Introduction
Diffuse large B-cell lymphoma (DLBCL) is a
clinically heterogeneous lymphoid malignancy and the most common
type of lymphoma, accounting for 35–40% of all cases in Japan
(1). The current standard first-line
treatment for DLBCL is a regimen that combines cyclophosphamide,
doxorubicin, vincristine and prednisolone (CHOP) with an anti-CD20
monoclonal antibody, such as rituximab (R-CHOP) (2). R-CHOP therapy is more effective than
chemotherapy alone and can cure more than half of the patients with
DLBCL; however, 30–40% of patients relapse or develop resistance to
this treatment (3). Therefore, the
establishment of novel target molecules is an important challenge
in treatment-resistant and relapse cases.
It has been reported that patients with DLBCL with
high expression levels of indoleamine 2,3-dioxygenase 1 (IDO1), the
rate-limiting enzyme in the tryptophan pathway, which normally
catalyzes tryptophan to kynurenine (KYN), and high KYN levels are
associated with a poor prognosis (4,5).
Furthermore, high levels of IDO1 and KYN are associated with poor
prognosis in other types of cancer, including acute myeloid
leukemia and adult T-cell leukemia-lymphoma (6–9).
Together, these findings indicate that the KYN pathway serves an
important role in regulating the immune response (10–12), and
thus helps cancer cells escape attacks by host immune cells.
However, the roles of metabolites acting downstream of KYN and
associated enzymes are not fully understood.
Kynurenine 3-monooxygenase (KMO), which catalyzes a
rate-limiting step in the KYN pathway, converts KYN to
3-hydroxykynurenine (3-HK), ultimately leading to the production of
NAD+ (Fig. S1) (13). KMO is found in the mitochondria and
is highly active in immune and tumor cells (14), macrophages, and various tissues, such
as liver and kidney tissues (15,16). KMO
inhibition is considered to have beneficial effects in several
cases. For example, the inhibition of KMO ameliorates
neurodegenerative disorders, such as Alzheimer's disease and
Huntington's disease (17,18). Furthermore, the absence of KMO
ameliorates symptoms in acute viral myocarditis, acute
pancreatitis-induced multi-organ dysfunction syndrome and acute
kidney allograft rejection (19–21).
Interestingly, it has been reported that 3-HK produced by KMO
activity induces effector T-cell apoptosis in vitro, thereby
regulating T-cell-dependent immune responses (22), and administration of 3-HK as part of
the treatment approach for sepsis reduces the overproduction of
IL-6, which is responsible for severe endotoxemia (23). Therefore, investigating the
relationship between KMO and 3-HK in DLBCL may be useful for
elucidating novel therapeutic mechanisms targeting the KYN
pathway.
The present study aimed to investigate the
association between prognosis and KMO activity using serum samples
from patients with DLBCL and human DLBCL cell lines with different
levels of KMO expression. The present study revealed that
KMO-targeted therapeutic strategies may help inhibit DLBCL cell
viability, and KMO activity and 3-HK levels may represent potential
biomarkers in patients with lymphoid malignancies.
Materials and methods
Patients
A total of 28 patients with DLBCL (18 men and 10
women; age range, 53–93 years; mean age, 72) and 34 healthy adult
volunteers serving as controls were included in the serum analyses.
The Ethics Committees of Fujita Health University (Toyoake, Japan;
approval no. HM20-268) and Gifu University (Gifu, Japan; approval
no. 2018-25) approved all procedures involving human subjects, and
the study was performed in accordance with the principles of the
Declaration of Helsinki. All patients and healthy volunteers signed
informed consent before study participation. The present study
investigated healthy volunteers and 28 patients, including 7
patients for Fig. S2 (3 men and 4
women; age range, 53-90 years), who were histologically diagnosed
with DLBCL according to the World Health Organization
classification of hematopoietic tumors (24) between April 2008 and November 2016.
Patients with DLBCL metastasis or without complete clinical
information were excluded from the present study. Serum samples
from patients with DLBCL were obtained before the initiation of
therapy. All serum samples were separated by centrifugation (1,500
× g; 15 min; 24°C) and stored at −80°C until analysis. Patients
<70 years of age were assigned to receive eight cycles of R-CHOP
or rituximab, pirarubicin, cyclophosphamide, vincristine and
prednisone (R-THP-COP) therapy in Gifu University Hospital (Gifu,
Japan) (25–27). Patients ≥70 years old received six
cycles of R-CHOP or R-THP-COP therapy in Gifu University Hospital,
which is recommended for elderly patients with DLBCL (28). The clinical characteristics of
patients with DLBCL at the time of diagnosis are summarized in
Table I. Concerning age as a
prognostic factor, a patient age cut-off of 60 was determined
according to the age cut-off of the International Prognostic Index
(IPI) and Revised International Prognostic Index (R-IPI) (29,30).
Healthy controls, without renal dysfunction, hepatic dysfunction,
pregnancy or breastfeeding, immune-mediated inflammatory disease or
medications, and blood dyscrasia or anemia, were sex-matched (20
male patients; 14 female patients) and age-matched (<60 years, 7
patients; ≥60 years, 27 patients; age range, 47–80 years; mean age,
66) to the patients with DLBCL. Blood samples from newly diagnosed
patients and age- and sex-matched controls were collected at the
inpatient and outpatient department (Gifu University Hospital,
Gifu, Japan), respectively. All follow-up dates were based on the
last entries on December 1, 2020. The sample size used in the
present study was determined by the number of samples collected
during the study period. Therefore, due to the small sample size,
statistical analysis was performed using non-parametric
analysis.
 | Table I.Characteristics of patients with
diffuse large B-cell lymphoma. |
Table I.
Characteristics of patients with
diffuse large B-cell lymphoma.
|
Characteristics | Cases, n (%) |
|---|
| Sex |
|
|
Male | 18 (64.2) |
|
Female | 10 (35.8) |
| Age, years |
|
|
<60 | 4 (14.3) |
|
≥60 | 24 (85.7) |
| PS |
|
| 0,
1 | 23 (82.1) |
|
2-4 | 5 (17.9) |
| LDH |
|
|
Normal | 10 (35.8) |
|
Increased | 18 (64.2) |
| sIL-2R, U/ml |
|
|
<2,000 | 15 (53.6) |
|
≥2,000 | 13 (46.4) |
| Extranodal
lesions |
|
| 0,
1 | 17 (60.7) |
| ≥2 | 11 (39.3) |
| Clinical stage |
|
|
I/II | 5 (17.9) |
|
III/IV | 23 (82.1) |
| B symptom |
|
|
Absent | 18 (64.2) |
|
Present | 10 (35.8) |
| IPI |
|
|
L/LI | 10 (35.8) |
|
HI/H | 18 (64.2) |
| R-IPI |
|
| Very
good | 1 (3.6) |
|
Good | 9 (32.1) |
|
Poor | 18 (64.2) |
| Molecular
subtypes |
|
|
GCB | 10 (35.7) |
|
ABC | 18 (64.3) |
Measurement of KYN pathway
metabolites
3-HK and KYN measurements were performed as
previously described (19,31). For KYN measurement, serum was diluted
(4:1, v/v) in 10% perchloric acid. After thorough mixing, the
precipitated proteins were removed by centrifugation (7,000 × g; 10
min; 4°C). A total of 50 µl of the resulting supernatant was
subjected to high-performance liquid chromatography (HPLC
Prominence; Shimadzu) analysis. KYN was isocratically eluted from a
reverse phase column [TSKgel ODS-100V; 3 µm, 4.6 mm (ID) × 150 mm
(L); Tosoh] using a mobile phase containing 10 mM sodium acetate
and 1% acetonitrile (pH adjusted to 4.5 with acetic acid) at a flow
rate of 0.9 ml/min. KYN was detected using an ultraviolet and
visible spectrophotometric apparatus (SPD-20A; Shimadzu) (UV
wavelength, 365 nm).
