Introduction
Cutaneous squamous cell carcinoma (CSCC) is a
malignant tumor originating from the epidermis or keratinocytes of
skin appendages (1). CSCC ranks
second to basal cell cancer as the most common type of skin cancer
in the USA (2). This global
incidence continues to increase annually with an estimated 50–200%
increase in the last three decades (3), CSCC severely endangers human health and
lives (4). Surgery is the preferred
treatment option for patients with CSCC (5). Although surgical resection can
eliminate small superficial tumor lesions, lymphadenectomy of the
removal of the regional draining lymph node is necessary for cancer
cases with high risks of lymph node invasion (6,7). The
risk of nodal metastasis (NM) in cohort and tumor registry studies
has ranged from 2.0 to 5.8%. A single cohort study reported a risk
of disease-specific death (DSD) of 1.5% (8), which is mainly attributed to cancer
cell infiltration and metastasis (9).
MicroRNA (miRNA/miR) is a type of endogenous small
non-coding RNA (10). miRNA
molecules can induce mRNA degradation, inhibit gene transcription
or lead to mRNA deadenylation by binding the 3′-untranslated region
(UTR) of target mRNA transcripts (11). A previous study have revealed the
crucial functions of miRNA in cancer development (12). Miao et al (13) demonstrated that miR-27b-3p played an
important role in the development of glioma and that Yes-associated
protein 1 (YAP1) was the downstream target of this miRNA. Moreover,
they also suggested that miR-27b-3p controlled the proliferation,
migration and apoptosis of glioma cells by regulating YAP1
(13). Yang et al (14) also reported overexpression of
miR-27b-3p in colorectal cancer and suggested that miR-27b-3p could
significantly promote the migration and invasion of colorectal
cancer by targeting homeobox A10.
EGFR is a transmembrane receptor tyrosine kinase
that initiates multiple intracellular signaling pathways (15). EGFR regulates the proliferation,
invasion, metastasis and apoptosis of cancer cells (15). MMP-13 is a matrix metalloproteinase
that participates in the degradation of the extracellular matrix
and is involved in tumor cell metastasis (16,17). Our
previous studies found that EGFR and MMP-13 are highly expressed in
CSCC (18,19). However, the potential functions of
EGFR and MMP-13 in the proliferation and metastasis of CSCC remain
largely unclear. The aim of the present study was to examine the
biological function of miR-27b-3p in CSCC and its underlying
mechanism to find potential molecular markers and therapeutic
targets for clinical treatment of CSCC.
Materials and methods
Cell culture
The human CSCC cell lines A-431 (cat. no. MZ-0014;
Ningbo Mingzhou Biotechnology Co., Ltd.), Colo-16 (cat. no.
MZ-1591; Ningbo Mingzhou Biotechnology Co., Ltd.), HSC-1 (cat. no.
MZ-1501; Ningbo Mingzhou Biotechnology Co., Ltd.) and SCL-1 (cat.
no. MZ-1504; Ningbo Mingzhou Biotechnology Co., Ltd.), human skin
keratinocyte cell line NHEK/SVTERT3-5 (cat. no. CE22072; Beijing
Crespo Biotechnology, Co., Ltd.) and the 293T cell line (cat. no.
MZ-0005; Ningbo Mingzhou Biotechnology Co., Ltd.) were provided by
Ningbo Mingzhou Biotechnology Co., Ltd. and Beijing Crespo
Biotechnology, Co., Ltd. NHEK/SVTERT3-5 cells were used as a
control.
The cells were cultured in Dulbecco's modified
Eagle's medium (DMEM) (Gibco; Thermo Fisher Scientific Inc.)
containing 10% fetal bovine serum (FBS) (Gibco; Thermo Fisher
Scientific Inc.) and 1% penicillin-streptomycin in a humidified
atmosphere with 5% CO2 at 37°C. When the cells reached
80% confluence, cell passage was performed using trypsin. The cells
were cryopreserved in 20% DMSO and at −80°C.
Cell transfection
The miR-27b-3p mimic (5′-CGUCUUGAAUCGGUGACACUU-3′)
and mimic negative control (NC) (5′-AAGUGUCACCGAUUCAAGACG-3′) were
synthesized by Guangzhou RiboBio Co., Ltd.. Cells were cultured in
serum-free Opti-DMEM™ (Gibco; Thermo Fisher Scientific) for 4-h
starvation. A mixture containing mimics or mimic-NC and
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was incubated for 20 min at room temperature and
added into each well at a final concentration of 100 nM in 5%
CO2 for 40 min at 37°C, and then, the Opti-MEM was then
replaced with DMEM containing 10% FBS and 1%
penicillin-streptomycin. After 24-h cell culture, reverse
transcription-quantitative PCR (RT-qPCR) was used to evaluate the
transfection efficacy.
RT-qPCR
Total RNA was extracted from cell lines using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.).
