Introduction
Annually, 230,000 women are diagnosed with ovarian
cancer and 150,000 succumb to this disease worldwide and the 5-year
survival rate of ovarian cancer is only 46% (1). Epithelial ovarian cancer has the
highest mortality rate among ovarian cancer types with a 5-year
relative survival rate of 29% (2).
It is characterized by a lack of clear symptoms at onset, late
initial diagnosis, early spread and metastasis, recurrence after
surgery, drug resistance and high mortality (3). Although great progress has been made in
the treatment of ovarian cancer with the introduction of tumor
reduction surgery and platinum chemotherapy combined with
paclitaxel, drug resistance remains an urgent issue to be resolved
(4). Therefore, it is necessary to
uncover the molecular mechanism of drug resistance.
Cisplatin (CDDP) has been widely used for the
treatment of a variety of tumors, including head and neck,
testicular, bladder, lung, colorectal and ovarian cancers (5,6). It is a
non-specific cytotoxic drug that inhibits the cell cycle and
distributes non-selectively to tumor tissues. Its main action is
the inhibition of tumor growth and induction of tumor cell death
via the inhibition of DNA replication and transcription (7). However, drug resistance seriously
limits the clinical use of CDDP (8).
DNA damage and repair systems serve a vital role in the response to
cancer treatment. The upregulated functioning of this system
results in an antagonistic response of tumor cells to
chemotherapeutic drugs and chemotherapy failure (9). It is well known that redox reactions
are important for oxidative stress-induced DNA damage (10). Oxidative stress results in
intracellular levels of reactive oxygen species (ROS) being
elevated, which causes damage to lipids, proteins and DNA (11). In response to the accumulation of ROS
induced by certain chemotherapies, cancer cells commonly produce
reducing substances to alleviate oxidative stress, which gradually
results in drug resistance (12).
Glutathione (GSH) is a reducing substance containing a sulfhydryl
group bound to the cysteine moiety of a tripeptide formed from
glutamic acid, cysteine and glycine, which has an ROS-scavenging
effect (13). Therefore, the drug
resistance of cancer cells may be closely associated with an
imbalance of GSH and ROS, resulting in a reduction in the DNA
damage induced by CDDP.
Growth arrest and DNA damage 45a (GADD45α) is
a downstream target gene of p53, which is involved in growth
arrest, apoptosis and DNA damage repair (14). GADD45α is closely associated with the
occurrence, development and prognosis of tumors, as it is able to
maintain genetic stability and inhibit the occurrence and
development of tumors (15).
However, some tumor cells have been revealed to avoid programmed
death by decreasing the expression of GADD45α (16). As a downstream target gene of p53,
GADD45α may be a key mediator of ovarian cancer chemosensitivity,
which can arrest the cell cycle to increase damaged DNA repair
(17). It has also been reported
that the decreased ability of cells to repair DNA caused by GADD45α
deletion is highly associated with DNA damage-induced tumorigenesis
(18). In addition, GADD45α has been
shown to alleviate multidrug resistance in tumor cells (19). In one study, the upregulation of
GADD45α expression increased the sensitivity of prostate cancer
cells to docetaxel (20). In another
study, GADD45α expression was induced by platinum-based
chemotherapy, which promoted the apoptosis of lung cancer cells and
increased their sensitivity to chemotherapy (21). Previous studies have also reported
that GADD45α can be upregulated by CDDP treatment in ovarian cancer
cells (22,23). It is thus very important to further
study the role of GADD45α in ovarian cancer in order to find a new
therapeutic target for its treatment. The present study constructed
a CDDP-resistant ovarian cancer cell line and performed RNA
sequencing, which revealed a difference in GADD45α gene expression
in the CDDP-resistant cell line compared with the parental cell
line. However, whether GADD45α increases the sensitivity of ovarian
cancer to CDDP has not yet been reported.
H2A histone family member X (H2AX) is a sensitive
marker for DNA damage that serves a crucial role in molecular and
cellular responses to DNA damage and in genome stability
maintenance (24). H2AX has been
demonstrated to be important for the inhibition of tumor growth, as
it increases genomic stability and reduces susceptibility to
tumorigenesis (25). H2AX prevents
the aberrant repair of programmed and general DNA breakage and
functions as a dosage-dependent suppressor of genomic instability
and tumors in mice (26). However,
the mechanism underlying its effects remains unknown.
The present study investigated the effect of redox
status on the drug resistance of ovarian cancer cells, and its
association with GADD45α. The effect of the overexpression of
GADD45α in CDDP-resistant ovarian cancer cells was investigated
in vitro and in vivo, and whether the role of GADD45α
in the CDDP resistance of ovarian cancer cells is mediated via
redox-mediated DNA damage was evaluated.
Materials and methods
Cell culture and reagents
SK-OV3 human ovarian cancer cells were purchased
from American Type Culture Collection. The cells were cultured in
complete RPMI-1640 medium (Thermo Fisher Scientific Inc.)
containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific
Inc.) and no antibiotics at 37°C and 5% humidified CO2.
Cells in the logarithmic growth phase were used for the
experiments. Thiazolyl blue tetrazolium bromide (MTT) was obtained
from Abcam (cat. no. ab211091). The primary antibodies GADD45α
(cat. no. ab203090), Ser-139 phosphorylated H2AX (γ-H2AX; cat. no.
ab26350), H2AX (cat. no. ab229914) and β-actin (cat. no. ab8226),
all 1:1,000, were purchased from Abcam. CDDP was acquired from
Sigma-Aldrich (Merck KGaA).
CDDP-induced SK-OV3/cddp cells
SK-OV3 cells were cultured in suspension in complete
RPMI-1640 medium containing 10% fetal bovine serum and passaged
every 3 days. Cells in the logarithmic growth phase were inoculated
in RPMI-1640 medium containing CDDP. Starting at a CDDP
concentration of 0.1 µg/ml and with the maintenance of positive
drug pressure, sensitive cells gradually died and drug-resistant
cells continued to be cultured for 3–4 weeks. The
high-concentration drug treatment was initiated when the number of
cells reached 1×107/ml. After repeated medium
replacement, passage and a step-wise increase in CDDP concentration
(0.1, 0.15, 0.2, 0.25, 0.375 and 0.5 µg/ml) over the course of 6
months, an SK-OV3/cddp cell line that was able to grow well in 0.5
µg/ml CDDP medium was obtained. The cells were frozen, stored for 3
months, and then recovered or grown in CDDP-free medium for nearly
6 months while maintaining their original drug resistance.
Treatment of cells
SK-OV3 and SK-OV3/cddp cells were treated with CDDP
(0, 1, 5, 10, 20, 40 and 80 µM) or H2O2 (0,
5, 10, 25 and 50 µM) at 37°C for 24 h for the cell viability assay.
The SK-OV3 and SK-OV3/cddp cells were treated with 80 µM CDDP and
50 µM H2O2 at 37°C for 24, 48 and 72 h for
the cell viability assay. The SK-OV3/cddp cells were treated with
80 µM CDDP and 50 µM H2O2 at 37°C for 2 weeks
for the soft agar colony formation assay. The SK-OV3/cddp cells
were treated with 50 µM H2O2 for 24 h at 37°C
in RT-qPCR and western blotting assays.
