Introduction
Pancreatic cancer (PC) is an aggressive type of
cancer with an increasing incidence rate worldwide, with 216,000
new cancer cases worldwide every year, causing more than 200,000
deaths each year (1,2). In recent years, PC has become the
fourth main cause of cancer-associated mortality and its long-time
survival rate is ≤5% due to its high degree of malignancy (3,4).
Furthermore, the intricate mechanisms underlying its pathological
progression and the complicated regulatory mechanisms represent a
considerable challenge in the early diagnosis of PC (5). Diagnosis and therapy have improved the
survival times of patients, with the advances in imaging and
clinical treatment methods (6);
however, the clinical outcome of patients with PC remains
unsatisfactory. Thus, it is crucial to further elucidate the
possible underlying molecular mechanisms and identify new
biomarkers for PC.
Long non-coding (lnc) RNAs are a type of ribose
nucleotide chain and are >200 nucleotides in length (7). lncRNAs were previously considered to
lack any biological functions, as they do not have protein-coding
ability; however, it was discovered that lncRNAs can exert their
biological functions via epigenetic regulation (8) at the transcriptional level,
post-transcriptional level (9) or
histone modification (10).
Accumulating evidence has proved the particular significance of
lncRNAs in the occurrence and progression of diverse diseases,
particularly cancer, such as gastric cancer (11), colorectal cancer (12) and glioma (13). lncRNAs have been demonstrated to
represent important elements of the competing endogenous (ce) RNA
network by combining with microRNAs (miRNAs/miRs) to neutralize
their inhibitory effects on target genes (14). In recent years, a number of lncRNAs
have been confirmed to indirectly modulate gene expression by
acting as ceRNAs in PC progression (15–17). The
lncRNA titin antisense RNA 1 (TTN-AS1) has been found to play an
oncogenic role in diverse types of cancer, such as gastric cancer
(18), papillary thyroid cancer
(19), cervical cancer (20) and hepatocellular carcinoma (21). Notably, TTN-AS1 has been shown to act
as a ceRNA and plays a regulatory role by sponging different miRNAs
(22,23). For example, TTN-AS1 enhanced breast
cancer cell invasion by regulating the miR-524-5p/ribonucleotide
reductase regulatory subunit M2 axis (24). However, to the best of our knowledge,
its significance in PC has not been investigated to date. The aim
of the present study was to determine whether TTN-AS1 induced by
forkhead box protein 1 (FOXP1) may function as an oncogene in PC
via the miR-589-5p/FOXP1 axis, to identify a novel regulatory axis
underlying PC progression.
Materials and methods
Tissue samples
A total of 78 paired specimens of PC and adjacent
normal tissues were obtained from patients who had received
surgical resection at Liyang People's Hospital (Liyang, China)
between March 2017 and December 2019. Prior to surgery, none of the
patients had received radiotherapy or chemotherapy. Patients that
had been treated with chemotherapy or radiotherapy prior to surgery
or lack of written informed consent were excluded from the study.
The protocol of the present study was approved by the Ethics
Committee of Liyang People's Hospital and written informed consent
was provided by each patient. Immediately after collection, the
tissues were frozen in liquid nitrogen and preserved at −80°C until
use.
Cell lines
The PC cell lines (BxPC-3, AsPC-1, CaPAN-2, PANC-1
and SW1990) were purchased from the American Type Culture
Collection and the human pancreatic duct epithelial (HPDE) cell
line was purchased from The Cell Bank of Type Culture Collection of
The Chinese Academy of Sciences. All the cell lines were cultured
in DMEM, supplemented with 10% FBS (both from Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin, and incubated at
37°C in a humidified incubator with 5% CO2.
Cell transfection
Short hairpin (sh) RNAs targeting TTN-AS1
(sh-TTN-AS1#1 and -#2) were designed to downregulate TTN-AS1 and
sh-negative control (NC) served as the control. miR-589-5p mimics
(5′-UUAUGGUUUGCCUGGGACUGAG-3′) were purchased to increase
miR-589-5p expression, with NC mimics (5′-UUCUCCGAACGUGUCACGUTT-3′)
as the control. To overexpress FOXP1, the pcDNA3.1/FOXP1 plasmid
was generated and empty pcDNA3.1 was the control. All the
aforementioned plasmids were purchased from Shanghai GenePharma
Co., Ltd., and Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to transfect 50 nM sh-TTN-AS1#1,
50 nM sh-TTN-AS1#2, 50 nM sh-NC, 50 nM miR-589 mimics, 50 nM NC
mimics, 4.0 µg pcDNA3.1/FOXP1 and 4.0 µg pcDNA3.1 into the BxPC-3
and AsPC-1 cell lines at room temperature for ~30 min. Reverse
transcription-quantitative (RT-q) PCR analysis was used to confirm
transfection efficiency. Subsequent experiments were performed 48 h
post-transfection.
RT-qPCR analysis
Total RNA was extracted from the PC tissues or cells
using TRIzol® (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Subsequently, total
RNA was reverse transcribed into cDNA using the ReverTra Ace qPCR
RT kit (Takara Biotechnology Co., Ltd.), according to the
manufacturer's protocol. The SYBR Green real-time PCR Master Mix
(Takara Biotechnology Co., Ltd.) was used for qPCR on an ABI 7500
real-time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The thermocycling conditions were as follows:
Pre-denaturation at 95°C for 1 min, followed by 40 cycles of 95°C
for 15 sec, 60°C for 30 sec and 72°C for 30 sec. GAPDH (for lncRNA
and mRNA) or U6 (for miRNA) served as the internal references.
Finally, the 2−ΔΔCq method (25) was used to calculate and analyze
relative target gene expression. The primer sequences were designed
as follows: TTN-AS1 forward, 5′-CGATACCATTGAACACGCTGC-3′ and
reverse, 5′-GGTTGAGGGTCCCAGTG-3′; miR-589-5p forward,
5′-CGCCTTGAATCGGTG-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′; FOXP1
forward, 5′-AGGACTTGCACAAGCAGAAC-3′ and reverse,
5′-GTTGGCGTACACGGGCGGCT-3′; GAPDH forward,
5′-AGCCACATCGCTCAGACAC-3′ and reverse, 5′-GCCCAATACGACCAAATCC-3′;
and U6 forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′.
Cell Counting Kit (CCK)-8 assay
To measure cell viability, the BxPC-3 and AsPC-1
cell lines were plated into 96-well plates (2×103
cells/well), and incubated for 0, 24, 48 and 72 h. Following which,
10 µl CCK-8 solution (Dojindo Molecular Technologies, Inc.) was
added per well and the samples were incubation for an additional 2
h until the cells adhered. Finally, the absorbance at 450 nm was
detected.
Colony formation assay
A total of 1,500 BxPC-3 and AsPC-1 cells per well
were seeded into 6-well plates and the transfected cells were
cultured for 2 weeks under normal culture conditions. Then, the
cells were fixed in 4% paraformaldehyde for 10 min and stained
using 0.5% crystal violet solution for 10 min at room temperature,
respectively. After washing three times with PBS, images of the
cell colonies were captured and counted under a light microscope
(magnification, ×20).
