Introduction
Lymphomatosis cerebri (LC) is a rare variant of
primary central nervous system lymphoma (PCNSL) (1–5) that is
pathologically characterized by diffuse cerebral infiltration
without a cohesive mass of malignant lymphoid cells around the
blood vessels along the white matter fiber tracts. It mostly occurs
in patients with normal immune function (2) and has a subacute onset, poor prognosis
even with surgical treatment and a high recurrence rate, but it is
sensitive to chemotherapy and radiotherapy (2,6).
Therefore, early diagnosis is significantly crucial for those
patients, which mostly depends on imaging. However, the
differentiated imaging features of LC have remained to be
established in detail (1–3,5,6).
The clinical and imaging findings of LC are
significantly different from those of common PCNSL and are similar
to those of central nervous system infection, inflammation,
poisoning, metabolic disorders, demyelinating encephalopathy,
autoimmune encephalitis and diffuse astrocytoma (1,2,5,6), which
easily leads to clinical misdiagnosis and unnecessary surgical
treatment in certain cases. A study by Li et al (5) first indicated that perivascular
curvilinear enhancement appears in diffuse infiltrative PCNSL,
which is similar to observations made by our group.
According to the American Association of
Neurological Surgeons, CT-guided stereotactic biopsy is a common
procedure that allows neurosurgeons to diagnose a brain lesion.
Performed in the operating room, the procedure involves the removal
of a small piece of tissue, most commonly from the brain, but may
include samples from the scalp, blood vessels or dura mater, and in
most cases, the neurosurgeon uses stereotactic equipment to
localize the preferable site for the biopsy (7). At present, early diagnosis of LC is
challenging and misdiagnosis is not uncommon (1–3,5,8–10), but specific epidemiological data are
lacking because it is a rare disease and most of the previous
studies are case reports (1–6).
In order to investigate the multimodality imaging
characteristics and clinical features of LC and the reasons for its
misdiagnosis in the clinic, the clinical data and cerebral
multimodality imaging findings from 11 patients with LC were
retrospectively analyzed. The present study indicated that
misdiagnosis mainly occurs due to limited knowledge of LC, since it
is rare and seldom reported. Furthermore, corticosteroid treatment
may cause the vanishing cancer phenomenon due to
corticosteroid-induced apoptosis and lymphoma cell lysis in a short
period of time (4,5,11), which
may result in false-negative pathology results of biopsies.
However, no abnormal enhancement was observed in adjacent meninges
or ependyma in any of those patients, which was not consistent with
inflammatory lesions. Whether this sign has differential diagnostic
value requires to be further studied and analyzed in a larger
sample. In addition, CT-guided stereotactic biopsy for LC with
extensive and diffuse lesions or posttreatment lesions has certain
shortcomings because plain CT scans are not able to effectively
distinguish active tumor areas from edema and necrotic areas, which
may lead to false-negative pathology results if the amount of
puncture specimen is limited. Inaccuracy of the puncture method,
location or timing may be secondary reasons for misdiagnosis.
To the best of our knowledge, the present study was
the first to summarize and discuss the clinical and multimodal
imaging characteristics of LC by analyzing a relatively large
number of cases, particularly in terms of the features of CT- and
MR-guided stereotactic biopsy, which is critical for the
comprehensive understanding and early diagnosis of LC and will
contribute to the reduction of misdiagnosis and avoid unnecessary
surgery.
Materials and methods
Clinical data
The clinical data and cerebral multimodality imaging
findings of 11 patients with LC (from November 2011 to December
2020) confirmed by pathology at The Affiliated Hospital of Guizhou
Medical University (Guiyang, China), including those confirmed
surgically (n=5) and via CT-guided stereotactic biopsy (n=6), were
retrospectively reviewed. The extracted information mainly included
clinical and histological data, cerebrospinal fluid findings,
treatment variables and multimodality imaging findings [mainly
including conventional CT and MR plain scan and contrast-enhanced
scan, diffusion-weighted imaging (DWI), 1H-magnetic
resonance spectroscopy (MRS) and 18F-fluorodeoxyglucose
(FDG)-positron emission tomography (PET)/CT scan]. All patients
were diagnosed based on the following criteria: i) All patients
presented with diffuse lesions of the bilateral cerebral white
matter on imaging; ii) all patients were diagnosed with lymphoma by
two experienced neuropathologists (each with 5 and 10 years of
experience, respectively) according to H&E staining and
immunohistochemical analysis; iii) All patients had complete
multimodality imaging and clinical data. Patients with concurrent
systemic lymphoma and intravascular lymphoma were excluded. All
patients provided informed consent for the multimodality imaging
examinations and for the use of personal data.
Radiological examination
methodology
128-slice CT scanning system (Toshiba Corporation)
and MRI scans using unenhanced and multiphase enhanced techniques,
(n=11), DWI (n=11), 1H-MRS (n=5) and
18F-FDG-PET/CT (n=3) were performed on patients with
lymphomatosis cerebri.
The parameters were as follows: Voltage, 120 kV;
current, 200 mA; scan thickness, 5 mm; interlayer spacing, 5 mm;
pitch, 0.5; and collimator, 16 slice × 0.625 mm. In order to make
the multiple planar reconstruction, the scan for coronal, sagittal
and other orientations were performed. A total of 60–100 ml of the
nonionic iodine contrast agent iohexol (iodine 300 mg/ml) were
injected into the cubital vein at a rate of 3 ml/sec by using a
high-pressure injector. The scan had 2 phase imaging: Arterial
(delay, 30 sec) and venous (delay, 60 sec).