Using the Takara Primescript™ RT Reagent Kit (Takara Bio, Inc.), 1
µg/µl of the RNA sample was purified with a gDNA eraser and reverse
transcribed to cDNA. The RT steps were as follows: 25°C for 5 min,
42°C for 15 min and 85°Cfor 5 sec. qPCR was carried out using SYBR
Premix Ex Taq™ II with Tli RNaseH (Takara Bio, Inc.). The
thermocycling conditions consisted of an initial denaturation at
95°C for 15 min, followed by 40 cycles at 95°C for 5 sec, 60°C for
30 sec and 72°C for 40 sec, then a final extension at 72°C for 10
min. The results was analyzed following the 2−ΔΔCq
method (20). GAPDH and U6 served as
the internal references. Primer sequences are listed in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Primer name | Primer sequence,
5′-3′ |
|---|
| miR-27b-3p-F |
AGTGGCTAAGTTCTGCGTCG |
| miR-27b-3p-R |
GTATCCAGTGCGTGTCGTGG |
| Akt-F |
TCTATGGCGCTGAGATTGTG |
| Akt-R |
CTTAATGTGCCCGTCCTTGT |
| Cyclin D1-F |
CGATGCCAACCTCCTCAACGA |
| Cyclin D1-R |
TCGCAGACCTCCAGCATCCA |
| N-CAD-F |
CCACAGACATGGAAGGCAATCC |
| N-CAD-R |
CACTGATTCTGTATGCCGCATTC |
| E-CAD-F |
GTACTTGTAATGACACATCTC |
| E-CAD-R |
TGCCAGTTTCTGCATCTTGC |
| GAPDH-F |
GAAGGTGAAGGTCGGAGT |
| GAPDH-R |
GAAGATGGTGATGGGATTTC |
| U6-F |
CTCGCTTCGGCAGCACA |
| U6-R |
AACGCTTCACGAATTTGCGT |
Western blot analysis
Total protein from CSCC cells was collected, and
first isolated by RIPA lysis buffer (Beyotime Institute of
Biotechnology), and protein samples was determined by BCA method at
1 µg/µl. The protein samples were then subjected to 5% (for EGFR,
N-CAD and E-CAD) or 10% (for MMP-13, AKT, p-AKT, cyclin D1 and
GAPDH) gel electrophoresis (50 µg/lane), then transferred to PVDF
membranes. Non-specific antigen binding was blocked using 5%
skimmed milk in room temperature for 1 h. Membranes were incubated
with primary antibodies (1:1,000) at 4°C overnight and then
incubated with secondary antibodies (1:1,000) at room temperature
for 1 h. Finally, the membrane was treated with chemiluminescent
horse radish peroxidase (HRP) Substrate (cat. no. WBKLS0500;
MilliporeSigma) to visualize the protein. Antibodies used in
western blot assay were purchased from ABclonal Biotech Co., Ltd.,
and the catalog numbers were as follows: EGFR (cat. no. A4929);
MMP-13 (cat. no. A11148); AKT (cat. no. A17909); p-AKT (cat. no.
AP0637); cyclin D1 (cat. no. A11022); N-CAD (cat. no. A19083);
E-CAD (A3044); GAPDH (cat. no. AC001) and the secondary antibody
HRP goat anti-rabbit (cat. no. AS014). Band exposure was achieved
using the enhanced chemiluminescence method and visualized by
Quantity One software v.4.6.9 (Bio-Rad Laboratories, Inc.).
Colony formation assay
CSCC cells were seeded in a 6-well plate at a
density of 2×102 cells/well, then cultured in DMEM
containing 10% FBS for 14 days until the formation of visible
colonies. The colonies were washed with PBS, fixed in 4%
paraformaldehyde for 30 min at room temperature and stained in 1%
crystal violet for 15 min at room temperature. Visible colonies
were captured under an inverted microscope and the number of
colonies were counted that had >50 cells.
MTT assay
MTT assay was conducted according to the
manufacturer's instructions of MTT Assay kit (ABclonal Biotech Co.,
Ltd.; cat. no. ab211091). CSCC cells were seeded in a 96-well plate
at a density of 6×103 cells/well. At the 12, 24, 36, 48
and 60-h time points, 20 µl MTT (5 mg/l) was added to each well at
37°C for 4 h. The supernatant was then discarded, and 150 µl DMSO
was added to each well. After gentle shaking for 10 min, the
optical density at 570 nm was measured using an ultraviolet
spectrophotometer.
Wound healing assay
At the bottom of a 24-well plate, an auxiliary line
(perpendicular to the cell scratch) was drawn every 0.5 cm to
ensure the consistency of each observation site. Cells were seeded
at 5×105 cells/well and cultured into a monolayer. An
artificial wound was created using a pipette tip, and the scratched
cells were washed in serum-free medium (21). After 24 h culture, cell migration was
observed using an inverted microscope. The scratch area was
calculated by ImageJ v.1.8.0 software (National Institutes of
Health).
Transwell assay
Matrigel diluted in DMEM at a 1:5 ratio (100 µl) was
used to coat in a Transwell chamber, then dried in cell incubator
at 37°C in 5% CO2; 24-well plates were used for cell
culture. On the following day, 200 µl cell suspension
(2.5×104 cells/ml) and 500 µl DMEM containing 10% FBS
were added to the top and bottom chambers, respectively. After 24-h
culture, the inner side cells that did not pass through the
membrane were discharged and stained with crystal violet for 5 min.
Photomicrographs were captured with an inverted fluorescence
microscope (magnification, ×100).
Dual-luciferase reporter assay
Target prediction for miR-27b-3p was carried out
using TargetScan v.7.2 (http://www.TargetScan.org/vert_72/). The 3′-UTRs of
EGFR and MMP-13 were cloned into pmir-GLO vectors (Promega
Corporation) to generate EGFR wild-type (wt) and MMP-13 wt vectors.
The EGFR mutant (mut) and MMP-13 mut vectors were generated using
the GeneTailer site-directed mutagenesis kit (Invitrogen; Thermo
Fisher Scientific Inc.). The aforementioned vectors were mixed with
the miR-27b-3p mimic or mimic-NC and Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) for 20 min at room
temperature. The mRNA/plasmid/Lipofectamine 2000 mixture was then
added to the cells for 40 min at 37°C in a humidified atmosphere
containing 5% CO2, the plasmids was transfected at 500
ng per well and the final concentration of mimic or mimic-NC was 20
nM. Cells were then cultured at 37°C and 5% CO2 for 48
h. Luciferase activity was then determined using a dual-luciferase
reporter assay system (Promega Corporation). Relative luciferase
activity was expressed as the ratio of firefly luciferase activity
to Renilla luciferase activity.
