Introduction
Breast cancer is the most common malignancy in young
women (20–39 year of age) worldwide (1). In the United States, a small but
significant increase in the incidence of metastatic breast cancer
in women aged 25–39 years has been recorded, without a
corresponding increase in the incidence in older women (2). Although the incidence and mortality
rates associated with hormone receptor (HR)-negative breast cancer
have been decreasing, those of HR-positive breast cancer have been
increasing in the United States, likely in part due to the
increasing prevalence of excess body weight and declining fertility
rates (3). On the other hand, young
adult patients with breast cancer (aged <35 years) have a poor
prognosis in Japan, independent of prognostic clinicopathological
factors (4). Therefore, basic and
translational studies for the development of prevention strategies
for breast cancer at younger ages are required.
Chemically-induced rats have frequently been used as
animal models of HR-positive breast cancer (5,6). In
addition to chemically-induced mammary carcinomas, mouse models of
estrogen receptor (ER)1, cyclin D1, prolactin, transforming growth
factor α, nuclear receptor coactivator 3, extra spindle pole bodies
like 1, separase and Wnt family member 1 overexpression,
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α
(Pik3ca) gain-of-function, and tumor protein p53
(Trp53) or signal transducer and activator of transcription
1 loss, were also found to be associated with ER-positive-mammary
carcinogenesis (7).
As aforementioned, several mammary tissue-targeting
genotoxic carcinogens have been used for the induction of mammary
carcinogenesis in rat models. The experimental polycyclic aromatic
hydrocarbon 7,12-dimethylbenz[a]anthracene (DMBA) is a potent and
well-established mammary gland carcinogen in rats (8,9), and
DMBA-induced adenocarcinomas were previously shown to originate
from both luminal and myoepithelial cells (5). DMBA was also reported to induce mammary
carcinomas in CD2F1 (BALB/c × DBA/2) (10) and B6D2F1 (C57BL/6 × DBA/2) mice
(11), and DMBA-induced mammary
adenocarcinomas were shown to be primarily composed of luminal
cells and residual myoepithelial-like cells (10,11), or
mainly of myoepithelial cells (11).
2-Amino-1-methyl-6-phenylimidazo(4,5-b)pyridine (PhIP) is a
heterocyclic amine found in cooked meat that potently induces
tumors not only in the mammary glands, but also in the colon and
prostate of rats (12–14). PhIP-induced mammary tumors exhibited
a dose-dependent increase in malignant phenotypes (12), and in combination with a high-fat
diet (HFD), PhIP was reported to enhance malignant alterations in
rats (15). In addition, HFD and
high-sucrose diets, administered from the start of animal growth
till the end of the study, were reported to stimulate mammary tumor
development in experimental rat and mouse models (16–18).
Furthermore, a limited-duration HFD (e.g., during fetal/suckling
stages) has been demonstrated to affect mammary tumorigenesis
(19,20). These carcinogens and nutrient factors
have also been epidemiologically reported as risk factors for
breast cancer (21–23).
The mutation and loss of function of p53 are common
features among types of human breast cancer, and heterozygous
BALB/c Trp53 knockout mice have been reported as a
spontaneous mammary carcinoma model (24,25). The
aim of the present study was to confirm whether DMBA or PhIP
promoted short-latency mammary carcinoma in heterozygous BALB/c
Trp53 knockout mice, and to investigate the phenotypic and
genotypic characteristics of the induced carcinomas with reference
to published data of human breast cancer.
Materials and methods
Mice
Heterozygous C3H Trp53 knockout mice, in
which the NcoI site in exon 2 of the p53 gene was
heterozygously inserted by a neomycin phosphotransferase gene
(26), were obtained from RIKEN
BioResource Center (identification no. RBRC00107) and crossed with
BALB/c mice (BALB/cAJcl; CLEA Japan, Inc.) for 5 (N5) or 9 (N9)
generations. A total of 34 N5 mice (body weight,14.0–24.9 g at 5
weeks of age) and 75 N9 mice (body weight, 18.9–29.0 g at 5 weeks
of age) were used in the present study. The mice were housed in
plastic cages with woodchip bedding (n≤5 per cage) in an
air-conditioned animal room maintained at 22°C (fluctuation range,
within 1°C) and 55% relative humidity (fluctuation range, within
10%) on a 12:12-h light-dark cycle, and given free access to the
standard chow diet CE2 or beef tallow-based HFD Quick Fat (both
CLEA Japan, Inc.), and tap water or 10% sucrose (Wako Pure Chemical
Industries, Ltd.) water (SW) during the period of 5–10 weeks of
age. However, as the number of animals in the control, HFD and SW
groups was small, no notable effects pertaining to nutrient factors
were observed among the mice; therefore, all groups were evaluated
as a whole in order to reach a conclusion regarding the phenotypic
and genotypic characteristics of DMBA- or PhIP-induced mammary
carcinomas in heterozygous BALB/c Trp53 knockout mice. Mouse
experiments were carried out in a specific pathogen-free
environment at the animal facility of the National Cancer Center
Research Institute (Tokyo, Japan), according to the institutional
guidelines, and following the approval of the National Cancer
Center Animal Ethics Committee in Japan (approval no.
T17-028-C02).
Induction of mammary carcinomas
To investigate the characteristics of mammary tumors
induced by DMBA (MilliporeSigma), BALB/c Trp53 heterozygous
N9 female knockout mice were administered a single dose of DMBA (50
mg/kg of bodyweight, n=20) and its vehicle, sesame oil (10 ml/kg
body weight, n=20; MilliporeSigma). N5 female mice were
administered PhIP (Wako Pure Chemical Industries, Ltd.) 6 times/2
weeks (50 mg/kg of bodyweight, n=34). Both chemicals were
administered by gavage at 7 weeks of age. The present dose setting
was based on reported oral doses for the induction of mammary
tumors in rats; a single oral dose of DMBA (50 mg/kg of body
weight) (27) and multiple oral
doses of PhIP (25–85 mg/kg of body weight) (28–30) to
adolescent female Sprague-Dawley rats induced mammary
adenocarcinomas within 20–30 weeks. In the present experiments,
groups of BALB/c Trp53 wild-type mice with (n=20) and
without (n=15) DMBA administration were used to confirm
susceptibility to mammary carcinogenesis. In our previous study, no
obvious changes in histopathological characteristics and the
ER-positive frequency of spontaneous mammary adenocarcinomas were
detected in generations F1 (first filial generation) to N4 in
BALB/c crossed with C3H heterozygous Trp53 knockout mice
(24). Therefore, the effects of
DMBA and PhIP on the characteristics of induced mammary
adenocarcinomas were independently evaluated from the numbers of
the backcross generations. During the experimental period, anatomic
regions containing mammary tissue, such as the cervix, thorax and
abdomen, were palpated weekly to detect subcutaneous nodules. When
nodules accounted for >10% of the body weight of mice [the
longest nodule diameter measured using calipers often exceeded 15
mm (31)] or general conditions were
poor, the mice were euthanized by isoflurane overdose, and the
inguinal mammary gland (fat pad), subcutaneous nodules and
organs/tissues with abnormalities in the abdominal and thoracic
cavities were excised, followed by fixing with 10% neutrally
buffered formalin. The pieces of subcutaneous nodules and fat pad
with mammary glands were stored in liquid nitrogen.
Histopathology and
immunohistochemistry (IHC)
The samples were fixed with 10% neutral buffered
formalin at room temperature for 1–2 days, and then cut at the
maximum cutting plane, processed routinely and embedded in paraffin
wax. Sections (thickness, 3 µm) were stained with hematoxylin and
eosin (H&E) and histopathologically evaluated using a
transmission light microscope (BX51; Olympus Corporation).
Histopathological phenotypes of the DMBA- or PhIP-induced
carcinomas were classified into the following subtypes: i)
Glandular/microacinar, tumor composed of glands and/or microacinar
formation; ii) papillary, tumor with finger-like projections
composed of epithelium covering a central fibrovascular core; and
iii) solid, tumor is composed of solid sheets of epithelial cells
with little or no glandular differentiation (32,33).