For 3-HK measurement, serum was diluted (1:4, v/v)
in 10% perchloric acid. After thorough mixing, the precipitated
proteins were removed by centrifugation (7,000 × g; 10 min; 4°C). A
total of 20 µl of the supernatant was applied to a 3-µm HPLC column
(HR-80; 80×4.6 mm; ESA), using a mobile phase consisting of 1.5%
acetonitrile, 0.9% triethylamine, 0.59% phosphoric acid, 0.27 mM
EDTA and 8.9 mM sodium heptane sulfonic acid, at a flow rate of 0.5
ml/min. 3-HK was detected electrochemically using an ECD 300
detector (oxidation potential: +0.55 V; Eicom). KMO activity was
calculated from the 3-HK/KYN ratio as previously described
(32,33). Furthermore, the present study
investigated the association between changes in 3-HK levels and KMO
activity and sex, age, performance status (PS), serum lactate
dehydrogenase (LDH) levels, soluble interleukin-2 receptor (sIL-2R)
levels, extranodal lesions, clinical stage (CS), B symptom, IPI and
R-IPI to establish a link between KMO activity and characteristics
of patients with DLBCL. Extranodal lesions, CS and B symptoms were
assessed according to the Ann Arbor criteria, and classification
was performed for all patients (34).
Cell lines and cultures
Human B-cell non-Hodgkin lymphoma cell lines (KML-1:
JCRB1347) and human DLBCL cell lines (STR-428: JCRB1384) were
purchased from the Japanese Collection of Research Bioresources
Cell Bank. KML-1 and STR-428 cells are positive for the surface
markers CD10, CD19, CD20 and human leukocyte antigen DR (HLA-DR)
(35,36). Therefore, both of these cell lines
are categorized as germinal center B-cell-like (GCB) types in the
Hans classification (37). All tumor
cells were cultured in RPMI-1640 medium (FUJIFILM Wako Pure
Chemical Corporation) supplemented with 10% FBS (HyClone; Cytiva),
50 U/ml penicillin (Sigma-Aldrich; Merck KGaA) and 50 µg/ml
streptomycin (Sigma-Aldrich; Merck KGaA) at 37°C with 5%
CO2.
RNA extraction, semi-quantitative PCR
and reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from KML-1 and STR-428 cells
using an Isogen RNA Isolation kit (Nippon Gene Co., Ltd.). The RNA
(250 ng) was then used for first-strand synthesis of cDNA with a
high-capacity cDNA reverse transcription kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Semi-quantitative RT-PCR in KML-1
and STR-428 cells was performed to detect KMO, IDO1 and β-actin
cDNA using the KAPA Taq Extra HotStart Readymix PCR Kit (Nippon
Gene Co., Ltd.) according to the manufacturer's protocol. The PCR
products were separated on a 2% agarose gel and stained with
ethidium bromide to visualize the amplified nucleic acid fragments.
The mRNA expression levels of kynureninase (KYNU),
3-hydroxyanthranilate-3,4-dioxygenase (3-HAO), quinolinate
phosphoribosyltransferase (QPRT) and β-actin KML-1 and STR-428
cells were quantified by qPCR on a 7900HT Fast Real-Time system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). KYNU-,
3-HAO-, QPRT- and β-actin-targeted reactions were performed using
Sso Advanced SYBR-Green Supermix (Bio-Rad Laboratories, Inc.)
according to the manufacturer's protocol (38), and data were analyzed using 7900HT
software (version 2.3; Applied Biosystems; Thermo Fisher
Scientific, Inc.). The PCR primers are listed in Table SI. Thermocycling conditions were as
follows: Initial denaturation, 95°C for 30 sec; 40 cycles of
amplification (denaturation, 95°C for 5 sec; annealing/extension,
58°C for 60 sec). Gene expression levels were normalized to β-actin
expression levels using a standard curve.
Western blotting
KML-1 and STR-428 cells were collected, 100 µl RIPA
extraction buffer (FUJIFILM Wako Pure Chemical Corporation) was
added per 1×106 cells, and the samples were sonicated
and centrifuged at 10,000 × g for 5 min at 4°C. The protein
concentration in the supernatant was measured using a BCA protein
assay. Subsequently, 10 μg of protein was loaded onto a 10%
Mini-PROTEAN TGX gel (Bio-Rad Laboratories, Inc.), separated by
SDS-PAGE and transferred to PVDF membranes. After blocking
non-specific reactions with 5% skimmed milk for 1 h at 24°C, the
membranes were first incubated with the primary antibodies for 12 h
at 4°C, including anti-KMO (dilution, 1:1,000; cat. no. 10698-1-AP;
ProteinTech Group, Inc.) and anti-β-actin (dilution, 1:1,000; cat.
no. A5441; Sigma-Aldrich; Merck KGaA). The membrane was then
incubated with horseradish peroxidase-conjugated secondary antibody
for 1 h at 24°C (dilution, 1:10,000; cat. nos. 115-035-144 and
115-006-020; Jackson ImmunoResearch Laboratories, Inc.). Detection
was performed using the Immunostar LD reagent (FUJIFILM Wako Pure
Chemical Corporation) and a WSE-6100 Lumino Graph I instrument
(ATTO Corporation).
Immunohistochemistry
The lymph node of a single patient with DLBCL was
fixed in 10% formalin in PBS overnight at 24°C and then embedded in
paraffin. Sections (thickness, 3-µm) were used for H&E staining
and KMO immunostaining. The primary antibody used was a mouse
anti-KMO antibody (dilution, 1:2,000; cat. no. 60029-1-Ig;
ProteinTech Group, Inc.). After deparaffinization and rehydration,
sections were heated at 121°C for 20 min in Histofine antigen
retrieval solution (pH 9.0; Nichirei Biosciences, Inc.). The
sections were soaked in 3% hydrogen peroxide in methanol for 30 min
to eliminate endogenous peroxidase activity. After nonspecific
binding was blocked for 15 min at 24°C with G-block (cat. no.
GB-01; GenoStaff Co., Ltd.), the sections were incubated with or
without (negative control) primary antibody overnight at 4°C.
Secondary antibody, conjugated with a peroxidase polymer (ready to
use; cat. no. MP-7500; ImmPRESS Reagent anti-rabbit IgG; Vector
Laboratories, Inc.) was added for 30 min at 24°C according to the
manufacturer's protocol, followed by the addition of the substrate
3,3′-diaminobenzidine tetrahydrochloride (Dako; Agilent
Technologies, Inc.). The sections were then counterstained with
hematoxylin for 10 sec at 24°C.
