Introduction
The ras homolog family member A (RHOA) gene
encodes a member of the Rho family of small GTPases and is known to
function in reorganization of the actin cytoskeleton, which is
associated with regulation of cell shape, attachment and motility.
RHOA has been found to be recurrently mutated in
gastrointestinal cancer, especially in diffuse-type gastric cancer
cases (1–3). In this cancer, residues p.Arg5,
p.Gly17, p.Tyr42 and p.Leu57 of RHOA are considered hotspot
missense mutations (1,2). However, the functional significance of
these mutations has not been consistently demonstrated. Kakiuchi
et al (1) suggested that the
hotspot mutations were gain-of-function mutations because
inhibiting the expression of the mutant RHOA induced the
suppression of proliferation of gastric cancer cells. In addition,
Zhang et al (4) showed that
RHOAp.Tyr42Cys was a gain-of-function mutation that
could sufficiently induce diffuse-type gastric cancer in a mouse
model. On the other hand, Wang et al (2) indicated that these were
loss-of-function mutations because the mutant RHOA protein showed
reduced small GTPase activity and lost the ability to mediate
anoikis. Sakata-Yanagimoto et al (5) also reported that the RHOA p.Gly17Val
mutation was a loss-of-function mutation because it showed loss of
GTP binding activity and inhibition of wild-type RHOA function.
Interestingly, knockdown of RHOA in gastric cancer cells
with intrinsic abundant expression of RHOA, irrespective of
its mutational status, results in inhibition of proliferation in
vitro (6). Downregulation of
RHOA via miR-31 inhibits cell proliferation and invasiveness
(7). Moreover, overexpression of
wild-type RHOA induces immortalization of human mammary epithelial
cells. However, these immortalized cells were anchorage-dependent
and were unable to form tumors when implanted in nude mice
(8). Although these pieces of
evidence have highlighted the different aspects of the molecular
functions of RHOA, the functional role of RHOA mutations in
the digestive tract cancers are yet to be determined. In the
present study, to understand the functional role of RHOA
mutations in digestive tract cancers, genotyping, transcriptome
analysis and proliferation assays were carried out in cell lines
expressing the mutant or wild-type RHOA, as well as in cells
where RHOA has been knocked down.
Materials and methods
Cell culture
The AGS cell line was obtained from American Type
Culture Collection. The GCIY, KATO III, HGC-27, MKN1 and MKN45 cell
lines were obtained from RIKEN BioResource Center. The OE19 and
SW948 cell lines were obtained from Public Health England
(Salisbury, UK). SNU16 and SNU719 cell lines were obtained from
Korean Cell Line Bank. CCK-81 cell line was obtained from Japanese
Collection of Research Bioresources Cell Bank. All cell lines were
cultured according to recommendations from suppliers. AGS cells
were cultured in F-12 Ham, Kaighn's Modification (Sigma-Aldrich;
Merck KGaA) supplemented with 10% FBS (Immuno-Biological
Laboratories Co., Ltd.). Minimum Essential Medium (MEM;
Sigma-Aldrich; Merck KGaA) supplemented with 15% FBS was used for
GCIY cells. RPMI-1640 (Sigma-Aldrich; Merck KGaA) supplemented with
10% FBS was used for KATO III, MKN1, MKN45, OE19, SNU16 and SNU719
cell culture. MEM supplemented with 10% FBS was used for HGC-27 and
CCK-81 cell maintenance. Leibovitz's L-15 (Thermo Fisher
Scientific, Inc.) supplemented with 2 mM Glutamine (Thermo Fisher
Scientific, Inc.) and 10% FBS was used for SW948 cell culture.
SW948 cells were maintained at 37°C with 100% air in a humidified
atmosphere; all other cell lines were cultured at 37°C with 5%
CO2 in a humidified atmosphere. These cell lines were
selected because of availability and of being characterized
previously as originating from digestive tract tumors (1,9–12).
Mutational analysis of the cell
lines
DNA was extracted from the cultured cells using
GenElute™ Mammalian Genomic DNA Miniprep Kit (Sigma-Aldrich; Merck
KGaA) according to the manufacturer's instructions. All coding
exons and splice sites of RHOA were amplified by using
AccuPrime™ Taq DNA Polymerase (Thermo Fisher Scientific, Inc.) and
paired primers shown in Table SI.
The amplified products were analyzed using Sanger sequencing, as
described previously (13). To
investigate whether RHOAp.Arg5Gln and
RHOAp.Tyr42Cys in the CCK-81 cell line were cis- or
trans-compound heterozygous mutations, mutation-specific primers,
wild-type specific primers and intron primers were designed as
shown in Table SII.