Inasmuch as distinct biphasic structures with luminal and
myoepithelial cells were observed in the DMBA-induced carcinomas, a
scoring system was generated in the present study, by
classification and grading based on the area occupied by the
biphasic structures: 0–4, 5–24, 25–49, 50–74 and 75–100% were
graded as 0, 1, 2, 3 and 4, respectively. Serial sections were
subjected to IHC, where a total of 10 DMBA- and PhIP-treated
samples obtained from heterozygous Trp53 knockout mice were
preferentially selected from large carcinomas. For antigen
retrieval, the sections were autoclaved at 121°C for 10 min in 0.01
M citrate buffer (pH 6.0), after deparaffinization. Then, the
sections were incubated with 3% hydrogen peroxide (at room
temperature for 10 min), 10% normal goat serum (at room temperature
for 20 min.; Nichirei Biosciences, Inc.), and with the following
primary antibodies 4°C overnight: Anti-human ERα mouse monoclonal
(1:50 dilution; clone 6F11; cat. no. NCL-ER-6F11; Novocastra),
anti-human/mouse phosphorylated-ERK1(pERK1; T202/Y204)/ERK2 (pERK2;
T185/Y187) rabbit polyclonal (1:500 dilution; cat. no. AF1018;
R&D Systems, Inc.), anti-human dual-specificity phosphatase
(DUSP)4 rabbit polyclonal (1:50 dilution, cat. no. ab72593; Abcam)
anti-human phosphorylated-AKT (pAKT; Ser473) rabbit monoclonal
(1:100 dilution; clone D9E; cat. no. #4060; Cell Signaling
Technology, Inc.), anti-mouse β-catenin mouse monoclonal (1:500
dilution; clone 14; cat. no. 610154; BD Transduction Laboratories),
anti-human cytokeratin 18 rabbit polyclonal (1:1,000 dilution; cat.
no. 10830-1-AP; ProteinTech Group, Inc.), anti-human α-smooth
muscle actin (αSMA) rabbit monoclonal (1:1,000 dilution; clone
EPR5368; cat. no. ab124964; Abcam) and anti-rat proliferating cell
nuclear antigen (PCNA) mouse monoclonal (1:800 dilution; clone
PC10; cat. no. M0879; DakoCytomation). The sections were incubated
with Histofine MAX PO solution (Nichirei Biosciences, Inc.).
Antibody binding was visualized using 3,3′-diaminobenzidine
tetrahydrochloride or New Fuchsin Substrate kit (Nichirei
Biosciences, Inc.), and sections were routinely counterstained with
hematoxylin at room temperature. Negative controls without primary
antibodies were set for each antigen.
Direct DNA sequencing
DNA was extracted from frozen normal mammary (fat
pad) and mammary carcinoma tissues with NucleoSpin Tissue (cat. no.
740952; Macherey-Nagel GmbH) according to the manufacturer's
instructions; 10 of the same samples used for IHC in each group
were analyzed. PCR products of HRas proto-oncogene (Hras)
exon 2, codon 61, which was expected to harbor DMBA-induced
mutations, as observed in rat mammary carcinomas (34,35), and
Pik3ca exons 6, 7, 9 and 20, in which hotspot mutations have
frequently been observed in human breast cancer (36), were obtained for mutation analysis
using the primers shown in Fig.
S1A. The sequencing reaction was performed using the BigDye
Terminator v3.1 Cycle Sequencing kit (cat. no. 4337455; Thermo
Fisher Scientific, Inc.) per the manufacturers protocol. For the
sequencing reaction of each target gene, PCR product (~0.1 pmoles,
which was estimated from the electrophoretic band density) was used
as a template, and the forward or reverse primer for the PCR
reaction was applied; however, an additional reverse sequencing
reaction was performed in cases where the obtained result in the
first reaction needed to be confirmed. The sequences were
determined using a 3130XL Genetic Analyzer (Thermo Fisher
Scientific, Inc.), and analyzed with SnapGene software version
3.1.4 (Insightful Science, LLC).
Reverse transcription-quantitative
(RT-q) PCR
RNA was extracted from frozen normal mammary or
carcinoma tissues using ISOGEN with Spin Column (Nippon Gene Co.,
Ltd.). cDNA was synthesized from RNA using the High-Capacity cDNA
Reverse Transcription kit (Thermo Fisher Scientific, Inc.). A total
of 10 samples of the same cases used for IHC in each group were
analyzed. qPCR was performed with TaqMan probes: The
dual-specificity phosphatase 4 (Dusp4) gene (Assay ID
Mm00723761_m1; cat. no. 4331182; Thermo Fisher Scientific, Inc.)
encoding DUSP4, actin β (Actb) gene (Assay ID Mm02619580_g1;
cat. no. 4331182; Thermo Fisher Scientific, Inc.) encoding β-actin
and TaqMan Fast Universal PCR master mix (Thermo Fisher Scientific,
Inc.) using the ABI-OneStep PCR system (Thermo Fisher Scientific,
Inc.). Thermocycling conditions were as follows: Initial
denaturation, 95°C for 20 sec; 40 cycles of amplification
(denaturation, 95°C for 1 sec; annealing/extension, 60°C for 20
sec). Values were normalized to the expression of the Actb
gene and calculated by the 2−ΔΔCq method (37).
Statistical analysis
Significant differences in the incidence of
histopathological findings were determined using Fisher's exact
test. For quantitative data from RT-qPCR for Dusp4 gene
expression, results are presented as the mean ± SEM, and
differences between groups were analyzed using the Kruskal-Wallis
test, followed by Steel's multiple comparison test using EZR (Easy
R) software, version 4.0.2 (38).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Accelerated induction of mammary
carcinomas by DMBA and PhIP
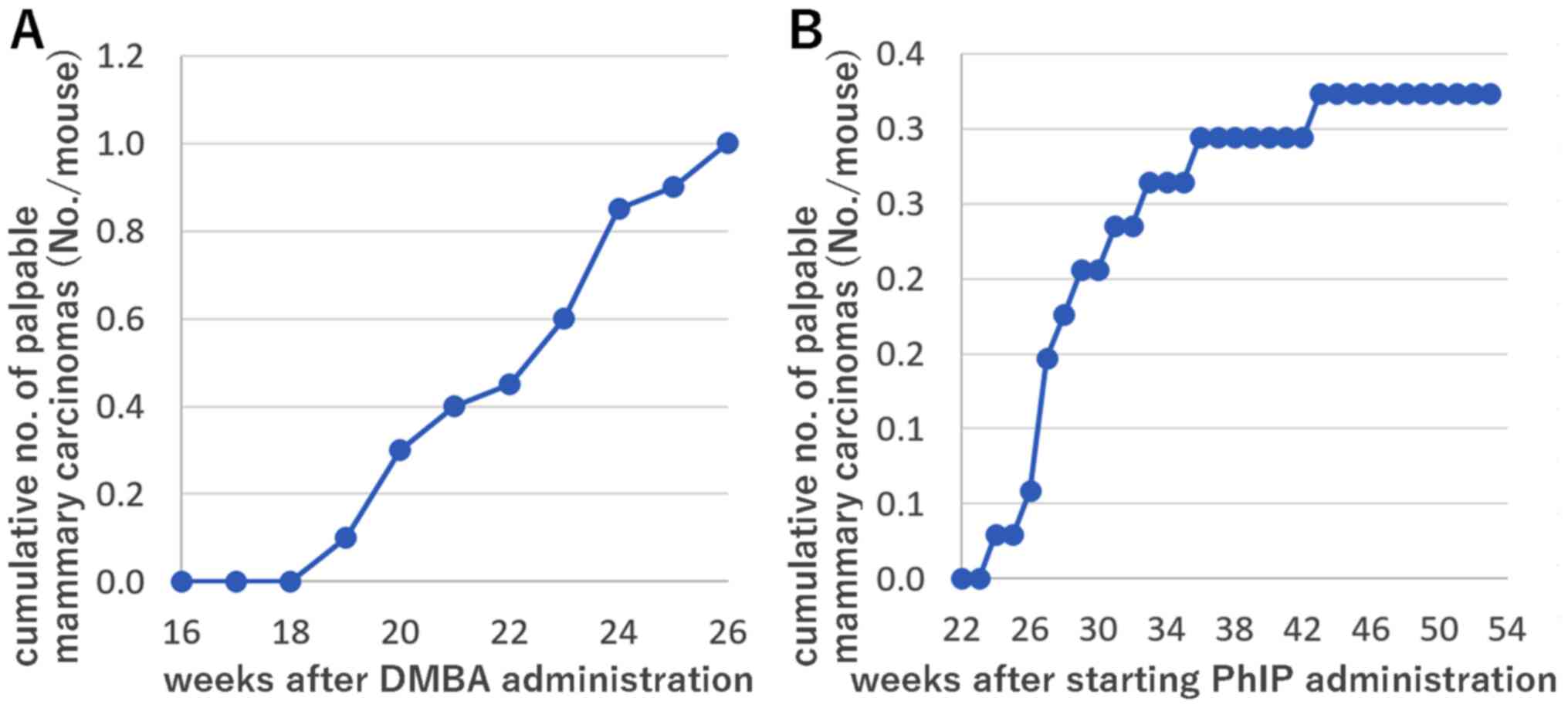
Mammary carcinomas were first identified by
palpation in the subcutaneous tissues of the cervix, thorax and/or
inguinal regions of 2/20 heterozygous Trp53 knockout mice on
week 19 post-DMBA administration, and the number of tumor-bearing
DMBA-treated mice gradually increased thereafter. The cumulative
numbers of palpable nodules histopathologically diagnosed as
mammary carcinomas are presented in Fig.