Xenograft experiments in mice
Female BALB/c nude mice of specific pathogen-free
(SPF) grade, aged 6 weeks and weighing 18–20 g were used in the
study. The 50 nude mice were purchased from Beijing Sibeifu
Biotechnology Co., Ltd. The animal experiments were performed in
accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals (1996) 7th Edition and approved
by the Animal Ethics Committee of Guizhou Medical University
(approval no. 2000470). The mice were provided with food and water
freely available and kept in a SPF-level environment with a
temperature of 22±2°C, a humidity of 40–60% and a 12-h light/dark
cycle. The mice were divided into five groups: Two control groups
(untreated SK-OV3 and SK-OV3/cddp cells, respectively); CDDP group
(CDDP treatment and SK-OV3/cddp cells); GADD45α group
(GADD45α-overexpressing SK-OV3/cddp cells) and CDDP + GADD45α group
(CDDP treatment and GADD45α-overexpressing SK-OV3/cddp cells), each
containing 10 mice. The mice were subcutaneously injected in the
left axillary region with a 100 µl single-cell suspension of
1.5×106 cells in saline, containing SK-OV3, SK-OV3/cddp
or GADD45α-overexpressing SK-OV3/cddp cells as appropriate. The
mice were continuously observed for 6 weeks. Once the tumor volume
reached 50 mm3, the mice in the CDDP group and CDDP +
GADD45α group were intraperitoneally injected with CDDP (4 mg/kg),
while the mice in the other groups were injected with saline. The
injection frequency was once every 3 days, for a total of five
injections. Measurements of the tumor were taken weekly, and tumor
volumes were calculated using the following formula: Tumor volume =
(AxB2)/2, where A and B are the tumor length and width
(in mm), respectively. When the maximum volume of the transplanted
tumor reached 1,500 mm3, the mice were euthanized by
cervical dislocation and tumor samples were taken. The tumor
tissues were frozen with liquid nitrogen and stored in a
refrigerator at −80°C.
Cell viability assay and 50%
inhibitory concentration (IC50) calculation
When cells reached 70% confluency in a 96-well
microplate, they were cultured in a medium containing CDDP (0, 1,
5, 10, 20, 40 and 80 µM) or H2O2 (0, 5, 10,
25 and 50 µM) at 37°C for 24 h. Then, 10 µl MTT, dissolved in PBS,
was added and the cells were incubated for 2 h at 37°C. The purple
formazan was dissolved in PBS. The absorbance values of the cells
were detected at 490 nm using a microplate reader (Omega Bio-Tek,
Inc.). The IC50 was calculated with GraphPad Prism
v.5.01 software (GraphPad software, Inc).
RNA sequencing
Total RNA from the CDDP-resistant ovarian cancer
cell line and its parental cell line was analyzed using Hiseq.
Agilent 2100 Bioanalyzer System (Agilent Technologies, Inc.) to
assess its quantity and quality. The differences of readcount data
were analyzed after being standardized by DESeq v.1.12.0 (Illumina
Inc.). Differentially expressed genes for CDDP-resistant SK-OV3
cells compared with the parental cell line were defined as genes
with an absolute log-transformed fold change [abs(log2FC)] >1
(P<0.005).
Intracellular GSH content
Intracellular GSH content was measured using
5,5-dithio-bis (2-nitrobenzoic acid) (DTNB). SK-OV3 and SK-OV3/cddp
cells were washed twice with PBS and the cell pellet
(1×106 cells) was lysed with 100 µl cell lysis buffer
(Beyotime Institute of Biotechnology). Thereafter, 15 µl HCl (0.1
N) and 15 µl 50% sulfosalicylic acid were added. Xenograft tissue
homogenate was prepared by perfusing the tissue with phosphate
buffer saline solution (pH 7.4) containing 0.16 mg/ml heparin to
remove red blood cells and clots. Then, the tissue was homogenized
in 5–10 ml cold buffer (50 mM potassium phosphate buffer, pH 7.5,
containing 1 mM EDTA) per gram of tissue. Supernatants were
collected after centrifugation for 15 min at 12,000 × g at 4°C. A
total of 25 µl cell lysate and 100 µl DTNB in sodium phosphate
buffer containing EDTA were mixed and the optical density at 412 nm
was measured immediately using a spectrophotometer (Varian Medical
Systems, Inc.). The protein level was measured using the Bradford
method (27).
ROS level analysis in cells
Cellular ROS levels were measured using
2,7-dichlorodihydrofluorescein diacetate (DCFH-DA; Beyotime
Institute of Biotechnology). SK-OV3 and SK-OV3/cddp cells were
washed twice with cold PBS, treated with 10 µM DCFH-DA and
incubated for 30 min at 37°C in a light-protected humidified
chamber. Following treatment with the probe, the cells were washed
at least twice with ice-cold PBS. The fluorescence emitted by the
cells was measured with a fluorescence detection instrument
(DTX800; Beckman Coulter, Inc.) using an excitation wavelength of
502 nm, and detection wavelength of 530 nm. The fluorescence
intensity was recorded and analyzed with GraphPad Prism v.5.01
(GraphPad software, Inc.).
ROS detection in dissected tumor
tissue
PBS was used to wash the excised tumor twice.
Ophthalmic scissors were used to cut the tumor tissue into pieces,
which were then treated with type I collagenase (1 mg/ml) to
destroy the extracellular matrix in the tissue at 37°C for 10 min.
The digestion fluid was filtered with a 200-mesh filter to obtain a
single-cell suspension. Then, 2×104 cells/well were
seeded in a 96-well plate, treated with 10 µM DCFH-DA and incubated
for 30 min at 37°C. The cells were washed with precooled PBS at
least twice and then examined using a fluorescence detection
instrument (DTX800). The excitation wavelength was 502 nm, and the
detection wavelength was 530 nm. The fluorescence intensity was
recorded and analyzed with GraphPad Prism v.5.01 (GraphPad
software, Inc.).
Soft agar colony formation assay
The lower gel was prepared from 2 ml RPMI-1640
medium (Thermo Fisher Scientific, Inc.) containing 20% fetal bovine
serum (Gibco; Thermo Fisher Scientific Inc.) with 0.4% agar. The
upper cell-containing layer was prepared by suspending
1×103 cells in 4 ml RPMI-1640 medium with 0.2% agar and
pouring it onto the lower gel. After 2 weeks, colonies were stained
with 0.5% crystal violet at room temperature for 1 h and counted
with an inverted microscope (CX43; Olympus Corporation).
Preparation and transfection of
lentiviral-GADD45α vector
Lentiviral vector (GV287; 4 µg) (Shanghai Genechem
Co., Ltd) containing the GADD45α gene was double digested using
AgeI (5 U/µl) and EcoRI (20 U/µl). Virus packaging
plasmid Mix:1 µg/µl (Mix=pMDL: VSV-G: REV=5:3:2). The product was
recovered, purified and mixed with T4 DNA ligase (1 µl) for 6 h at
16°C. It was then transformed into competent DH5α cells (Shanghai
Ji Kai Biotechnology Co., Ltd) with Lipofectamine 2000®
(Thermo Fisher Scientific, Inc.). Recombinant positive clones were
preliminarily identified using PCR and restriction analysis.
Plasmid sequencing was performed by Shanghai Ji Kai Biotechnology
Co., Ltd. The primer sequences used were as follows: GADD45α
forward, 5′-AGUCGCUACAUGGAUCAAUTT-3′ and reverse,
5′-AUUGAUCCAUGUAGCGACUTT-3′; GAPDH forward,
5′-GCAGGGGGGAGCCAAAAGGGT-3′ and reverse,
5′-TGGGTGGCAGTGATGGCATGG-3′. SK-OV3/cddp cells in the logarithmic
growth phase were seeded in 6-well plates at a concentration of
2×105 cells/ml, cultured for 24 h, mixed with the 4ug
lentiviral-GADD45α vector with a multiplicity of infection value of
80 for 12 h, and then replenished with fresh medium. Transfection
efficiency was detected using western blotting and reverse
transcription-quantitative PCR (RT-qPCR) after 6 days of
transfection. GFP lentivirus control plasmid (Shanghai Ji Kai
Biotechnology Co., Ltd.) was used as control for the GADD45α
vector.