Small interfering RNA (siRNA)
targeting RHOA
Knockdown of RHOA using siRNA was conducted,
as previously reported (1). The
validated RHOA siRNA used included: i) RHOA siRNA2 sense,
5′-CUAUGAUUAUUAACGAUGUTT-3′ and antisense,
5′-ACAUCGUUAAUAAUCAUAGTT-3′; and ii) RHOA siRNA3 sense,
5′-GGCUUUACUCCGUAACAGATT-3′ and antisense,
5′-UCUGUUACGGAGUAAAGCCCT-3′. The negative control (NC) siRNA
sequences were: sense, 5′-GUACCGCACGUCAUUCGUAUC-3′ and antisense,
5′-UACGAAUGACGUGCGGUACGU-3′. For the cellular proliferation assay,
1.0×104 cells/well were seeded into a 96-well clear flat
bottom ultra-low attachment plate (Corning, Inc.) with 100 µl
growth medium containing 1 nM of siRNA and 0.16% (vol/vol) RNAiMAX
(Thermo Fisher Scientific, Inc.) according to the manufacture's
instruction. The cells were incubated at 37°C with 5%
CO2 in humidified conditions, except for SW948 cells
that were incubated at 37°C with 100% air. Cells were assayed 24 h
later (Day 1) and then every 48 h (Day 3 and 5) until Day 7. For
immunoblotting, 2.5×105 cells/well were seeded into a
6-well clear flat-bottom ultra-low attachment plate with 1 ml
medium containing 1 nM siRNA and 0.16% (vol/vol) of RNAiMAX. The
transfected cells were incubated as aforementioned, and collected
48 h later. The low attachment plates were used to allow
proliferation in three-dimensional spheroid conditions, a method
that is more suitable for in vitro bioassays than
conventional two-dimensional assays (14).
Three-dimensional cell proliferation
assay
Following RHOA-knockdown, cell viability was
assessed using the CellTiter-Glo® 3D Cell Viability
Assay (Promega Corporation) according to the manufacturer's
instruction. The viability of the cells transfected with NC siRNA
was used as the control. Cell viability was calculated after
subtraction of background absorbance as follows: Cell viability
(%)=(absorbance of the sample/absorbance of the control) ×100.
Immunoblotting
Cells were harvested and lysed in modified RIPA
buffer containing 1X complete mini protease inhibitor cocktail
(Sigma-Aldrich; Merck KGaA) and 1X PhosSTOP phosphatase inhibitor
cocktail (Sigma-Aldrich; Merck KGaA). Protein concentration was
determined by using the Bradford Protein Assay Kit (Bio-Rad
Laboratories, Inc.) according to the manufacture's instruction.
Cell extracts containing 40 µg protein were separated by
electrophoresis on a 10–20% gradient polyacrylamide gel and blotted
onto a polyvinylidene difluoride membrane (ATTO Corporation) using
the XV Pantera MP System (DRC Co., Ltd.), according to the
manufacturer's instructions. Blocking was performed for 1 h using
the ECL Blocking Agent (Amersham Biosciences; Cytiva) at room
temperature (RT), and the membrane was incubated with primary
antibodies overnight at 4°C. Primary antibodies used were the
rabbit monoclonal anti-RHOA antibody (clone 67B9; 1:1,000 dilution;
cat. no. 2117; Cell Signaling Technology, Inc.) and the mouse
monoclonal anti-β-actin antibody (clone AC-15; 1:1,000 dilution;
cat. no. A5441; Sigma-Aldrich; Merck KGaA). The membrane was
incubated with a corresponding secondary antibody for 1 h at RT.
The secondary antibodies used were horseradish
peroxidase-conjugated anti-mouse and anti-rabbit immunoglobulin
antibody (1:10,000 dilution) (cat. nos. NA931 and NA934; GE
Healthcare). The signals were visualized using the ECL Prime
Western Blotting Detection Reagent (Cytiva) and LAS 4000 Mini
system (Fujifilm Wako Pure Chemical Corporation).
Microarray analysis
Total RNA was isolated from the cultured cells using
the RNeasy Mini kit (Qiagen GmbH) and was subjected to microarray
analysis for transcriptome. The microarray analysis was performed
by RIKEN Genesis, using Agilent SurePrint G3 Human GE Microarray
8×60k Ver3.0 (G4851C) (Agilent Technologies, Inc.). Gene Ontology
analysis was performed (http://geneontology.org) using the PANTHER
Classification System (http://pantherdb.org/) (15).
Statistical analysis
The cell growth rate was represented in terms of
mean and standard error and was compared using one-way ANOVA and
Tukey's test. In microarray analysis, only genes whose expression
levels were detected were considered for further analysis. Changes
in gene expression levels were compared using unpaired two-tailed
Student's t-tests. Hierarchical clustering analysis was performed
using absolute values of fold changes of genes by the following
conditions: Clustering Algorithm, Hierarchical; Clustered By,
Normalized intensity values; Similarity Measure, Euclidean; Linkage
Rule, Wards. P<0.05 was considered statistically significant,
except for gene ontology analysis, in which GeneSpring corrected
P-value <0.1 was considered statistically significant. The
statistical analyses of cell viability assay were performed using
JMP Pro 13 (Cary). The statistical analyses of microarray results
were performed using GeneSpring 14.8 (Agilent Technologies,
Inc.).