1A. The DMBA-treated and vehicle control groups were euthanized
at 26 weeks. The incidence of histopathologically diagnosed mammary
carcinomas, including intraductal carcinomas in the DMBA group, was
15/20 (75.0%), and additional dysplastic ductal hyperplasia was
observed in 3/20 (15.0%) mice on week 26 post-DMBA administration
(Table I). The number of identified
mammary carcinomas were 1, 2, 3 and 4/mouse in 4, 2, 3 and 6
DMBA-treated mice, respectively. On the other hand, mammary
carcinomas were not observed in the vehicle sesame oil-treated
control group without DMBA administration until 26 weeks after
vehicle administration (Table I).
The incidence of mammary tumors was significantly higher in
DMBA-treated heterozygous Trp53 knockout mice than in
DMBA-treated wild-type mice (P<0.001). In addition to the
identified mammary carcinomas, malignant lymphomas were found in
3/20 (15.0%), and lung adenocarcinomas in 1/20 (5%) heterozygous
Trp53 knockout mice each with and without DMBA
administration, respectively; other tumors, including endometrial
stromal sarcomas and granulosa cell tumors, were found in
DMBA-treated Trp53 heterozygous knockout or wild-type mice
with DMBA administration. The results indicate that the incidence
of mammary carcinomas was significantly increased in DMBA-treated
Trp53 heterozygous knockout mice, compared with the vehicle
controls, by week 26 post-DMBA administration.
 | Table I.Incidences of mammary carcinomas and
hyperplastic lesions in BALB/c-Trp53 heterozygous knockout
mice as well as BALB/c-Trp53-wild-type mice with and without
DMBA/PhIP administration. |
Table I.
Incidences of mammary carcinomas and
hyperplastic lesions in BALB/c-Trp53 heterozygous knockout
mice as well as BALB/c-Trp53-wild-type mice with and without
DMBA/PhIP administration.
|
| Trp53
heterogenous knockout mice, n (%) |
Trp53-wild-type mice, n (%) | Trp53
heterogenous knockout mice, n (%) |
|---|
|
|
|
|
|
|---|
| Group | DMBA N=20 | Vehicle N=20 | DMBA N=20 | Vehicle N=15 | PhIPa N=34 |
|---|
| Mammary
carcinoma | 14
(70)b | 0 | 0 (0) | 1 (7) | 12
(35) |
| Intraductal
carcinoma | 1 (5)b | 0 | 3
(15) | 0 | 2
(6) |
| Dysplastic ductal
hyperplasia | 3
(15) | 0 | 0 | 0 | 0 |
In PhIP-treated BALB/c heterozygous Trp53
knockout mice, palpable mammary carcinomas were observed in only
2/34 (6%) mice 26 weeks from the commencement of PhIP
administration. Therefore, lifetime observations were conducted for
the PhIP-treated group. The cumulative numbers of palpable nodules
histopathologically diagnosed as mammary carcinomas are presented
in Fig. 1B. Palpable mammary
carcinomas and tumors in other organs and tissues were identified
on weeks 24–53 from the commencement of PhIP administration. The
incidence of histopathologically diagnosed mammary carcinomas,
including intraductal carcinomas, was 14/34 (41%; Table I) and the median latency period was
33–34 weeks from the commencement of PhIP administration. A single
mammary carcinoma was diagnosed per mouse. The incidence of tumors
in other organs/tissues [adrenal subcapsular cell carcinomas in
9/34 (26%) mice, malignant lymphomas in 6/34 (18%) mice,
histiocytic sarcomas and osteosarcomas in 4/34 (12%) mice] was
cumulatively higher than that of mammary carcinomas in the
PhIP-treated groups. In summary, the median latency period from the
commencement of PhIP administration was shorter than that for
spontaneous cases during the lifetime observation of heterozygous
BALB/c Trp53 knockout mice without PhIP administration.
DMBA-induced mammary carcinomas are
histopathologically characterized by biphasic structures and
PhIP-induced solid/microacinar-type carcinomas
Mammary carcinomas induced in the DMBA-treated
heterozygous Trp53 knockout mice histopathologically
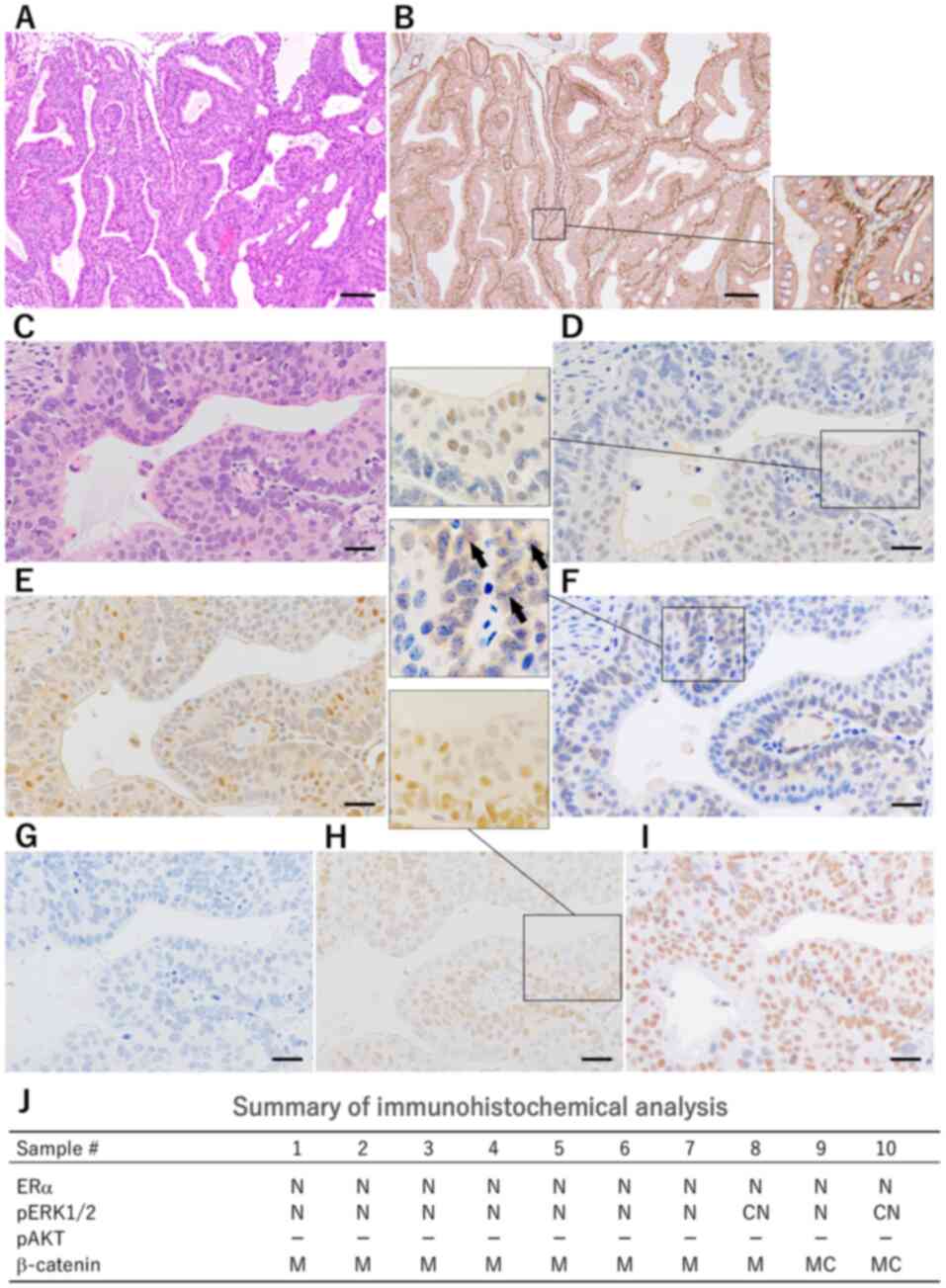
exhibited highly differentiated glandular structures (Fig. 2A). They featured a characteristic
biphasic structure consisting of two differentiation types of inner
luminal and outer myoepithelial cells. Immunohistochemically, inner
cells were positive for cytokeratin 18 and negative for αSMA. Outer
cells were positive for αSMA (Fig.