Transwell assay
A 24-well Transwell chamber (Corning, Inc.)
containing a polycarbonate membrane filter (8-µm pore size) was
used for the Transwell assays. To investigate invasion, the chamber
was precoated with 100 µg Matrigel for 1 h at room temperature,
whereas this step was omitted for the migration assay. Briefly,
5×104 cells in serum-free medium were plated in the top
chamber and the bottom chamber was filled with medium (500 µl),
supplemented with 10% FBS. After incubating for 24 h at 37°C, the
cells remaining in the upper side of the filter were removed using
cotton-tipped swabs. The cells that had migrated or invaded into
the lower side of the filter were fixed with 4% methanol for 20 min
and stained with 0.1% crystal violet for 20 min, both at room
temperature, and finally counted under an light microscope
(magnification, ×200).
Bioinformatic analysis
The StarBase 2.0 database (http://starbase.sysu.edu.cn/) was used to predict the
binding sites between miR-589-5p and TTN-AS1 or FOXP1. Santa Cruz
Genome Browser (http://genome.ucsc.edu) was used to predict the
potential transcription factor of TTN-AS1.
Luciferase reporter assay
The corresponding full-length sequence of TTN-AS1 or
FOXP1 3′-untranslated region (UTR) with wild-type (WT) or mutated
(Mut) miR-589-5p binding sites was ligated into the pmirGLO vector
(Promega Corporation) to form pmirGLO-TTN-AS1-WT/Mut or
pmirGLO-FOXP1-WT/Mut reporter vectors. Then, the constructed
reporter vectors were co-transfected with miR-589-5p mimics or NC
mimics into the BxPC-3 and AsPC-1 cells, using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After 48 h, the relative luciferase activities
were measured using a Dual-Luciferase Reporter Assay System
(Promega Corporation). Firefly luciferase activity was normalized
to Renilla luciferase activity.
The fragments of the TTN-AS1 promoter containing the
FOXP1-binding site (WT or Mut) were ligated into the pGL3-basic
vector (Promega Corporation). Subsequently, the recombinant
construct was co-transfected with pcDNA3.1/FOXP1 plasmids into the
BxPC-3 and AsPC-1 cell lines, using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). After 48 h, the relative
luciferase activity was examined using the Dual Luciferase Reporter
Gene Assay kit (BioTek Instruments, Inc.) to analyze firefly and
Renilla luciferase activities.
Chromatin immunoprecipitation (ChIP)
assay
The BxPC-3 and AsPC-1 cell lines were collected and
fixed with 1% formaldehyde for 10 min at 37°C for cross-linking DNA
and protein. Next, ultrasonication was used to generate DNA
fragments (200–500 bp). Then, the cell lysates, with the DNA
fragments, were immunoprecipitated with anti-FOXP1 (1:1,000; cat.
no. ab93807) or anti-IgG (1:10,000; cat. no. ab172730) (both from
Abcam). Subsequently, magnetic beads were used to capture the
precipitated DNA fragments and the precipitated DNA was quantified
using RT-qPCR.
Western blot analysis
Total protein was isolated from the PC cells using
RIPA lysis buffer (Thermo Fisher Scientific, Inc.). Protein
concentration was detected using a BCA assay kit (Sangon Biotech
Co., Ltd.). The total protein (20 µg) was separated with 10%
SDS-PAGE, then transferred to a PVDF membrane. After blocking with
5% skimmed milk for 2 h, the membrane was incubated with the
primary antibodies against E-cadherin (cat. no. ab1416; 1:1,000),
N-cadherin (cat. no. ab18203; 1: 1,000) and GAPDH (cat. no. ab9485;
1:1,000) overnight at 4°C. Subsequently, the membrane was incubated
with goat anti-rabbit IgG H&L (HRP) secondary antibody
(1:1,000; cat. no. ab205718; Abcam) and the bands were evaluated
using an enhanced chemiluminescence kit (Pierce; Thermo Fisher
Scientific, Inc.).
Statistical analysis
Data from independent triplicate experiments were
analyzed via SPSS 20.0 (IBM Corp.) and are shown as the mean ± SD.
The comparison between two groups was conducted using paired or
unpaired Student's t-test and between multiple groups using one-way
ANOVA followed by Tukey's post hoc test. The correlation between
mRNA expression levels was analyzed using Pearson's correlation
analysis. The χ2 test was used to assess the
associations between TTN-AS1 expression and the clinicopathological
characteristics. P<0.05 was considered to indicate a
statistically significant difference.
Results
TTN-AS1 silencing inhibits PC cell
proliferation
To examine the function of TTN-AS1 in PC, its mRNA
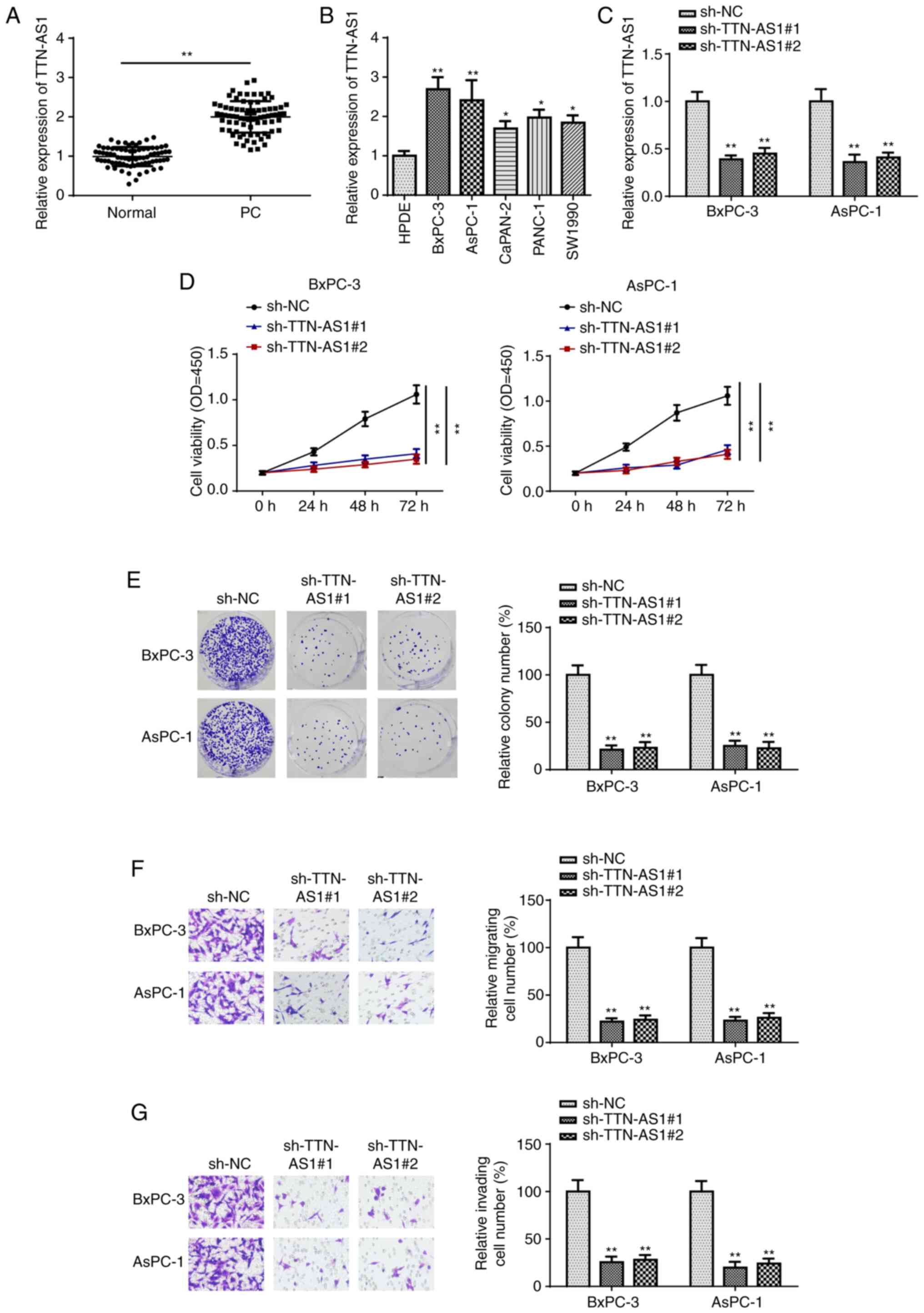
expression levels were first detected using RT-qPCR. As shown in
Fig. 1A, TTN-AS1 was found to be
significantly increased in PC tissues. Furthermore, clinical data
demonstrated that TTN-AS1 expression was associated with TNM stage
and lymph node metastasis, while there was no significant
association between TTN-AS1 mRNA expression level and age or sex
(Table I). Then, TTN-AS1 mRNA
expression level was analyzed in the PC and HPDE cell lines, the
latter was used as the NC. The results of RT-qPCR indicated that
the PC cell lines exhibited higher TTN-AS1 mRNA expression levels
compared with that in the HPDE cell line (Fig. 1B). Furthermore, the BxPC-3 and AsPC-1
cell lines exhibited the highest TTN-AS1 mRNA expression level;
thus, these two cell lines were selected for further experiments.
