Introduction
Osteosarcoma is a common primary bone malignancy
(1). Originating from mesenchymal
tissue, osteosarcoma is the most prevalent bone tumor in childhood
and adolescence and has high malignancy (1). The incidence of osteosarcoma is high,
with a sex ratio of 3:2 (male to female), and the 5-year survival
rate is only 20–30% in patients undergoing surgical treatment alone
(2). Although chemotherapy extends
the 5-year survival rate to 60–80%, ~50% of patients with
osteosarcoma do not respond to chemotherapy (1). Locally, osteosarcoma exhibits
aggressive growth and is prone to recurrence (3). Hematogenous metastasis to the lungs is
the most common type of osteosarcoma metastasis, and the main cause
of mortality is pulmonary metastasis (4). Early clinical symptoms primarily
include local pain or swelling, related joint dysfunction, and in a
small number of cases, pathological fractures (5). The majority of patients present with
lung or systemic metastasis, low survival rate, and high mortality,
and highly malignant osteosarcoma (unpublished data). However, the
etiology and pathogenesis of osteosarcoma remain unclear (6). Clinically, it is important to improve
the survival outcomes of patients with osteosarcoma and maintain
limb function as much as possible to avoid the psychological trauma
of amputation. Thus, it is critical to identify novel methods for
diagnosing and treating osteosarcoma.
The growth and metastasis of a tumor are dependent
on the formation of new blood vessels, and this process is under
the joint control of oncogenic and tumor-suppressive factors of
angiogenesis (7). Vascular
endothelial growth factor (VEGF) is the most important stimulatory
factor of tumor angiogenesis (8,9). Thus,
its concentration can reflect the formation, progression and
regression of tumors. Tumor growth and metastasis are rapid
(7). During the transformation of a
tumor cell mass into a solid tumor, tumor cells produce a large
amount of VEGF to promote angiogenesis. At this point, the tumor is
usually at an early stage, which is the best time for tumor
screening, and can be diagnosed by existing clinical methods
(8). Early screening can improve
patient survival rate and prolong survival (9). A change in an angiogenesis trend is
accompanied by a change in VEGF levels (8). Thus, VEGF can be employed for the
screening of patients for almost any solid tumors, and its broad
spectrum of specificity cannot be matched by other tumor indicators
(10). In the clinic, several
malignant tumors lack specific tumor markers, whereas VEGF has high
sensitivity and broad specificity (11). An anomalous serum VEGF concentration
can indicate the risk of a solid tumor (12). In addition, monitoring the level of
VEGF regularly can help to determine the stage of tumor progression
and to make an auxiliary judgment on the prognosis of the tumor
(12). Thus, the higher the VEGF
level, the higher the tumor malignancy and the worse the prognosis
within the same tumor type. Generally, VEGF can be found at an
early stage of cancer, and the detection of cancer at this stage
greatly affects the survival rate and survival time of patients
(12). VEGF is a broad-spectrum
tumor marker suitable for the screening of several tumors (12) and can be combined with other tumor
markers, thereby effectively improving the accuracy of cancer
screening. The sensitivity of VEGF for tumor detection is high
(better than that of traditional tumor markers), and the limit of
detection of this assay is at the picogram level (12).
Photothermal therapy (PTT), an effective
non-invasive treatment for different types of diseases, has been
extensively investigated as a cancer treatment due to its unique
low invasiveness, relatively simple administration and convenient
direct heating in tumors (13–15).
Several types of photosensitizers that strongly absorb
near-infrared (NIR) light have been used as imaging agents
(16). An example is indocyanine
green (ICG), which is the only substance approved by the U.S. Food
and Drug Administration (FDA) for NIR imaging that has been used
clinically for retinal imaging and for evaluation of liver
function, as well as for guiding biopsies (17,18).
However, ICG has low photothermal conversion efficiency (16). Thus, there is an urgent requirement
for materials with better photothermal conversion to replace ICG.
Among the relevant nanoparticles (NPs), gold NPs (AuNPs) have been
investigated due to their favorable biocompatibility and ability to
convert NIR light into local heat (19). A combination of PTT and chemotherapy
should greatly improve the clinical outcomes of osteosarcoma. The
present study conjugated doxorubicin (DOX) with AuNPs to achieve
combined photothermal and chemotherapeutic anticancer effects to
inhibit the growth of the primary tumor and to suppress its
metastasis.
Multifunctional core-shell nanostructures as a drug
carrier were recently created to overcome the limitations of tumor
imaging and therapy (20–23). Inorganic nanocrystals, such as those
of Au and Ag, possess excellent heat conduction properties
(19,24). In the process of ablation, the
generated heat is rapidly transferred through inorganic crystals
(AuNPs), which improve the heat transfer efficiency and contribute
to the goal of killing tumor cells (23). Thus, there is an urgent requirement
for in-depth investigation of suitability of AuNPs and other NPs
for cancer cell imaging and therapy (25,26).
Furthermore, researchers have proposed targeting nanocarriers that
can be used for improving the stability of NPs or drugs and for
targeted imaging (27,28), such as mesoporous silica, owing to
its large surface area, large accessible pore volume and
well-defined surface properties (28). As a shell (SiO2 or
mSiO2), this substance may be widely used as a drug
carrier to protect inorganic nanocrystals from aggregating
(29–31). Thus, the present study investigated
Au@SiO2 as a core-shell nanocarrier for combined PTT and
chemotherapy of osteosarcoma.
Given their unique applications in cancer imaging
and therapy (32,33), a series of nanomaterials have been
successfully developed, including polymeric NPs, quantum dots, and
graphene, gold and magnetic nanomaterials. AuNPs possess several
attractive features, such as low toxicity, biocompatibility, high
chemical stability, and sonocatalytic properties (32,33).
Core-shell nanostructures are an important way to protect NPs from
aggregation (23). Therefore,
mesoporous silica-coated Au nanorods (AuNP@SiO2) have
received much attention in the fields of PTT (34) and sonodynamic cancer therapy
(35,36). However, there have been no reports on
the application of AuNP@SiO2 in osteosarcoma animal
models for both imaging and therapy; such a study would be a
critical step towards understanding how these materials behave
in vivo for further clinical applications against
osteosarcoma. Thus, the present study designed a probe composed of
SiO2-coated AuNPs incorporating ICG or DOX
(Au@SiO2_ICG or Au@SiO2_DOX).
The present study investigated a multifunctional
core-shell nanostructure composed of ICG/DOX-loaded SiO2
(Au@SiO2-drug/VEGF) for diagnosing osteosarcoma and for
combined PTT and cytotoxic chemotherapy.
Au@SiO2-ICG/VEGF was used to determine tumor
localization via fluorescence imaging (NIR) technology.
Au@SiO2-DOX/VEGF was used for the combined PTT and
chemotherapy. The results demonstrated that targeted combination
therapy is more effective against osteosarcoma compared with DOX
chemotherapy alone. This technology can substantially contribute to
the development and understanding of novel techniques for the
detection of malignant tumors.
Materials and methods
Materials
Au@SiO2 NPs were purchased from Hangzhou
Xinqiao Biotechnology Co., Ltd., whereas ICG and DOX were purchased
from the Alfa Aesar Company. DMEM, fetal bovine serum (FBS),
penicillin-streptomycin, trypsin-EDTA and Hoechst were purchased
from Gibco; Thermo Fisher Scientific, Inc. All other reagents used
in the present study were purchased from Sinopharm Group Co., Ltd.
and were of certified analytical reagent grade.