2B). Each H&E-stained specimen was classified into 5
grades, based on the area occupied by the biphasic structures: 0–4,
5–24, 25–49, 50–74 and 75–100% were graded as 0, 1, 2, 3 and 4,
respectively. Among the selected 10 large carcinoma samples from
the DMBA-treated heterozygous Trp53 knockout mice, 0, 2, 2,
1 and 5 samples were classified as grades 0, 1, 2, 3 and 4,
respectively.
The results of IHC for signaling molecules in the
DMBA-treated heterozygous Trp53 knockout mice are shown in
Fig. 2D-2I and summarized in
Fig. 2J. In H&E-stained
specimens, inner luminal cells in each biphasic glandular structure
exhibited an abundant eosinophilic cytoplasm and large nucleus.
Outer myoepithelial cells exhibited a scant basophilic cytoplasm
(Fig. 2C). IHC for ERα revealed
nuclear (N) positivity in the inner cells (Fig. 2D). ERα-positive carcinomas were
identified in 10/10 DMBA-treated mice. pERK showed N positivity and
cytoplasmic and nuclear (CN) positivity in the peripheral area of
the nodules, with N positivity prominent (8/10; Fig. 2E). IHC for β-catenin revealed
membranous (M) and membranous and cytoplasmic (MC) positivity in
the carcinoma cells, with M positivity prominent (8/10; Fig. 2F). No DMBA-treated mice were
pAKT-positive (Fig. 2G). In all
DMBA-treated mice, PCNA positivity was primarily detected in the
outer cells (Fig. 2H) and pERK
positivity in inner mammary carcinoma cells (Fig. 2E). This indicates that DMBA-induced
mammary carcinomas in heterozygous Trp53 knockout mice were
distinctly characterized by histological biphasic structures, and
predominant ERα- and pERK-positivity (Fig. 2J).
Mammary carcinomas induced in the PhIP-treated
heterozygous Trp53 knockout mice demonstrated moderately
differentiated solid/microacinar structures (Fig. 3A) and partly papillary patterns,
resembling those of spontaneous mammary carcinomas in the
heterozygous Trp53 knockout mice (Fig. S2A) and one carcinoma case from the
Trp53 wild-type group without DMBA administration (data not
shown). Immunohistochemically, αSMA-positive myoepithelial cells
were not observed or found to be scattered in various degrees among
luminal cells (Fig. 3B). Among the
10 selected large carcinoma samples in the PhIP-treated
heterozygous Trp53 knockout mice, 6, 4, 0, 0 and 0 cases
were classified into grades 0, 1, 2, 3 and 4, respectively, based
on the biphasic characteristics.
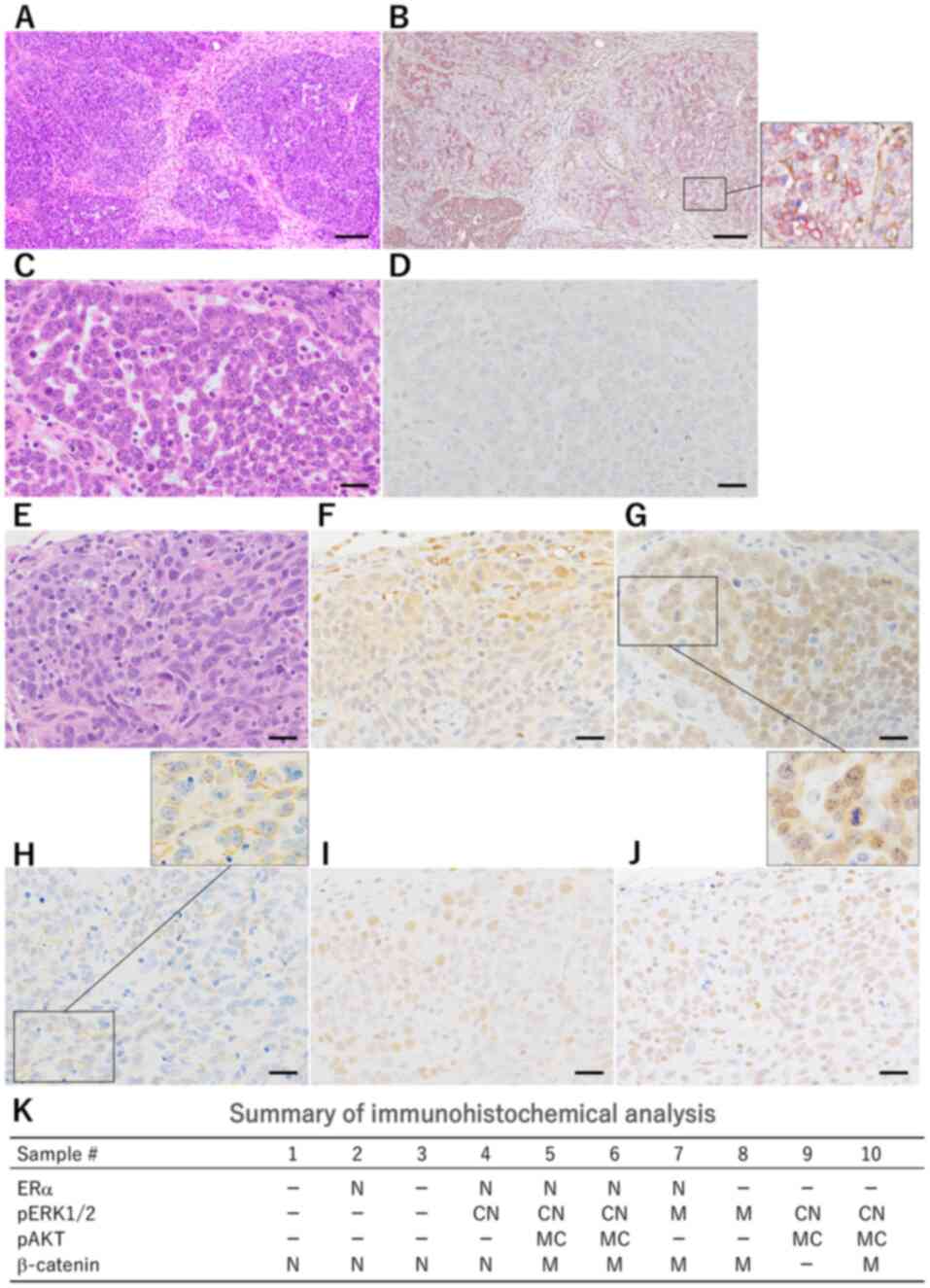 | Figure 3.(A) Mammary carcinomas induced in
PhIP-treated Trp53 heterozygous knockout mice demonstrated
moderately differentiated solid/microacinar structures (H&E
staining; scale bar, 50 µm). (B) Myoepithelial cells positive for
αSMA (brown) were scattered among luminal cells; the lower right
box is a higher magnification image of the lower right box. (C)
Microacinar type with small ductules. H&E staining; scale bar,
20 µm. (D) Immunohistochemistry for estrogen receptor α was
negative in 5/10 cases. (E) Solid type. H&E staining; scale
bar, 20 µm. (F) CN positivity for pERK was detected in the
peripheral area of the nodules. (G) CN positivity for β-catenin was
detected; the lower right box is a higher magnification image of
the upper left box. (H) M positivity for pAKT was diffusely
scattered. The box in the upper right is a higher magnification
image of the lower left box. (I) Proliferating cell nuclear antigen
is diffusely positive. (J) N positivity for dual-specificity
phosphatase is weak. (K) Summary of immunohistochemical analysis of
mammary carcinomas in PhIP-treated Trp53 heterozygous
knockout mice. PhIP,
2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine; CN, cytoplasmic
and nuclear; N, nuclear; M, membranous; H&E, hematoxylin and
eosin; αSMA, α-smooth muscle actin. |
The results of IHC for signaling molecules in the
PhIP-treated heterozygous Trp53 knockout mice are shown in
Fig. 3D and F-3J and summarized in
Fig. 3K. In H&E-stained
specimens, cellular atypia with nuclear enlargement and
pleomorphism in the PhIP-treated heterozygous Trp53 knockout
mice was more conspicuous (Fig. 3C and
E) than in mice without chemical treatment in the heterozygous
Trp53 knockout mice (Fig.
S2A) and one carcinoma case from the Trp53 wild-type
group without DMBA administration (data not shown). IHC for ERα
revealed N positivity, and the incidence of ERα-positive and
-negative carcinomas was 5/10 each in PhIP-treated mice (Fig. 3D). pERK exhibited CN (5/10) (Fig. 3F) and M (2/10) positivity in the
peripheral area of the nodules. IHC for β-catenin revealed M (5/10)
and N (4/10) positivity in the carcinoma cells (Fig. 3G). With regards to pAKT, M or C
positivity was observed in 4/10 PhIP-treated mice (Fig. 3H). PCNA positivity was diffusely
detected (Fig. 3I) in the carcinoma
tissues of PhIP-treated mice. No obvious association between
histopathological types (solid, microacinar structures or papillary
patterns) and IHC characteristics was observed in mammary
carcinomas from the PhIP group. In summary, mammary carcinomas
induced in PhIP-treated heterozygous Trp53 knockout mice
exhibited different molecular markers, indicating Wnt/β-catenin
signaling and/or PI3K/AKT signaling activation (Fig. 3K).