A Philips Achieva 3.0-T whole-body MRI system
(Philips Healthcare) and head phased array coil (an 8-channel
phased array coil) were used. Before the scan, all patients fasted
4–8 h. T2-weighted fluid-attenuated inversion recovery (FLAIR),
T1-weighted images (T1WI) and T2-weighted images (T2WI) were
collected as baseline MRI data. After intravenous administration of
0.1 mmol/kg gadopentetate dimeglumine (Beilu Inc.), all patients
took the postcontrast T1WI. DWI was performed by using B-values of
0 sec/mm2 and 1,000 sec/mm2. The mean
apparent diffusion coefficient (ADC) values in both the lesions and
normal-appearing white matter were measured by two experienced
neuroradiologists (each with 5 and 10 years of experience). MR
spectroscopy was performed with a long echo time (135 msec) as a
multivoxel 2D exam encompassing the lesion. Major metabolites were
detected, such as choline (Cho), N-acetylaspartate (NAA), creatine
(Cr), lipids (Lip), and lactate (Lac) peaks. In order to quantify
other metabolites for each spectrum, the peak height of total
creatine (Cr) was used as the internal reference.
A Philips GEMINI TF 64 PET/CT machine (Philips
Healthcare) with 18F-FDG (purity, >95%) was
performed. Before the scan, patients were requested to fast for 6 h
and the fingertip fasting blood glucose level needed to be <11
mmol/l (normal range, 3.9–6.1 mmol/l). After 18F-FDG
(3.7 MBq/kg body weight) was injected into the cubital vein, the
scan was performed in the supine position for 1 h in a quiet dark
room. Parameters for CT were as follows: Voltage=120 kV;
current=120 mA; CT reconstruction thickness=5 mm; and interval=5.0
mm. CT data were used to attenuate and correct the PET images.
Regarding PET image reconstruction, ordered subset iterative
expectation maximization for was performed with a thickness of 5 mm
and an interval distance of 5 mm. In order to get cross-sectional
PET, sagittal PET, coronal PET, CT and PET/CT fusion images,
reconstructed images of CT and PET were transferred to a dedicated
workstation and analyzed using IntelliSpace Portal v.6.5 (Philips
Healthcare). Head lesions were outlined as the region of interest
using a semiquantitative method to measure the maximum standardized
uptake value (SUVmax) of radioactivity.
Analysis of the multimodal imaging
data
Imaging data were reviewed independently by two
expert radiologists (with 10 and 15 years of experience) using a
double-blind method and any disagreements were resolved by
consensus. All scans were reviewed with a focus on the observation
of the lesion location in the brain, patterns of contrast
enhancement, DWI signal and the corresponding ADC map, MRS
metabolic patterns and the SUVmax of radioactivity on PET/CT. The
lesion distribution included the deep cerebral white matter,
cortical and subcortical white matter, basal ganglia and the
thalamus, subtentorial cerebellum and brainstem. Contrast
enhancement was defined as patchy when a minimally or moderately
heterogeneous, poorly defined area of contrast enhancement was
present, regardless of the size. The imaging analysis and diagnosis
of each patient were compared with the pathological findings.
H&E staining
After the sections (5 µm) were deparaffinized in
xylene and rehydrated with an alcohol gradient, they were stained
with hematoxylin solution and differentiated in 1% hydrochloric
alcohol, followed by dehydration in 95% ethanol. The sections were
counterstained with 1% eosin solution.
Immunohistochemical analysis
Paraffin sections (5 µm) were deparaffinized in
xylene, rehydrated with an alcohol gradient and processed for
antigen retrieval by boiling in 10 mM citrate buffer (pH 6.0) for
2–3 min. The sections were incubated in 1%
H2O2 in PBS for 15 min at room temperature to
quench endogenous peroxidase. To block nonspecific binding,
sections were incubated in 3% bovine serum albumin (Wuhan Boster
Biological Technology, Ltd.) for 10 min. Sections were then
incubated with anti-CD20 (cat. no. OTI4B4) and anti-CD3 (cat. no.
OTI3E10; both used at 1:150 dilution; OriGene Technologies, Inc.)
overnight at room temperature. After washing, antibodies were
visualized using the HRP-polymer kit (Wuhan Boster Biological
Technology) with incubation for 15–20 min at room temperature,
followed by processing with 3,3′-diaminobenzidine
tetrahydrochloride as the chromogen for 1–3 min at room
temperature.
Results
Clinical features
The present case series consisted of 7 males and 4
females with an age ranging from 28 to 78 years (average age,
53.2±13.3 years; median age, 56 years). The common symptoms
included cognitive decline (n=2, 72.73%), gait disturbance (n=2,
81.82%) and behavioral disturbance (n=2, 45.45%) with subacute
onset (Table I). Examination of the
cerebrospinal fluid indicated that the number of cells and the
protein level increased (n=8, 80%), while the sugar content (n=2,
20%) and chloride levels decreased (n=4, 40%). A total of five
patients were treated with surgery and the remaining six patients
were subjected to CT-guided stereotactic biopsy. Among the
patients, 5 cases were misdiagnosed as having inflammatory lesions,
3 as metabolic or toxic lesions and 3 as diffuse astrocytoma based
on clinic symptoms and imaging results. The general and clinical
information of these patients is summarized in Table I.
 | Table I.General and clinical information of
the 11 patients with lymphomatosis cerebri. |
Table I.