Results
The mutations in the entire coding exons and splice
sites of RHOA were examined in one esophageal cancer cell
line (OE19), eight gastric cancer cell lines (AGS, GCIY, HGC-27,
KATO III, MKN1, MKN45, OE19, SNU16 and SNU719) and two colon cancer
cell lines (CCK-81 and SW948) using Sanger sequencing. Mutations
were identified as p.Arg5Gln and p.Tyr42Cys in CCK-81, p.Arg5Trp
and p.Phe39Leu in SNU16, p.Gly17Glu in SW948, p.Tyr42Ser in OE19,
p.Ala61Val in SNU719 and p.Glu64del in AGS in RHOA, some of
which were consistent with published reports (Table SIII and Fig. S1A) (1,16,17). All
these mutations were heterozygous. Among them, p.Arg5Gln and
p.Tyr42Cys in CCK-81 were compound heterozygous mutations in a
trans configuration (Fig. S1B and
C). Although AGS had been used as a cell line with wild-type
RHOA in a report published elsewhere (1), the AGS line used in the present study
harbored an in-frame deletion, p.Glu64del, which was consistent
with the data in the COSMIC database (COSM2849889, http://cancer.sanger.ac.uk/cell_lines/mutation/overview?id=122450537).
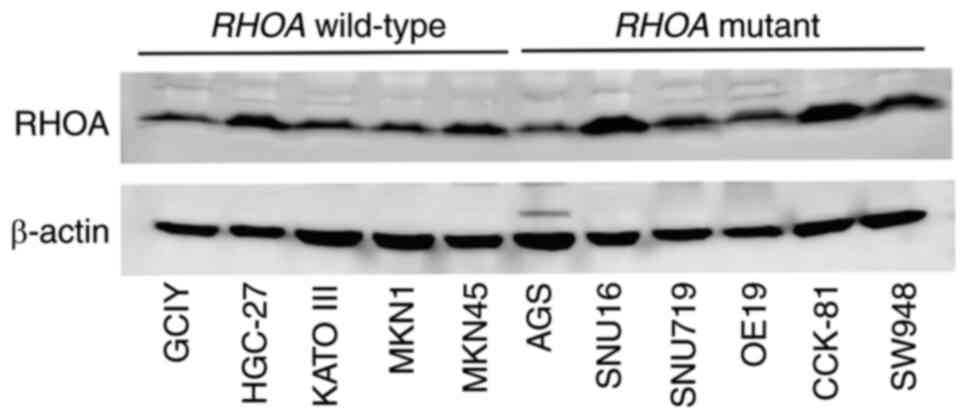
No RHOA mutation was found in GCIY, HGC-27, KATO III, MKN1
and MKN45. The expression of RHOA was examined in all cell lines.
RHOA protein was markedly expressed, although at different levels,
regardless of the presence or absence of mutations (Fig. 1).
To understand the functional significance of RHOA in
these cancer cell lines, 3-dimensional cell proliferation assays
were conducted in cell lines expressing the protein and in cell
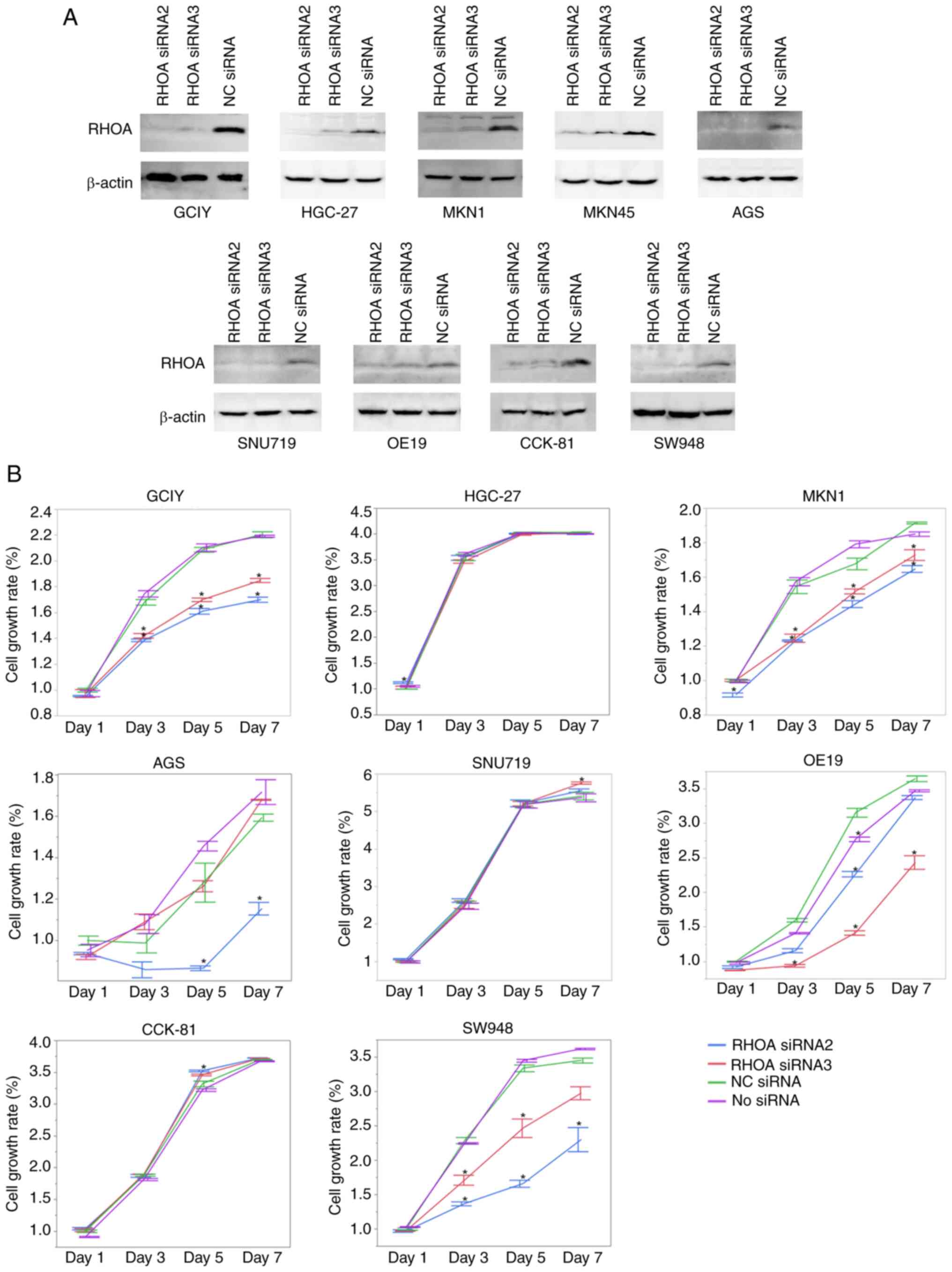
lines where RHOA had been knocked down. The knockdown of
RHOA was carried out in 9 adherent cell lines (AGS, CCK-81,
GCIY, HGC-27, MKN1, MKN45, OE19, SNU719 and SW948) by RNA
interference using two siRNAs that were previously validated and
used elsewhere (1). After two days
of transfection, knockdown of RHOA was confirmed in all the
examined cells by immunoblotting (Fig.