DMBA-induced mammary tumors exhibit
high-frequency Hras mutations and stimulate MAPK signaling
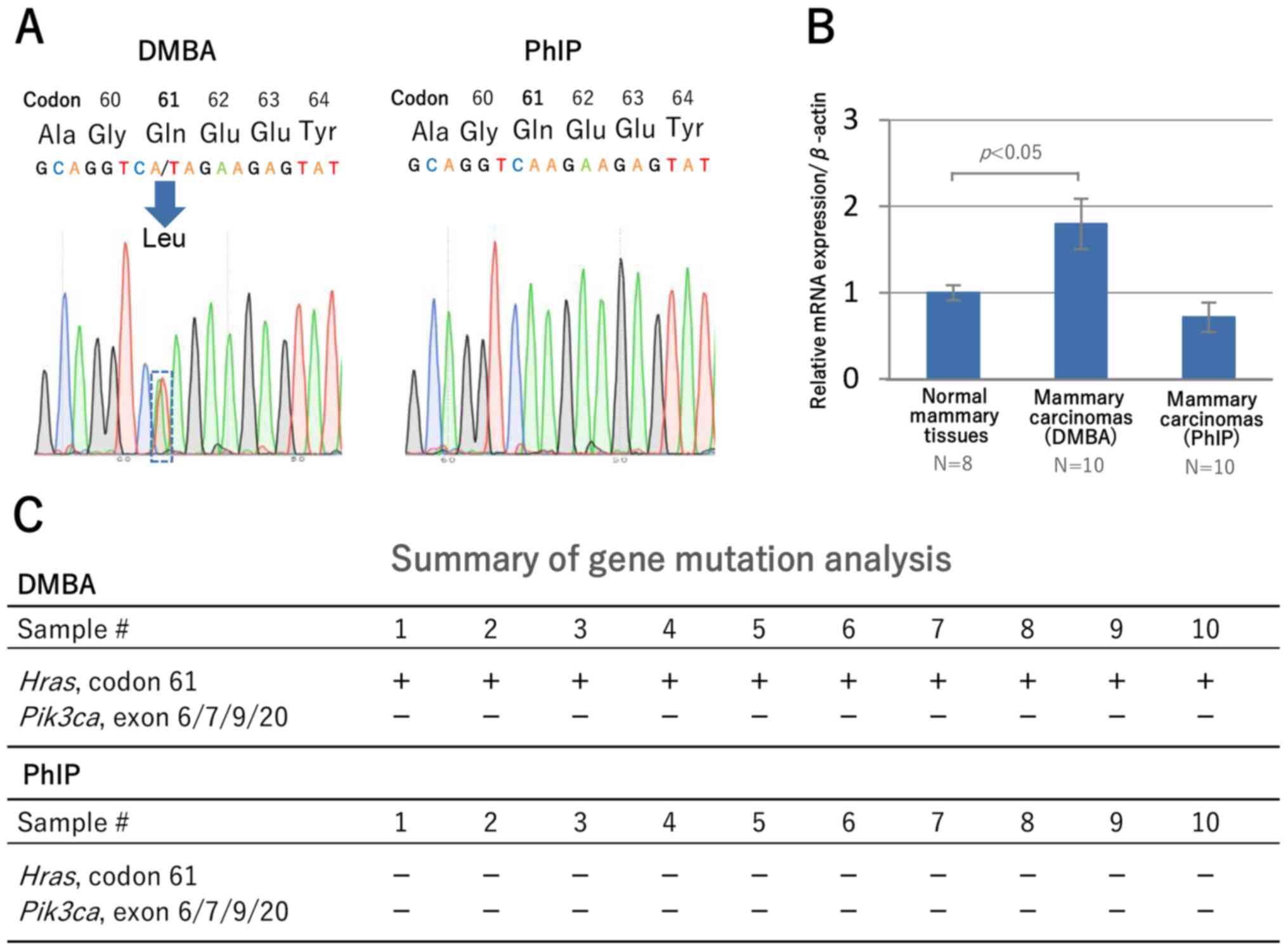
Direct DNA sequencing revealed a missense mutation
from CAA to CTA transversion (Gln to Leu) at exon 2, codon 61 of
the Hras gene (Fig. 4A) in
the mammary carcinomas of DMBA-treated mice (10/10), which was
consistent with the findings of a previous study (39). PhIP-treated mice exhibited no such
Hras mutation.
To confirm the activation of the MAPK signaling
pathway in DMBA-induced mammary carcinomas, the expression levels
of Dusp4, a negative regulator of the MAPK signaling
pathway, were examined. RT-qPCR revealed higher Dusp4
expression in carcinoma tissues from DMBA-treated mice than in
normal mammary tissues (P<0.05) from mice without DMBA
administration (Fig. 4B). Also in
IHC for DUSP4, diffuse N positivity in 10/10 DMBA-treated mice
(Fig. 2I) and weak N positivity in
PhIP-treated mice (Fig. 3J) were
observed. PIK3CA (human)/Pik3ca (mouse) mutations at
several hot spots, which are found in human breast carcinoma
(Fig. S1B), were reported in
DMBA-treated CD2F1 mice (10), but
no Pik3ca mutation was detected in the carcinomas of DMBA-
and PhIP-treated heterozygous BALB/c Trp53 knockout mice
(Fig. 4C). These results showed that
DMBA-induced mammary carcinomas were clearly characterized by
Hras mutation at a high frequency, and gene/protein
expression indicating MAPK stimulation.
Discussion
The primary aim of the present study was to confirm
whether DMBA or PhIP promoted mammary carcinogenesis in
heterozygous BALB/c Trp53 knockout mice. It was found that
DMBA administration significantly accelerated the induction of
mammary carcinomas in heterozygous Trp53 knockout mice, and
the terminal incidence in wild-type mice was lower than that in
heterozygous Trp53 knockout mice. A previous study reported
no differences in susceptibility to mammary tumors following DMBA
administration between heterozygous BALB/c Trp53 knockout
and Trp53 wild-type mice (40), but the doses and treatment schedule,
as well as internal hormonal conditions, differed from those in the
present study. A significant reduction in p53 function has been
observed in heterozygous Trp53 knockout mice compared with
wild-type mice (41). In addition,
the study indicated that carcinogenesis in the Trp53
heterozygous knockout mice was likely to involve numerous
carcinogen-tissue interactions that determine the tumor origin site
and latency to tumor formation, and the wild-type Trp53
allele was frequently retained in induced tumors (41). The tumor suppressor p53 plays an
essential role during the cellular response to DNA damage by
regulating several cellular processes, such as cell cycle arrest,
apoptosis and DNA repair (42).
Genomic instability associated with heterozygous Trp53
knockout is considered to cause DMBA-induced gene mutations. In the
PhIP group, the median latency period of mammary carcinomas was
40–41 weeks of age, and it was slightly shorter at ≥50 weeks of age
for spontaneous cases during the lifetime observation of
heterozygous BALB/c Trp53 knockout mice (24,25). In
wild-type BALB/c mice, the survival period for mice with mammary
carcinomas was >65 and >75 weeks in the PhIP-treated and
non-treated control groups, respectively (43). The present study demonstrated that
the increased susceptibility to mammary carcinogenesis was due to
the combination of Trp53 gene function deficiency and
exogenous factors.
Another important aim of the present study was to
investigate the phenotypic and genotypic characteristics of the
induced carcinomas with reference to published data on human breast
cancer. DMBA-induced mammary carcinomas histopathologically
exhibited distinct biphasic structures with luminal and
myoepithelial cells. The majority (62%) of spontaneous mammary
tumors identified during the lifetime observation of heterozygous
BALB/c Trp53 knockout mice were reported to exhibit a
mixture of luminal and myoepithelial cells, while other tumors were
reported to exhibit luminal or basal/myoepithelial cells only
(25). Furthermore, DMBA-induced
mammary tumors in B6D2F1 mice were reported to exhibit bicellular
characteristics at a rate of 29% (11), suggesting that the distinct biphasic
structures of DMBA-induced mammary tumors are enhanced in
heterozygous Trp53 knockout mice. Among human breast tumors,
adenomyoepithelioma is characterized as a stereotypical
epithelial-myoepithelial tumor composed of myoepithelial cells
surrounding epithelial tissue-lined spaces (44,45).