RT-qPCR
The primers used for RT-qPCR validation are as
described above for PCR. Total cell RNA samples were isolated using
Total RNA Kit I (Omega Bio-Tek, Inc.). Then, 500 ng RNA was reverse
transcribed to cDNA using the Takara PrimeScript™ RT Reagent kit
with gDNA Eraser (Takara Bio, Inc.) in a total reaction volume of
20 µl. The RT conditions used were 25°C for 5 min, 42°C for 30 min,
85°C for 5 min followed by storage at 4°C. The cDNA (2 µl) was used
as a template for qPCR conducted using a Real-Time PCR System
(Bio-Rad Laboratories, Inc.). The qPCR steps were conducted in
triplicate using SYBR Green Supermix according to the
manufacturer's protocol (Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: 3 min at 95°C followed by
40 cycles of 10 sec at 95°C and 30 sec at 60°C. Following
amplification, an additional thermal denaturizing cycle
(temperature range, 65–95°C in 0.5°C increments) was performed to
obtain the melting curves of the RT-qPCR products and verify
amplification specificity. The relative expression of the gene of
interest was normalized to GAPDH expression in each sample. The
target gene expression level was calculated using the
2−ΔΔCq method (28) and
the values for each gene are expressed as fold changes.
Western blot analysis
SK-OV3 cells were treated with lysis buffer
containing 20 mmol/l Tris (pH 7.5), 150 mmol/l NaCl, 1% Triton
X-100 and protease and phosphatase inhibitors on ice for 30 min.
The supernatant was harvested after centrifuging the cell lysis
products for 10 min at 12,700 × g and 4°C. The total protein
concentration was measured using a BCA Protein Assay kit (Thermo
Fisher Scientific, Inc.). Total protein samples were boiled for 5
min. Then, 30 µg/lane total protein was electrophoresed on an 8%
SDS-PAGE gel, transferred onto PVDF membranes and blocked with 5%
BSA for 2 h at room temperature before incubation with the
aforementioned primary antibodies overnight at 4°C. The membranes
were then washed with Tris-buffered saline containing 0.1%
Tween-20, and incubated with goat anti-mouse IgG-horseradish
peroxidase (HRP)-conjugated secondary antibody (1:10,000; cat. no.
sc-2031; Santa Cruz Biotechnology, Inc.), for 2 h at room
temperature. Protein immunoreactivity was visualized using
Immobilon Western Chemilum HRP substrate (EMD Millipore) and
quantified using Quantity One software 6.0 (Bio-Rad Laboratories,
Inc).
Immunofluorescence assay
Precooled PBS was used for washing SK-OV3 and
SK-OV3/cddp cells (10,000 cells/well) cultured in 6-well plates for
3 min each time for 3 times. Subsequently, 4% paraformaldehyde was
used to fix cells at room temperature for 15 min. Next, the cells
were washed twice with PBS, and permeabilized for 15 min at room
temperature with 0.5% Triton X-100 to and blocked with 3% BSA to
block the cells at room temperature for 40 min. The blocked SK-OV3
and SK-OV3 /cddp cells were incubated with primary antibody against
γ-H2AX (1:200; Abcam; cat. no. ab81299) at 4°C for 12 h. The cells
were washed 3 times with PBS prior to incubation with a Goat
polyclonal Secondary Antibody to Rabbit IgG (1:100; Abcam; cat. no.
ab150077) dilution for 40 min at room temperature. Subsequently,
the nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI;
Thermo Fisher Scientific Inc.) for 5 min at room temperature. The
cells were observed using a fluorescence microscope at (CX43;
Olympus Corporation) (magnification, ×400).
Immunohistochemistry (IHC)
IHC analysis was performed using an SP-HRP kit
(Santa Cruz Biotechnology, Inc.). The tumor tissue was cut into
3-µm thick sections. Sections of tumor tissue were blocked with 5%
Goat Serum (Beyotime Institute of Biotechnology) for 30 min at
37°C, incubated with anti-KI67 primary antibody (1:100; cat. no.
sc-23900; Santa Cruz Biotechnology, Inc.) at 4°C overnight, then
incubated with secondary antibody at dilution (1:5,000; cat. no.
sc-2031; Santa Cruz Biotechnology, Inc.), for 2 h at room
temperature, developed with 3,3′-diaminobenzidine and
counterstained with hematoxylin for 5 min at room temperature and
observation was performed using a light microscope (CX43; Olympus
Corporation).
Statistical analysis
All experiments were performed in triplicate and
data are expressed as the mean ± SD. Statistical significance was
analyzed with one-way analysis of variance or two-way analysis of
variance followed by Dunnett's test when groups were compared with
a single control group or Tukey's test when analyzing the
differences for all pairs of groups. Student's t-test was used to
compare the differences between the SK-OV3 group and SK-OV3/cddp
group or the NC group and GADD45α group. GraphPad Prism version
5.01 was used to perform the statistical analysis (GraphPad
software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Redox status affects the drug
resistance of ovarian cancer cells
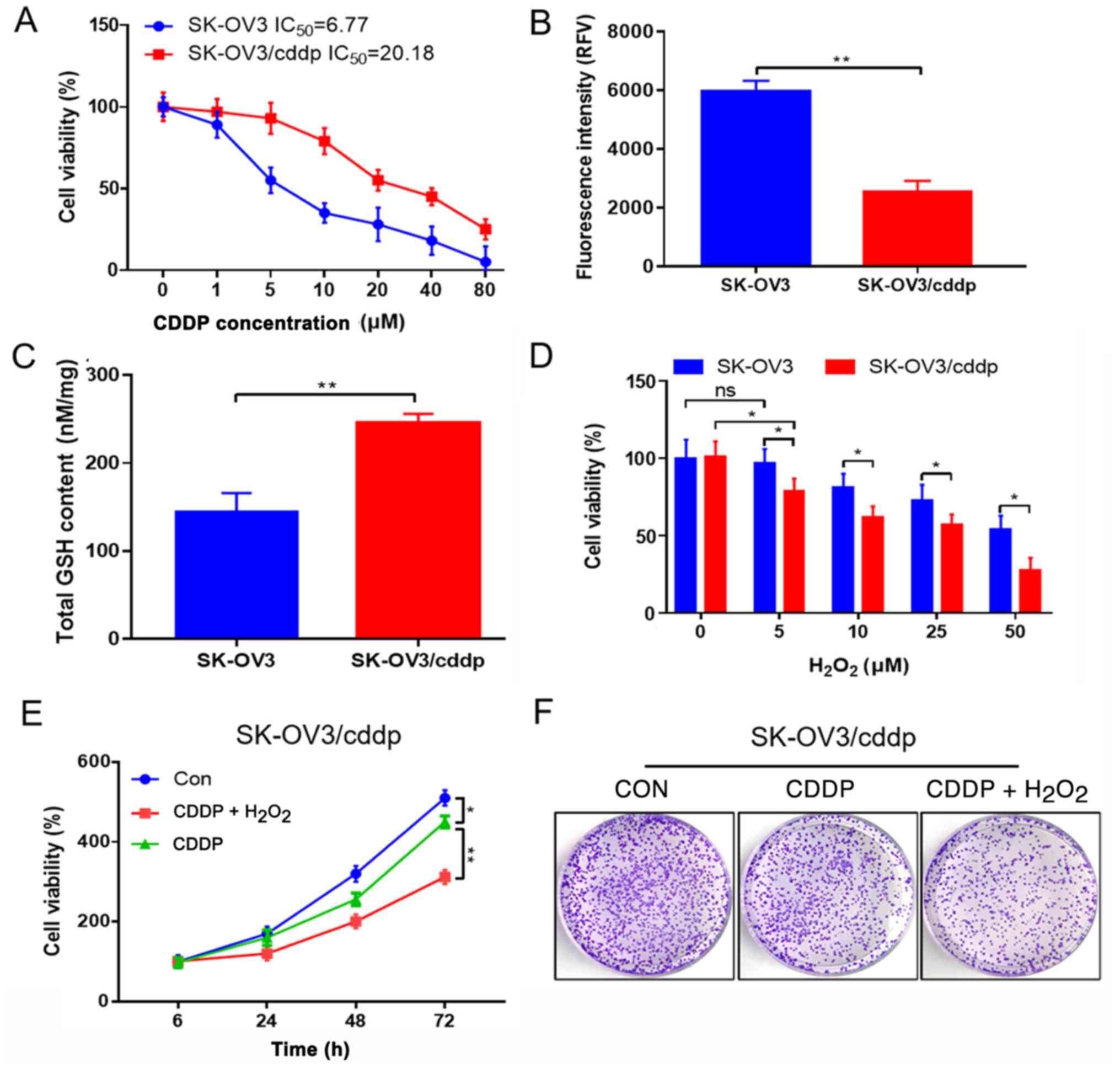
To determine the drug resistance of ovarian cancer
cells, the 50% inhibitory concentration (IC50) values of
SK-OV3 and SK-OV3/cddp cells were detected. The IC50 of
the SK-OV3 cells was lower than that of the SK-OV3/cddp cells
(Fig. 1A). Then, the ROS and GSH
levels in the SK-OV3 and SK-OV3/cddp cells were detected.