Lentivirus transduction
A 2nd generation system was used to the package of
lentivirus. EGFR and MMP-13 overexpression plasmids were generated
by cloning the sequences of these two genes into the GV287 plasmid
(Shanghai GeneChem Co., Ltd.), the lentiviral plasmid was mixed
with packaging vector (Shanghai GeneChem Co., Ltd.) and envelope
vector (Shanghai GeneChem Co., Ltd.) at a 4:3:2 ratio for a total
DNA mass of 20 µg, the mixture was then incubated with Lenti-Easy
Packaging Mix (cat. no. LPK001; Shanghai GeneChem Co., Ltd.) at
37°C for 15 min and then incubated incubation with
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific Inc.) for another 20 min. The mixture were added into
293T cells (cat. no. MZ-0005; Ningbo Mingzhou Biotechnology Co.,
Ltd.) for 6 h at 37°C. The transfection medium was replaced with
fresh DMEM medium, 293T cells were cultured overnight to 80%
confluence. Opti-MEM was replaced for another 4 h culture. The
lentiviral plasmid, packaging vector and envelope vector were mixed
at a 4:3:2 ratio for a total DNA mass of 20 µg and incubated with 1
ml Lenti-Easy Packaging Mix for 15 min. The mixture was then
incubated with Lipofectamine® 2000(Invitogen; Thermo
Fisher Scientific Inc.) for another 20 min. Finally, the mixture
was incubated with 293T cells for 6 h at 37°C (2.5×105
cells/plate in a 10-cm plate), which were previously incubated in
Opti-MEM for 4 h. Then, the medium was then replaced with DMEM
containing 10% FBS and 1% penicillin-streptomycin on the following
day. At the 72-h time point, the supernatant of the transfected
293T cells was collected and centrifuged via 4.5 µm filter, then
concentrated by ultracentrifugation at 70,000 × g at 4°C for 2 h.
The suspension was filtered to determine the viral titers. The
A-431 and Colo-16 cells were cultured at 80% confluence, then
infected with lentivirus at a multiplicity of infection of 5 and
with polybrene (Sigma-Aldrich; Merck KGaA) for 24 h. Fresh culture
medium was then used to replace the old medium. After 3 days, the
fluorescence intensity was subsequently observed to screen stable
cell lines and the transfection efficiency was determined by
RT-qPCR.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 8 (GraphPad Software, Inc.). Data were normally distributed
and are presented as the mean ± SD. Comparisons between multiple
groups were analyzed using one-way ANOVA followed by Tukey's post
hoc test or Bonferroni correction. P<0.05 was considered to
indicate a statistically significant difference. All experiments
were performed in triplicate and repeated three times.
Results
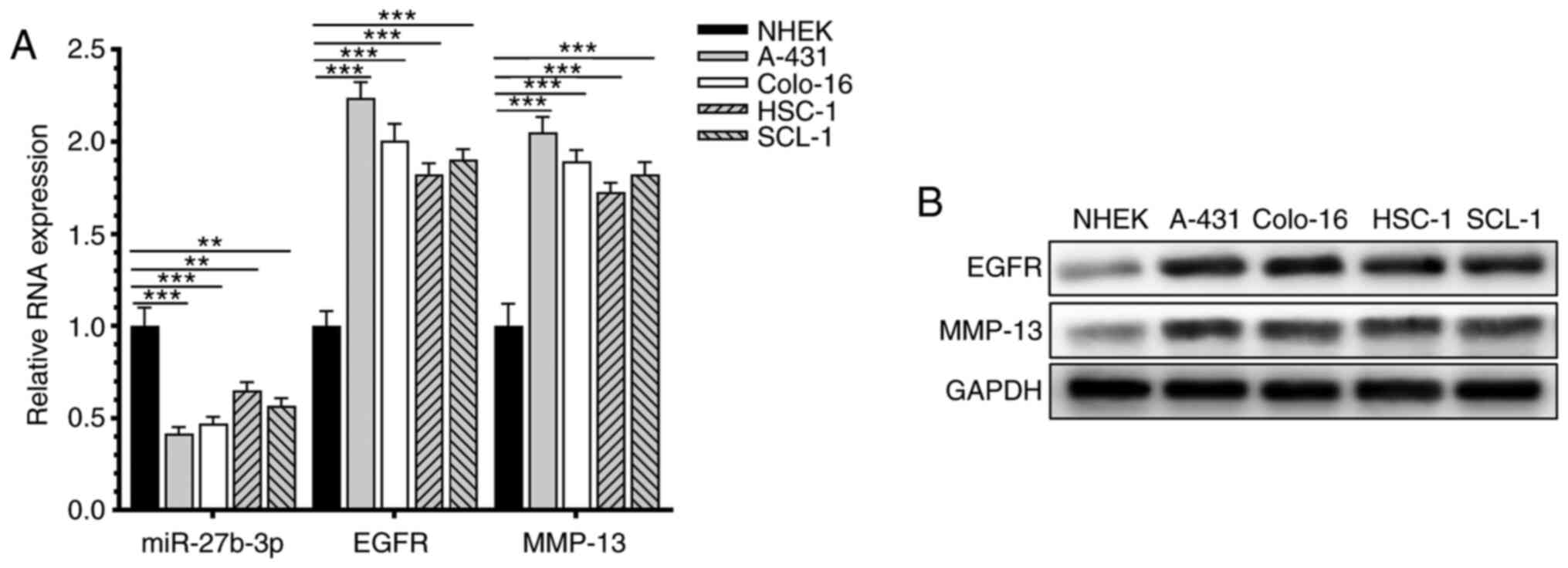
miR-27b-3p is downregulated in CSCC
cells
The relative expression levels of miR-27b-3p, EGFR
and MMP-13 in human CSCC cell lines and human normal skin
fibroblasts were detected (Fig. 1).
miR-27b-3p was downregulated in CSCC cell lines compared with the
normal skin keratinocyte cell line NHEK/SVTERT3-5.
miR-27b-3p inhibits the proliferation,
migration and invasion of CSCC cells
Compared with untransfected cells and cells
transfected with mimic-NC, transfection of A-431 and Colo-16 cells
with miR-27b-3p mimic upregulated the expression of miR-27b-3p, but
downregulated the mRNA expression levels of EGFR and MMP-13
(Fig. 2A). The results of colony
formation and MTT assays indicated that overexpression of
miR-27b-3p significantly inhibited the proliferative ability of the
CSCC cells (Fig. 2B and C).