KML-1 and STR-428 cells (2×105 cells/200
µl) were seeded on 6-well coverslips and fixed with 4%
paraformaldehyde at 24°C for 30 min. Fixed cells were permeabilized
in 0.1% Triton X-100 in 1X PBS (FUJIFILM Wako Pure Chemical
Corporation) for 30 min at 24°C, and blocked with 5% horse serum
(Vector Laboratories, Inc.) for 1 h at 24°C. Anti-KMO antibody
(dilution, 1:100; cat. no. 10698-1-AP; ProteinTech Group, Inc.) was
used as the primary antibody, which was hybridized with the samples
for 1 h at 24°C. The cells were then stained with Northern Lights
anti-rabbit IgG-NL557 as the secondary antibody (dilution, 1:1,000;
cat. no. NL004; R&D Systems, Inc.) for 1 h at 24°C in the dark.
Nuclei were stained with DAPI (dilution, 1:1,000; cat. no. BS04;
Dojindo Molecular Technologies, Inc.) for 5 min at 24°C. All
immunostained slides were observed under a BX51 fluorescence
microscope equipped with a DP74 digital camera (Olympus
Corporation).
Cell viability assay
KML-1 and STR-428 cells were seeded in 96-well
plates at a density of 1×104 cells/well, and 10 µl 3-HK
(1 and 10 µM; Sigma-Aldrich; Merck KGaA) or KMO inhibitor Ro61-8048
(10 and 100 nM, Sigma-Aldrich; Merck KGaA) was added. As a control,
10 µl PBS was added to the cells. After 24 h at 37°C with 5%
CO2, 10 µl water-soluble tetrazolium salt (WST)-1
reagent (Premix WST-1 Cell Proliferation Assay System; Takara Bio
Inc.) was added and the cells were incubated for 1.5 h at 37°C with
5% CO2. Absorbance measurements were performed on a
microplate reader (main wavelength, 450 nm; auxiliary wavelength,
620 nm). The number of viable cells was quantified based on the
absorbance.
Measurement of NAD+
levels
NAD+ levels in KML-1 and STR-428 cells
were measured using the NAD+/NADH assay kit-WST (cat.
no. N509; Dojindo Laboratories Inc.), which allows for the
determination of intracellular amounts of total
NAD+/NADH and NADH alone. Intracellular NAD+
levels were determined by subtracting the levels of NADH from total
NAD+/NADH levels.
Statistical analysis
GraphPad Prism software version 6 (GraphPad
Software, Inc.) was used for all statistical analyses. Experiments
were repeated at least three times. All data are presented as the
mean ± SD. The Mann-Whitney U test (Fig.
1) and two-sided paired t-test (Fig. S2) was used to test for significant
differences between two groups for patient data. Correlations
between serum KYN and 3-HK levels in patients with DLBCL were
analyzed using Pearson's product-moment correlation coefficient
(r). Categorical variables in Tables
II and III were presented as
numbers, and the groups were compared using the χ2 or
Fisher's exact test, as appropriate. For in vitro
experiments, unpaired Student's t-test was used to identify
significant differences between two groups. One-way analysis of
variance was used for significance testing of three groups. Tukey's
multiple comparison test was used for post-hoc pairwise analyses of
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
 | Table II.Associations between the levels of
serum 3-HK and clinical characteristics of patients with diffuse
large B-cell lymphoma. |
Table II.
Associations between the levels of
serum 3-HK and clinical characteristics of patients with diffuse
large B-cell lymphoma.
|
| 3-HK levels,
nM |
|
|---|
|
|
|
|
|---|
|
Characteristics | <21.9, n | ≥21.9, n | P-value |
|---|
| Sex |
|
|
|
|
Male | 7 | 11 | 0.6979 |
|
Female | 5 | 5 |
|
| Age, years |
|
|
|
|
<60 | 3 | 1 | 0.2850 |
|
≥60 | 9 | 15 |
|
| PS |
|
|
|
| 0,
1 | 10 | 13 | >0.9999 |
|
2-4 | 2 | 3 |
|
| LDH |
|
|
|
|
Normal | 6 | 4 | 0.2425 |
|
Increased | 6 | 12 |
|
| sIL-2R, U/ml |
|
|
|
|
<2,000 | 8 | 7 | 0.4120 |
|
≥2,000 | 4 | 9 |
|
| Extranodal
lesions |
|
|
|
| 0,
1 | 9 | 8 | 0.2530 |
| ≥2 | 3 | 8 |
|
| Clinical stage |
|
|
|
|
I/II | 5 | 0 | 0.0081 |
|
III/IV | 7 | 16 |
|
| B symptom |
|
|
|
|
Absent | 10 | 8 | 0.1144 |
|
Present | 2 | 8 |
|
| IPI |
|
|
|
|
L/LI | 7 | 3 | 0.0497 |
|
HI/H | 5 | 13 |
|
| R-IPI |
|
|
|
| Very
good/good | 7 | 3 | 0.0497 |
|
Poor | 5 | 13 |
|
| Molecular
subtypes |
|
|
|
|
GCB | 4 | 6 | >0.9999 |
|
ABC | 8 | 10 |
 | Table III.Associations between the levels of
serum KMO activity and clinical characteristics of patients with
diffuse large B-cell lymphoma. |
Table III.
Associations between the levels of
serum KMO activity and clinical characteristics of patients with
diffuse large B-cell lymphoma.