There are histopathological differences between DMBA-induced rodent
adenocarcinomas and human adenomyoepithelioma. For example, in the
former, inner epithelial components are predominant and
multilayered, and the outer myoepithelium is single or bilayered;
while in the latter, myoepithelial components are predominant, and
surround simple ductal structures. As compared with spontaneous
mammary carcinomas in another lifetime study of BALB/c-Trp53
heterozygous knockout mice without DMBA/PhIP administration
(24), PhIP-induced mammary
carcinomas demonstrated solid/microacinar structures with more
prominent cellular atypia and pleomorphism. Therefore, both the
DMBA-induced cases, and the modestly accelerated induction of
mammary carcinomas by PhIP, were suspected to be influenced by
genetic or epigenetic events.
Of note, Hras mutation at exon 2, codon 61
was frequently observed in the DMBA-induced mammary carcinomas in
heterozygous Trp53 knockout mice in the present study. A
characteristically active mutation, A to T transversion, at the
middle adenosine nucleotide in codon 61 following DMBA treatment in
the BALB/c mouse mammary gland, has previously been reported
(46). In CDF1 mice, the Hras
mutation in codon 61 was detected in 1/9 (11%) of mammary
carcinomas during long-latency periods following DMBA
administration (10), and the
facilitation of Hras mutation at a frequency of 10/10 (100%)
in the present study suggested molecular mechanisms underlying the
acceleration of the induction or selection of the mutation in
Trp53 heterozygous knockout mice compared with wild-type
(39,46). Indeed, when the p53 R273H mutation
occurred within RasV12-transformed epithelia, cell death was
strongly suppressed, and the majority of p53 R273H-expressing cells
survived (47). Mutations in the
Hras oncogenes in mammary tumors and mammary hyperplasic
outgrowth from DMBA-treated mice were confined to codon 61
substitutions of A to T or G (39).
An incidence of ~20% of the Hras mutations at codon 61 were
also reported in DMBA-induced mammary carcinomas in rats (34,35), but
the frequency in heterozygous Trp53 knockout mice in the
present study was higher.
HRAS activation is uncommon, and PIK3CA
mutations, which were detected in 45% of the luminal A
[HR-positive, progesterone receptor-high, HER2-negative] subtype,
were associated with the induction and/or poor outcome of human
breast cancer (36,48). On the other hand, mutations in both
HRAS and PI3K/AKT pathway genes, including PIK3CA,
were recently reported as drivers of breast adenomyoepitheliomas
(49,50). The associations between HRAS
(human)/Hras (mouse) mutations and the composition of
carcinoma cells with luminal and myoepithelial lineages in human
adenomyoepitheliomas and DMBA-induced mouse mammary carcinomas are
not clear. However, in the present study, DMBA-induced mammary
carcinomas with a high-frequency Hras mutation may partly
function as a model of adenomyoepithelioma. The IHC results of the
DMBA-induced mammary carcinomas in heterozygous Trp53
knockout mice showed a high frequency of ERα-positive cells
(suggesting HR-positive breast cancer) and N positivity for pERK,
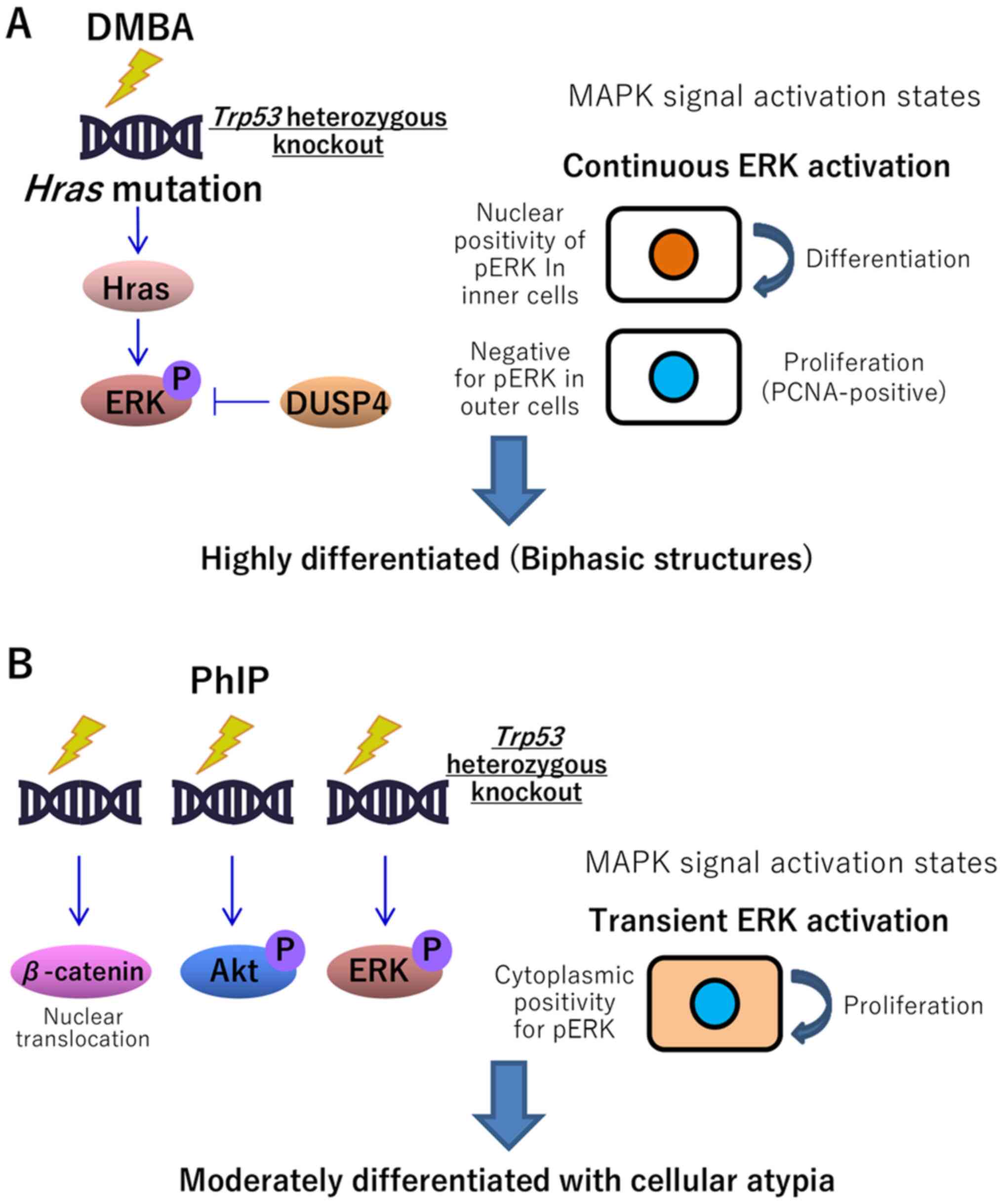
indicating the continuous stimulation of the MAPK signaling pathway
(51). The continuous stimulation of
MAPK leads to cellular differentiation, while transient stimulation
induces cellular proliferation (Fig.
5) (52). In the present
DMBA-induced carcinomas, PCNA was positively expressed primarily in
pERK-negative cells, which was consistent with a previous report
(52). In a previous cDNA microarray
analysis of DMBA-induced mammary carcinomas in female
Sprague-Dawley rats, the MAPK-modulation gene DUSP, which functions
in the dephosphorylation and/or sequestration of ERK, JNK and p38
kinases (53), was the predominantly
expressed clone (54). The present
RT-qPCR analysis revealed that the expression level of Dusp4
was high in DMBA-induced mammary carcinomas, suggesting that the
activation of the MAPK signaling pathway is negatively regulated by
increased Dusp4 expression. As for the mutations in
Pik3ca exons 6, 7, 9 and 20, no mutation was found in the
DMBA-induced mammary carcinomas in heterozygous BALB/c Trp53
knockout mice. Abba et al (10) demonstrated that Pik3ca
mutations at a hot spot in human breast carcinomas were also
detected in mammary carcinomas induced in CDF1 mice during
long-latency periods following DMBA administration, but not in
mammary carcinomas induced in the same mouse strain after a
short-latency period following progestin medroxyprogesterone
acetate plus DMBA administration.
Based on the IHC findings of mammary carcinomas in
the present DMBA-treated heterozygous BALB/c Trp53 knockout
mice, the incidence of ERα-positive carcinomas appeared to be
increased, compared with that in spontaneous mammary carcinomas
(Fig. S2) (24), and no obvious differences were
observed in the positivity of pERK, β-catenin and pAKT. On the
other hand, in PhIP-treated heterozygous BALB/c Trp53
knockout mice, multiple molecular mechanisms for carcinogenesis
(Fig. 5), including the activation
of the Wnt/β-catenin, PI3K/AKT and MAPK signaling pathways, e.g., N
positivity for β-catenin, MC positivity for pAKT, and CN positivity
for pERK, respectively, were exhibited in comparison with those in
spontaneous mammary carcinomas (Fig.