Moreover, wound healing and Transwell assays demonstrated that
miR-27b-3p could attenuate the migratory and invasive abilities of
CSCC cells (Fig. 2D and E). The
protein expression levels of p-Akt/total AKT, cyclin D1 and N-CAD
were downregulated in CSCC cells overexpressing miR-27b-3p, whereas
E-CAD was upregulated (Fig. 2F).
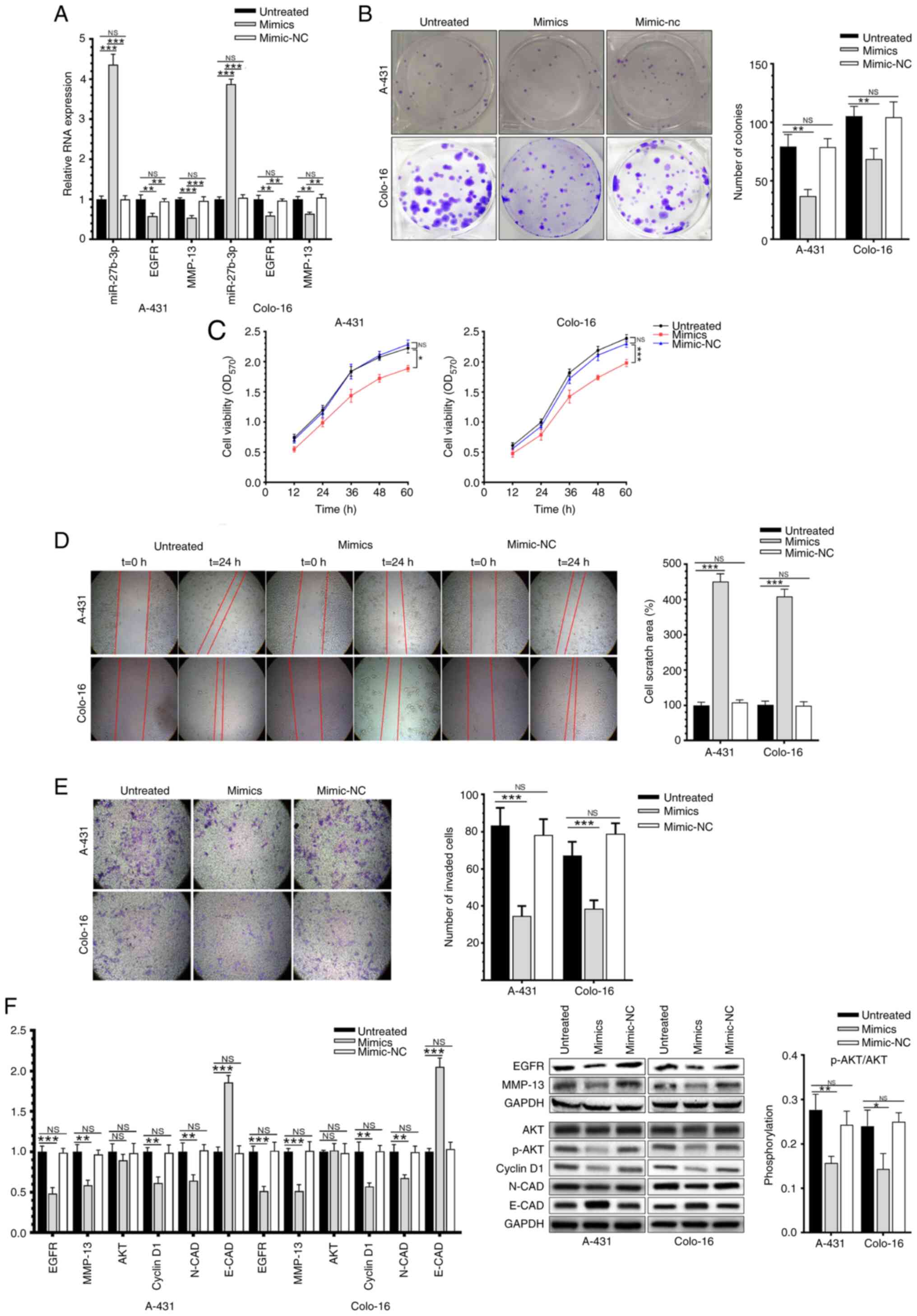 | Figure 2.miR-27b-3p inhibits the
proliferation, migration and invasion of CSCC cells. (A) miR-27b-3p
mimic transfection regulates the mRNA expression levels EGFR and
MMP-13 level in CSCC cells. (B) Colony formation, (C) cell
viability, (D) migration and (E) invasion of CSCC cells following
transfection with the miR-27b-3p mimic (magnification, ×100). (F)
mRNA and protein expression levels of key molecules associated with
proliferation and invasion in CSCC cells following transfection.
Data were presented as the mean ± SD, and performed in triplicate.