|
| KMO activity
(3-HK/KYN ratio) |
|
|---|
|
|
|
|
|---|
|
Characteristics | <9.2 | ≥9.2 | P-value |
|---|
| Sex |
|
|
|
|
Male | 9 | 9 | >0.9999 |
|
Female | 5 | 5 |
|
| Age, years |
|
|
|
|
<60 | 3 | 1 | 0.5956 |
|
≥60 | 11 | 13 |
| PS |
|
|
|
| 0,
1 | 11 | 12 | >0.9999 |
|
2-4 | 3 | 2 |
|
| LDH |
|
|
|
|
Normal | 6 | 4 | 0.6946 |
|
Increased | 8 | 10 |
|
| sIL-2R, U/ml |
|
|
|
|
<2,000 | 8 | 7 | >0.9999 |
|
≥2,000 | 6 | 7 |
|
| Extranodal
lesions |
|
|
|
| 0,
1 | 11 | 6 | 0.1217 |
| ≥2 | 3 | 8 |
|
| Clinical stage |
|
|
|
|
I/II | 5 | 0 | 0.0407 |
|
III/IV | 9 | 14 |
|
| B symptom |
|
|
|
|
Absent | 11 | 7 | 0.2365 |
|
Present | 3 | 7 |
|
| IPI |
|
|
|
|
L/LI | 7 | 3 | 0.2365 |
|
HI/H | 7 | 11 |
|
| R-IPI |
|
|
|
| Very
good/good | 7 | 3 | 0.2365 |
|
Poor | 7 | 11 |
|
| Molecular
subtypes |
|
|
|
|
GCB | 5 | 5 | >0.9999 |
|
ABC | 9 | 9 |
Results
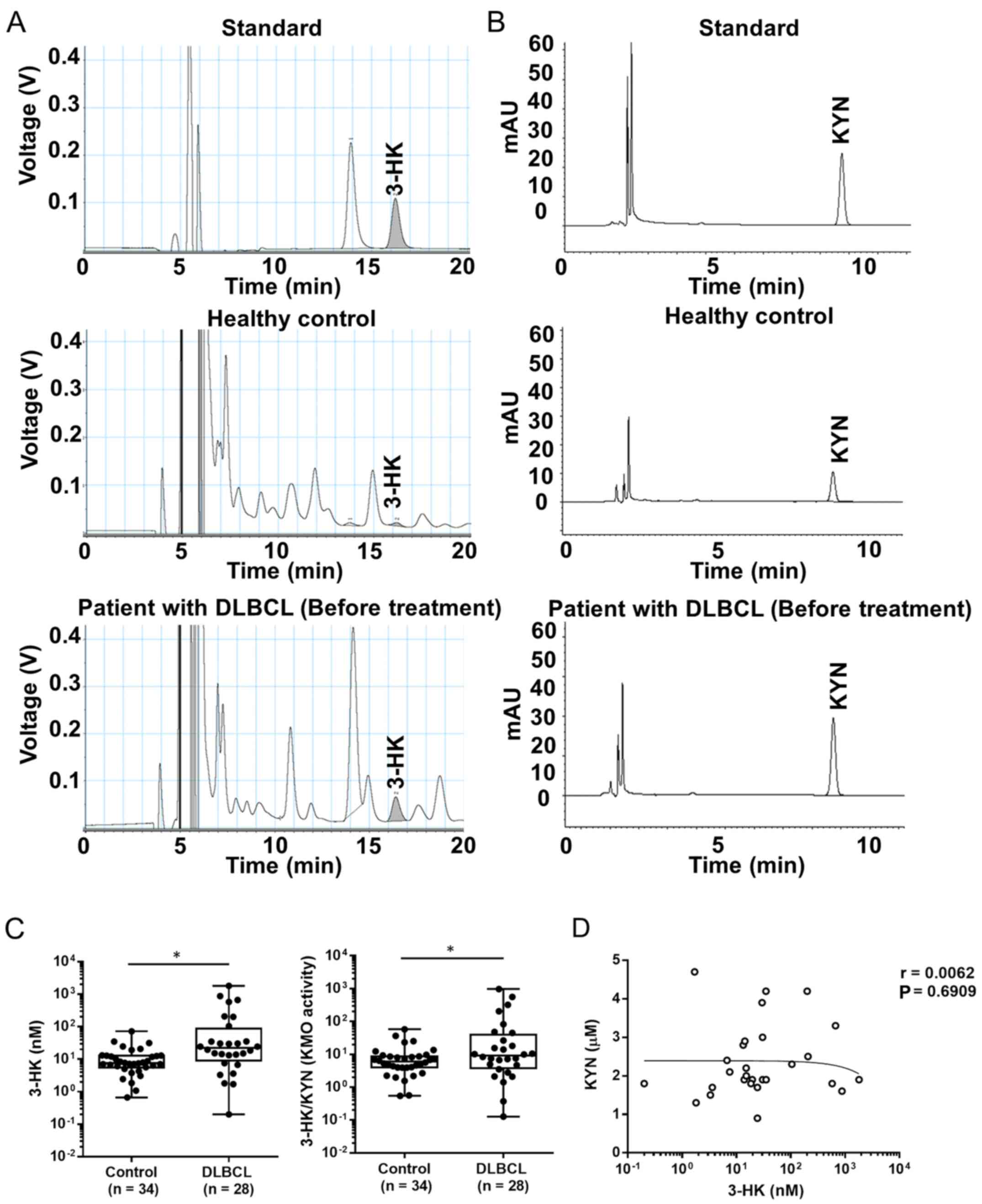
Levels of serum 3-HK and KMO activity
are increased in patients with DLBCL
The levels of serum 3-HK and the ratio of 3-HK/KYN,
as an indicator of KMO activity (32,33),
were significantly higher in patients with DLBCL before treatment
than in healthy controls (Fig.
1A-C). Similarly, it has been reported that the levels of KYN
in patients with DLBCL are increased compared with those in healthy
controls (4). However, there was no
correlation between KYN and 3-HK levels in patients with DLBCL
(Fig. 1D). Therefore, the levels of
serum 3-HK in patients with DLBCL were altered independently of KYN
levels.
Serum 3-HK levels and KMO activity in
patients with DLBCL are associated with disease progression
To investigate associations between the serum 3-HK
levels or KMO activity and clinical characteristics of patients
with DLBCL, patients with DLBCL were classified into low or high
groups based on the median serum 3-HK levels (cut-off value, 21.9
nM) or KMO activity (cut-off value, 9.2). No significant
associations between 3-HK levels or KMO activity and sex, age, PS,
LDH, sIL-2R, molecular subtypes, extranodal lesions or B symptoms
were observed. Importantly, 3-HK levels were significantly
associated with CS, IPI and R-IPI. KMO activity was significantly
associated with CS, but not IPI and R-IPI (Tables II and III). Furthermore, the levels of serum
3-HK and KMO activity in patients after treatment tended to be
decreased compared with those measured before treatment (Fig. S2). These results suggested that
serum 3-HK levels and KMO activity may reflect tumor
progression.
KMO-mediated NAD+ synthesis
regulates DLBCL cell viability
To confirm KMO expression and its roles in DLBCL
cells, the present study investigated KMO expression in KML-1 and
STR-428 cells, and lymph nodes of patients with DLBCL. KML-1
(B-cell non-Hodgkin lymphoma) and STR-428 (DLBCL) cell lines
exhibited a difference in KMO expression despite being derived from
patients with the same disease and pathological classification (GCB
type). Notably, STR-428 cells exhibited high levels of KMO
expression (KMOhigh), whereas KML-1 cells exhibited low
levels of KMO expression (KMOlow; Fig. 2A-C). Both cell lines were negative
for IDO1 mRNA using RT-PCR (data not shown). High levels of KMO
expression were also observed in the lymph nodes of patients with
DLBCL compared with those in the negative controls (Fig. 2D).