General and clinical information of
the 11 patients with lymphomatosis cerebri.
|
|
|
| Clinical
manifestations | CSF examination |
|---|
|
|
|
|
|
|
|---|
| Case no. | Sex/age, years | Pathological
diagnosis | Cognitive
decline | Gait disturbance | Behavioral
disturbance | Personality
abnormalities | Headache | Cell increase | Protein increase | Sugar decrease | Chloride
decrease |
|---|
| 1 | M/45 | T | + | + | + | + | − | + | + | − | + |
| 2 | M/56 | B | + | − | − | − | − | + | + | + | − |
| 3 | M/59 | B | − | + | − | − | + | − | − | − | + |
| 4 | M/78 | T | + | + | + | + | − | + | + | − | − |
| 5 | F/28 | B | + | + | + | + | − | + | + | − | − |
| 6 | M/54 | B | − | − | − | − | − | / | / | / | / |
| 7 | F/58 | B | + | + | + | + | − | + | − | − | + |
| 8 | M/49 | B | + | + | + | + | − | + | + | − | − |
| 9 | M/37 | B | + | + | − | − | − | + | + | − | − |
| 10 | F/59 | B | + | + | − | − | + | + | + | − | + |
| 11 | F/62 | T | − | + | − | − | + | − | + | + | − |
Multi-modality imaging features
All patients had both extensive deep and lobar