2A). A total of eight cell lines which showed sufficient
knockdown of RHOA were assayed for their proliferation (Fig. 2B). Proliferation was attenuated in
the GCIY, MKN1, OE19 and SW948 cell lines, but not in the HGC-27,
SNU719 or CCK-81 cell lines. AGS cell line showed a conflicting
result of decreased proliferation with one siRNA but no change with
the other siRNA, although both siRNAs resulted in the same level of
RHOA knockdown.
To investigate the gene expression profiles
underlying the proliferation phenotypes, transcriptome analyses of
cells with RHOA knockdown and mock transfectants were
performed using microarray. The cell lines used for transcriptome
analysis include: i) HGC-27, harboring the wild-type RHOA,
with no growth alteration following RHOA knockdown; ii) AGS,
harboring RHOAp.Glu64del, with reduced
proliferation following RHOA knockdown; iii) CCK-81,
harboring RHOAp.Arg5Gln and
RHOAp.Tyr42Cys, with no growth change following
RHOA knockdown; and iv) SW948, harboring
RHOAp.Gly17Glu, with reduced proliferation
following RHOA knockdown. Significantly knocked down of
RHOA was confirmed at its transcriptional level by the
microarray analysis in HGC-27, AGS, CCK-81 and SW948, with fold
changes in expression of 4.13×10−2,
4.67×10−2, 1.46×10−1 and
4.91×10−2, respectively.
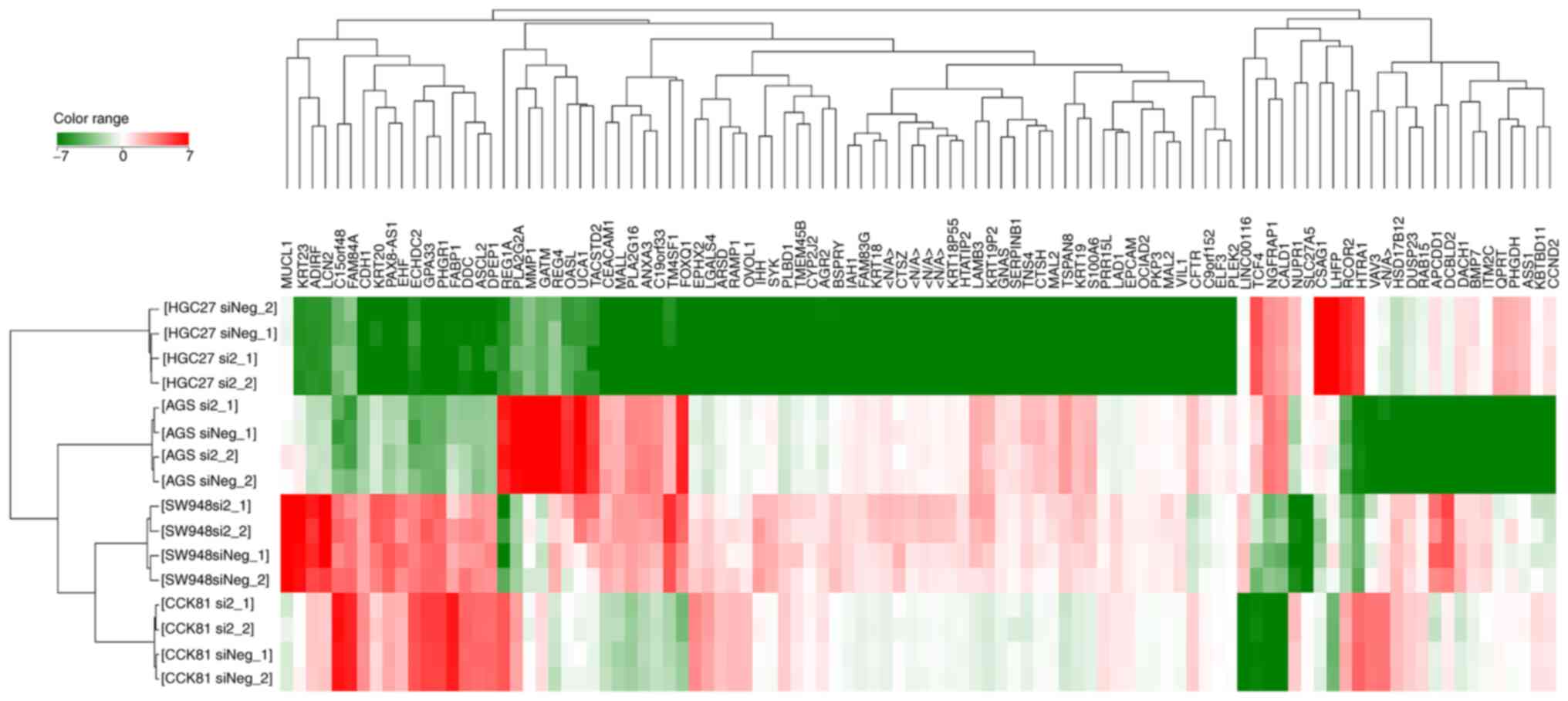
A hierarchical clustering analysis of the
transcriptomes showed that the expression profiles clustered
specific to the cell type rather than the knockdown of RHOA
(Fig. 3). On a detailed comparison
between the transcriptomes of cells with RHOA knockdown and
those without the knockdown, numerous genes were identified that
were significantly downregulated (<0.5-fold) or upregulated
(>2.0-fold) (Tables I and
SIV). lnc-DERA-1 was
significantly downregulated after RHOA knockdown in cells
with mutated RHOA.
 | Table I.Genes of significantly altered
expression following RHOA knockdown. |
Table I.
Genes of significantly altered
expression following RHOA knockdown.
| Cell line | Downregulated
gene | Upregulated
gene |
|---|
| AGS | CLDN18, CYP26C1,
KRT28, LGSN, LINC00909, LINC00933, lnc-ARRDC3-1, lnc-DERA-1,
LOC155060, RHOA, SLC26A1, STK31 | GATS, KDR,
KRT39, LINC00113, lnc-DHX34-1, lnc-EIF2B5-2, lnc-GABARAPL3-4,
lnc-RIC3-1, LOC399900, LOC643339, OR4C15, SMIM24, SZT2 |