Significantly lower ROS and higher GSH levels were observed in the
SK-OV3/cddp cells compared with the SK-OV3 cells (Fig. 1B and C). In order to verify the role
of GSH in SK-OV3/cddp cells, different concentrations of
H2O2 were added to SK-OV3/cddp and SK-OV3
cells for 24 h to deplete GSH. Cell viability was then detected.
The viability of SK-OV3/cddp cells was significantly decreased
following H2O2 treatment (Fig. 1D). It was hypothesized that a high
level of reduction and low level of oxidation in SK-OV3/cddp cells
may contribute to their drug resistance. To investigate this
hypothesis, the effect of H2O2 on CDDP
resistance was detected. Cell viability and colony formation were
detected in SK-OV3/cddp cells treated with CDDP alone or in
combination with H2O2.
H2O2 combined with CDDP significantly
inhibited the viability and colony formation of SK-OV3/cddp cells
compared with that of CDDP alone (Fig.
1E and F), indicating that redox status affected the drug
resistance of these ovarian cancer cells.
Redox regulation of ovarian cancer
cell resistance is associated with GADD45α
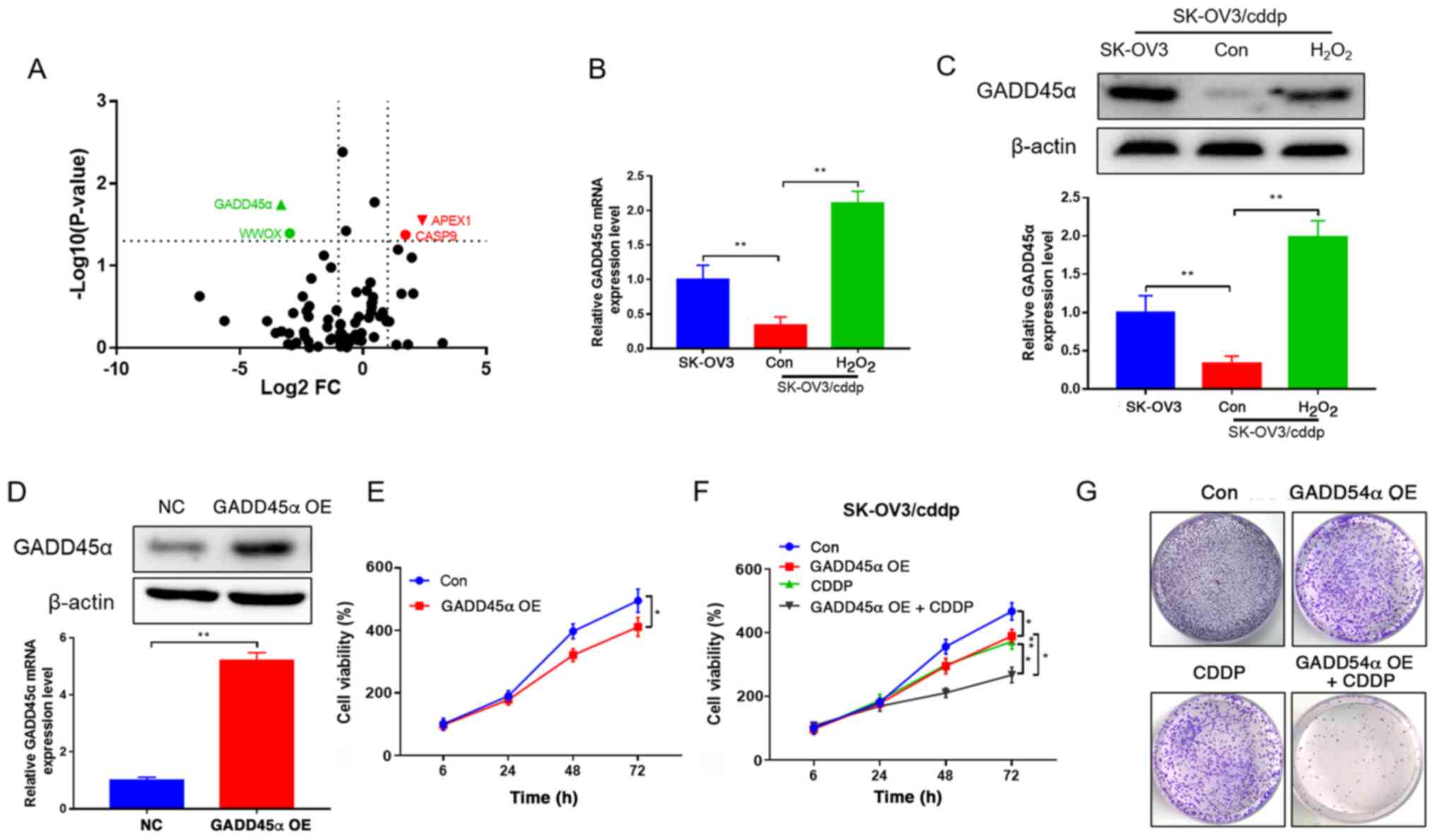
Differentially expressed genes between the SK-OV3
and SK-OV3/cddp cells were detected using RNA sequencing. The two
genes presented in red were upregulated 2-fold, and those in green
were downregulated 2-fold (P<0.05), and GADD45α exhibited the
most notable reduction, as it had the smallest P-value and lowest
negative FC (Fig. 2A). To
investigate the mechanism by which redox status regulates drug
resistance in ovarian cancer cells, the expression of GADD45α,
which is involved in the redox process, was detected (29). GADD45α mRNA and protein expression
levels were significantly lower in SK-OV3/cddp cells compared with
SK-OV3 cells. However, H2O2 significantly
increased the expression of GADD45α in the SK-OV3/cddp cells
(Fig. 2B and C). In order to explore
the role of GADD45 in drug resistance, GADD45α was overexpressed in
SK-OV3 cells (Fig. 2D), which led to
a reduction in the viability of the cells (Fig. 2E). Cell viability and colony
formation were also detected in GADD45α-overexpressing SK-OV3/cddp
cells following CDDP treatment. Treatment with CDDP significantly
inhibited GADD45α viability and markedly reduced colony formation
in the GADD45α-overexpressing SK-OV3/cddp cells (Fig. 2F and G). The results indicated that
the overexpression of GADD45α reversed the CDDP resistance of
SK-OV3/cddp cells, which is associated with redox regulation.
GADD45α regulates DNA damage repair in
SK-OV3/cddp cells
GADD45α is involved in DNA damage repair (18), while γ-H2AX is a sensitive molecular
marker for monitoring DNA damage initiation (30). Whether GADD45α has a role in the
regulation of H2AX is unclear. To clarify this, the expression of
GADD45α and H2AX and the levels of γ-H2AX were detected in SK-OV3
and SK-OV3/cddp cells. Western blot and immunofluorescence assays
indicated that GADD45α expression and γ-H2AX levels in SK-OV3/cddp
cells were markedly lower compared with those in SK-OV3 cells
(Fig. 3A and B). Then,
GADD45α-overexpressing SK-OV3/cddp cells were treated with CDDP to
determine the role of GADD45α in DNA damage repair. Western
blotting results revealed that CDDP treatment significantly
increased the expression of GADD45α in the SK-OV3/cddp cells.