Synthesis of Au@SiO2-drug
and Au@SiO2-drug/VEGF NPs
First, 0.5 ml of Au@SiO2 solution was
added into a flask and NaOH solution (100 µl; 0.1 M) was
subsequently added. Tetraethyl orthosilicate (30 µl; 20% in
methanol) was added at 30 min intervals. Either ICG (200 µl; 5
mg/ml) or DOX (200 µl; 5 mg/ml) were added to the solution. After
30 min, the final dose of tetraethyl orthosilicate was added and
the mixture was subjected to a lucifugal reaction, which was
allowed to proceed for 2 days in the dark at room temperature. The
Au@SiO2-drug nanostructure was separated via centrifugation at
335.4 × g at room temperature and by decantation after the
reaction. Finally, 10 mg/ml NH2-polyethylene glycol
(PEG)-VEGF (>3500 Da) was added to modify the surface of the
NPs. The product, Au@SiO2-drug/VEGF NPs, was stored at
4°C.
Characterization
Transmission electron microscopy (TEM, HITACHI
JEM-1011; Hitachi, Ltd.) and scanning electron microscopy (SEM,
EM-30AX; COXEM Co., Ltd.) were performed to evaluate the morphology
and size of Au@SiO2-ICG NPs (in an aqueous dispersion)
at 120 kV acceleration voltage. The hydrodynamic particle size
distribution was determined on a Malvern Zetasizer (ZEN 3600;
Malvern Instruments, Ltd.). Absorbance spectra of ICG and
Au@SiO2-ICG were recorded via ultraviolet-visible
spectroscopy (UV-2450; Shimadzu Corporation), and fluorescence
spectra were acquired on a fluorescence spectrofluorometer (F-7000;
Hitachi, Ltd.), with excitation at wavelengths of 780 and 808 nm,
respectively.
Photothermal effects in vitro
Aqueous solutions (1 ml) of free ICG (1 mg/ml),
Au@SiO2 (4 mg/ml) and Au@SiO2-ICG (4 mg of
Au@SiO2 per 1 mg of ICG) were irradiated with an 808 nm
continuous laser at a power density of 8 W/cm2 for 7
min. The temperature was registered every minute by means of a
Fluke infrared thermal imager (TI400; FLUKE). Each assay was
repeated three times. As a control, 1 ml of PBS was irradiated to
record the temperature at the same laser settings.
Cell culture
The human osteosarcoma cell line, MG63-Luc (Procell
Life Science & Technology Co., Ltd.) was used for both in
vitro and in vivo experiments. MG63-Luc cells express
green fluorescent protein and firefly D-luciferin, and are
compatible with a standard transfection protocol. Cells were
cultured in Minimum Essential Medium supplemented with 10% FBS and
1% penicillin/streptomycin in a CO2 incubator (Heracell)
at 37°C (36), according to the
manufacturer's recommendations.
Cytotoxicity assessment
The cytotoxicity of Au@SiO2-ICG and
Au@SiO2-ICG/VEGF NPs was assessed via the MTT assay, in
the dark. MG63-Luc cells were seeded into 96-well plates at a
density of 1×104 cells/well and cultured for 24 h at
37°C. Cells were incubated with different concentrations of the
probe. Concentrations of Au@SiO2-ICG and
Au@SiO2-ICG/VEGF NPs in 0, 6.25, 12.5, 25, 50, 100, 200,
300 and 400 µg/ml were assessed. The viability of the treated cells
was determined via the MTT assay. Briefly, 10 µl of the MTT reagent
(5 mg/ml) was added into each well and incubated for 4 h. DMSO was
subsequently added to dissolve the formazan crystals and absorbance
was measured at 570 nm wavelength using a microplate absorbance
reader (Bio-Rad iMARK™; Bio-Red Laboratories, Inc.).
Animal experiments
A total of 20 BALB/c nude male mice (athymic,
5-weeks-old, ~20 g) were provided by Beijing Vital River Laboratory
Animal Technology Co., Ltd. All animal experiments were approved by
the Institutional Animal Care and Use Committee of the Capital
Medical University (Beijing, China; approval no. CMU097230). Animal
health and behavior were monitored every 2 days. The following
housing conditions were applied: Temperature, 20°C; humidity, 55%;
air exchange frequency, 15/h; bedding was cleaned every 3 days;
light/dark cycle, 12/12 h; mice had free access to food and water.
A suspension of ~1×106 MG63-Luc cancer cells in 100 µl
of phosphate buffer (0.01 mol/l; pH 7.2) was subcutaneously
injected into the axillary fossa of each mouse to establish the
subcutaneous tumor model. The tumors were allowed to grow for 2–3
weeks until a signal of a 0.8 cm diameter was detectable via in
vivo Imaging System (IVIS). The tumor-bearing mice were
injected with a probe (200 µl) for in vivo detection (n=5
for each imaging probe or dye). Each mouse was anesthetized with 2%
isoflurane prior to IVIS imaging. Cervical dislocation was applied
to all mice for euthanasia 25 days post-probe injection. The
duration of the animal experiment (from injection to euthanasia)
was ~50 days.
NIR fluorescence imaging in vivo
For in vivo fluorescence imaging, six
subcutaneous osteosarcoma tumors were selected when the diameter of
each tumor reached 5–6 mm. The mice were randomly divided into two
groups (ICG and Au@SiO2-ICG; n=3/group). Each mouse was
anesthetized with 2% isoflurane. A solution of either free ICG or
Au@SiO2-ICG in 10 mM phosphate buffer pH 7.2 (150 µl)
was injected into mice via the tail vein. The region of interest
was examined after 10 min and 24, 48, 72 and 96 h using an IVIS
Spectrum imaging system (PerkinElmer, Inc.), with an excitation
wavelength of 745 nm and an emission wavelength of 840 nm. The data
were analyzed using IVIS Living Imaging 3.0 software (PerkinElmer,
Inc.).
Photothermal therapy in vivo
Mice bearing a tumor were injected with
Au@SiO2/VEGF in the tail vein and irradiated for 12 min
post-injection under laser. Probe concentration was 2, 4, 6, 8
mg/ml under 1.4 W and 0, 1, 2, 4 mg/ml under 2 W. The
irradiation-induced temperature rise was recorded by a Fluke
infrared thermal imager.
Tumor xenografts and antitumor
therapy
To determine the most appropriate laser power and
probe concentration, the subcutaneous-tumor model mice were divided
into different groups, receiving either PBS or different
concentrations of probe prior to 1.4/2 W laser power PTT. Each
mouse was anesthetized with 2% isoflurane prior to PTT. The laser
irradiation time was limited to 5 min to avoid possible tissue
damage by hyperthermia. The temperature and photothermal images of
the tumor surface during the laser irradiation were recorded every
minute via the infrared thermal imaging system. Once the optimal
laser power and probe concentration were confirmed (1.4 W with 4
mg/ml), tumor bearing mice were treated with DOX (24 mg/kg) + 808
nm laser or Au@SiO2-DOX/VEGF (injection volume, 200 µl
and probe concentration, 4 mg/ml) + 808 nm laser to assess the
effect of PTT combined with chemotherapy. Tumor size was measured
using a vernier caliper, and body weight was measured every 5 days
using an electronic balance. On day 25, the mice model was assessed
using a small animal optical molecular imaging system (IVIS
Spectrum imaging system; PerkinElmer, Inc.) and analyzed using IVIS
Living Imaging 3.0 software (PerkinElmer, Inc.). The mice received
80 µl of D-Luciferin solution (15 mg/ml) via intraperitoneal
injection 8 min before bioluminescence imaging was performed.
During imaging, the mice were anesthetized with 2% isoflurane and
placed in the supine position. The parameters for the
bioluminescence imaging system were as follows: Binning 4 and
exposure time 1 sec.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 7.05 software (GraphPad Software, Inc.). All experiments were
performed in triplicate and data are presented as the mean ± SD.
One-way ANOVA was used to compare differences between two groups.
P<0.05 was considered to indicate a statistically significant
difference by t-test.