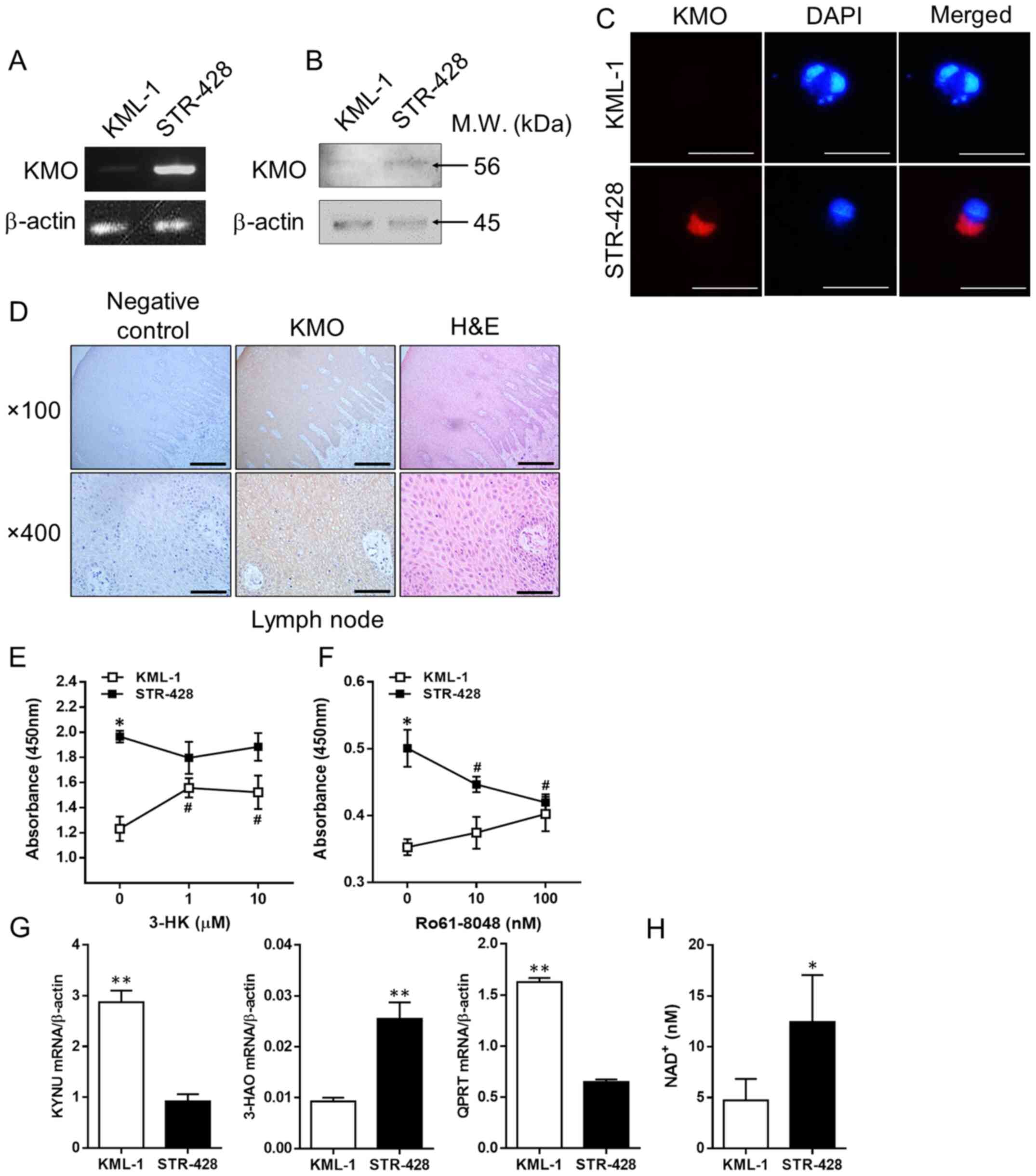 | Figure 2.3-HK produced by KMO activity
increases viability of DLBCL cells. (A) mRNA expression levels of
KMO in each DLBCL cell line determined by reverse transcription
PCR. (B) Protein expression levels of KMO in each DLBCL cell line
determined by western blotting. (C) Immunofluorescence staining of
KMO in KML-1 and STR-428 cells. The cytoplasmic staining of KMO is
shown in red and DAPI-stained nuclei are shown in blue. Scale bar,
20 µm. (D) Immunohistochemical staining of KMO in the lymph nodes
of patients with DLBCL [magnification, ×100 (upper panels) or ×400
(lower panels)]. Negative control (left), KMO (middle) and H&E
staining (right). Scale bar, 200 µm (upper panels) or 50 µm (lower
panels). (E) Viability of each DLBCL cell line after addition of
3-HK. (F) Viability of each DLBCL cell line after the addition of
Ro61-8048. (G) mRNA expression levels of KYNU, 3-HAO and QPRT in
each DLBCL cell line measured by quantitative RT-PCR. (H)
NAD+ levels in the culture supernatants of DLBCL cell
lines were measured using a NAD+ assay. Data are
presented as the mean ± SD (n=3 per group). *P<0.05,
**P<0.001 for KML-1 compared with STR-428 (unpaired Student's
t-test). #P<0.05 compared with the control (0 µM for
3-HK and 0 nM for Ro61-8048) (one-way ANOVA followed by Tukey's
multiple comparison test). 3-HAO,
3-hydroxyanthranilate-3,4-dioxygenase; 3-HK, 3-hydroxykynurenine;
DLBCL, diffuse large B-cell lymphoma; KMO, kynurenine
3-monooxygenase; KYNU, kynureninase; QPRT, quinolinate
phosphoribosyl transferase. |
To investigate the role of KMO expression in DLBCL
cells, the present study examined cell viability upon KMO
inhibition or 3-HK addition. Spontaneous cell viability of
KMOhigh STR-428 cells, which have the ability to produce
higher 3-HK levels, was much higher than that of KMOlow
KML-1 cells, which have the ability to produce lower 3-HK levels
(untreated ‘0’ in Fig. 2). Notably,
although the addition of 3-HK to KMOlow KML-1 cells
significantly improved cell viability, KMOhigh STR-428
cells were not affected (Fig. 2E).
By contrast, KMO inhibition in KMOhigh STR-428 cells
using Ro61-8048 reduced cell viability, but KMOlow KML-1
cells were not affected (Fig. 2F).
These results suggested that the viability of DLBCL cells may be
regulated by the production of 3-HK through KMO activity.
NAD+ is an essential coenzyme involved in
cell redox reactions and is required for cell proliferation as a
substrate for NAD+-dependent enzymes (39). Therefore, it was hypothesized that
the difference in viability of these DLBCL cell lines resulted from
an increase in the levels of NAD+, the final product of
the KYN pathway. To test this hypothesis, the present study
investigated the mRNA expression levels of KYNU, 3-HAO and QPRT,
which encode metabolic enzymes downstream of KMO. The mRNA
expression levels of KYNU and QPRT were significantly higher in
KML-1 cells than in STR-428 cells. Furthermore, the mRNA expression
levels of 3-HAO were significantly lower in KML-1 cells than in
STR-428 cells (Fig. 2G). Notably,
NAD+ levels in KMOhigh STR-428 cells were
significantly increased compared with those in KMOlow
KML-1 cells (Fig. 2H).
Discussion
The present study demonstrated that serum 3-HK
levels varied independently of KYN levels in patients with DLBCL,
and increased serum 3-HK levels and KMO activity served as
indicators of disease progression. Furthermore, the addition of KMO
inhibitors or 3-HK could regulate the viability of DLBCL cells
in vitro.
Previous studies have suggested that increased KYN
levels in IDO1high-expressing DLBCL cells were
associated with poor prognosis compared with that in
IDOlow-expressing DLBCL cells (4,5).
Therefore, it was hypothesized that KYN produced by DLBCL cells
affects the surrounding immune cells, resulting in immune escape.