Moreover, the level of γ-H2AX in the SK-OV3/cddp cells was also
significantly increased after CDDP treatment, GADD45α
overexpression and CDDP combined with GADD45α overexpression. CDDP
and GADD45α cooperated in increasing the level of γ-H2AX (Fig. 3C and D). These results indicate that
GADD45α overexpression increased DNA damage in SK-OV3/cddp cells,
which may be involved in the alleviation of drug resistance.
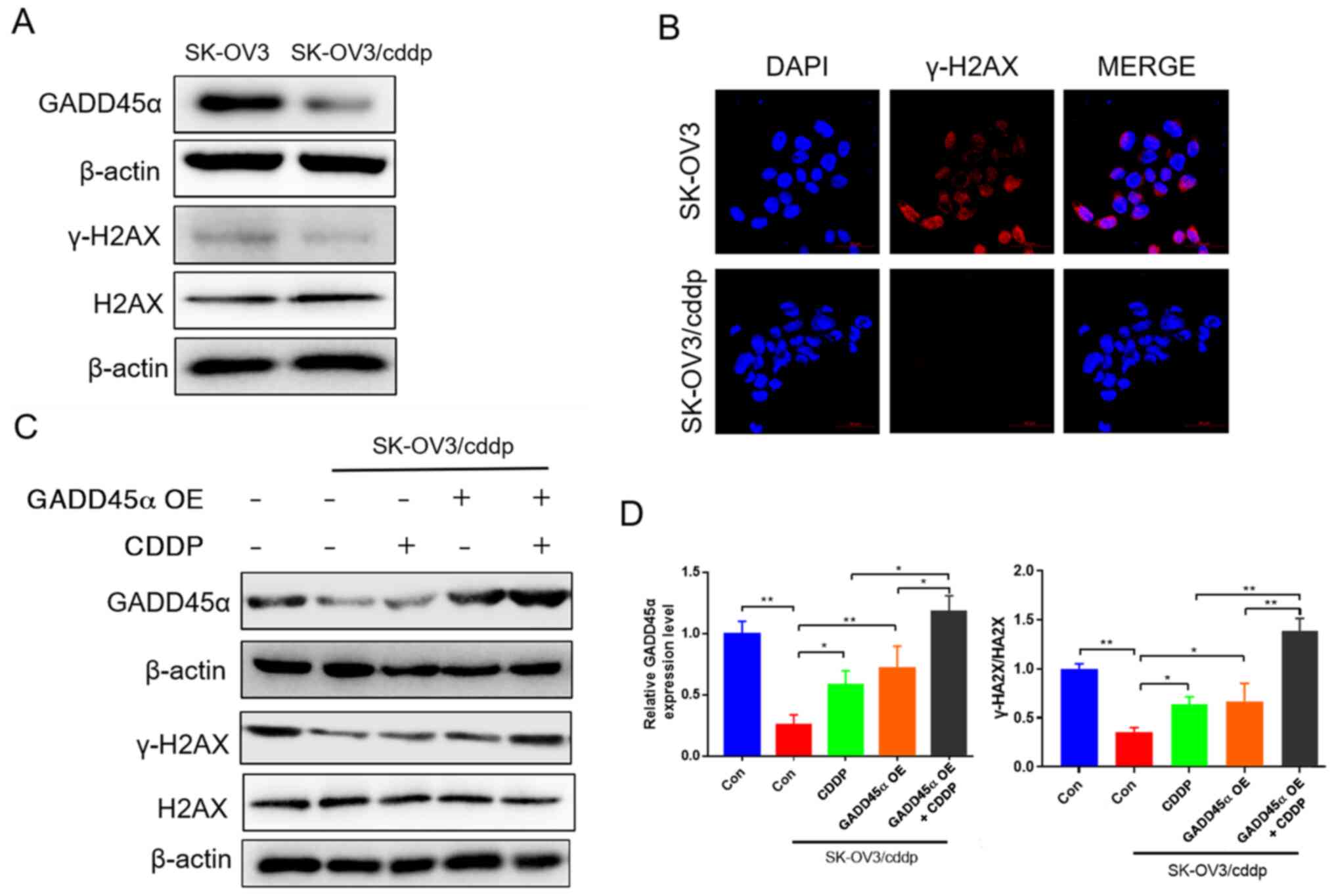 | Figure 3.GADD45α regulates DNA damage repair.
(A) Western blotting results showing the expression of GADD45α and
H2AX, and the phosphorylation of H2AX in SK-OV3 and SK-OV3/cddp
cells. (B) Immunofluorescence images of γ-H2AX (magnification,
×400) to assess cell damage. (C and D) Western blotting results
showing the expression of GADD45α, and H2AX, and phosphorylation of
H2AX in SK-OV3 and SK-OV3/cddp cells with CDDP treatment, GADD45α
overexpression or CDDP combined with GADD45α overexpression; (C)
representative blots and (D) quantified data. *P<0.05 and
**P<0.01. GADD45α, growth arrest and DNA damage 45; H2AX, H2A
histone family member X; γ-, Ser-139 phosphorylated; SK-OV3/cddp,
Sk-OV3 cells resistant to CDDP; CDDP, cisplatin; OE,
overexpression; Con, control. |
Regulation of CDDP resistance by
GADD45α is associated with redox status in vivo
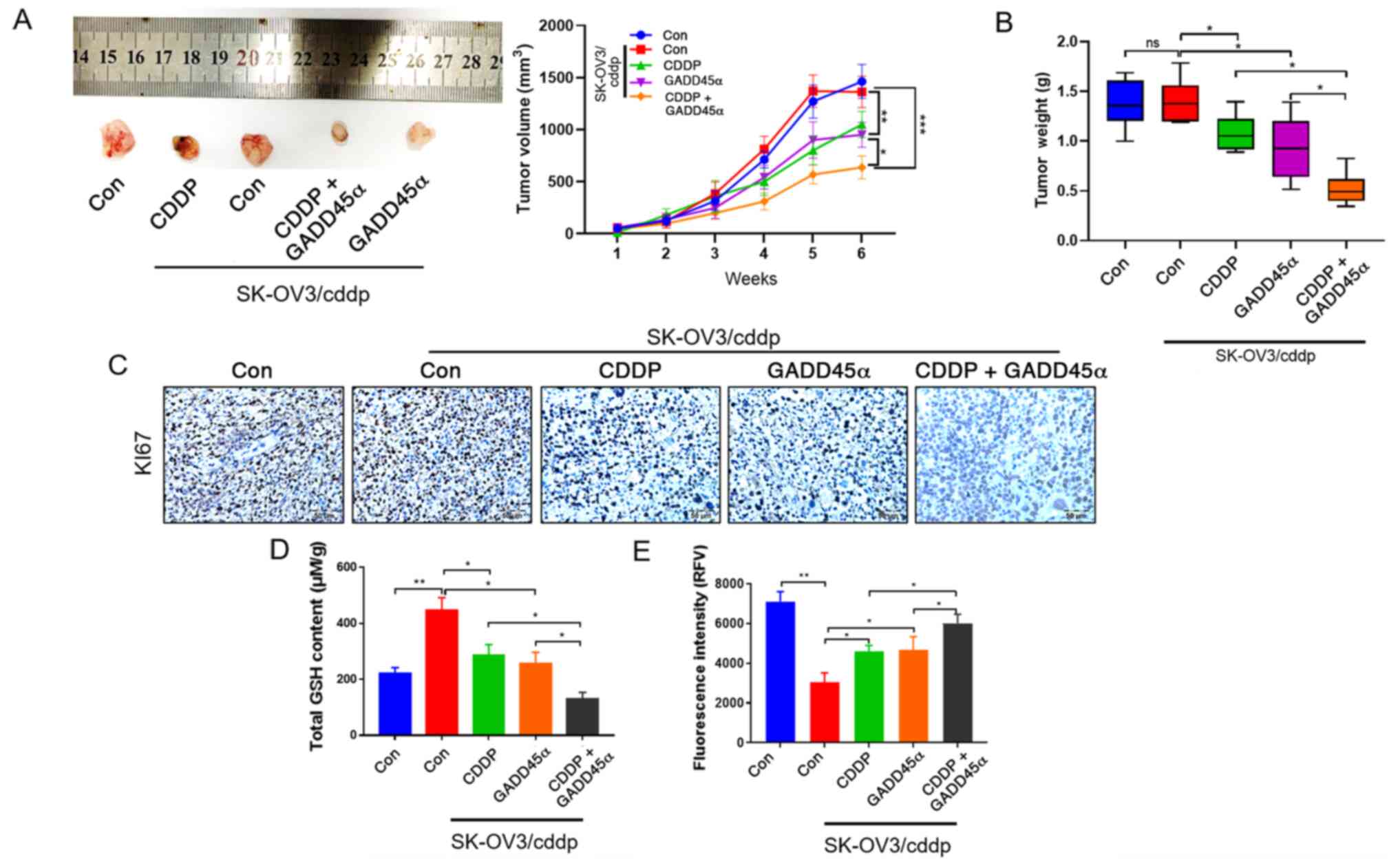
To investigate the role of GADD45α in CDDP
resistance in vivo, SK-OV3, SK-OV3/cddp and
GADD45α-overexpressing SK-OV3/cddp cells were implanted in the
axillary subcutaneous tissue of nude mice, and the tumor volume and
weight were subsequently evaluated. The results revealed that the
tumor volume (Fig. 4A) and weight
(Fig. 4B) for the SK-OV3/cddp
cell-based tumors were significantly decreased by GADD45α
overexpression, and further decreased by GADD45α overexpression
combined with CDDP. IHC analysis was performed to detect the
expression of the cell proliferation marker KI67 in the nude mouse
tumor tissue. KI67 expression in the SK-OV3/cddp-based tissue was
markedly decreased by CDDP treatment and further decreased by
GADD45α overexpression combined with CDDP treatment (Fig. 4C), indicating that GADD45α alleviated
the CDDP resistance of the SK-OV3/cddp cells in vivo. As the
reversal of CDDP resistance by GADD45α was demonstrated to be
associated with redox regulation (Fig.