Results
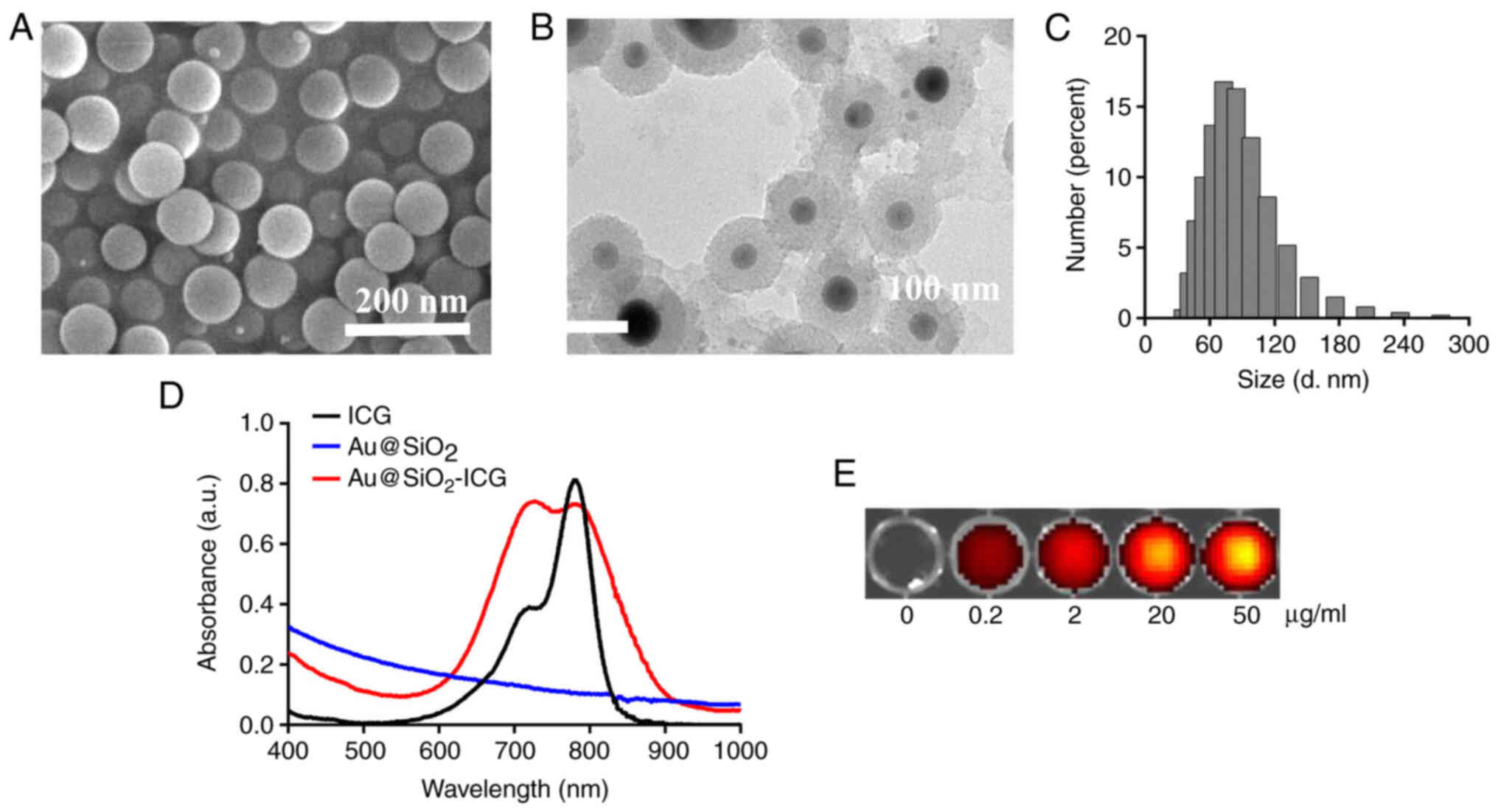
Characterization of
Au@SiO2-drug/VEGF
Characterization was performed via SEM and size
measurement. SEM analysis demonstrated that Au@SiO2-drug/VEGF NPs
were successfully synthesized. Au@SiO2-drug/VEGF NPs had a good
shape and a uniform size of ~90 nm (Fig.
1A). TEM analysis demonstrated homogeneous microstructure of
the Au@SiO2-drug/VEGF NPs, consistently with the SEM
results (Fig. 1B). Hydrated-particle
size was determined to verify the successful preparation of
Au@SiO2-drug/VEGF NPs. The size distribution was mainly
in the range of 60–120 nm, and the average particle size of the
bubbles was ~90 nm (Fig. 1C).
Fig. 1D conveys that the absorption
peak of free ICG was ~780 nm, while SiO2 had no
absorption peak. Notably, SiO2 conjugated with ICG
yielded two absorption peaks, at 700 and 780 nm. Furthermore,
fluorescence intensity of the peak at 700 nm was stronger than that
of free ICG, suggesting that Au@SiO2-ICG exhibits better
optical performance compared with free ICG did. Qualitative
analyses of Au@SiO2-ICG indicated that the fluorescence
intensity increased in a dose-dependent manner (Fig. 1E).
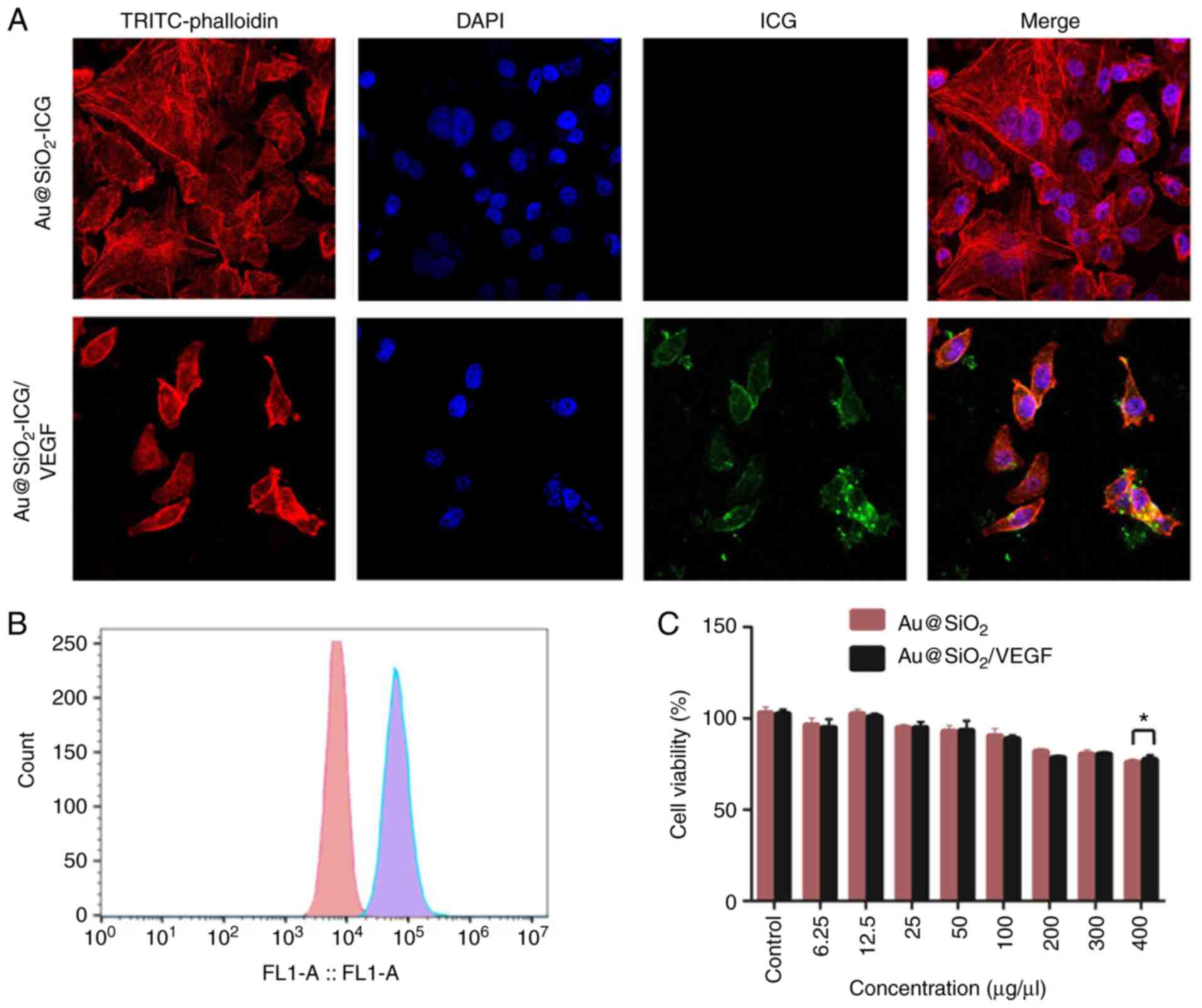
Au@SiO2-drug/VEGF in vivo
biodistribution and the potential underlying mechanism
The expected cell-targeting mechanism was supported
by the observed endocytosis of different NPs
(Au@SiO2-ICG/VEGF and Au@SiO2-ICG) in the
cancer cell line. This was confirmed via confocal fluorescence
microscopy of MG63-Luc cells (Fig.