cerebral lesion distribution in the bilateral cerebral white matter
(images of representative cases in Fig.
1,Fig. 2,Fig. 3,4),
which involved the cerebral cortex and subcortical white matter in
8 cases (72.72%), basal ganglia in 7 cases (63.64%), the thalamus
in 5 cases (45.45%), cerebellum in 6 cases (54.54%) and the
brainstem in 6 cases (54.54%). A total of six patients underwent
spinal cord examination and 1 patient (16.67%) presented with
cervical spinal cord and thoracic spinal cord infiltration
(Fig. 1L and Q). The lesions were
mostly limited and asymmetrical in the early stage and the number
and range of lesions increased along with disease progression
(Figs. 1 and 2). However, the lesions exhibited
significant improvement in the short term after treatment with
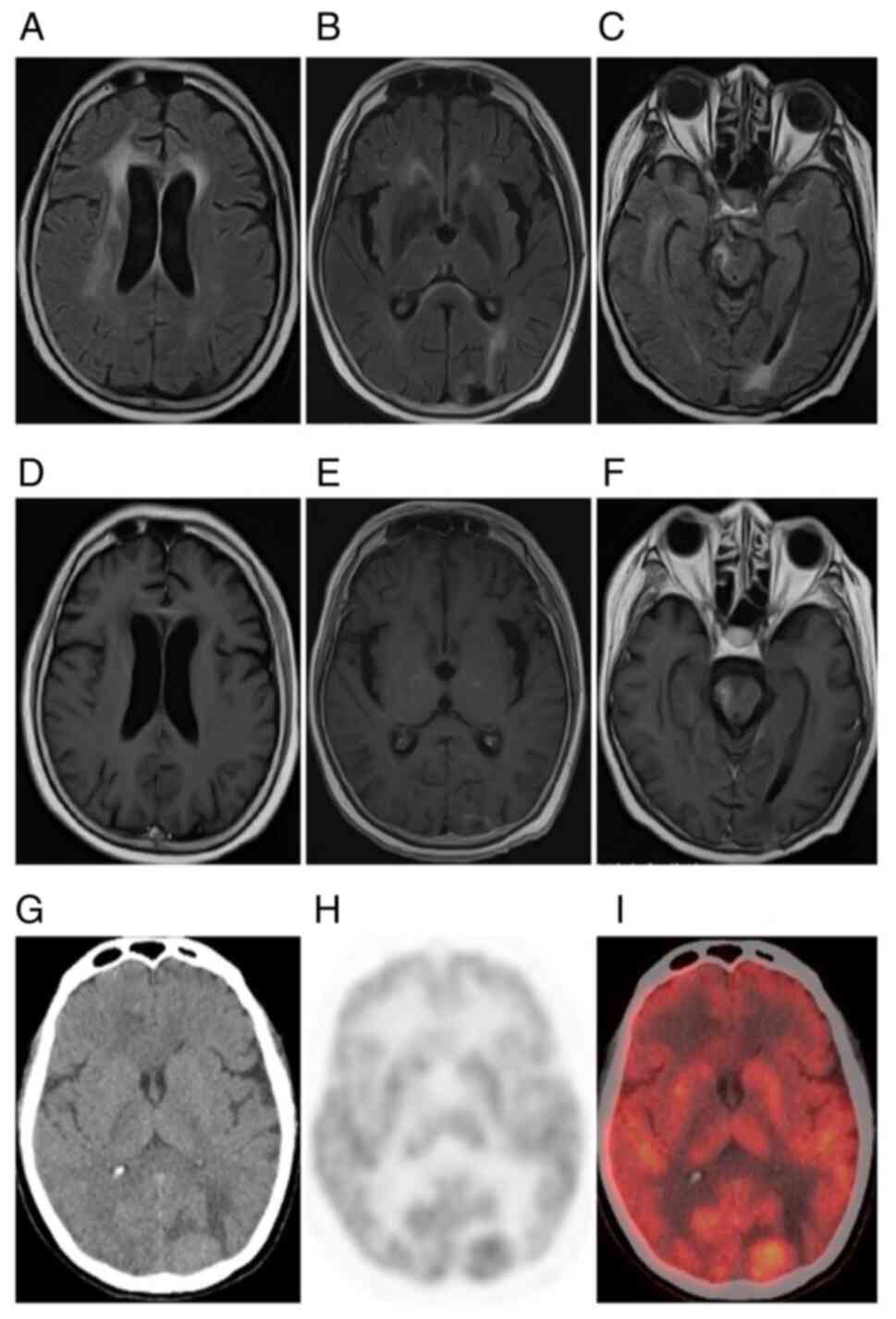
high-dose corticosteroid pulse therapy (Fig. 2H) but exhibit recurrence and
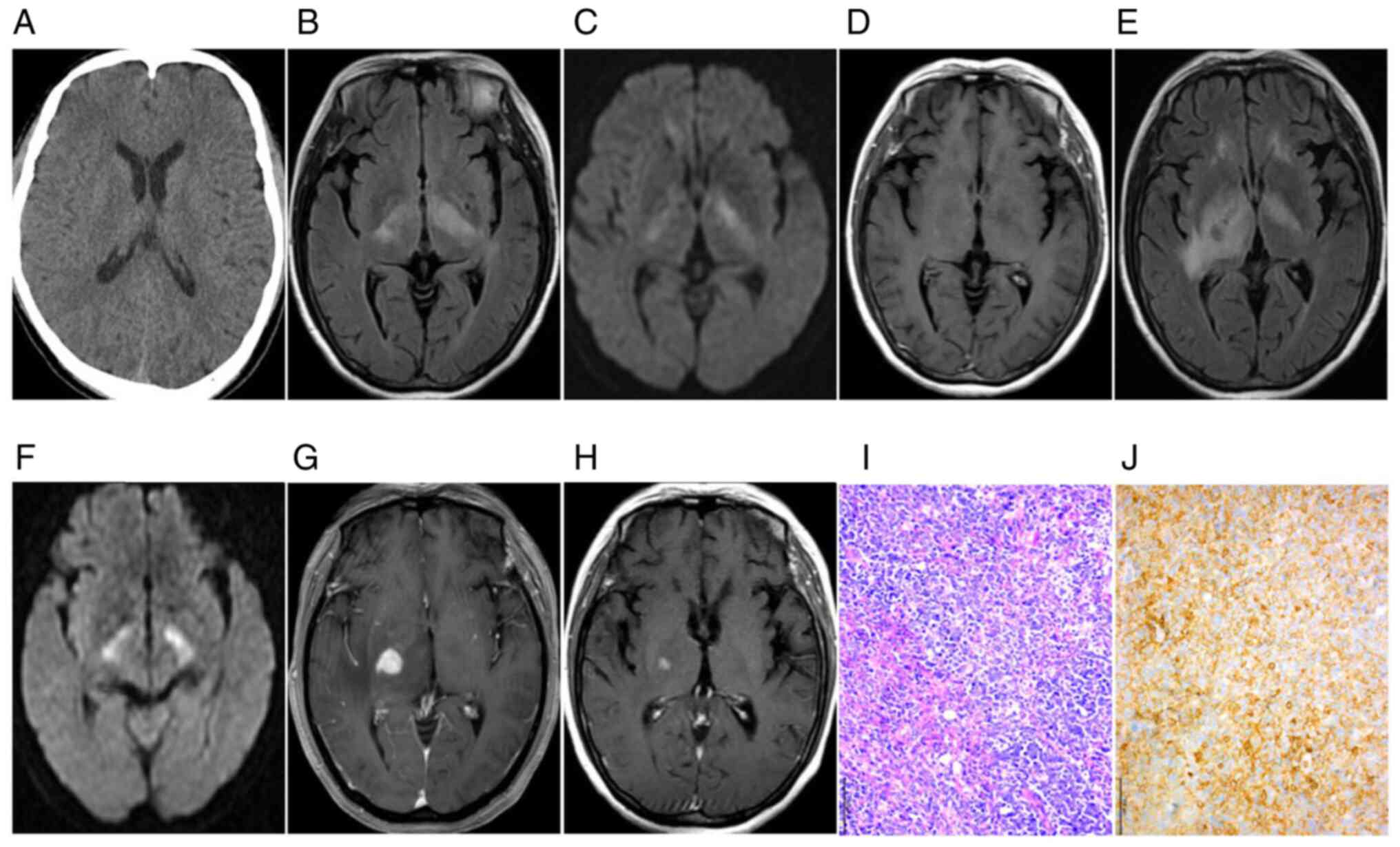
progressive aggravation in the long term (Fig. 1H-L). Plain CT scans exhibited equal
or slight low-density shadows (Figs.