*P<0.05, **P<0.01 and ***P<0.001. CSCC, cutaneous squamous
cell carcinoma; miR, microRNA; NS, not significant; NC, negative
control; OD, optical density; CAD, cadherin. |
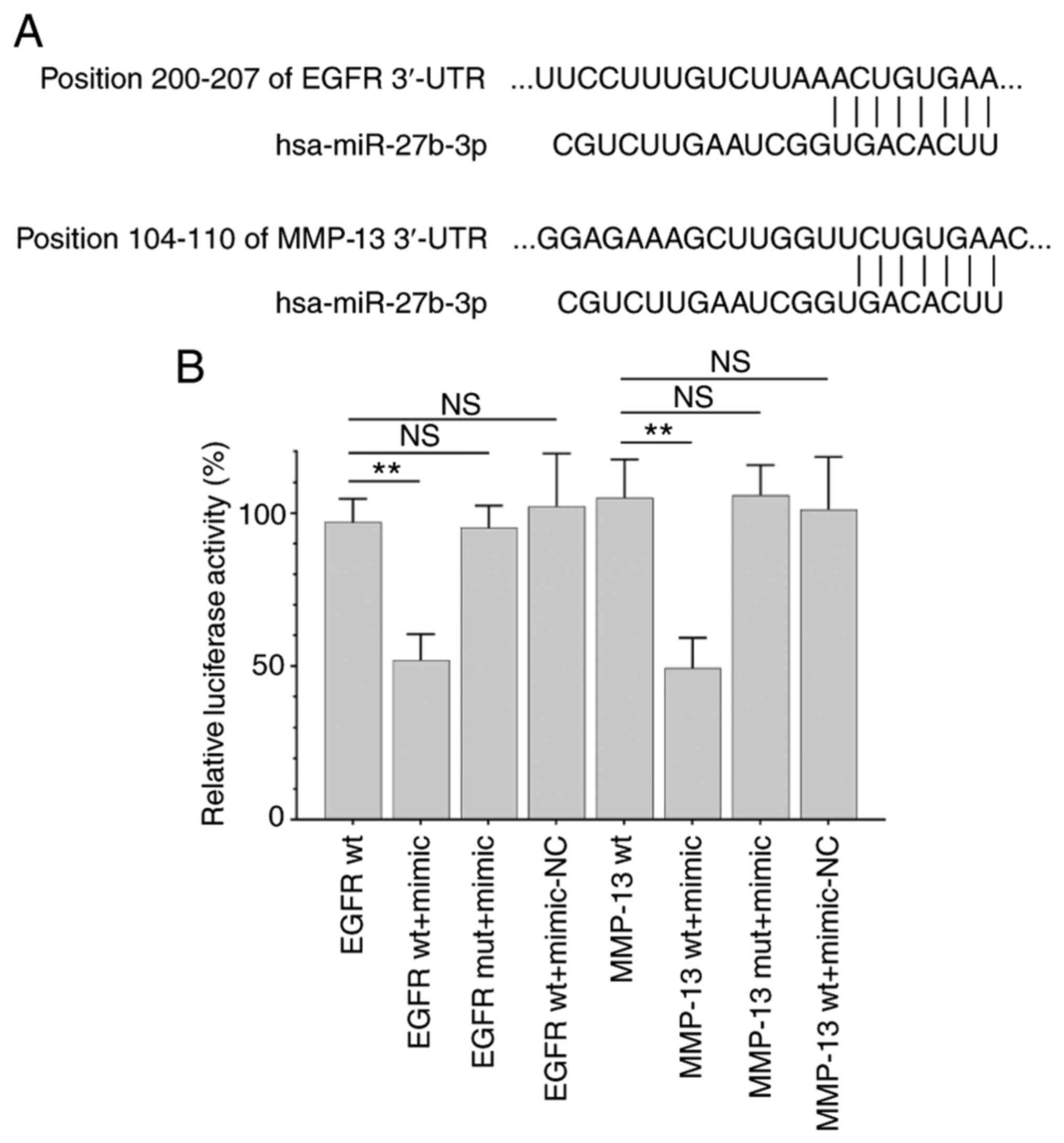
miR-27b-3p binds to the 3′-UTRs of
EGFR and MMP-13
Target prediction with TargetScan indicated that
miR-27b-3p contained sequences that could interact with the 3′-UTRs
of EGFR and MMP-13 (Fig. 3A). The
dual-luciferase reporter assay revealed that co-transfection with
the miR-27b-3p mimic significantly decreased luciferase activity in
EGFR wt and MMP-13 wt, but not EGFR mut and MMP-13 mut (Fig. 3B). This finding confirmed that
miR-27b-3p targeted EGFR and MMP13.
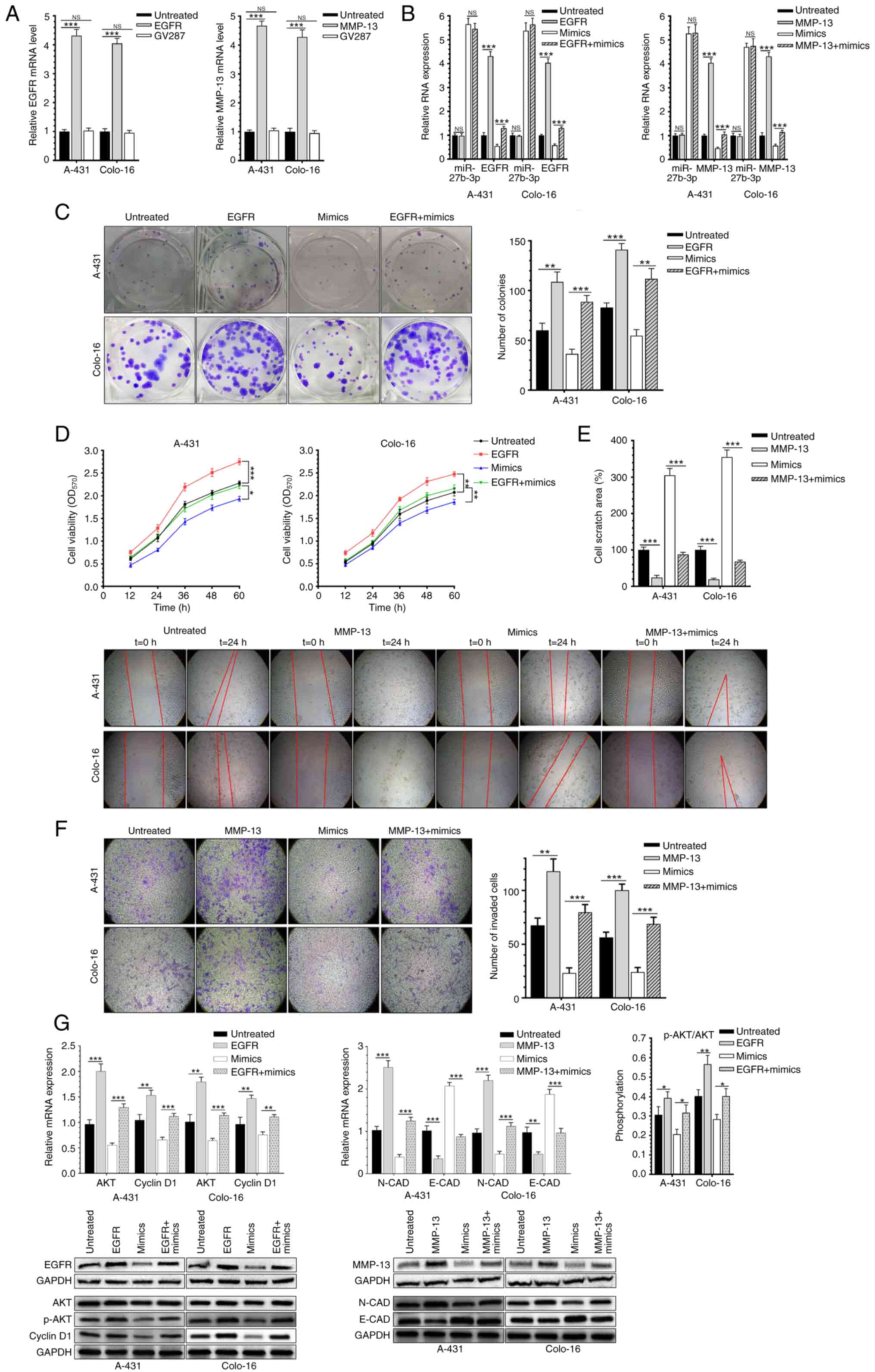
Overexpression of EGFR or MMP-13
reverses the effects of miR-27b-3p on CSCC cells
A-431 and Colo-16 cells were co-transfected with the
miR-27b-3p mimic and lentivirus infection. Transfection efficiency
is shown in Fig. 4A and relevant RNA
expression was shown as Fig. 4B.
Notably, overexpression of EGFR significantly reversed the
inhibitory effect of miR-27b-3p on CSCC proliferation (Fig. 4C and D). Overexpression of MMP-13 and
the miR-27b-3p mimic increased the migration and invasion of CSCC
cells, compared with cells transfected with the mimic alone
(Fig. 4E and F). Co-overexpression
of EGFR markedly promoted the viability and proliferation of CSCC
cell lines, while overexpression of MMP-13 markedly enhanced the
migration and invasion of CSCC cells (Fig. 4C-F). Moreover, overexpression of EGFR
upregulated p-Akt/total AKT expression and cyclin D1.
Overexpression of MMP-13 downregulated N-CAD and upregulated E-CAD
levels compared with untreated or mimic-group (Fig. 4G). Therefore, overexpression of EGFR
and MMP-13 reversed the inhibitory effect of miR-27b-3p on CSCC
cells.
Discussion
The prognosis of CSCC is closely associated with
clinical manifestations and histopathology, including tumor size,
infiltration depth, nerve involvement, previous therapeutic
effects, histological differentiation and immune status (22). The American Joint Committee on Cancer
uses criteria including tumor diameter larger than 2 cm, poor
cellular differentiation, depth of invasion more than 2 mm or to
the reticular dermis (Clark level IV), perineural invasion, or ear