2A). There was an obvious difference in uptake between
Au@SiO2-ICG/VEGF and Au@SiO2-ICG NPs
(Fig. 2A). In the band of ICG, there
were endocytosed Au@SiO2-ICG/VEGF NPs and staining of
the plasma membrane of MG63 cells. Furthermore, through
quantification of cellular endocytosis of
Au@SiO2-ICG/VEGF and Au@SiO2-ICG (200 mg/ml)
via flow cytometry, NP uptake efficiency was determined in MG63
cells. A distinct change in the fluorescence intensity of
Au@SiO2-ICG/VEGF-fed MG63 cells in the fla-1 channel
(563 nm) was observed (Fig. 2B). The
results demonstrated that the VEGF protein had high efficiency of
cancer cell targeting. To confirm that Au@SiO2-ICG/VEGF
and Au@SiO2-ICG are biocompatible agents,
biodistribution and toxicology assays at different concentrations
(0, 6.25, 12.5, 25, 50, 100, 200, 300 and 400 µg/µl) were performed
with irradiation via the MTT assay (Fig.
2C). The assays revealed notable decreases in cell viability at
concentrations ranging from 0–400 µg/ml. Without a marked
difference, Au@SiO2-ICG/VEGF and Au@SiO2-ICG
yielded the same cell viability trends as ICG, which is approved by
the FDA. Thus, Au@SiO2-ICG/VEGF NPs appear to be a
biocompatible and nontoxic agent for in vivo imaging
use.
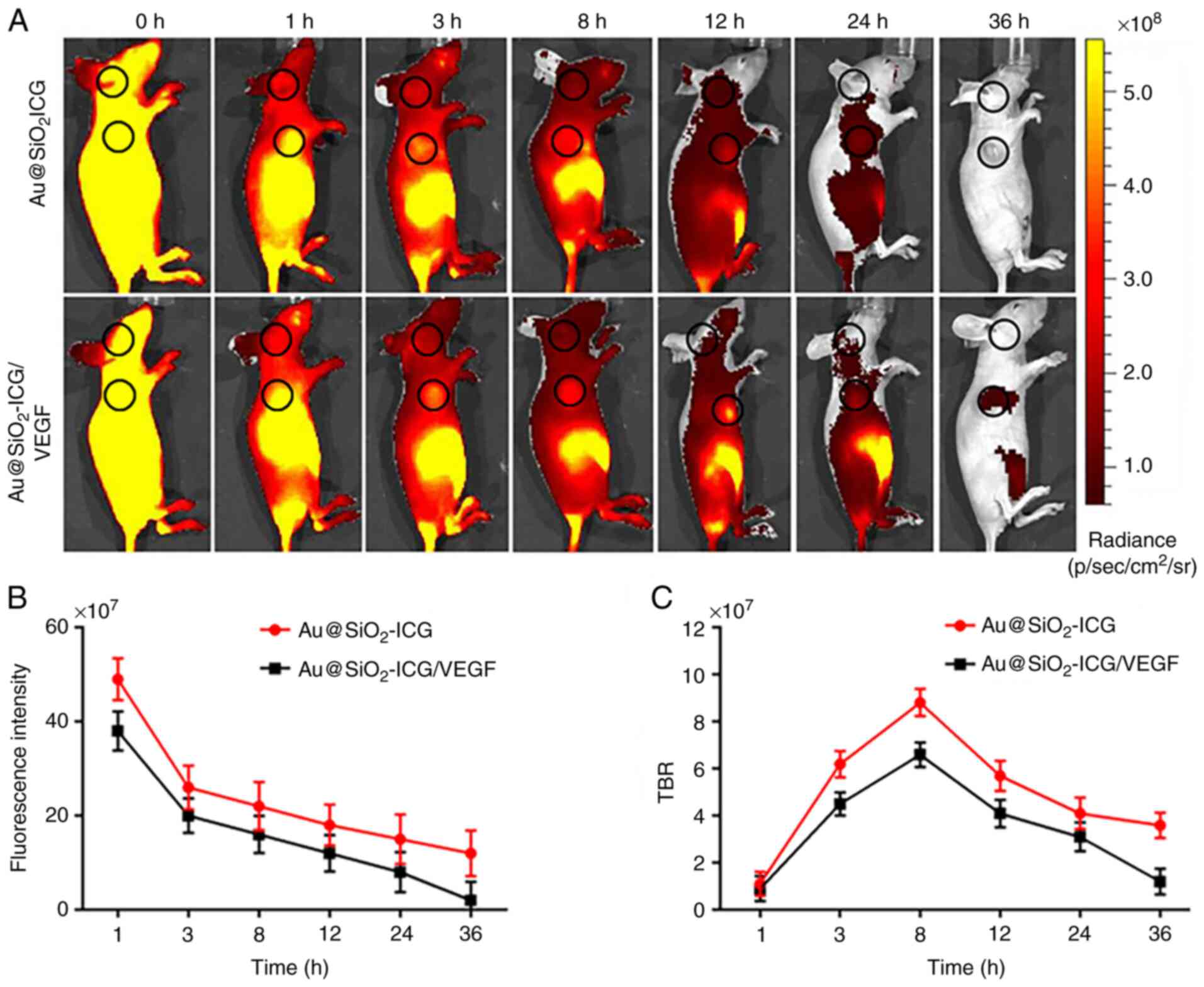
In vivo metabolism of the probe
Fluorescence imaging represents a non-invasive
highly sensitive tissue distribution assay in real-time (18). Our method of fluorescence imaging
(37) enabled highly sensitive
real-time imaging of osteosarcoma to assess the metabolism of
Au@SiO2-ICG/VEGF and its accumulation by the
subcutaneous tumor. The mice carried subcutaneous osteosarcoma
tumors derived from MG63 cells. The Au@SiO2-ICG/VEGF
probe generated a strong fluorescence signal in the tumor region
(Fig. 3) according to the IVIS
Spectrum imaging system (PerkinElmer, Inc.), and the data were
analyzed using IVIS Living Imaging 3.0 software (PerkinElmer,
Inc.). Following fluorescence imaging in vivo, the mice were
analyzed for fluorescence at 24 h post injection, and both prone
and supine views of the NIR data were obtained (Fig. 3A). As presented in Fig. 3A, it is easy to see that the probe
accumulated at the site of the subcutaneous tumor and outlined the
tumor boundary. The probe was found to be metabolized by the liver
and kidneys for 18 h. In addition, the present study quantitated
the probe distribution in vivo in the whole-body tissue
distribution analysis presented in Fig.