For example, KYN inhibits T cell proliferation, promotes T cell
apoptosis, and induces the differentiation of naive T cells into
regulatory T cells by activating the aryl hydrocarbon receptor
(11,12). In the present study, despite the fact
that the serum 3-HK levels in patients with DLBCL varied
independently of KYN levels, increased serum 3-HK levels and KMO
activity in patients with DLBCL were significantly associated with
worse CS, IPI and R-IPI grades. Since KMO is an intracellular
enzyme (13), 3-HK and KYN levels in
the serum were measured instead to indirectly determine KMO
activity. In this context, some metabolites produced in the
cytoplasm can be secreted and diffuse rapidly into various tissues
via blood circulation (40). The
present results showed that KMO activity could be determined based
on the levels of 3-HK and KYN in the sera. It has been reported
that local concentrations within tumors are likely to be far higher
than those in serum (5,41). Based on the present results and
information, the 3-HK levels in the serum were associated with
tumorigenesis. Therefore, these results suggested that there is not
merely an immune escape mechanism based on increased KYN in disease
progression of DLBCL, such as T cell suppression, but also another
mechanism through KMO activity. The levels of 3-HK and KMO activity
were low in stage I/II, and lower KMO activity and 3-HK levels were
not associated with T cell suppression. Therefore, KMO-derived 3-HK
in DLBCL cells may affect disease progression and elucidating the
role of 3-HK produced through KMO activation may provide a novel
target for DLBCL diagnosis and treatment.
In the present study, the differences in 3-HK levels
between healthy volunteers and patients were small but significant.
In this context, it was considered that patients with DLBCL are
divided into at least three types: KMOlow-predominant
type, KMOhigh-predominant type and both. Therefore, it
is possible that patients with DLBCL with lower 3-HK have the
KMOlow type. Therefore, KMOlow KML-1 and
KMOhigh STR-428 cells were used to evaluate the roles of
3-HK and KMO in DLBCL. Interestingly, the addition of KMO inhibitor
negatively regulated the viability of the KMOhigh
STR-428 cells, whereas the addition of 3-HK positively regulated
the viability of the KMOlow KML-1 cells. However,
although the addition of 3-HK in KML-1 cells resulted in changes,
these were not dose-dependent. 3-HK is known as a free radical
generator (42). Therefore, a high
dose of 3-HK may cause cell death in KML-1 cells. Future studies on
KMO-overexpressing cells are required to further investigate the
role of 3-HK in cell proliferation. Furthermore, the KMO inhibitor
Ro61-8048 acted only on KMOhigh STR-428 cells and
inhibited their viability. However, KMOlow KML-1 cells
were able to escape the inhibitory effect of Ro61-8048 to maintain
viability. Therefore, although the de novo synthesis pathway
of NAD+ via KMO was inhibited, the nicotinic acid
contained in the medium allowed the KMOlow KML cells to
survive. By contrast, it was hypothesized that the de novo
synthesis pathway was more activated in KMOhigh STR
cells than in KMOlow KML cells, which accentuated the
effect of KMO inhibition. NAD+ regulates numerous
metabolic pathways, including transcription, DNA repair, cell cycle
and apoptosis (43), and is
essential for tumor cell proliferation (39). Therefore, DLBCL cells may also
require NAD+ for their proliferation.
NAD+-dependent enzymes, such as GAPDH and the sirtuin
family of ADP-ribosyltransferases, have been reported to be
involved in the proliferation of DLBCL cells (44,45). One
pathway for NAD+ synthesis is the KYN pathway, of which
KMO is the rate-limiting enzyme (13). The addition of 3-HK increases
NAD+ levels in human primary astrocytes and neurons
(46). By contrast, the addition of
KMO inhibitors results in decreased NAD+ levels in HeLa
cells (47). The present study
revealed that KMO expression positively regulated NAD+
levels in DLBCL cells in vitro. Although the verification of
the in vitro results in primary cells as a potential
limitation of the present study requires further investigation, the
present study provided insights into the biological function of KMO
in DLBCL cells. The present results revealed that the mRNA levels
of KMO and 3-HAO were increased in STR-428 cells but not in KML-1
cells. Therefore, it was hypothesized that KMO and 3-HAO are
involved in regulating NAD+ levels, at least in DLBCL
cells. By contrast, KYNU and QPRT, which were highly expressed in
KML-1 cells, did not affect NAD+ levels. In this
respect, although it was speculated that the lowered substrate
levels of KYNU and QPRT were associated with low KMO expression,
further studies are required to clarify these results.
In conclusion, patients with DLBCL with advanced CS,
IPI and R-IPI had increased levels of 3-HK. An in vitro
study with human DLBCL cell lines revealed constitutively different
KMO expression (KMOlow KML-1 cells and
KMOhigh STR-428 cells), suggesting that differential
expression affects NAD+ synthesis levels. Furthermore,
the addition of KMO inhibitors reduced the viability of
KMOhigh STR-428 cells. Therefore, high KMO activity in
patients with DLBCL is associated with advanced tumor growth and
CS. Although treatments targeting the KYN pathway, such as IDO1,
alone have not been sufficiently effective (48,49), the
present results suggested that KMO may be a potential target for
novel therapeutic strategies. Notably, although patients with
GCB-type DLBCL are generally known to have an improved prognosis
compared with those with the activated B cell type (50), the present study demonstrated that
the prognosis and response to treatment may differ depending on the
expression levels of KMO in patients with the GCB type. However,
further studies evaluating more cases are required to clarify the
association between KMO activity and prognosis.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Hiroyuki Tezuka
(Department of Cellular Function Analysis, Research Promotion and
Support Headquarters, Fujita Health University, Toyoake, Japan) and
Dr Naoe Goto (Department of Hematology, Fujita Health University
Hospital, Toyoake, Japan) for their advice on this experiment.
Funding
The present study was supported by a Fujita Health
University Grant (grant no. MH-2020).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MH and KS planned the experiments. NM, MH, SN and TH
performed the experiments. NM, MH and TH were responsible for data
integrity and data analysis. NM, MH, SN, TE, MY, TH, HT and KS
discussed the results. NM and MH wrote the manuscript. MH, TH, TE,
MY, HT and KS conducted the research. NM and MH confirm the
authenticity of all the raw data. KS had primary responsibility for
the final content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Committee of Fujita Health University (Toyoake, Japan; approval no.