Subsequently, TTN-AS1 expression was knocked down using shRNA in
the BxPC-3 and AsPC-1 cell lines, and the transfection efficiency
was confirmed using RT-qPCR (Fig.
1C). CCK-8 assays revealed that knock down of TTN-AS1
significantly suppressed the viability of both the BxPC-3 and
AsPC-1 cell lines (Fig. 1D). In
addition, colony formation assays further verified the inhibitory
effect of TTN-AS1 knockdown on BxPC-3 and AsPC-1 cell proliferation
(Fig. 1E). Lastly, Transwell and
Matrigel assays demonstrated that TTN-AS1 knockdown reduced the
migration and invasion abilities of the BxPC-3 and AsPC-1 cell
lines (Fig. 1F and G, respectively).
The aforementioned findings indicated that TTN-AS1 may act as an
oncogene in PC.
 | Table I.Association between TTN-AS1 and the
clinicopathological characteristics in patients with pancreatic
cancer. |
Table I.
Association between TTN-AS1 and the
clinicopathological characteristics in patients with pancreatic
cancer.
|
| TTN-AS1 expression
level |
|
|---|
|
|
|
|
|---|
| Characteristic | High | Low | P-value |
|---|
| Age, years |
|
|
|
|
≥60 | 25 | 21 | 0.621 |
|
<60 | 20 | 12 |
|
| Sex |
|
|
|
|
Male | 23 | 17 | 0.932 |
|
Female | 22 | 16 |
|
| TNM stage |
|
|
|
|
I–II | 14 | 20 | 0.021 |
|
III–IV | 31 | 13 |
|
| Lymph node
metastasis |
|
|
|
|
Negative | 13 | 27 | <0.001 |
|
Positive | 32 | 6 |
|
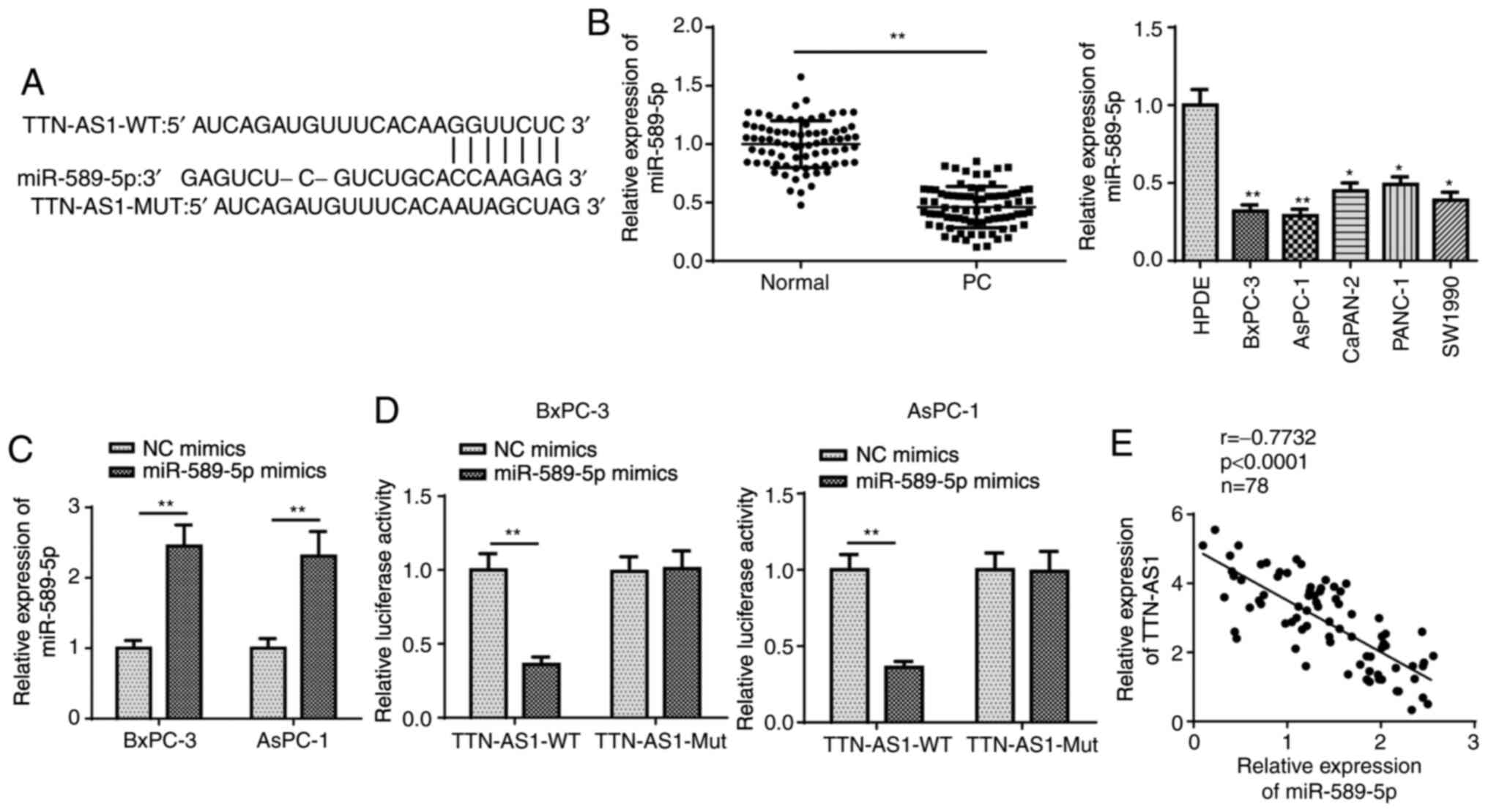
TTN-AS1 sponges miR-589-5p in PC
A number of studies have found that lncRNAs play key
roles in cancer by combining with miRNAs (26–28);
thus, it was investigated whether potential miRNAs could interact
with TTN-AS1. To predict potential miRNAs, StarBase database
(http://starbase.sysu.edu.cn/) was used.