3A. The Au@SiO2_ICG probe, which was found to be
distributed uniformly throughout the whole body initially, was
slowly metabolized over time (Fig.
3B). The time point corresponding to the optimal tumor
accumulation of Au@SiO2-ICG/VEGF in vivo was at 8
h, according to the tumor/background signal ratio (Fig. 3C).
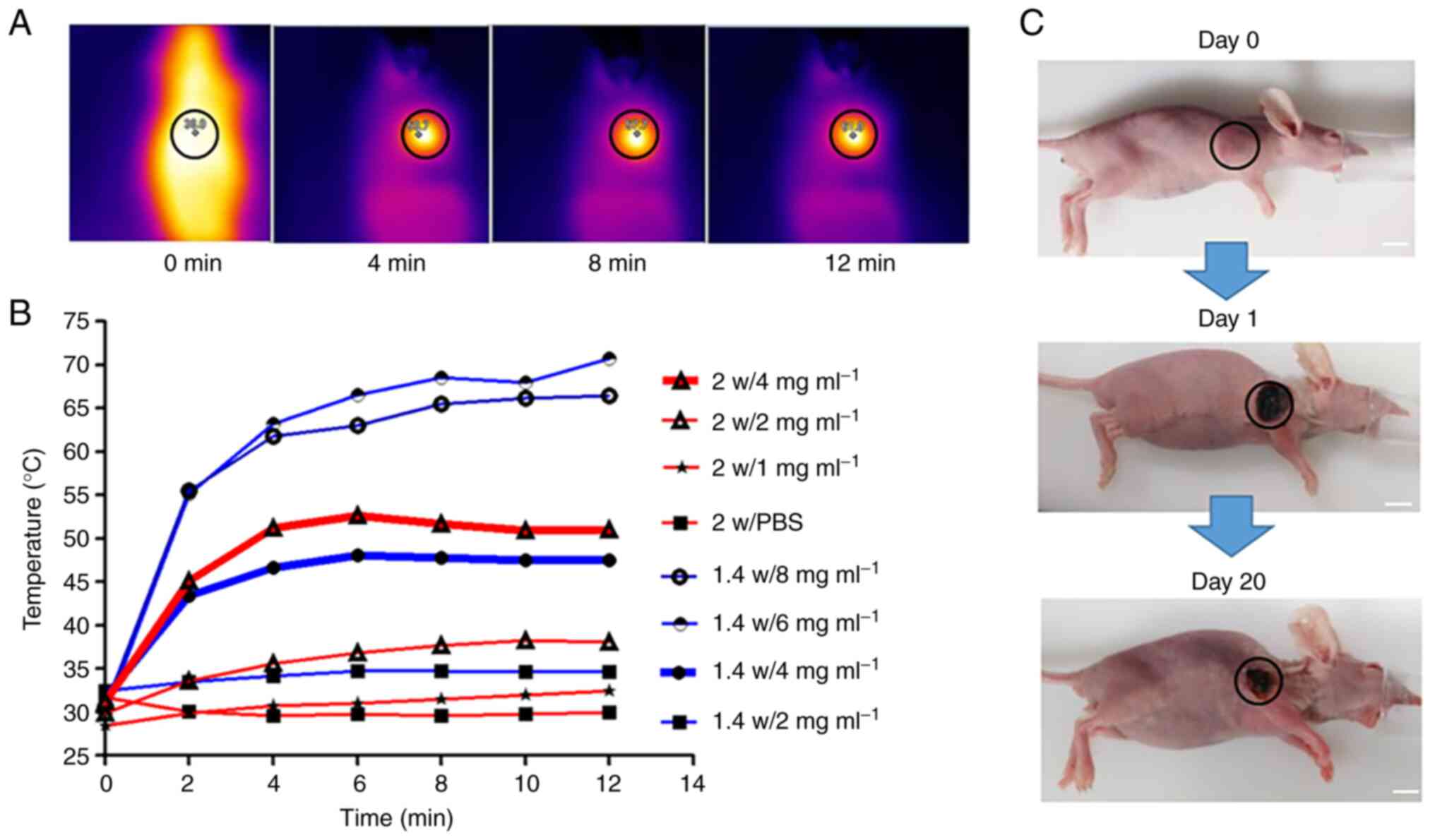
Probe based PTT of osteosarcoma
To investigate the most appropriate laser power and
probe concentration of Au@SiO2/VEGF-based PTT in
vivo, the photothermal effect of Au@SiO2/VEGF was
monitored using an infrared thermal imaging camera post 1.4/2 W
laser power irradiation, with different probe concentrations. As
the laser power increases, the temperature curve should also
increase more rapidly, thus the concentration range assessed under
2 W is narrow compared with 1.4 W. The laser irradiation time was
limited to 5 min to avoid possible tissue damage by hyperthermia.
The mice were conscious, and the healthy epidermis was not burned
during the laser irradiation. The temperature and photothermal
images of the tumor surface during the laser irradiation were
recorded every minute by the infrared thermal imaging system
(Fig. 4A), and tumor temperature
gradually increased with the time of laser radiation as recorded by
thermal camera. A notable increase in tumor temperature was
observed in Au@SiO2/VEGF-injected mice, whereas the
tumor temperature increase was minimal in mice injected with PBS
(Fig. 4B). In addition, the
temperature was higher and increased more rapidly following
treatment with greater laser power and higher probe concentration.
Furthermore, the temperature was higher as the probe concentration
increased under the same laser power. A similar trend was observed
when the laser power increased under the same probe concentration
(Fig. 4B). According to a previous
study (13), tumor cells are
effectively eliminated at 42°C, thus the present study selected 1.4
W laser power with 4 mg/ml probe concentration for subsequent
experimentation. Images of mice treated with 1.4 W laser power and
4 mg/ml probe concentration were taken on days 0, 1 and 20. Tumor
shrinkage was observed; however, tumor residual remained, thus a
more effective strategy against osteosarcoma, such as combination
therapy, is required.
Combined PTT and cytotoxic
chemotherapy of osteosarcoma
Dual anticancer effects of
Au@SiO2-DOX/VEGF were assessed in tumor-bearing nude
mice. Athymic nude mice (5-weeks-old) were subcutaneously injected
with 2×106 MG63-Luc cells into the dorsal right side.
The tumor formed after 14 days, and its volume was calculated via
multiplication of the largest and smallest dimensions. A total of
12 mice were randomly divided into two groups to assess the
photothermal properties of Au@SiO2-DOX/VEGF.