S2) (24). However, no mutations
were found in Pik3ca exons 6, 7, 9 and 20, or in Hras exon
2, codon 61. This may have been due to the fact that pAKT, a
typical marker of PI3K/AKT pathway activation, was not increased in
Pik3ca-mutated luminal A subtype cancer. It was instead
highly prevalent in the basal-like (HR-negative, HER2-negative) and
HER2-enriched (HR-negative, HER2-positive) subtypes (36,55). To
the best of our knowledge, there are no reports suggesting an
association between the Wnt/β-catenin signaling pathway and mammary
carcinogenesis. In human breast cancer, no gene mutation associated
with the Wnt signaling pathway has been reported (36), but the epigenetic inactivation of Wnt
antagonist genes, such as secreted frizzled-related protein 1, has
been reported (56,57). In-depth evaluation to clarify the
underlying genetic mechanisms was not included in the present
study. Therefore, further investigation of the carcinoma tissues
(including genome-wide mutation sequencing or methylation
analysis), is required to clarify the molecular mechanisms
underlying the acceleration of mammary carcinogenesis, particularly
by PhIP administration.
In conclusion, the present mouse mammary
carcinogenesis models induced by a combination of genetic and
exogenous factors may be utilized (such as the DMBA-induced model
with Trp53 gene function deficiency as a model of
adenomyoepithelioma characterized by distinct biphasic cell
constituents and Hras mutations), but PhIP-induced models
are required to further analyze the other genetic/epigenetic
mechanisms promoting mammary carcinomas.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank the Animal Core
Facility of the National Cancer Center Research Institute for
maintaining the mice, and for their technical support in
histopathological evaluations. The Core Facility was supported by
the National Cancer Center Research and Development Fund
(2020-J-002). In addition, the authors would like to thank Ms. Sudo
Yukiko, Mr. Shun Ito, Ms. Ruri Nakanishi, Ms. Naoko Higashijima,
Mr. Naoaki Uchiya and Ms. Yurika Shiotani (Central Animal Division
National Cancer Center Research Institute, Tokyo, Japan), for their
technical assistance.
Funding
The present study was supported in part by a Health
and Labour Sciences Research Grant from the Ministry of Health,
Labour and Welfare of Japan (grant no. H30-Food-003), the National
Cancer Center Research and Development Fund (grant no. 2020-J-002),
and Grants-in-Aid for Scientific Research (JSPS KAKENHI, grant no.
JP19H01610).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YM carried out the experiments, contributed to the
analysis and interpretation of data, and drafted, read and approved
the final manuscript. TI contributed to developing the overall
strategies and concepts, and was responsible for the study design,
data analysis and manuscript finalization. YM and TI confirmed the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments and procedures were approved
by the National Cancer Center Animal Ethics Committee (approval no.
T17-028-C02).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fidler MM, Gupta S, Soerjomataram I,
Ferlay J, Steliarova-Foucher E and Bray F: Cancer incidence and
mortality among young adults aged 20–39 years worldwide in 2012: A
population-based study. Lancet Oncol. 18:1579–1589. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnson RH, Chien FL and Bleyer A:
Incidence of breast cancer with distant involvement among women in
the United States, 1976 to 2009. JAMA. 309:800–805. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeSantis CE, Ma J, Gaudet MM, Newman LA,
Miller KD, Goding Sauer A, Jemal A and Siegel RL: Breast cancer
statistics, 2019. CA Cancer J Clin. 69:438–451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kataoka A, Iwamoto T, Tokunaga E, Tomotaki
A, Kumamaru H, Miyata H, Niikura N, Kawai M, Anan K, Hayashi N, et
al: Young adult breast cancer patients have a poor prognosis
independent of prognostic clinicopathological factors: A study from
the Japanese Breast Cancer Registry. Breast Cancer Res Treat.
160:163–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Imai T, Cho YM, Takahashi M, Kitahashi T,
Takami S, Nishikawa A and Ogawa K: High susceptibility of
heterozygous (+/fa) lean Zucker rats to
7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis.
Oncol Rep. 29:1914–1922. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaga C, Takagi A, Kano M, Kado S, Kato I,
Sakai M, Miyazaki K, Nanno M, Ishikawa F, Ohashi Y, et al:
Lactobacillus casei Shirota enhances the preventive efficacy
of soymilk in chemically induced breast cancer. Cancer Sci.
104:1508–1514. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dabydeen SA and Furth PA: Genetically
engineered ERα-positive breast cancer mouse models. Endocr Relat
Cancer. 21:R195–R208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huggins C, Grand LC and Brillantes FP:
Mammary cancer induced by a single feeding of polymucular
hydrocarbons, and its suppression. Nature. 189:204–207. 1961.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Russo J, Gusterson BA, Rogers AE, Russo
IH, Wellings SR and van Zwieten MJ: Comparative study of human and
rat mammary tumorigenesis. Lab Invest. 62:244–278. 1990.PubMed/NCBI
|
|
10
|
Abba MC, Zhong Y, Lee J, Kil H, Lu Y,
Takata Y, Simper MS, Gaddis S, Shen J and Aldaz CM: DMBA induced
mouse mammary tumors display high incidence of activating
Pik3caH1047 and loss of function Pten mutations. Oncotarget.
7:64289–64299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rehm S: Chemically induced mammary gland
adenomyoepitheliomas and myoepithelial carcinomas of mice.
Immunohistochemical and ultrastructural features. Am J Pathol.
136:575–584. 1990.PubMed/NCBI
|
|
12
|
Hasegawa R, Sano M, Tamano S, Imaida K,
Shirai T, Nagao M, Sugimura T and Ito N: Dose-dependence of
2-amino-1-methyl-6-phenylimidazo[4,5-b]-pyridine (PhIP)
carcinogenicity in rats. Carcinogenesis. 14:2553–2557. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shirai T, Sano M, Tamano S, Takahashi S,
Hirose M, Futakuchi M, Hasegawa R, Imaida K, Matsumoto K,
Wakabayashi K, et al: The prostate: A target for carcinogenicity of
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) derived from
cooked foods. Cancer Res. 57:195–198. 1997.PubMed/NCBI
|
|
14
|
Nakagama H, Ochiai M, Ubagai T, Tajima R,
Fujiwara K, Sugimura T and Nagao M: A rat colon cancer model
induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, PhIP.
Mutat Res. 506-507:137–144. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghoshal A, Preisegger KH, Takayama S,
Thorgeirsson SS and Snyderwine EG: Induction of mammary tumors in
female Sprague-Dawley rats by the food-derived carcinogen
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and effect of
dietary fat. Carcinogenesis. 15:2429–2433. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ip C, Carter CA and Ip MM: Requirement of
essential fatty acid for mammary tumorigenesis in the rat. Cancer
Res. 45:1997–2001. 1985.PubMed/NCBI
|
|
17
|
Welsch CW: Relationship between dietary
fat and experimental mammary tumorigenesis: A review and critique.
Cancer Res. 52 (Suppl 7):2040s–2048s. 1992.PubMed/NCBI
|
|
18
|
Jiang Y, Pan Y, Rhea PR, Tan L, Gagea M,
Cohen L, Fischer SM and Yang P: A sucrose-enriched diet promotes
tumorigenesis in mammary gland in part through the 12-Lipoxygenase
pathway. Cancer Res. 76:24–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lo CY, Hsieh PH, Chen HF and Su HM: A
maternal high-fat diet during pregnancy in rats results in a
greater risk of carcinogen-induced mammary tumors in the female
offspring than exposure to a high-fat diet in postnatal life. Int J
Cancer. 125:767–773. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grassi TF, Bidinotto LT, Lopes GA,
Zapaterini JR, Rodrigues MA and Barbisan LF: Maternal western-style
diet enhances the effects of chemically-induced mammary tumors in
female rat offspring through transcriptome changes. Nutr Res.
61:41–52. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Macacu A, Autier P, Boniol M and Boyle P:
Active and passive smoking and risk of breast cancer: A
meta-analysis. Breast Cancer Res Treat. 154:213–224. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hecht SS: Tobacco smoke carcinogens and
breast cancer. Environ Mol Mutagen. 39:119–126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Engin A: Obesity-associated breast cancer:
Analysis of risk factors. Adv Exp Med Biol. 960:571–606. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Machida Y, Sudo Y, Uchiya N and Imai T:
Increased susceptibility to mammary carcinogenesis and an opposite
trend in endometrium in Trp53 heterozygous knockout female mice by
backcrossing the BALB/c strain onto the background C3H strain. J
Toxicol Pathol. 32:197–203. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan H, Blackburn AC, McLary SC, Tao L,
Roberts AL, Xavier EA, Dickinson ES, Seo JH, Arenas RB, Otis CN, et
al: Pathways contributing to development of spontaneous mammary
tumors in BALB/c-Trp53+/− mice. Am J Pathol.