HM19-182) and Gifu University (Gifu, Japan; approval no. 2018-25),
and written informed consent was provided by all patients and
healthy controls prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DLBCL
|
diffuse large B-cell lymphoma
|
|
KYN
|
kynurenine
|
|
3-HK
|
3-hydroxykynurenine
|
|
IDO1
|
indoleamine 2,3-dioxygenase 1
|
|
KMO
|
kynurenine 3-monooxygenase
|
|
KYNU
|
kynureninase
|
|
3-HAO
|
3-hydroxyanthranilate-3,4-dioxygenase
|
|
QPRT
|
quinolinate
phosphoribosyltransferase
|
|
PS
|
performance status
|
|
CS
|
clinical stage
|
|
IPI
|
International Prognostic Index
|
|
R-IPI
|
Revised International Prognostic
Index
|
References
|
1
|
No authors listed. The world health
organization classification of malignant lymphomas in japan:
Incidence of recently recognized entities. Lymphoma Study Group of
Japanese Pathologist. Pathol Int. 50:696–702. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Flowers CR, Sinha R and Vose JM: Improving
outcomes for patients with diffuse large B-cell lymphoma. CA Cancer
J Clin. 60:393–408. 2010.PubMed/NCBI
|
|
3
|
Feugier P, Van Hoof A, Sebban C,
Solal-Celigny P, Bouabdallah R, Fermé C, Christian B, Lepage E,
Tilly H, Morschhauser F, et al: Long-term results of the R-CHOP
study in the treatment of elderly patients with diffuse large
B-cell lymphoma: A study by the Groupe d'Etude des Lymphomes de
l'Adulte. J Clin Oncol. 23:4117–4126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoshikawa T, Hara T, Tsurumi H, Goto N,
Hoshi M, Kitagawa J, Kanemura N, Kasahara S, Ito H, Takemura M, et
al: Serum concentration of L-kynurenine predicts the clinical
outcome of patients with diffuse large B-cell lymphoma treated with
R-CHOP. Eur J Haematol. 84:304–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ninomiya S, Hara T, Tsurumi H, Goto N,
Saito K, Seishima M, Takami T and Moriwaki H: Indoleamine
2,3-dioxygenase expression and serum kynurenine concentrations in
patients with diffuse large B-cell lymphoma. Leuk Lymphoma.
53:1143–1145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Corm S, Berthon C, Imbenotte M, Biggio V,
Lhermitte M, Dupont C, Briche I and Quesnel B: Indoleamine
2,3-dioxygenase activity of acute myeloid leukemia cells can be
measured from patients' sera by HPLC and is inducible by IFN-gamma.
Leuk Res. 33:490–494. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Masaki A, Ishida T, Maeda Y, Suzuki S, Ito
A, Takino H, Ogura H, Totani H, Yoshida T, Kinoshita S, et al:
Prognostic significance of tryptophan catabolism in adult T-cell
leukemia/lymphoma. Clin Cancer Res. 21:2830–2839. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moretti S, Menicali E, Voce P, Morelli S,
Cantarelli S, Sponziello M, Colella R, Fallarino F, Orabona C,
Alunno A, et al: Indoleamine 2,3-dioxygenase 1 (IDO1) is
up-regulated in thyroid carcinoma and drives the development of an
immunosuppressant tumor microenvironment. J Clin Endocrinol Metab.
99:E832–E840. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hoshi M, Ito H, Fujigaki H, Takemura M,
Takahashi T, Tomita E, Ohyama M, Tanaka R, Saito K and Seishima M:
Indoleamine 2,3-dioxygenase is highly expressed in human adult
T-cell leukemia/lymphoma and chemotherapy changes tryptophan
catabolism in serum and reduced activity. Leuk Res. 33:39–45. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Munn DH, Shafizadeh E, Attwood JT,
Bondarev I, Pashine A and Mellor AL: Inhibition of T cell
proliferation by macrophage tryptophan catabolism. J Exp Med.
189:1363–1372. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fallarino F, Grohmann U, Vacca C, Orabona
C, Spreca A, Fioretti MC and Puccetti P: T cell apoptosis by
kynurenines. Adv Exp Med Biol. 527:183–190. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mezrich JD, Fechner JH, Zhang X, Johnson
BP, Burlingham WJ and Bradfield CA: An interaction between
kynurenine and the aryl hydrocarbon receptor can generate
regulatory T cells. J Immunol. 185:3190–3198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kolodziej LR, Paleolog EM and Williams RO:
Kynurenine metabolism in health and disease. Amino Acids.
41:1173–1183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chiu YH, Lei HJ, Huang KC, Chiang YL and
Lin CS: Overexpression of kynurenine 3-monooxygenase correlates
with cancer malignancy and predicts poor prognosis in canine
mammary gland tumors. J Oncol. 2019:62017642019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Castro FT, Brown RR and Price JM: The
intermediary metabolism of tryptophan by cat and rat tissue
preparations. J Biol Chem. 228:777–784. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heyes MP, Chen CY, Major EO and Saito K:
Different kynurenine pathway enzymes limit quinolinic acid
formation by various human cell types. Biochem J. 326:351–356.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thevandavakkam MA, Schwarcz R, Muchowski
PJ and Giorgini F: Targeting kynurenine 3-monooxygenase (KMO):
Implications for therapy in Huntington's disease. CNS Neurol Disord
Drug Targets. 9:791–800. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zwilling D, Huang SY, Sathyasaikumar KV,
Notarangelo FM, Guidetti P, Wu HQ, Lee J, Truong J,
Andrews-Zwilling Y, Hsieh EW, et al: Kynurenine 3-monooxygenase
inhibition in blood ameliorates neurodegeneration. Cell.
145:863–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kubo H, Hoshi M, Mouri A, Tashita C,
Yamamoto Y, Nabeshima T and Saito K: Absence of kynurenine
3-monooxygenase reduces mortality of acute viral myocarditis in
mice. Immunol Lett. 181:94–100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mole DJ, Webster SP, Uings I, Zheng X,
Binnie M, Wilson K, Hutchinson JP, Mirguet O, Walker A, Beaufils B,
et al: Kynurenine-3-monooxygenase inhibition prevents multiple
organ failure in rodent models of acute pancreatitis. Nat Med.
22:202–209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Merchen TD, Fang X, Lassiter R, Ho
CS, Jajosky R, Kleven D, Thompson T, Mohamed E, Yu M, et al:
Regulation of indoleamine 2,3 dioxygenase and its role in a porcine
model of acute kidney allograft rejection. J Investig Med.
66:1109–1117. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Terness P, Bauer TM, Röse L, Dufter C,
Watzlik A, Simon H and Opelz G: Inhibition of allogeneic T cell
proliferation by indoleamine 2,3-dioxygenase-expressing dendritic
cells: Mediation of suppression by tryptophan metabolites. J Exp
Med. 196:447–457. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hoshi M, Kubo H, Ando T, Tashita C,
Nakamoto K, Yamamoto Y, Tezuka H and Saito K: 3-Hydroxykynurenine
regulates lipopolysaccharide-stimulated IL-6 production and
protects against endotoxic shock in mice. Immunohorizons.
5:523–534. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sabattini E, Bacci F, Sagramoso C and
Pileri SA: WHO classification of tumours of haematopoietic and
lymphoid tissues in 2008: An overview. Pathologica. 102:83–87.