As a result, TTN-AS1 was found to potentially combine with
miR-589-5p under strict screening conditions (Pan-Cancer, 10 cancer
types), and the binding sequence of TTN-AS1 on miR-589-5p is
illustrated in Fig. 2A.
Subsequently, miR-589-5p mRNA expression level was determined and
found to be low in PC tissues and cell lines (Fig. 2B). In further experiments, miR-589-5p
mRNA expression level was increased following transfection with
miR-589-5p mimics (Fig. 2C).
Subsequently, the plasmids containing the WT (TTN-AS1-WT) and Mut
(TTN-AS1-Mut) miR-589-5p binding site were generated and ligated
into dual-luciferase reporter vectors for luciferase activity
assay. The results revealed that the luciferase activity of
TTN-AS1-WT was inhibited by miR-589-5p overexpression, but that of
TTN-AS1-Mut was unaffected (Fig.
2D), suggesting the direct binding of TTN-AS1 to miR-589-5p.
Furthermore, Pearson's correlation analysis demonstrated the
inverse association between TTN-AS1 and miR-589-5p expression
levels in the PC tissues (Fig. 2E).
Collectively, these findings indicated that TTN-AS1 directly
interacted with miR-589-5p in PC.
FOXP1 is the downstream target of
miR-589-5p in PC
To further verify the ceRNA hypothesis, the
downstream target genes of miR-589-5p were investigated. Using
StarBase, 10 potential candidate targets were identified (Fig. 3A) and the mRNA expression level of
these genes in the miR-589-5p mimics-transfected cells was examined
using RT-qPCR. The results revealed that FOXP1 expression was lower
compared with that in the other 9 genes when miR-589-5p was
overexpressed (Fig. 3B). Thus, FOXP1
was selected for subsequent analyses. As shown in Fig. 3C, PC tissues expressed higher
expression levels of FOXP1 compared with that in adjacent normal
tissues. RT-qPCR and western blot analyses also indicated that the
mRNA and protein expression level of FOXP1 was upregulated in the
PC cell lines compared with that in the HPDE cell lines (Fig. 3D and E, respectively). Furthermore,
it was demonstrated that the luciferase activity of FOXP1-WT, but
not that of FOXP1-Mut, was significantly reduced in the miR-589-5p
mimics-transfected cells (Fig. 3F).
In addition, FOXP1 expression in the PC tissues was found to be
negatively correlated with miR-589-5p and positively correlated
with TTN-AS1 mRNA expression levels using Pearson's correlation
analysis (Fig. 3G). Taken together,
these findings indicated that miR-589-5p directly targeted FOXP1 in
PC.
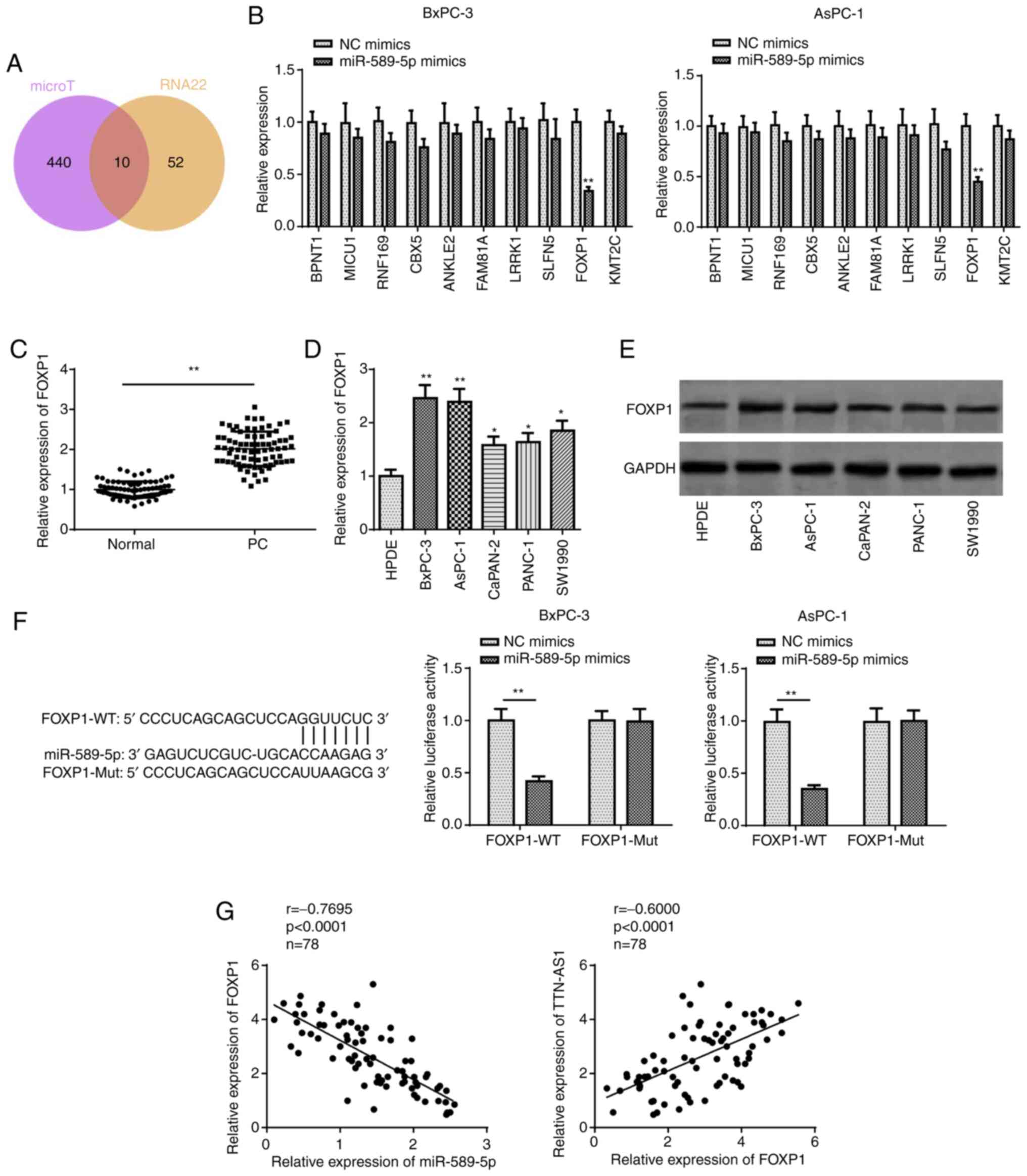 | Figure 3.FOXP1 is targeted by miR-589-5p. (A)
The potential target genes for miR-589-5p were predicted using
StarBase. (B) The mRNA expression level of the predicted targets in
the BxPC-3 and AsPC-1 cell lines transfected with miR-589-5p
mimics. (C) Reverse transcription-quantitative PCR analysis of
FOXP1 expression in PC and adjacent normal tissues. The (D) mRNA
and (E) protein expression levels of FOXP1 were measured in the PC
cell lines and the human pancreatic duct epithelial cell line. (F)
The interaction between miR-589-5p and FOXP1 was confirmed using a
luciferase reporter assay. (G) Pearson correlation analysis
revealed the correlation between FOXP1 and miR-589-5p, and with
TTN-AS1. *P<0.05, **P<0.01, PC or experiment groups vs.