| CCK81 | FOXQ1,
lnc-DERA-1, lnc-FAM189A1-3, lnc-OXNAD1-2, lnc-RP11, 181C3.1.1–1,
METTL6, RHOA, SP5 | CDK15, CYSLTR1,
KLF2, lnc-C5orf38-3, lnc-NTRK2-3, MXRA7, RHOB, ZG16 |
| HGC27 | EGFR, IGFBP3,
lnc-AL020996.1–2, lnc-CPSF7-1, lnc-ZNF730-1, MEIS1, OPN1SW, RHOA,
SPIN3, TRIAP1 | CDK19, COL5A1,
CSRNP3, LINC01529, lnc-ANLN-4, LOC102724301, PABPC1L2B, SLC36A1,
SLC4A4, SWAP70, ZDHHC20 |
| SW948 | ACSL6, AIFM3,
CAMKK1, CERKL, CMKLR1, GPR128, KCNMB4, lnc-C9orf80-1, lnc-CILP-1,
lnc-DERA-1, lnc-RNF219-3, LOC102724484, LOC729732, NCKAP5,
PNLIPRP2, PTPN20B, PTPRO, RHOA, RIIAD1, SEMA3C, SMPX, SNX22,
TAS2R45, XLOC_l2_010029 | ADM, AMOTL2,
ANO1, ARL14, ATP2B4, ATP8B3, CACNB4, CAV1, CDRT1, CITED2, CPE,
CRYGC, CTGF, CXCL1, CYR61, DOCK4, DOK7, EDN1, EPHA2, GALNT5, GJB3,
GNGT2, GPR37L1, GRPR, GULP1, HDAC5, IL1RN, KCNK9, KRT34, KRTAP1-5,
KRTAP3-1, LAMA3, LIMCH1, LIMS2, LINC00520, LINC00592, LINC00704,
LINC01468, LMO1, lnc-ACTBL2-1, lnc-ANKRD10-1, lnc-ARFGEF2-2,
lnc-CEP44-1, lnc-COL1A1-4, lnc-COX4NB-1, lnc-MRP63-6, lnc-MYO1D-1,
lnc-OR10H5-2, lnc-PAX4-1, lnc-RP11-582J16.5.1–3,
lnc-RP11-817J15.3.1–2, lnc-SNURF-3, lnc-YPEL5-3, LOC101927260,
LOC101928620, LOC101928666, MAFF, MYL9, NT5DC4, OR1S2, PAG1, PDGFB,
PLK2, PPP1R15A, PTPRR, PXDN, RGCC, S100A2, SCARA3, SH2D5, SH3RF1,
SLC1A3, SLC26A9, SLC2A14, SLC2A3, SLC6A20, SPANXA1, SPTSSB, SSUH2,
TAGLN, TCTEX1D4, TM4SF1, TM4SF1-AS1, TMCC3, TNNC1, UCA1, WBSCR28,
WFDC2, WWTR1, XLOC_l2_009441 |
The functional relationship among differentially
expressed genes was analyzed using the Gene Ontology database and
the PANTHER Classification System (Table SV and Fig. S2). According to interpretations of
the biological process terms from the Gene Ontology database, it
was inferred that genes associated with ‘small molecule metabolic
process (GO:0044281)’ and ‘oxidation-reduction process
(GO:0055114)’ were downregulated, while genes associated with
‘vasculogenesis (GO:0001570)’, ‘positive regulation of endothelial
cell proliferation (GO:0001938)’, ‘cyclin-dependent protein
serine/threonine kinase activity (GO:0004693)’, ‘transmembrane
signaling receptor activity (GO:0004888)’ and ‘olfactory receptor
activity (GO:0004984)’ were upregulated. This altered expression
profile was common only in cells with attenuated proliferation
in vitro due to RHOA knockdown.