2), the effect of redox on CDDP resistance in vivo was
verified by detecting the GSH and ROS levels in the SK-OV3/cddp
cell xenograft model. The GSH level was significantly decreased and
the ROS level was increased by CDDP treatment, and these changes
were significantly stronger when the CDDP treatment was
administered in combination with GADD45α overexpression CDDP
(Fig. 4D and E). The results
indicated that the regulation of CDDP resistance by GADD45α is
associated with the redox status in vivo.
Mechanism by which reduction-induced
CDDP resistance in ovarian cells is regulated by GADD45α
expression
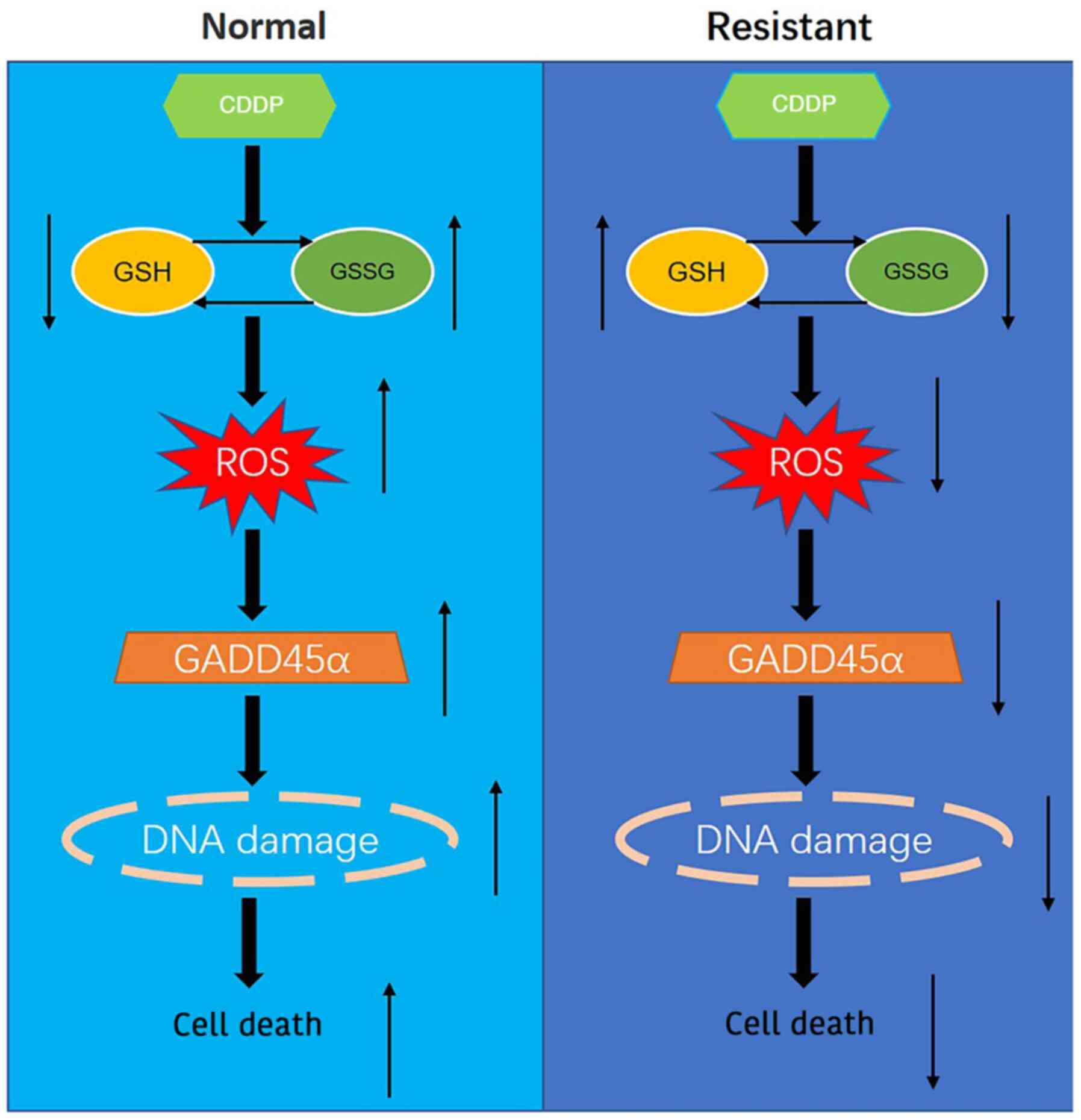
The present study explored the mechanism of redox
reactions in normal and CDDP-resistant ovarian cells by regulating
GADD45α expression, leading to the following hypothesis: In normal
ovarian cancer cells, CDDP promotes the oxidation of GSH to
glutathione disulfide (GSSG) which increases ROS levels, and
thereby upregulates the expression of GADD45α, induces DNA damage
and increases tumor cell death. However, in CDDP-resistant ovarian
cells, CDDP promotes the reduction of GSSG to GSH, which inhibits
the production of ROS, and thereby downregulates the expression of
GADD45α, decreases DNA damage and reduces tumor cell death
(Fig. 5).
Discussion
The present study demonstrated that the effects of
redox status on drug resistance are associated with GADD45α, which
regulates DNA damage repair in SK-OV3/cddp cells. The effect of
GADD45α on CDDP resistance was also verified in vivo. The
results indicated that the regulation of CDDP resistance by GADD45α
was associated with the redox status of the cells.