1A and 3G) and 4 cases (36.36%)
had isodensity and correct diagnoses were missed. MRI displayed
slightly longer T1WI and T2WI signals in all patients (n=11, 100%)
(Fig. 1,Fig. 2,Fig.
3,4). At the initial visit, a
lack of contrast enhancement and subtle patchy enhancement patterns
were observed in 6 patients (n=6, 54.55%) (Figs. 1F and 2D) and 5 patients (n=5, 45.45%) (Fig. 3D-F), respectively, and the follow-up
imaging in the 5 patients who lacked enhancement indicated obvious
enhancement of multiple patches and nodules or masses (n=5, 45.45%)
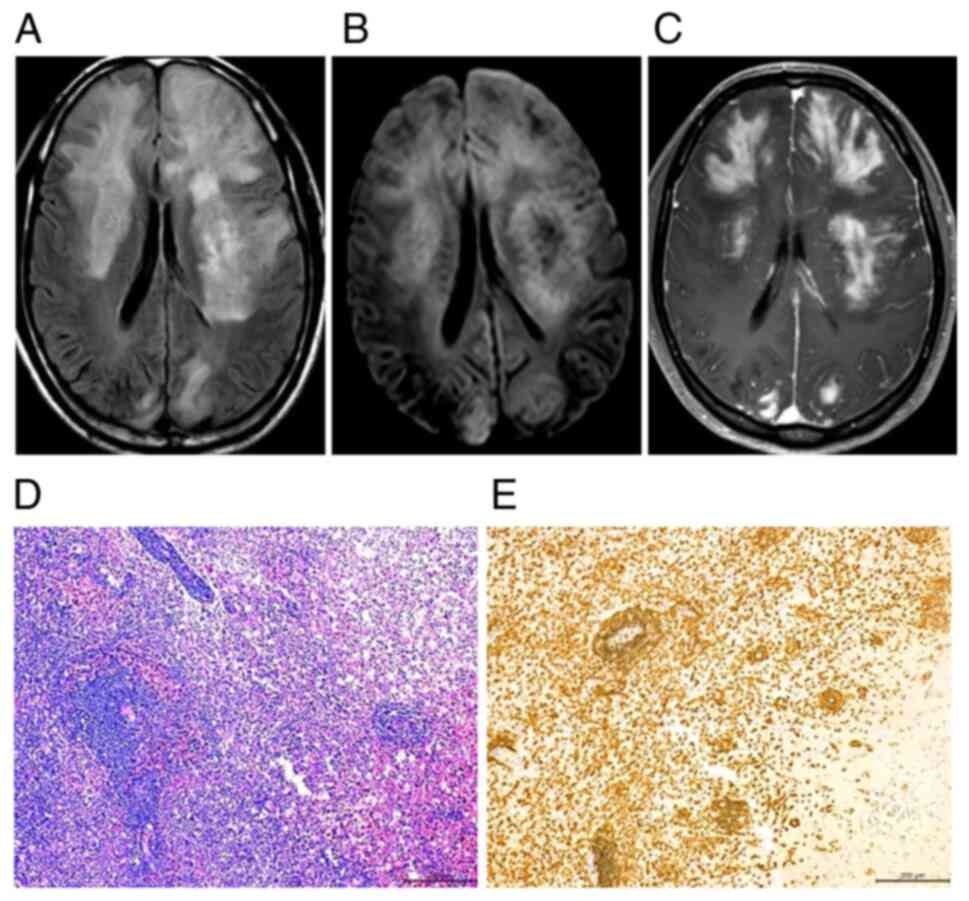
(Figs. 2G and 4C). Most of the lesions (n=9, 81.82%)
displayed with isointensity or slight hyperintensity compared to
normal brain tissue on DWI and hyperintensity on ADC maps (Figs. 1E and 2D). Follow-up observations gradually
indicated extensive diffusion limitation (Figs. 2F and 4B). A total of five patients (n=5, 100%)
presented with marked decreases in NAA/Cr and increases in Cho/Cr
and Lip/Cr (n=3, 60%) on 1H-MRS. Among the three
patients who underwent 18F-FDG PET/CT examination, 1
(33.33%) exhibited no abnormal increase in lesion metabolism, while
2 (66.67%) had slightly increased tumor metabolism (Fig. 3G-I). The multimodality imaging
findings of the patients are summarized in Table II.