or mucosal lip location to classify high-risk tumors (8). Gore et al (9) analyzed 57 patients with CSCC and found
that patients with lymphatic metastasis experienced poor prognosis
and high mortality. Among them, 8 patients presented lymphatic
metastasis, with infiltration of nerves and lymphatic vessels as
the main reasons affecting their prognosis. During the follow-up
period of 19.4 months, 9 patients experienced recurrence, including
6 deaths. Thus, infiltration and metastasis may be the leading
causes of deterioration and poor prognosis in CSCC.
miR-27b-3p, which exhibits anticancer effects, is
downregulated in several types of cancer, such as breast cancer,
lung carcinoma, esophageal carcinoma and colorectal carcinoma
(14,23–25). Han
et al (24) determined that
miR-27b-3p was downregulated in esophageal squamous cell carcinoma
(ESCC) tissue samples and cell lines and was associated with poor
cell differentiation, TNM staging and lymphatic metastasis. The
transcription factor nuclear-related factor 2 is the direct target
of miR-27b-3p, as evidenced by dual-luciferase reporter assays
(24). In a study conducted by Zeng
et al (26), IL-10 induced
the upregulation of miR-27b-3p and reduced the mRNA stability of
proliferating cell nuclear antigen, which inhibited the development
of hemangioma cavernosum. As the target of the long non-coding RNA
HLA complex P5, miR-27b-3p drives the malignant development of
gastric cancer, including proliferation and
epithelial-to-mesenchymal transition (EMT) (27). However, the potential functions of
miR-27b-3p in the development of CSCC have rarely been studied. The
results of the present study revealed that miR-27b-3p was
significantly downregulated in CSCC cell lines. Overexpression of
miR-27b-3p attenuated the proliferative, migratory and invasive
abilities of A-431 and Colo-16 cells compared with untreated group.
In addition, the relative expression levels of EGFR, MMP-13, Akt,
p-Akt and cyclin D1 were downregulated by miR-27b-3p
overexpression, while E-CAD was upregulated compared with untreated
group. These findings suggested that miR-27b-3p exerted an
inhibitory role on the growth of CSCC cells.
EGFR is a transmembrane glycoprotein encoded by the
proto-oncogene C-erbB-1 (28).