Discussion
The present study identified RHOA mutations
in digestive tract cancer cell lines and showed that the protein
was evidently but varyingly expressed in these cells regardless of
the genotype. The mutations included missense mutations and one
in-frame deletion (p.Arg5Gln, p.Arg5Trp, p.Gly17Glu, p.Phe39Leu,
p.Tyr42Cys, p.Tyr42Ser, p.Ala61Val and p.Glu64del). According to
the COSMIC database, p.Arg5Gln, p.Arg5Trp, p.Gly17Glu, p.Tyr42Cys
and p.Tyr42Ser are common hotspot mutations while p.Phe39Leu,
p.Ala61Val and p.Glu64del are rare mutations. It is indicated that
the frequencies of the p.Arg5Gln, p.Arg5Trp, p.Gly17Glu, p.Tyr42Cys
and p.Tyr42Ser represented 4, 10, 7, 23 and 4% of 99 nonsynonymous
mutations detected in 1,854 gastric cancer samples, respectively
(COSMIC database; accessed on 2019.1.15). However, p.Phe39Leu, and
Ala61Val have not been identified in the gastric cancer samples,
but in the hematopoietic system (p.Phe39Leu) and large intestine
(p.Ala61Val), in the COSMIC database. In the present study,
knockdown of RHOA inhibited the proliferation of some cell
lines. The inhibition was observed in two of the three cell lines
expressing wild-type RHOA and three of the five cell lines
with mutant RHOA (AGS with p.Glu64del, OE19 with p.Tyr42Cys
and SW948 with p.Gly17Glu). This suggested that RHOA
promoted cell proliferation depending on some intrinsic nature of
the cells. The AGS cell line showed the conflicting result of
decreased proliferation with one siRNA but no change with the other
siRNA, although both siRNAs resulted in the same level of RHOA
knockdown, which is different from the result of a similar
experiment using the same siRNAs, performed by Kakiuchi et
al (1) (showing no significant
change by either siRNA). Knockdown of RHOA in AGS cells was shown
to inhibit cell proliferation in a previously published study by
Liu et al (18), which is
partially consistent with the findings of the current study. The
biological reason for these conflicting results is obscure, and
requires further investigation. The knockdown of RHOA in the
current experiments were not specific to mutated transcripts, but
specific to both the mutated and the wild-type transcripts in cells
with heterologous alleles. The cell cycle and apoptosis of RHOA
knockdown cells were not examined; therefore, it is unclear whether
the inhibition of proliferation was due to attenuation of cell
cycle or increase of apoptosis.
Furthermore, the present study also evaluated the
change in the expression profile of other genes associated with
RHOA. Hence, the transcriptome of RHOA knockdown cells was
analyzed. It was hypothesized that genes that were down- and
upregulated following RHOA knockdown would represent genes
promoted and inhibited by RHOA expression, respectively.
lnc-DERA-1 was commonly downregulated in examined cells with
RHOA mutation. According to LNCipedia (https://hg19.lncipedia.org; accessed 2019.1.21),
lnc-DERA-1 is a non-coding RNA encoded by a gene at
chr12:16573561-16573994, whose function has not been uncovered yet.
In the gene ontological analysis, small molecule metabolic process
and oxidation-reduction process were commonly downregulated
biological processes in cells with the attenuated proliferation,
which could be associated with in vitro cell proliferation.
Protein kinases play a critical role in cell proliferation.
