiao HF, Yeh YC, Chen TW and Li
TK: Inflammatory interferon activates HIF-1α-mediated
epithelial-to-mesenchymal transition via PI3K/AKT/mTOR pathway. J
Exp Clin Cancer Res. 37:702018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang J, Zhang Q, Lou Y, Fu Q, Chen Q, Wei
T, Yang J, Tang J, Wang J, Chen Y, et al: Hypoxia-inducible
factor-1alpha/interleukin-1beta signaling enhances hepatoma
epithelial-mesenchymal transition through macrophages in a
hypoxic-inflammatory microenvironment. Hepatology. 67:1872–1889.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Palumbo A Jr, Meireles Da Costa N, Pontes
B, Leite de Oliveira F, Lohan Codeço M, Ribeiro Pinto LF and
Nasciutti LE: Esophageal cancer development: crucial clues arising
from the extracellular matrix. Cells. 9:4552020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Uraoka N, Oue N, Sakamoto N, Sentani K, Oo
HZ, Naito Y, Noguchi T and Yasui W: NRD1, which encodes nardilysin
protein, promotes esophageal cancer cell invasion through induction
of MMP2 and MMP3 expression. Cancer Sci. 105:134–140. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li L, Yue GG, Lee JK, Wong EC, Fung KP, Yu
J, Lau CB and Chiu PW: The adjuvant value of Andrographis
paniculata in metastatic esophageal cancer treatment-from
preclinical perspectives. Sci Rep. 7:8542017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xuan X, Li S, Lou X, Zheng X, Li Y, Wang
F, Gao Y, Zhang H, He H and Zeng Q: Stat3 promotes invasion of
esophageal squamous cell carcinoma through up-regulation of MMP2.
Mol Biol Rep. 42:907–915. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kromer C, Xu J, Ostrom QT, Gittleman H,
Kruchko C, Sawaya R and Barnholtz-Sloan JS: Estimating the annual
frequency of synchronous brain metastasis in the United States
2010–2013: A population-based study. J Neurooncol. 134:55–64. 2017.
View Article : Google Scholar : PubMed/NCBI
|