Binding of ligands to the extracellular domain of EGFR triggers
conformational changes in its transmembrane region and activates
the intracellular region to bind to ATPase, leading to
autophosphorylation and transphosphorylation. Consequently,
multiple cellular signaling pathways are induced (29). For instance, EGFR can affect tumor
development, metastasis and drug resistance mainly by activating
the RAS-RAF-MAPK, the PI3K-PTEN-Akt and the JAK/STAT pathways
(30). Diego Carrillo-Beltrán et
al (31) analyzed relevant
signaling pathways that mediate the carcinogenesis of oral cancer
induced by high-risk human papillomavirus infection and
demonstrated that HPV16 E7 activated the EGFR/PI3K/Akt1/NRF2, which
in turn induced the activation of pirin/NF-κB signaling in oral
cancer. In addition, Tang et al (32) demonstrated that knockdown of
circ_0081143 suppressed hypoxia-induced migration, invasion and EMT
in gastric cancer cells via the miR-497-5p/EGFR axis. Xiong et
al (33) reported that WAP
four-disulfide core domain 2 (WFDC2) levels negatively correlated
to the Gleason score and incidence of metastasis in patients with
prostate cancer. WFDC2 binds to the extracellular domain of EGFR
and inhibits the EGFR/Akt/GSK3β/Snail signaling pathway, thus
blocking the metastasis of prostate cancer (33). In the present study, EGFR was the
direct target of miR-27b-3p. By targeting EGFR, miR-27b-3p
attenuated CSCC proliferation through the inhibition of Akt
phosphorylation and cyclin D1 downregulation.
The MMP family is closely related to tumor migration
and invasion (34). Wang et
al (35) suggested that
clusterin (CLU) promotes cell proliferation and survival in
hepatocellular carcinoma (HCC) and that CLU levels are associated
with poor survival of patients with HCC and relapse. CLU
accelerates HCC metastasis by activating the EIF3I/Akt/MMP13
signaling pathway (35).
Overexpressed TLR-9 promoted the expression of MMP-13 and triggered
the migratory and invasive abilities of prostate cancer (36). Zhang et al (37) reported that sirtuin 1 (SIRT1) is
significantly upregulated in gastric cancer tissue samples, and via
activating the STAT3/MMP-13 signaling pathway, SIRT1 deficiency
facilitates the migration of gastric cancer both in vivo and
in vitro. MMP-13 was validated as a target gene of
miR-27b-3p in the present study. Overexpression of miR-27b-3p
upregulated E-CAD in A-431 and Colo-16 cells by downregulating
MMP-13, thus inhibiting the metastasis and EMT of CSCC cells.
In conclusion, miR-27b-3p reduces the proliferation,
migration and invasion of CSCC cells by binding to the 3′-UTRs of
EGFR and MMP-13. Thus, this miRNA may represent a potential
diagnostic marker and therapeutic option for CSCC.
Acknowledgements
Not applicable.
Funding
This project was funded by Guangdong medical science
and Technology Research Fund (grant no. A2019289).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DL and ZZ made substantial contributions to
conception and design, acquisition, analysis and interpretation of
data. DL and ZZ performed the experiments. ZZ drafted the
manuscript and revised it critically for important intellectual
content. DL and ZZ confirmed the authenticity of the raw data. Both
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Que SKT, Zwald FO and Schmults CD:
Cutaneous squamous cell carcinoma: Incidence, risk factors,
diagnosis, and staging. J Am Acad Dermatol. 78:237–247. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Asgari MM, Warton EM and Whittemore AS:
Family history of skin cancer is associated with increased risk of
cutaneous squamous cell carcinoma. Dermatol Surg. 41:481–486. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Waldman A and Schmults C: Cutaneous
squamous cell carcinoma. Hematol Oncol Clin North Am. 33:1–12.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perera E, Gnaneswaran N, Staines C, Win AK
and Sinclair R: Incidence and prevalence of non-melanoma skin
cancer in Australia: A systematic review. Australas J Dermatol.
56:258–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee AY and Berman RS: The landmark series:
Non-melanoma skin cancers. Ann Surg Oncol. 27:22–27. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Newlands C and Gurney B: Management of
regional metastatic disease in head and neck cutaneous malignancy.
2. Cutaneous malignant melanoma. Br J Oral Maxillofac Surg.
52:301–307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bowe CM, Gurney B, Whitaker S and Newlands
C: Management of regional metastatic disease in cutaneous
malignancy of the head and neck. 3. Merkel cell carcinoma. Br J
Oral Maxillofac Surg. 57:847–856. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmults CD, Karia PS, Carter JB, Han J
and Qureshi AA: Factors predictive of recurrence and death from
cutaneous squamous cell carcinoma: A 10-year, single-institution
cohort study. JAMA Dermatol. 149:541–547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gore SM, Shaw D, Martin RC, Kelder W, Roth
K, Uren R, Gao K, Davies S, Ashford BG, Ngo Q, et al: Prospective
study of sentinel node biopsy for high-risk cutaneous squamous cell
carcinoma of the head and neck. Head Neck. 38 (Suppl 1):E884–E889.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sempere LF, Azmi AS and Moore A:
MicroRNA-based diagnostic and therapeutic applications in cancer
medicine. Wiley Interdiscip Rev RNA. May 17–2021.(Epub ahead of
print). doi: 10.1002/wrna.1662. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iqbal MA, Arora S, Prakasam G, Calin GA
and Syed MA: MicroRNA in lung cancer: Role, mechanisms, pathways
and therapeutic relevance. Mol Aspects Med. 70:3–20. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Acunzo M and Croce CM: MicroRNA in cancer
and cachexia-A mini-review. J Infect Dis. 212 (Suppl 1):S74–S77.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miao W, Li N, Gu B, Yi G, Su Z and Cheng
H: miR-27b-3p suppresses glioma development via targeting YAP1.
Biochem Cell Biol. 98:466–473. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang X, Chen J, Liao Y, Huang L, Wen C,
Lin M, Li W, Zhu Y, Wu X, Iwamoto A, et al: miR-27b-3p promotes
migration and invasion in colorectal cancer cells by targeting
HOXA10. Biosci Rep. 39:BSR201910872019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, Wang P, Zhang C and Ma Z: Epidermal
growth factor receptor (EGFR): A rising star in the era of
precision medicine of lung cancer. Oncotarget. 8:50209–50220. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan Q, Yuan Y, Yankui L, Jingjie F,
Linfang J, Yong P, Dong H and Xiaowei Q: The expression and
significance of CXCR5 and MMP-13 in colorectal cancer. Cell Biochem
Biophys. 73:253–259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang SH, Law CH, Kuo PH, Hu RY, Yang CC,
Chung TW, Li JM, Lin LH, Liu YC, Liao EC, et al: MMP-13 is involved
in oral cancer cell metastasis. Oncotarget. 7:17144–17161. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Gao L, Ma S, Ma J, Wang Y, Li S,
Hu X, Han S, Zhou M, Zhou L and Ding Z: MALAT1-KTN1-EGFR regulatory
axis promotes the development of cutaneous squamous cell carcinoma.