Au@SiO2-DOX/VEGF group (n=6) was treated with the 808 nm
laser for 5 min at 1.4 W/cm2 after injection of the
probe, while DOX group (n=6) received DOX and 808 nm laser
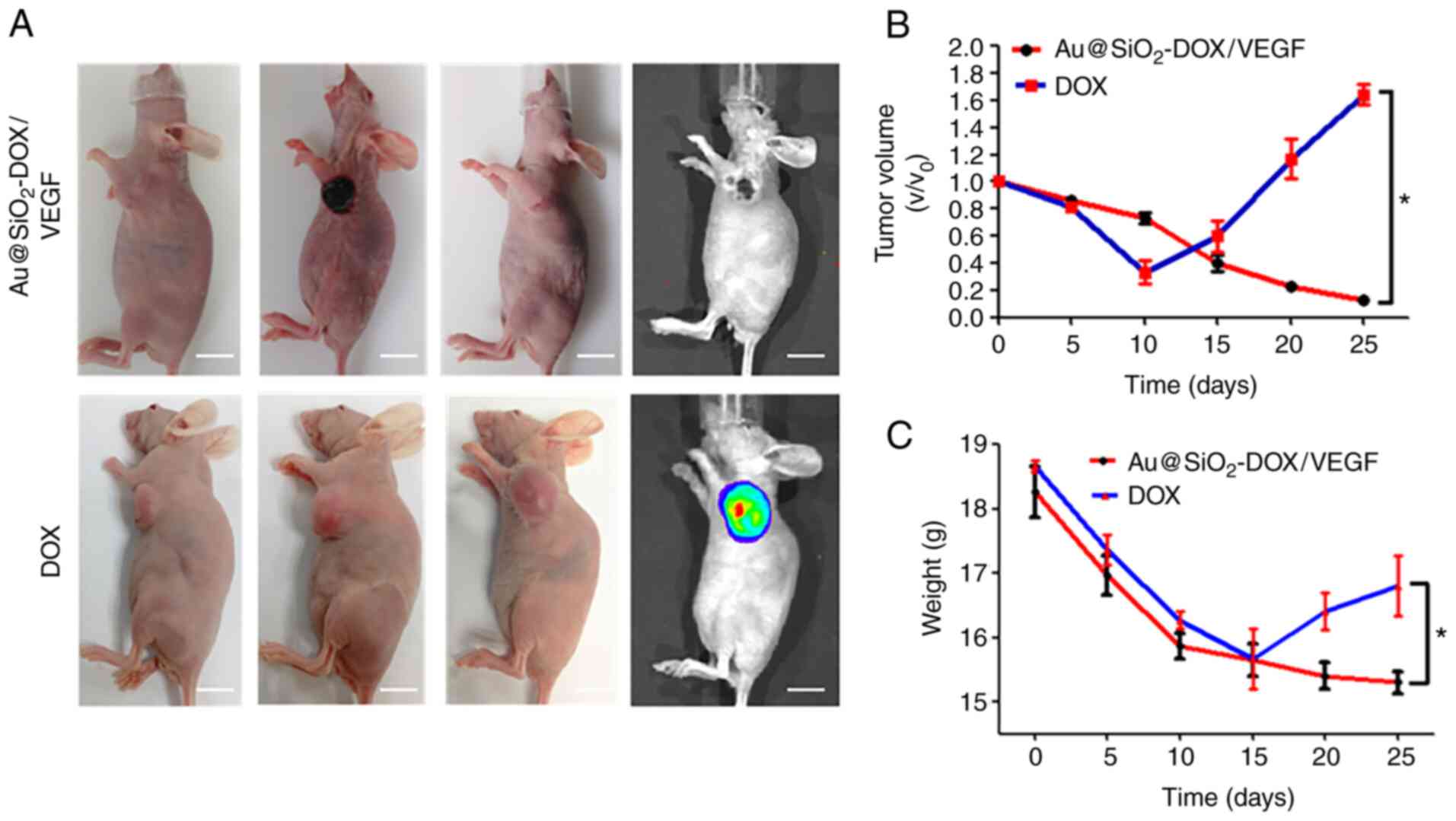
treatment for 5 min at 1.4 W/cm2 (Fig. 5A). Across the 25 days, the tumors had
disparate growth trends in both treatment groups, imaging of tumor
region was taken at day 0, 10 and day 25 of both groups and tumor
regrowth was detected by IVIS at day 25. A strong synergistic
antitumor effect was achieved when combining PTT and chemotherapy,
and the tumor growth in Au@SiO2-DOX/VEGF group was
almost completely inhibited on day 25 (upper row in Fig. 5A). Notably, both treatments caused
major cellular damage in cancerous tissues but not in normal
tissues. Regrowth of the tumor was detected in the DOX-treated
tumor group start from day 10 (lower row in Fig. 5A). Furthermore, in contrast to the
slow linear decline of tumor volume in the Au@SiO2-DOX/VEGF group,
tumor volume in the DOX group manifested linear growth after 10
days of treatment (Fig. 5B). Body
weight remained stable in the Au@SiO2-DOX/VEGF group after 15 days,
suggesting that mice in this group responded well to treatment and
exhibited improved quality of life, including greater food and
water consumption. Conversely, body weight increased in the DOX
group after 15 days, which was associated with rapid tumor growth
(Fig. 5C). Taken together, these
results suggest that Au@SiO2-DOX/VEGF and the laser are
a safer and more effective method of eliminating tumor cells
compared with DOX alone.
Discussion
The present study successfully synthesized a
nanoscale probe Au@SiO2-drug/VEGF and proved that the
probe can specifically bind to osteosarcoma cells. Notably,
Au@SiO2-drug/VEGF and DOX exhibited enhanced antitumor
activity when combined with NIR laser irradiation compared with DOX
plus laser treatment in vivo. The antitumor effect of
Au@SiO2-DOX/VEGF may be attributed to cell-targeting
endocytosis of the probe and the synergistic interaction between
chemotherapy and PTT (22). In this
nanoconstruct, VEGF peptide with high tumor binding affinity and
high plasma stability was conjugated to Au@SiO2 via a
PEG linker, which ensured availability of the peptide to the target
receptor. This strategy has been successfully used for the
formulation of other targeted NPs (38–41).
Characterization of probe demonstrated that the probe NPs had a
good nanosphere shape and a uniform size of ~90 nm. The probe
exhibited optimal performance compared with free ICG, suggesting
that the NP can potentially be used for tumor in vivo
detection.
Both in vitro and in vivo data
demonstrated that Au@SiO2-drug/VEGF can be
cell-targeting endocytosed. In vivo,
Au@SiO2-drug/VEGF displayed significantly higher
accumulation compared with nontargeted Au@SiO2-drug/VEGF
in human osteosarcoma cells. Thus, the results of the present study
support successful VEGF receptor-mediated targeted delivery of
Au@SiO2-drug/VEGF after intravenous injection. In
addition to active targeting mediated by receptor ligands
exemplified in the present study, physiological and physical
methods, such as tumor priming, vascular disruption, degradation of
the extracellular matrix and vessel normalization may be used to
improve tumor distribution of NPs (42,43).
These studies suggest that targeted NPs, such as
Au@SiO2-drug/VEGF, may achieve further improvement in
tumor deposition when combined with physiological and physical
methods.
The temperature of tumors in mice that received
intravenous injection of probe reached ~47°C after 5 min of
continuous wave NIR laser exposure at 1.4 W/cm2. This
temperature is sufficient for inducing irreversible damage to
cancer cells (44). In the present
study, 1.4 W increased the temperature only when probe
concentration was higher than 2 W. Au in the probe is known to have
good photothermal conversion performance (44). The higher the probe concentration,
the more Au and more heat generated (45,46). As
expected, there was no temperature change in the tumors of mice
that receives PBS injection followed by NIR irradiation. Therefore,
Au@SiO2-drug/VEGF mediates efficient photothermal
effects. Among the different types of inorganic NPs, gold NPs are
the most widely used for drug delivery and other biological
applications, due to their non-toxic and biocompatible properties,
their size- and shape-controllable synthesis, the ease of surface
modification with functional thiolate ligands and their extremely
rich and versatile optical heterogeneous and peculiar nature of
individual cancers and the inability to target therapeutics to
cancer cells without damaging normal tissues (43). There are studies using various
nanostructures to medicate PTT, such as nanorod, nanoshell capsules
and nancages (46–48), excellent photothermic conversion and
promising antitumor activity have been observed. However, further
studies are required to identify other nanostructured particles for
the treatment of osteosarcoma.