176:1421–1432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsukada T, Tomooka Y, Takai S, Ueda Y,
Nishikawa S, Yagi T, Tokunaga T, Takeda N, Suda Y, Abe S, et al:
Enhanced proliferative potential in culture of cells from
p53-deficient mice. Oncogene. 8:3313–3322. 1993.PubMed/NCBI
|
|
27
|
Ueda M, Imai T, Takizawa T, Onodera H,
Mitsumori K, Matsui T and Hirose M: Possible enhancing effects of
atrazine on growth of 7,12-dimethylbenz(a) anthracene-induced
mammary tumors in ovariectomized Sprague-Dawley rats. Cancer Sci.
96:19–25. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
el-Bayoumy K, Chae YH, Upadhyaya P,
Rivenson A, Kurtzke C, Reddy B and Hecht SS: Comparative
tumorigenicity of benzo[a]pyrene, 1-nitropyrene and
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine administered by
gavage to female CD rats. Carcinogenesis. 16:431–434. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Snyderwine EG, Thorgeirsson UP, Venugopal
M and Roberts-Thomson SJ: Mammary gland carcinogenicity of
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in Sprague-Dawley
rats on high- and low-fat diets. Nutr Cancer. 31:160–167. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Imaoka T, Nishimura M, Doi K, Tani S,
Ishikawa K, Yamashita S, Ushijima T, Imai T and Shimada Y:
Molecular characterization of cancer reveals interactions between
ionizing radiation and chemicals on rat mammary carcinogenesis. Int
J Cancer. 134:1529–1538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Workman P, Aboagye EO, Balkwill F, Balmain
A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham DA,
Glennie MJ, et al Committee of the National Cancer Research
Institute, : Guidelines for the welfare and use of animals in
cancer research. Br J Cancer. 102:1555–1577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cardiff RD, Anver MR, Gusterson BA,
Hennighausen L, Jensen RA, Merino MJ, Rehm S, Russo J, Tavassoli
FA, Wakefield LM, et al: The mammary pathology of genetically
engineered mice: The consensus report and recommendations from the
Annapolis meeting. Oncogene. 19:968–988. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rudmann D, Cardiff R, Chouinard L, Goodman
D, Küttler K, Marxfeld H, Molinolo A, Treumann S and Yoshizawa K;
INHAND Mammary, Zymbal's, Preputial, and Clitoral Gland Organ
Working Group, : Proliferative and nonproliferative lesions of the
rat and mouse mammary, Zymbal's, preputial, and clitoral glands.
Toxicol Pathol. 40 (Suppl 6):7S–39S. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kito K, Kihana T, Sugita A, Murao S, Akehi
S, Sato M, Tachibana M, Kimura S and Ueda N: Incidence of p53 and
Ha-ras gene mutations in chemically induced rat mammary carcinomas.
Mol Carcinog. 17:78–83. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
El-Sohemy A and Archer MC: Inhibition of
N-methyl-N-nitrosourea- and 7,12-dimethylbenz[a] anthracene-induced
rat mammary tumorigenesis by dietary cholesterol is independent of
Ha-Ras mutations. Carcinogenesis. 21:827–831. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cancer Genome Atlas Network, .
Comprehensive molecular portraits of human breast tumours. Nature.
490:61–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kumar R, Medina D and Sukumar S:
Activation of H-ras oncogenes in preneoplastic mouse mammary
tissues. Oncogene. 5:1271–1277. 1990.PubMed/NCBI
|
|
40
|
Jerry DJ, Butel JS, Donehower LA, Paulson
EJ, Cochran C, Wiseman RW and Medina D: Infrequent p53 mutations in
7,12-dimethylbenz[a]anthracene-induced mammary tumors in BALB/c and
p53 hemizygous mice. Mol Carcinog. 9:175–183. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Venkatachalam S, Tyner SD, Pickering CR,
Boley S, Recio L, French JE and Donehower LA: Is p53
haploinsufficient for tumor suppression? Implications for the
p53+/− mouse model in carcinogenicity testing. Toxicol
Pathol. 29 (Suppl 1):147–154. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hollstein M and Hainaut P: Massively
regulated genes: The example of TP53. J Pathol. 220:164–173. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nagao M, Ushijima T, Watanabe N, Okochi E,
Ochiai M, Nakagama H and Sugimura T: Studies on mammary
carcinogenesis induced by a heterocyclic amine,
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, in mice and rats.
Environ Mol Mutagen. 39:158–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tan PH and Ellis IO: Myoepithelial and
epithelial-myoepithelial, mesenchymal and fibroepithelial breast
lesions: Updates from the WHO Classification of Tumours of the
Breast 2012. J Clin Pathol. 66:465–470. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
McLaren BK, Smith J, Schuyler PA, Dupont
WD and Page DL: Adenomyoepithelioma: Clinical, histologic, and
immunohistologic evaluation of a series of related lesions. Am J
Surg Pathol. 29:1294–1299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cardiff RD, Gumerlock PH, Soong MM,
Dandekar S, Barry PA, Young LJ and Meyers FJ: c-H-ras-1 expression
in 7,12-dimethyl benzanthracene-induced Balb/c mouse mammary
hyperplasias and their tumors. Oncogene. 3:205–213. 1988.PubMed/NCBI
|
|
47
|
Watanabe H, Ishibashi K, Mano H, Kitamoto
S, Sato N, Hoshiba K, Kato M, Matsuzawa F, Takeuchi Y, Shirai T, et
al: Mutant p53-expressing cells undergo necroptosis via cell
competition with the neighboring normal epithelial cells. Cell Rep.
23:3721–3729. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ramirez-Ardila D, Timmermans AM, Helmijr
JA, Martens JW, Berns EM and Jansen MP: Increased MAPK1/3
phosphorylation in luminal breast cancer related with PIK3CA
hotspot mutations and prognosis. Transl Oncol. 10:854–866. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Geyer FC, Li A, Papanastasiou AD, Smith A,
Selenica P, Burke KA, Edelweiss M, Wen HC, Piscuoglio S, Schultheis
AM, et al: Recurrent hotspot mutations in HRAS Q61 and PI3K-AKT
pathway genes as drivers of breast adenomyoepitheliomas. Nat
Commun. 9:18162018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ginter PS, McIntire PJ, Kurtis B,
Mirabelli S, Motanagh S, Hoda S, Elemento O, Shin SJ and Mosquera
JM: Adenomyoepithelial tumors of the breast: Molecular
underpinnings of a rare entity. Mod Pathol. 33:1764–1772. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kato S, Endoh H, Masuhiro Y, Kitamoto T,
Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H,
et al: Activation of the estrogen receptor through phosphorylation
by mitogen-activated protein kinase. Science. 270:1491–1494. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Marshall CJ: Specificity of receptor
tyrosine kinase signaling: Transient versus sustained extracellular
signal-regulated kinase activation. Cell. 80:179–185. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Owens DM and Keyse SM: Differential
regulation of MAP kinase signalling by dual-specificity protein
phosphatases. Oncogene. 26:3203–3213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shan L, He M, Yu M, Qiu C, Lee NH, Liu ET
and Snyderwine EG: cDNA microarray profiling of rat mammary gland
carcinomas induced by
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and
7,12-dimethylbenz[a]anthracene. Carcinogenesis. 23:1561–1568. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sakr RA, Weigelt B, Chandarlapaty S,
Andrade VP, Guerini-Rocco E, Giri D, Ng CK, Cowell CF, Rosen N,
Reis-Filho JS, et al: PI3K pathway activation in high-grade ductal
carcinoma in situ - implications for progression to invasive breast
carcinoma. Clin Cancer Res. 20:2326–2337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Veeck J, Niederacher D, An H, Klopocki E,
Wiesmann F, Betz B, Galm O, Camara O, Dürst M, Kristiansen G, et
al: Aberrant methylation of the Wnt antagonist SFRP1 in breast
cancer is associated with unfavourable prognosis. Oncogene.
25:3479–3488. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Suzuki H, Toyota M, Carraway H, Gabrielson
E, Ohmura T, Fujikane T, Nishikawa N, Sogabe Y, Nojima M, Sonoda T,
et al: Frequent epigenetic inactivation of Wnt antagonist genes in
breast cancer. Br J Cancer. 98:1147–1156. 2008. View Article : Google Scholar : PubMed/NCBI
|