CDDP resistance is the primary cause of chemotherapy
failure in ovarian cancer. GSH is a major intracellular regulator
of redox conditions (31), which is
present at high levels in numerous CDDP-resistant cell lines. For
example, a recent study reported that elevated GSH levels are
associated with the CDDP resistance of non-small cell lung cancer
cell lines (32). High cellular GSH
levels have also been reported in CDDP-resistant head and neck
cancer cells (33). Furthermore,
intracellular GSH content has been demonstrated to be much higher
in CDDP-resistant human ovarian cancer cells than in CDDP-sensitive
cells (34). The present study
observed that lower ROS and higher GSH levels were present in
CDDP-resistant human ovarian cancer cells (SK-OV3/cddp) compared
with parental SK-OV3 cells. Lan et al (32) demonstrated that exogenous GSH
promotes the CDDP resistance of A549 lung cancer cells. In
addition, Cadoni et al (35)
revealed that CDDP is able to react with GSH, which results in
deactivation of the drug. In the present study, SK-OV3 and
SK-OV3/cddp cells were treated with H2O2,
which would be reduced by GSH, resulting in GSH depletion (36). The viability and colony formation of
SK-OV3/cddp cells were significantly inhibited by combined CDDP and
H2O2 treatment compared with CDDP treatment
alone. The results indicated that GSH levels serve an important
role in the drug resistance of SK-OV3/cddp cells, which is in
accordance with previous research.
Yang et al (37) reported that
H2O2 leads to the accumulation of ROS in
cells, which increases the expression of GADD45α. GADD45α is
involved in DNA repair, cell cycle arrest and apoptosis in response
to physiological or environmental stresses (38). Grossi et al (29) demonstrated that GADD45α serves an
important role in promoting the removal of ROS and sustaining redox
balance. There is evidence indicating that GADD45α is involved in
the resistance to anticancer drugs. For example, Liu et al
(38) revealed that the
downregulation of GADD45α increased the sensitivity of melanoma to
CDDP. However, Wang et al (39) demonstrated that overexpression
GADD45α attenuated the drug resistance of hepatocellular carcinoma
cells. The present study demonstrated that GADD45α mRNA and protein
expression levels were lower in CDDP-resistant SK-OV3 cells than in
the parental cell line. However, these levels were increased by
H2O2 treatment, and the overexpression of
GADD45α-enhanced the CDDP chemosensitivity of SK-OV3/cddp cells.
The dual role of GADD45α in drug resistance may be attributed to
differences in the drug resistance mechanism among different types
of tumors.
The γ-H2AX protein is involved in the regulation of
DNA damage repair (40). The
expression of H2AX is increased upon the initiation of DNA damage,
which serves as an indicator of DNA damage (41). It has been reported that H2AX serves
a role in the drug resistance of cancer cells. Wang et al
(42) reported that the
gemcitabine-induced drug resistance of pancreatic cancer cells was
associated with the inhibition of DNA repair protein γ-H2AX. The
present study demonstrated that the expression of GADD45α and
phosphorylation level of H2AX in SK-OV3/cddp cells was
significantly lower compared with that in SK-OV3 cells. As
mentioned above, GADD45α is also involved in DNA damage repair
(14). The current results are in
accordance with the viewpoint that increased DNA repair ability
contributes to chemotherapy resistance (43). The present study observed that CDDP
treatment significantly increased the expression of GADD45α and the
phosphorylation of H2AX. Moreover, the phosphorylation level of
H2AX was significantly increased following GADD45α overexpression
or treatment with CDDP combined with GADD45α overexpression in
SK-OV3/cddp cells. The results demonstrated that GADD45α increases
DNA damage and alleviates CDDP resistance in SK-OV3/cddp cells, and
these in vitro findings were verified in the in vivo
experiment.
In conclusion, the present study demonstrated that
GADD45α alleviated the CDDP resistance of SK-OV3/cddp cells, which
was associated with redox-mediated DNA damage. Therefore, GADD45α
may be a potential therapeutic target for CDDP-resistant ovarian
cancer treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Department of Guizhou Province [grant no. (2017)7196];
Guiyang Science and Technology Bureau [grant no. (2018)1-91]; Key
Talent Introduction Project of Education, Science, Culture and
Health of State Administration of Foreign Affairs (grant no.
20175200037); and the Science and Technology Fund Project of
Guizhou Health and Family Planning Commission (grant no.
H-2017-05).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZZ and FW performed experiments and the data
analysis. HLY, YYC, RGP, YMW, LN, YKQ, JJW and XZ contributed to
performing experiments and data analysis. DZ contributed to study
design. QZZ and DZ confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This research was approved by the Ethics Committee
of Guizhou Medical University (ethics approval no. 2000470).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lheureux S, Gourley C, Vergote I and Oza
AM: Epithelial ovarian cancer. Lancet. 393:1240–1253. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reid BM, Permuth JB and Sellers TA:
Epidemiology of ovarian cancer: A review. Cancer Biol Med. 14:9–32.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deng J, Wang L, Chen H, Hao J, Ni J, Chang
L, Duan W, Graham P and Li Y: Targeting epithelial-mesenchymal
transition and cancer stem cells for chemoresistant ovarian cancer.
Oncotarget. 7:55771–55788. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feng RM, Zong YN, Cao SM and Xu RH:
Current cancer situation in China: Good or bad news from the 2018
Global Cancer Statistics? Cancer Commun (Lond). 39:222019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chu YH, Sibrian-Vazquez M, Escobedo JO,
Phillips AR, Dickey DT, Wang Q, Ralle M, Steyger PS and Strongin
RM: Systemic Delivery and Biodistribution of Cisplatin in Vivo. Mol
Pharm. 13:2677–2682. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raudenska M, Balvan J, Fojtu M, Gumulec J
and Masarik M: Unexpected therapeutic effects of cisplatin.
Metallomics. 11:1182–1199. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trimmer EE and Essigmann JM: Cisplatin.
Essays Biochem. 34:191–211. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qi R, Xiao H, Wu S, Li Y, Zhang Y and Jing
X: Design and delivery of camplatin to overcome cisplatin drug
resistance. J Mater Chem B Mater Biol Med. 3:176–179. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Telli ML, Stover DG, Loi S, Aparicio S,
Carey LA, Domchek SM, Newman L, Sledge GW and Winer EP: Homologous
recombination deficiency and host anti-tumor immunity in
triple-negative breast cancer. Breast Cancer Res Treat. 171:21–31.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kurz T, Leake A, von Zglinicki T and Brunk
UT: Lysosomal redox-active iron is important for oxidative
stress-induced DNA damage. Ann N Y Acad Sci. 1019:285–288. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schieber M and Chandel NS: ROS function in
redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang P, Du W and Wu M: Regulation of the
pentose phosphate pathway in cancer. Protein Cell. 5:592–602. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kwon DH, Lee H, Park C, Hong SH, Hong SH,
Kim GY, Cha HJ, Kim S, Kim HS, Hwang HJ, et al: Glutathione Induced
Immune-Stimulatory Activity by Promoting M1-Like Macrophages
Polarization via Potential ROS Scavenging Capacity. Antioxidants.