The antitumor efficacy of
Au@SiO2-DOX/VEGF plus laser and free DOX plus laser was
investigated in a 25-day follow up. The follow-up did not continue
pass this point as the mice receiving free DOX plus laser has
significantly weight loss and tumor size growth on day 25. The
results demonstrated that mice receiving
Au@SiO2-DOX/VEGF plus laser had a significantly better
prognosis. Thus, PTT and cell toxicity combined therapy may be a
more effective therapy module compared with chemotherapy alone. In
addition, a previous study reported that NP loaded cytotoxicity
drug can reduce the side effects of chemotherapy due to controlled
release (49). Osteosarcomas are
deep-seated, bone tumors and their metastasis is associated with
the lungs, which are deep-seated organs (1), so the clinical application of
probe-based PTT for osteosarcomas may be tumor residual elimination
following tumor resection. However, laser radiation before any
incision is made considering the penetration of laser in human
tissues.
In conclusion, the present study successfully
developed Au@SiO2-drug/VEGF NPs enabling simultaneous
NIR hyperthermia and drug delivery. The
Au@SiO2-drug/VEGF NPs exhibited optimal antitumor
efficacy and satisfactory biocompatibility. In addition,
Au@SiO2-drug/VEGF exhibited good targeting performance
towards osteosarcoma due to the targeting agent, VEGF. It was
confirmed that the combined treatment (cytotoxic chemotherapy and
PTT) can be administered to mice and successfully suppress the
cancer and prolong the survival time. Taken together, these results
suggest that Au@SiO2-drug/VEGF delivers targeted heating
and drugs to tumor tissues and minimizes collateral damage to
healthy tissues. Thus, this approach has great potential for
effective treatment of different types of tumors. Given that this
therapy is both feasible and effective, it may be incorporated into
clinical practice in the near future.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Nature
Science Foundation of China (grant no. 81473502).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TC and TL made substantial contributions to
conception and design, acquisition of data and analysis and
interpretation of data. TC was involved in drafting the manuscript
and revising it critically for important intellectual content. JW
made substantial contributions to conception and design and gave
final approval of the version to be published. TC and JW confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee of the Capital Medical
University (Beijing, China; approval no. CMU097230).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bramwell VH, Burgers M, Sneath R, Souhami
R, van Oosterom AT, Voûte PA, Rouesse J, Spooner D, Craft AW,
Somers R, et al: A comparison of two short intensive adjuvant
chemotherapy regimens in operable osteosarcoma of limbs in children
and young adults: The first study of the European Osteosarcoma
Intergroup. J Clin Oncol. 10:1579–1591. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roberts RD, Lizardo MM, Reed DR, Hingorani
P, Glover J, Allen-Rhoades W, Fan T, Khanna C, Sweet-Cordero EA,
Cash T, et al: Provocative questions in osteosarcoma basic and
translational biology: A report from the Children's Oncology Group.
Cancer. 125:3514–3525. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fauske L, Lorem G, Grov EK and Bondevik H:
Changes in the body image of bone sarcoma survivors following
surgical treatment-A qualitative study. J Surg Oncol. 113:229–234.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guerra RB, Tostes MD, da Costa Miranda L,
Pires de Camargo O, Baptista AM, Caiero MT, Dos Santos Machado TM,
Abadi MD, Mendes de Oliveira CR and Filippi RZ: Comparative
analysis between osteosarcoma and Ewing's sarcoma: Evaluation of
the time from onset of signs and symptoms until diagnosis. Clinics
(Sao Paulo). 61:99–106. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang D, Niu X, Wang Z, Song CL, Huang Z,
Chen KN, Duan J, Bai H, Xu J, Zhao J, et al: Multiregion sequencing
reveals the genetic heterogeneity and evolutionary history of
osteosarcoma and matched pulmonary metastases. Cancer Res. 79:7–20.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Y, Tian W, Yang L, Zhang Q, Zhu M,
Liu Y, Li J, Yang L, Liu J, Shen Y and Qi Z: Gemcitabine
potentiates anti-tumor effect of resveratrol on pancreatic cancer
via down-regulation of VEGF-B. J Cancer Res Clin Oncol. 147:93–103.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scarpellino G, Munaron L, Cantelmo AR and
Fiorio Pla A: Calcium-permeable channels in tumor vascularization:
Peculiar sensors of microenvironmental chemical and physical cues.
Rev Physiol Biochem Pharmacol. Aug 19–2020.(Epub ahead of print).
doi: 10.1007/112_2020_32. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu C, Dou T, Liu Y and Liu R: Clinical
value of TV-CDS combined with serum tumor markers in diagnosis of
ovarian cancer. Oncol Lett. 20:2028–2034. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mu L, Guan B, Tian J, Li X, Long Q, Wang
M, Wang W, She J, Li X, Wu D and Du Y: MicroRNA-218 inhibits tumor
angiogenesis of human renal cell carcinoma by targeting GAB2. Oncol
Rep. 44:1961–1970. 2020.PubMed/NCBI
|
|
11
|
Sanhueza C, Bennett JC,
Valenzuela-Valderrama M, Contreras P, Lobos-González L, Campos A,
Wehinger S, Lladser Á, Kiessling R, Leyton L and Quest AFG:
Caveolin-1-mediated tumor suppression is linked to reduced HIF1α
S-nitrosylation and transcriptional activity in hypoxia. Cancers
(Basel). 12:23492020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Apte RS, Chen DS and Ferrara N: VEGF in
signaling and disease: Beyond discovery and development. Cell.
176:1248–1264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Q, Hu Q, Dukhovlinova E, Chen G, Ahn
S, Wang C, Ogunnaike EA, Ligler FS, Dotti G and Gu Z: Photothermal
therapy promotes tumor infiltration and antitumor activity of CAR T
cells. Adv Mater. 31:e19001922019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin X, Fang Y, Tao Z, Gao X, Wang T, Zhao
M, Wang S and Liu Y: Tumor-microenvironment-induced All-in-One
nanoplatform for multimodal imaging-guided chemical and
photothermal therapy of cancer. ACS Appl Mater Interfaces.
11:25043–25053. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Z, Zhen X, Upputuri PK, Jiang Y, Lau
J, Pramanik M, Pu K and Xing B: Redox-activatable and Acid-enhanced
nanotheranostics for second Near-infrared photoacoustic tomography
and combined photothermal tumor therapy. ACS Nano. 13:5816–5825.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng Z, Yu X, Jiang M, Zhu L, Zhang Y,
Yang W, Xi W, Li G and Qian J: Excretable IR-820 for in vivo NIR-II
fluorescence cerebrovascular imaging and photothermal therapy of
subcutaneous tumor. Theranostics. 9:5706–5719. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bramos A, Perrault D, Yang S, Jung E, Hong
YK and Wong AK: Prevention of postsurgical lymphedema by 9-cis
retinoic acid. Ann Surg. 264:353–361. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ogawa M, Kosaka N, Choyke PL and Kobayashi
H: In vivo molecular imaging of cancer with a quenching
near-infrared fluorescent probe using conjugates of monoclonal
antibodies and indocyanine green. Cancer Res. 69:1268–1272. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang F, Han X, Hu Y, Wang S, Liu S, Pan
X, Wang H, Ma J, Wang W, Li S, et al: Interventional photothermal
therapy enhanced brachytherapy: A new strategy to fight deep
pancreatic cancer. Adv Sci (Weinh). 6:18015072019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mickoleit F, Lanzloth C and Schuler D: A
versatile toolkit for controllable and highly selective
multifunctionalization of bacterial magnetic nanoparticles. Small.