2010.PubMed/NCBI
|
|
25
|
Miller AA and Salewski E: Prospects for
pirarubicin. Med Pediatr Oncol. 22:261–268. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takagi T and Oguro M:
(2″-R)-4′-o-tetrahydropyranyladriamycin, a new anthracycline
derivative; its effectiveness in lymphoid malignancies. Cancer
Chemother Pharmacol. 20:151–154. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsurumi H, Yamada T, Sawada M, Kasahara S,
Kanemura N, Kojima Y, Fukuno K, Hara T, Saio M, Takahashi T, et al:
Biweekly CHOP or THP-COP regimens in the treatment of newly
diagnosed aggressive non-Hodgkin's lymphoma. A comparison of
doxorubicin and pirarubicin: A randomized phase II study. J Cancer
Res Clin Oncol. 130:107–113. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsurumi H, Hara T, Goto N, Kanemura N,
Kasahara S, Sawada M, Yasuda I, Yamada T, Shimizu M, Takami T, et
al: A phase II study of a THP-COP regimen for the treatment of
elderly patients aged 70 years or older with diffuse large B-cell
lymphoma. Hematol Oncol. 25:107–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
International Non-Hodgkin's Lymphoma
Prognostic Factors Project, . A predictive model for aggressive
non-Hodgkin's lymphoma. N Engl J Med. 329:987–994. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sehn LH, Berry B, Chhanabhai M, Fitzgerald
C, Gill K, Hoskins P, Klasa R, Savage KJ, Shenkier T, Sutherland J,
et al: The revised International Prognostic Index (R-IPI) is a
better predictor of outcome than the standard IPI for patients with
diffuse large B-cell lymphoma treated with R-CHOP. Blood.
109:1857–1861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Saito K, Quearry BJ, Saito M, Nowak TS Jr,
Markey SP and Heyes MP: Kynurenine 3-hydroxylase in brain: Species
activity differences and effect of gerbil cerebral ischemia. Arch
Biochem Biophys. 307:104–109. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Skouras C, Zheng X, Binnie M, Homer NZ,
Murray TB, Robertson D, Briody L, Paterson F, Spence H, Derr L, et
al: Increased levels of 3-hydroxykynurenine parallel disease
severity in human acute pancreatitis. Sci Rep. 6:339512016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hajsl M, Hlavackova A, Broulikova K,
Sramek M, Maly M, Dyr JE and Suttnar J: Tryptophan metabolism,
inflammation, and oxidative stress in patients with neurovascular
disease. Metabolites. 10:2082020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Carbone PP, Kaplan HS, Musshoff K,
Smithers DW and Tubiana M: Report of the committee on Hodgkin's
disease staging classification. Cancer Res. 31:1860–1861.
1971.PubMed/NCBI
|
|
35
|
Ikezoe T, Miyagi T, Kubota T, Taguchi T,
Ohtsuki Y, Miyake K, Inokuchi K, Nomura T, Koeffler HP and Miyoshi
I: Inactivation of the DCC tumor suppressor gene in a B-cell
lymphoma cell line with the alteration of chromosome 18. Am J
Hematol. 50:124–132. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Taira T, Nagasaki A, Tomoyose T, Miyagi J,
Kakazu N, Makino S, Shinjyo T, Taira N, Masuda M and Takasu N:
Establishment of a human herpes virus-8-negative malignant effusion
lymphoma cell line (STR-428) carrying concurrent translocations of
BCL2 and c-MYC genes. Leuk Res. 31:1285–1292. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hans CP, Weisenburger DD, Greiner TC,
Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E,
Braziel RM, Jaffe ES, et al: Confirmation of the molecular
classification of diffuse large B-cell lymphoma by
immunohistochemistry using a tissue microarray. Blood. 103:275–282.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hoshi M, Saito K, Hara A, Taguchi A,
Ohtaki H, Tanaka R, Fujigaki H, Osawa Y, Takemura M, Matsunami H,
et al: The absence of IDO upregulates type I IFN production,
resulting in suppression of viral replication in the
retrovirus-infected mouse. J Immunol. 185:3305–3312. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Garten A, Schuster S, Penke M, Gorski T,
de Giorgis T and Kiess W: Physiological and pathophysiological
roles of NAMPT and NAD metabolism. Nat Rev Endocrinol. 11:535–546.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kaper T, Looger LL, Takanaga H, Platten M,
Steinman L and Frommer WB: Nanosensor detection of an
immunoregulatory tryptophan influx/kynurenine efflux cycle. PLoS
Biol. 5:e2572007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ninomiya S, Hara T, Tsurumi H, Hoshi M,
Kanemura N, Goto N, Kasahara S, Shimizu M, Ito H, Saito K, et al:
Indoleamine 2,3-dioxygenase in tumor tissue indicates prognosis in
patients with diffuse large B-cell lymphoma treated with R-CHOP.
Ann Hematol. 90:409–416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vazquez S, Garner B, Sheil MM and Truscott
RJ: Characterisation of the major autoxidation products of
3-hydroxykynurenine under physiological conditions. Free Radic Res.
32:11–23. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chiarugi A, Dölle C, Felici R and Ziegler
M: The NAD metabolome - a key determinant of cancer cell biology.
Nat Rev Cancer. 12:741–752. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chiche J, Pommier S, Beneteau M, Mondragón
L, Meynet O, Zunino B, Mouchotte A, Verhoeyen E, Guyot M, Pagès G,
et al: GAPDH enhances the aggressiveness and the vascularization of
non-Hodgkin's B lymphomas via NF-κB-dependent induction of HIF-1α.
Leukemia. 29:1163–1176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chowdhury S, Sripathy S, Webster A, Park
A, Lao U, Hsu JH, Loe T, Bedalov A and Simon JA: Discovery of
selective SIRT2 inhibitors as therapeutic agents in B-cell lymphoma
and other malignancies. Molecules. 25:4552020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Braidy N, Grant R, Brew BJ, Adams S,
Jayasena T and Guillemin GJ: Effects of kynurenine pathway
metabolites on intracellular NAD synthesis and cell death in human
primary astrocytes and neurons. Int J Tryptophan Res. 2:61–69.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pittelli M, Formentini L, Faraco G,
Lapucci A, Rapizzi E, Cialdai F, Romano G, Moneti G, Moroni F and
Chiarugi A: Inhibition of nicotinamide phosphoribosyltransferase:
Cellular bioenergetics reveals a mitochondrial insensitive NAD
pool. J Biol Chem. 285:34106–34114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kristeleit R, Davidenko I, Shirinkin V,
El-Khouly F, Bondarenko I, Goodheart MJ, Gorbunova V, Penning CA,
Shi JG, Liu X, et al: A randomised, open-label, phase 2 study of
the IDO1 inhibitor epacadostat (INCB024360) versus tamoxifen as
therapy for biochemically recurrent (CA-125 relapse)-only
epithelial ovarian cancer, primary peritoneal carcinoma, or
fallopian tube cancer. Gynecol Oncol. 146:484–490. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Beatty GL, O'Dwyer PJ, Clark J, Shi JG,
Bowman KJ, Scherle PA, Newton RC, Schaub R, Maleski J, Leopold L,
et al: First-in-human phase I study of the oral inhibitor of
indoleamine 2,3-dioxygenase-1 epacadostat (INCB024360) in patients
with advanced solid malignancies. Clin Cancer Res. 23:3269–3276.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Alizadeh AA, Eisen MB, Davis RE, Ma C,
Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, et al:
Distinct types of diffuse large B-cell lymphoma identified by gene
expression profiling. Nature. 403:503–511. 2000. View Article : Google Scholar : PubMed/NCBI
|