8:E4132019. View Article : Google Scholar
|
|
14
|
Li J, Dong J, Li S, Xia W, Su X, Qin X,
Chen Y, Ding H, Li H, Huang A, et al: An alternative
microRNA-mediated post-transcriptional regulation of GADD45A by p53
in human non-small-cell lung cancer cells. Sci Rep. 7:71532017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu LQ, Tian FJ, Xiong Y, Zhao Y and Song
JB: Gadd45a gene silencing by RNAi promotes cell proliferation and
inhibits apoptosis and senescence in skin squamous cell carcinoma
through the p53 signaling pathway. J Cell Physiol. 233:7424–7434.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhan Q, Chen IT, Antinore MJ and Fornace
AJ Jr: Tumor suppressor p53 can participate in transcriptional
induction of the GADD45 promoter in the absence of direct DNA
binding. Mol Cell Biol. 18:2768–2778. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wingert S, Thalheimer FB, Haetscher N,
Rehage M, Schroeder T and Rieger MA: DNA-damage response gene
GADD45A induces differentiation in hematopoietic stem cells without
inhibiting cell cycle or survival. Stem Cells. 34:699–710. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hollander MC, Kovalsky O, Salvador JM, Kim
KE, Patterson AD, Haines DC and Fornace AJ Jr:
Dimethylbenzanthracene carcinogenesis in Gadd45a-null mice is
associated with decreased DNA repair and increased mutation
frequency. Cancer Res. 61:2487–2491. 2001.PubMed/NCBI
|
|
19
|
Chen H, Shan J, Chen D, Wang R, Qi W, Wang
H, Ke Y, Liu W and Zeng X: CtIP promotes G2/M arrest in
etoposide-treated HCT116 cells in a p53-independent manner. J Cell
Physiol. 234:11871–11881. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Yuan Y, Liang P, Zhang Z, Guo X,
Xia L, Zhao Y, Shu XS, Sun S, Ying Y, et al: Overexpression of a
novel candidate oncogene KIF14 correlates with tumor progression
and poor prognosis in prostate cancer. Oncotarget. 8:45459–45469.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krushkal J, Zhao Y, Hose C, Monks A,
Doroshow JH and Simon R: Concerted changes in transcriptional
regulation of genes involved in DNA methylation, demethylation, and
folate-mediated one-carbon metabolism pathways in the NCI-60 cancer
cell line panel in response to cancer drug treatment. Clin
Epigenetics. 8:732016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
De Feudis P, Debernardis D, Beccaglia P,
Valenti M, Graniela Siré E, Arzani D, Stanzione S, Parodi S,
D'Incalci M, Russo P, et al: DDP-induced cytotoxicity is not
influenced by p53 in nine human ovarian cancer cell lines with
different p53 status. Br J Cancer. 76:474–479. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Delmastro DA, Li J, Vaisman A, Solle M and
Chaney SG: DNA damage inducible-gene expression following platinum
treatment in human ovarian carcinoma cell lines. Cancer Chemother
Pharmacol. 39:245–253. 1997.PubMed/NCBI
|
|
24
|
Tarrade S, Bhardwaj T, Flegal M, Bertrand
L, Velegzhaninov I, Moskalev A and Klokov D: Histone H2AX Is
Involved in FoxO3a-Mediated Transcriptional Responses to Ionizing
Radiation to Maintain Genome Stability. Int J Mol Sci.
16:29996–30014. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Celeste A, Difilippantonio S,
Difilippantonio MJ, Fernandez-Capetillo O, Pilch DR, Sedelnikova
OA, Eckhaus M, Ried T, Bonner WM and Nussenzweig A: H2AX
haploinsufficiency modifies genomic stability and tumor
susceptibility. Cell. 114:371–383. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bassing CH, Suh H, Ferguson DO, Chua KF,
Manis J, Eckersdorff M, Gleason M, Bronson R, Lee C and Alt FW:
Histone H2AX: A dosage-dependent suppressor of oncogenic
translocations and tumors. Cell. 114:359–370. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Banerjee K, Ganguly A, Chakraborty P,
Sarkar A, Singh S, Chatterjee M, Bhattacharya S and Choudhuri SK:
ROS and RNS induced apoptosis through p53 and iNOS mediated pathway
by a dibasic hydroxamic acid molecule in leukemia cells. Eur J
Pharm Sci. 52:146–164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen H, Liao K, Cui-Zhao L, Qiang-Wen F,
Feng-Zeng X, Ping-Wu F, Liang-Guo S and Juan-Chen Y: Cigarette
smoke extract induces apoptosis of rat alveolar Type II cells via
the PLTP/TGF-β1/Smad2 pathway. Int Immunopharmacol. 28:707–714.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grossi V, Forte G, Sanese P, Peserico A,
Tezil T, Lepore Signorile M, Fasano C, Lovaglio R, Bagnulo R,
Loconte DC, et al: The longevity SNP rs2802292 uncovered: HSF1
activates stress-dependent expression of FOXO3 through an intronic
enhancer. Nucleic Acids Res. 46:5587–5600. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mah LJ, El-Osta A and Karagiannis TC:
gammaH2AX: A sensitive molecular marker of DNA damage and repair.
Leukemia. 24:679–686. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen HHW, Song IS, Hossain A, Choi MK,
Yamane Y, Liang ZD, Lu J, Wu LY, Siddik ZH, Klomp LW, et al:
Elevated glutathione levels confer cellular sensitization to
cisplatin toxicity by up-regulation of copper transporter hCtr1.
Mol Pharmacol. 74:697–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lan D, Wang L, He R, Ma J, Bin Y, Chi X,
Chen G and Cai Z: Exogenous glutathione contributes to cisplatin
resistance in lung cancer A549 cells. Am J Transl Res.
10:1295–1309. 2018.PubMed/NCBI
|
|
33
|
Tonigold M, Rossmann A, Meinold M, Bette
M, Märken M, Henkenius K, Bretz AC, Giel G, Cai C, Rodepeter FR, et
al: A cisplatin-resistant head and neck cancer cell line with
cytoplasmic p53(mut) exhibits ATP-binding cassette transporter
upregulation and high glutathione levels. J Cancer Res Clin Oncol.
140:1689–1704. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Okuno S, Sato H, Kuriyama-Matsumura K,
Tamba M, Wang H, Sohda S, Hamada H, Yoshikawa H, Kondo T and Bannai
S: Role of cystine transport in intracellular glutathione level and
cisplatin resistance in human ovarian cancer cell lines. Br J
Cancer. 88:951–956. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cadoni E, Valletta E, Caddeo G, Isaia F,
Cabiddu MG, Vascellari S and Pivetta T: Competitive reactions among
glutathione, cisplatin and copper-phenanthroline complexes. J Inorg
Biochem. 173:126–133. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mailloux RJ, Craig Ayre D and Christian
SL: Induction of mitochondrial reactive oxygen species production
by GSH mediated S-glutathionylation of 2-oxoglutarate
dehydrogenase. Redox Biol. 8:285–297. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang Y, Su Y, Wang D, Chen Y, Wu T, Li G,
Sun X and Cui L: Tanshinol Attenuates the Deleterious Effects of
Oxidative Stress on Osteoblastic Differentiation via Wnt/FoxO3a
Signaling. Oxid Med Cell Longev. 2013:3518952013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu J, Jiang G, Mao P, Zhang J, Zhang L,
Liu L, Wang J, Owusu L, Ren B, Tang Y, et al: Down-regulation of
GADD45A enhances chemosensitivity in melanoma. Sci Rep. 8:41112018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang X, Zou F, Zhong J, Yue L, Wang F, Wei
H, Yang G, Jin T, Dong X, Li J, et al: Secretory Clusterin Mediates
Oxaliplatin Resistance via the Gadd45a/PI3K/Akt Signaling Pathway
in Hepatocellular Carcinoma. J Cancer. 9:1403–1413. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sekhar SC, Venkatesh J, Cheriyan VT, Muthu
M, Levi E, Assad H, Meister P, Undyala VV, Gauld JW and Rishi AK: A
H2AX-CARP-1 Interaction Regulates Apoptosis Signaling Following DNA
Damage. Cancers (Basel). 11:E2212019. View Article : Google Scholar
|
|
41
|
Salzano M, Sanz-García M, Monsalve DM,
Moura DS and Lazo PA: VRK1 chromatin kinase phosphorylates H2AX and
is required for foci formation induced by DNA damage. Epigenetics.
10:373–383. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang Y, Kuramitsu Y, Kitagawa T, Tokuda K,
Baron B, Akada J and Nakamura K: The Histone Deacetylase Inhibitor
Valproic Acid Sensitizes Gemcitabine-Induced Cytotoxicity in
Gemcitabine-Resistant Pancreatic Cancer Cells Possibly Through
Inhibition of the DNA Repair Protein Gamma-H2AX. Target Oncol.
10:575–581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tang XH, Li H, Zheng XS, Lu MS, An Y and
Zhang XL: CRM197 reverses paclitaxel resistance by inhibiting the
NAC-1/Gadd45 pathway in paclitaxel-resistant ovarian cancer cells.
Cancer Med. 8:6426–6436. 2019. View Article : Google Scholar : PubMed/NCBI
|