control group. miR, microRNA; NC, negative control; PC, pancreatic
cancer; TTN-AS1, titin antisense RNA 1; WT, wild-type; Mut, mutant;
FOXP1, forkhead box protein 1. |
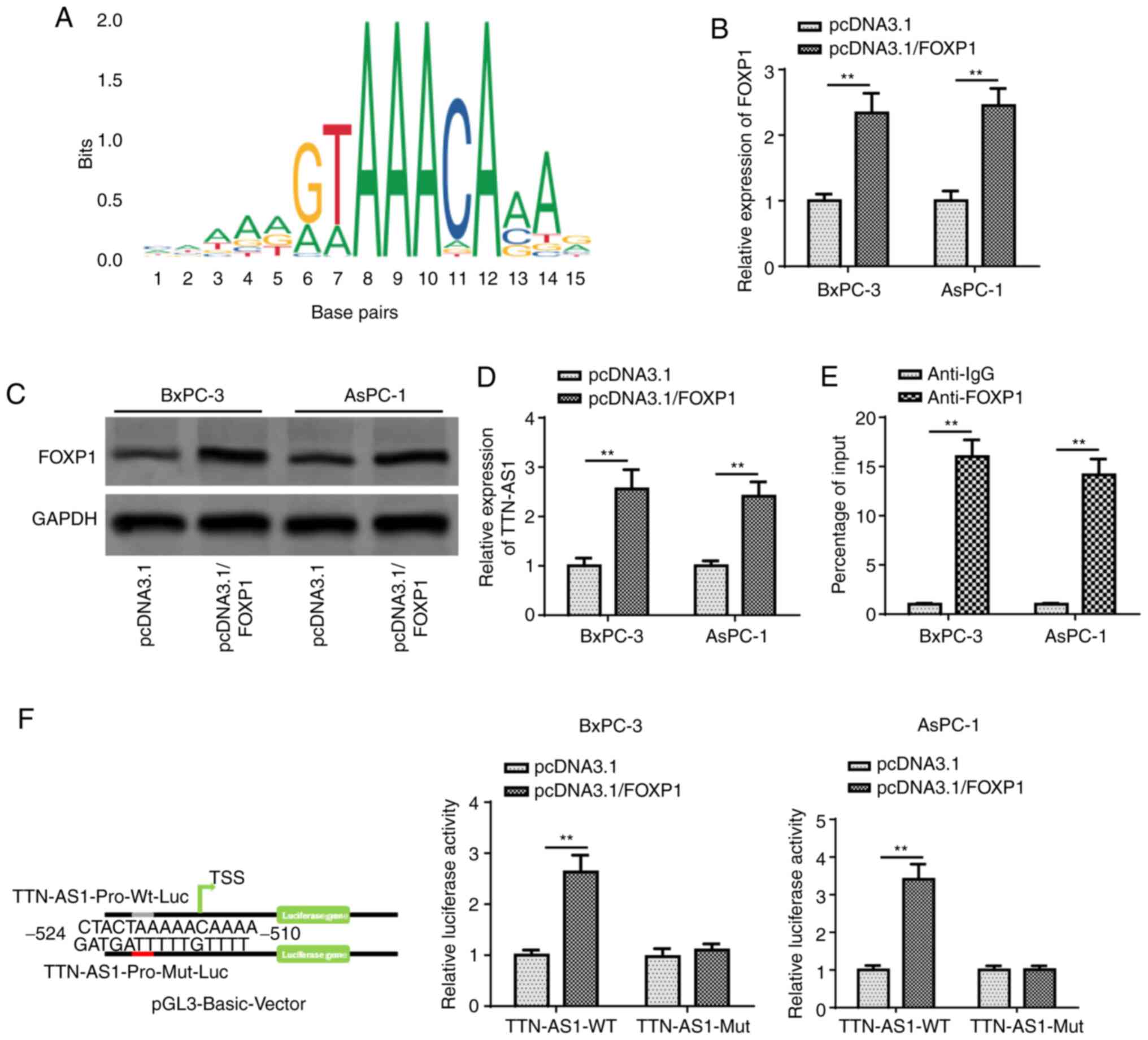
TTN-AS1 is transcriptionally activated
by FOXP1
According to previous reports, FOXP1 may act as a
transcription factor and promote the transcription of lncRNAs
(29,30). However, to the best of our knowledge,
whether FOXP1 can transcriptionally activate the expression of
TTN-AS1 has not been investigated to date. Using the University of
California, Santa Cruz Genome Browser (http://genome.ucsc.edu/), FOXP1 was found to act as a
potential transcription factor by binding to the TTN-AS1 promoter,
and its DNA motif was obtained from the JASPAR database (http://jaspar.genereg.net) (Fig. 4A). Then, the pcDNA3.1/FOXP1 plasmid
was transfected into the BxPC-3 and AsPC-1 cell lines to increase
FOXP1 expression (Fig. 4B and C).
Subsequently, TTN-AS1 mRNA expression level was found to be
significantly increased by pcDNA3.1/FOXP1 transfection (Fig. 4D). ChIP assay revealed the direct
interaction between FOXP1 and the TTN-AS1 promoter (Fig. 4E). Subsequently, the WT and Mut
binding sites between FOXP1 and TTN-AS1 promoter were obtained, and
a luciferase reporter assay revealed that FOXP1 overexpression
increased the luciferase activity of the WT TTN-AS1 promoter
reporter construct, while no notable changes were observed with the
Mut TTN-AS1 promoter reporter (Fig.
4F). All these data indicated that FOXP1 directly binds to the
TTN-AS1 promoter.