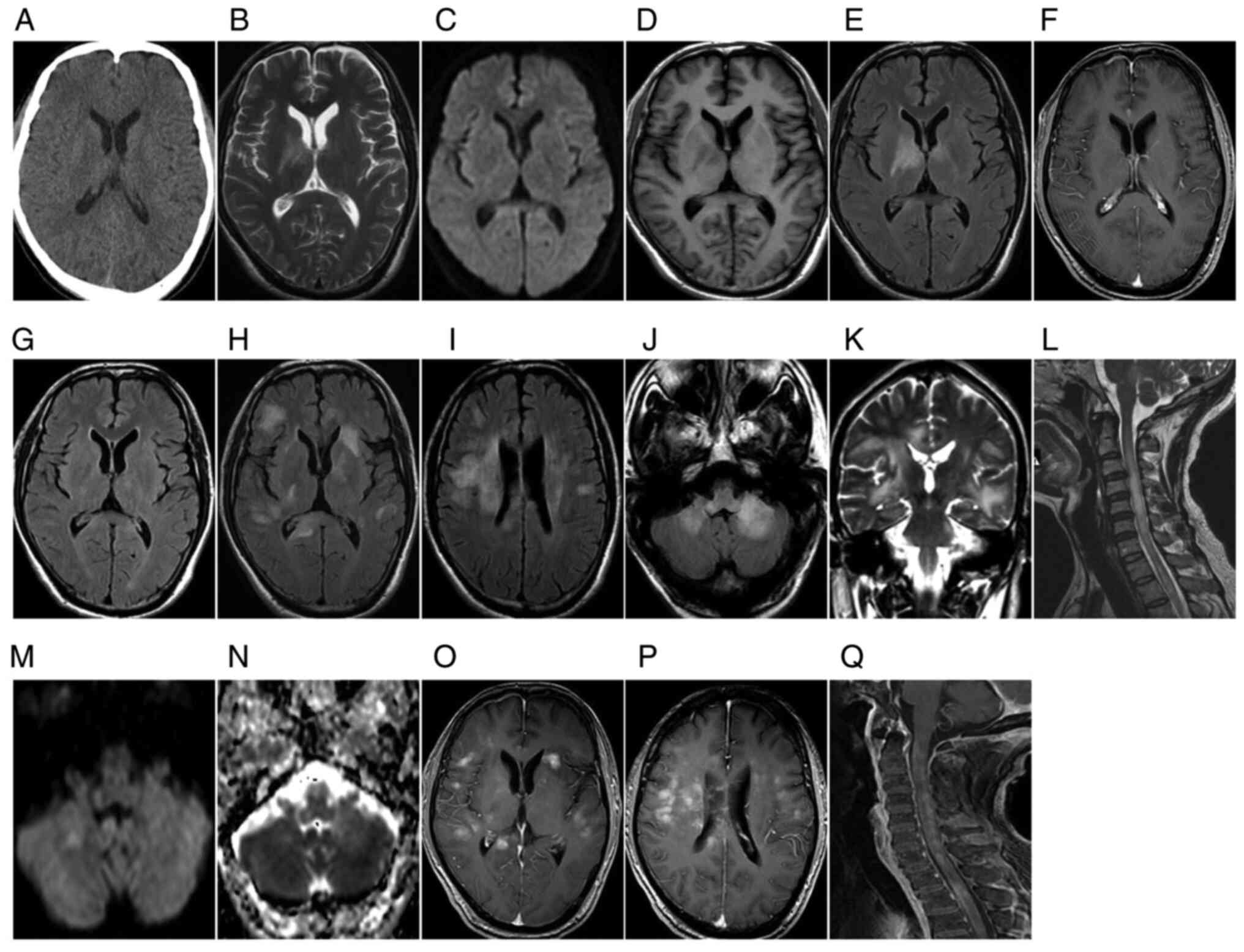 | Figure 1.A 45-year-old male with diffuse T-cell
lymphomatosis cerebri presented with memory loss, fatigue, lethargy
and slow response for 4 months, which had been aggravated for 10
days. (A-F) Initial examination: (A) Plain axial CT scan indicated
no obvious abnormalities. MRI: Patchy bilateral basal ganglia
signals with slightly longer signals on (B) T1WI and (C) T2WI, and
(D) high FLAIR signal. (E) DWI exhibited no diffusion limitation
and (F) the T1WI enhanced scan revealed no enhancement. Re
examination after 1 month of cortisol treatment: (G) FLAIR sequence
indicated that the original abnormal patchy bilateral basal ganglia
signals were significantly less pronounced on re-examination.
Symptom recurrence re-examination after 2 months: (H-J) Axial FLAIR
sequence indicated that the bilateral basal ganglia area had
slightly higher signals than at the initial examination and the
frontal lobe, temporal lobe, parietal lobe, radiation coronal area,
corpus callosum and bilateral cerebellar hemispheres had new,
similar lesions along the line; (K) the corticospinal tract was
observed to infiltrate the cerebral feet and pons. (L) Sagittal
T2WI revealed new, similar lesions in the cervical and upper
thoracic spinal cord. (M) Cerebellar and brainstem lesions on DWI
produced equal or slightly high signals and (N) apparent diffusion
coefficient images displayed mainly slightly high signals. (O-Q)
T1WI enhanced scan indicated that the abovementioned brain lesions
were patchy and nodular with obvious enhancement. FLAIR,
fluid-attenuated inversion recovery; T1WI, T1-weighted imaging;
DWI, diffusion-weighted imaging. |
 | Table II.Multimodality imaging findings of the
11 patients with lymphomatosis cerebri. |
Table II.