16:e19069222020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun Z, Cheng K, Yao Y, Wu F, Fung J, Chen
H, Ma X, Tu Y, Xing L, Xia L and Cheng Z: Controlled Nano-bio
interface of functional nanoprobes for in vivo monitoring enzyme
activity in tumors. ACS Nano. 13:1153–1167. 2019.PubMed/NCBI
|
|
22
|
Kim KS, Han JH, Park JH, Kim HK, Choi SH,
Kim GR, Song H, An HJ, Han DK, Park W and Park KS: Multifunctional
nanoparticles for genetic engineering and bioimaging of natural
killer (NK) cell therapeutics. Biomaterials. 221:1194182019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shang W, Zeng C, Du Y, Hui H, Liang X, Chi
C, Wang K, Wang Z and Tian J: Core-shell gold Nanorod@Metal-Organic
framework nanoprobes for multimodality diagnosis of glioma. Adv
Mater. Nov 18–2016.(Epub ahead of print). doi:
10.1002/adma.201604381.
|
|
24
|
Zhu P, Gao S, Lin H, Lu X, Yang B, Zhang
L, Chen Y and Shi J: Inorganic nanoshell-stabilized liquid metal
for targeted photonanomedicine in NIR-II biowindow. Nano Lett.
19:2128–2137. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Imani R, Dillert R, Bahnemann DW, Pazoki
M, Apih T, Kononenko V, Repar N, Kralj-Iglič V, Boschloo G, Drobne
D, et al: Multifunctional Gadolinium-doped mesoporous TiO2
nanobeads: Photoluminescence, enhanced spin relaxation, and
reactive oxygen species photogeneration, beneficial for cancer
diagnosis and treatment. Small. Apr 4–2017.(Epub ahead of print).
doi: 10.1002/smll.201700349. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kumar A, Huo S, Zhang X, Liu J, Tan A, Li
S, Jin S, Xue X, Zhao Y, Ji T, et al: Neuropilin-1-targeted gold
nanoparticles enhance therapeutic efficacy of platinum(IV) drug for
prostate cancer treatment. ACS Nano. 8:4205–4220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deng X, Liang S, Cai X, Huang S, Cheng Z,
Shi Y, Pang M, Ma P and Lin J: Yolk-shell structured au
Nanostar@Metal-Organic framework for synergistic chemo-photothermal
therapy in the second Near-infrared window. Nano Lett.
19:6772–6780. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao J, Wang F, Wang S, Liu L, Liu K, Ye Y,
Wang Z, Wang H, Chen B, Jiang J, et al: Hyperthermia-triggered
On-demand biomimetic nanocarriers for synergetic photothermal and
chemotherapy. Adv Sci. 7:19036422020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng D, Du Y, Shi Y, Mao D, Jia X, Li H,
Zhu Y, Wang K and Tian J: Precise diagnosis in different scenarios
using photoacoustic and fluorescence imaging with dual-modality
nanoparticles. Nanoscale. 8:14480–14488. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abbasi Pour S and Shaterian HR: Design and
characterization of lisinopril-loaded superparamagnetic
nanoparticles as a new contrast agent for in vitro, in vivo MRI
imaging, diagnose the tumors and drug delivery system. J Mater Sci
Mater Med. 28:912017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu P, Wang Y, Liu Y, Tan F, Li J and Li
N: S-nitrosothiols loaded mini-sized Au@silica nanorod elicits
collagen depletion and mitochondrial damage in solid tumor
treatment. Theranostics. 10:6774–6789. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Huang Q, He X, Chen H, Zou Y, Li
Y, Lin K, Cai X, Xiao J, Zhang Q and Cheng Y: Multifunctional
melanin-like nanoparticles for bone-targeted chemo-photothermal
therapy of malignant bone tumors and osteolysis. Biomaterials.
183:10–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han HS, Choi KY, Lee H, Lee M, An JY, Shin
S, Kwon S, Lee DS and Park JH: Gold-nanoclustered hyaluronan
Nano-assemblies for photothermally maneuvered photodynamic tumor
ablation. ACS Nano. 10:10858–10868. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jin R, Yang J, Zhao D, Hou X, Li C, Chen
W, Zhao Y, Yin Z and Liu B: Hollow gold nanoshells-incorporated
injectable genetically engineered hydrogel for sustained
chemo-photothermal therapy of tumor. J Nanobiotechnology.
17:992019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deepagan VG, You DG, Um W, Ko H, Kwon S,
Choi KY, Yi GR, Lee JY, Lee DS, Kim K, et al: Long-circulating
Au-TiO2 nanocomposite as a sonosensitizer for
ROS-mediated eradication of cancer. Nano Lett. 16:6257–6264. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin X, Liu S, Zhang X, Zhu R, Chen S, Chen
X, Song J and Yang H: An ultrasound activated vesicle of janus
Au-MnO nanoparticles for promoted tumor penetration and
sono-chemodynamic therapy of orthotopic liver cancer. Angew Chem
Int Ed Engl. 59:1682–1688. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Deng H, Shang W, Lu G, Guo P, Ai T, Fang C
and Tian J: Targeted and multifunctional technology for
identification between hepatocellular carcinoma and liver
cirrhosis. ACS Appl Mater Interfaces. 11:14526–14537. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Du Y, Liu X, Liang Q, Liang XJ and Tian J:
Optimization and design of magnetic ferrite nanoparticles with
uniform tumor distribution for highly sensitive MRI/MPI performance
and improved magnetic hyperthermia therapy. Nano Lett.
19:3618–3626. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Davis ME, Zuckerman JE, Choi CH, Seligson
D, Tolcher A, Alabi CA, Yen Y, Heidel JD and Ribas A: Evidence of
RNAi in humans from systemically administered siRNA via targeted
nanoparticles. Nature. 464:1067–1070. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Petros RA and DeSimone JM: Strategies in
the design of nanoparticles for therapeutic applications. Nat Rev
Drug Disco. 9:615–627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lu W, Zhang G, Zhang R, Flores LG II,
Huang Q, Gelovani JG and Li C: Tumor site-specific silencing of
NF-kappaB p65 by targeted hollow gold nanosphere-mediated
photothermal transfection. Cancer Res. 70:3177–3188. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lu D, Wientjes MG, Lu Z and Au JL: Tumor
priming enhances delivery and efficacy of nanomedicines. J
Pharmacol Exp Ther. 322:80–88. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Melancon MP, Elliott A, Ji X, Shetty A,
Yang Z, Tian M, Taylor B, Stafford RJ and Li C: Theranostics with
multifunctional magnetic gold nanoshells: Photothermal therapy and
t2* magnetic resonance imaging. Invest Radiol. 46:132–140. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu X, Feng W, Chang J, Tan YW, Li J, Chen
M, Sun Y and Li F: Temperature-feedback upconversion nanocomposite
for accurate photothermal therapy at facile temperature. Nat
Commun. 7:104372016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sanna V and Sechi M: Nanoparticle
therapeutics for prostate cancer treatment. Nanomedicine. 1 (Suppl
1):S31–S36. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liao J, Li W, Peng J, Yang Q, Li H, Wei Y,
Zhang X and Qian Z: Combined cancer photothermal-chemotherapy based
on doxorubicin/gold nanorod-loaded polymersomes. Theranostics.
5:345–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang H, Zhao R, Li Y, Liu H, Li F, Zhao Y
and Nie G: Aspect ratios of gold nanoshell capsules mediated
melanoma ablation by synergistic photothermal therapy and
chemotherapy. Nanomedicine. 12:439–448. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dong L, Li Y, Li Z, Xu N, Liu P, Du H,
Zhang Y, Huang Y, Zhu J, Ren G, et al: Au Nanocage-strengthed
dissolving microneedles for chemo-photothermal combined therapy of
superficial skin tumors. ACS Appl Mater Interfaces. 10:9247–9256.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
You J, Zhang R, Xiong C, Zhong M, Melancon
M, Gupta S, Nick AM, Sood AK and Li C: Effective photothermal
chemotherapy using doxorubicin-loaded gold nanospheres that target
EphB4 receptors in tumors. Cancer Res. 72:4777–4786. 2012.
View Article : Google Scholar : PubMed/NCBI
|