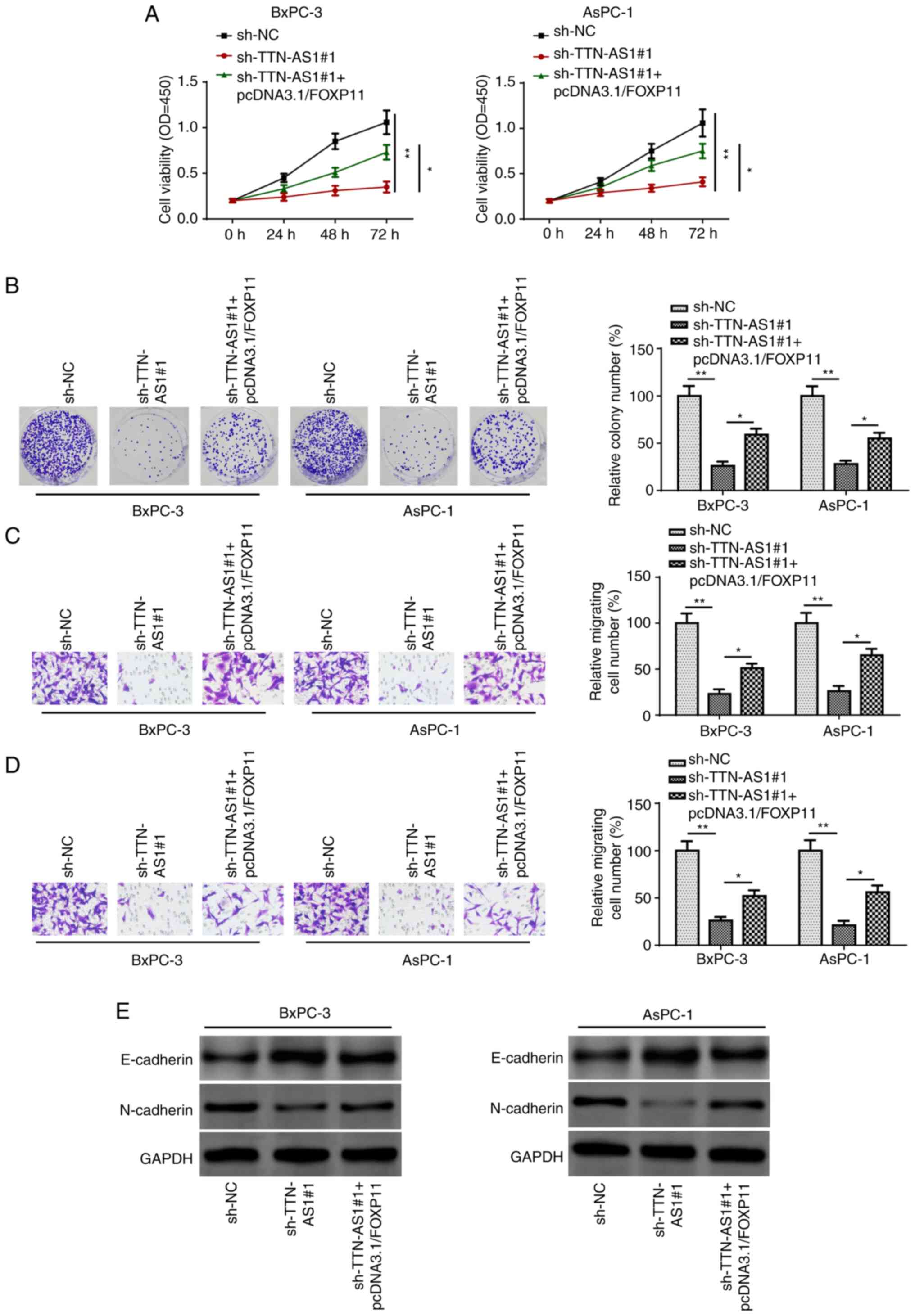
TTN-AS1 is associated with PC cell
line migration and invasion by upregulating FOXP1
To verify whether TTN-AS1 promoted PC progression
via FOXP1, rescue experiments were performed. Based on the results
of the CCK-8 assay, FOXP1 upregulation counteracted the inhibitory
effect of TTN-AS1 knockdown in PC cell viability (Fig. 5A). The results of the colony
formation assay suggested that the suppressed proliferative ability
in TTN-AS1 knockdown cells was restored by increasing the
expression level of FOXP1 (Fig. 5B).
Furthermore, TTN-AS1 knockdown inhibited the migration and invasion
of the BxPC-3 and AsPC-1 cells, while the overexpression of FOXP1
recovered this effect (Fig. 5C and
D). In addition, the results of western blot analysis
demonstrated that TTN-AS1 knockdown notably increased and decreased
E-cadherin and N-cadherin protein expression levels, while FOXP1
overexpression partially reversed this effect (Fig. 5E). In conclusion, TTN-AS1 upregulated
FOXP1 to facilitate PC cell line migration and invasion.
Discussion
PC is an aggressive malignancy, and its development
and progression are intricate processes involving the accumulation
of epigenetic or genetic variations. Further elucidating the
mechanisms underlying PC tumorigenesis is crucial for decreasing
the PC-associated mortality rate (31,32).
Extensive evidence has indicated the important role of lncRNAs in
cancer progression (33–35). Thus, the regulatory mechanisms
underlying the roles of lncRNAs in mediating malignant or abnormal
biological behavior must be further investigated. Various lncRNAs
have been associated with PC (36,37);
however, to the best of our knowledge, the detailed role and
mechanism of TTN-AS1 in PC has not been elucidated. Previously,
TTN-AS1 was confirmed to facilitate cervical cancer growth and
metastasis via the miR-573/E2F3 axis (20). Furthermore, TTN-AS1 was found to act
as a tumor promoter in papillary thyroid cancer by enhancing cell
proliferation and migration via the miR-153-3p/ZNRF2 axis (19). In addition, TTN-AS1 was also reported
to upregulate KLF12 and accelerate gastric cancer progression by
sponging miR-376b-3p (18). In the
present study, the lncRNA TTN-AS1 was found to be upregulated in PC
tissues and cell lines, whereas TTN-AS1 knockdown significantly
reduced the proliferation, migration and invasion abilities of the
PC cell lines. Collectively, these findings support the oncogenic
properties of TTN-AS1 in PC.
It was previously revealed that the majority of the
genome is transcribed as non-coding RNAs, including lncRNAs and
miRNAs (38). miRNAs, which are
21–24 nucleotides in length, are single-stranded RNAs that can
target mRNA 3′-UTRs to trigger translation inhibition or
degradation (39). The functional
role of miRNAs in cancer has also been widely reported. For
example, miRNA-129-5p was shown to inhibit lymph node metastasis
and lymphangiogenesis in nasopharyngeal carcinoma cell lines
(40). miR-127-3p and miR-376a-3p
exerted suppressive effects on cell proliferation in osteosarcoma
cell lines (41). Of note, multiple
miRNAs were found to be decreased and play biologically significant
roles in PC, such as miR-15a (42),
miR-3924 (15) and miR-30a-3p
(43). Interacting with miRNAs to
indirectly modulate target gene expression is a common mechanism of
action of lncRNAs (44). miR-589-5p
was previously demonstrated to serve as a tumor inhibitor in
endometrial carcinoma cell lines (45), and was associated with the ceRNA
mechanism in hepatocellular carcinoma cell lines (46). In the present study, miR-589-5p was
found to be sponged by TTN-AS1 in PC cell lines, and there was an
inverse correlation between TTN-AS1 and miR-589-5p mRNA expression
level.