Multimodality imaging findings of the
11 patients with lymphomatosis cerebri.
|
| Distribution of
lesions |
|
|
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Case no. | Cerebral deep white
matter | Cerebral cortex and
subcortical white matter | Basal ganglion and
thalamus | Brainstem | Cerebellum | Spinal cord | CT plain | T1WI | T2WI/FLAIR | Enhanced scan | DWI | MRS | PET/CT |
|---|
| 1 | + | + | + | + | + | + | − | + | ++ | − | − | Cho/Cr↑,
Lip/Cr↑ | / |
| 2 | + | + | + | + | − | / | − | + | ++ | − | − | / | − |
| 3 | + | + | + | + | + | / | + | + | ++ | + | − | Cho/Cr↑ | / |
| 4 | + | + | + | + | + | − | + | + | ++ | + | − | Cho/Cr↑,
Lip/Cr↑ | / |
| 5 | + | − | − | + | − | − | + | + | ++ | − | + | / | / |
| 6 | + | + | + | − | − | − | − | + | ++ | + | − | / | / |
| 7 | + | + | + | + | − | / | + | + | ++ | − | + | Cho/Cr↑,
Lip/Cr↑ | / |
| 8 | + | + | + | − | + | / | + | + | ++ | + | − | / | / |
| 9 | + | − | − | − | + | − | − | + | ++ | + | − | Cho/Cr↑ | / |
| 10 | + | + | − | − | − | − | + | + | ++ | − | − | / | + |
| 11 | + | − | − | − | + | / | + | + | ++ | − | − | / | + |
Pathological findings
A total of eight patients were diagnosed with
diffuse large B-cell lymphoma and 3 patients were diagnosed with
T-cell lymphoma. Microscopically, high proliferation of large
lymphoid cells with perivascular infiltration and high mitotic
index were observed. Tumor cells were permeated by reactive small T
and B lymphocytes, macrophages, activated microglial cells and
reactive astrocytes (Figs. 2I and
4D). Of note, lymphoid cells in all
those patient sections expressed CD20 (11/11) (Fig. 2J). In sections from certain patients
(6/11), a small number of reactive CD3+ T cells were
present in the vicinity of neoplastic B cells (Fig. 4E).
Discussion
LC predominantly occurs in middle-aged and elderly
males. The common clinical manifestations include progressive
cognitive dysfunction and gait instability, as well as behavioral
abnormalities and personality changes (1,5,7,9,11). In the present study, 8 cases were
large B-cell lymphoma and 3 cases were T-cell lymphoma.
Immunohistochemistry analysis indicated that these lymphoid cells
expressed CD20 in the majority of the cases and a small number of
reactive CD3+ T cells were observed in the vicinity of
neoplastic B cells, in line with the previous literature (4,7–10).
Most of the cases of the present study were
supratentorial with bilateral onset and the most common lesion
location was the deep white matter of the bilateral cerebral
hemispheres. The lesions were mostly localized and asymmetric in
the early stage, increasing in size as the disease progressed. They
also tended to be bilaterally symmetric, with a propensity to
infiltrate the entire central nervous system along the
corticospinal tract (5,12). The distribution and development
characteristics of the lesions were consistent with the biological
properties of lymphoma cells, which have a tendency to spread along
the white matter fiber tracts (5,13),
supporting the theory that the disease is a whole-brain disease
(13). Therefore, the progressive
development of LC suggests the importance of dynamic clinical
imaging observations in earlier diagnosis of this disease (14,15).
Most cases of the present study had no enhancement
at the early stage but then presented with enhancement at follow-up
and the enhancement mode changed as the lesions progressed, similar
to previous findings (5,7,10,11,13,16).
This observation is related to the progressive pathological
features of lymphoma cells diffusely infiltrating the brain tissue
surrounding the blood vessels. Histopathological analysis indicated
infiltration of tumor cells and destruction of microvessels by
small round lymphocytes, providing supportive evidence of
blood-brain barrier disruption with the appearance of enhancement
on imaging.
In addition, most of the patients in this group
(81.82%) exhibited no limited diffusion on the initial DWI but
follow-up observation gradually indicated extensive limited
diffusion, which was consistent with previous reports (2,7,9). It may be speculated that this was a
result of the cell density in the tumor area continuing to increase
and the intercellular space becoming narrower as the disease
progressed, eventually leading to restricted local water molecule
diffusion. While three patients underwent 18F-FDG-PET/CT
examination, it remained challenging to differentiate between early
negative PET/CT results, non-neoplastic lesions such as
inflammation and late high uptake from other malignant lesions. At
present, the characteristics of LC-related PET/CT findings are
poorly understood and the characteristic findings require to be
summarized in larger samples.
In summary, despite the small sample size of the
cohort, the present results still indicated that the clinical
manifestations and multimodal imaging findings of LC have certain
characteristic features, including extensive diffuse bilateral
cerebral white matter lesions, particularly in deep and lobar
cerebral regions, with a tendency to infiltrate the entire central
nervous system along the corticospinal tract, along with no obvious
diffusion limitation on DWI and no obvious enhancement or with
varied enhancement patterns as the lesions progressed. Prompt
recognition of these features by clinicians and radiologists is
important for the early diagnosis and improved prognosis of LC.
Apart from that, stereotactic biopsy guided by MRI or enhanced CT
is another ideal choice (7,17), while puncturing biopsy should be
avoided after corticosteroid treatment.
Acknowledgements
Not applicable.
Funding
This research was supported by grants from the
Science and Technology Program of Guizhou Province [platform
talents (2018)5779-72 and LC (2017) no. 7206].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ZR, LC and YH contributed to the extraction and
review of patient data from the clinical records and images, as
well as the conceptualization of the study and writing of the
manuscript. LC and CL interpreted the patients' clinical data. YH
performed the analysis for the DWI and MRS parts and wrote part of
the manuscript and edited it. JH interpreted the histological
examination results of the specimens. ZR and LC confirmed the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Guizhou Medical University
(Guizhou, China) and was performed according to the Declaration of
Helsinki guidelines. Written informed consent was obtained from all
participants for inclusion in the study.