Downregulated genes encoding protein kinases in cells with
attenuated proliferation were STK31 in AGS and CAMKK1
in SW948. STK31 is a cancer-associated gene that encodes a
serine/threonine protein kinase known to play a role in microtubule
assembly that is necessary for cell cycle progression (19). CAMKK1 encodes
calcium/calmodulin dependent protein kinase kinase 1 that activates
calcium/calmodulin dependent protein kinase (CAMK). CAMK plays a
central role in calcium/calmodulin-dependent signaling cascades
implicated in cell survival and carcinogenesis (20). The genes associated with the
metabolic process of small molecules which were downregulated
include CYP26C1 and SLC26A1 in AGS and ACSL6
and PNLIPRP2 in SW948 cell lines. CYP26C1 encodes a
member of the cytochrome P450 superfamily of enzymes, which is
involved in several processes, including drug metabolism and lipid
synthesis (Entrez Gene; http://www.ncbi.nlm.nih.gov/gene). SLC26A1
encodes a sulfate/anion transporter that functions in transporting
of glucose and other sugars, bile salts and organic acids, metal
ions and cytochrome P450-arranged by substrate type (GeneCards;
http://www.genecards.org). ACSL6 encodes
Acyl-CoA synthase that catalyzes the formation of acyl-CoA from
fatty acids, ATP and CoA (Entrez Gene: http://www.ncbi.nlm.nih.gov/gene). PNLIPRP2
encodes pancreatic lipase that hydrolyzes galactolipids (Entrez
Gene; http://www.ncbi.nlm.nih.gov/gene). The downregulated
genes associated with oxidation-reduction included CYP26C1
and AIFM3 in the AGS and SW948 cell line, respectively.
AIFM3 encodes apoptosis inducing factor mitochondria
associated 3 that has a pyridine nucleotide-disulfide
oxidoreductase domain and mediates apoptosis (21). Downregulation of these genes may
induce metabolic stress. However, the mechanistic relationship
between the inhibition of RHOA and the altered expression of
these genes was not evaluated in the present study. Recently, one
clue potentially associated with the transcriptional regulation by
RHOA has emerged. Regulation of the actin cytoskeleton by RHOA is
associated with nuclear translocation of Yes-associated protein 1
(YAP) and WW-domain-containing transcription regulator 1
(WWTR1/TAZ) that are known to be an important transcriptional
regulator (22). Interestingly, the
transcription analysis in the present study demonstrated that
WWTR1/TAZ was upregulated after RHOA knockdown in SW948, which
potentially indicates some negative feedback regulations.
One limitation of the present study was that the
transcriptome analyses were not performed for all the cell lines.
The functional significance of RHOA mutations was not
evaluated. Further study on the regulation of transcription by
RHOA including the upregulation of lnc-DERA-1 may be
needed for improved understanding of the mechanism of association
between RHOA and cell proliferation.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank the depositors of
the cell lines used in this study, namely, Dr Mutsumi Nozue,
University of Tsukuba, Ibaraki, Japan, for depositing GCIY cells to
RIKEN BRC; Dr Masuo Obinata, Tohoku University, Sendai, Japan, for
depositing KATO III cells to RIKEN BRC; Dr Tetsuo Kimoto, Okayama
University, Okayama, Japan, for depositing HGC-27 cells to RIKEN
BRC; Dr Teiichi Motoyama, Yamagata University School of Medicine,
Yamagata, Japan, depositing MKN1 and MKN45 cells to RIKEN BRC; Drs
J.C. Rockett and A. Morriss, University of Warwick, Coventry, UK,
and Dr S.J. Darnton, Birmingham Heartlands Hospital, Birmingham,
UK, for depositing OE19 to the European Collection of Authenticated
Cell Cultures, Public Health England; Dr. Jae-Gahb Park, Cancer
Research Institute, Korean Cell Line Bank, Korean Cell Line
Research Foundation, Seoul, Republic of Korea, for depositing SNU16
and SNU719 cells to the Korean Cell Line Bank; and Dr Isaka,
Hidehiko, Kagoshima University, Kagoshima, Japan, for depositing
CCK-81 cells to the Japanese Collection of Research Bioresources
Cell Bank.
Funding
The present study was supported by the Japan Society
for the Promotion of Science-KAKENHI (grant no. JP16K10518).
Availability of data and materials
The microarray data are available in the Gene
Expression Omnibus repository under the accession number GSE110237
(http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE110237).
All data generated or analyzed during this study are included in
this published article.
Authors' contributions
NI, AS and TF conceived the study and designed the
experiments. NI and ET performed the experiments. NI, AS, MY and TF
performed the bioinformatics data analysis. NI, AS, MY and TF
contributed to drafting and critical review of manuscript. NI and
TF confirm the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
RHOA
|
ras homolog family member A
|
|
YAP
|
Yes-associated protein 1
|
|
TAZ
|
WW-domain-containing transcription
regulator 1
|
References
|
1
|
Kakiuchi M, Nishizawa T, Ueda H, Gotoh K,
Tanaka A, Hayashi A, Yamamoto S, Tatsuno K, Katoh H, Watanabe Y, et
al: Recurrent gain-of-function mutations of RHOA in diffuse-type
gastric carcinoma. Nat Genet. 46:583–587. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang K, Yuen ST, Xu J, Lee SP, Yan HH, Shi
ST, Siu HC, Deng S, Chu KM, Law S, et al: Whole-genome sequencing
and comprehensive molecular profiling identify new driver mutations
in gastric cancer. Nat Genet. 46:573–582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ushiku T, Ishikawa S, Kakiuchi M, Tanaka
A, Katoh H, Aburatani H, Lauwers GY and Fukayama M: RHOA mutation
in diffuse-type gastric cancer: A comparative clinicopathology
analysis of 87 cases. Gastric Cancer. 19:403–411. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang H, Schaefer A, Wang Y, Hodge RG,
Blake DR, Diehl JN, Papageorge AG, Stachler MD, Liao J, Zhou J, et
al: Gain-of-function RHOA mutations promote focal adhesion kinase
activation and dependency in diffuse gastric cancer. Cancer Discov.