FOXP1 has been associated with B-cell survival and
differentiation (47,48). It was also found to have a positive
association with the mRNA expression level of BCL2 and to prevent
cell apoptosis (49,50). Notably, FOXP1 was found to act as a
transcription factor to activate the transcription of lncRNAs,
thereby increasing their expression (30,51).
Based on the results of the present study, FOXP1 was shown to
combine with miR-589-5p, and its mRNA expression level was
inversely correlated with miR-589-5p and directly correlated with
TTN-AS1 mRNA expression level. Furthermore, FOXP1 was confirmed to
interact with the TTN-AS1 promoter and upregulate TTN-AS1 mRNA
expression level.
To the best of our knowledge, the present study was
the first to examine the function of TTN-AS1 in PC cell lines and
investigate the underlying mechanism. The results demonstrated that
FOXP1-mediated upregulation of TTN-AS1, promoted PC progression by
sponging miR-589-5p and targeting FOXP1, uncovering the presence of
a TTN-AS1/miR-589-5p/FOXP1 feedback loop in PC cell lines. These
findings may prove to be of value in the research of PC treatment.
However, the lack of in vivo nude mouse tumor formation
experiments constitutes a limitation of the present study.
Therefore, further in vivo experiments will be conducted to
verify the regulatory role of the TTN-AS1/miR-589-5p/FOXP1 feedback
loop in PC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JZ and JY designed the present study. JZ, FW and JY
performed the experiments, analyzed the data and prepared the
figures. JZ and JY drafted the initial manuscript. JZ and JY
confirmed the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Liyang People's Hospital (Liyang, China) and written
informed consent was provided by all patients prior to the study
start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Abel EV and Simeone DM: Biology and
clinical applications of pancreatic cancer stem cells.
Gastroenterology. 144:1241–1248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McGuire S: World cancer report 2014.
Geneva, Switzerland: World health organization, international
agency for research on cancer, WHO press, 2015. Adv Nutr.
7:418–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ansari D, Tingstedt B, Andersson B,
Holmquist F, Sturesson C, Williamsson C, Sasor A, Borg D, Bauden M
and Andersson R: Pancreatic cancer: Yesterday, today and tomorrow.
Future Oncol. 12:1929–1946. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shin SJ, Park H, Sung YN, Yoo C, Hwang DW,
Park JH, Kim KP, Lee SS, Ryoo BY, Seo DW, et al: Prognosis of
pancreatic cancer patients with synchronous or metachronous
malignancies from other organs is better than those with pancreatic
cancer only. Cancer Res Treat. 50:1175–1185. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han Q, Li J, Xiong J and Song Z: Long
noncoding RNA LINC00514 accelerates pancreatic cancer progression
by acting as a ceRNA of miR-28-5p to upregulate Rap1b expression. J
Exp Clin Cancer Res. 39:1512020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Halbrook CJ and Lyssiotis CA: Employing
metabolism to improve the diagnosis and treatment of pancreatic
cancer. Cancer Cell. 31:5–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beermann J, Piccoli MT, Viereck J and Thum
T: Non-coding RNAs in development and disease: Background,
mechanisms, and therapeutic approaches. Physiol Rev. 96:1297–1325.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei JW, Huang K, Yang C and Kang CS:
Non-coding RNAs as regulators in epigenetics (Review). Oncol Rep.
37:3–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dykes IM and Emanueli C: Transcriptional
and post-transcriptional gene regulation by long non-coding RNA.
Genomics Proteomics Bioinformatics. 15:177–186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsai MC, Manor O, Wan Y, Mosammaparast N,
Wang JK, Lan F, Shi Y, Segal E and Chang HY: Long noncoding RNA as
modular scaffold of histone modification complexes. Science.
329:689–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cao Y, Xiong JB, Zhang GY, Liu Y, Jie ZG
and Li RZ: Long noncoding RNA UCA1 regulates PRL-3 expression by
sponging microRNA-495 to promote the progression of gastric cancer.
Mol Ther Nucleic Acids. 19:853–864. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luan Y, Li X, Luan Y, Zhao R, Li Y, Liu L,
Hao Y, Vladimir BO and Jia L: Circulating lncRNA UCA1 promotes
malignancy of colorectal cancer via the miR-143/MYO6 axis. Mol Ther
Nucleic Acids. 19:790–803. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Malik SS, Zia A, Mubarik S, Masood N,
Rashid S, Sherrard A, Khan MB and Khadim MT: Correlation of MLH1
polymorphisms, survival statistics, in silico assessment and gene
downregulation with clinical outcomes among breast cancer cases.
Mol Biol Rep. 47:683–692. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The rosetta stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan S, Shen M, Zhou M, Shi X, He R, Yin T,
Wang M, Guo X and Qin R: Long noncoding RNA LINC01111 suppresses
pancreatic cancer aggressiveness by regulating DUSP1 expression via
microRNA-3924. Cell Death Dis. 10:8832019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lei S, He Z, Chen T, Guo X, Zeng Z, Shen Y
and Jiang J: Long noncoding RNA 00976 promotes pancreatic cancer
progression through OTUD7B by sponging miR-137 involving EGFR/MAPK
pathway. J Exp Clin Cancer Res. 38:4702019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun Y, Zhu Q, Yang W, Shan Y, Yu Z, Zhang
Q and Wu H: LncRNA H19/miR-194/PFTK1 axis modulates the cell
proliferation and migration of pancreatic cancer. J Cell Biochem.
120:3874–3886. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dong MM, Peng SJ, Yuan YN and Luo HP:
LncRNA TTN-AS1 contributes to gastric cancer progression by acting
as a competing endogenous RNA of miR-376b-3p. Neoplasma.
66:564–575. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cui Z, Luo Z, Lin Z, Shi L, Hong Y and Yan
C: Long non-coding RNA TTN-AS1 facilitates tumorigenesis of
papillary thyroid cancer through modulating the miR-153-3p/ZNRF2
axis. J Gene Med. 21:e30832019. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen P, Wang R, Yue Q and Hao M: Long
non-coding RNA TTN-AS1 promotes cell growth and metastasis in
cervical cancer via miR-573/E2F3. Biochem Biophys Res Commun.
503:2956–2962. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou Y, Huang Y, Dai T, Hua Z, Xu J, Lin
Y, Han L, Yue X, Ho L, Lu J and Ai X: LncRNA TTN-AS1 intensifies
sorafenib resistance in hepatocellular carcinoma by sponging
miR-16-5p and upregulation of cyclin E1. Biomed Pharmacother.
133:1110302021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jia Y, Duan Y, Liu T, Wang X, Lv W, Wang
M, Wang J and Liu L: LncRNA TTN-AS1 promotes migration, invasion,
and epithelial mesenchymal transition of lung adenocarcinoma via
sponging miR-142-5p to regulate CDK5. Cell Death Dis. 10:5732019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu SW, Zhang Y, Li S, Shi ZY, Zhao J and
He QL: LncRNA TTN-AS1 promotes the progression of oral squamous
cell carcinoma via miR-411-3p/NFAT5 axis. Cancer Cell Int.