Patient consent for publication
Written informed consent was obtained from all
participants for publication of their images in this
manuscript.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
LC
|
lymphomatosis cerebri
|
|
PCNSL
|
primary central nervous system
lymphoma
|
|
FLAIR
|
fluid-attenuated inversion
recovery
|
|
DWI
|
diffusion-weighted imaging
|
|
ADC
|
apparent diffusion coefficient
|
|
MRS
|
magnetic resonance spectroscopy
|
|
NAA
|
N-acetylaspartate
|
|
Cho
|
choline
|
|
Cr
|
creatine
|
|
Lac
|
lactate
|
|
Lip
|
lipid
|
|
PET
|
positron emission tomography
|
References
|
1
|
Hatanpaa KJ, Fuda F, Koduru P, Young K,
Lega B and Chen W: Lymphomatosis cerebri: A diagnostic challenge.
JAMA Neurol. 72:1066–1067. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tao J, Zhang Y, Huang Z, Li H and Wang L:
A case of lymphomatosis cerebral with the features characterized by
bilateral cerebral diffuse white matter lesions. Chin J Nerv Ment
Dis. 45:53–56. 2019.
|
|
3
|
Yamaura G, Ogasawara A, Ito T, Ohsugi S,
Kanatsuka Y, Hayashi R, Iwashita H, Hayashi H, Koyano S, Yamaguchi
S and Tanaka F: Pathologically proven gadolinium-enhanced MRI
lesions in the bilateral corticospinal tracts in lymphomatosis
cerebri. Intern Med. 59:2931–2934. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gametchu B: Glucocorticoid receptor-like
antigen in lymphoma cell membranes: Correlation to cell lysis.
Science. 236:456–461. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li L, Rong JH and Feng J:
Neuroradiological features of lymphomatosis cerebri: A systematic
review of the English literature with a new case report. Oncol
Lett. 16:1463–1474. 2018.PubMed/NCBI
|
|
6
|
Duan L, Wang X, Zhang M and Wu J: Clinical
analysis of 75 cases of primary central nervous system lymphoma.
Chin J Oncol. 45:88–91. 2018.
|
|
7
|
American Association of Neurological
Surgeons, . Available from:. https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Stereotactic-Brain-BiopsyMay
11–2021
|
|
8
|
Yu H, Gao B, Liu J, Yu YC, Shiroishi MS,
Huang MM, Yang WX and Guan ZZ: Lymphomatosis cerebri: A rare
variant of primary central nervous system lymphoma and MR imaging
features. Cancer Imaging. 17:262017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kerbauy MN, Pasqualin DDC, Smid J, Iquizli
R, Kerbauy LN, Nitrini R, Ribas GC, Neder L and Hamerschlak N:
Diffuse large B-cell lymphoma of the central nervous system
presenting as ‘lymphomatosis cerebri’ and dementia in elderly man:
Case report and review of the literature. Medicine (Baltimore).
98:e143672019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen YF, Qiu QD, Wu H, Liu XM, Wu MM and
Yan ZH: Imaging and pathological study of primary central nervous
system lymphoma in special sites. Chin J Neurol. 53:700–705.
2020.
|
|
11
|
Izquierdo C, Velasco R, Vidal N, Sánchez
JJ, Argyriou AA, Besora S, Graus F and Bruna J: Lymphomatosis
cerebri: A rare form of primary central nervous system lymphoma.
Analysis of 7 cases and systematic review of the literature. Neuro
Oncol. 18:707–715. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mora P, Majós C, Castañer S, Sánchez JJ,
Gabarrós A, Muntané A, Aguilera C and Arús C: (1)H-MRS is useful to
reinforce the suspicion of primary central nervous system lymphoma
prior to surgery. Eur Radiol. 24:2895–2905. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Imataki O, Uchida S, Yokokura S, Uemura M
and Kadowaki N: Central nervous system peripheral T cell lymphoma
manifesting as lymphomatosis cerebri that was misdiagnosed as
neuro-behçet's disease: A case report. Case Rep Oncol. 11:806–813.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Imperiale D, Taraglio S, Atzori C and
Testi R: Diffuse leukoencephalopathy due to lymphomatosis cerebri:
A clinicopathological report. Neurol Sci. 36:1071–1073. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sato H, Takahashi Y, Wada M, Shiono Y,
Suzuki I, Kohno K, Kato Y, Kawanami T, Sakurada K, Kayama T and
Kato T: Lymphomatosis cerebri with intramedullary spinal cord
involvement. Intern Med. 52:2561–2565. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murakami T, Yoshida K, Segawa M, Yoshihara
A, Hoshi A, Nakamura K, Ichikawa M, Suzuki O, Yokoyama Y, Toyoshima
Y, et al: A case of lymphomatosis cerebri mimicking inflammatory
diseases. BMC Neurol. 16:1282016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giannini C, Dogan A and Salomão DR: CNS
lymphoma: A practical diagnostic approach. J Neuropathol Exp
Neurol. 73:478–494. 2014. View Article : Google Scholar : PubMed/NCBI
|