Cell Death Differ. 26:2061–2073. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rahmati Nezhad P, Riihilä P, Piipponen M,
Kallajoki M, Meri S, Nissinen L and Kähäri VM: Complement factor I
upregulates expression of matrix metalloproteinase-13 and −2 and
promotes invasion of cutaneous squamous carcinoma cells. Exp
Dermatol. Apr 4–2021.(Epub ahead of print). doi: 10.1111/exd.14349.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rodriguez LG, Wu X and Guan JL:
Wound-healing assay. Methods Mol Biol. 294:23–29. 2005.PubMed/NCBI
|
|
22
|
Saito Y, Fujikawa H, Takatsuka S, Abe R
and Takenouchi T: Risk factors for lymph node metastasis in
cutaneous squamous cell carcinoma: A long-term retrospective study
of Japanese patients. Int J Clin Oncol. 26:606–612. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Z, Chen X, Lu B, Gu Y, Chen Q, Lei T,
Nie F, Gu J, Huang J, Wei C, et al: Up-regulated LINC01234 promotes
non-small-cell lung cancer cell metastasis by activating VAV3 and
repressing BTG2 expression. J Hematol Oncol. 13:72020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han M, Li N, Li F, Wang H and Ma L:
miR-27b-3p exerts tumor suppressor effects in esophageal squamous
cell carcinoma by targeting Nrf2. Hum Cell. 33:641–651. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen SJ, Song Y, Ren XY, Xu YL, Zhou YD,
Liang ZY and Sun Q: MicroRNA-27b-3p promotes tumor progression and
metastasis by inhibiting peroxisome proliferator-activated receptor
gamma in triple-negative breast cancer. Front Oncol. 10:13712020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zeng Z, Chen H, Cai J, Huang Y and Yue J:
IL-10 regulates the malignancy of hemangioma-derived endothelial
cells via regulation of PCNA. Arch Biochem Biophys. 688:1084042020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen S, Ren C, Zheng H, Sun X and Dai J:
The effect of long non-coding RNA (lncRNA) HCP5 on regulating
epithelial-mesenchymal transition (EMT)-related markers in gastric
carcinoma is partially reversed by miR-27b-3p. Med Sci Monit.
26:e9213832020.PubMed/NCBI
|
|
28
|
Hoffmann TK, Balló H, Braunstein S, Van
Lierop A, Wagenmann M and Bier H: Serum level and tissue expression
of c-erbB-1 and c-erbB-2 proto-oncogene products in patients with
squamous cell carcinoma of the head and neck. Oral Oncol. 37:50–56.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yun CH, Boggon TJ, Li Y, Woo MS, Greulich
H, Meyerson M and Eck MJ: Structures of lung cancer-derived EGFR
mutants and inhibitor complexes: Mechanism of activation and
insights into differential inhibitor sensitivity. Cancer Cell.
11:217–227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hirsch FR, Dziadziuszko R, Thatcher N,
Mann H, Watkins C, Parums DV, Speake G, Holloway B, Bunn PA Jr and
Franklin WA: Epidermal growth factor receptor immunohistochemistry:
Comparison of antibodies and cutoff points to predict benefit from
gefitinib in a phase 3 placebo-controlled study in advanced
nonsmall-cell lung cancer. Cancer. 112:1114–1121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carrillo-Beltrán D, Muñoz JP,
Guerrero-Vásquez N, Blanco R, León O, de Souza Lino V, Tapia JC,
Maldonado E, Dubois-Camacho K, Hermoso MA, et al: Human
papillomavirus 16 E7 promotes EGFR/PI3K/AKT1/NRF2 signaling pathway
contributing to PIR/NF-κB activation in oral cancer cells. Cancers
(Basel). 12:19042020. View Article : Google Scholar
|
|
32
|
Tang J, Zhu H, Lin J and Wang H: Knockdown
of Circ_0081143 mitigates hypoxia-induced migration, invasion, and
EMT in gastric cancer cells through the miR-497-5p/EGFR axis.
Cancer Biother Radiopharm. 36:333–346. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiong Y, Yuan L, Chen S, Xu H, Peng T, Ju
L, Wang G, Xiao Y and Wang X: WFDC2 suppresses prostate cancer
metastasis by modulating EGFR signaling inactivation. Cell Death
Dis. 11:5372020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Matrisian LM: Metalloproteinases and their
inhibitors in matrix remodeling. Trends Genet. 6:121–125. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang C, Jin G, Jin H, Wang N, Luo Q, Zhang
Y, Gao D, Jiang K, Gu D, Shen Q, et al: Clusterin facilitates
metastasis by EIF3I/Akt/MMP13 signaling in hepatocellular
carcinoma. Oncotarget. 6:2903–2916. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kalantari E, Abolhasani M, Roudi R,
Farajollahi MM, Farhad S, Madjd Z, Askarian-Amiri S and
Mohsenzadegan M: Co-expression of TLR-9 and MMP-13 is associated
with the degree of tumour differentiation in prostate cancer. Int J
Exp Pathol. 100:123–132. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang S, Yang Y, Huang S, Deng C, Zhou S,
Yang J, Cao Y, Xu L, Yuan Y, Yang J, et al: SIRT1 inhibits gastric
cancer proliferation and metastasis via STAT3/MMP-13 signaling. J
Cell Physiol. 234:15395–15406. 2019. View Article : Google Scholar : PubMed/NCBI
|