20:4152020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feng H, Wang Q, Xiao W, Zhang B, Jin Y and
Lu H: LncRNA TTN-AS1 regulates miR-524-5p and RRM2 to promote
breast cancer progression. Onco Targets Ther. 13:4799–4811. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang W, Lou W, Ding B, Yang B, Lu H, Kong
Q and Fan W: A novel mRNA-miRNA-lncRNA competing endogenous RNA
triple sub-network associated with prognosis of pancreatic cancer.
Aging (Albany NY). 11:2610–2627. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chan JJ and Tay Y: Noncoding RNA:RNA
regulatory networks in cancer. Int J Mol Sci. 19:13102018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lai XN, Li J, Tang LB, Chen WT, Zhang L
and Xiong LX: MiRNAs and LncRNAs: Dual roles in TGF-β
signaling-regulated metastasis in lung cancer. Int J Mol Sci.
21:11932020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang C, Tan C, Wen Y, Zhang D, Li G, Chang
L, Su J and Wang X: FOXP1-induced lncRNA CLRN1-AS1 acts as a tumor
suppressor in pituitary prolactinoma by repressing the autophagy
via inactivating Wnt/β-catenin signaling pathway. Cell Death Dis.
10:4992019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gong T, Li Y, Feng L, Fang MZ, Dai G,
Huang X, Yang Y and Liu S: CASC21, a FOXP1 induced long non-coding
RNA, promotes colorectal cancer growth by regulating CDK6. Aging
(Albany NY). 12:12086–12106. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan J, Jia Y, Chen H, Chen W and Zhou X:
Long non-coding RNA PXN-AS1 suppresses pancreatic cancer
progression by acting as a competing endogenous RNA of miR-3064 to
upregulate PIP4K2B expression. J Exp Clin Cancer Res. 38:3902019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yao GW, Bai JR and Zhang DP: P21 activated
kinase 2 promotes pancreatic cancer growth and metastasis. Oncol
Lett. 17:3709–3718. 2019.PubMed/NCBI
|
|
33
|
Dugimont T, Curgy JJ, Wernert N, Delobelle
A, Raes MB, Joubel A, Stehelin D and Coll J: The H19 gene is
expressed within both epithelial and stromal components of human
invasive adenocarcinomas. Biol Cell. 85:117–124. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Verkerk AJ, Ariel I, Dekker MC, Schneider
T, van Gurp RJ, de Groot N, Gillis AJ, Oosterhuis JW, Hochberg AA
and Looijenga LH: Unique expression patterns of H19 in human
testicular cancers of different etiology. Oncogene. 14:95–107.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ji P, Diederichs S, Wang W, Böing S,
Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, et
al: MALAT-1, a novel noncoding RNA, and thymosin beta4 predict
metastasis and survival in early-stage non-small cell lung cancer.
Oncogene. 22:8031–8041. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tahira AC, Kubrusly MS, Faria MF, Dazzani
B, Fonseca RS, Maracaja-Coutinho V, Verjovski-Almeida S, Machado
MCC and Reis EM: Long noncoding intronic RNAs are differentially
expressed in primary and metastatic pancreatic cancer. Mol Cancer.
10:1412011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim K, Jutooru I, Chadalapaka G, Johnson
G, Frank J, Burghardt R, Kim S and Safe S: HOTAIR is a negative
prognostic factor and exhibits pro-oncogenic activity in pancreatic
cancer. Oncogene. 32:1616–1625. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guttman M, Amit I, Garber M, French C, Lin
MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, et al:
Chromatin signature reveals over a thousand highly conserved large
non-coding RNAs in mammals. Nature. 458:223–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vasudevan S, Tong Y and Steitz JA:
Switching from repression to activation: MicroRNAs can up-regulate
translation. Science. 318:1931–1934. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu D, Han GH, Zhao X, Liu X, Xue K, Wang D
and Xu CB: MicroRNA-129-5p suppresses nasopharyngeal carcinoma
lymphangiogenesis and lymph node metastasis by targeting ZIC2. Cell
Oncol (Dordr). 43:249–261. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fellenberg J, Lehner B, Saehr H, Schenker
A and Kunz P: Tumor suppressor function of miR-127-3p and
miR-376a-3p in osteosarcoma cells. Cancers (Basel). 11:20192019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guo S, Fesler A, Huang W, Wang Y, Yang J,
Wang X, Zheng Y, Hwang GR, Wang H and Ju J: Functional significance
and therapeutic potential of miR-15a mimic in pancreatic ductal
adenocarcinoma. Mol Ther Nucleic Acids. 19:228–239. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Georgikou C, Yin L, Gladkich J, Xiao X,
Sticht C, de la Torre C, Gretz N, Gross W, Schäfer M, Karakhanova S
and Herr I: Inhibition of miR30a-3p by sulforaphane enhances gap
junction intercellular communication in pancreatic cancer. Cancer
Lett. 469:238–245. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cesana M, Cacchiarelli D, Legnini I,
Santini T, Sthandier O, Chinappi M, Tramontano A and Bozzoni I: A
long noncoding RNA controls muscle differentiation by functioning
as a competing endogenous RNA. Cell. 147:358–369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang Y, Dong L and Liu Y: Targeting
thyroid receptor interacting protein 6 by MicroRNA-589-5p inhibits
cell proliferation, migration, and invasion in endometrial
carcinoma. Cancer Biother Radiopharm. 34:529–536. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu Q, Luo Z, Lu G, Gui F, Wu J, Li F and
Ni Y: LncRNA FABP5P3/miR-589-5p/ZMYND19 axis contributes to
hepatocellular carcinoma cell proliferation, migration and
invasion. Biochem Biophys Res Commun. 498:551–558. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hu H, Wang B, Borde M, Nardone J, Maika S,
Allred L, Tucker PW and Rao A: Foxp1 is an essential
transcriptional regulator of B cell development. Nat Immunol.
7:819–826. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
48
|
Craig VJ, Cogliatti SB, Imig J, Renner C,
Neuenschwander S, Rehrauer H, Schlapbach R, Dirnhofer S, Tzankov A
and Müller A: Myc-mediated repression of microRNA-34a promotes
high-grade transformation of B-cell lymphoma by dysregulation of
FoxP1. Blood. 117:6227–6236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yu B, Zhou X, Li B, Xiao X, Yan S and Shi
D: FOXP1 expression and its clinicopathologic significance in nodal
and extranodal diffuse large B-cell lymphoma. Ann Hematol.
90:701–708. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sagaert X, de Paepe P, Libbrecht L,
Vanhentenrijk V, Verhoef G, Thomas J, Wlodarska I and De
Wolf-Peeters C: Forkhead box protein P1 expression in
mucosa-associated lymphoid tissue lymphomas predicts poor prognosis
and transformation to diffuse large B-cell lymphoma. J Clin Oncol.
24:2490–2497. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wu M, Huang Y, Chen T, Wang W, Yang S, Ye
Z and Xi X: LncRNA MEG3 inhibits the progression of prostate cancer
by modulating miR-9-5p/QKI-5 axis. J Cell Mol Med. 23:29–38. 2019.
View Article : Google Scholar : PubMed/NCBI
|