10:288–305. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sakata-Yanagimoto M, Enami T, Yoshida K,
Shiraishi Y, Ishii R, Miyake Y, Muto H, Tsuyama N, Sato-Otsubo A,
Okuno Y, et al: Somatic RHOA mutation in angioimmunoblastic T cell
lymphoma. Nat Genet. 46:171–175. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang HR, Nam S, Lee J, Kim JH, Jung HR,
Park HS, Park S, Ahn YZ, Huh I, Balch C, et al: Systematic approach
identifies RHOA as a potential biomarker therapeutic target for
Asian gastric cancer. Oncotarget. 7:81435–81451. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Korourian A, Roudi R, Shariftabrizi A and
Madjd Z: MicroRNA-31 inhibits RhoA-mediated tumor invasion and
chemotherapy resistance in MKN-45 gastric adenocarcinoma cells. Exp
Biol Med (Maywood). 242:1842–1847. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao X, Lu L, Pokhriyal N, Ma H, Duan L,
Lin S, Jafari N, Band H and Band V: Overexpression of RhoA induces
preneoplastic transformation of primary mammary epithelial cells.
Cancer Res. 69:483–491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ideo H, Seko A and Yamashita K: Galectin-4
binds to sulfated glycosphingolipids and carcinoembryonic antigen
in patches on the cell surface of human colon adenocarcinoma cells.
J Biol Chem. 280:4730–4737. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma C, Xie J, Luo C, Yin H, Li R, Wang X,
Xiong W, Zhang T, Jiang P, Qi W, et al: OxLDL promotes
lymphangiogenesis and lymphatic metastasis in gastric cancer by
upregulating VEGF-C expression and secretion. Int J Oncol.
54:572–584. 2019.PubMed/NCBI
|
|
11
|
Ohashi N, Kodera Y, Nakanishi H, Yokoyama
H, Fujiwara M, Koike M, Hibi K, Nakao A and Tatematsu M: Efficacy
of intraperitoneal chemotherapy with paclitaxel targeting
peritoneal micrometastasis as revealed by GFP-tagged human gastric
cancer cell lines in nude mice. Int J Oncol. 27:637–644.
2005.PubMed/NCBI
|
|
12
|
Kim YI, Lee HJ, Khang I, Cho BN and Lee
HK: Selective inhibition of cell growth by activin in SNU-16 cells.
World J Gastroenterol. 12:3000–3005. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuboki Y, Shimizu K, Hatori T, Yamamoto M,
Shibata N, Shiratori K and Furukawa T: Molecular biomarkers for
progression of intraductal papillary mucinous neoplasm of the
pancreas. Pancreas. 44:227–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fennema E, Rivron N, Rouwkema J, van
Blitterswijk C and de Boer J: Spheroid culture as a tool for
creating 3D complex tissues. Trends Biotechnol. 31:108–115. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mi H, Huang X, Muruganujan A, Tang H,
Mills C, Kang D and Thomas PD: PANTHER version 11: Expanded
annotation data from gene ontology and reactome pathways, and data
analysis tool enhancements. Nucleic Acids Res. 45:D183–D189. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu J, McCleland M, Stawiski EW, Gnad F,
Mayba O, Haverty PM, Durinck S, Chen YJ, Klijn C, Jhunjhunwala S,
et al: Integrated exome and transcriptome sequencing reveals ZAK
isoform usage in gastric cancer. Nat Commun. 5:38302014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mouradov D, Sloggett C, Jorissen RN, Love
CG, Li S, Burgess AW, Arango D, Strausberg RL, Buchanan D, Wormald
S, et al: Colorectal cancer cell lines are representative models of
the main molecular subtypes of primary cancer. Cancer Res.
74:3238–3247. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu N, Bi F, Pan Y, Sun L, Xue Y, Shi Y,
Yao X, Zheng Y and Fan D: Reversal of the malignant phenotype of
gastric cancer cells by inhibition of RhoA expression and activity.
Clin Cancer Res. 10:6239–6247. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuo PL, Huang YL, Hsieh CC, Lee JC, Lin BW
and Hung LY: STK31 is a cell-cycle regulated protein that
contributes to the tumorigenicity of epithelial cancer cells. PLoS
One. 9:e933032014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsu WC, Le HN, Lin YJ, Chen MC, Wang TF,
Li CC, Kuo WW, Mahalakshmi B, Singh CH, Chen MC and Huang CY:
Calmodulin/CaMKII-γ mediates prosurvival capability in
apicidin-persistent hepatocellular carcinoma cells via
ERK1/2/CREB/c-fos signaling pathway. J Cell Biochem. 122:612–625.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie Q, Lin T, Zhang Y, Zheng J and Bonanno
JA: Molecular cloning and characterization of a human AIF-like gene
with ability to induce apoptosis. J Biol Chem. 280:19673–19681.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kofler M, Speight P, Little D, Di
Ciano-Oliveira C, Szászi K and Kapus A: Mediated nuclear import and
export of TAZ and the underlying molecular requirements. Nat
Commun. 9:49662018. View Article : Google Scholar : PubMed/NCBI
|